SERS-Based Local Field Enhancement in Biosensing Applications
Abstract
1. Introduction

2. Nanostructures (Colloids) in SERS Sensors
2.1. Single Crystalline Nanoparticles
2.1.1. Nanorods and Nanowires
2.1.2. Nano Spikes
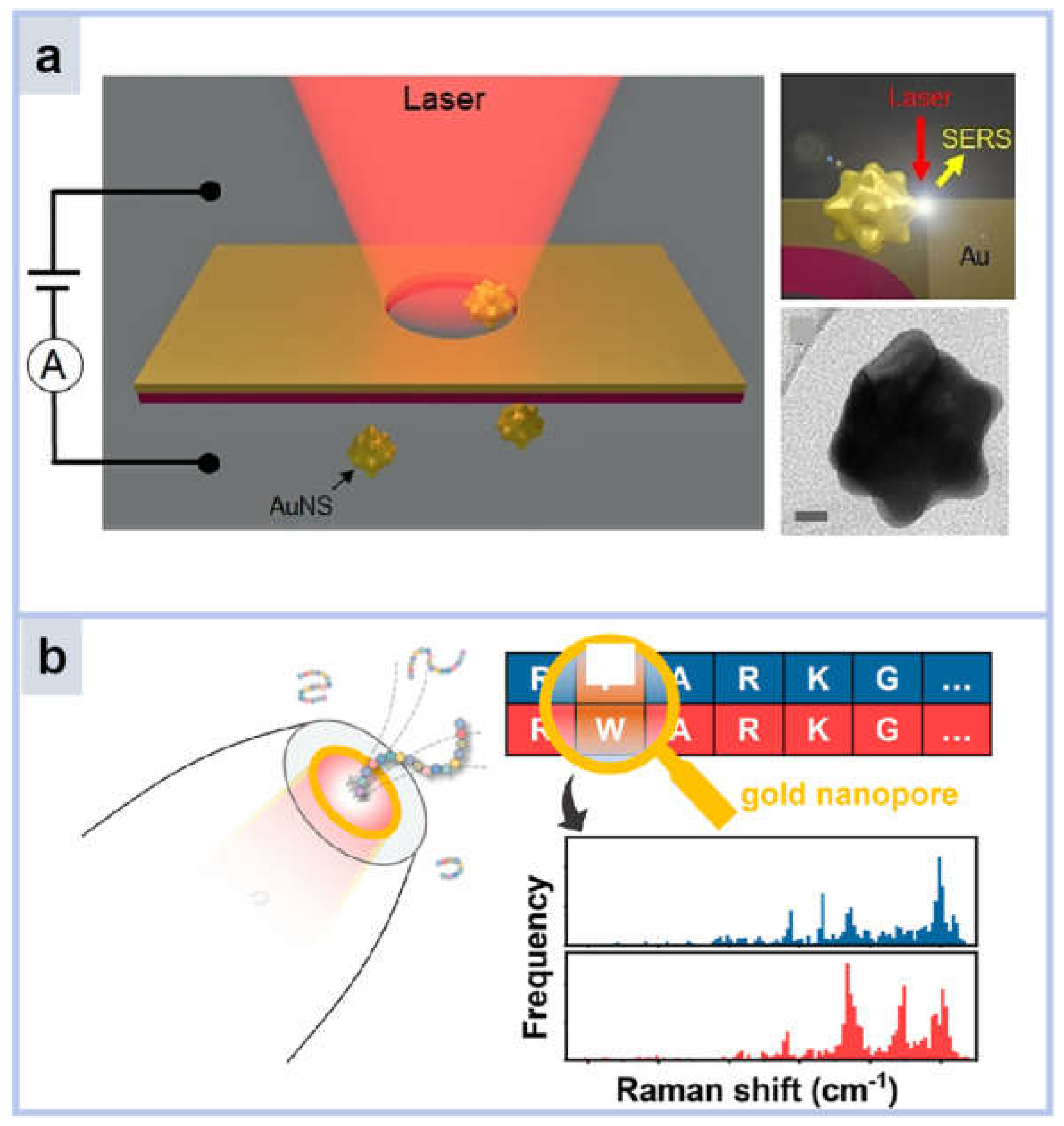
2.1.3. Nanopores
2.2. Core–Shell Nanoparticles
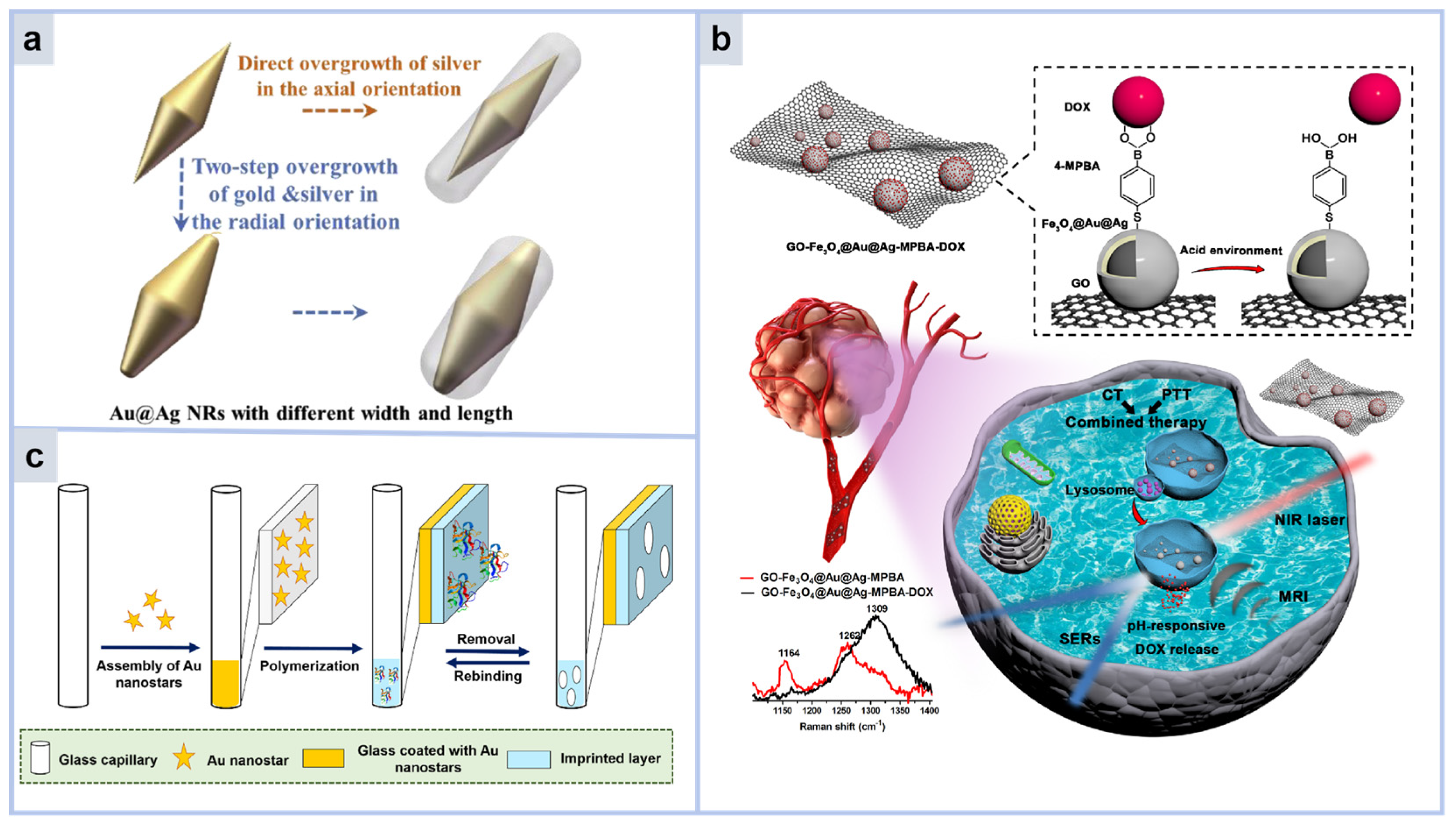
2.2.1. SERS Sensor with Metal Core–Shell Structure
2.2.2. SERS Sensor with Non-Metallic Core–Shell Structure
2.3. SERS Sensor with MOF Structure
3. The Substrate in the SERS Sensor
3.1. Self-Assembly of Nanoparticles
3.2. 2D Substrate Materials
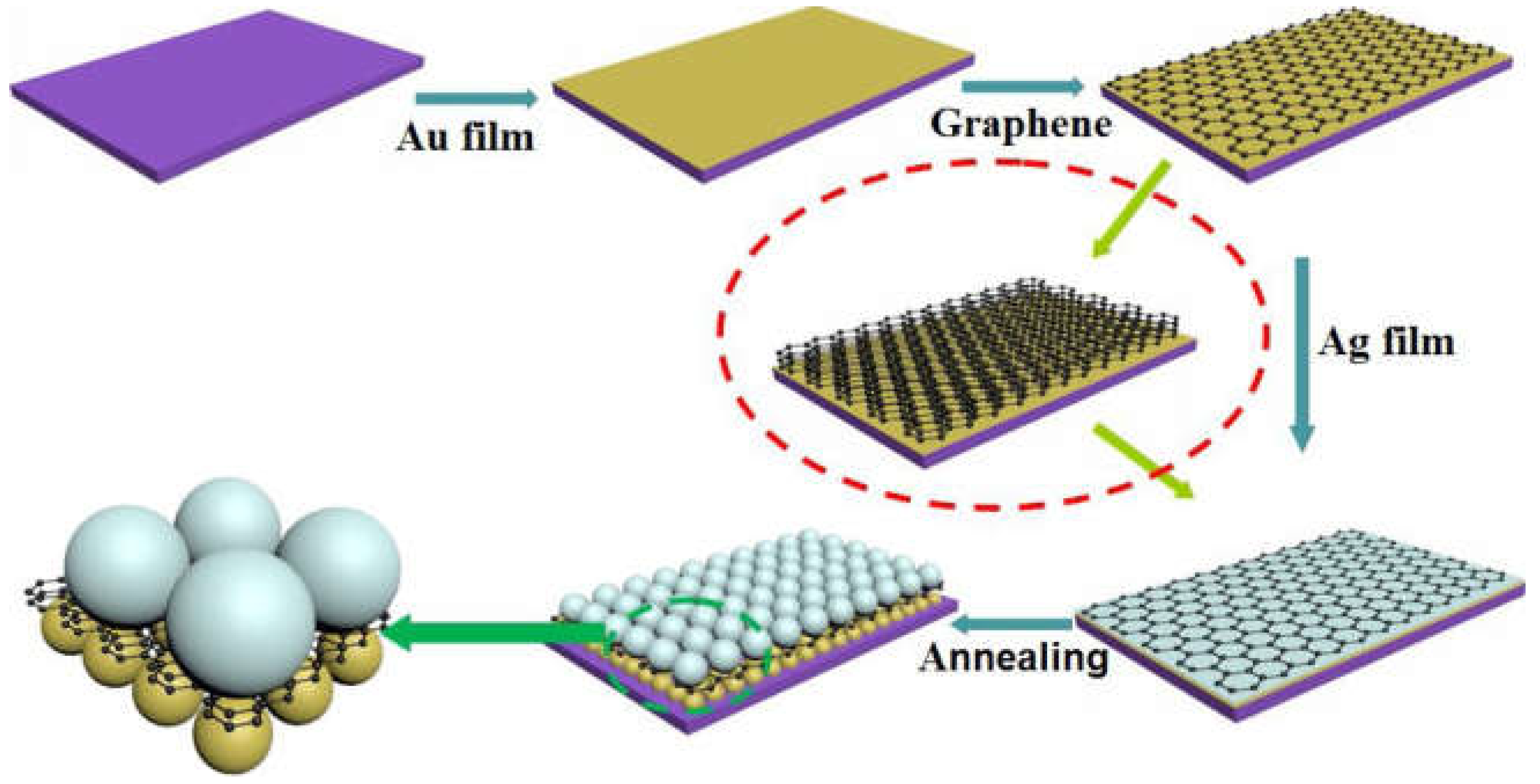
3.2.1. Graphene, h-BN, g-C3N4
3.2.2. Black Scales (BP)
3.2.3. MXenes
3.3. 3D Substrate Materials
3.3.1. Three-Dimensional Nanocavities
3.3.2. 3D Multilayer Substrate
3.3.3. 3D Microporous Substrates
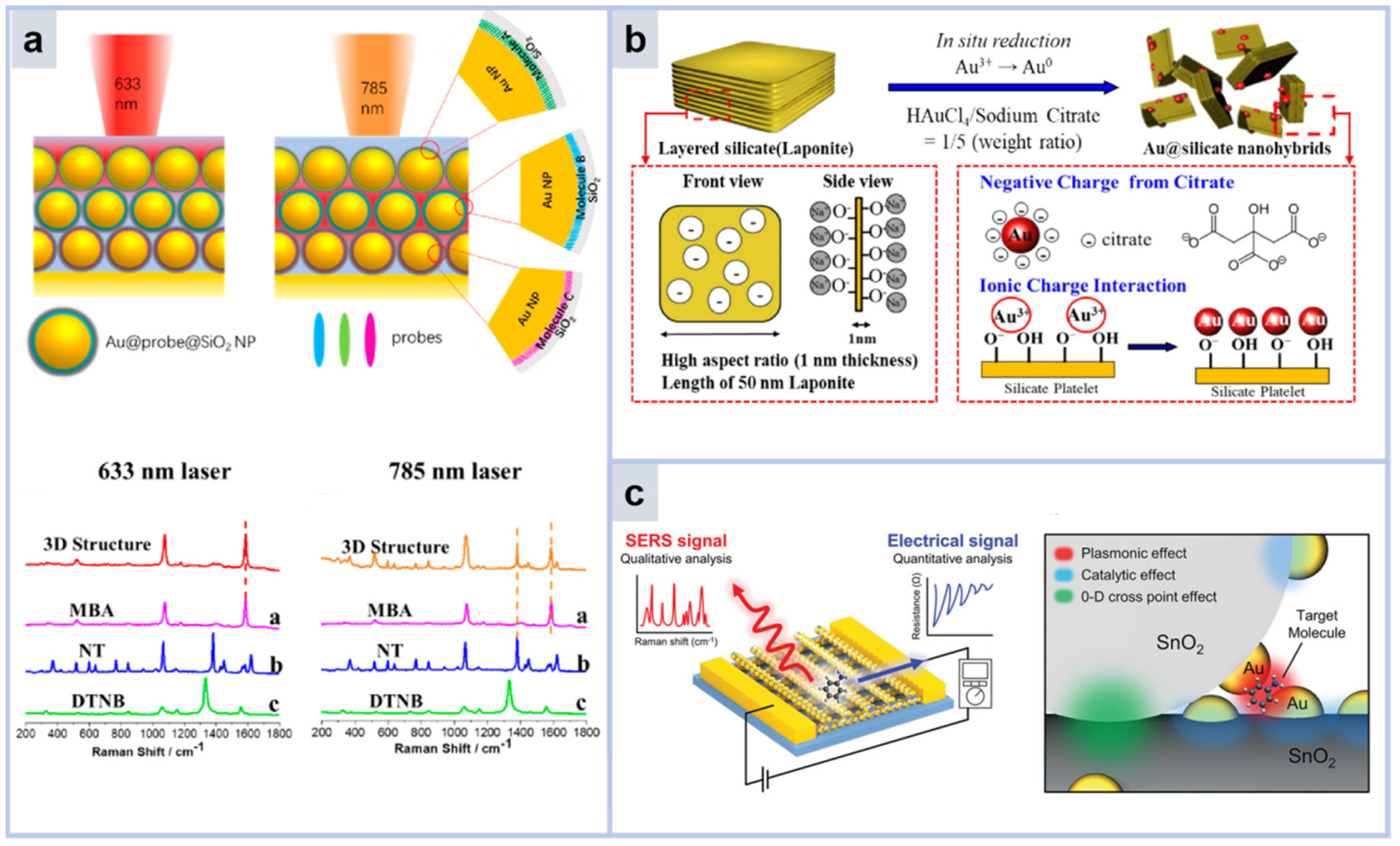
3.3.4. 3D Array Substrate
3.4. Optical Layer, Dielectric Layer Regulation Substrate
3.4.1. Photonic Crystal Substrate
3.4.2. Dielectric Layer
3.4.3. Bragg Mirror
4. Tip Enhanced Raman Spectroscopy (TERS)
4.1. Single-Molecule Detection
4.2. Chemical Reaction Detection and Imaging Detection
4.3. Biomolecular Analysis
5. Conclusions and Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Su, X.-Y.; Ma, Y.-L.; Zhai, C.; Li, Y.-L.; Ma, Q.-Y.; Sun, J.-F.; Wang, W.-X. Research Progress of Surface Enhanced Raman Spectroscopy in Quality and Safety Detection of Liquid Food. Spectrosc. Spectr. Anal. 2023, 43, 2657–2666. [Google Scholar]
- Bantz, K.C.; Meyer, A.F.; Wittenberg, N.J.; Im, H.; Kurtulus, O.; Lee, S.H.; Lindquist, N.C.; Oh, S.-H.; Haynes, C.L. Recent progress in SERS biosensing. Phys. Chem. Chem. Phys. 2011, 13, 11551–11567. [Google Scholar] [CrossRef] [PubMed]
- Fu, C.; Liang, L.; Qi, G.; Xu, S.; Xu, W. Biomolecule-assisted Surface-enhanced Raman Scattering (SERS) Technology and SERS Biosensing. Chem. J. Chin. Univ.-Chin. 2015, 36, 2134–2147. [Google Scholar] [CrossRef]
- Jones, R.R.; Hooper, D.C.; Zhang, L.; Wolverson, D.; Valev, V.K. Raman Techniques: Fundamentals and Frontiers. Nanoscale Res. Lett. 2019, 14, 231. [Google Scholar] [CrossRef]
- Hess, C. New advances in using Raman spectroscopy for the characterization of catalysts and catalytic reactions. Chem. Soc. Rev. 2021, 50, 3519–3564. [Google Scholar] [CrossRef]
- Fleischmann, M.; Hendra, P.J.; McQuillan, A.J. Raman spectra of pyridine adsorbed at a silver electrode. Chem. Phys. Lett. 1974, 26, 163–166. [Google Scholar] [CrossRef]
- Liu, H.; He, Y.; Cao, K. Flexible Surface-Enhanced Raman Scattering Substrates: A Review on Constructions, Applications, and Challenges. Adv. Mater. Interfaces 2021, 8, 2100982. [Google Scholar] [CrossRef]
- Song, G.; Gong, W.B.; Cong, S.; Zhao, Z.G. Ultrathin Two-Dimensional Nanostructures: Surface Defects for Morphology-Driven Enhanced Semiconductor SERS. Angew. Chem.-Int. Ed. 2021, 60, 5505–5511. [Google Scholar] [CrossRef]
- Mosier-Boss, P.A. Review of SERS Substrates for Chemical Sensing. Nanomaterials 2017, 7, 142. [Google Scholar] [CrossRef]
- Li, Y.; Sun, J.; Wu, L.; Ji, J.; Sun, X.; Qian, Y. Surface-enhanced fluorescence immunosensor using Au nano-crosses for the detection of microcystin-LR. Biosens. Bioelectron. 2014, 62, 255–260. [Google Scholar] [CrossRef]
- Willets, K.A.; Van Duyne, R.P. Localized surface plasmon resonance spectroscopy and sensing. Annu. Rev. Phys. Chem. 2007, 58, 267–297. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.X.; Bjerneld, E.J.; Käll, M.; Börjesson, L. Spectroscopy of single hemoglobin molecules by surface enhanced Raman scattering. Phys. Rev. Lett. 1999, 83, 4357–4360. [Google Scholar] [CrossRef]
- Fang, Y.; Seong, N.H.; Dlott, D.D. Measurement of the distribution of site enhancements in surface-enhanced Raman scattering. Science 2008, 321, 388–392. [Google Scholar] [CrossRef]
- Feng, S.-L.; Wang, J.-Y.; Chen, S.; Meng, L.-Y.; Shen, S.-X.; Yang, Z.-L. Surface plasmon resonance “hot spots” and near-field enhanced spectroscopy at interfaces. Acta Phys. Sin. 2019, 68, 147801–147815. [Google Scholar] [CrossRef]
- Zheng, X.L.; Ye, Z.W.; Akmal, Z.; He, C.; Zhang, J.L.; Wang, L.Z. Recent progress in SERS monitoring of photocatalytic reactions. Chem. Soc. Rev. 2024, 53, 656–683. [Google Scholar] [CrossRef]
- Li, J.F.; Huang, Y.F.; Ding, Y.; Yang, Z.L.; Li, S.B.; Zhou, X.S.; Fan, F.R.; Zhang, W.; Zhou, Z.Y.; Wu, D.Y.; et al. Shell-isolated nanoparticle-enhanced Raman spectroscopy. Nature 2010, 464, 392–395. [Google Scholar] [CrossRef]
- Zhao, Y. On the Measurements of the Surface-Enhanced Raman Scattering Spectrum: Effective Enhancement Factor, Optical Configuration, Spectral Distortion, and Baseline Variation. Nanomaterials 2023, 13, 2998. [Google Scholar] [CrossRef]
- Li, J.; Chen, X.; Zhu, J.-J. DNA functionalized plasmonic nanoassemblies as SERS sensors for environmental analysis. Aggregate 2023, 4, e271. [Google Scholar] [CrossRef]
- Wang, J.A.; Zhu, T.; Zhang, X.; Liu, Z.F. The SERS intensity vs. the size of Au nanoparticles. Acta Phys.-Chim. Sin. 1999, 15, 476–480. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, X.; Ma, Q.; Zhang, Q.; Bai, H.; Yi, W.; Liu, J.; Han, J.; Xi, G. A metallic molybdenum dioxide with high stability for surface enhanced Raman spectroscopy. Nat. Commun. 2017, 8, 14903. [Google Scholar] [CrossRef]
- Ding, Q.; Wang, J.; Chen, X.; Liu, H.; Li, Q.; Wang, Y.; Yang, S. Quantitative and Sensitive SERS Platform with Analyte Enrichment and Filtration Function. Nano Lett. 2020, 20, 7304–7312. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Zheng, Y.; Yin, L.; Xue, S.; Ma, L.; Zhou, R.; El-Seedi, H.R.; Zhang, Y.; Yosri, N.; Jayan, H.; et al. Flexible Au@AgNRs/MAA/PDMS-based SERS sensor coupled with intelligent algorithms for in-situ detection of thiram on apple. Sens. Actuators B-Chem. 2024, 404, 135303. [Google Scholar] [CrossRef]
- Armelles, G.; Bautista Gonzalez-Diaz, J.; Garcia-Martin, A.; Miguel Garcia-Martin, J.; Cebollada, A.; Ujue Gonzalez, M.; Acimovic, S.; Cesario, J.; Quidant, R.; Badenes, G. Localized surface plasmon resonance effects on the magneto-optical activity of continuous Au/Co/Au trilayers. Opt. Express 2008, 16, 16104–16112. [Google Scholar] [CrossRef] [PubMed]
- Nishijima, Y.; Rosa, L.; Juodkazis, S. Long-range interaction of localized surface plasmons in periodic and random patterns of Au nanoparticles. Appl. Phys. A-Mater. Sci. Process. 2014, 115, 409–414. [Google Scholar] [CrossRef]
- Albarazanchi, A.K.H.; Al-Haddad, A.; Sultan, M.F. Plasmonic Enhancement Mechanism of Template-Based Synthesized Au@TiO2 Nanodiscs. Chemnanomat 2021, 7, 27–33. [Google Scholar] [CrossRef]
- Niu, W.; Chua, Y.A.A.; Zhang, W.; Huang, H.; Lu, X. Highly Symmetric Gold Nanostars: Crystallographic Control and Surface-Enhanced Raman Scattering Property. J. Am. Chem. Soc. 2015, 137, 10460–10463. [Google Scholar] [CrossRef]
- Gao, J.; Sanchez-Purra, M.; Huang, H.; Wang, S.; Chen, Y.; Yu, X.; Luo, Q.; Hamad-Schifferli, K.; Liu, S. Synthesis of different-sized gold nanostars for Raman bioimaging and photothermal therapy in cancer nanotheranostics. Sci. China-Chem. 2017, 60, 1219–1229. [Google Scholar] [CrossRef]
- Tang, Y.; Kuzume, A.; Yamamoto, K. Structural Effect of Polyvinylpyrrolidone-stabilized Au Nanostars for SERS Application. Chem. Lett. 2021, 50, 248–251. [Google Scholar] [CrossRef]
- Guo, L.; Jackman, J.A.; Yang, H.-H.; Chen, P.; Cho, N.-J.; Kim, D.-H. Strategies for enhancing the sensitivity of plasmonic nanosensors. Nano Today 2015, 10, 213–239. [Google Scholar] [CrossRef]
- Su, X.Y.; Chen, X.Y.; Sun, C.B.; Zhao, B.; Ruan, W.D. Preparation of Au Nanoparticles with Different Morphologies and Study of Their Property as Surface Enhanced Raman Scattering Substrates. Spectrosc. Spectr. Anal. 2017, 37, 7–12. [Google Scholar]
- Wen, C.C.; Wang, L.P.; Liu, L.; Shen, X.C.; Chen, H. Surface-enhanced Raman Probes Based on Gold Nanomaterials for in vivo Diagnosis and Imaging. Chem.-Asian J. 2022, 17, e202200014. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Yang, K.; He, S.; Zhu, F.; Kang, S.; Liu, B.; Sun, C.; Pang, W.; Wang, Y. Dual-functional gold nanorods micro pattern guiding cell alignment and cellular microenvironment monitoring. J. Colloid Interface Sci. 2023, 647, 429–437. [Google Scholar] [CrossRef] [PubMed]
- Jeon, Y.; Kim, D.; Kwon, G.; Lee, K.; Oh, C.-S.; Kim, U.-J.; You, J. Detection of nanoplastics based on surface-enhanced Raman scattering with silver nanowire arrays on regenerated cellulose films. Carbohydr. Polym. 2021, 272, 118470. [Google Scholar] [CrossRef] [PubMed]
- Jiang, R.; Li, J.; Li, L.; Lu, Y.; Zhang, H.; Li, M. Engineering Near-Infrared Plasmonic Spiky Gold Nanostructures for Highly Efficient Surface-Enhanced Raman Spectroscopy-Guided Cancer Hyperthermia Therapy. Adv. Funct. Mater. 2024, 34, 2307631. [Google Scholar] [CrossRef]
- Pang, Y.; Jin, M. Self-Assembly of Silver Nanowire Films for Surface-Enhanced Raman Scattering Applications. Nanomaterials 2023, 13, 1358. [Google Scholar] [CrossRef]
- MacKenzie, M.; Argyropoulos, C. An Introduction to Nanopore Sequencing: Past, Present, and Future Considerations. Micromachines 2023, 14, 459. [Google Scholar] [CrossRef]
- Huang, J.A.; Mousavi, M.Z.; Giovannini, G.; Zhao, Y.Q.; Hubarevich, A.; Soler, M.A.; Rocchia, W.; Garoli, D.; De Angelis, F. Multiplexed Discrimination of Single Amino Acid Residues in Polypeptides in a Single SERS Hot Spot. Angew. Chem.-Int. Ed. 2020, 59, 11423–11431. [Google Scholar] [CrossRef]
- Ma, L.; Li, J.; Zou, S.; Zhang, Z. Ag Nanorods-Oxide Hybrid Array Substrates: Synthesis, Characterization, and Applications in Surface-Enhanced Raman Scattering. Sensors 2017, 17, 1895. [Google Scholar] [CrossRef]
- Li, J.-F.; Zhang, Y.-J.; Ding, S.-Y.; Panneerselvam, R.; Tian, Z.-Q. Core-Shell Nanoparticle-Enhanced Raman Spectroscopy. Chem. Rev. 2017, 117, 5002–5069. [Google Scholar] [CrossRef]
- Chen, J.; Wu, Y.; Fu, C.; Cao, H.; Tan, X.; Shi, W.; Wu, Z. Ratiometric SERS biosensor for sensitive and reproducible detection of microRNA based on mismatched catalytic hairpin assembly. Biosens. Bioelectron. 2019, 143, 111619. [Google Scholar] [CrossRef]
- Song, C.; Zhang, J.; Jiang, X.; Gan, H.; Zhu, Y.; Peng, Q.; Fang, X.; Guo, Y.; Wang, L. SPR/SERS dual-mode plasmonic biosensor via catalytic hairpin assembly-induced AuNP network. Biosens. Bioelectron. 2021, 190, 113376. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Li, Y.; Peng, Y.; Zhao, S.; Xu, M.; Zhang, L.; Huang, Z.; Shi, J.; Yang, Y. Recent development of surface-enhanced Raman scattering for biosensing. J. Nanobiotechnol. 2023, 21, 149. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, J.; Fu, Z.-W.; Qin, D. Galvanic Replacement-Free Deposition of Au on Ag for Core-Shell Nanocubes with Enhanced Chemical Stability and SERS Activity. J. Am. Chem. Soc. 2014, 136, 8153–8156. [Google Scholar] [CrossRef]
- Xue, S.; Yin, L.; Gao, S.; Zhou, R.; Zhang, Y.; Jayan, H.; El-Seedi, H.R.; Zou, X.; Guo, Z. A film-like SERS aptasensor for sensitive detection of patulin based on GO@Au nanosheets. Food Chem. 2024, 441, 138364. [Google Scholar] [CrossRef]
- Luo, Z.; Zheng, K.; Xie, J. Engineering ultrasmall water-soluble gold and silver nanoclusters for biomedical applications. Chem. Commun. 2014, 50, 5143–5155. [Google Scholar] [CrossRef]
- Lin, S.; Lin, X.; Han, S.; He, L.; Zhao, H.Y.; Zhang, J.; Hasi, W.L.J.; Wang, L. Width and length dependent SERS performance of core-shell Au@Ag nanorod self-assembled monolayers. J. Alloys Compd. 2019, 805, 318–326. [Google Scholar] [CrossRef]
- Chen, C.; Zhang, Y.F.; Wang, X.M.; Qiao, X.G.; Waterhouse, G.I.N.; Xu, Z.X. A core-satellite self-assembled SERS aptasensor containing a “biological-silent region” Raman tag for the accurate and ultrasensitive detection of histamine. Food Sci. Hum. Wellness 2024, 13, 1029–1039. [Google Scholar] [CrossRef]
- Tu, J.; Wu, T.; Yu, Q.; Li, J.X.; Zheng, S.; Qi, K.Z.; Sun, G.H.; Xiao, R.; Wang, C.W. Introduction of multilayered magnetic core-dual shell SERS tags into lateral flow immunoassay: A highly stable and sensitive method for the simultaneous detection of multiple veterinary drugs in complex samples. J. Hazard. Mater. 2023, 448, 130912. [Google Scholar] [CrossRef]
- Huang, X.; Sheng, B.; Tian, H.; Chen, Q.; Yang, Y.; Bui, B.; Pi, J.; Cai, H.; Chen, S.; Zhang, J.; et al. Real-time SERS monitoring anticancer drug release along with SERS/MR imaging for pH-sensitive chemo-phototherapy. Acta Pharm. Sin. B 2023, 13, 1303–1317. [Google Scholar] [CrossRef]
- Zhang, M.Z.; Meng, L.; Kalyinur, K.; Dong, S.Y.; Chang, X.Y.; Yu, Q.; Wang, R.; Pang, B.; Kong, X.M. Fabrication and Application of Ag@SiO2/Au Core-Shell SERS Composite in Detecting Cu2+ in Water Environment. Molecules 2024, 29, 1503. [Google Scholar] [CrossRef]
- Yin, Z.; Wang, Y.; Song, C.; Zheng, L.; Ma, N.; Liu, X.; Li, S.; Lin, L.; Li, M.; Xu, Y.; et al. Hybrid Au-Ag Nanostructures for Enhanced Plasmon-Driven Catalytic Selective Hydrogenation through Visible Light Irradiation and Surface-Enhanced Raman Scattering. J. Am. Chem. Soc. 2018, 140, 864–867. [Google Scholar] [CrossRef] [PubMed]
- Bock, S.; Choi, Y.S.; Kim, M.; Yun, Y.; Pham, X.H.; Kim, J.; Seong, B.; Kim, W.; Jo, A.; Ham, K.M.; et al. Highly sensitive near-infrared SERS nanoprobes for in vivo imaging using gold-assembled silica nanoparticles with controllable nanogaps. J. Nanobiotechnol. 2022, 20, 130. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Yao, A.H.; Zhou, T.; Wang, D.P. Surface Enhanced Raman Scattering of Au@SiO2 Nanoparticles with Different Shell Thicknesses. Rare Met. Mater. Eng. 2016, 45, 1066–1070. [Google Scholar]
- Schiller, T.L.; Keddie, D.J.; Blakey, I.; Fredericks, P.M. Surface-enhanced Raman encoded polymer stabilized gold nanoparticles: Demonstration of potential for use in bioassays. Eur. Polym. J. 2017, 87, 508–518. [Google Scholar] [CrossRef]
- Arabi, M.; Ostovan, A.; Wang, Y.; Mei, R.; Fu, L.; Li, J.; Wang, X.; Chen, L. Chiral molecular imprinting-based SERS detection strategy for absolute enantiomeric discrimination. Nat. Commun. 2022, 13, 5757. [Google Scholar] [CrossRef]
- van der Hoeven, J.E.S.; Gurunarayanan, H.; Bransen, M.; de Winter, D.A.M.; de Jongh, P.E.; van Blaaderen, A. Silica-Coated Gold Nanorod Supraparticles: A Tunable Platform for Surface Enhanced Raman Spectroscopy. Adv. Funct. Mater. 2022, 32, 2200148. [Google Scholar] [CrossRef]
- Arabi, M.; Ostovan, A.; Zhang, Z.Y.; Wang, Y.Q.; Mei, R.C.; Fu, L.W.; Wang, X.Y.; Ma, J.P.; Chen, L.X. Label-free SERS detection of Raman-Inactive protein biomarkers by Raman reporter indicator: Toward ultrasensitivity and universality. Biosens. Bioelectron. 2021, 174, 112825. [Google Scholar] [CrossRef]
- Strozyk, M.S.; Jimenez de Aberasturi, D.; Liz-Marzan, L.M. Composite Polymer Colloids for SERS-Based Applications. Chem. Rec. 2018, 18, 807–818. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, M.; Geng, H.; Zhang, P.; Zheng, Z.; Zhou, Y.; He, W. NIR enhanced peroxidase-like activity of Au@CeO2 hybrid nanozyme by plasmon-induced hot electrons and photothermal effect for bacteria killing. Appl. Catal. B Environ. 2021, 295, 120317. [Google Scholar] [CrossRef]
- Chen, P.; Peng, Y.; Lin, L.; Yuan, Y.; Chen, J.; Mo, J.; Miao, J.; He, H.; Jin, Y.; Zhang, L.; et al. Au/CeO2 NR Restricted Inside Cu-MOFs: A Three-in-One Artificial Enzyme with Synergistically Enhanced Peroxidase-Like Activity for Dual-Mode Sensing of Multiple Biomarkers. ACS Sustain. Chem. Eng. 2023, 11, 8106–8119. [Google Scholar] [CrossRef]
- Yang, D.; Li, H.; Li, Q.; Li, K.; Xiao, F.; Yang, Y. Highly selective histamine assay via SERS: Based on the signal enhancement of carbon dots and the fluorescence quenching of gold nanoparticles. Sens. Actuators B-Chem. 2022, 350, 130866. [Google Scholar] [CrossRef]
- Lee, S.; Zhao, Q.; Lee, S.; Lee, Y.; Jung, I.; Park, S. Plasmonic Nanotrenches with 1 nm Nanogaps for Surface-Enhanced Raman Scattering-Based Screening of His-Tagged Proteins. Nano Lett. 2024, 24, 12315–12322. [Google Scholar] [CrossRef] [PubMed]
- Lai, H.S.; Li, G.K.; Xu, F.G.; Zhang, Z.M. Metal-organic frameworks: Opportunities and challenges for surface-enhanced Raman scattering—A review. J. Mater. Chem. C 2020, 8, 2952–2963. [Google Scholar] [CrossRef]
- Allegretto, J.A.; Dostalek, J. Metal-Organic Frameworks in Surface Enhanced Raman Spectroscopy-Based Analysis of Volatile Organic Compounds. Adv. Sci. 2024, 11, 2401437. [Google Scholar] [CrossRef]
- Xu, Y.; Dong, Q.; Cong, S.; Zhao, Z. SERS Materials with Small-Molecule Sensitivity for Biological Diagnosis. Anal. Sens. 2024, 4, e202300067. [Google Scholar] [CrossRef]
- Broza, Y.Y.; Vishinkin, R.; Barash, O.; Nakhleh, M.K.; Haick, H. Synergy between nanomaterials and volatile organic compounds for non-invasive medical evaluation. Chem. Soc. Rev. 2018, 47, 4781–4859. [Google Scholar] [CrossRef]
- Qiao, X.Z.; Su, B.S.; Liu, C.; Song, Q.; Luo, D.; Mo, G.; Wang, T. Selective Surface Enhanced Raman Scattering for Quantitative Detection of Lung Cancer Biomarkers in Superparticle@MOF Structure. Adv. Mater. 2018, 30, 1702275. [Google Scholar] [CrossRef]
- Fu, J.H.; Zhong, Z.; Xie, D.; Guo, Y.J.; Kong, D.X.; Zhao, Z.X.; Zhao, Z.X.; Li, M. SERS-Active MIL-100 (Fe) Sensory Array for Ultrasensitive and Multiplex Detection of VOCs. Angew. Chem.-Int. Ed. 2020, 59, 20489–20498. [Google Scholar] [CrossRef]
- Wei, S.; Li, R.; Li, L.; Huang, D.; Wu, L.; Hou, X. Photoinduced defective MIL-100-NH 2 as SERS substrate with charge-transfer resonances for direct, sensitive and simultaneous detection of small-molecule biothiols. Sens. Actuators B-Chem. 2024, 417, 136076. [Google Scholar] [CrossRef]
- Xin, M.; Fu, Y.; Zhou, Y.; Han, J.; Mao, Y.; Li, M.; Liu, J.; Huang, M. The surface-enhanced Raman scattering of all-inorganic perovskite quantum dots of CsPbBr3 encapsulated in a ZIF-8 metal-organic framework. New J. Chem. 2020, 44, 17570–17576. [Google Scholar] [CrossRef]
- Qin, L.; Gu, H.; Shen, H.; Luo, M.; Zhang, T.; Kang, S.-Z.; Li, X. A portable architectonics of Al/carbon nitride/metal-organic frameworks anchored Ag nanoparticles for SERS detection and photocatalytic degradation of fungicide. Spectrochim. Acta Part A-Mol. Biomol. Spectrosc. 2023, 285, 121897. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yang, Z.; Zou, Y.; Farooq, S.; Qin, Z.; Zhang, H. In situ synthesized highly sensitive AANS@ZIF-8@GZEF constructed flexible SERS sensor for CPS determination. Sens. Actuators B-Chem. 2024, 414, 135936. [Google Scholar] [CrossRef]
- Barbillon, G. Latest Novelties on Plasmonic and Non-Plasmonic Nanomaterials for SERS Sensing. Nanomaterials 2020, 10, 1200. [Google Scholar] [CrossRef] [PubMed]
- Eskandari, V.; Sahbafar, H.; Zeinalizad, L.; Marashipour, R.; Hadi, A. A Review of Paper-Based Substrates as Surface-Enhanced Raman Spectroscopy (SERS) Biosensors and Microfluidic Paper-Based SERS Platforms. J. Comput. Appl. Mech. 2022, 53, 132–146. [Google Scholar] [CrossRef]
- Kitaw, S.L.; Birhan, Y.S.; Tsai, H.-C. Plasmonic surface-enhanced Raman scattering nano-substrates for detection of anionic environmental contaminants: Current progress and future perspectives. Environ. Res. 2023, 221, 115247. [Google Scholar] [CrossRef]
- Wang, J.; Cong, L.; Shi, W.; Xu, W.; Xu, S. Single-Cell Analysis and Classification according to Multiplexed Proteins via Microdroplet-Based Self-Driven Magnetic Surface-Enhanced Raman Spectroscopy Platforms Assisted with Machine Learning Algorithms. Anal. Chem. 2023, 95, 11019–11027. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Y.; Yu, J.; Xie, X.; Deng, R.; Min, C.; Yuan, X. Plasmonic-Thermoelectric Nanotweezers for Immersive SERS Mapping. ACS Nano 2022, 16, 18621–18629. [Google Scholar] [CrossRef]
- Qi, J.; Zhou, W.; Yang, C.; Liu, W.; Guan, C.; Zhang, C.; Han, Q.; Gao, W.; Zhu, L.; Dong, J. Fabrication of sandwich nanostructured substrates with Au@Ag NCs/Graphene/AgMP for ultrasensitive SERS detection. Mater. Res. Bull. 2025, 181, 113107. [Google Scholar] [CrossRef]
- Xie, T.; Li, P.; Wang, J.; Dong, R.; Yang, L. Three-Phase Catassembly of 10 nm Au Nanoparticles for Sensitive and Stable Surface-Enhanced Raman Scattering Detection. Anal. Chem. 2023, 95, 15293–15301. [Google Scholar] [CrossRef]
- Xing, X.; Zhao, Y.; Zhao, H.; Zhang, Y.; Chen, Z.; Liu, B.; Ren, B. Label-Free Detection of DNA Base Mutation and Hybridized DNA by an Electrostatically Modified 3D Plasmonic Array. ACS Sens. 2024, 9, 4119–4126. [Google Scholar] [CrossRef]
- Zhao, T.; Wang, R.; Wei, S.; Zhang, L.; Liu, Z.; Wang, M.; Jiao, T. Preparation of Langmuir-Blodgett composite films based on sericin protein and silk fibroin with good surface-enhanced Raman scattering and photovoltaic properties. Colloids Surf. A-Physicochem. Eng. Asp. 2024, 688, 133669. [Google Scholar] [CrossRef]
- Huang, R.; Liu, X.; Mao, Y.; Qian, C.; Qin, Y.; Jin, J.; Xue, S.; Mao, Q. Quantitative detection of tapered fiber SERS probes prepared in batches by electrostatic adsorption self-assembly method. Measurement 2025, 239, 115512. [Google Scholar] [CrossRef]
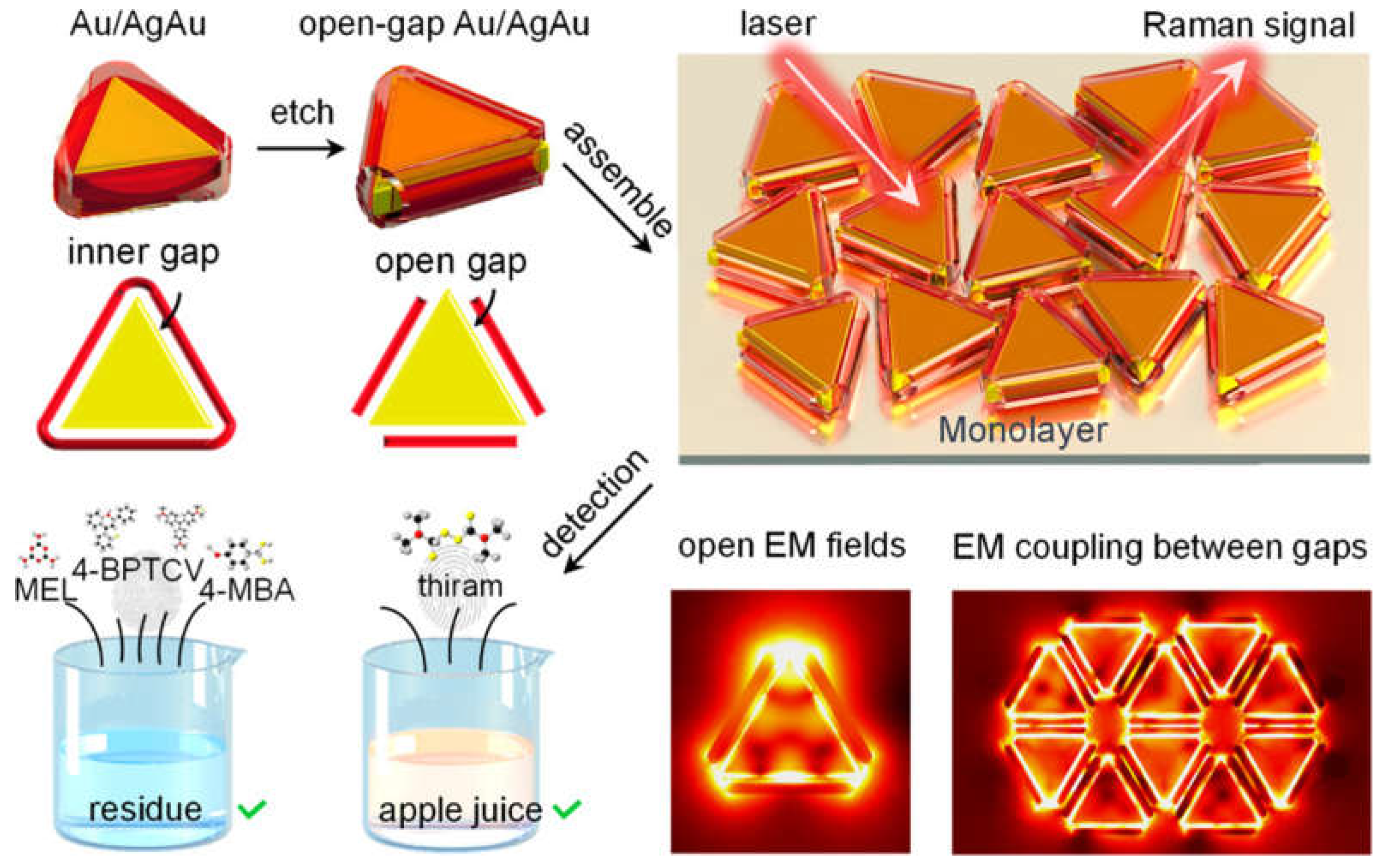
- Zhao, Y.X.; Liang, X.; Chen, Y.L.; Chen, Y.T.; Ma, L.; Ding, S.J.; Chen, X.B.; Wang, Q.Q. Open-Nanogap-Induced Strong Electromagnetic Enhancement in Au/AgAu Monolayer as a Stable and Uniform SERS Substrate for Ultrasensitive Detection. Anal. Chem. 2024, 96, 8416–8423. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Yan, W.; Liu, B.; Yang, J. Fabrication of Long-Range Film of Flower-Like Ag Nanoparticles for Highly Sensitive SERS Substrate. Plasmonics 2024. [Google Scholar] [CrossRef]
- Chen, M.; Liu, D.; Du, X.; Lo, K.H.; Wang, S.; Zhou, B.; Pan, H. 2D materials: Excellent substrates for surface-enhanced Raman scattering (SERS) in chemical sensing and biosensing. TrAC Trends Anal. Chem. 2020, 130, 115983. [Google Scholar] [CrossRef]
- Kumar, R.; Sahoo, S.; Joanni, E.; Singh, R.K.; Yadav, R.M.; Verma, R.K.; Pratap Singh, D.; Tan, W.K.; Perez del Pino, A.; Moshkalev, S.A.; et al. A review on synthesis of graphene, h-BN and MoS2 for energy storage applications: Recent progress and perspectives. Nano Res. 2019, 12, 2655–2694. [Google Scholar] [CrossRef]
- Wang, J.; Ma, F.; Sun, M. Graphene, hexagonal boron nitride, and their heterostructures: Properties and applications. RSC Adv. 2017, 7, 16801–16822. [Google Scholar] [CrossRef]
- Weng, Q.; Wang, X.; Wang, X.; Bando, Y.; Golberg, D. Functionalized hexagonal boron nitride nanomaterials: Emerging properties and applications. Chem. Soc. Rev. 2016, 45, 3989–4012. [Google Scholar] [CrossRef]
- Murali, G.; Modigunta, J.K.R.; Park, Y.H.; Lee, J.-H.; Rawal, J.; Lee, S.-Y.; In, I.; Park, S.-J. A Review on MXene Synthesis, Stability, and Photocatalytic Applications. ACS Nano 2022, 16, 13370–13429. [Google Scholar] [CrossRef]
- Zhang, C.; Li, C.; Yu, J.; Jiang, S.; Xu, S.; Yang, C.; Liu, Y.J.; Gao, X.; Liu, A.; Man, B. SERS activated platform with three-dimensional hot spots and tunable nanometer gap. Sens. Actuators B Chem. 2018, 258, 163–171. [Google Scholar] [CrossRef]
- Ling, X.; Fang, W.; Lee, Y.-H.; Araujo, P.T.; Zhang, X.; Rodriguez-Nieva, J.F.; Lin, Y.; Zhang, J.; Kong, J.; Dresselhaus, M.S. Raman Enhancement Effect on Two-Dimensional Layered Materials: Graphene, h-BN and MoS2. Nano Lett. 2014, 14, 3033–3040. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Huang, H.; Guo, W.; Zhang, C.; Chen, Z.; Li, S.; Ma, L.; Deng, Y. Recent Progress in Black Phosphorus Sensors. J. Biomed. Nanotechnol. 2020, 16, 1045–1064. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Liang, S.; Peng, Y.; Long, L.; Li, Y.; Huang, Z.; Nguyen Viet, L.; Luo, X.; Liu, J.; Li, Z.; et al. Visualized SERS Imaging of Single Molecule by Ag/Black Phosphorus Nanosheets. Nano-Micro Lett. 2022, 14, 75. [Google Scholar] [CrossRef] [PubMed]
- Du, T.; Han, X.; Yan, X.; Shang, J.; Li, Y.; Song, J. MXene-Based Flexible Sensors: Materials, Preparation, and Applications. Adv. Mater. Technol. 2023, 8, 2202029. [Google Scholar] [CrossRef]
- Satheeshkumar, E.; Makaryan, T.; Melikyan, A.; Minassian, H.; Gogotsi, Y.; Yoshimura, M. Author Correction: One-step Solution Processing of Ag, Au and Pd@MXene Hybrids for SERS (vol 6, 32049, 2016). Sci. Rep. 2020, 10, 16069. [Google Scholar] [CrossRef]
- Xie, H.; Li, P.; Shao, J.; Huang, H.; Chen, Y.; Jiang, Z.; Chu, P.K.; Yu, X.-F. Electrostatic Self-Assembly of Ti3C2Tx MXene and Gold Nanorods as an Efficient Surface-Enhanced Raman Scattering Platform for Reliable and High-Sensitivity Determination of Organic Pollutants. ACS Sens. 2019, 4, 2303–2310. [Google Scholar] [CrossRef]
- Guselnikova, O.; Lim, H.; Kim, H.-J.; Kim, S.H.; Gorbunova, A.; Eguchi, M.; Postnikov, P.; Nakanishi, T.; Asahi, T.; Na, J.; et al. New Trends in Nanoarchitectured SERS Substrates: Nanospaces, 2D Materials, and Organic Heterostructures. Small 2022, 18, 202107182. [Google Scholar] [CrossRef]
- Hu, W.; Xia, L.; Hu, Y.; Li, G. Recent progress on three-dimensional substrates for surface-enhanced Raman spectroscopic analysis. Microchem. J. 2022, 172, 106908. [Google Scholar] [CrossRef]
- Heydaryan, K.; Aspoukeh, P.; Mehmandoust, S.; Abbas, A.H.; Khojasteh, H.; Hadi, M.S.; Eskandari, V.; Sahbafar, H. Nanopore/Nanocavity-Based Structures as Surface-Enhanced Raman Spectroscopy (SERS) Platforms. Plasmonics 2024. [Google Scholar] [CrossRef]
- Liu, L.; Gao, T.; Zhao, Q.; Xue, Z.; Wu, Y. Self-assembled bimetallic plasmonic nanocavity substrate for supersensitive SERS. Opt. Laser Technol. 2025, 181, 111827. [Google Scholar] [CrossRef]
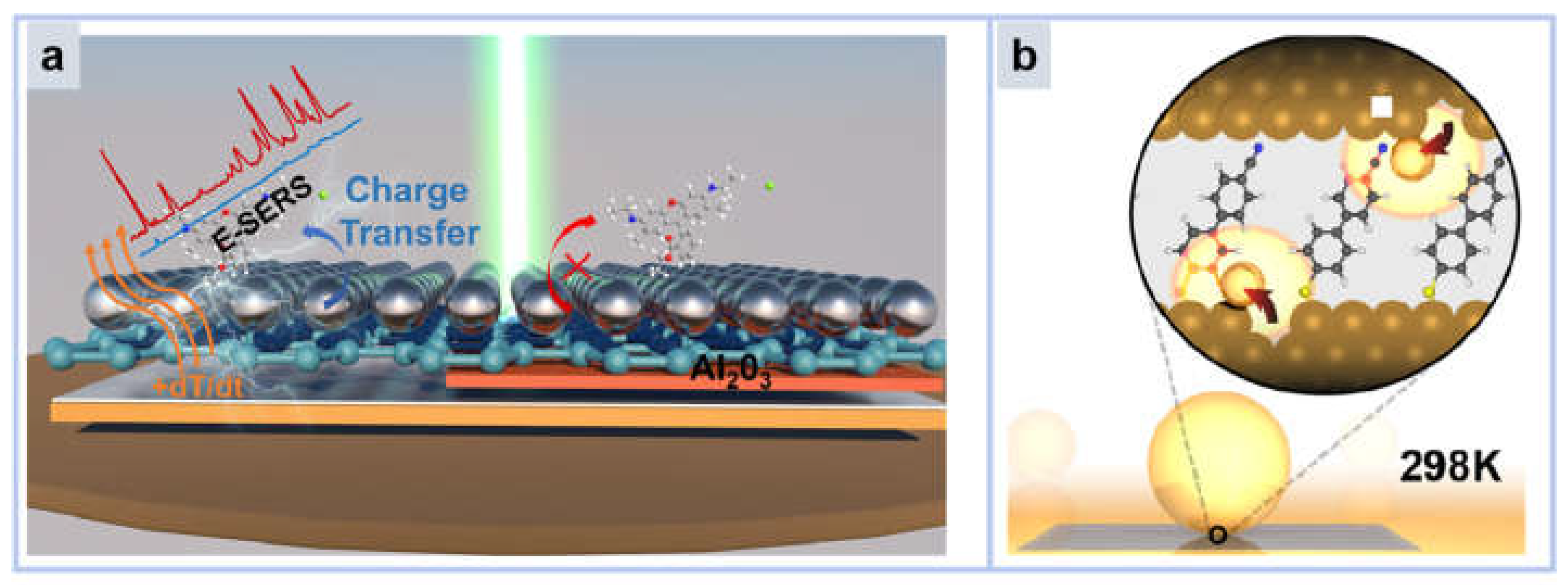
- Zhang, C.; Tan, J.; Du, B.; Ji, C.; Pei, Z.; Shao, M.; Jiang, S.; Zhao, X.; Yu, J.; Man, B.; et al. Reversible Thermoelectric Regulation of Electromagnetic and Chemical Enhancement for Rapid SERS Detection. ACS Appl. Mater. Interfaces 2024, 16, 12085–12094. [Google Scholar] [CrossRef] [PubMed]
- Carnegie, C.; Griffiths, J.; de Nijs, B.; Readman, C.; Chikkaraddy, R.; Deacon, W.M.; Zhang, Y.; Szabó, I.; Rosta, E.; Aizpurua, J.; et al. Room-Temperature Optical Picocavities below 1 nm3 Accessing Single-Atom Geometries. J. Phys. Chem. Lett. 2018, 9, 7146–7151. [Google Scholar] [CrossRef] [PubMed]
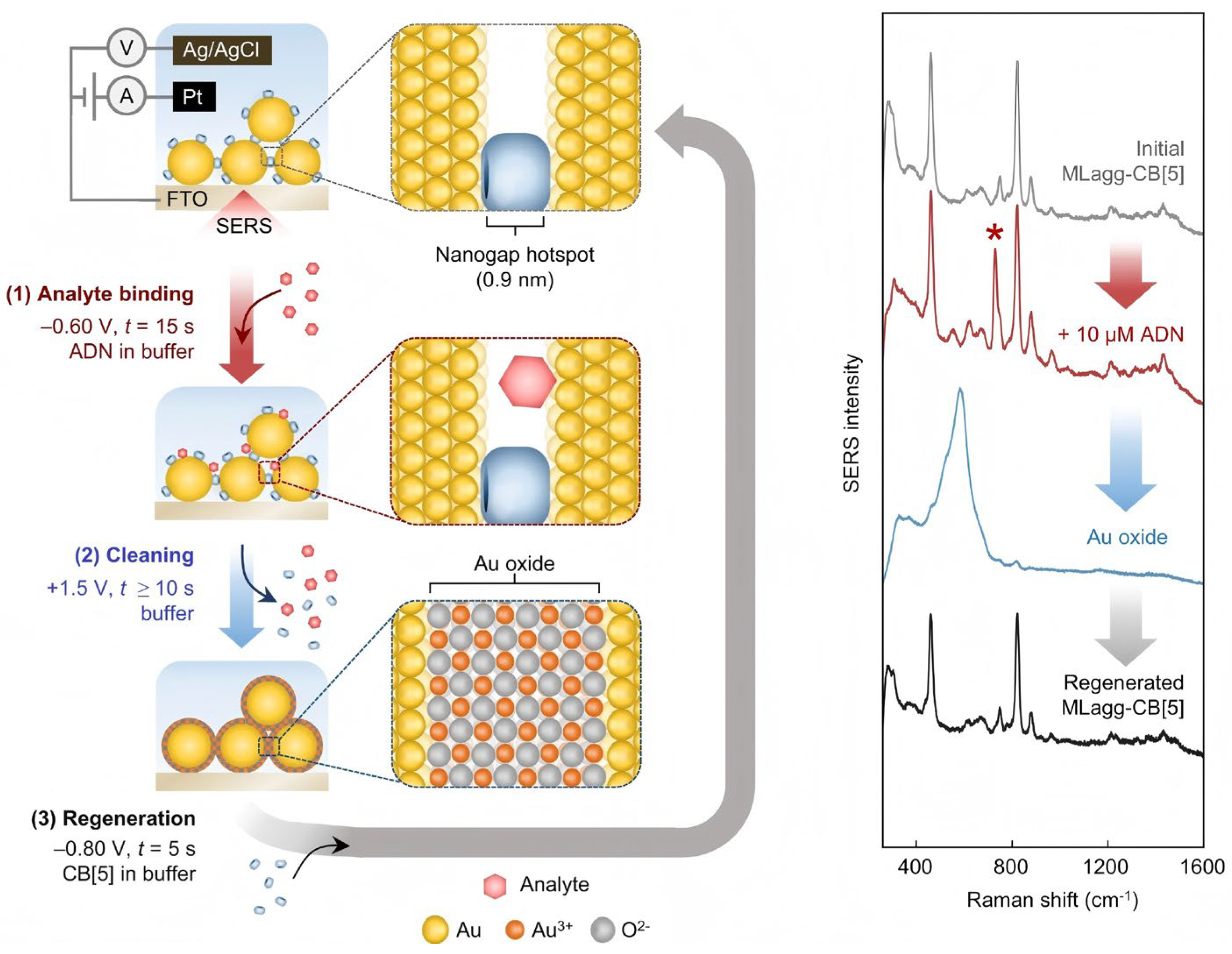
- Sibug-Torres, S.M.; Grys, D.B.; Kang, G.; Niihori, M.; Wyatt, E.; Spiesshofer, N.; Ruane, A.; de Nijs, B.; Baumberg, J.J. In situ electrochemical regeneration of nanogap hotspots for continuously reusable ultrathin SERS sensors. Nat. Commun. 2024, 15, 2022. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-J.; Chen, S.; Radjenovic, P.; Bodappa, N.; Zhang, H.; Yang, Z.-L.; Tian, Z.-Q.; Li, J.-F. Probing the Location of 3D Hot Spots in Gold Nanoparticle Films Using Surface-Enhanced Raman Spectroscopy. Anal. Chem. 2019, 91, 5316–5322. [Google Scholar] [CrossRef]
- Qu, L.-L.; Ying, Y.-L.; Yu, R.-J.; Long, Y.-T. In situ food-borne pathogen sensors in a nanoconfined space by surface enhanced Raman scattering. Microchim. Acta 2021, 188, 201. [Google Scholar] [CrossRef]
- Chen, Y.F.; Lee, Y.C.; Lee, J.C.M.; Li, J.W.; Chiu, C.W. Coaxial electrospinning of Au@silicate/poly(vinyl alcohol) core/shell composite nanofibers with non-covalently immobilized gold nanoparticles for preparing flexible, freestanding, and highly sensitive SERS substrates amenable to large-scale fabrication. Adv. Compos. Hybrid Mater. 2024, 7, 124. [Google Scholar] [CrossRef]
- Zhang, L.; Lang, X.; Hirata, A.; Chen, M. Wrinkled Nanoporous Gold Films with Ultrahigh Surface-Enhanced Raman Scattering Enhancement. ACS Nano 2011, 5, 4407–4413. [Google Scholar] [CrossRef]
- Ham, J.-H.; Park, J.S.; Oh, M.-K.; Kim, J.H. Reusable Wrinkled Nanoporous Silver Film Fabricated by Plasma Treatment for Surface-Enhanced Raman Scattering Applications. ACS Omega 2023, 8, 47146–47152. [Google Scholar] [CrossRef]
- Han, H.J.; Cho, S.H.; Han, S.; Jang, J.S.; Lee, G.R.; Cho, E.N.; Kim, S.J.; Kim, I.D.; Jang, M.S.; Tuller, H.L.; et al. Synergistic Integration of Chemo-Resistive and SERS Sensing for Label-Free Multiplex Gas Detection. Adv. Mater. 2021, 33, 2105199. [Google Scholar] [CrossRef]
- Chen, Q.; Wang, Z.; Liao, H.; Li, R.; Feng, Z.; Li, Z.; Lin, L.; Li, J.; Chen, G. EM and CT synergistic SERS enhancement in Si/TiO2/Ag heterostructures via local interfacial effect. Chem. Eng. J. 2024, 493, 152316. [Google Scholar] [CrossRef]
- Wang, C.; Gao, Y.; Hu, S.; Zhu, A.; Ying, Y.; Guo, X.; Wu, Y.; Wen, Y.; Yang, H. MnO2 coated Au nanoparticles advance SERS detection of cellular glutathione. Biosens. Bioelectron. 2022, 215, 114388. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Xiao, J.; Zhang, J.; Sun, Q.; Liu, D.; Liu, Y.; Gao, X. Python-assisted detection and photothermal inactivation of Salmonella typhimurium and Staphylococcus aureus on a background-free SERS chip. Biosens. Bioelectron. 2024, 247, 115913. [Google Scholar] [CrossRef] [PubMed]
- Qin, C.; Zhang, D.; Wu, Z.; Ni, D.; Yu, Z.; Liang, P. Construction of an efficient molecular enrichment and magnetic separation flow with Fe3O4@Ag@COF for ratiometric SERS detection of methamidophos. Chem. Eng. J. 2024, 496, 154177. [Google Scholar] [CrossRef]
- Qu, C.; Fang, H.; Yu, F.; Chen, J.; Su, M.; Liu, H. Artificial nose of scalable plasmonic array gas sensor for Multi-Dimensional SERS recognition of volatile organic compounds. Chem. Eng. J. 2024, 482, 148773. [Google Scholar] [CrossRef]
- Li, J.; Lin, X.; Wu, J.; Ying, D.; Duan, N.; Wang, Z.; Wu, S. Multifunctional magnetic composite nanomaterial for Colorimetric-SERS Dual-Mode detection and photothermal sterilization of Vibrio parahaemolyticus. Chem. Eng. J. 2023, 477, 147113. [Google Scholar] [CrossRef]
- Barveen, N.R.; Chinnapaiyan, S.; Zeng, C.-W.; Huang, C.-H.; Lin, Y.-Y.; Cheng, Y.-W. Plasmonic Au-NPs photodecorated on NiCoLDH nanosheets as a flexible SERS sensor for the real-time detection of fipronil. J. Hazard. Mater. 2024, 480, 135907. [Google Scholar] [CrossRef]
- Zhang, T.; Li, Y.; Lv, X.; Jiang, S.; Jiang, S.; Sun, Z.; Zhang, M.; Li, Y. Ultra-Sensitive and Unlabeled SERS Nanosheets for Specific Identification of Glucose in Body Fluids. Adv. Funct. Mater. 2024, 34, 202315668. [Google Scholar] [CrossRef]
- Lv, B.; Wang, Y.; Mei, R.; Li, Y.; Zhao, X.; Yuan, Q.; Xu, H.; Chen, L. Enhanced Superhydrophobic Substrate-Based 3D SERS Platform for a Portable Raman Spectrometer-Assisted Drug Detection in Plasma. ACS Appl. Nano Mater. 2024, 7, 14665–14672. [Google Scholar] [CrossRef]
- Wang, M.; Lou, Z.; Hou, Y.; Song, L.; Zhang, L.; Zhao, Y.; Ruan, L.; Huang, Y. 3D hotspot engineering and analytes strategy enabled ultrasensitive SERS platform for biosensing of depression biomarker. Biosens. Bioelectron. 2024, 250, 116059. [Google Scholar] [CrossRef]
- Li, Y.; Tang, S.; Xu, S.; Duan, Z.; Wang, Z.; Zhang, Y. Ag Nanoframes Deposited on Au Films Generate Optical Cavities for Surface-Enhanced Raman Scattering. ACS Appl. Nano Mater. 2020, 3, 5116–5122. [Google Scholar] [CrossRef]
- Liu, W.; Li, Y.; Li, Z.; Du, X.; Xie, S.; Liu, C.; Jiang, S.; Li, Z. 3D flexible compositing resonant cavity system for high-performance SERS sensing. Opt. Express 2023, 31, 6925–6937. [Google Scholar] [CrossRef] [PubMed]
- Mandal, P.; Tewari, B.S. Near-Field Manipulation and Metallic Cavity Assisted High Surface Enhanced Raman Scattering Detection. In Proceedings of the 64th DAE Solid State Physics Symposium (DAE-SSPS), Jodhpur, India, 18–22 December 2019; Indian Institute of Technology Jodhpur: Jodhpur, India, 2020. [Google Scholar]
- Kong, X.; Xi, Y.; Le Duff, P.; Chong, X.; Li, E.; Ren, F.; Rorrer, G.L.; Wang, A.X. Detecting explosive molecules from nanoliter solution: A new paradigm of SERS sensing on hydrophilic photonic crystal biosilica. Biosens. Bioelectron. 2017, 88, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Boginskaya, I.A.; Nechepurenko, I.A.; Shishkov, V.Y.; Dorofeenko, A.V.; Bykov, I.V.; Afanasyev, K.N.; Ryzhikov, I.A.; Vinogradov, A.P.; Budashov, I.A.; Kurochkin, I.M.; et al. Additional enhancement of SERS effect by a surface wave in photonic crystal. J. Raman Spectrosc. 2019, 50, 1452–1461. [Google Scholar] [CrossRef]
- Skrabic, M.; Kosovic, M.; Gotic, M.; Mikac, L.; Ivanda, M.; Gamulin, O. Near-Infrared Surface-Enhanced Raman Scattering on Silver-Coated Porous Silicon Photonic Crystals. Nanomaterials 2019, 9, 421. [Google Scholar] [CrossRef] [PubMed]
- Song, D.; Wang, T.; Zhuang, L. Preparation of SiO2@Au Nanoparticle Photonic Crystal Array as Surface-Enhanced Raman Scattering (SERS) Substrate. Nanomaterials 2023, 13, 2156. [Google Scholar] [CrossRef]
- Chen, G.J.; Zhang, K.L.; Luo, B.B.; Hong, W.; Chen, J.; Chen, X.D. Plasmonic-3D photonic crystals microchip for surface enhanced Raman spectroscopy. Biosens. Bioelectron. 2019, 143, 111596. [Google Scholar] [CrossRef]
- Qiao, X.Z.; Xue, Z.J.; Liu, L.; Liu, K.Y.; Wang, T. Superficial-Layer-Enhanced Raman Scattering (SLERS) for Depth Detection of Noncontact Molecules. Adv. Mater. 2019, 31, 201804275. [Google Scholar] [CrossRef]
- Kumar, S.; Kanagawa, M.; Namura, K.; Fukuoka, T.; Suzuki, M. Multilayer thin-film flake dispersion gel for surface-enhanced Raman spectroscopy. Appl. Nanosci. 2023, 13, 155–163. [Google Scholar] [CrossRef]
- Safar, W.; Azziz, A.; Edely, M.; de la Chapelle, M.L. Conventional Raman, SERS and TERS Studies of DNA Compounds. Chemosensors 2023, 11, 399. [Google Scholar] [CrossRef]
- Sasso, A.; Capaccio, A.; Rusciano, G. Exploring Reliable and Efficient Plasmonic Nanopatterning for Surface- and Tip-Enhanced Raman Spectroscopies. Int. J. Mol. Sci. 2023, 24, 16164. [Google Scholar] [CrossRef]
- Ferreira, R.C.d.C.; Sagwal, A.; Dolezal, J.; Canola, S.; Merino, P.; Neuman, T.; Svec, M. Resonant Tip-Enhanced Raman Spectroscopy of a Single-Molecule Kondo System. Acs Nano 2024, 18, 13164–13170. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Cooney, G.S.; Talaga, D.; Vallee, R.A.L.; Quinzi, R.; Bouffier, L.; Lecomte, S.; Bonhommeau, S. Nanoscale Chemical Imaging of Amyloid Fibrils in Water Using Total-Internal-Reflection Tip-Enhanced Raman Spectroscopy. J. Phys. Chem. Lett. 2024, 15, 10190–10197. [Google Scholar] [CrossRef] [PubMed]
- Meng, B.; Xie, Y.; Chen, L.; Wang, H.; Li, M.; Dong, Z. Apex-Confined Plasmonic Tip for High Resolution Tip-Enhanced Raman Spectroscopic Imaging of Carbon Nanotubes. ACS Appl. Mater. Interfaces 2023, 15, 16984–16990. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Sheng, S.X.; Pisarra, M.; Martin-Jimenez, A.; Martin, F.; Kern, K.; Garg, M. Selective excitation of vibrations in a single molecule. Nat. Commun. 2024, 15, 6983. [Google Scholar] [CrossRef]
- Li, L.F.; Schultz, J.F.; Mahapatra, S.; Liu, X.L.; Zhang, X.; Hersam, M.C.; Jiang, N. Atomic-Scale Insights into the Interlayer Characteristics and Oxygen Reactivity of Bilayer Borophene. Angew. Chem.-Int. Ed. 2023, 62, 202306590. [Google Scholar] [CrossRef]
- Giergiel, M.; Veettil, T.C.P.; Rossetti, A.; Kochan, K. Advanced Vibrational Spectroscopy and Bacteriophages Team Up: Dynamic Synergy for Medical and Environmental Applications. Int. J. Mol. Sci. 2024, 25, 8148. [Google Scholar] [CrossRef]
- Han, Y.; Dong, L.; Zhu, L.-Y.; Hu, C.-R.; Li, H.; Zhang, Y.; Zhang, C.; Zhang, Y.; Dong, Z.-C. Real-Space Spectral Determination of Short Single-Stranded DNA Sequence Structures. J. Am. Chem. Soc. 2024, 146, 33865–33873. [Google Scholar] [CrossRef]


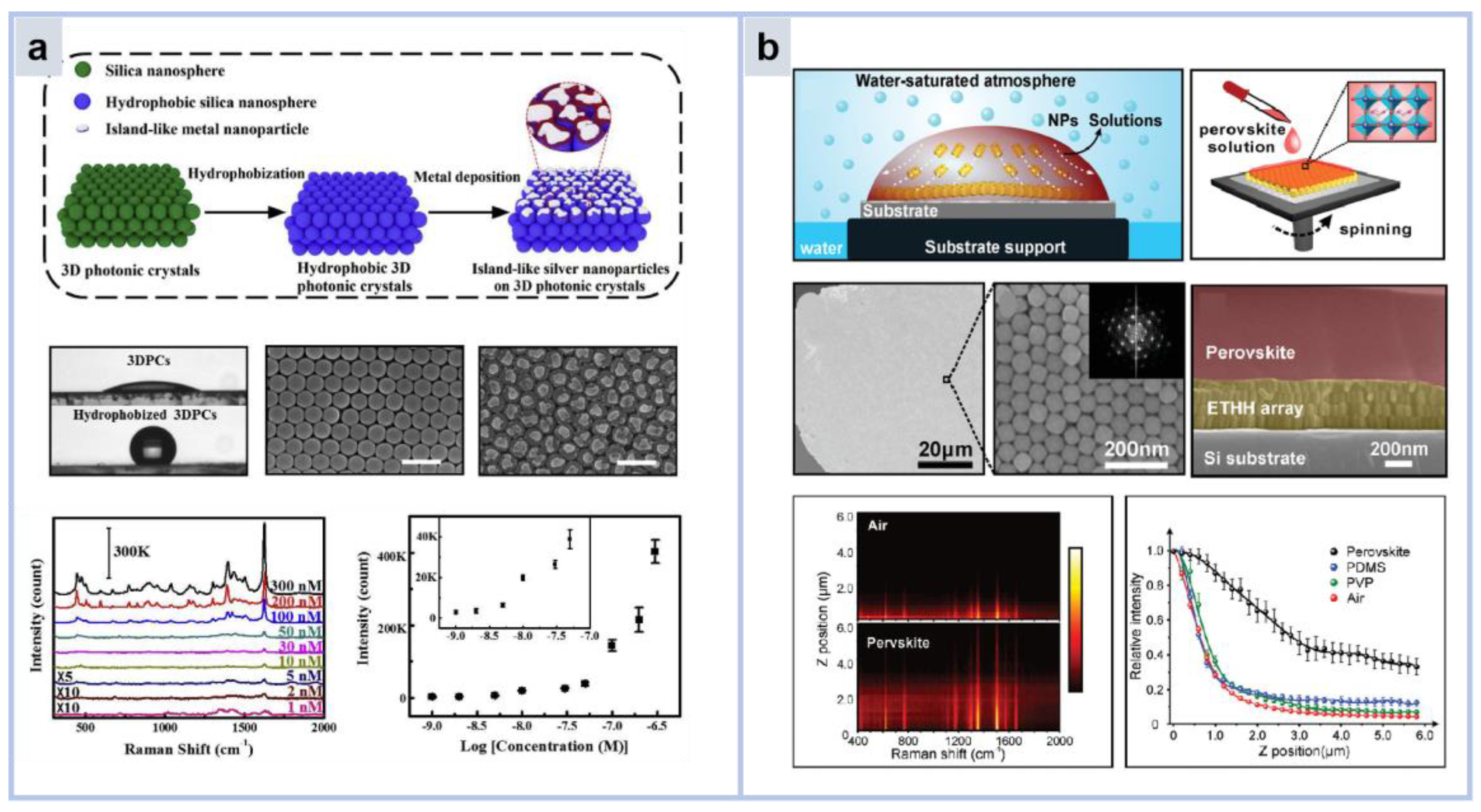
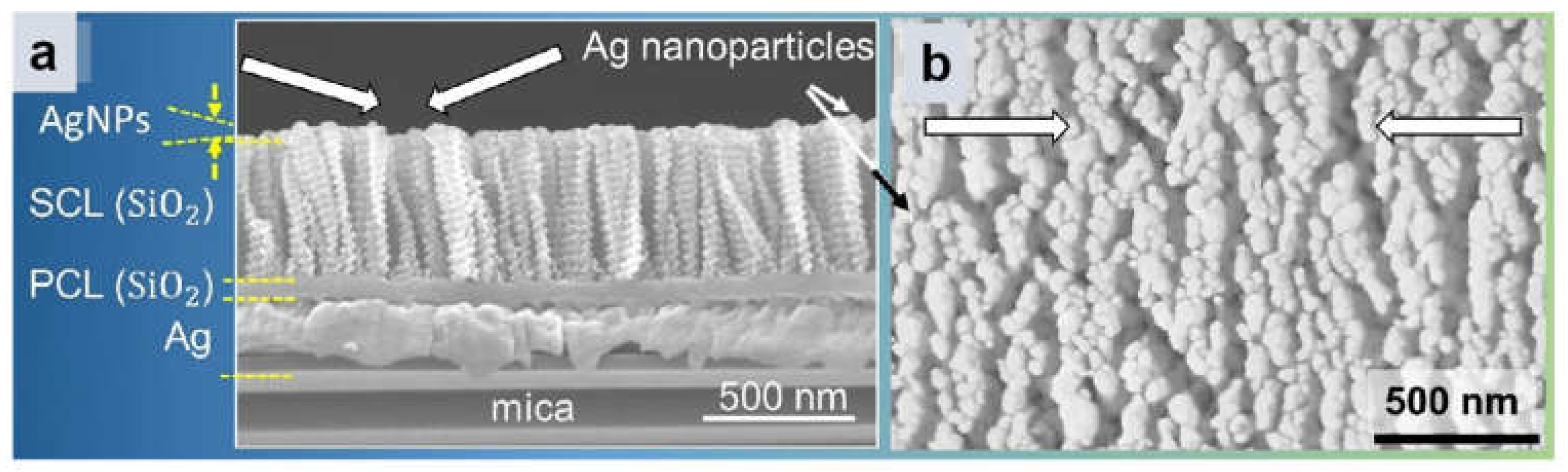
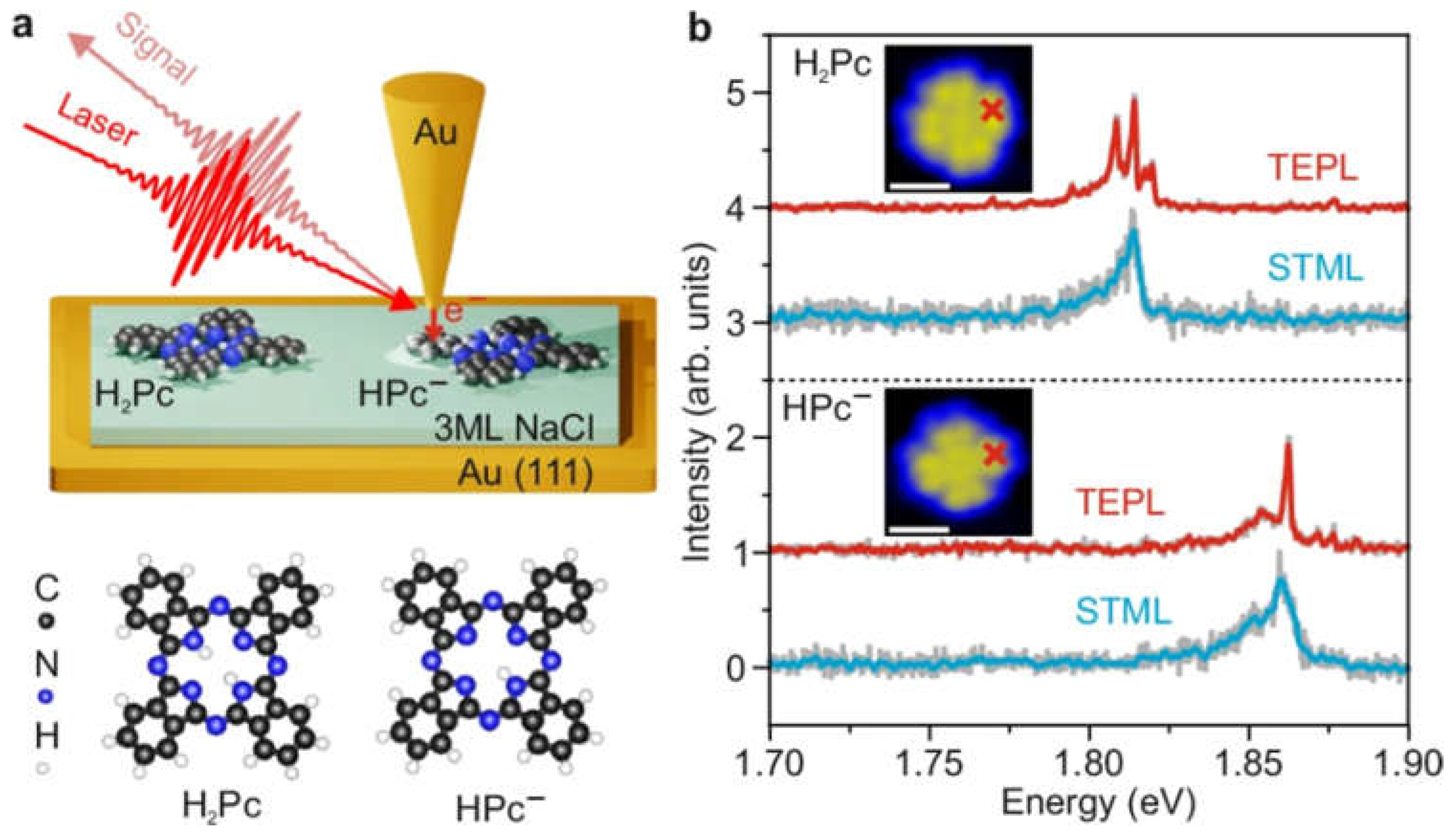
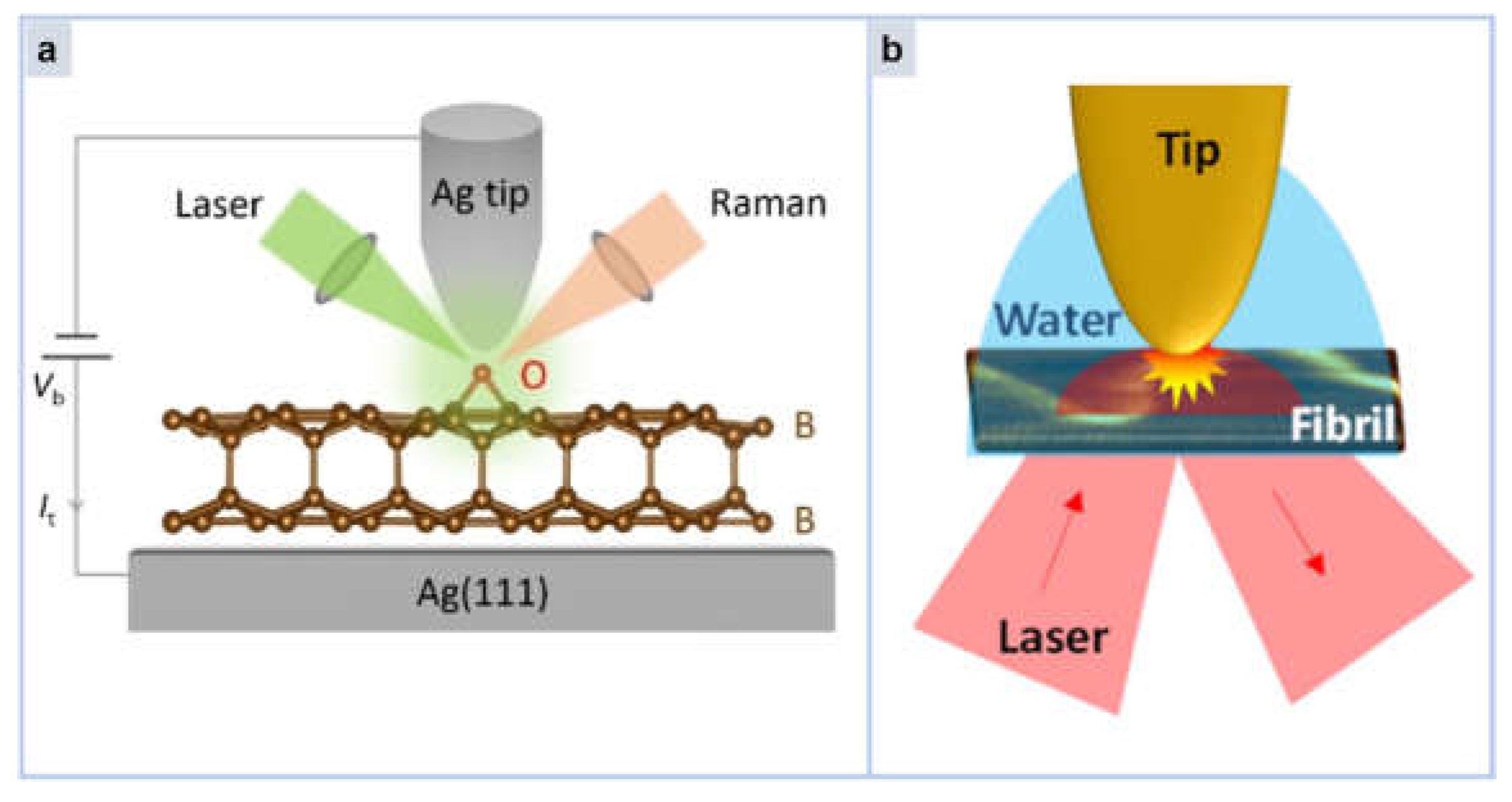

| Ref. | Platform | Substrate | Material | Target | Description | LOD |
|---|---|---|---|---|---|---|
| [33] | Single crystal | Hydrogel | Ag NWs@RC | Nano plastic | Ag NWs@RC was used for the detection of nanoplastics in water | 0.1 mg/mL |
| [110] | Core shell | Si | Si/TiO2@Ag | R6G | EM and CT synergistic SERS enhancement in Si/TiO2/Ag heterostructures via local interfacial effect | 0.1 pM |
| [111] | Core shell | - | Au NPs@MnO2 | GSH | MnO2-coated Au nanoparticles advance SERS detection of cellular glutathione | 1 μM |
| [112] | Core shell | GO | pAu/G/PBA | S. aureus | Detecting and inactivating Salmonella and Staphylococcus with a background-free SERS chip | 10 CFU/mL |
| [113] | MOF | - | Fe3O4@Ag@COF | MTD | Molecules were enriched using a magnetic metal shell and detected by SERS | 0.1 nM |
| [114] | MOF | - | Au NPs@ZIF-8 | VOC | Detection of volatile organic compound gas by a scalable plasma gas sensor | 1 nM |
| [115] | MOF | Au NPs@MIL-100 | V. parahaemolyticus | Colorimetric SERS dual-mode was used to detect Vibrio parahaemolyticus | 9 cfu/mL | |
| [116] | Single crystal | NiCoLDH | Au-NPs/NiCoLDH/CC | FP | Au NPs/NiCoLDH flexible SERS substrate for real-time detection of fipronil | 3.78 nM |
| [117] | Single crystal | AgNS600 | AgNS600 | glucose | A simple physical scratch fabrication of SERS substrate | 0.5 aM |
| [118] | Array | Silicon wafer | Ag NPs | MTO MB | Self-assembled superhydrophobic silver film with interfacial assembly | 8 × 10−10 M 3 × 10−8 M |
| [119] | Array | PDMS | Au NSs | estrogen | 3D micro/nano plasma substrate binding azotization based on Au NSs monolayer films | 10−11 mg/mL |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, Y.; Xu, J.; Shao, D.; Liu, Y.; Qu, X.; Hu, S.; Dong, B. SERS-Based Local Field Enhancement in Biosensing Applications. Molecules 2025, 30, 105. https://doi.org/10.3390/molecules30010105
Xie Y, Xu J, Shao D, Liu Y, Qu X, Hu S, Dong B. SERS-Based Local Field Enhancement in Biosensing Applications. Molecules. 2025; 30(1):105. https://doi.org/10.3390/molecules30010105
Chicago/Turabian StyleXie, Yangdong, Jiling Xu, Danyang Shao, Yuxin Liu, Xuzhou Qu, Songtao Hu, and Biao Dong. 2025. "SERS-Based Local Field Enhancement in Biosensing Applications" Molecules 30, no. 1: 105. https://doi.org/10.3390/molecules30010105
APA StyleXie, Y., Xu, J., Shao, D., Liu, Y., Qu, X., Hu, S., & Dong, B. (2025). SERS-Based Local Field Enhancement in Biosensing Applications. Molecules, 30(1), 105. https://doi.org/10.3390/molecules30010105







