Carvacrol Essential Oil as a Neuroprotective Agent: A Review of the Study Designs and Recent Advances
Abstract
1. Introduction
2. Methodology
3. Results
4. Discussion
4.1. Neuroprotective Ability of CV
4.1.1. Antioxidant Activity of CV
4.1.2. Anti-Inflammatory Activity of CV
4.1.3. Anti-Apoptotic Activity of CV
4.2. Need of Suitable Dosage Forms for CV
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Asadi, S.; Nayeri-Fasaei, B.; Zahraei-Salehi, T.; Yahya-Rayat, R.; Shams, N.; Sharifi, A. Antibacterial and Anti-Biofilm Properties of Carvacrol Alone and in Combination with Cefixime against Escherichia coli. BMC Microbiol. 2023, 23, 55. [Google Scholar] [CrossRef]
- Imran, M.; Aslam, M.; Alsagaby, S.A.; Saeed, F.; Ahmad, I.; Afzaal, M.; Arshad, M.U.; Abdelgawad, M.A.; El-Ghorab, A.H.; Khames, A.; et al. Therapeutic Application of Carvacrol: A Comprehensive Review. Food Sci. Nutr. 2022, 10, 3544–3561. [Google Scholar] [CrossRef]
- Irshad, M.; Subhani, M.A.; Ali, S.; Hussain, A.; Irshad, M.; Subhani, M.A.; Ali, S.; Hussain, A. Biological Importance of Essential Oils; IntechOpen: London, UK, 2019; ISBN 978-1-78984-641-6. [Google Scholar]
- Baptista-Silva, S.; Borges, S.; Ramos, O.L.; Pintado, M.; Sarmento, B. The Progress of Essential Oils as Potential Therapeutic Agents: A Review. J. Essent. Oil Res. 2020, 32, 279–295. [Google Scholar] [CrossRef]
- Chaachouay, N.; Zidane, L. Plant-Derived Natural Products: A Source for Drug Discovery and Development. Drugs Drug Candidates 2024, 3, 184–207. [Google Scholar] [CrossRef]
- Swain, S.S.; Paidesetty, S.K.; Padhy, R.N.; Hussain, T. Nano-Technology Platforms to Increase the Antibacterial Drug Suitability of Essential Oils: A Drug Prospective Assessment. OpenNano 2023, 9, 100115. [Google Scholar] [CrossRef]
- Cimino, C.; Maurel, O.M.; Musumeci, T.; Bonaccorso, A.; Drago, F.; Souto, E.M.B.; Pignatello, R.; Carbone, C. Essential Oils: Pharmaceutical Applications and Encapsulation Strategies into Lipid-Based Delivery Systems. Pharmaceutics 2021, 13, 327. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.R.; Wang, C.H.; Flores, N.J.C.; Su, Y.Y. Targeting Open Market with Strategic Business Innovations: A Case Study of Growth Dynamics in Essential Oil and Aromatherapy Industry. J. Open Innov. Technol. Mark. Complex. 2019, 5, 7. [Google Scholar] [CrossRef]
- Fernandes, L.C.B.; Costa, I.M.; Freire, M.A.M.; Lima, F.O.V.; Neta, F.I.; Lucena, E.E.d.S.; Alves, R.D.; Cavalcanti, J.R.L.P.; Pinheiro, F.I.; de Azevedo, E.P.; et al. Essential Oils in Experimental Models of Neuropsychiatric Disorders: A Systematic Review. Curr. Neuropharmacol. 2021, 19, 1738–1759. [Google Scholar] [CrossRef]
- Zheng, J.C.; Chen, S. Translational Neurodegeneration in the Era of Fast Growing International Brain Research. Transl. Neurodegener. 2022, 11, 1. [Google Scholar] [CrossRef] [PubMed]
- Dati, L.M.; Ulrich, H.; Real, C.C.; Feng, Z.P.; Sun, H.S.; Britto, L.R. Carvacrol Promotes Neuroprotection in the Mouse Hemiparkinsonian Model. Neuroscience 2017, 356, 176–181. [Google Scholar] [CrossRef]
- Lins, L.C.R.F.; Souza, M.F.; Bispo, J.M.M.; Gois, A.M.; Melo, T.C.S.; Andrade, R.A.S.; Quintans-Junior, L.J.; Ribeiro, A.M.; Silva, R.H.; Santos, J.R.; et al. Carvacrol Prevents Impairments in Motor and Neurochemical Parameters in a Model of Progressive Parkinsonism Induced by Reserpine. Brain Res. Bull. 2018, 139, 9–15. [Google Scholar] [CrossRef]
- Hamzehloei, L.; Rezvani, M.E.; Rajaei, Z. Effects of Carvacrol and Physical Exercise on Motor and Memory Impairments Associated with Parkinson’s Disease. Arq. Neuropsiquiatr. 2019, 77, 493–500. [Google Scholar] [CrossRef]
- Akan, T.; Aydın, Y.; Korkmaz, O.T.; Ulupınar, E.; Saydam, F. The Effects of Carvacrol on Transient Receptor Potential (TRP) Channels in an Animal Model of Parkinson’s Disease. Neurotox. Res. 2023, 41, 660–669. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.; Pushpa Tryphena, K.; Singh, G.; Kulkarni, A.; Pinjala, P.; Kumar Khatri, D. Neuroprotective Role of Carvacrol via Nrf2/HO-1/NLRP3 Axis in Rotenone-Induced PD Mice Model. Brain Res. 2024, 1836, 148954. [Google Scholar] [CrossRef]
- Manouchehrabadi, M.; Farhadi, M.; Azizi, Z.; Torkaman-Boutorabi, A. Carvacrol Protects Against 6-Hydroxydopamine-Induced Neurotoxicity in In Vivo and In Vitro Models of Parkinson’s Disease. Neurotox. Res. 2020, 37, 156–170. [Google Scholar] [CrossRef] [PubMed]
- Azizi, Z.; Salimi, M.; Amanzadeh, A.; Majelssi, N.; Naghdi, N. Carvacrol and Thymol Attenuate Cytotoxicity Induced by Amyloid Β25-35 via Activating Protein Kinase C and Inhibiting Oxidative Stress in PC12 Cells. Iran. Biomed. J. 2020, 24, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Caputo, L.; Amato, G.; De Martino, L.; De Feo, V.; Nazzaro, F. Anti-Cholinesterase and Anti-α-Amylase Activities and Neuroprotective Effects of Carvacrol and p-Cymene and Their Effects on Hydrogen Peroxide Induced Stress in SH-SY5Y Cells. Int. J. Mol. Sci. 2023, 24, 6073. [Google Scholar] [CrossRef]
- Medhat, D.; El-mezayen, H.A.; El-Naggar, M.E.; Farrag, A.R.; Abdelgawad, M.E.; Hussein, J.; Kamal, M.H. Evaluation of Urinary 8-Hydroxy-2-Deoxyguanosine Level in Experimental Alzheimer’s Disease: Impact of Carvacrol Nanoparticles. Mol. Biol. Rep. 2019, 46, 4517–4527. [Google Scholar] [CrossRef]
- Azizi, Z.; Choopani, S.; Salimi, M.; Majlessi, N.; Naghdi, N. Protein Kinase C Involvement in Neuroprotective Effects of Thymol and Carvacrol Against Toxicity Induced by Amyloid-β in Rat Hippocampal Neurons. Basic Clin. Neurosci. 2022, 13, 295–304. [Google Scholar] [CrossRef]
- Kazemi, S.; Safari, S.; Komaki, S.; Karimi, S.A.; Golipoor, Z.; Komaki, A. The Effects of Carvacrol and P-Cymene on Aβ1-42-Induced Long-Term Potentiation Deficit in Male Rats. CNS Neurosci. Ther. 2024, 30, e14459. [Google Scholar] [CrossRef]
- Celik Topkara, K.; Kilinc, E.; Cetinkaya, A.; Saylan, A.; Demir, S. Therapeutic Effects of Carvacrol on Beta-Amyloid-Induced Impairments in in Vitro and in Vivo Models of Alzheimer’s Disease. Eur. J. Neurosci. 2022, 56, 5714–5726. [Google Scholar] [CrossRef] [PubMed]
- Mahmoodi, M.; Amiri, H.; Ayoobi, F.; Rahmani, M.; Taghipour, Z.; Ghavamabadi, R.T.; Jafarzadeh, A.; Sankian, M. Carvacrol Ameliorates Experimental Autoimmune Encephalomyelitis through Modulating Pro- and Anti-Inflammatory Cytokines. Life Sci. 2019, 219, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, M.; Eidi, A.; Khaksarian, M.; Ahmadvand, H.; Sotoodehnejadnematalahi, F. Effect of Carvacrol on Inflammatory Factors and Myelin Repair in Experimental Autoimmune Encephalomyelitis on Female Lewis Rats. Res. Sq. 2021; preprint. [Google Scholar] [CrossRef]
- Ahmadi, M.; Eidi, A.; Ahmadvand, H.; Khaksarian, M.; Sotoodehnejadnematalahi, F. Effect of Carvacrol on Histological Analysis and Expression of Genes Involved in an Animal Model of Multiple Sclerosis. Mult. Scler. Relat. Disord. 2023, 70, 104471. [Google Scholar] [CrossRef]
- Li, W.T.; Zhang, S.Y.; Zhou, Y.F.; Zhang, B.F.; Liang, Z.Q.; Liu, Y.H.; Wei, Y.; Li, C.K.; Meng, X.J.; Xia, M.; et al. Carvacrol Attenuates Traumatic Neuronal Injury through Store-Operated Ca2+ Entry-Independent Regulation of Intracellular Ca2+ Homeostasis. Neurochem. Int. 2015, 90, 107–113. [Google Scholar] [CrossRef]
- Peters, M.; Trembovler, V.; Alexandrovich, A.; Parnas, M.; Birnbaumer, L.; Minke, B.; Shohami, E. Carvacrol Together with TRPC1 Elimination Improve Functional Recovery after Traumatic Brain Injury in Mice. J. Neurotrauma 2011, 29, 2831–2834. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Lee, S.H.; Choi, S.; Choi, B.Y.; Suh, S.W. Carvacrol Inhibits Expression of Transient Receptor Potential Melastatin 7 Channels and Alleviates Zinc Neurotoxicity Induced by Traumatic Brain Injury. Int. J. Mol. Sci. 2022, 23, 13840. [Google Scholar] [CrossRef] [PubMed]
- Abbasloo, E.; Amiresmaili, S.; Shirazpour, S.; Khaksari, M.; Kobeissy, F.; Thomas, T.C. Satureja khuzistanica Jamzad Essential Oil and Pure Carvacrol Attenuate TBI-Induced Inflammation and Apoptosis via NF-ΚB and Caspase-3 Regulation in the Male Rat Brain. Sci. Rep. 2023, 13, 4780. [Google Scholar] [CrossRef] [PubMed]
- Abbasloo, E.; Khaksari, M.; Sanjari, M.; Kobeissy, F.; Thomas, T.C. Carvacrol Decreases Blood–Brain Barrier Permeability Post-Diffuse Traumatic Brain Injury in Rats. Sci. Rep. 2023, 13, 14546. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.S.; Pu, Z.C.; Hao, Z.H. Carvacrol Protects against Spinal Cord Injury in Rats via Suppressing Oxidative Stress and the Endothelial Nitric Oxide Synthase Pathway. Mol. Med. Rep. 2015, 12, 5349–5354. [Google Scholar] [CrossRef]
- Sol Park, C.; Youn Lee, J.; Young Choi, H.; Gun Ju, B.; Young Yune, T.; Hee, K. Suppression of TRPM7 by Carvacrol Protects against Injured Spinal Cord by Inhibiting Blood-Spinal Cord Barrier Disruption. J. Neurotrauma 2021, 39, 9–10. [Google Scholar] [CrossRef]
- Khalil, A.; Kovac, S.; Morris, G.; Walker, M.C. Carvacrol after Status Epilepticus (SE) Prevents Recurrent SE, Early Seizures, Cell Death, and Cognitive Decline. Epilepsia 2017, 58, 263–273. [Google Scholar] [CrossRef]
- Sadegh, M.; Sakhaie, M.H. Carvacrol Mitigates Proconvulsive Effects of Lipopolysaccharide, Possibly through the Hippocampal Cyclooxygenase-2 Inhibition. Metab. Brain Dis. 2018, 33, 2045–2050. [Google Scholar] [CrossRef] [PubMed]
- Anwar, S.; Khan, A.-u.; Irshad, N. Pharmacological Evaluation of Carvacrol Anti-Migraine Potential. Naunyn Schmiedebergs Arch. Pharmacol. 2023, 396, 1309–1324. [Google Scholar] [CrossRef]
- Li, Z.; Hua, C.; Pan, X.; Fu, X.; Wu, W. Carvacrol Exerts Neuroprotective Effects Via Suppression of the Inflammatory Response in Middle Cerebral Artery Occlusion Rats. Inflammation 2016, 39, 1566–1572. [Google Scholar] [CrossRef]
- Hong, D.K.; Choi, B.Y.; Kho, A.R.; Lee, S.H.; Jeong, J.H.; Kang, B.S.; Kang, D.H.; Park, K.H.; Suh, S.W. Carvacrol Attenuates Hippocampal Neuronal Death after Global Cerebral Ischemia via Inhibition of Transient Receptor Potential Melastatin 7. Cells 2018, 7, 231. [Google Scholar] [CrossRef] [PubMed]
- Guan, X.; Li, X.; Yang, X.; Yan, J.; Shi, P.; Ba, L.; Cao, Y.; Wang, P. The Neuroprotective Effects of Carvacrol on Ischemia/Reperfusion-Induced Hippocampal Neuronal Impairment by Ferroptosis Mitigation. Life Sci. 2019, 235, 116795. [Google Scholar] [CrossRef]
- Çetinkaya, A.; Çamsari, Ç. Effects of Carvacrol Administration on Motor Function Following Spinal Ischemia Andreperfusion. Turk. J. Zool. 2020, 44, 440–445. [Google Scholar] [CrossRef]
- Shahrokhi Raeini, A.; Hafizibarjin, Z.; Rezvani, M.E.; Safari, F.; Afkhami Aghda, F.; Zare Mehrjerdi, F. Carvacrol Suppresses Learning and Memory Dysfunction and Hippocampal Damages Caused by Chronic Cerebral Hypoperfusion. Naunyn Schmiedebergs Arch. Pharmacol. 2020, 393, 581–589. [Google Scholar] [CrossRef]
- Chenet, A.L.; Duarte, A.R.; de Almeida, F.J.S.; Andrade, C.M.B.; de Oliveira, M.R. Carvacrol Depends on Heme Oxygenase-1 (HO-1) to Exert Antioxidant, Anti-Inflammatory, and Mitochondria-Related Protection in the Human Neuroblastoma SH-SY5Y Cells Line Exposed to Hydrogen Peroxide. Neurochem. Res. 2019, 44, 884–896. [Google Scholar] [CrossRef]
- Yılmaz, A.; Hacımüftüoğlu, A.; Çiçek, B.; Isık, M.; Necip, A.; tgzd Tgzd, A. Carvacrol Protect Hippocampal Neurons Against Hydroxychloroquine-Induced Damage: In Vitro Study. Recent Trends Pharmacol. 2023, 1, 16–26. [Google Scholar]
- Celik, F.; Gocmez, C.; Bozkurt, M.; Kaplan, I.; Kamasak, K.; Akil, E.; Dogan, E.; Guzel, A.; Uzar, E. Neuroprotective Effects of Carvacrol and Pomegranate against Methotrexate-Induced Toxicity in Rats. Eur. Rev. Med. Pharmacol. Sci. 2013, 17, 2988–2993. [Google Scholar] [PubMed]
- Wang, P.; Luo, Q.; Qiao, H.; Ding, H.; Cao, Y.; Yu, J.; Liu, R.; Zhang, Q.; Zhu, H.; Qu, L. The Neuroprotective Effects of Carvacrol on Ethanol-Induced Hippocampal Neurons Impairment via the Antioxidative and Antiapoptotic Pathways. Oxid. Med. Cell Longev. 2017, 2017, 4079425. [Google Scholar] [CrossRef]
- Mohebbati, R.; Jalili-Nik, M.; Paseban, M.; Shafei, M.N.; Rad, A.K. Effects of Zataria Multiflora Extract and Carvacrol on Doxorubicin-Induced Oxidative Stress in Rat Brain. Pharm. Sci. 2018, 24, 187–192. [Google Scholar] [CrossRef]
- Noshy, P.A.; Elhady, M.A.; Khalaf, A.A.A.; Kamel, M.M.; Hassanen, E.I. Ameliorative Effect of Carvacrol against Propiconazole-Induced Neurobehavioral Toxicity in Rats. Neurotoxicology 2018, 67, 141–149. [Google Scholar] [CrossRef]
- El-Far, A.H.; Mohamed, H.H.; Elsabagh, D.A.; Mohamed, S.A.; Noreldin, A.E.; Al Jaouni, S.K.; Alsenosy, A.A. Eugenol and Carvacrol Attenuate Brain D-Galactose-Induced Aging-Related Oxidative Alterations in Rats. Environ. Sci. Pollut. Res. 2022, 29, 47436–47447. [Google Scholar] [CrossRef]
- Yıldız, M.O.; Çelik, H.; Caglayan, C.; Genç, A.; Doğan, T.; Satıcı, E. Neuroprotective Effects of Carvacrol against Cadmium-Induced Neurotoxicity in Rats: Role of Oxidative Stress, Inflammation and Apoptosis. Metab. Brain Dis. 2022, 37, 1259–1269. [Google Scholar] [CrossRef]
- Durmus, H.; Burak, A.M.; Goktug, S.; Aysegul, B. Metabolomic Modelling and Neuroprotective Effects of Carvacrol against Acrylamide Toxicity in Rat’s Brain and Sciatic Nerve. Clin. Exp. Pharmacol. Physiol. 2024, 51, e13841. [Google Scholar] [CrossRef]
- Babak, F.; Sadegh, M.; Jalali-Mashayekhi, F.; Sakhaie, M.H. Effects of Carvacrol on Cognitive Function and Apoptotic Gene Expression in Trimethyltin- Induced Hippocampal Injury in Rats. Cell J. (Yakhteh) 2024, 26, 277–284. [Google Scholar] [CrossRef]
- Melo, F.H.C.; Venâncio, E.T.; De Sousa, D.P.; De França Fonteles, M.M.; De Vasconcelos, S.M.M.; Viana, G.S.B.; De Sousa, F.C.F. Anxiolytic-like Effect of Carvacrol (5-Isopropyl-2-Methylphenol) in Mice: Involvement with GABAergic Transmission. Fundam. Clin. Pharmacol. 2010, 24, 437–443. [Google Scholar] [CrossRef]
- Vilmosh, N.; Delev, D.; Kostadinov, I.; Zlatanova, H.; Kotetarova, M.; Kandilarov, I.; Kostadinova, I. Anxiolytic Effect of Satureja Montana Dry Extract and Its Active Compounds Rosmarinic Acid and Carvacrol in Acute Stress Experimental Model. J. Integr. Neurosci. 2022, 21, 124. [Google Scholar] [CrossRef] [PubMed]
- Melo, F.H.C.; Moura, B.A.; de Sousa, D.P.; de Vasconcelos, S.M.M.; Macedo, D.S.; Fonteles, M.M.d.F.; Viana, G.S.d.B.; de Sousa, F.C.F. Antidepressant-like Effect of Carvacrol (5-Isopropyl-2-Methylphenol) in Mice: Involvement of Dopaminergic System. Fundam. Clin. Pharmacol. 2011, 25, 362–367. [Google Scholar] [CrossRef] [PubMed]
- Samarghandian, S.; Farkhondeh, T.; Samini, F.; Borji, A. Protective Effects of Carvacrol against Oxidative Stress Induced by Chronic Stress in Rat’s Brain, Liver, and Kidney. Biochem. Res. Int. 2016, 2016, 2645237. [Google Scholar] [CrossRef]
- Elhady, M.A.; Khalaf, A.A.A.; Kamel, M.M.; Noshy, P.A. Carvacrol Ameliorates Behavioral Disturbances and DNA Damage in the Brain of Rats Exposed to Propiconazole. Neurotoxicology 2019, 70, 19–25. [Google Scholar] [CrossRef]
- Bayraktar, D.; Ertas, B.; Aydın, Y.; Sener, G. Carvacrol Improves Cognitive Dysfunction by Decreasing Amyloid-ß Accumulation and Regulating Neuroinflammation in Ovariectomized Renovascular Hypertensive Rats. 2023. Available online: https://ssrn.com/abstract=4361585 (accessed on 20 August 2024).
- Hakimi, Z.; Salmani, H.; Marefati, N.; Arab, Z.; Gholamnezhad, Z.; Beheshti, F.; Shafei, M.N.; Hosseini, M. Protective Effects of Carvacrol on Brain Tissue Inflammation and Oxidative Stress as Well as Learning and Memory in Lipopolysaccharide-Challenged Rats. Neurotox. Res. 2020, 37, 965–976. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Yeom, M.; Shim, I.; Lee, H.; Hahm, D.H. Inhibitory Effect of Carvacrol on Lipopolysaccharide-Induced Memory Impairment in Rats. Korean J. Physiol. Pharmacol. 2020, 24, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Salmani, H.; Hakimi, Z.; Arab, Z.; Marefati, N.; Mahdinezhad, M.R.; RezaeiGolestan, A.; Beheshti, F.; Soukhtanloo, M.; Mahnia, A.A.; Hosseini, M. Carvacrol Attenuated Neuroinflammation, Oxidative Stress and Depression and Anxiety like Behaviors in Lipopolysaccharide-Challenged Rats. Avicenna J. Phytomed. 2022, 12, 514–526. [Google Scholar] [CrossRef]
- Amooheydari, Z.; Rajaei, Z.; Alaei, H.; Esmaeil, N. Supplementation of Carvacrol Attenuates Hippocampal Tumor Necrosis Factor-Alpha Level, Oxidative Stress, and Learning and Memory Dysfunction in Lipopolysaccharide-Exposed Rats. Adv. Biomed. Res. 2022, 11, 33. [Google Scholar] [CrossRef]
- Gabrielle, A.; Dantas, B.; Limongi De Souza, R.; Rodrigues De Almeida, A.; Humberto, F.; Júnior, X.; Galdino Da Rocha Pitta, M.; Barreto, J.; Rêgo, M.; Oliveira, E.E.; et al. Development, Characterization, and Immunomodulatory Evaluation of Carvacrol-Loaded Nanoemulsion. Molecules 2021, 26, 3899. [Google Scholar] [CrossRef]
- Sisti, F.M.; dos Santos, N.A.G.; do Amaral, L.; dos Santos, A.C. The Neurotrophic-Like Effect of Carvacrol: Perspective for Axonal and Synaptic Regeneration. Neurotox. Res. 2021, 39, 886–896. [Google Scholar] [CrossRef]
- Yu, H.; Zhang, Z.L.; Chen, J.; Pei, A.; Hua, F.; Qian, X.; He, J.; Liu, C.F.; Xu, X. Carvacrol, a Food-Additive, Provides Neuroprotection on Focal Cerebral Ischemia/Reperfusion Injury in Mice. PLoS ONE 2012, 7, e33584. [Google Scholar] [CrossRef]
- Zotti, M.; Colaianna, M.; Morgese, M.G.; Tucci, P.; Schiavone, S.; Avato, P.; Trabace, L. Carvacrol: From Ancient Flavoring to Neuromodulatory Agent. Molecules 2013, 18, 6161–6172. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Z.; Wang, B.; Dai, M.; Sun, Y.; Sun, Q.; Yang, G.; Bian, L. Carvacrol Alleviates Cerebral Edema by Modulating AQP4 Expression after Intracerebral Hemorrhage in Mice. Neurosci. Lett. 2013, 555, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Chun, Y.L.; Kim, M.; Kim, Y.H.; Kim, N.; Yang, H.; Park, C.; Huh, Y.; Jung, J. Carvacrol Effectively Protects Demyelination by Suppressing Transient Receptor Potential Melastatin 7 (TRPM7) in Schwann Cells. Anat. Sci. Int. 2020, 95, 230–239. [Google Scholar] [CrossRef]
- Zare Mehrjerdi, F.; Niknazar, S.; Yadegari, M.; Akbari, F.A.; Pirmoradi, Z.; Khaksari, M. Carvacrol Reduces Hippocampal Cell Death and Improves Learning and Memory Deficits Following Lead-Induced Neurotoxicity via Antioxidant Activity. Naunyn Schmiedebergs Arch. Pharmacol. 2020, 393, 1229–1237. [Google Scholar] [CrossRef]
- Forqani, M.A.; Akbarian, M.; Amirahmadi, S.; Soukhtanloo, M.; Hosseini, M.; Forouzanfar, F. Carvacrol Improved Learning and Memory and Attenuated the Brain Tissue Oxidative Damage in Aged Male Rats. Int. J. Neurosci. 2023, 134, 1242–1249. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Xu, B.; Xiao, A.; Liu, L.; Fang, X.; Liu, R.; Turlova, E.; Barszczyk, A.; Zhong, X.; Sun, C.L.F.; et al. TRPM7 Inhibitor Carvacrol Protects Brain from Neonatal Hypoxic-Ischemic Injury. Mol. Brain 2015, 8, 11. [Google Scholar] [CrossRef]
- Buso, P.; Manfredini, S.; Ahmadi-Ashtiani, H.R.; Sciabica, S.; Buzzi, R.; Vertuani, S.; Baldisserotto, A. Iranian Medicinal Plants: From Ethnomedicine to Actual Studies. Medicina 2020, 56, 97. [Google Scholar] [CrossRef]
- Nooshkam, A.; Mumivand, H.; Hadian, J.; Alemardan, A.; Morshedloo, M.R. Drug Yield and Essential Oil and Carvacrol Contents of Two Species of Satureja (S. khuzistanica Jamzad and S. rechingeri Jamzad) Cultivated in Two Different Locations. J. Appl. Res. Med. Aromat. Plants 2017, 6, 126–130. [Google Scholar] [CrossRef]
- Assaei, R.; Mostafavi-Pour, Z.; Pajouhi, N.; Hossein, G.; Omrani, R.; Sepehrimanesh, M.; Zal, F.; Mostafavi, Z. Effects of Essential Oil of Satureja Khuzestanica on the Oxidative Stress in Experimental Hyperthyroid Male Rat. Vet. Res. Forum 2015, 6, 233–238. [Google Scholar] [PubMed]
- Sarrazin, S.L.F.; Da Silva, L.A.; Oliveira, R.B.; Raposo, J.D.A.; Da Silva, J.K.R.; Salimena, F.R.G.; Maia, J.G.S.; Mourão, R.H.V. Antibacterial Action against Food-Borne Microorganisms and Antioxidant Activity of Carvacrol-Rich Oil from Lippia origanoides Kunth. Lipids Health Dis. 2015, 14, 145. [Google Scholar] [CrossRef]
- Wang, L. The Shennong’s Herbal Canon for Health Management of Herbal Foods. Nat. Anthropol. 2024, 3, 10019. [Google Scholar] [CrossRef]
- Zhang, S.; He, L.; Shang, J.; Chen, L.; Xu, Y.; Chen, X.; Li, X.; Jiao, Q.; Jin, S.; Hu, X.; et al. Carvacrol Suppresses Human Osteosarcoma Cells via the Wnt/β-Catenin Signaling Pathway. Anticancer Agents Med. Chem. 2022, 22, 1714–1722. [Google Scholar] [CrossRef] [PubMed]
- Zheng, K.; Wu, S.Z.; Lv, Y.W.; Pang, P.; Deng, L.; Xu, H.C.; Shi, Y.C.; Chen, X.Y. Carvacrol Inhibits the Excessive Immune Response Induced by Influenza Virus A via Suppressing Viral Replication and TLR/RLR Pattern Recognition. J. Ethnopharmacol. 2021, 268, 113555. [Google Scholar] [CrossRef] [PubMed]
- Al Shoyaib, A.; Archie, S.R.; Karamyan, V.T. Intraperitoneal Route of Drug Administration: Should It Be Used in Experimental Animal Studies? Pharm. Res. 2020, 37, 12. [Google Scholar] [CrossRef]
- Jones, C.P.; Boyd, K.L.; Wallace, J.M. Evaluation of Mice Undergoing Serial Oral Gavage While Awake or Anesthetized. J. Am. Assoc. Lab. Anim. Sci. 2016, 55, 805–810. [Google Scholar] [PubMed]
- Larcombe, A.N.; Wang, K.C.W.; Phan, J.A.; Berry, L.J.; Noble, P.B. Confounding Effects of Gavage in Mice: Impaired Respiratory Structure and Function. Am. J. Respir. Cell Mol. Biol. 2019, 61, 791–794. [Google Scholar] [CrossRef] [PubMed]
- Bédard, P.; Gauvin, S.; Ferland, K.; Caneparo, C.; Pellerin, È.; Chabaud, S.; Bolduc, S. Innovative Human Three-Dimensional Tissue-Engineered Models as an Alternative to Animal Testing. Bioengineering 2020, 7, 115. [Google Scholar] [CrossRef]
- Olufunmilayo, E.O.; Gerke-Duncan, M.B.; Holsinger, R.M.D. Oxidative Stress and Antioxidants in Neurodegenerative Disorders. Antioxidants 2023, 12, 517. [Google Scholar] [CrossRef]
- De Almeida, A.J.P.O.; De Oliveira, J.C.P.L.; Da Silva Pontes, L.V.; De Souza Júnior, J.F.; Gonçalves, T.A.F.; Dantas, S.H.; De Almeida Feitosa, M.S.; Silva, A.O.; De Medeiros, I.A. ROS: Basic Concepts, Sources, Cellular Signaling, and Its Implications in Aging Pathways. Oxid. Med. Cell Longev. 2022, 2022, 1225578. [Google Scholar] [CrossRef]
- Kumar, S.; Saxena, J.; Srivastava, V.K.; Kaushik, S.; Singh, H.; Abo-EL-Sooud, K.; Abdel-Daim, M.M.; Jyoti, A.; Saluja, R. The Interplay of Oxidative Stress and ROS Scavenging: Antioxidants as a Therapeutic Potential in Sepsis. Vaccines 2022, 10, 1575. [Google Scholar] [CrossRef]
- Jena, A.B.; Samal, R.R.; Bhol, N.K.; Duttaroy, A.K. Cellular Red-Ox System in Health and Disease: The Latest Update. Biomed. Pharmacother. 2023, 162, 114606. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Jones, D.P. Reactive Oxygen Species (ROS) as Pleiotropic Physiological Signalling Agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, A.G.; Oliveira, G.F.; Melo, M.S.; Cavalcanti, S.C.H.; Antoniolli, A.R.; Bonjardim, L.R.; Silva, F.A.; Santos, J.P.A.; Rocha, R.F.; Moreira, J.C.F.; et al. Bioassay-Guided Evaluation of Antioxidant and Antinociceptive Activities of Carvacrol. Basic Clin. Pharmacol. Toxicol. 2010, 107, 949–957. [Google Scholar] [CrossRef]
- Ali, T.; Majeed, S.T.; Majeed, R.; Bashir, R.; Mir, S.A.; Jan, I.; Bader, G.N.; Andrabi, K.I. Recent Advances in the Pharmacological Properties and Molecular Mechanisms of Carvacrol. Rev. Bras. Farmacogn. 2024, 34, 35–47. [Google Scholar] [CrossRef]
- Gąssowska-Dobrowolska, M.; Chlubek, M.; Kolasa, A.; Tomasiak, P.; Korbecki, J.; Skowrońska, K.; Tarnowski, M.; Masztalewicz, M.; Baranowska-Bosiacka, I. Microglia and Astroglia—The Potential Role in Neuroinflammation Induced by Pre- and Neonatal Exposure to Lead (Pb). Int. J. Mol. Sci. 2023, 24, 9903. [Google Scholar] [CrossRef]
- Kwon, H.S.; Koh, S.H. Neuroinflammation in Neurodegenerative Disorders: The Roles of Microglia and Astrocytes. Transl. Neurodegener. 2020, 9, 42. [Google Scholar] [CrossRef]
- Cicalău, G.I.P.; Babes, P.A.; Calniceanu, H.; Popa, A.; Ciavoi, G.; Iova, G.M.; Ganea, M.; Scrobotă, I. Anti-Inflammatory and Antioxidant Properties of Carvacrol and Magnolol, in Periodontal Disease and Diabetes Mellitus. Molecules 2021, 26, 6899. [Google Scholar] [CrossRef] [PubMed]
- Somensi, N.; Rabelo, T.K.; Guimarães, A.G.; Quintans-Junior, L.J.; de Souza Araújo, A.A.; Moreira, J.C.F.; Gelain, D.P. Carvacrol Suppresses LPS-Induced pro-Inflammatory Activation in RAW 264.7 Macrophages through ERK1/2 and NF-KB Pathway. Int. Immunopharmacol. 2019, 75, 105743. [Google Scholar] [CrossRef] [PubMed]
- Avola, R.; Furnari, A.G.; Graziano, A.C.E.; Russo, A.; Cardile, V. Management of the Brain: Essential Oils as Promising Neuroinflammation Modulator in Neurodegenerative Diseases. Antioxidants 2024, 13, 178. [Google Scholar] [CrossRef]
- Nguyen, T.T.M.; Gillet, G.; Popgeorgiev, N. Caspases in the Developing Central Nervous System: Apoptosis and Beyond. Front. Cell Dev. Biol. 2021, 9, 702404. [Google Scholar] [CrossRef]
- Fricker, M.; Tolkovsky, A.M.; Borutaite, V.; Coleman, M.; Brown, G.C. Neuronal Cell Death. Physiol. Rev. 2018, 98, 813–880. [Google Scholar] [CrossRef]
- Zhang, H.; Li, N.; Li, Z.; Li, Y.; Yu, Y.; Zhang, L. The Involvement of Caspases in Neuroinflammation and Neuronal Apoptosis in Chronic Pain and Potential Therapeutic Targets. Front. Pharmacol. 2022, 13, 898574. [Google Scholar] [CrossRef]
- Qin, C.; Bai, L.; Li, Y.; Wang, K. The Functional Mechanism of Bone Marrow-Derived Mesenchymal Stem Cells in the Treatment of Animal Models with Alzheimer’s Disease: Crosstalk between Autophagy and Apoptosis. Stem Cell Res. Ther. 2022, 13, 90. [Google Scholar] [CrossRef] [PubMed]
- Trinetta, V.; Morgan, M.T.; Coupland, J.N.; Yucel, U. Essential Oils Against Pathogen and Spoilage Microorganisms of Fruit Juices: Use of Versatile Antimicrobial Delivery Systems. J. Food Sci. 2017, 82, 471–476. [Google Scholar] [CrossRef] [PubMed]
- Majeed, H.; Bian, Y.-Y.; Ali, B.; Jamil, A.; Majeed, U.; Khan, Q.F.; Iqbal, K.J.; Shoemaker, C.F.; Fang, Z. Essential Oil Encapsulations: Uses, Procedures, and Trends. RSC Adv. 2015, 5, 58449–58463. [Google Scholar] [CrossRef]
- Liao, W.; Badri, W.; Dumas, E.; Ghnimi, S.; Elaissari, A.; Saurel, R.; Gharsallaoui, A. Nanoencapsulation of Essential Oils as Natural Food Antimicrobial Agents: An Overview. Appl. Sci. 2021, 11, 5778. [Google Scholar] [CrossRef]
- Sousa, V.I.; Parente, J.F.; Marques, J.F.; Forte, M.A.; Tavares, C.J. Microencapsulation of Essential Oils: A Review. Polymers 2022, 14, 1730. [Google Scholar] [CrossRef] [PubMed]
- Yammine, J.; Chihib, N.E.; Gharsallaoui, A.; Ismail, A.; Karam, L. Advances in Essential Oils Encapsulation: Development, Characterization and Release Mechanisms. Polym. Bull. 2023, 81, 3837–3882. [Google Scholar] [CrossRef]
- de Souza, R.L.; Dantas, A.G.B.; Melo, C.d.O.; Felício, I.M.; Oliveira, E.E. Nanotechnology as a Tool to Improve the Biological Activity of Carvacrol: A Review. J. Drug Deliv. Sci. Technol. 2022, 76, 103834. [Google Scholar] [CrossRef]
- Bellettato, C.M.; Scarpa, M. Possible Strategies to Cross the Blood-Brain Barrier. Ital. J. Pediatr. 2018, 44, 131. [Google Scholar] [CrossRef]

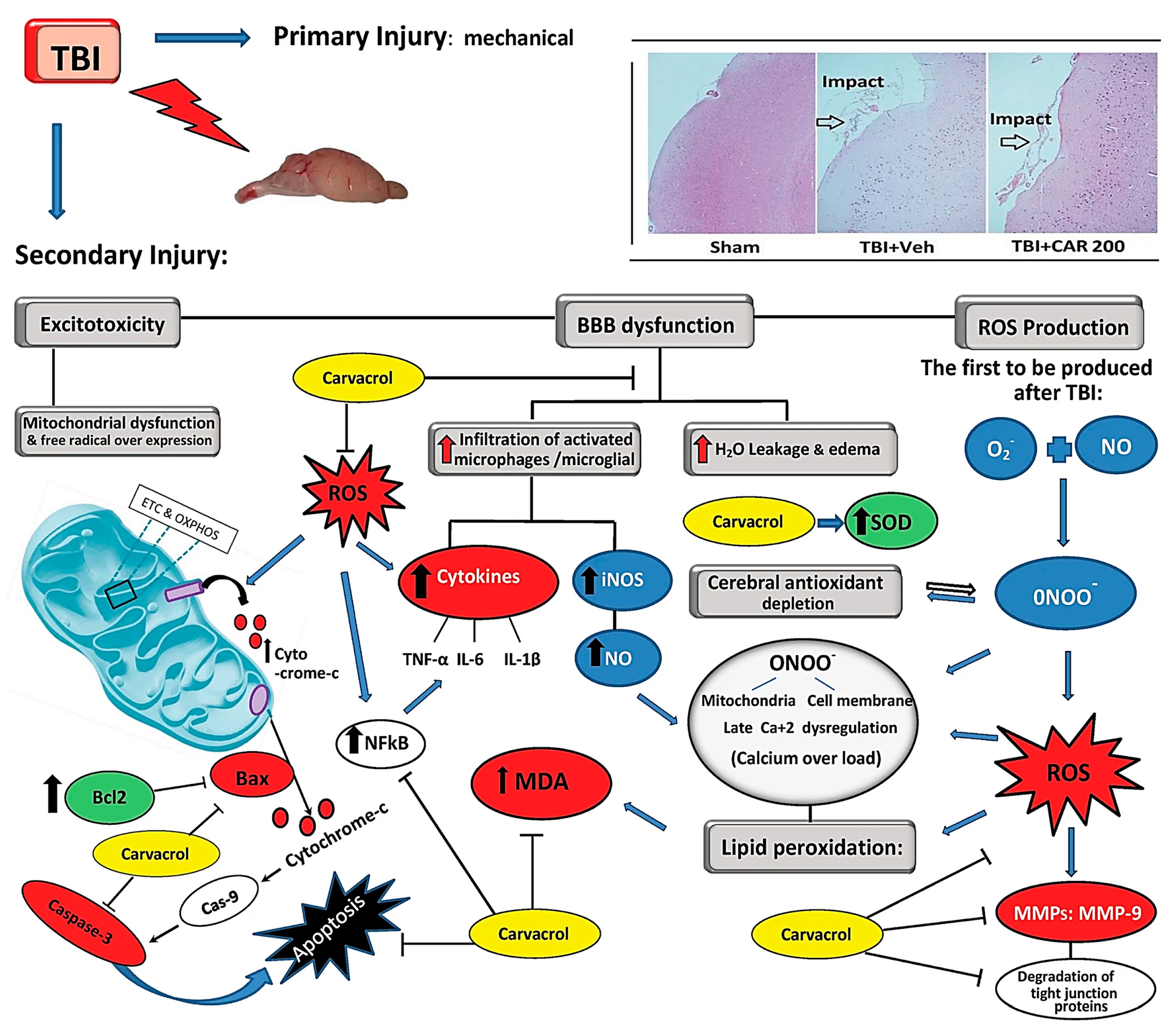

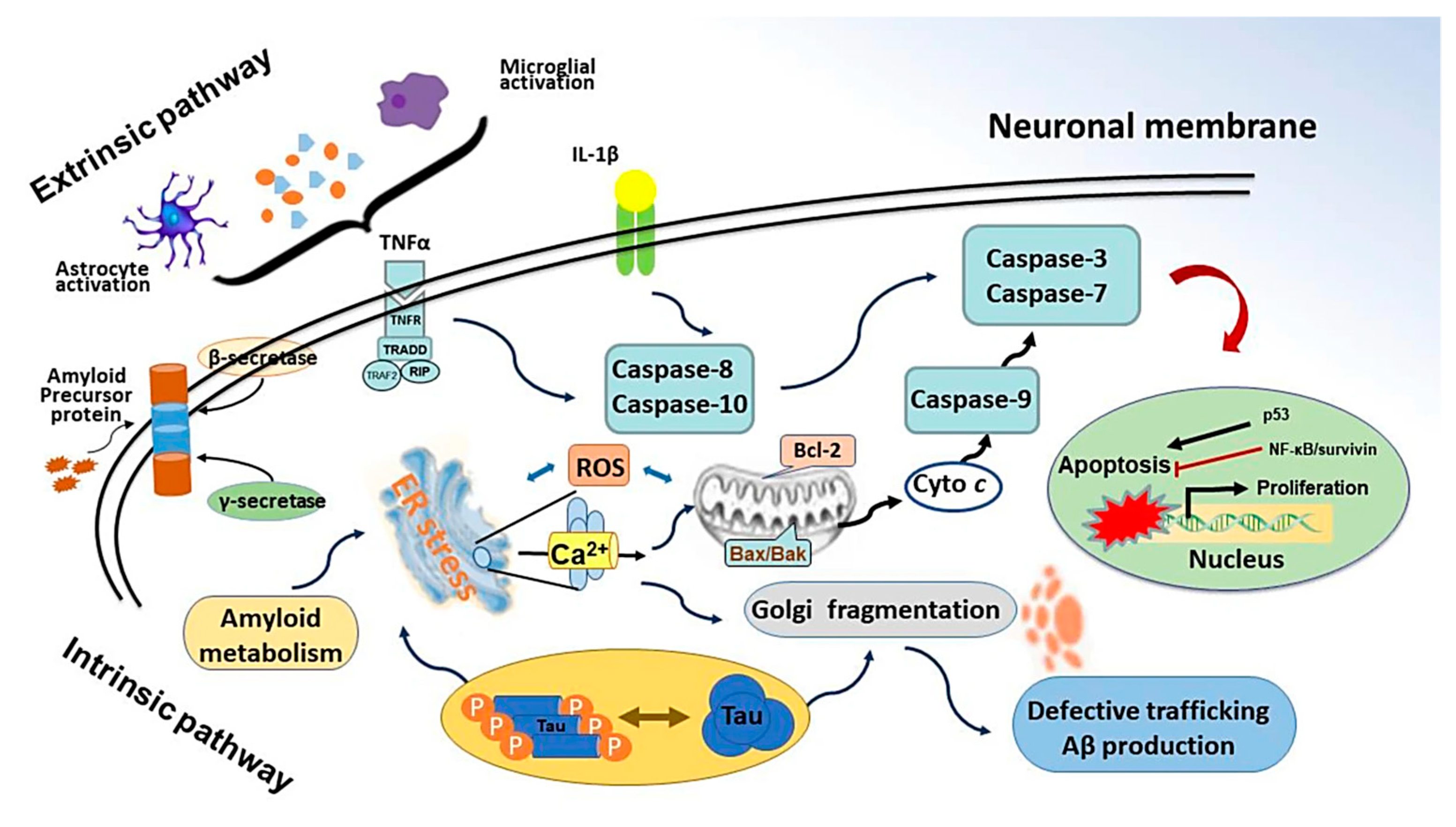
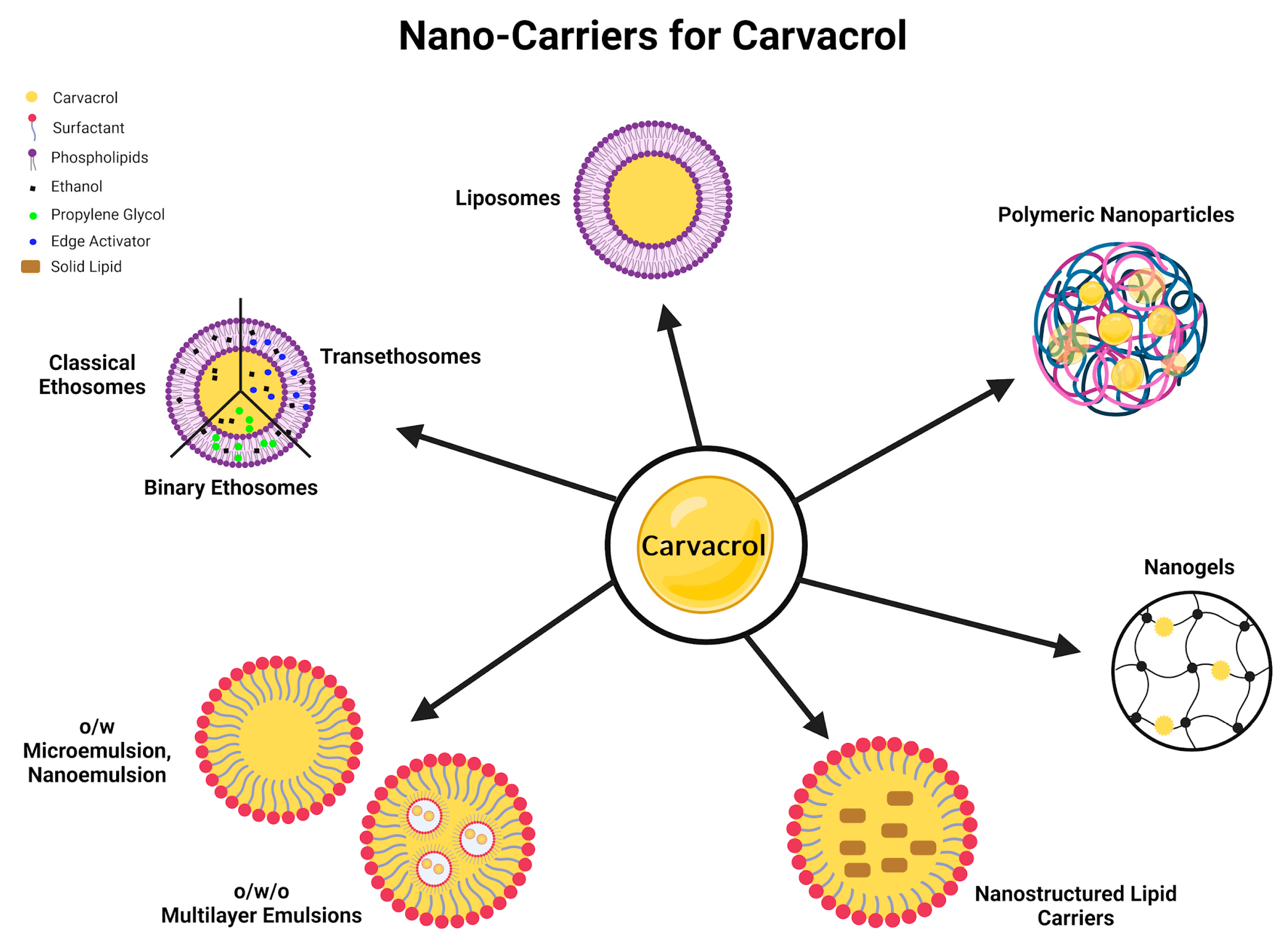
| No. | Study Type | Ref. | Formulation Type | Doses of CV | Experiment Timespan (Days) | Test Subject | Route | Analyses Techniques | Key Findings and Remarks |
|---|---|---|---|---|---|---|---|---|---|
| 1 | In vivo | [11] | Solution (saline) | 40 mg/kg | 15 | Male mice (C57BL/6) | IP |
|
|
| 2 | [12] | Solution | 12.5 and 25 mg/kg | 28 | Male Wistar Rats | IP |
|
| |
| 3 | [13] | Solution | 25 mg/kg | 49 | Male Wistar Rats | IP |
|
| |
| 4 | [14] | Solution | 10 mg/kg | 14 | Male Spraque-Dawley rats | IP |
|
| |
| 5 | [15] | Solution | 25 mg/kg, 50 mg/kg, 100 mg/kg | 21 | Swiss Albino Mice | PO |
|
| |
| 6 | In-vitro and in vivo | [16] | Solution | 10, 15, and 20 mg/kg | 15 | PC12 cell-based neuronal model and Male Albino Wistar Rats | IP |
|
|
| No. | Study Type | Ref. | Formulation Type | Doses of CV | Experiment Timespan (Days) | Test Subject | Route | Analyses Techniques | Key Findings and Remarks |
|---|---|---|---|---|---|---|---|---|---|
| 1 | In vitro | [17] | Solution | 1 to 1000 µM | 2 | PC12 cell-based neuronal model | - |
MTT cell viability assay Measurement of ROS generation Kinase activity assay |
CV along with thymol demonstrated a protective effect in PC12 cells against Aβ25–35. Moreover, increased antioxidant activity and expression of protein kinases C (PKC) were proposed to be related to the protection of memory and cognitive functioning. |
| 2 | [18] | Solution | 5, 10, 25, 50, and 100 µg/mL | 1 | Human neuroblastoma (SH-SY5Y) cancer cells | - |
Analysis of cholinesterase and a-amylase inhibition Enzyme activity Hydrogen peroxide scavenging assay MTT assay | p-cymene and CV exhibited anti-enzymatic properties and may function as neuroprotective agents against oxidative stress in AD patients. The inhibitory impact of CV on acetylcholinesterase (AChE) via the reduction in caspase-3 expression was found to be fourfold greater than that of p-cymene. The authors reported that the activity of CV was due to presence of an OH group in its structure. | |
| 3 | In vivo | [19] | Oil and nanoemulsion | 20 µL/kg of either oil or nanoemulsion | 30 | Male Wistar Albino Rats | PO |
Analysis of brain cholinesterase (quantitative colorimetric kinetic assay) Analysis of brain monoamines Determination of urinary 8-hydroxy-2′-deoxyguanosine (8-OHdG), immunohistochemistry of brain cyclooxygenase |
CV oil and CV nanoemulsion were found to be significant in their ability to reverse AlCl3-induced brain AD, which could be attributed to the antioxidant and anti-inflammatory properties of CV, modifying the effects of oxidative stress. In addition, it was noted that CV nanoemulsions provided a more effective and efficient method of delivering CV across the blood–brain barrier and ameliorating any brain alterations as compared to oil. |
| 4 | [20] | Solution | 0.5, 1, or 2 mL/kg | 5 | Male Wistar Albino rats | IP |
Behavioural test (Morris water maze test) Kinase activity assay Western blot Histopathological examination |
In an AD model of rat brains, thymol and CV enhanced learning and memory deficits by stimulating hippocampal PKC signalling. As the modulation of PKC activity has the potential to improve cognitive function and potentially alter the pathophysiology of AD, this upregulation of PKC by CV and thymol could prove to be potential therapeutic strategies in AD. | |
| 5 | [21] | Solution | 50 mg/kg | 56 | Male Wistar rats | PO | Determination of population spike (PS) amplitude and field excitatory postsynaptic potentials slope |
CV or p-cymene alone was found to be effective in preventing synaptic plasticity impairment in an AD model. A potential interaction is reported between CV and p-cymene, as their combined therapy did not prevent the adverse outcomes of Aβ1–42 on synaptic plasticity. | |
| 6 | In vitro and in vivo | [22] | Solution | 100, 200, and 300 µM for cell lines and 1 mg/kg IP injection for rats twice daily | 6 | SH-SY5Y neuroblastoma cells and Male Wistar Rats | IP |
MTT assay Determination of oxidative stress-related biomarkers and Tau peptide in cell culture supernatant Assay procedures for SOD, MDA, and Tau peptide Assay procedure for H2O2 |
CV prevented the release of LDH. CV controlled the levels of MDH and H2O2 in vitro; however, it had no effect on these parameters in vivo. CV-treated rats demonstrated memory impairment in vivo. In a nutshell, CV is a multitarget pharmacological agent that shows potential in treating AD by inhibiting AChE activity, neuronal toxicity, oxidative stress, neuroinflammation, and memory problems linked to the disease’s aetiology. |
| No. | Study Type | Ref. | Formulation Type | Doses of CV | Experiment Timespan (Days) | Test Subject | Route | Analyses Techniques | Key Findings and Remarks |
|---|---|---|---|---|---|---|---|---|---|
| 1 | In vivo | [23] | Solution | 5 and 10 mg/kg | 21 | Female C57BL/6 mice | IP |
|
|
| 2 | [24] | Solution | 20 mg/kg | 28 | Female Lewis Rats | IP |
|
| |
| 3 | [25] | Solution | 25 mg/kg | 29 | Female Lewis Rats | IP |
|
|
| No. | Study Type | Ref. | Formulation Type | Doses of CV | Experiment Timespan (Days) | Test Subject | Route | Analyses Techniques | Key Findings and Remarks |
|---|---|---|---|---|---|---|---|---|---|
| 1 | In vitro | [26] | Solution | 0.5 and 1 mM | <1 | Cortical neurons cell culture | - |
|
|
| 2 | In vivo | [27] | Solution | 75, 750 mg/kg, and 3.75 g/kg | >21 | Sabra Mice and C57BL/6 mice (wild type) | IP |
|
|
| 3 | [28] | Solution | 50 mg/kg | 7 | Male Sprague Dawley rats | IP |
|
| |
| 4 | [29] | Solution | 100 and 200 mg/kg | 1 | Male Wistar Rats | IP |
|
| |
| 5 | [30] | Solution | 100–200 mg/kg | 1 | Male Wister rats | IP |
|
| |
| 6 | [31] | Solution | 25, 50, and 100 mg/kg | 46 | Wistar Rats | IP |
|
| |
| 7 | [32] | Solution | 50 mg/kg | 7 | Male Sprague Dawley rats | IP |
|
|
| No. | Study Type | Ref. | Formulation Type | Doses of CV | Experiment Timespan (Days) | Test Subject | Route | Analyses Techniques | Key Findings and Remarks |
|---|---|---|---|---|---|---|---|---|---|
| 1 | In vivo | [33] | Solution | 75 mg/kg | 10 | Male Sprague–Dawley rats | IP |
|
|
| 2 | [34] | Solution | 100 mg/kg | <1 | Male Wistar rats | IP |
|
| |
| 3 | [35] | Solution | 25 and 50 mg/kg | 14 | Female Sprague–Dawley rats | - |
|
| |
| 4 | [36] | Solution | 10, 20, and 40 mg/kg | <1 | Male Sprague–Dawley rats | IP |
|
| |
| 5 | [37] | Solution | 50 mg/kg | 3 | Male Sprague Dawley rats | IP |
|
| |
| 6 | [38] | Solution | 25, 50, and 100 mg/kg | 14 | Male gerbils | IP |
|
| |
| 7 | [39] | Solution | 100 mg/kg | 2 | Wistar rats | IV |
|
| |
| 8 | [40] | Solution | 25 and 50 mg/kg | 56 | Male Wistar rats | PO |
|
|
| No. | Study Type | Ref. | Formulation Type | Doses of CV | Experiment Timespan (Days) | Test Subject | Route | Analyses Techniques | Key Findings and Remarks |
|---|---|---|---|---|---|---|---|---|---|
| 1 | In vitro | [41] | Solution | 10 to 1000 µM | <1 | Human neuroblastoma SH-SY5Y cell line | - |
|
|
| 2 | [42] | Solution | 10, 25, 50, and 100 mg/mL | 2 | Cortex neurons cell culture from Sprague Dawley rats; 24 h after birth | - |
|
| |
| 3 | In vivo | [43] | Solution | 73 mg/kg | 2 | Male Wistar albino rats | IP |
|
|
| 4 | [44] | Solution | 25, 50, and 100 mg/kg | 28 | Male C57BL/6 mice | IP |
|
| |
| 5 | [45] | Solution | 20 mg/kg | 28 | Male Wistar rats | PO |
|
| |
| 6 | [46] | Solution | 50 mg/kg | 56 | Male Albino rats | PO |
|
| |
| 7 | [47] | Solution | 40 and 80 mg/kg | 42 | Male Wistar rats | PO |
|
| |
| 8 | [48] | Solution | 25 and 50 mg/kg | 7 | Male Sprague Dawley rats | PO |
|
| |
| 9 | [49] | Solution | 50 mg/kg | 15 | Male Wister rats | IP |
|
| |
| 10 | [50] | Solution | 40 and 70 mg/kg | 21 | Male Wistar rats | IP |
|
|
| No. | Study Type | Ref. | Formulation Type | Doses of CV | Experiment Timespan (Days) | Test Subject | Route | Analyses Techniques | Key Findings and Remarks |
|---|---|---|---|---|---|---|---|---|---|
| 1 | In vivo | [51] | Solution | 12.5, 25, and 50 mg/ kg | <1 | Male Swiss Mice | PO |
|
|
| 2 | [52] | Solution (olive oil) | 500 mg/kg | 14 | Wistar Rats | PO |
|
| |
| 3 | [53] | Solution | 12.5, 25, and 50 mg/ kg | <1 | Male Swiss Mice | PO |
|
| |
| 4 | [54] | Solution | 20, 30, and 40 mg/kg | 21 | Albino Wistar Rats | IP |
|
| |
| 5 | [55] | Solution | 50 mg/kg | 60 | Sprague Dawley Rats | PO |
|
| |
| 6 | [56] | Solution | 40 mg/kg | 7 | Female Sprague-Dawley Rats | PO |
|
|
| No. | Study Type | Ref. | Formulation Type | Doses of CV | Experiment Timespan (Days) | Test Subject | Route | Analyses Techniques | Key Findings and Remarks |
|---|---|---|---|---|---|---|---|---|---|
| 1 | In vivo | [57] | Solution | 25, 50, and 100 mg/kg | 7 | Male Wistar Rats | IP |
|
|
| 2 | [58] | Solution | 25, 50, and 100 mg/kg | 28 | Male Sprague-Dawley Rats | IP |
|
| |
| 3 | [59] | Solution | 25, 50, and 100 mg/kg | 7 | Rats | IP |
|
| |
| 4 | [60] | Solution | 25 and 50 mg/kg | 19 | Male Wistar Rats | IP |
|
|
| No. | Study Type | Ref. | Formulation Type | Doses of CV | Experiment Timespan (Days) | Test Subject | Route | Analyses Techniques | Key Findings and Remarks |
|---|---|---|---|---|---|---|---|---|---|
| 1 | In vitro | [61] | Nanoemulsion | 25 and 50 µM | 90 | Peripheral blood mononuclear cell (PBMC) culture supernatants | - |
|
|
| 2 | [62] | Solution | 12.5 to 800 µM | 3 | PC12 cell-based neuronal model | - |
|
| |
| 3 | In vivo | [63] | Solution | 10 µg (i.c.v) and 5, 25, and 50 mg/kg (IP) | <1 | Male ICR mice | ICV and IP |
|
|
| 4 | [64] | Solution (peanut oil) | 12.5 mg /kg for 7 days and 150 or 450 mg/kg for acute single doses | 7 | Male Wistar rats | PO |
|
| |
| 5 | [65] | Solution | 25, 50, 75, and 100 mg/kg | 7 | Male C57BL/6 mice | IP |
|
| |
| 6 | [66] | Solution | 3 mM | 3 | Male Sprague-Dawley rats | - |
|
| |
| 7 | [67] | Solution | 25, 50, and 100 mg/kg | 40 | Male Wistar rats | PO |
|
| |
| 8 | [68] | Solution | 15–30 mg/kg | 28 | Rats | IP |
|
| |
| 9 | In vitro and in vivo | [69] | Solution | >200 mM in cell cultures and 30 or 50 mg/kg in vivo | 7 | Timed pregnant CD1 mice and HEK293 cell cultures | IP |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tareen, F.K.; Catenacci, L.; Perteghella, S.; Sorrenti, M.; Bonferoni, M.C. Carvacrol Essential Oil as a Neuroprotective Agent: A Review of the Study Designs and Recent Advances. Molecules 2025, 30, 104. https://doi.org/10.3390/molecules30010104
Tareen FK, Catenacci L, Perteghella S, Sorrenti M, Bonferoni MC. Carvacrol Essential Oil as a Neuroprotective Agent: A Review of the Study Designs and Recent Advances. Molecules. 2025; 30(1):104. https://doi.org/10.3390/molecules30010104
Chicago/Turabian StyleTareen, Fahad Khan, Laura Catenacci, Sara Perteghella, Milena Sorrenti, and Maria Cristina Bonferoni. 2025. "Carvacrol Essential Oil as a Neuroprotective Agent: A Review of the Study Designs and Recent Advances" Molecules 30, no. 1: 104. https://doi.org/10.3390/molecules30010104
APA StyleTareen, F. K., Catenacci, L., Perteghella, S., Sorrenti, M., & Bonferoni, M. C. (2025). Carvacrol Essential Oil as a Neuroprotective Agent: A Review of the Study Designs and Recent Advances. Molecules, 30(1), 104. https://doi.org/10.3390/molecules30010104









