Reconstructing Kaolinite Compounds for Remarkably Enhanced Adsorption of Congo Red
Abstract
1. Introduction
2. Results and Discussion
2.1. Characterization
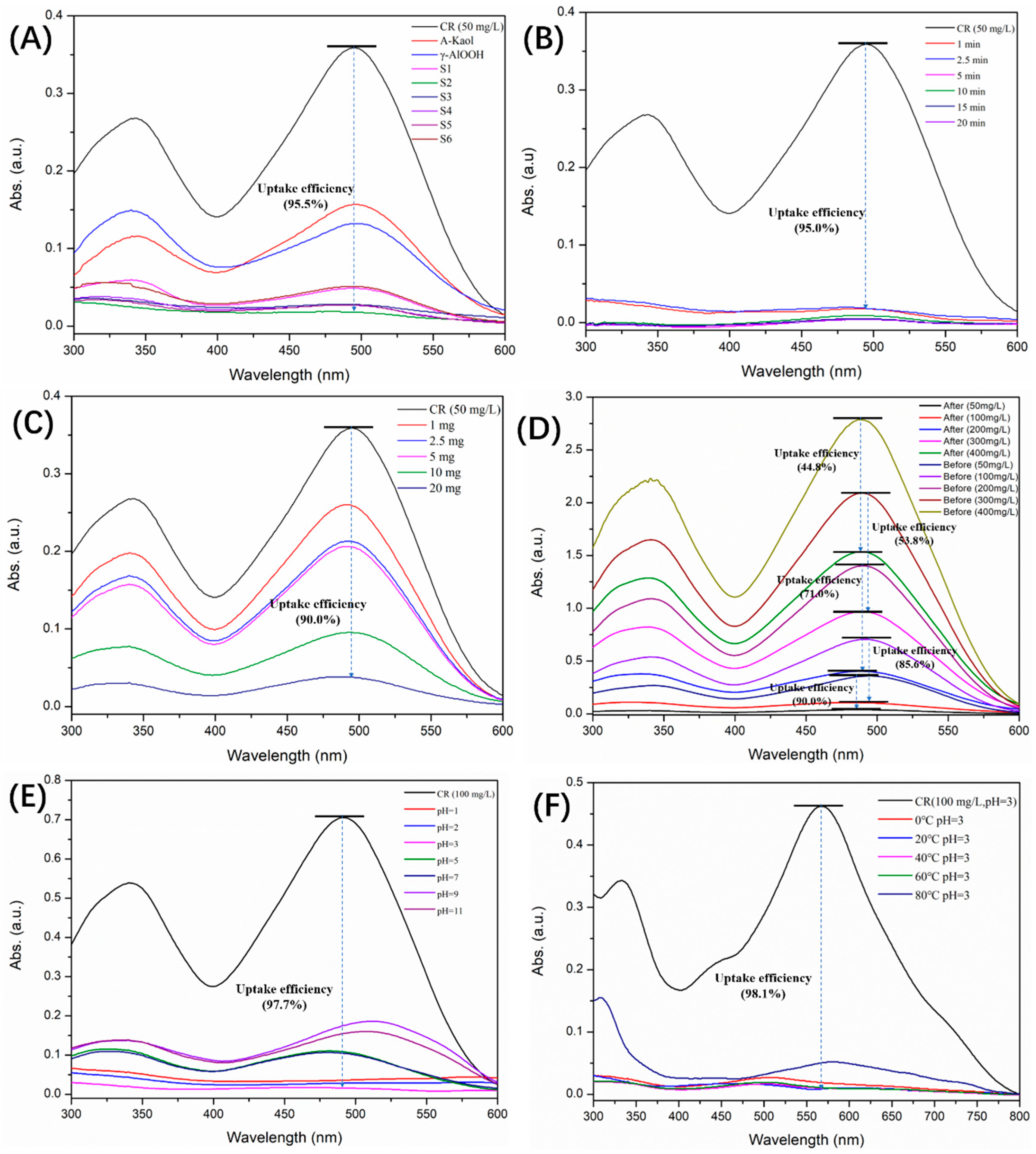
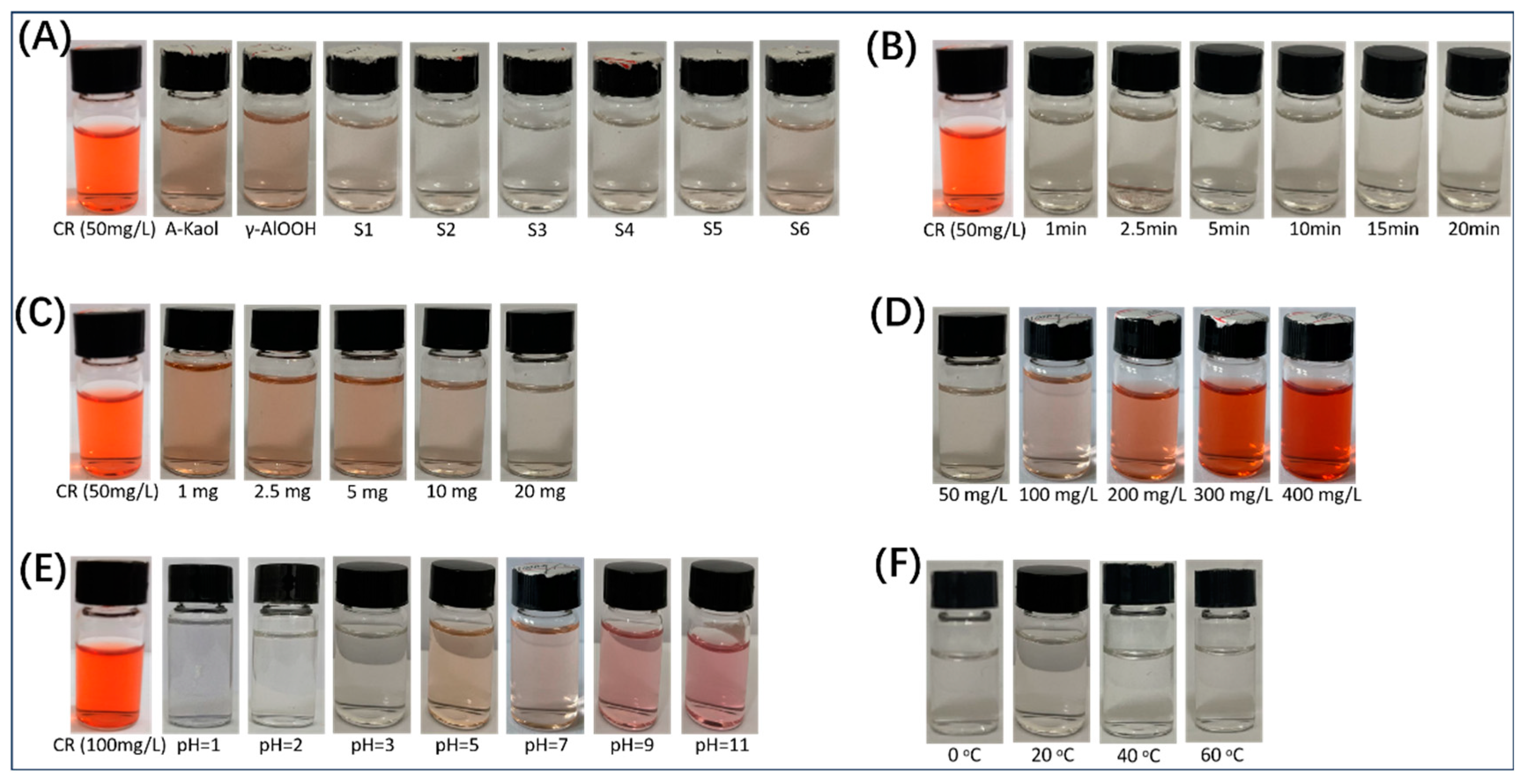
2.2. Adsorption Properties of γ-AlOOH@A-Kaol
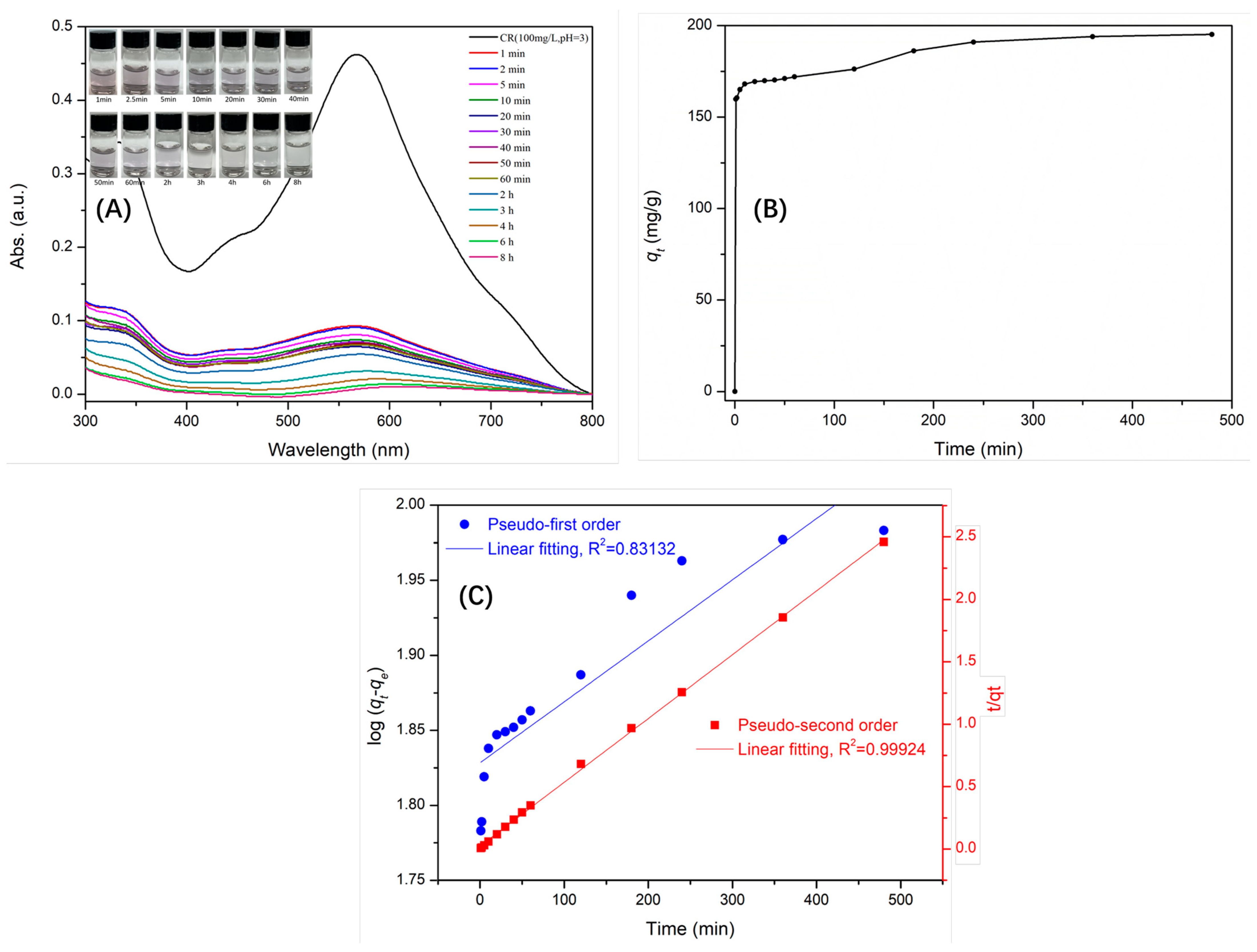
2.3. Adsorption Isotherm and Kinetic Models
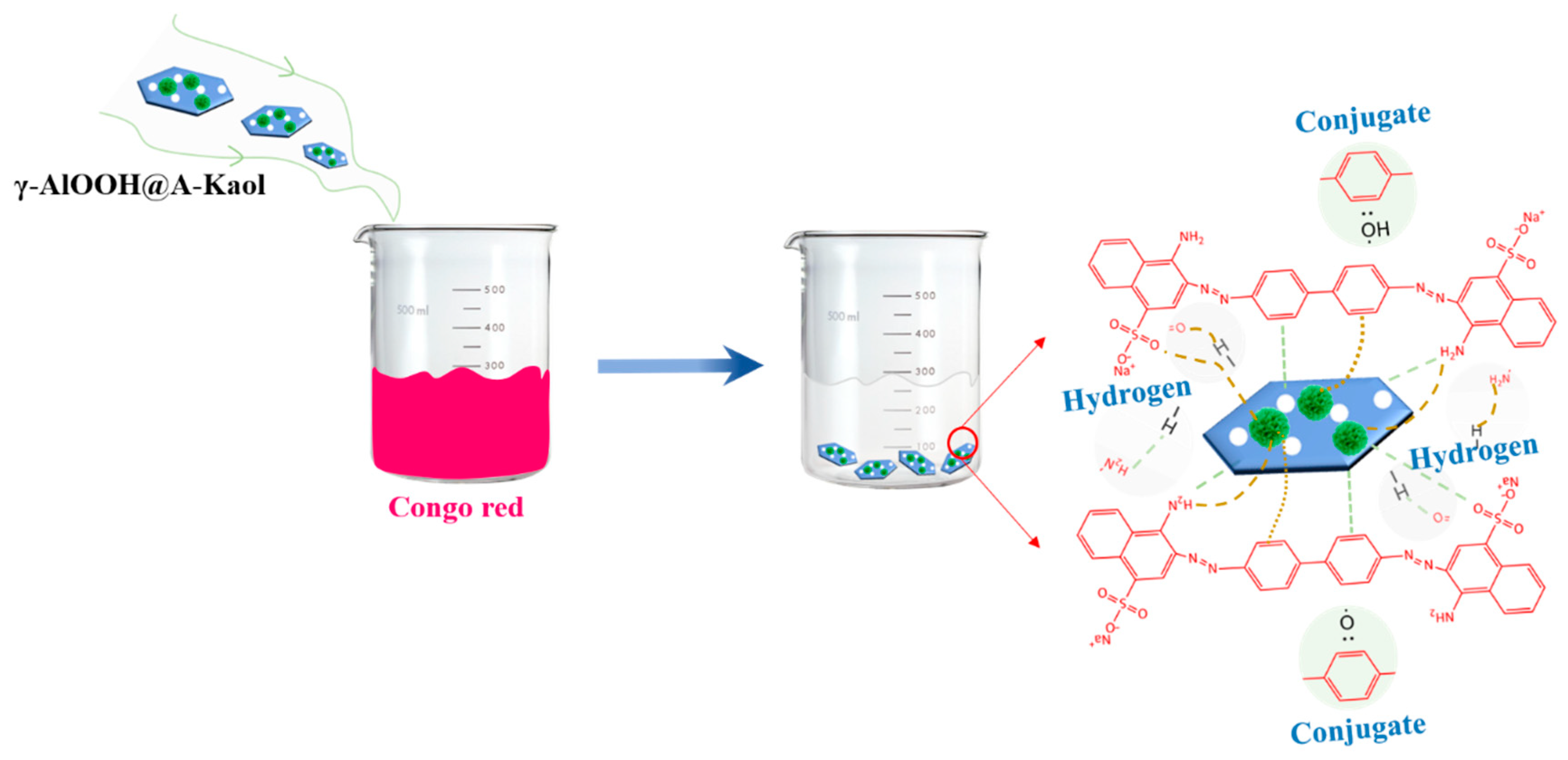
2.4. Adsorption Mechanism
3. Experimental
3.1. Materials
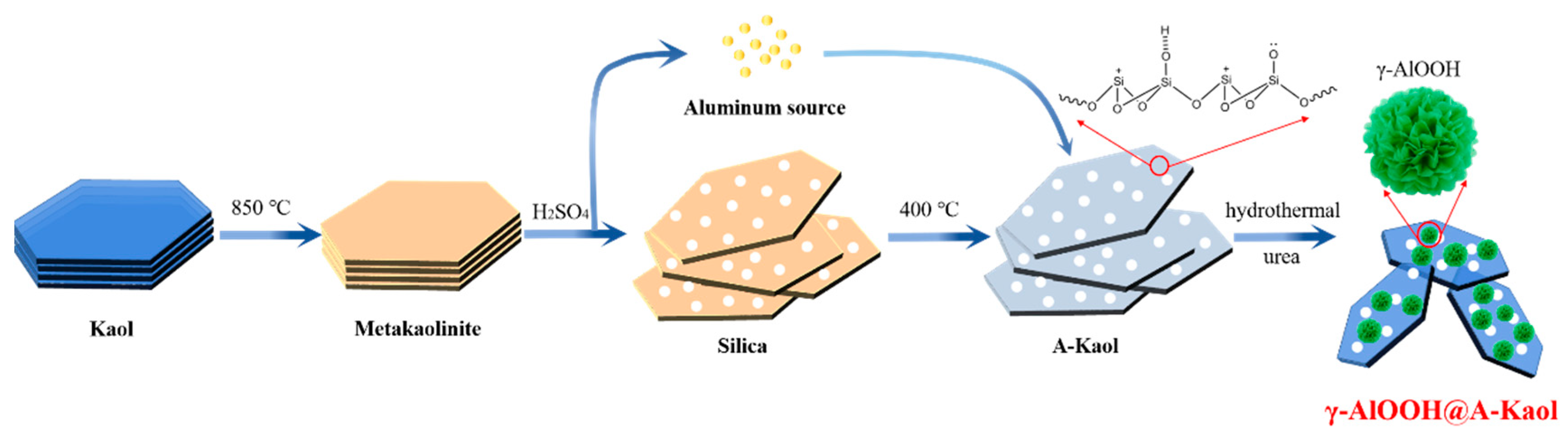
3.2. Preparation of γ-AlOOH@A-Kaol
3.3. Congo Red Adsorption Experiments
3.4. Standard Curve
3.5. Characterization of γ-AlOOH@A-Kaol
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gherca, D.; Porcescu, M.; Herea, D.D.; Chiriac, H.; Lupu, N.; Buema, G. Superior efficacies adsorptions on hydrotalcite-like compound as dual-functional clay nanomaterial for heavy metals and anionic dyes. Appl. Clay Sci. 2023, 233, 106841. [Google Scholar] [CrossRef]
- Xia, M.; Liu, H.; Wang, H.; Sun, F.; Zou, X.; Chen, T.; Chu, Z.; Chen, D.; Zhou, Y.; Xie, Q. Impact of the interaction between hematite and halloysite on environmental fate of organic pollutants. Appl. Clay Sci. 2021, 209, 106123. [Google Scholar] [CrossRef]
- Shang, Y.R.; Cui, Y.P.; Shi, R.X.; Che, Q.D.; Zhang, A.Y.; Yang, P. Depositing Ag3PO4 on WO3 hollow microspheres at room temperature for rapid photocatalytic degradation of Rhodamine B. Prog. Nat. Sci. 2022, 32, 282–288. [Google Scholar] [CrossRef]
- Mehdizadeh, P.; Orooji, Y.; Amiri, O.; Salavati-Niasari, M.; Moayedi, H. Green synthesis using cherry and orange juice and characterization of TbFeO3 ceramic nanostructures and their application as photocatalysts under UV light for removal of organic dyes in water. J. Clean. Prod. 2020, 252, 119765. [Google Scholar] [CrossRef]
- Wang, Q.; Yu, Z.; Liu, Y.; Zhu, X.; Long, R.; Li, X. Co-intercalation of TiO2 and LDH to reduce graphene oxide photocatalytic composite membrane for purification of dye wastewater. Appl. Clay Sci. 2022, 216, 106359. [Google Scholar] [CrossRef]
- Verma, M.; Lee, I.; Hong, Y.; Kumar, V.; Kim, H. Multifunctional β-Cyclodextrin-EDTA-Chitosan polymer adsorbent synthesis for simultaneous removal of heavy metals and organic dyes from wastewater. Environ. Pollut. 2022, 292, 118447. [Google Scholar] [CrossRef]
- Han, G.; Du, Y.; Huang, Y.; Wang, W.; Su, S.; Liu, B. Study on the removal of hazardous Congo red from aqueous solutions by chelation flocculation and precipitation flotation process. Chemosphere 2022, 289, 133109. [Google Scholar] [CrossRef] [PubMed]
- Maruthupandy, M.; Muneeswaran, T.; Chackaravarthi, G.; Vennila, T.; Anand, M.; Cho, W.S.; Quero, F. Synthesis of chitosan/SnO2 nanocomposites by chemical precipitation for enhanced visible light photocatalytic degradation efficiency of congo red and rhodamine-B dye molecules. J. Photochem. Photobiol. A Chem. 2022, 430, 113972. [Google Scholar] [CrossRef]
- Liu, X.; Chen, Z.; Du, W.; Liu, P.; Zhang, L.; Shi, F. Treatment of wastewater containing methyl orange dye by fluidized three dimensional electrochemical oxidation process integrated with chemical oxidation and adsorption. J. Environ. Manag. 2022, 311, 114775. [Google Scholar] [CrossRef]
- Li, X.; Yi, K.; Ran, Q.; Fan, Z.; Liu, C.; Liu, X.; Jia, K. Selective removal of cationic organic dyes via electrospun nanofibrous membranes derived from polyarylene ethers containing pendent nitriles and sulfonates. Sep. Purif. Technol. 2022, 301, 121942. [Google Scholar] [CrossRef]
- Lahiri, S.; Zhang, C.; Sillanpää, M.; Liu, L. Nanoporous NiO@SiO2 photo-catalyst prepared by ion-exchange method for fast elimination of reactive dyes from wastewater. Mater. Today Chem. 2022, 23, 100677. [Google Scholar] [CrossRef]
- Ahmadijokani, F.; Molavi, H.; Rezakazemi, M.; Tajahmadi, S.; Bahi, A.; Ko, F.; Aminabhavi, T.M.; Li, J.R.; Arjmand, M. UiO-66 metal—organic frameworks in water treatment: A critical review. Prog. Mater. Sci. 2022, 125, 100904. [Google Scholar] [CrossRef]
- Vo, T.S.; Vo, T.T.B.C. Organic dye removal and recycling performances of graphene oxide-coated biopolymer sponge. Prog. Nat. Sci. 2022, 32, 634–642. [Google Scholar] [CrossRef]
- Jeon, M.; Jun, B.M.; Kim, S.; Jang, M.; Park, C.M.; Snyder, S.A.; Yoon, Y. A review on MXene-based nanomaterials as adsorbents in aqueous solution. Chemosphere 2020, 261, 127781. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, A. Graphene-based nanomaterials for the removal of organic pollutants: Insights into linear versus nonlinear mathematical models. J. Environ. Manag. 2020, 270, 110911. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yu, E.; Sun, S.; Liu, W.; Hu, R.; Xu, L. Fast and highly efficient adsorption of cationic dyes by phytic acid crosslinked β-cyclodextrin. Carbohydr. Polym. 2022, 284, 119231. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Zhang, A.; Cheng, Y.; Dessie, W.; Liao, Y.; Chen, H.; Qin, Z.; Wang, X.; Jin, X. Fabrication and application of chitosan-based biomass composites with fire safety, water treatment and antibacterial properties. Int. J. Biol. Macromol. 2023, 225, 266–276. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Zhu, H.; Che, J.; Xu, Y.; Tan, Q.; Zhao, Y. Stem cell niche-inspired microcarriers with ADSCs encapsulation for diabetic wound treatment. Bioact. Mater. 2023, 26, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Tian, X.; Wei, W.; Xu, X.; Li, J.; Guo, Y.; Zhou, Z. Wheat straw-core hydrogel spheres with polypyrrole nanotubes for the removal of organic dyes. J. Clean. Prod. 2022, 344, 131100. [Google Scholar] [CrossRef]
- Liu, Z.; Khan, T.A.; Islam, M.A.; Tabrez, U. A review on the treatment of dyes in printing and dyeing wastewater by plant biomass carbon. Bioresour. Technol. 2022, 354, 127168. [Google Scholar] [CrossRef]
- Wang, Q.; Lai, Z.; Luo, C.; Zhang, J.; Cao, X.; Liu, J.; Mu, J. Honeycomb-like activated carbon with microporous nanosheets structure prepared from waste biomass cork for highly efficient dye wastewater treatment. J. Hazard. Mater. 2021, 416, 125896. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Yi, W.; Yin, C.; Li, K.; Feng, L.; Zhou, Q.; Yi, Z.; Zhang, X.; Wang, Y.; Yu, Y.; et al. 2D-3D magnetic NiFe layered double hydroxide decorated diatomite as multi-function material for anionic, cationic dyes, arsenate, and arsenite adsorption. Appl. Clay Sci. 2022, 229, 106664. [Google Scholar] [CrossRef]
- Ewis, D.; Ba-Abbad, M.M.; Benamor, A.; El-Naas, M.H. Adsorption of organic water pollutants by clays and clay minerals composites: A comprehensive review. Appl. Clay Sci. 2022, 229, 106686. [Google Scholar] [CrossRef]
- Jawad, A.H.; Abdulhameed, A.S. Mesoporous Iraqi red kaolin clay as an efficient adsorbent for methylene blue dye: Adsorption kinetic, isotherm and mechanism study. Surf. Interfaces 2020, 18, 100422. [Google Scholar] [CrossRef]
- Li, D.; Li, Y.; Yang, F.; Tian, X.; Che, S.; Wang, Y.; Bao, W.; Lv, G.; Xu, C.; Sun, Y.; et al. Green production of silica hydroxyl riched palygorskite by shear-assisted supercritical CO2 separation process for dye adsorption and heavy oil viscosity reduction. Appl. Clay Sci. 2021, 212, 106207. [Google Scholar] [CrossRef]
- Fan, H.; Gu, X.; Zhang, S.; Liu, F.; Liao, Y.; Tang, W. Synergistic effect between novel triazine-based charring agent and modified kaolinite: An efficient system for fire hazard and aging suppression of epoxy resin. Polym. Degrad. Stabil. 2022, 204, 110109. [Google Scholar] [CrossRef]
- Tang, W.; Song, L.; Liu, F.; Dessie, W.; Qin, Z.; Zhang, S.; Gu, X. Improving the flame retardancy and thermal stability of polypropylene composites via introducing glycine intercalated kaolinite compounds. Appl. Clay Sci. 2022, 217, 106411. [Google Scholar] [CrossRef]
- Zhang, Q.; Yan, Z.; Ouyang, J.; Zhang, Y.; Yang, H.; Chen, D. Chemically modified kaolinite nanolayers for the removal of organic pollutants. Appl. Clay Sci. 2018, 157, 283–290. [Google Scholar] [CrossRef]
- Yan, Z.; Fu, L.; Yang, H.; Ouyang, J. Amino-functionalized hierarchical porous SiO2-AlOOH composite nanosheets with enhanced adsorption performance. J. Hazard. Mater. 2018, 344, 1090–1100. [Google Scholar] [CrossRef]
- Yu, Y.; Yang, M.; Yan, Z.; Li, T.; Jing, Q.; Liu, P.; Xu, B.; Cao, J. Regulation of hierarchically porous structures based on multi-scale nanosheets derived from kaolinite for enhanced adsorption. Appl. Clay Sci. 2021, 200, 105895. [Google Scholar] [CrossRef]
- Tang, W.; Fan, H.; Liu, F.; Liao, Y.; Xiao, Q.; Yan, J.; Zhang, S.; Gu, X. MoS2 supported on acid activated kaolinite used in the kaolinite-epoxy resin composites for suppressing smoke toxicity and degradation. Appl. Clay Sci. 2023, 233, 1106822. [Google Scholar] [CrossRef]
- Hérisson, T.; Estournès, C. Translucent γ-AlOOH and γ-Al2O3 glass-ceramics using the cold sintering process. Scripta. Mater. 2021, 194, 113650. [Google Scholar] [CrossRef]
- Romero, R.; Anaya, L.; Martínez, J. The Role of the Aluminum Source on the Physicochemical Properties of γ-AlOOH Nanoparticles. Maced. J. Chem. Chem. Eng. 2020, 39, 89–99. [Google Scholar] [CrossRef]
- Wang, D.; Li, Z.; Lv, F.; Guan, M.; Chen, J.; Wu, C.; Li, Y.; Li, Y.; Zhang, W. Characterization of microspheres γ-AlOOH and the excellent removal efficiency of Congo red. J. Phys. Chem. Solids. 2023, 174, 111043. [Google Scholar] [CrossRef]
- Tang, W.; Zhang, S.; Sun, J.; Li, H.; Liu, X.; Gu, X. Effects of surface acid-activated kaolinite on the fire performance of polypropylene composite. Thermochim. Acta 2017, 648, 1–12. [Google Scholar] [CrossRef]
- Tang, W.; Zhang, H.; Liu, T.; Li, X.; Yang, H.; Huang, W.; Liao, Y.; Gu, X.; Zhang, S. Improving the flame retardancy and smoke suppression of ethylene vinyl acetate composites by introducing the hybrid material of Zn/TiO2@AKaol. Polym. Degrad. Stabil. 2024, 221, 110673. [Google Scholar] [CrossRef]
- Zuhra, Z.; Zhao, Z.; Qin, L.; Zhou, Y.; Zhang, L.; Ali, S.; Tang, F.; Ping, E. In situ formation of a multiporous MOF(Al)@γ-AlOOH composite material: A versatile adsorbent for both N- and S-heterocyclic fuel contaminants with high selectivity. Chem. Eng. J. 2019, 360, 1623–1632. [Google Scholar] [CrossRef]


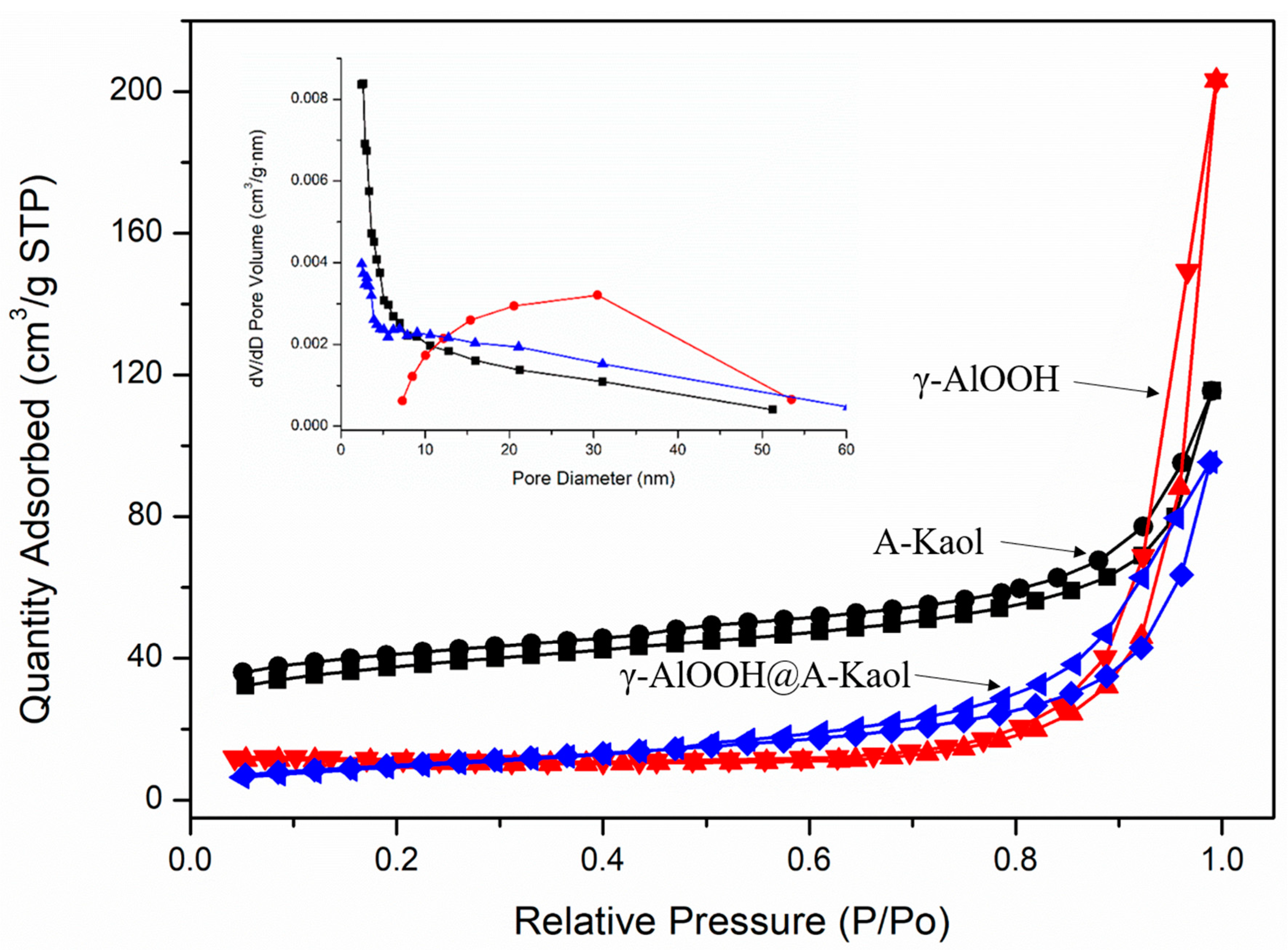



| Samples | S (m2g−1) a | V (cm3g−1) b | D (nm) c |
|---|---|---|---|
| A-Kaol | 127.4 | 0.127 | 4.28 |
| γ-AlOOH | 34.1 | 0.315 | 21.5 |
| γ-AlOOH@A-Kaol | 36.5 | 0.146 | 13.0 |
| PFO | PSO | ||||
|---|---|---|---|---|---|
| qe cal (mg/g) | K1 | R2 | qe cal (mg/g) | K2 | R2 |
| 67.3 | 9.36 × 10−4 | 0.83132 | 195.3 | 0.0013 | 0.99934 |
| Samples | m/Al2(SO4)3·18H2O | m/Urea | MM * | m/A-Kaol |
|---|---|---|---|---|
| A-Kaol | 0 | 0 | 0 | 0 |
| γ-AlOOH | 3 g | 1.08 g | 0 | 0 |
| S1 | 3 g | 1.08 g | 1/5 | 0.816 g |
| S2 | 3 g | 1.08 g | 1/10 | 0.408 g |
| S3 | 3 g | 1.08 g | 1/20 | 0.204 g |
| S4 | 3 g | 1.08 g | 1/40 | 0.102 g |
| S5 | 3 g | 1.08 g | 1/80 | 0.051 g |
| S6 | 3 g | 1.08 g | 1/160 | 0.0255 g |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, T.; Li, X.; Wang, H.; Li, M.; Yang, H.; Liao, Y.; Tang, W.; Li, Y.; Liu, F. Reconstructing Kaolinite Compounds for Remarkably Enhanced Adsorption of Congo Red. Molecules 2024, 29, 2121. https://doi.org/10.3390/molecules29092121
Liu T, Li X, Wang H, Li M, Yang H, Liao Y, Tang W, Li Y, Liu F. Reconstructing Kaolinite Compounds for Remarkably Enhanced Adsorption of Congo Red. Molecules. 2024; 29(9):2121. https://doi.org/10.3390/molecules29092121
Chicago/Turabian StyleLiu, Ting, Xinle Li, Hao Wang, Mingyang Li, Hua Yang, Yunhui Liao, Wufei Tang, Yong Li, and Fang Liu. 2024. "Reconstructing Kaolinite Compounds for Remarkably Enhanced Adsorption of Congo Red" Molecules 29, no. 9: 2121. https://doi.org/10.3390/molecules29092121
APA StyleLiu, T., Li, X., Wang, H., Li, M., Yang, H., Liao, Y., Tang, W., Li, Y., & Liu, F. (2024). Reconstructing Kaolinite Compounds for Remarkably Enhanced Adsorption of Congo Red. Molecules, 29(9), 2121. https://doi.org/10.3390/molecules29092121






