Study on the Skincare Effects of Red Rice Fermented by Aspergillus oryzae In Vitro
Abstract
1. Introduction
2. Results
2.1. Composition Analysis of RRFA
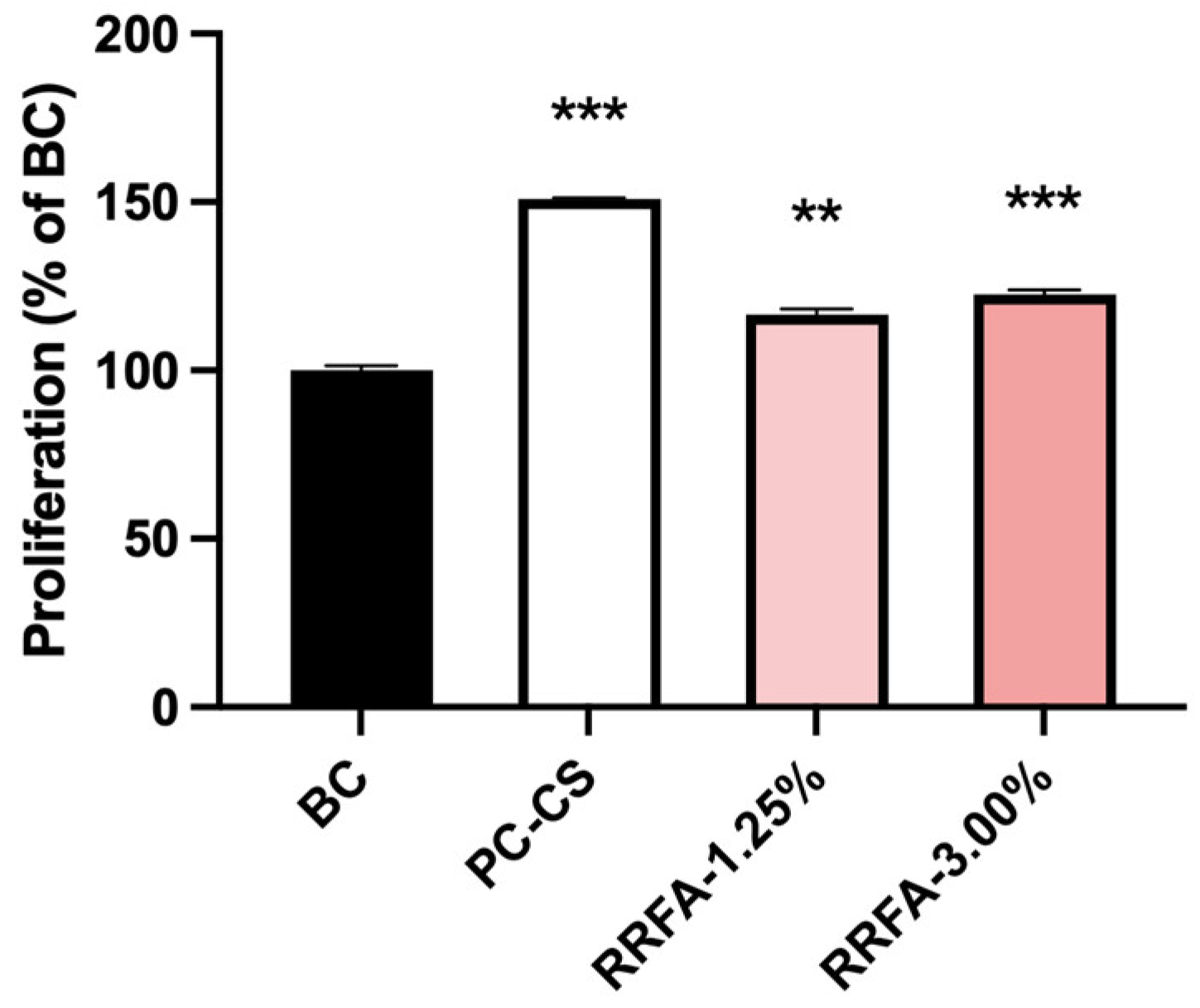
2.2. Effect of RRFA on Cell Proliferation
2.3. Effect of RRFA on the Expression of Type I Collagen, Type III Collagen, and Elastin mRNA in FB
2.4. Antioxidant Effect of RRFA
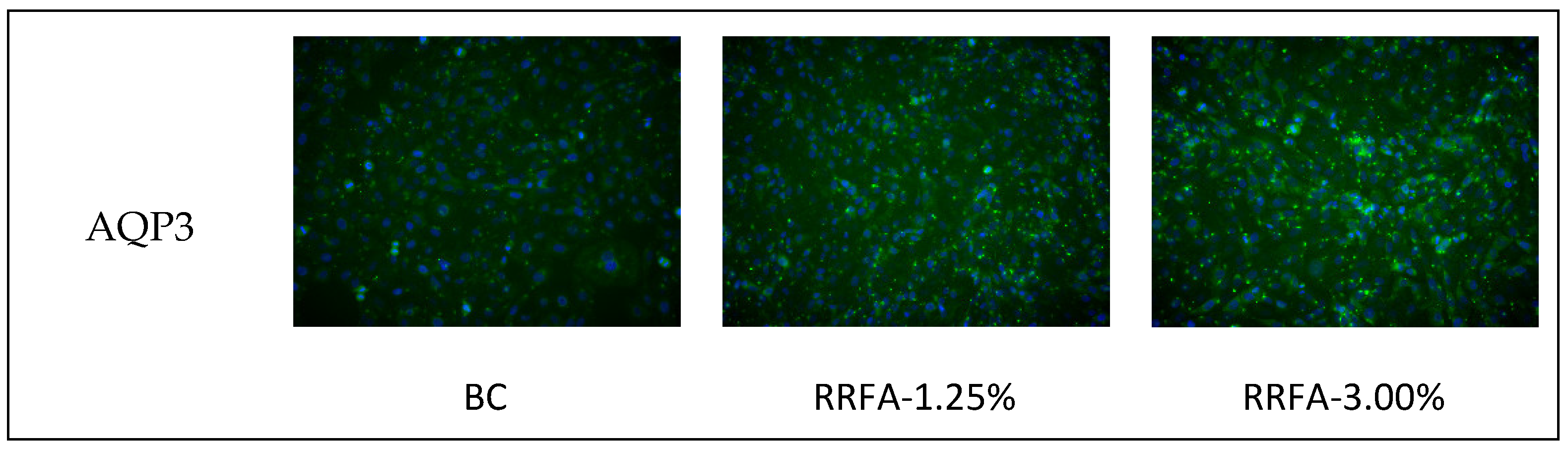
2.5. Effect of RRFA on AQP3 and FLG Protein Content
2.6. Effects of RRFA on the mRNA Expression of HAS1, CLDN1, IVL and ZO-1 in HaCaT Cells
2.7. Effects of RRFA on Skin Moisture Content
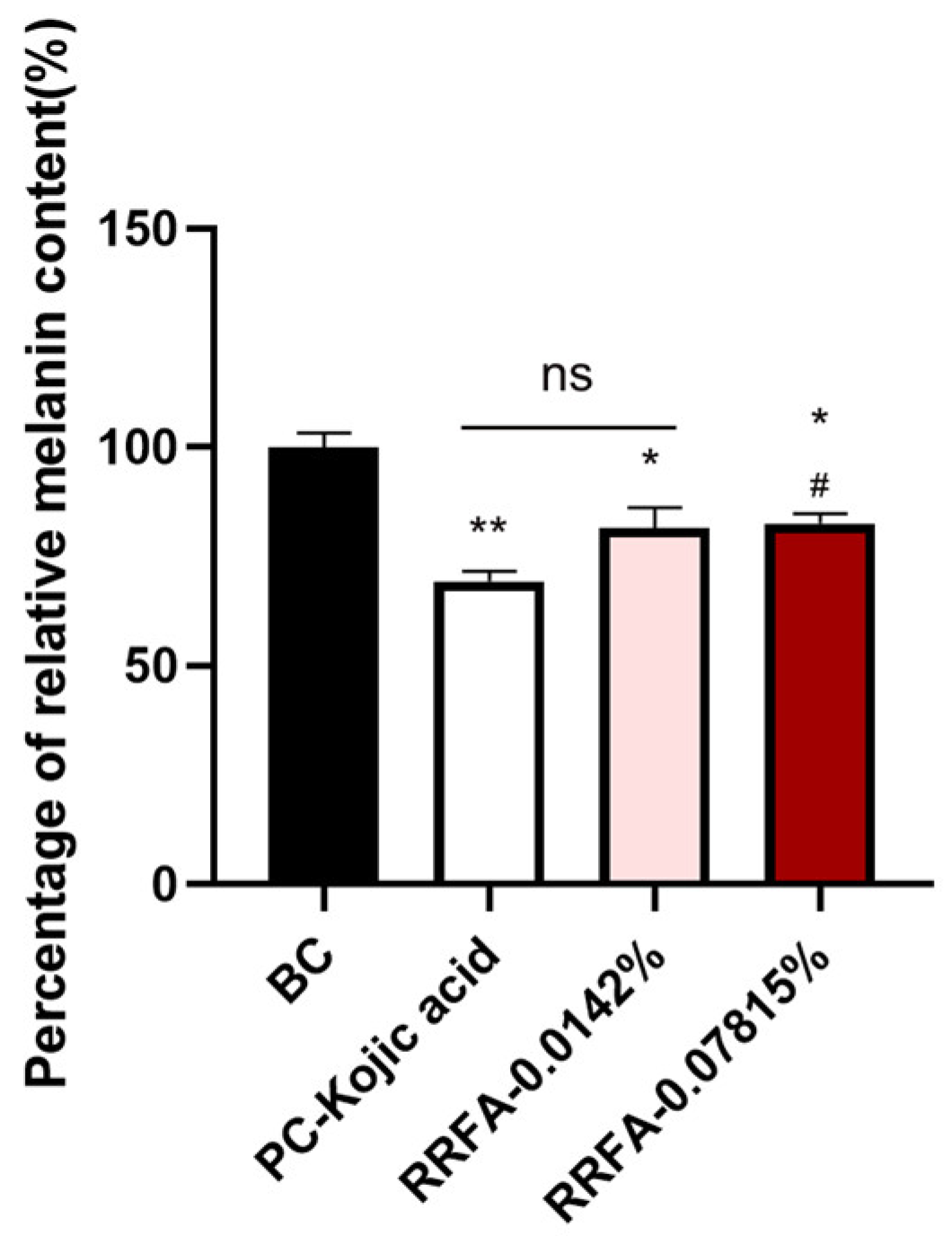
2.8. The Whitening Effect of RRFA
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. The Preparation Procedure of RRFA
4.3. Cell Viability
4.4. Component Analysis of RRFA
4.5. qRT-PCR Analysis
4.6. Cellular Reactive Oxygen Species (ROS) Detection Assay
4.7. Cell Immunofluorescence Staining
4.8. Three-Dimensional Epidermal Model Moisture Content Test
4.9. Melanin Content Test Based on Human Melanocytes
5. Statistical Analysis
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ferreira, M.S.; Magalhães, M.C.; Oliveira, R.; Sousa-Lobo, J.M.; Almeida, I.F. Trends in the Use of Botanicals in Anti-Aging Cosmetics. Molecules 2021, 26, 3584. [Google Scholar] [CrossRef]
- Ribeiro, A.S.; Estanqueiro, M.; Oliveira, M.B.; Sousa Lobo, J.M. Main benefits and applicability of plant extracts in skin care products. Cosmetics 2015, 2, 48–65. [Google Scholar] [CrossRef]
- Zamil, D.H.; Khan, R.M.; Braun, T.L.; Nawas, Z.Y. Dermatological uses of rice products: Trend or true? J. Cosmet. Dermatol. 2022, 21, 6056–6060. [Google Scholar] [CrossRef] [PubMed]
- Kanlayavattanakul, M.; Lourith, N.; Chaikul, P. Jasmine rice panicle: A safe and efficient natural ingredient for skin aging treatments. J. Ethnopharmacol. 2016, 193, 607–616. [Google Scholar] [CrossRef] [PubMed]
- Palungwachira, P.; Tancharoen, S.; Phruksaniyom, C.; Klungsaeng, S.; Srichan, R.; Kikuchi, K.; Nararatwanchai, T. Antioxidant and Anti-Inflammatory Properties of Anthocyanins Extracted from Oryza sativa, L. in Primary Dermal Fibroblasts. Oxidative Med. Cell. Longev. 2019, 2019, 2089817. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.Y.; Seo, Y.K.; Park, J.M.; Seo, M.J.; Park, J.K.; Kim, J.W.; Park, C.S. Fermented rice bran downregulates MITF expression and leads to inhibition of alpha-MSH-induced melanogenesis in B16F1 melanoma. Biosci. Biotechnol. Biochem. 2009, 73, 1704–1710. [Google Scholar] [CrossRef]
- Seo, Y.-K.; Jung, S.-H.; Song, K.-Y.; Park, J.-K. Anti-photoaging effect of fermented rice bran extract on UV-induced normal skin fibroblasts. Eur. Food Res. Technol. 2010, 231, 163–169. [Google Scholar] [CrossRef]
- Kovach, M.J.; Sweeney, M.T.; McCouch, S.R. New insights into the history of rice domestication. Trends Genet. 2007, 23, 578–587. [Google Scholar] [CrossRef] [PubMed]
- Piazzon, A.; Forte, M.; Nardini, M. Characterization of phenolics content and antioxidant activity of different beer types. J. Agric. Food Chem. 2010, 58, 10677–10683. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Fan, Z.; Fawen, W.; Yan, L.; Haifeng, Q.; Hui, Z.; Xiguang, Q. The healthy benefits and applications of red rice. Food Mach. 2019, 35, 226–232. [Google Scholar]
- Mehra, R.; Kumar, H.; Kumar, N.; Kaushik, R. Red rice conjugated with barley and rhododendron extracts for new variant of beer. J. Food Sci. Technol. 2020, 57, 4152–4159. [Google Scholar] [CrossRef] [PubMed]
- Munkong, N.; Somnuk, S.; Jantarach, N.; Ruxsanawet, K.; Nuntaboon, P.; Kanjoo, V.; Yoysungnoen, B. Red Rice Bran Extract Alleviates Hih-Fat Diet-Induced Non-Alcoholic Fatty Liver Disease and Dyslipidemia in Mice. Nutrients 2023, 15, 246. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhao, Z.; Guo, S.; Li, Y.; Yin, H.; Tian, L.; Cheng, G.; Li, Y. Red Rice Seed Coat Targeting SPHK2 Ameliorated Alcoholic Liver Disease via Restored Intestinal Barrier and Improved Gut Microbiota in Mice. Nutrients 2023, 15, 4176. [Google Scholar] [CrossRef] [PubMed]
- Dou, J.; Feng, N.; Guo, F.; Chen, Z.; Liang, J.; Wang, T.; Guo, X.; Xu, Z. Applications of Probiotic Constituents in Cosmetics. Molecules 2023, 28, 6765. [Google Scholar] [CrossRef] [PubMed]
- Hahm, K.M.; Park, S.H.; Oh, S.W.; Kim, J.H.; Yeom, H.S.; Lee, H.J.; Yang, S.; Cho, J.Y.; Park, J.O.; Lee, J. Aspergillus oryzae-Fermented Wheat Peptone Enhances the Potential of Proliferation and Hydration of Human Keratinocytes through Activation of p44/42 MAPK. Molecules 2021, 26, 6074. [Google Scholar] [CrossRef] [PubMed]
- Ha, C.W.; Sohn, E.H.; Kim, S.H.; Jang, S.; Park, M.R.; Kim, Y.K.; Bae, I.Y. Fermented Angelicae tenussimae with Aspergillus oryzae Improves Skin Barrier Properties, Moisturizing, and Anti-Inflammatory Responses. Int. J. Mol. Sci. 2022, 23, 12072. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M. Research Progress on the Mechanism of Fibroblasts in Skin Aging. Adv. Clin. Med. 2022, 12, 3481–3486. [Google Scholar] [CrossRef]
- Xu, Y. The relationship between the molecular mechanism of fibroblast aging and skin aging and research progress. Chin. Aesthetic Med. 2023, 32, 199–202. [Google Scholar]
- Binic, I.; Lazarevic, V.; Ljubenovic, M.; Mojsa, J.; Sokolovic, D. Skin ageing: Natural weapons and strategies. Evid.-Based Complement. Altern. Med. 2013, 2013, 827248. [Google Scholar] [CrossRef]
- Xiong, Z.M.; O’Donovan, M.; Sun, L.; Choi, J.Y.; Ren, M.; Cao, K. Anti-Aging Potentials of Methylene Blue for Human Skin Longevity. Sci. Rep. 2017, 7, 2475. [Google Scholar] [CrossRef]
- Harris, A.K.; Stopak, D.; Wild, P. Fibroblast traction as a mechanism for collagen morphogenesis. Nature 1981, 290, 249–251. [Google Scholar] [CrossRef] [PubMed]
- Bollag, W.B.; Aitkens, L.; White, J.; Hyndman, K.A. Aquaporin-3 in the epidermis: More than skin deep. Am. J. Physiol. Cell Physiol. 2020, 318, C1144–C1153. [Google Scholar] [CrossRef] [PubMed]
- Kezic, S.; O’Regan, G.M.; Yau, N.; Sandilands, A.; Chen, H.; Campbell, L.E.; Kroboth, K.; Watson, R.; Rowland, M.; McLean, W.H.; et al. Levels of filaggrin degradation products are influenced by both filaggrin genotype and atopic dermatitis severity. Allergy 2011, 66, 934–940. [Google Scholar] [CrossRef]
- Chen, H.; Hossain, M.A.; Kim, J.H.; Cho, J.Y. Kahweol Exerts Skin Moisturizing Activities by Upregulating STAT1 Activity. Int. J. Mol. Sci. 2021, 22, 8864. [Google Scholar] [CrossRef]
- Kuo, I.H.; Carpenter-Mendini, A.; Yoshida, T.; McGirt, L.Y.; Ivanov, A.I.; Barnes, K.C.; Gallo, R.L.; Borkowski, A.W.; Yamasaki, K.; Leung, D.Y.; et al. Activation of epidermal toll-like receptor 2 enhances tight junction function: Implications for atopic dermatitis and skin barrier repair. J. Investig. Dermatol. 2013, 133, 988–998. [Google Scholar] [CrossRef]
- Kanwal, S.; Singh, S.K.; Soman, S.P.; Choudhury, S.; Kumari, P.; Ram, P.K.; Garg, S.K. Expression of barrier proteins in the skin lesions and inflammatory cytokines in peripheral blood mononuclear cells of atopic dogs. Sci. Rep. 2021, 11, 11418. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Chen, S.; Yu, B. Poly-γ-glutamic acid: Recent achievements, diverse applications and future perspectives. Trends Food Sci. Technol. 2022, 119, 1–12. [Google Scholar] [CrossRef]
- Uehara, E.; Hokazono, H.; Hida, M.; Sasaki, T.; Yoshioka, H.; Matsuo, N. GABA promotes elastin synthesis and elastin fiber formation in normal human dermal fibroblasts (HDFs). Biosci. Biotechnol. Biochem. 2017, 81, 1198–1205. [Google Scholar] [CrossRef]
- Chen, A.C.; Damian, D.L. Nicotinamide and the skin. Australas. J. Dermatol. 2014, 55, 169–175. [Google Scholar] [CrossRef]
- Uchida, Y.; Park, K. Ceramides in Skin Health and Disease: An Update. Am. J. Clin. Dermatol. 2021, 22, 853–866. [Google Scholar] [CrossRef]
- Jadoon, S.; Karim, S.; Bin Asad, M.H.; Akram, M.R.; Khan, A.K.; Malik, A.; Chen, C.; Murtaza, G. Anti-Aging Potential of Phytoextract Loaded-Pharmaceutical Creams for Human Skin Cell Longetivity. Oxid. Med. Cell Longev. 2015, 2015, 709628. [Google Scholar] [CrossRef] [PubMed]
- Slominski, R.M.; Sarna, T.; Płonka, P.M.; Raman, C.; Brożyna, A.A.; Slominski, A.T. Melanoma, Melanin, and Melanogenesis: The Yin and Yang Relationship. Front. Oncol. 2022, 14, 842496. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Zhu, P. Research progress on melanin synthesis and whitening products. J. East China Norm. Univ. (Nat. Sci. Ed.) 2016, 2, 1–8. [Google Scholar]
- Ren, Q.; Wu, H.; Jin, J. Cosmetic plant raw materials (IV)—Research and development of plant whitening raw materials that inhibit the melanin synthesis signaling pathway. Dly. Chem. Ind. 2021, 51, 590–597. [Google Scholar]
- Cheng, Q.; Gao, L.; Deng, F.; Zhong, Y. Application progress of antioxidants in the cosmetics industry. Dly. Chem. Sci. 2019, 42, 32–38+40. [Google Scholar]
- Hakozaki, T.; Minwalla, L.; Zhuang, J.; Chhoa, M.; Matsubara, A.; Miyamoto, K.; Greatens, A.; Hillebrand, G.G.; Bissett, D.L.; Boissy, R.E. The effect of niacinamide on reducing cutaneous pigmentation and suppression of melanosome transfer. Br. J. Dermatol. 2002, 147, 20–31. [Google Scholar] [CrossRef]
- GB/T 30987-2020; Determination of Free Amino Acids in Plant. Standardization Administration of the People’s Republic of China: Beijing, China, 2020.
- GB 5009.8-2016; Determination of Fructose, Glucose, Sucrose, Maltose and Lactose in Foods. National Standard of the People’s Republic of China: Beijing, China, 2016.
- QB/T 4587-2013; Gamma. Aminobutyric Acid. Light Industry Industry Standard: Beijing, China,, 2013.
- GB 5009.89-2016; Determination of Niacin and Nicotinamide in Foods. National Standard of the People’s Republic of China: Beijing, China, 2016.
- GB/T 15672-2009; Determination of Total Saccharide in Edible Mushroom. National Standard of the People’s Republic of China: Beijing, China, 2009.
- GB 5009.5-2016; National Food Safety Standard Determination of Protein in Foods. National Standard of the People’s Republic of China: Beijing, China, 2016.
- T/AHFIA 005-2018; Determination of Total Polyphenol Content in Plant Extracts and their Products Spectrophotometry Method. Anhui Food Industry Association Group Standard of China: Anhui, China, 2018.
- Xie, J.P.; Zhang, M.L.; Liu, Z. Research progress on ceramides and their analysis and separation techniques. Fine Chem. 2002, 19, 381–384, 387. [Google Scholar]





| Ingredient | Content |
|---|---|
| Total protein | 0.27 wt% |
| Total polyphenols | 0.01 wt% |
| Total sugar | 9.9 wt% |
| Peptide | 0.22 wt% |
| Gamma-aminobutyric acid | 13.21 mg/L |
| VB3 | 17.61 mg/L |
| Ceramide | 330.66 pg/mL |
| Name | Forward Primer (5′-3′) Reverse Primer (5′-3′) |
|---|---|
| Collagen I | GTGGCAGTGATGGAAGTGTG AGGACCAGCGTTACCAACAG |
| Collagen III | ACCAGGAGCTAACGGTCTCA TCTGATCCAGGGTTTCCATC |
| Elastin | ACCCCTGACTCACGACCTCA CGCTCCCCTCTTGTTTCCTT |
| HAS1 | ACTCGGACACAAGGTTGGAC TTAGGAAGCTGACCCAGGAG |
| CLDN1 | GCATGAAGTGTATGAAGTGCTTGGA CCATACCATGCTGTGGCAACTAA |
| IVL | CCACTTATTTCGGGTCCGCT CTGAGGTTGGGATTGGGGTC |
| ZO-1 | CTCAGCCTGTGAGGCGTAGT GCTGTGCTCTTACTGTGGCA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, M.; Sun, Y.; Zhu, L.; Li, L.; Zhao, Y. Study on the Skincare Effects of Red Rice Fermented by Aspergillus oryzae In Vitro. Molecules 2024, 29, 2066. https://doi.org/10.3390/molecules29092066
Chen M, Sun Y, Zhu L, Li L, Zhao Y. Study on the Skincare Effects of Red Rice Fermented by Aspergillus oryzae In Vitro. Molecules. 2024; 29(9):2066. https://doi.org/10.3390/molecules29092066
Chicago/Turabian StyleChen, Mo, Yi Sun, Le Zhu, Lingyu Li, and Ya Zhao. 2024. "Study on the Skincare Effects of Red Rice Fermented by Aspergillus oryzae In Vitro" Molecules 29, no. 9: 2066. https://doi.org/10.3390/molecules29092066
APA StyleChen, M., Sun, Y., Zhu, L., Li, L., & Zhao, Y. (2024). Study on the Skincare Effects of Red Rice Fermented by Aspergillus oryzae In Vitro. Molecules, 29(9), 2066. https://doi.org/10.3390/molecules29092066







