Shoots and Turions of Aquatic Plants as a Source of Fatty Acids
Abstract
1. Introduction
1.1. The Roles and Sources of Unsaturated Fatty Acids
1.2. Turions of Aquatic Plants
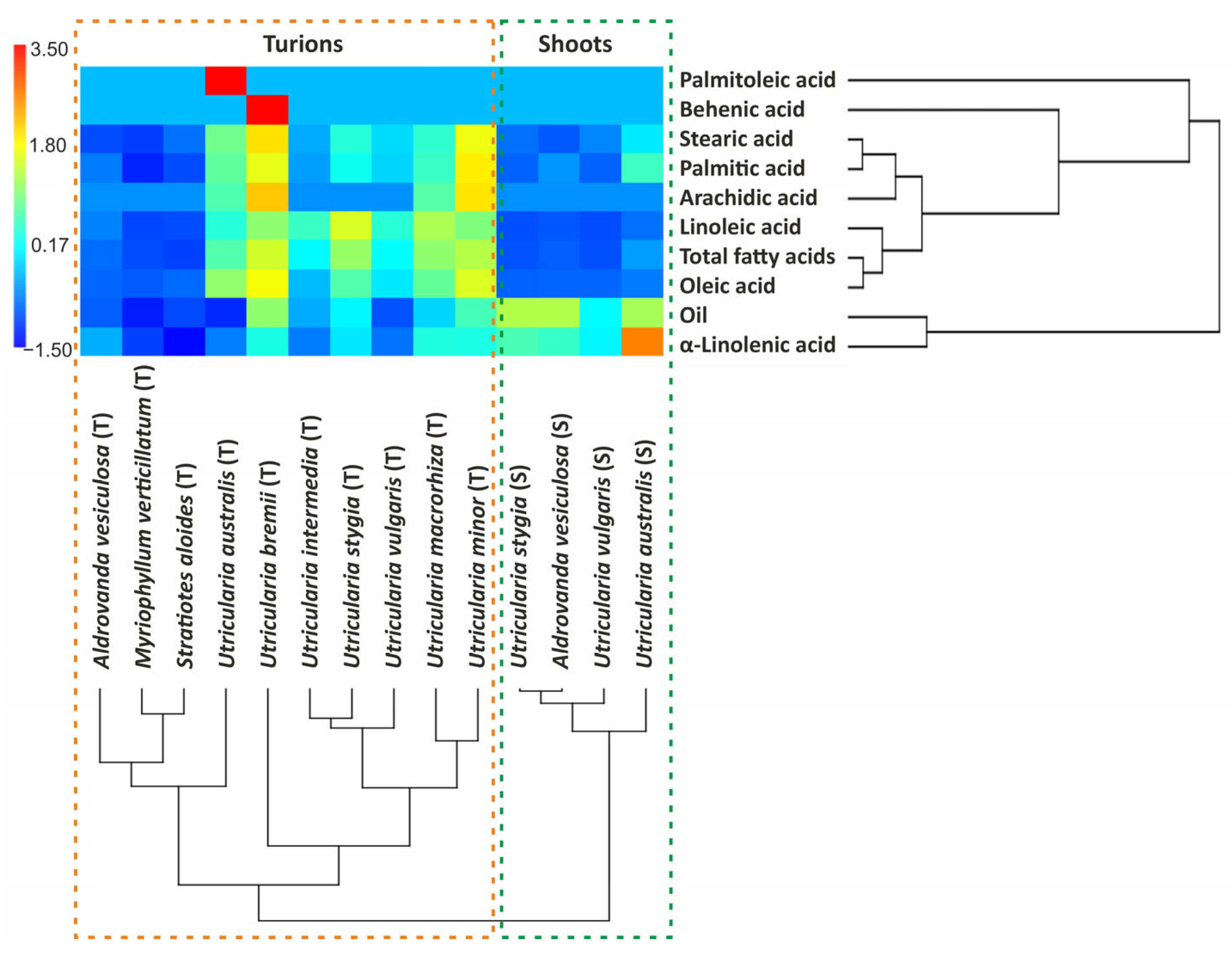
2. Results
2.1. Contents and Composition of Oils
2.2. Principal Component Analysis
3. Discussion
4. Materials and Methods
4.1. Plant Material and Sample Collection
4.2. Isolation of Oil and Preparation of Samples for GC Analysis
4.3. Fatty Acid Analysis
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- He, M.; Qin, C.X.; Wang, X.; Ding, N.Z. Plant unsaturated fatty acids: Biosynthesis and regulation. Front. Plant Sci. 2020, 11, 390. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Fats and Fatty Acids in Human Nutrition: Report of an Expert Consultation; Food and Agriculture Organization of the United Nations: Rome, Italy, 2010; Volume 91, ISBN 9789251067338. [Google Scholar]
- Carvalho, I.S.; Miranda, I.; Pereira, H. Evaluation of oil composition of some crops suitable for human nutrition. Ind. Crops Prod. 2006, 24, 75–78. [Google Scholar] [CrossRef]
- Porokhovinova, E.A.; Matveeva, T.V.; Khafizova, G.V.; Bemova, V.D.; Doubovskaya, A.G.; Kishlyan, N.V.; Podolnaya, L.P.; Gavrilova, V.A. Fatty acid composition of oil crops: Genetics and genetic engineering. Genet. Resour. Crop Evol. 2022, 69, 2029–2045. [Google Scholar] [CrossRef]
- Branquinho, R.G.; Alves, J.M.; da Silva, M.A.; Barbosa, K.F.; Travagin, E.L. New oil and forage producing plant species evaluated on phosphorus doses and row spacing. Biofuels 2023, 14, 157–163. [Google Scholar] [CrossRef]
- Kodahl, N.; Frandsen, H.B.; Lütken, H.; Petersen, I.L.; Paredes Andrade, N.J.; García-Davila, C.; Sørensen, M. Lipid composition of the Amazonian ‘Mountain Sacha Inchis’ including Plukenetia carolis-vegae Bussmann, Paniagua & C. Téllez. Sci. Rep. 2022, 12, 6450. [Google Scholar] [CrossRef] [PubMed]
- Mandim, F.; Petropoulos, S.A.; Santos-Buelga, C.; Ferreira, I.C.F.R.; Barros, L. Effect of harvesting time on the chemical composition of Cynara cardunculus L. var. altilis blades. Agronomy 2022, 12, 1705. [Google Scholar] [CrossRef]
- Strzemski, M.; Płachno, B.J.; Mazurek, B.; Kozłowska, W.; Sowa, I.; Lustofin, K.; Załuski, D.; Rydzik, Ł.; Szczepanek, D.; Sawicki, J.; et al. Morphological, anatomical, and phytochemical studies of Carlina acaulis L. cypsela. Int. J. Mol. Sci. 2020, 21, 9230. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Du, S.; Yuan, J.; Hu, Y. Fatty acid profile in the seeds and seed tissues of Paeonia L. species as new oil plant resources. Sci. Rep. 2016, 6, 26944. [Google Scholar] [CrossRef]
- Adamec, L. Ecophysiological characteristics of turions of aquatic plants: A review. Aquat. Bot. 2018, 148, 64–77. [Google Scholar] [CrossRef]
- Sculthorpe, C.D. The Biology of Aquatic Vascular Plants; Edward Arnold Ltd.: London, UK, 1967; 610p. [Google Scholar]
- Adamec, L. Dark respiration and photosynthesis of dormant and sprouting turions of aquatic plants. Fundam. Appl. Limnol. 2011, 179, 151–158. [Google Scholar] [CrossRef]
- Adamec, L. Respiration of turions and winter apices in aquatic carnivorous plants. Biologia 2008, 63, 515–520. [Google Scholar] [CrossRef]
- Adamec, L.; Kučerová, A.; Janeček, Š. Mineral nutrients, photosynthetic pigments and storage carbohydrates in turions of 21 aquatic plant species. Aquat. Bot. 2020, 165, 103238. [Google Scholar] [CrossRef]
- Janauer, G.A. Elodea canadensis and its dormant apices: An investigation of organic and mineral constituents. Aquat. Bot. 1981, 11, 231–243. [Google Scholar] [CrossRef]
- Ley, S.; Dölger, K.; Appenroth, K.J. Carbohydrate metabolism as a possible physiological modulator of dormancy in turions of Spirodela polyrhiza (L.) Schleiden. Plant Sci. 1997, 129, 1–7. [Google Scholar] [CrossRef]
- Płachno, B.J.; Adamec, L.; Kozieradzka-Kiszkurno, M.; Świątek, P.; Kamińska, I. Cytochemical and ultrastructural aspects of aquatic carnivorous plant turions. Protoplasma 2014, 251, 1449–1454. [Google Scholar] [CrossRef] [PubMed]
- Villanueva, V.R.; Simolat, L.K.; Mardon, M. Polyamines in turions and young plants of hydrocharis morsus-ranae and Utricularia intermedia. Phytochemistry 1985, 24, 171–172. [Google Scholar] [CrossRef]
- Adamec, L. Seasonal growth dynamics and overwintering of the aquatic carnivorous plant Aldrovanda vesiculosa at experimental field sites. Folia Geobot. 1999, 34, 287–297. [Google Scholar] [CrossRef]
- de Dios Barajas-Lopez, J.; Tiwari, A.; Zarza, X.; Shaw, M.W.; Pascual, J.; Punkkinen, M.; Bakowska, J.C.; Munnik, T.; Fujii, H. Early response to dehydration 7 remodels cell membrane lipid composition during cold stress in Arabidopsis. Plant Cell Physiol. 2021, 62, 80–91. [Google Scholar] [CrossRef] [PubMed]
- Gigon, A.; Matos, A.R.; Laffray, D.; Zuily-Fodil, Y.; Pham-Thi, A.T. Effect of drought stress on lipid metabolism in the leaves of Arabidopsis thaliana (ecotype Columbia). Ann. Bot. 2004, 94, 345–351. [Google Scholar] [CrossRef]
- He, M.; Ding, N.Z. Plant unsaturated fatty acids: Multiple roles in stress response. Front. Plant Sci. 2020, 11, 562785. [Google Scholar] [CrossRef]
- Ivanova, T.V.; Maiorova, O.V.; Orlova, Y.V.; Kuznetsova, E.I.; Khalilova, L.A.; Myasoedov, N.A.; Balnokin, Y.V.; Tsydendambaev, V.D. Cell ultrastructure and fatty acid composition of lipids in vegetative organs of Chenopodium album L. under salt stress conditions. Russ. J. Plant Physiol. 2016, 63, 763–775. [Google Scholar] [CrossRef]
- Upchurch, R.G. Fatty acid unsaturation, mobilization, and regulation in the response of plants to stress. Biotechnol. Lett. 2008, 30, 967–977. [Google Scholar] [CrossRef] [PubMed]
- Barrero-Sicilia, C.; Silvestre, S.; Haslam, R.P.; Michaelson, L.V. Lipid remodelling: Unravelling the response to cold stress in Arabidopsis and its extremophile relative Eutrema salsugineum. Plant Sci. 2017, 263, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Nokhsorov, V.V.; Dudareva, L.V.; Petrov, K.A.; Senik, S.V.; Chirikova, N.K. Influence of extremely low temperatures of the pole of cold on the lipid and fatty-acid composition of aerial parts of the horsetail family (Equisetaceae). Plants 2021, 10, 996. [Google Scholar] [CrossRef] [PubMed]
- Skoczowski, A.; Filek, M.; Dubert, F. The long-term effect of cold on the metabolism of winter wheat seedlings. II. composition of fatty acids of phospholipids. J. Therm. Biol. 1994, 19, 171–176. [Google Scholar] [CrossRef]
- Tian, J.; Tian, L.; Chen, M.; Chen, Y.; Wei, A. Low temperature affects fatty acids profiling and key synthesis genes expression patterns in Zanthoxylum bungeanum Maxim. Int. J. Mol. Sci. 2022, 23, 2319. [Google Scholar] [CrossRef]
- Voronkov, A.; Ivanova, T. Significance of lipid fatty acid composition for resistance to winter conditions in Asplenium scolopendrium. Biology 2022, 11, 507. [Google Scholar] [CrossRef]
- Wang, J.; Liu, Z.; Liu, H.; Peng, D.; Zhang, J.; Chen, M. Linum usitatissimum FAD2A and FAD3A enhance seed polyunsaturated fatty acid accumulation and seedling cold tolerance in Arabidopsis thaliana. Plant Sci. 2021, 311, 111014. [Google Scholar] [CrossRef]
- Wang, X.; Yu, C.; Liu, Y.; Yang, L.; Li, Y.; Yao, W.; Cai, Y.; Yan, X.; Li, S.; Cai, Y.; et al. GmFAD3A, A ω-3 fatty acid desaturase gene, enhances cold tolerance and seed germination rate under low temperature in rice. Int. J. Mol. Sci. 2019, 20, 3796. [Google Scholar] [CrossRef]
- Adamec, L.; Kučerová, A. Overwintering temperatures affect freezing temperatures of turions of aquatic plants. Flora Morphol. Distrib. Funct. Ecol. Plants 2013, 208, 497–501. [Google Scholar] [CrossRef]
- Cannavacciuolo, C.; Pagliari, S.; Frigerio, J.; Giustra, C.M.; Labra, M.; Campone, L. Natural Deep Eutectic Solvents (NADESs) combined with sustainable extraction techniques: A review of the green chemistry approach in food analysis. Foods 2023, 12, 56. [Google Scholar] [CrossRef] [PubMed]
- Whelan, J.; Fritsche, K. Linoleic acid. Adv. Nutr. 2013, 4, 311–312. [Google Scholar] [CrossRef] [PubMed]
- Wójciak, M.; Feldo, M.; Stolarczyk, P.; Płachno, B.J. Carnivorous plants from Nepenthaceae and Droseraceae as a source of secondary metabolites. Molecules 2023, 28, 2155. [Google Scholar] [CrossRef] [PubMed]
- Wójciak, M.; Feldo, M.; Stolarczyk, P.; Płachno, B.J. Biological potential of carnivorous plants from Nepenthales. Molecules 2023, 28, 3639. [Google Scholar] [CrossRef] [PubMed]
- Płachno, B.J.; Kapusta, M.; Stolarczyk, P.; Świątek, P. Arabinogalactan proteins in the digestive glands of Dionaea muscipula J. Ellis Traps. Cells 2022, 11, 586. [Google Scholar] [CrossRef] [PubMed]
- Miclea, I. Secondary metabolites with biomedical applications from plants of the Sarraceniaceae family. Int. J. Mol. Sci. 2022, 23, 9877. [Google Scholar] [CrossRef] [PubMed]
- Fassio, A.; Cozzolino, D. Non-destructive prediction of chemical composition in sunflower seeds by near infrared spectroscopy. Ind. Crop. Prod. 2004, 20, 321–329. [Google Scholar] [CrossRef]
- Gonzalez-Martin, I.; Villaescusa-Garcia, V.; Lopez-González, F.; Oiz-Jiménez, C.; Lobos-Ortega, I.A.; Gordillo, A.B.; Hernández-Hierro, J.M. Control of quality and silo storage of sunflower seeds using near infrared technology. Grasas Aceites 2013, 64, 1–124. [Google Scholar] [CrossRef]
- Ergun, Z. The effects of plant growth substances on the oil content and fatty acid composition of Ricinus communis L.: An in vitro study. Mol. Biol. Rep. 2022, 49, 5241–5249. [Google Scholar] [CrossRef]
- Román-Figueroa, C.; Cea, M.; Paneque, M.; González, M.E. Oil content and fatty acid composition in castor bean naturalized accessions under mediterranean conditions in Chile. Agronomy 2020, 10, 1145. [Google Scholar] [CrossRef]
- Sharafi, Y.; Majidi, M.M.; Goli, S.A.H.; Rashidi, F. Oil content and fatty acids composition in Brassica species. Int. J. Food Prop. 2015, 18, 2145–2154. [Google Scholar] [CrossRef]
- Cartea, E.; De Haro-Bailón, A.; Padilla, G.; Obregón-Cano, S.; Del Rio-Celestino, M.; Ordás, A. Seed oil quality of Brassica napus and Brassica rapa germplasm from northwestern Spain. Foods 2019, 27, 292. [Google Scholar] [CrossRef] [PubMed]
- Adamec, L. How to grow Aldrovanda vesiculosa outdoors. Carniv. Plant Newsl. 1997, 26, 85–88. [Google Scholar] [CrossRef]
- Elansary, H.O.M.; Adamec, L.; Štorchová, H. Uniformity of organellar DNA in Aldrovanda vesiculosa, an endangered aquatic carnivorous species, distributed across four continents. Aquat. Bot. 2010, 92, 214–220. [Google Scholar] [CrossRef]
- Płachno, B.J.; Strzemski, M.; Dresler, S.; Adamec, L.; Wojas-Krawczyk, K.; Sowa, I.; Danielewicz, A.; Miranda, V.F.O. A chemometry of Aldrovanda vesiculosa L. (waterwheel, Droseraceae) populations. Molecules 2021, 26, 72. [Google Scholar] [CrossRef]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. Past: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 4. [Google Scholar]


| Species | Oil (% of Dry Weight Plant Material) | % of Dry Weight Plant Material | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Palmitic Acid | Palmitoleic Acid | Stearic Acid | Oleic Acid | Linoleic Acid | Arachidic Acid | α-Linolenic Acid | Behenic Acid | Total Fatty Acids | ||
| Shoots | ||||||||||
| Aldrovanda vesiculosa | 58.5 ± 1.00 | 2.10 ± 0.05 | n.d. | 0.21 ± 0.18 | 0.69 ± 0.07 | 3.24 ± 0.04 | n.d. | 2.06 ± 0.10 | n.d. | 8.30 ± 0.09 |
| Utricularia australis | 58.0 ± 6.87 | 4.7 ± 0.60 | n.d. | 0.88 ± 0.06 | 1.42 ± 0.14 | 5.24 ± 0.62 | n.d. | 4.27 ± 0.33 | n.d. | 16.51 ± 0.35 |
| Utricularia stygia | 58.5 ± 2.55 | 1.35 ± 0.22 | n.d. | 0.31 ± 0.05 | 0.60 ± 0.17 | 2.47 ± 0.15 | n.d. | 2.19 ± 0.59 | n.d. | 6.92 ± 0.24 |
| Utricularia vulgaris | 50.3 ± 8.75 | 1.34 ± 0.01 | n.d. | 0.41 ± 0.05 | 0.76 ± 0.09 | 2.33 ± 0.18 | n.d. | 1.63 ± 0.35 | n.d. | 6.47 ± 0.14 |
| Turions | ||||||||||
| Aldrovanda vesiculosa | 43.0 | 1.72 | n.d. | 0.15 | 0.75 | 6.62 | n.d. | 1.13 | n.d. | 10.37 |
| Myriophyllum verticillatum | 40.0 | 0.38 | n.d. | 0.08 | 0.40 | 1.78 | n.d. | 0.39 | n.d. | 6.03 |
| Stratiotes aloides | 42.0 | 0.97 | n.d. | 0.32 | 0.89 | 2.37 | n.d. | n.d. | n.d. | 4.55 |
| Utricularia australis | 40.7 | 5.21 | 1.60 | 1.48 | 10.69 | 19.75 | 0.58 | 0.81 | n.d. | 40.12 |
| Utricularia bremii | 57.0 | 7.27 | n.d. | 2.26 | 13.95 | 28.04 | 1.29 | 1.86 | 1.89 | 56.56 |
| Utricularia intermedia | 46.5 | 2.29 | n.d. | 0.58 | 3.61 | 21.47 | n.d. | 0.80 | n.d. | 28.75 |
| Utricularia macrorhiza | 48.5 | 4.64 | n.d. | 1.18 | 9.17 | 30.44 | 0.60 | 1.90 | n.d. | 47.93 |
| Utricularia minor | 53.5 | 7.90 | n.d. | 2.05 | 13.06 | 26.93 | 1.20 | 1.87 | n.d. | 53.01 |
| Utricularia stygia | 50.0 | 3.96 | n.d. | 1.14 | 8.55 | 34.13 | n.d. | 1.52 | n.d. | 49.30 |
| Utricularia vulgaris | 42.5 | 3.12 | n.d. | 0.80 | 5.13 | 20.03 | n.d. | 0.73 | n.d. | 29.81 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Strzemski, M.; Adamec, L.; Dresler, S.; Mazurek, B.; Dubaj, K.; Stolarczyk, P.; Feldo, M.; Płachno, B.J. Shoots and Turions of Aquatic Plants as a Source of Fatty Acids. Molecules 2024, 29, 2062. https://doi.org/10.3390/molecules29092062
Strzemski M, Adamec L, Dresler S, Mazurek B, Dubaj K, Stolarczyk P, Feldo M, Płachno BJ. Shoots and Turions of Aquatic Plants as a Source of Fatty Acids. Molecules. 2024; 29(9):2062. https://doi.org/10.3390/molecules29092062
Chicago/Turabian StyleStrzemski, Maciej, Lubomir Adamec, Sławomir Dresler, Barbara Mazurek, Katarzyna Dubaj, Piotr Stolarczyk, Marcin Feldo, and Bartosz J. Płachno. 2024. "Shoots and Turions of Aquatic Plants as a Source of Fatty Acids" Molecules 29, no. 9: 2062. https://doi.org/10.3390/molecules29092062
APA StyleStrzemski, M., Adamec, L., Dresler, S., Mazurek, B., Dubaj, K., Stolarczyk, P., Feldo, M., & Płachno, B. J. (2024). Shoots and Turions of Aquatic Plants as a Source of Fatty Acids. Molecules, 29(9), 2062. https://doi.org/10.3390/molecules29092062