Exploring the Potential of Biochar Derived from Chinese Herbal Medicine Residue for Efficient Removal of Norfloxacin
Abstract
1. Introduction
2. Results and Discussion
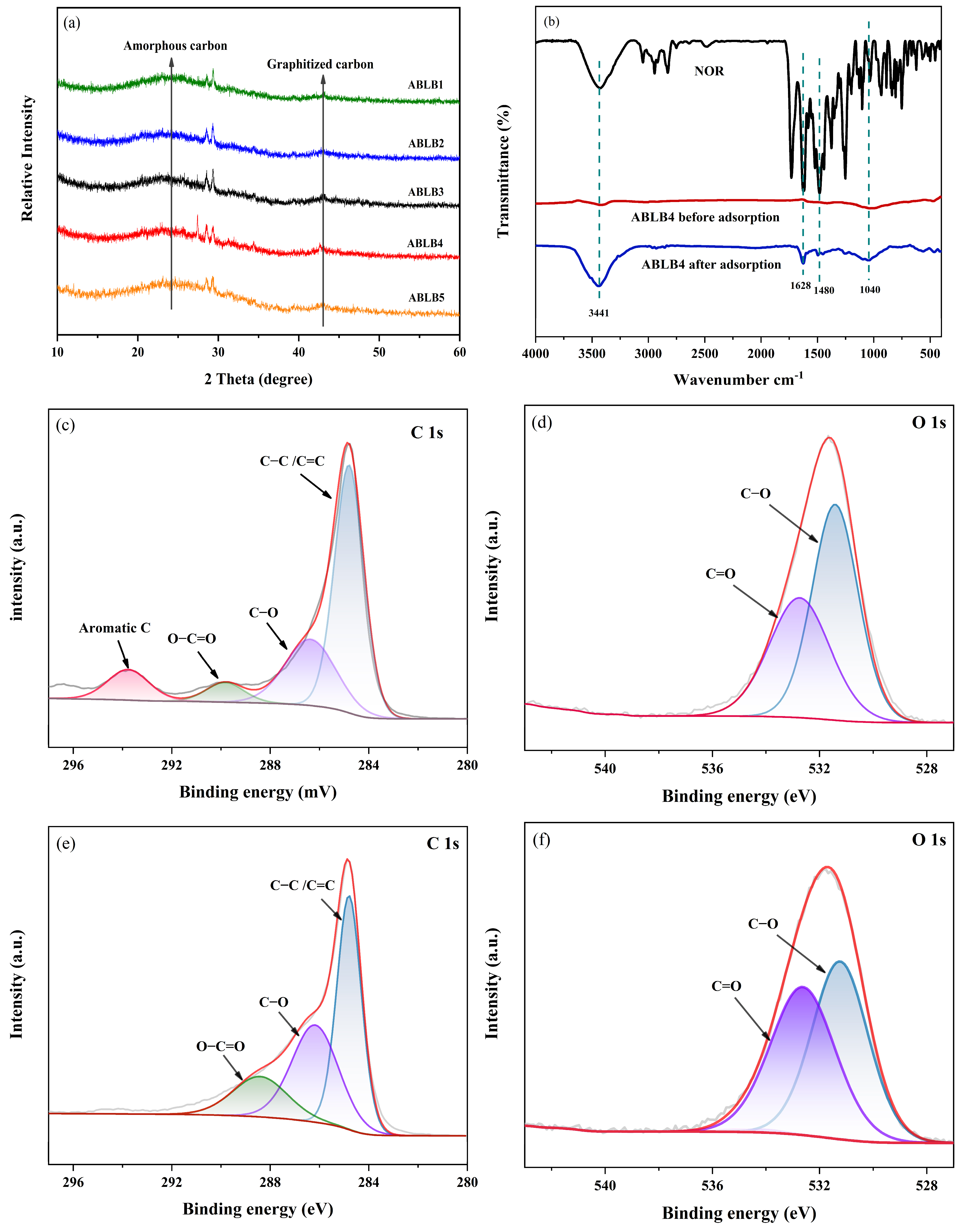
2.1. Characterization
2.2. Adsorption Performance
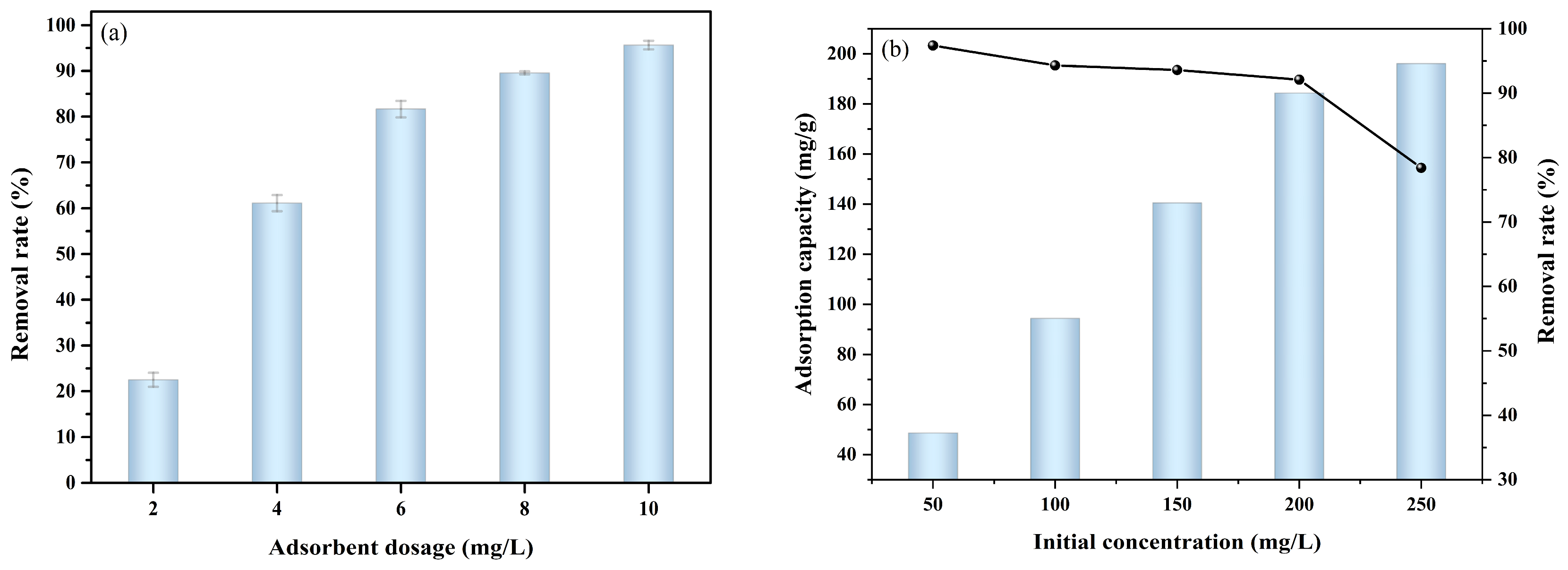
2.2.1. Effect of Adsorbent Dosage
2.2.2. Effect of Initial NOR Concentration
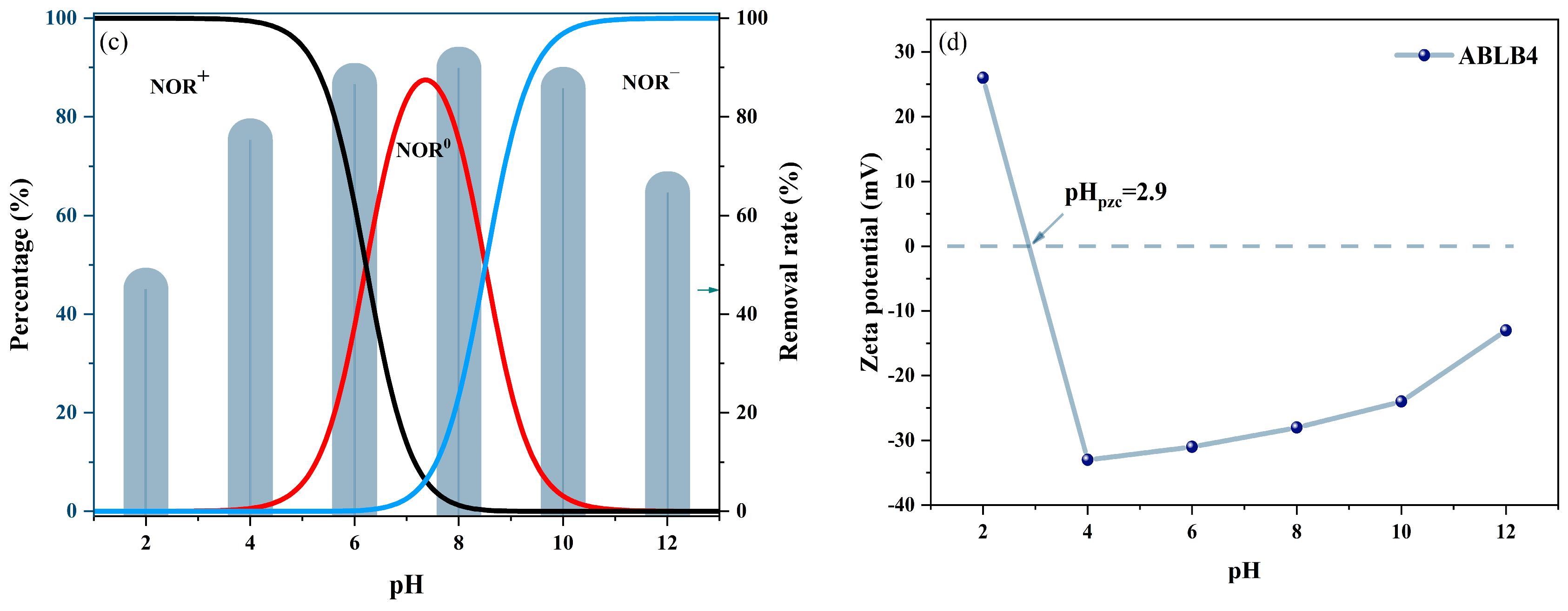
2.2.3. Effect of Solution pH
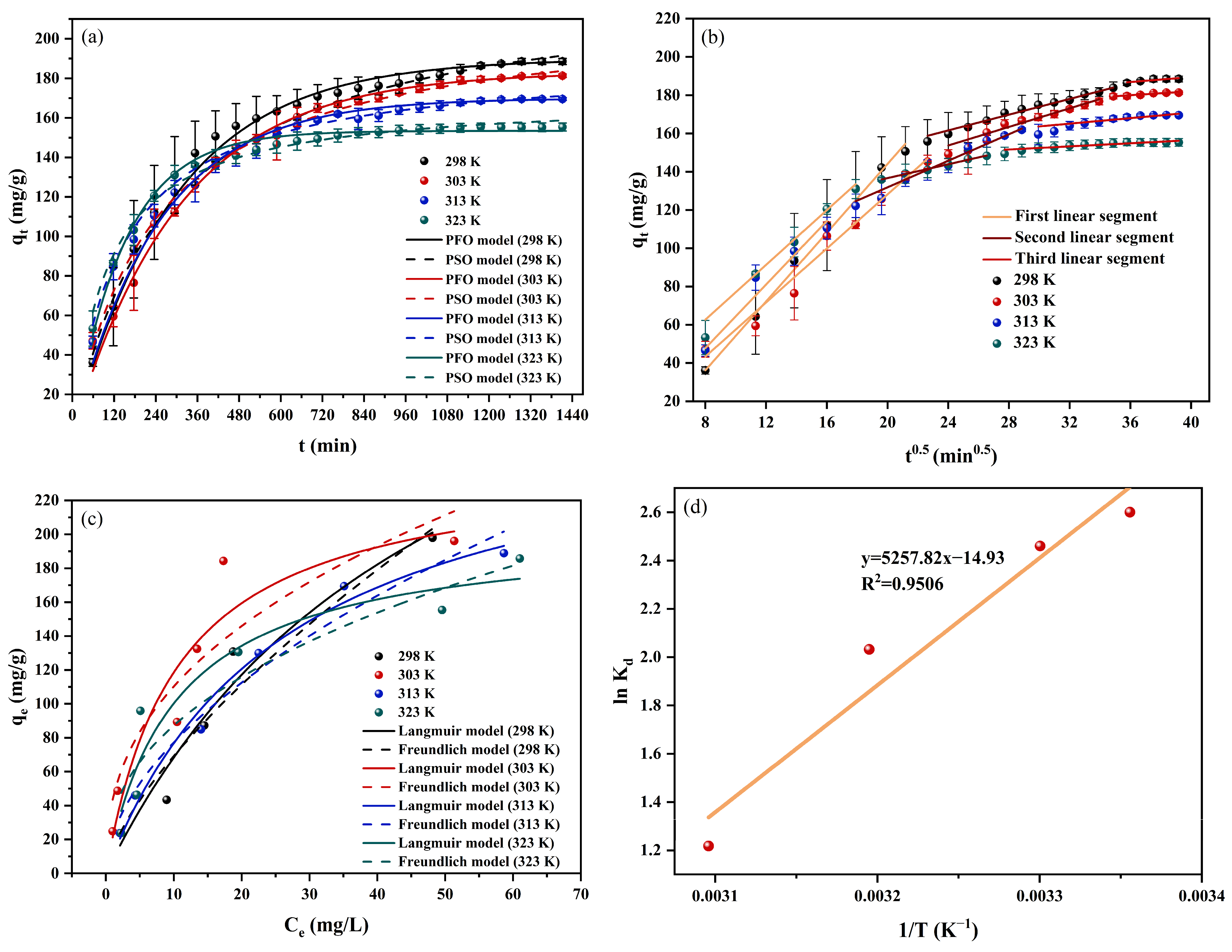
2.2.4. Adsorption Kinetics
2.2.5. Adsorption Isotherm
2.2.6. Thermodynamic Analysis
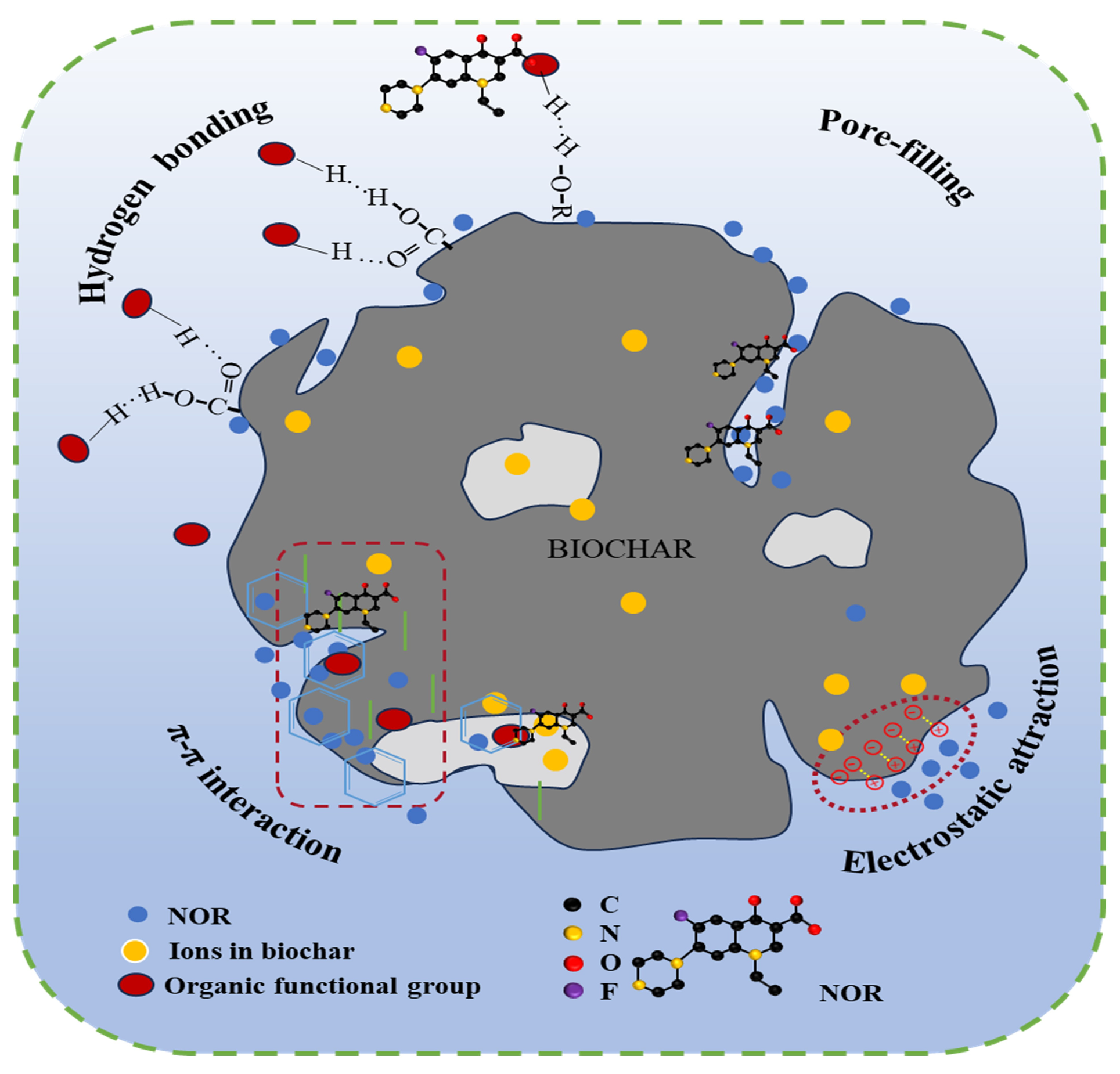
2.3. Adsorption Mechanism
2.4. Industrial Economic Feasibility Analysis
2.5. Response Surface Method (RSM) Regression Analysis
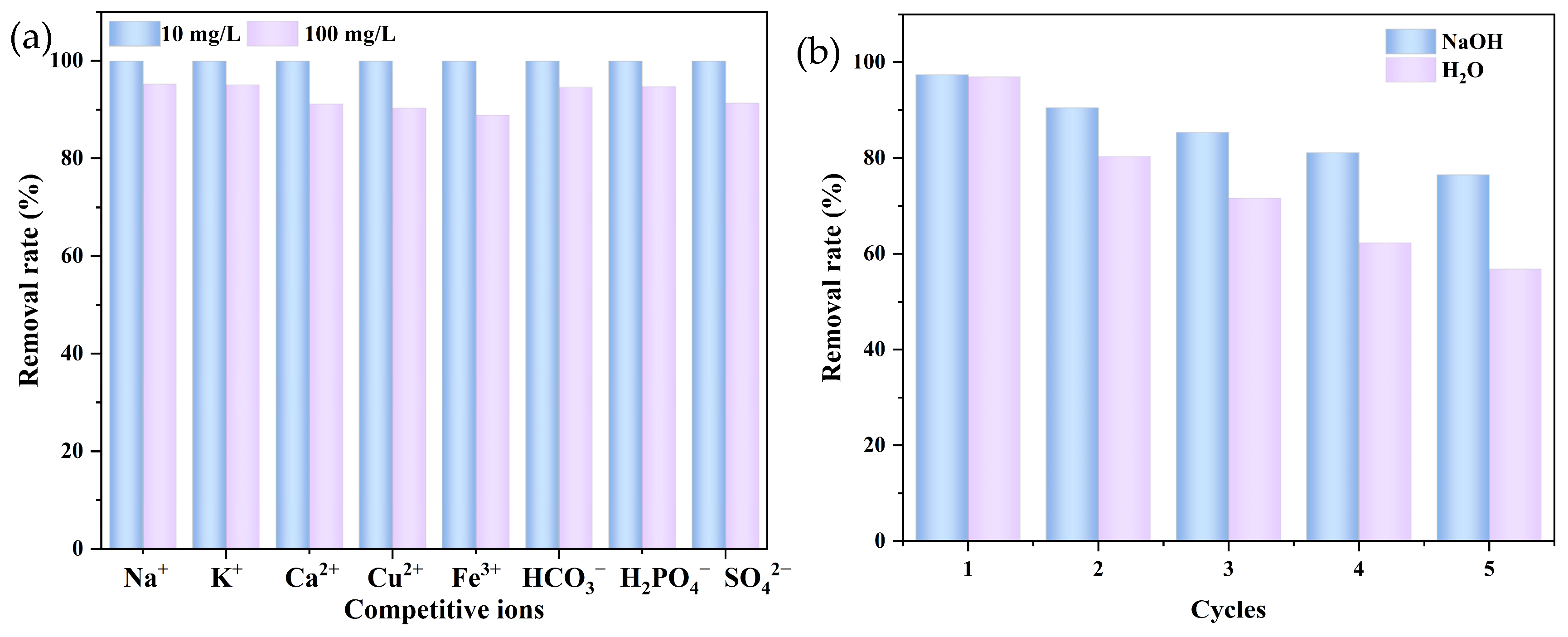
2.6. Influence of Other Coexisting Ions
2.7. Regeneration Ability of ABLB4 Biochar
3. Materials and Methods
3.1. Chemicals and Materials
3.2. Synthesis of ABL Biochar
3.3. NOR Removal Study
3.3.1. Adsorption Studies
3.3.2. Adsorption Kinetics, Isotherms, and Thermodynamics
3.4. Reusability Performance
3.5. Response Surface Method (RSM)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yan, B.; Niu, C.H.; Wang, J. Kinetics, electron-donor-acceptor interactions, and site energy distribution analyses of norfloxacin adsorption on pretreated barley straw. Chem. Eng. J. 2017, 330, 1211–1221. [Google Scholar] [CrossRef]
- Yao, N.; Zhang, X.; Yang, Z.; Yang, W.; Tian, Z.; Zhang, L. Norfloxacin and Bisphenol-A Removal Using Temperature-Switchable Graphene Oxide. ACS Appl. Mater. Interfaces 2018, 10, 29083–29091. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Dong, Y.H.; Wang, H. Residues of veterinary antibiotics in manures from feedlot livestock in eight provinces of China. Sci. Total Environ. 2010, 408, 1069–1075. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Wang, Z.; Gao, C.; Sun, Q.; Liu, J.; She, D. Synthesis of honeycomb lignin-based biochar and its high-efficiency adsorption of norfloxacin. Bioresour. Technol. 2023, 369, 128402. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Zhao, N.; Wei, Y.; Wang, S.; Liu, D.; Yuan, P. Efficient adsorption and separation of norfloxacin from water by allophane aerogel microspheres. Sep. Purif. Technol. 2023, 327, 124808. [Google Scholar] [CrossRef]
- Yang, Y.; Zhong, Z.; Li, J.; Du, H.; Li, Z. Efficient with low-cost removal and adsorption mechanisms of norfloxacin, ciprofloxacin and ofloxacin on modified thermal kaolin: Experimental and theoretical studies. J. Hazard. Mater. 2022, 430, 128500. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Gu, Q.; Shao, H.; Liu, H.; Luan, J.; Yan, Z.; Liu, W.; Ke, X. How PPY/CMC aerogels possess selective adsorption capacity for norfloxacin: Coupling molecular scale interpretation with experiments. Chem. Eng. J. 2023, 464, 142485. [Google Scholar] [CrossRef]
- Pap, S.; Boyd, K.G.; Taggart, M.A.; Turk Sekulic, M. Circular economy based landfill leachate treatment with sulphur-doped microporous biochar. Waste Manag. 2021, 124, 160–171. [Google Scholar] [CrossRef]
- Jia, S.; Yang, Z.; Ren, K.; Tian, Z.; Dong, C.; Ma, R.; Yu, G.; Yang, W. Removal of antibiotics from water in the coexistence of suspended particles and natural organic matters using amino-acid-modified-chitosan flocculants: A combined experimental and theoretical study. J. Hazard. Mater. 2016, 317, 593–601. [Google Scholar] [CrossRef]
- Lan, Y.; Luo, Y.; Yu, S.; Ye, H.; Zhang, Y.; Xue, M.; Sun, Q.; Yin, Z.; Li, X.; Xie, C.; et al. Cornstalk hydrochar produced by phosphoric acid-assisted hydrothermal carbonization for effective adsorption and photodegradation of norfloxacin. Sep. Purif. Technol. 2024, 330, 125543. [Google Scholar] [CrossRef]
- Sharma, V.; Vinoth Kumar, R.; Pakshirajan, K.; Pugazhenthi, G. Integrated adsorption-membrane filtration process for antibiotic removal from aqueous solution. Powder Technol. 2017, 321, 259–269. [Google Scholar] [CrossRef]
- Maged, A.; Elgarahy, A.M.; Hlawitschka, M.W.; Haneklaus, N.H.; Gupta, A.K.; Bhatnagar, A. Synergistic mechanisms for the superior sorptive removal of aquatic pollutants via functionalized biochar-clay composite. Bioresour. Technol. 2023, 387, 12953. [Google Scholar] [CrossRef] [PubMed]
- Cai, P.; Zhao, J.; Zhang, X.; Zhang, T.; Yin, G.; Chen, S.; Dong, C.-L.; Huang, Y.-C.; Sun, Y.; Yang, D.; et al. Synergy between cobalt and nickel on NiCo2O4 nanosheets promotes peroxymonosulfate activation for efficient norfloxacin degradation. Appl. Catal. B Environ. 2022, 306, 121091. [Google Scholar] [CrossRef]
- Liu, W.; Dong, Y.; Yang, D.; Zhang, C.; Zhang, L.; Lu, Y.; Jin, Q.; Liu, Z.; Liu, J.; Lin, H. Unraveling the highly efficient synergy of adsorption and degradation for norfloxacin elimination by Mo/Fe anchored carbon fiber aerogel via peroxydisulfate activation. Chem. Eng. J. 2023, 464, 142667. [Google Scholar] [CrossRef]
- Hou, M.; He, Y.; Yang, X.; Yang, Y.; Lin, X.; Feng, Y.; Kan, H.; Hu, H.; He, X.; Liu, C. Preparation of Biomass Biochar with Components of Similar Proportions and Its Methylene Blue Adsorption. Molecules 2023, 28, 6261. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Duan, L.; Zhang, X.; Li, Z.; Qiu, P.; He, Y.; Shang, P. Selective Adsorption and Recovery of Silver from Acidic Solution Using Biomass-Derived Sulfur-Doped Porous Carbon. ACS Appl. Mater. Interfaces 2023, 15, 40088–40099. [Google Scholar] [CrossRef]
- Ji, D.; Liu, Y.; Ma, H.; Bai, Z.; Qiao, Z.; Ji, D.; Yan, C.; Yan, Y.; Wu, H. Study on Uranium Adsorption Property of Carbon Nanotubes Prepared by Molten Salt Electrolysis. ACS Sustain. Chem. Eng. 2022, 10, 11990–11999. [Google Scholar] [CrossRef]
- Ding, W.-Q.; Xu, L.; Li, X.-Y.; Fu, M.-L.; Yuan, B. 3D-Printed MOFs/Polymer Composite as a Separatable Adsorbent for the Removal of Phenylarsenic Acid in the Aqueous Solution. ACS Appl. Mater. Interfaces 2023, 15, 49181–49194. [Google Scholar] [CrossRef] [PubMed]
- Srinivasa Raghavan, D.S.; Qiu, G.; Ting, Y.-P. Fate and removal of selected antibiotics in an osmotic membrane bioreactor. Chem. Eng. J. 2018, 334, 198–205. [Google Scholar] [CrossRef]
- Jing, F.; Guan, J.; Tang, W.; Chen, J. Mechanistic insight into adsorptive removal of ionic NOR and nonionic DEP organic contaminates by clay-biochar composites. Environ. Pollut. 2022, 310, 119881. [Google Scholar] [CrossRef]
- Lauwers, A.; Vercammen, J.; De Vos, D. Adsorption of PFAS by All-Silica Zeolite β: Insights into the Effect of the Water Matrix, Regeneration of the Material, and Continuous PFAS Adsorption. ACS Appl. Mater. Interfaces 2023, 15, 52612–52621. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.; Zhang, B.; Tong, H.; Liu, Y.; Wang, S.; Wei, S.; Wang, L.; Wang, Y.; Zhang, Y. High-efficiency decontamination of Pb(II) and tetracycline in contaminated water using ball-milled magnetic bone derived biochar. J. Clean. Prod. 2023, 385, 135683. [Google Scholar] [CrossRef]
- Charmas, B.; Zięzio, M.; Jedynak, K. Assessment of the Porous Structure and Surface Chemistry of Activated Biocarbons Used for Methylene Blue Adsorption. Molecules 2023, 28, 4922. [Google Scholar] [CrossRef] [PubMed]
- Cheng, N.; Wang, B.; Chen, M.; Feng, Q.; Zhang, X.; Wang, S.; Zhao, R.; Jiang, T. Adsorption and photocatalytic degradation of quinolone antibiotics from wastewater using functionalized biochar. Environ. Pollut. 2023, 336, 122409. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Li, X.; Ge, C.; Müller, K.; Yu, H.; Deng, H.; Shaheen, S.M.; Tsang, D.C.W.; Bolan, N.S.; Rinklebe, J.; et al. Preparation of ammonium-modified cassava waste-derived biochar and its evaluation for synergistic adsorption of ternary antibiotics from aqueous solution. J. Environ. Manag. 2021, 298, 113530. [Google Scholar] [CrossRef] [PubMed]
- Rueda-Márquez, J.J.; Moreno-Andrés, J.; Rey, A.; Corada-Fernández, C.; Mikola, A.; Manzano, M.A.; Levchuk, I. Post-treatment of real municipal wastewater effluents by means of granular activated carbon (GAC) based catalytic processes: A focus on abatement of pharmaceutically active compounds. Water Res. 2021, 192, 116833. [Google Scholar] [CrossRef] [PubMed]
- Monisha, R.S.; Mani, R.L.; Sivaprakash, B.; Rajamohan, N.; Vo, D.-V.N. Green remediation of pharmaceutical wastes using biochar: A review. Environ. Chem. Lett. 2021, 20, 681–704. [Google Scholar] [CrossRef]
- Xiao, J.; Xu, X.; Wang, F.; Ma, J.; Liao, M.; Shi, Y.; Fang, Q.; Cao, H. Analysis of exposure to pesticide residues from Traditional Chinese Medicine. J. Hazard. Mater. 2019, 365, 857–867. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Deng, Y.; Dai, M.; Jiang, X.; Li, S.; Fu, H.; Peng, C. Migration and transformation of heavy metals in Chinese medicine residues during the process of traditional pyrolysis and solar pyrolysis. Chemosphere 2022, 293, 133658. [Google Scholar] [CrossRef]
- Liu, R.; Yang, Z.; Wang, G.; Xian, J.; Li, T.; Pu, Y.; Jia, Y.; Zhou, W.; Cheng, Z.; Zhang, S.; et al. Simultaneous removal of ammonium and phosphate in aqueous solution using Chinese herbal medicine residues: Mechanism and practical performance. J. Clean. Prod. 2021, 313, 127945. [Google Scholar] [CrossRef]
- Liu, X.; Zhou, Y.; Wang, C.-L.; Liu, Y.; Tao, D.-J. Solvent-free self-assembly synthesis of N-doped ordered mesoporous carbons as effective and bifunctional materials for CO2 capture and oxygen reduction reaction. Chem. Eng. J. 2022, 427, 130878. [Google Scholar] [CrossRef]
- Luo, M.; Wang, L.; Li, H.; Bu, Y.; Zhao, Y.; Cai, J. Hierarchical porous biochar from kelp: Insight into self-template effect and highly efficient removal of methylene blue from water. Bioresour. Technol. 2023, 372, 128676. [Google Scholar] [CrossRef] [PubMed]
- Richard, A.J.; Chen, Z.; Islamoglu, T.; Farha, O.K.; El-Kaderi, H.M. Heteroatom-Doped Porous Carbons as Effective Adsorbers for Toxic Industrial Gasses. ACS Appl. Mater. Interfaces 2022, 14, 33173–33180. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.-X.; Zhang, C.-Y.; Tian, Z.-W.; Cai, J.-J. Large-surface-area carbons derived from lotus stem waste for efficient CO2 capture. New Carbon Mater. 2018, 33, 252–261. [Google Scholar] [CrossRef]
- Shen, J.; Estevez, L.; Barpaga, D.; Zheng, J.; Shutthanandan, V.; McGrail, B.P.; Motkuri, R.K. Structure–Property Correlation of Hierarchically Porous Carbons for Fluorocarbon Adsorption. ACS Appl. Mater. Interfaces 2021, 13, 54266–54273. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Zhao, T.; Zhao, Z.; Tang, H.; Feng, W.; Zhang, Z. Biochar Derived from Chinese Herb Medicine Residues for Rhodamine B Dye Adsorption. ACS Omega 2023, 8, 4813–4825. [Google Scholar] [CrossRef] [PubMed]
- Pawar, A.; Panwar, N.L. A comparative study on morphology, composition, kinetics, thermal behaviour and thermodynamic parameters of Prosopis Juliflora and its biochar derived from vacuum pyrolysis. Bioresour. Technol. Rep. 2022, 18, 101053. [Google Scholar] [CrossRef]
- Liu, Z.; Zang, C.; Ju, Z.; Hu, D.; Zhang, Y.; Jiang, J.; Liu, C. Consistent preparation, chemical stability and thermal properties of a shape-stabilized porous carbon/paraffin phase change materials. J. Clean. Prod. 2020, 247, 119565. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhou, X.; Wang, L.; Tan, M.; Tan, Z.; Huang, Z.; Li, C.; Wu, Z.; Qin, X.; Li, H. Effect of oxidative torrefaction on the pyrolysis of Clitocybe maxima stipe: Pyrolysis behaviour, and products’ properties. J. Anal. Appl. Pyrolysis 2024, 177, 106311. [Google Scholar] [CrossRef]
- Zhou, H.; Li, X.; Jin, H.; She, D. Mechanism of a double-channel nitrogen-doped lignin-based carbon on the highly selective removal of tetracycline from water. Bioresour. Technol. 2022, 346, 126652. [Google Scholar] [CrossRef]
- Navarathna, C.M.; Bombuwala Dewage, N.; Keeton, C.; Pennisson, J.; Henderson, R.; Lashley, B.; Zhang, X.; Hassan, E.B.; Perez, F.; Mohan, D.; et al. Biochar Adsorbents with Enhanced Hydrophobicity for Oil Spill Removal. ACS Appl. Mater. Interfaces 2020, 12, 9248–9260. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhong, J.; Liu, B. Effective removal of methylene blue with zero-valent iron/tea residual biochar composite: Performance and mechanism. Bioresour. Technol. 2023, 371, 128592. [Google Scholar] [CrossRef] [PubMed]
- Martins, A.C.; Pezoti, O.; Cazetta, A.L.; Bedin, K.C.; Yamazaki, D.A.S.; Bandoch, G.F.G.; Asefa, T.; Visentainer, J.V.; Almeida, V.C. Removal of tetracycline by NaOH-activated carbon produced from macadamia nut shells: Kinetic and equilibrium studies. Chem. Eng. J. 2015, 260, 291–299. [Google Scholar] [CrossRef]
- Sadeghi, M.; Moradian, M.; Tayebi, H.-A.; Mirabi, A. Removal of Penicillin G from aqueous medium by PPI@SBA-15/ZIF-8 super adsorbent: Adsorption isotherm, thermodynamic, and kinetic studies. Chemosphere 2023, 311, 136887. [Google Scholar] [CrossRef] [PubMed]
- Sutar, S.; Otari, S.; Jadhav, J. Biochar based photocatalyst for degradation of organic aqueous waste: A review. Chemosphere 2022, 287, 132200. [Google Scholar] [CrossRef]
- Nayak, A.; Bhushan, B.; Kotnala, S. Fabrication of chitosan-hydroxyapatite nano-adsorbent for removal of norfloxacin from water: Isotherm and kinetic studies. Mater. Today Proc. 2022, 61, 143–149. [Google Scholar] [CrossRef]
- Jain, M.; Khan, S.A.; Sahoo, A.; Dubey, P.; Pant, K.K.; Ziora, Z.M.; Blaskovich, M.A.T. Statistical evaluation of cow-dung derived activated biochar for phenol adsorption: Adsorption isotherms, kinetics, and thermodynamic studies. Bioresour. Technol. 2022, 352, 127030. [Google Scholar] [CrossRef]
- Kwoczynski, Z.; Čmelík, J. Characterization of biomass wastes and its possibility of agriculture utilization due to biochar production by torrefaction process. J. Clean. Prod. 2021, 280, 124302. [Google Scholar] [CrossRef]
- Streletskiy, O.A.; Zavidovskiy, I.A.; Nuriahmetov, I.F.; Nishchak, O.Y.; Pavlikov, A.V.; Savchenko, N.F. Resistive Gas Sensors Based on Porous Sp-Containing Films Obtained by Dehydrohalogenation of PVDC and PVDC-PVC Copolymer. C 2023, 9, 82. [Google Scholar] [CrossRef]
- Nipun, T.S.; Khatib, A.; Ahmed, Q.U.; Redzwan, I.E.; Ibrahim, Z.; Khan, A.Y.F.; Primaharinastiti, R.; Khalifa, S.A.M.; El-Seedi, H.R. Alpha-Glucosidase Inhibitory Effect of Psychotria malayana Jack Leaf: A Rapid Analysis Using Infrared Fingerprinting. Molecules 2020, 25, 4161. [Google Scholar] [CrossRef]
- Zhao, J.; Yu, L.; Ma, H.; Zhou, F.; Yang, K.; Wu, G. Corn stalk-based activated carbon synthesized by a novel activation method for high-performance adsorption of hexavalent chromium in aqueous solutions. J. Colloid Interface Sci. 2020, 578, 650–659. [Google Scholar] [CrossRef] [PubMed]
- Zhao, N.; Zhao, C.; Lv, Y.; Zhang, W.; Du, Y.; Hao, Z.; Zhang, J. Adsorption and coadsorption mechanisms of Cr(VI) and organic contaminants on H3PO4 treated biochar. Chemosphere 2017, 186, 422–429. [Google Scholar] [CrossRef] [PubMed]
- Ding, D.; Zhou, L.; Kang, F.; Yang, S.; Chen, R.; Cai, T.; Duan, X.; Wang, S. Synergistic Adsorption and Oxidation of Ciprofloxacin by Biochar Derived from Metal-Enriched Phytoremediation Plants: Experimental and Computational Insights. ACS Appl. Mater. Interfaces 2020, 12, 53788–53798. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.K.; Nguyen, T.B.; Chen, W.H.; Chen, C.W.; Kumar Patel, A.; Bui, X.T.; Chen, L.; Singhania, R.R.; Dong, C.D. Phosphoric acid-activated biochar derived from sunflower seed husk: Selective antibiotic adsorption behavior and mechanism. Bioresour. Technol. 2023, 371, 128593. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wang, X.; Fang, D. A review on C1s XPS-spectra for some kinds of carbon materials. Fuller. Nanotub. Carbon Nanostruct. 2020, 28, 1048–1058. [Google Scholar] [CrossRef]
- Streletskiy, O.A.; Nishchak, O.Y.; Zavidovskiy, I.A.; Maslakov, K.I.; Pavlikov, A.V. Sp-based thin films synthesized by magnetron sputtering of dehydrohalogenated Polyvinylidenchloride. Thin Solid Films 2021, 739, 138993. [Google Scholar] [CrossRef]
- Tang, J.; Ma, Y.; Deng, Z.; Li, P.; Qi, X.; Zhang, Z. One-pot preparation of layered double oxides-engineered biochar for the sustained removal of tetracycline in water. Bioresour. Technol. 2023, 381, 129119. [Google Scholar] [CrossRef] [PubMed]
- Sulistiyo, C.D.; Cheng, K.-C.; Su’andi, H.J.; Yuliana, M.; Hsieh, C.-W.; Ismadji, S.; Angkawijaya, A.E.; Go, A.W.; Hsu, H.Y.; Tran-Nguyen, P.L.; et al. Removal of hexavalent chromium using durian in the form of rind, cellulose, and activated carbon: Comparison on adsorption performance and economic evaluation. J. Clean. Prod. 2022, 380, 135010. [Google Scholar] [CrossRef]
- Nguyen, V.-T.; Vo, T.-D.-H.; Nguyen, T.-B.; Dat, N.D.; Huu, B.T.; Nguyen, X.-C.; Tran, T.; Le, T.-N.-C.; Duong, T.-G.-H.; Bui, M.-H.; et al. Adsorption of norfloxacin from aqueous solution on biochar derived from spent coffee ground: Master variables and response surface method optimized adsorption process. Chemosphere 2022, 288, 132577. [Google Scholar] [CrossRef]
- Patel, J.; Singh, A.K.; Carabineiro, S.A.C. Assessing the Photocatalytic Degradation of Fluoroquinolone Norfloxacin by Mn:ZnS Quantum Dots: Kinetic Study, Degradation Pathway and Influencing Factors. Nanomaterials 2020, 10, 964. [Google Scholar] [CrossRef]
- Liu, L.; Wang, X.; Fang, W.; Li, X.; Shan, D.; Dai, Y. Adsorption of metolachlor by a novel magnetic illite–biochar and recovery from soil. Environ. Res. 2022, 204, 111919. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, P.; Huang, Y.; Rene, E.R.; Kumar, A.J.; Chen, S. Mechanism of allethrin biodegradation by a newly isolated Sphingomonas trueperi strain CW3 from wastewater sludge. Bioresour. Technol. 2020, 305, 123074. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Liu, Q.; Yu, Y.; Kong, Q.; Zhou, L.-L.; Du, Y.-D.; Wang, X.-F. Norfloxacin removal from aqueous solution using biochar derived from luffa sponge. J. Water Supply Res. Technol.-Aqua 2018, 67, 703–714. [Google Scholar] [CrossRef]
- Liu, P.; Li, H.; Liu, X.; Wan, Y.; Han, X.; Zou, W. Preparation of magnetic biochar obtained from one-step pyrolysis of salix mongolica and investigation into adsorption behavior of sulfadimidine sodium and norfloxacin in aqueous solution. J. Dispers. Sci. Technol. 2019, 41, 214–226. [Google Scholar] [CrossRef]
- Yang, W.; Lu, Y.; Zheng, F.; Xue, X.; Li, N.; Liu, D. Adsorption behavior and mechanisms of norfloxacin onto porous resins and carbon nanotube. Chem. Eng. J. 2012, 179, 112–118. [Google Scholar] [CrossRef]
- Qin, T.; Wang, Z.; Xie, X.; Xie, C.; Zhu, J.; Li, Y. A novel biochar derived from cauliflower (Brassica oleracea L.) roots could remove norfloxacin and chlortetracycline efficiently. Water Sci. Technol. 2017, 76, 3307–3318. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Z.; Xie, X.; Zhu, J.; Li, R.; Qin, T. Removal of Norfloxacin from aqueous solution by clay-biochar composite prepared from potato stem and natural attapulgite. Colloids Surf. Physicochem. Eng. Aspects 2017, 514, 126–136. [Google Scholar] [CrossRef]
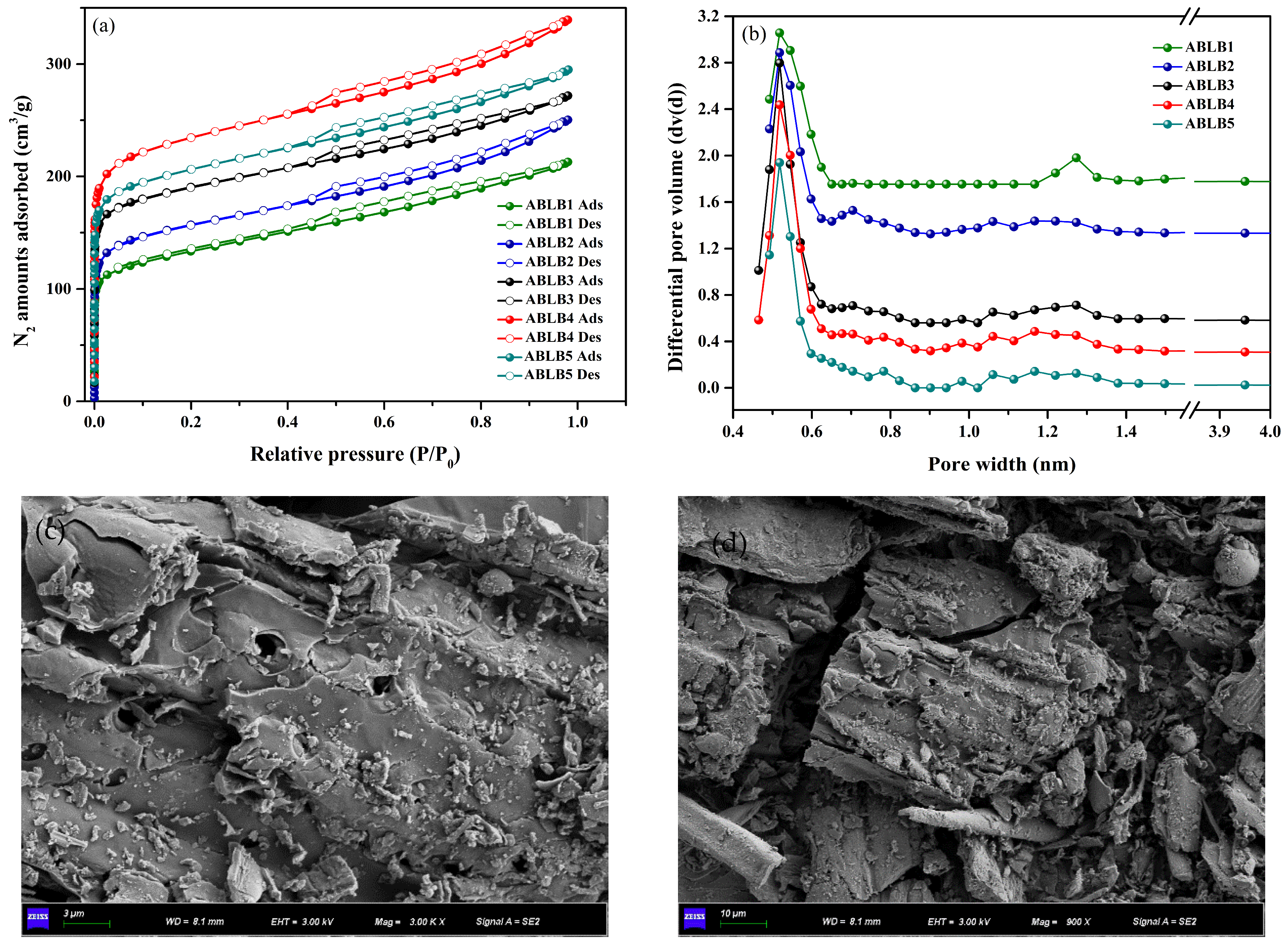

| Samples | SBET (m2/g) | SLangmuir (m2/g) | VMicro (cm3/g) | VTotal (cm3/g) | Average Pore Diameter (nm) |
|---|---|---|---|---|---|
| ABLB1 | 499 | 639 | 0.12 | 0.33 | 2.69 |
| ABLB2 | 665 | 828 | 0.20 | 0.42 | 2.51 |
| ABLB3 | 664 | 800 | 0.20 | 0.40 | 2.40 |
| ABLB4 | 812 | 1002 | 0.24 | 0.50 | 2.40 |
| ABLB5 | 712 | 882 | 0.21 | 0.43 | 2.42 |
| Samples | C (%) | H (%) | O (%) | N (%) | S (%) | H/C | O/C | (O + N)/C |
|---|---|---|---|---|---|---|---|---|
| ABLB1 | 71.85 | 1.09 | 8.82 | 0.44 | 0.15 | 0.02 | 0.12 | 0.13 |
| ABLB2 | 67.91 | 0.72 | 7.53 | 0.34 | 0.06 | 0.01 | 0.11 | 0.12 |
| ABLB3 | 71.31 | 0.58 | 6.72 | 0.00 | 0.92 | 0.01 | 0.09 | 0.09 |
| ABLB4 | 70.99 | 0.58 | 5.91 | 0.00 | 0.16 | 0.01 | 0.08 | 0.08 |
| ABLB5 | 71.68 | 0.43 | 6.52 | 0.34 | 0.04 | 0.01 | 0.09 | 0.10 |
| Temperature | Langmuir | Freundlich | Temkin | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| qe,exp (mg/g) | qe,cal (mg/g) | KL (L/mg) | R2 | KF ((mg1−n Ln)/g) | n | R2 | KT (L/g) | b (J/mol) | R2 | |
| 298 K | 192.4 | 251.97 | 0.07 | 0.98 | 33.94 | 2.17 | 0.93 | 0.44 | 41.44 | 0.8526 |
| 303 K | 183.37 | 242.88 | 0.10 | 0.88 | 43.51 | 2.48 | 0.83 | 0.56 | 56.26 | 0.8654 |
| 313 K | 164.28 | 220.58 | 0.06 | 0.99 | 28.91 | 2.22 | 0.95 | 0.60 | 50.79 | 0.9456 |
| 323 K | 156.93 | 202.98 | 0.10 | 0.90 | 34.05 | 2.45 | 0.86 | 0.91 | 60.43 | 0.9132 |
| Source | SS | DF | MS | F-Value | p-Value |
|---|---|---|---|---|---|
| Model | 5769.83 | 9 | 641.09 | 9.00 | 0.0042 |
| A-temperature (K) | 6.25 | 1 | 6.25 | 0.088 | 0.7757 |
| B-initial concentration (mg/L) | 2191.22 | 1 | 2191.22 | 30.75 | 0.0009 |
| C-pH | 203.31 | 1 | 203.31 | 2.85 | 0.1351 |
| AB | 0.90 | 1 | 0.90 | 0.013 | 0.9136 |
| AC | 7.225 × 10−3 | 1 | 7.225 × 10−3 | 1.014 × 10−3 | 0.9922 |
| BC | 19.80 | 1 | 19.80 | 0.28 | 0.6144 |
| A2 | 233.77 | 1 | 233.77 | 3.28 | 0.1130 |
| B2 | 369.38 | 1 | 369.38 | 5.18 | 0.0569 |
| C2 | 2781.28 | 1 | 2781.28 | 39.03 | 0.0004 |
| Residual | 498.83 | 7 | 71.26 | ||
| Lack of Fit | 494.16 | 3 | 164.72 | 141.03 | 0.0002 |
| Pure Error | 4.67 | 4 | 1.17 | ||
| Cor Total | 6268.66 | 16 | |||
| Removal rate (%) = 85.06 − 0.88 × A − 16.55 × B + 50.4 × C − 7.45 × A2 + 9.37 × B2 − 25.70 × C2 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, P.; Zhao, Z.; Zhang, M.; Su, H.; Zhao, T.; Feng, W.; Zhang, Z. Exploring the Potential of Biochar Derived from Chinese Herbal Medicine Residue for Efficient Removal of Norfloxacin. Molecules 2024, 29, 2063. https://doi.org/10.3390/molecules29092063
Li P, Zhao Z, Zhang M, Su H, Zhao T, Feng W, Zhang Z. Exploring the Potential of Biochar Derived from Chinese Herbal Medicine Residue for Efficient Removal of Norfloxacin. Molecules. 2024; 29(9):2063. https://doi.org/10.3390/molecules29092063
Chicago/Turabian StyleLi, Pengwei, Ziheng Zhao, Miaomiao Zhang, Hang Su, Ting Zhao, Weisheng Feng, and Zhijuan Zhang. 2024. "Exploring the Potential of Biochar Derived from Chinese Herbal Medicine Residue for Efficient Removal of Norfloxacin" Molecules 29, no. 9: 2063. https://doi.org/10.3390/molecules29092063
APA StyleLi, P., Zhao, Z., Zhang, M., Su, H., Zhao, T., Feng, W., & Zhang, Z. (2024). Exploring the Potential of Biochar Derived from Chinese Herbal Medicine Residue for Efficient Removal of Norfloxacin. Molecules, 29(9), 2063. https://doi.org/10.3390/molecules29092063








