Theoretical Study of p-Block Metal Single-Atom-Loaded Carbon Nitride Catalyst for Photocatalytic Water Splitting
Abstract
1. Introduction
2. Results and Discussion
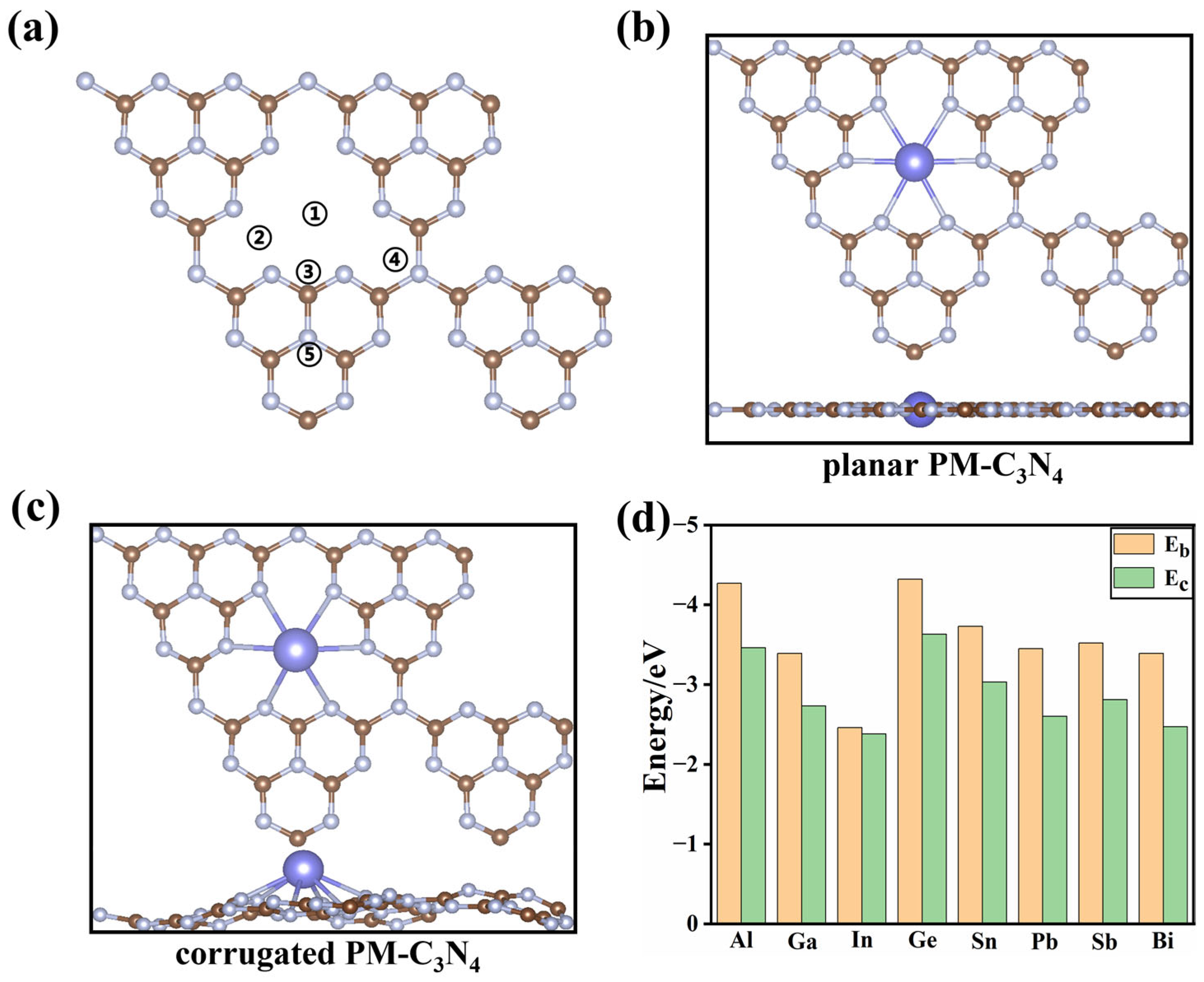
2.1. Structures and Stability
2.2. Photocatalytic Water Splitting Reaction
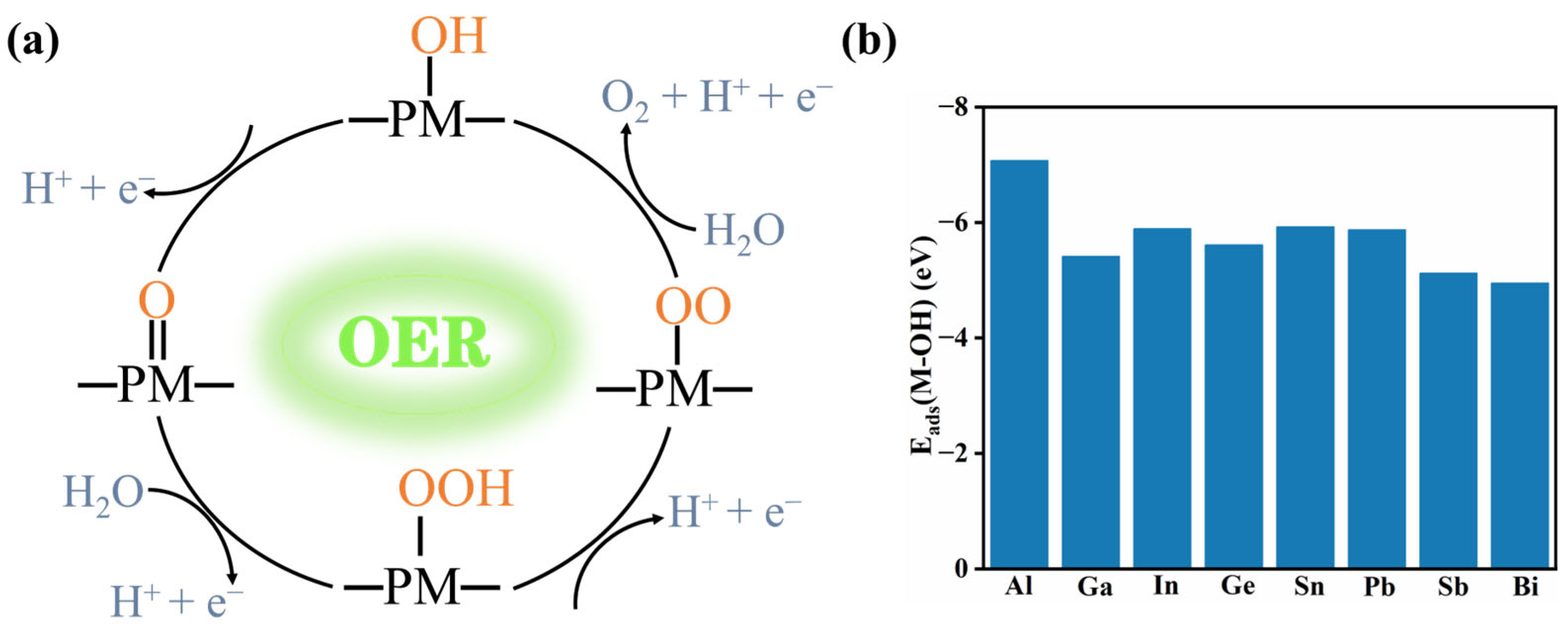
2.2.1. OER Reactivity
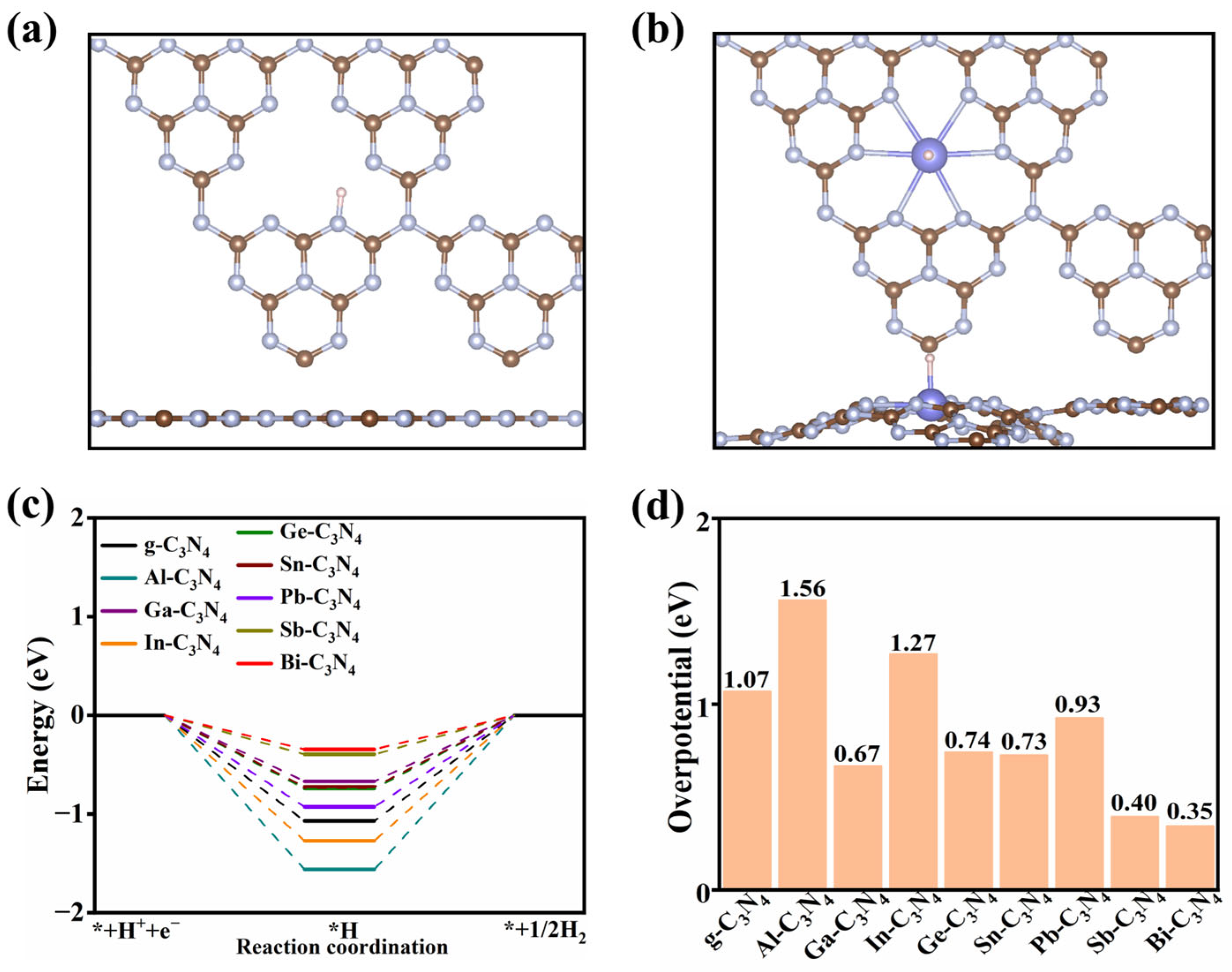
2.2.2. The HER Reactivity
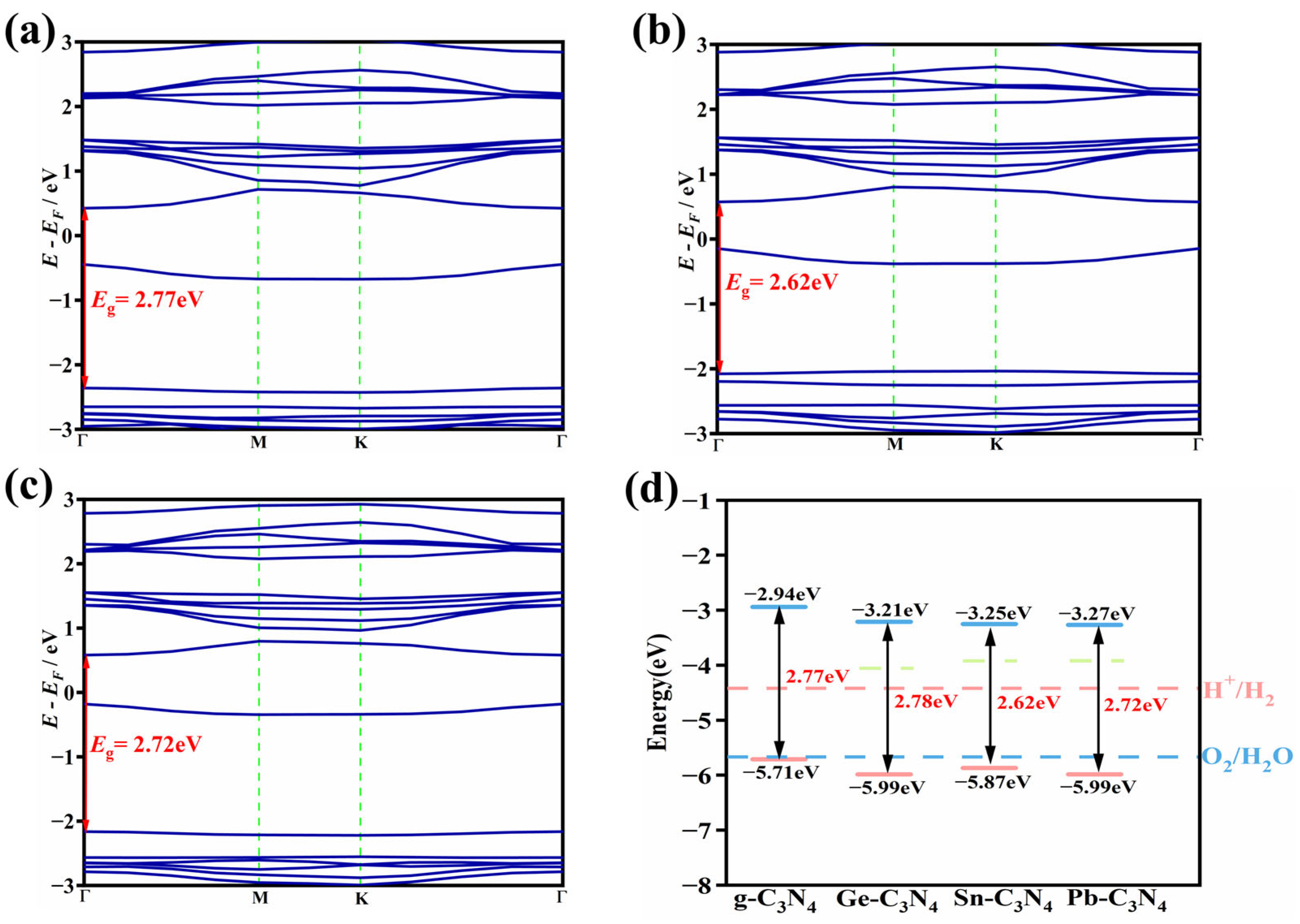
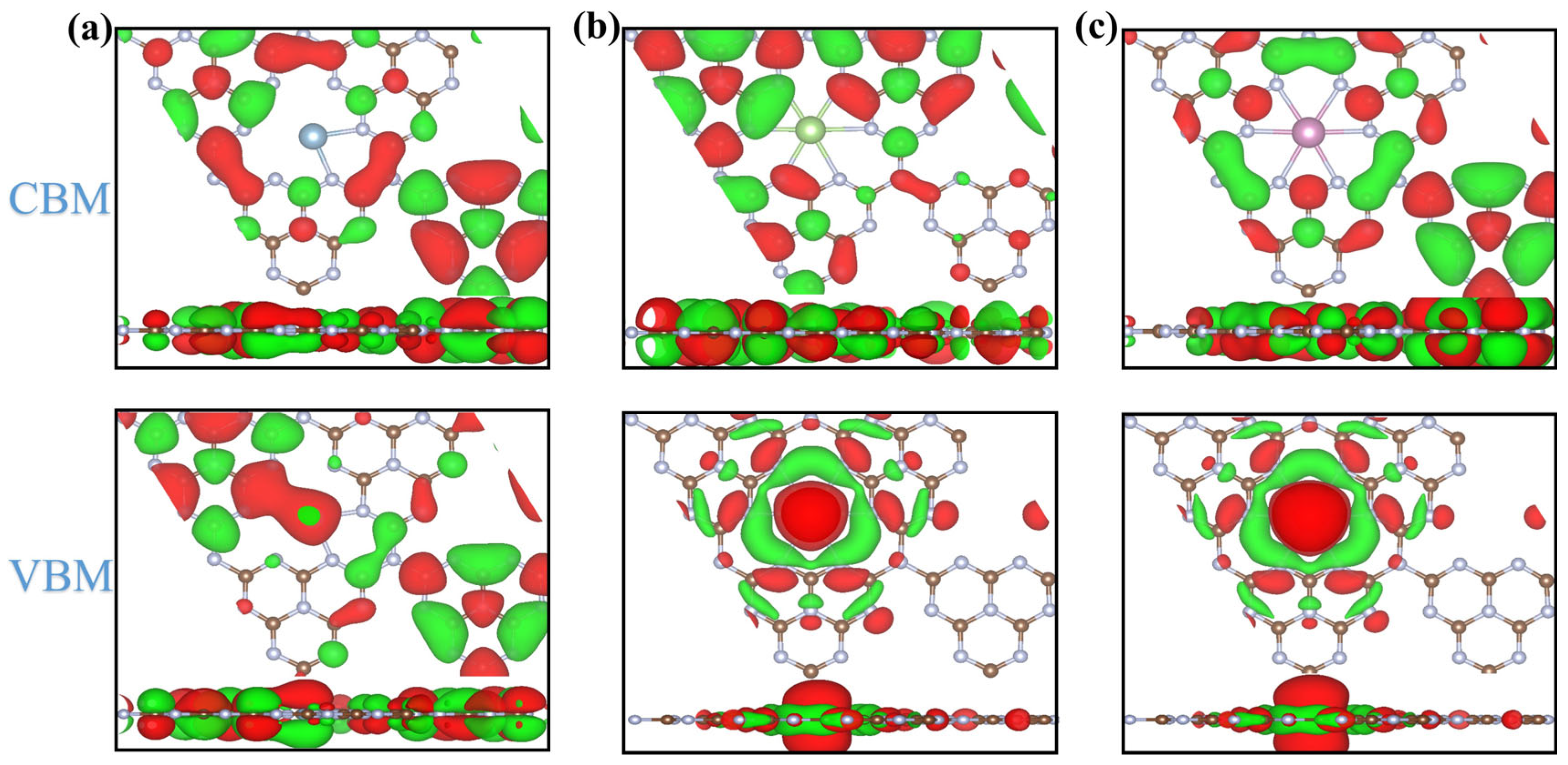
2.3. Electronic Structure of Candidates
3. Conclusions
4. Computational Method
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lin, L.; Lin, Z.; Zhang, J.; Cai, X.; Lin, W.; Yu, Z.; Wang, X. Molecular-level insights on the reactive facet of carbon nitride single crystals photocatalysing overall water splitting. Nat. Catal. 2020, 3, 649–655. [Google Scholar] [CrossRef]
- Liu, D.; Dai, L.; Lin, X.; Chen, J.F.; Zhang, J.; Feng, X.; Müllen, K.; Zhu, X.; Dai, S. Chemical Approaches to Carbon-Based Metal-Free Catalysts. Adv. Mater. 2019, 31, 1804863. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Xue, S.; Qin, Z.; Nazari, M.; Yang, G.; Yue, S.; Tong, T.; Ghasemi, H.; Hernandez, F.C.R.; Xue, S.; et al. Making g-C3N4 ultra-thin nanosheets active for photocatalytic overall water splitting. Appl. Catal. B Environ. 2021, 282, 119557–119564. [Google Scholar] [CrossRef]
- Hisatomi, T.; Domen, K. Reaction systems for solar hydrogen production via water splitting with particulate semiconductor photocatalysts. Nat. Catal. 2019, 2, 387–399. [Google Scholar] [CrossRef]
- Wang, Z.; Li, C.; Domen, K. Recent developments in heterogeneous photocatalysts for solar-driven overall water splitting. Chem. Soc. Rev. 2019, 48, 2109–2125. [Google Scholar] [CrossRef] [PubMed]
- Navarro Yerga, R.M.; Álvarez Galván, M.C.; del Valle, F.; Villoria de la Mano, J.A.; Fierro, J.L.G. Water Splitting on Semiconductor Catalysts under Visible-Light Irradiation. ChemSusChem 2009, 2, 471–485. [Google Scholar] [CrossRef] [PubMed]
- Qin, S.; Denisov, N.; Sarma, B.B.; Hwang, I.; Doronkin, D.E.; Tomanec, O.; Kment, S.; Schmuki, P. Pt Single Atoms on TiO2 Polymorphs—Minimum Loading with a Maximized Photocatalytic Efficiency. Adv. Mater. Interfaces 2022, 9, 2200808–2200817. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, C.; Huang, M.; Zhang, J.; Zhang, J.; Liu, H.; Wang, G.; Wang, R. The activation of porous atomic layered MoS2 basal-plane to induce adjacent Mo atom pairs promoting high efficiency electrochemical N2 fixation. Appl. Catal. B Environ. 2021, 285, 119810–119818. [Google Scholar] [CrossRef]
- Zhou, X.; Li, M.; Wang, P.; Wu, M.; Jin, B.; Luo, J.; Chen, M.; Zhou, X.; Zhang, Y.; Zhou, X. Synergistic effect of phosphorus doping and MoS2 co-catalysts on g-C3N4 photocatalysts for enhanced solar water splitting. J. Mater. Sci. Technol. 2023, 158, 171–179. [Google Scholar] [CrossRef]
- Wu, H.; Irani, R.; Zhang, K.; Jing, L.; Dai, H.; Chung, H.Y.; Abdi, F.F.; Ng, Y.H. Unveiling Carrier Dynamics in Periodic Porous BiVO4 Photocatalyst for Enhanced Solar Water Splitting. ACS Energy Lett. 2021, 6, 3400–3407. [Google Scholar] [CrossRef]
- Xue, Z.-H.; Luan, D.; Zhang, H.; Lou, X.W. Single-atom catalysts for photocatalytic energy conversion. Joule 2022, 6, 92–133. [Google Scholar] [CrossRef]
- Zhang, D.; Li, Y.; Li, Y.; Zhan, S. Towards single-atom photocatalysts for future carbon-neutral application. SmartMat 2022, 3, 417–446. [Google Scholar] [CrossRef]
- Ye, D.; Liu, L.; Peng, Q.; Qiu, J.; Gong, H.; Zhong, A.; Liu, S. Effect of Controlling Thiophene Rings on D-A Polymer Photocatalysts Accessed via Direct Arylation for Hydrogen Production. Molecules 2023, 28, 4507. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.A.; Xian, J.Y.; Rong, L.R.; Qin, H.; Jie, Z. Theoretical study of metal ion impact on geometric and electronic properties of terbutaline compounds. Monatsh. Chem. 2019, 150, 1355–1364. [Google Scholar] [CrossRef]
- Wang, K.; He, X.; Rong, C.; Zhong, A.; Liu, S.; Zhao, D. On the origin and nature of internal methyl rotation barriers: An information-theoretic approach study. Theor. Chem. Acc. 2022, 141, 68. [Google Scholar] [CrossRef]
- Li, X.; Chen, Y.; Tao, Y.; Shen, L.; Xu, Z.; Bian, Z.; Li, H. Challenges of photocatalysis and their coping strategies. Chem. Catal. 2022, 2, 1315–1345. [Google Scholar] [CrossRef]
- Wang, Z.; Zhao, J.; Wan, Q.; Lin, S. Halogen-driven bandgap opening in graphdiyne for overall photocatalytic water splitting. Chin. J. Chem. Phys. 2021, 34, 805–813. [Google Scholar] [CrossRef]
- Xu, J.; Wan, Q.; Wang, Z.; Lin, S. The band structure engineering of fluorine-passivated graphdiyne nanoribbons via doping with BN pairs for overall photocatalytic water splitting. Phys. Chem. Chem. Phys. 2020, 22, 26995–27001. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Wan, Q.; Anpo, M.; Lin, S. Bandgap Opening of Graphdiyne Monolayer via B, N-Codoping for Photocatalytic Overall Water Splitting: Design Strategy from DFT Studies. J. Phys. Chem. C 2020, 124, 6624–6633. [Google Scholar] [CrossRef]
- Wan, Q.; Wei, F.; Ma, Z.; Anpo, M.; Lin, S. Novel Porous Boron Nitride Nanosheet with Carbon Doping: Potential Metal-Free Photocatalyst for Visible-Light-Driven Overall Water Splitting. Adv. Theory Simul. 2019, 2, 1800174. [Google Scholar] [CrossRef]
- Fang, Z.; Weng, S.; Ye, X.; Feng, W.; Zheng, Z.; Lu, M.; Lin, S.; Fu, X.; Liu, P. Defect Engineering and Phase Junction Architecture of Wide-Bandgap ZnS for Conflicting Visible Light Activity in Photocatalytic H2 Evolution. ACS Appl. Mater. Interfaces 2015, 7, 13915–13924. [Google Scholar] [CrossRef] [PubMed]
- Godin, R.; Wang, Y.; Zwijnenburg, M.A.; Tang, J.; Durrant, J.R. Time-Resolved Spectroscopic Investigation of Charge Trapping in Carbon Nitrides Photocatalysts for Hydrogen Generation. J. Am. Chem. Soc. 2017, 139, 5216–5224. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q. Exploring the Nanoconfinement Effect Using 2D Capillaries. Acc. Mater. Res. 2022, 4, 1–3. [Google Scholar] [CrossRef]
- Wang, X.; Maeda, K.; Thomas, A.; Takanabe, K.; Xin, G.; Carlsson, J.M.; Domen, K.; Antonietti, M. A metal-free polymeric photocatalyst for hydrogen production from water under visible light. Nat. Mater. 2009, 8, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Zhao, Z.; Liu, Q. Regulating effect on photocatalytic water splitting performance of g-C3N4 via confinement of single atom Pt based on energy band engineering: A first principles investigation. Appl. Surf. Sci. 2022, 577, 151916. [Google Scholar] [CrossRef]
- Vasilchenko, D.; Zhurenok, A.; Saraev, A.; Gerasimov, E.; Cherepanova, S.; Kovtunova, L.; Tkachev, S.; Kozlova, E. Platinum deposition onto g-C3N4 with using of labile nitratocomplex for generation of the highly active hydrogen evolution photocatalysts. Int. J. Hydrogen Energy 2022, 47, 11326–11340. [Google Scholar] [CrossRef]
- Sidorenko, N.D.; Topchiyan, P.A.; Saraev, A.A.; Gerasimov, E.Y.; Zhurenok, A.V.; Vasilchenko, D.B.; Kozlova, E.A. Bimetallic Pt-IrOx/g-C3N4 Photocatalysts for the Highly Efficient Overall Water Splitting under Visible Light. Catalysts 2024, 14, 225. [Google Scholar] [CrossRef]
- Vasilchenko, D.; Tkachev, S.; Tkachenko, P.; Berdyugin, S.; Popovetskiy, P.; Gerasimov, E.; Zhurenok, A.; Kozlova, E. Platinum(IV) Carbonato Complexes: Formation via the Addition of CO2 to the [Pt(OH)6]2− Anion and Generation of Platinum(IV) Oxide Nanoparticles for the Preparation of Catalysts. Inorg. Chem. 2023, 62, 9732–9748. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Cao, L.; Cheng, W.; Cao, Y.; Liu, X.; Zhang, W.; Mou, X.; Jin, L.; Zheng, X.; Che, W.; et al. Single-Site Active Cobalt-Based Photocatalyst with a Long Carrier Lifetime for Spontaneous Overall Water Splitting. Angew. Chem. Int. Ed. 2017, 56, 9312–9317. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Chen, X.; Yang, Z.; Yu, T.; Liu, L.; Ye, J. Precisely Tailoring Nitrogen Defects in Carbon Nitride for Efficient Photocatalytic Overall Water Splitting. ACS Appl. Mater. Interfaces 2022, 14, 3970–3979. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.-F.; Song, J.; Wang, X.; Pan, L.; Li, K.; Zhang, X.; Wang, L.; Zou, J.-J. Switching charge transfer of C3N4/W18O49 from type-II to Z-scheme by interfacial band bending for highly efficient photocatalytic hydrogen evolution. Nano Energy 2017, 40, 308–316. [Google Scholar] [CrossRef]
- Ding, K.; Wen, L.; Huang, M.; Zhang, Y.; Lu, Y.; Chen, Z. How does the B,F-monodoping and B/F-codoping affect the photocatalytic water-splitting performance of g-C3N4? Phys. Chem. Chem. Phys. 2016, 18, 19217–19226. [Google Scholar] [CrossRef]
- Wu, J.; Liu, Z.; Lin, X.; Jiang, E.; Zhang, S.; Huo, P.; Yan, Y.; Zhou, P.; Yan, Y. Breaking through water-splitting bottlenecks over carbon nitride with fluorination. Nat. Commun. 2022, 13, 6999–7007. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Shen, J.; Yu, X.; Yang, X.; Liu, W.; Yang, J.; Tang, H.; Xu, H.; Li, H.; Li, Y.; et al. Unveiling the origin of boosted photocatalytic hydrogen evolution in simultaneously (S, P, O)-Codoped and exfoliated ultrathin g-C3N4 nanosheets. Appl. Catal. B Environ. 2019, 248, 84–94. [Google Scholar] [CrossRef]
- Qiao, B.; Wang, A.; Yang, X.; Allard, L.F.; Jiang, Z.; Cui, Y.; Liu, J.; Li, J.; Zhang, T. Single-atom catalysis of CO oxidation using Pt1/FeOx. Nat. Chem. 2011, 3, 634–641. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zheng, X.; Feng, Z.; Lu, Z.; Zhang, Z.; Huang, W.; Li, Y.; Vuckovic, D.; Li, Y.; Dai, S.; et al. Organic wastewater treatment by a single-atom catalyst and electrolytically produced H2O2. Nat. Sustain. 2020, 4, 233–241. [Google Scholar] [CrossRef] [PubMed]
- He, T.; Matta, S.K.; Will, G.; Du, A. Transition-Metal Single Atoms Anchored on Graphdiyne as High-Efficiency Electrocatalysts for Water Splitting and Oxygen Reduction. Small Methods 2019, 3, 1800419. [Google Scholar] [CrossRef]
- Jin, H.; Zhou, K.; Zhang, R.; Cui, H.; Yu, Y.; Cui, P.; Song, W.; Cao, C. Regulating the electronic structure through charge redistribution in dense single-atom catalysts for enhanced alkene epoxidation. Nat. Commun. 2023, 14, 2494–2503. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Zhao, J.; Wang, S.; Chen, R.; Ding, J.; Tsai, H.J.; Zeng, W.J.; Hung, S.F.; Xu, W.; Wang, J.; et al. Operando Spectroscopic Analysis of Axial Oxygen-Coordinated Single-Sn-Atom Sites for Electrochemical CO2 Reduction. J. Am. Chem. Soc. 2023, 145, 7242–7251. [Google Scholar] [CrossRef]
- Lin, S.; Ye, X.; Gao, X.; Huang, J. Mechanistic insight into the water photooxidation on pure and sulfur-doped g-C3N4 photocatalysts from DFT calculations with dispersion corrections. J. Mol. Catal. A Chem. 2015, 406, 137–144. [Google Scholar] [CrossRef]
- Su, H.; Che, W.; Tang, F.; Cheng, W.; Zhao, X.; Zhang, H.; Liu, Q. Valence Band Engineering via PtII Single-Atom Confinement Realizing Photocatalytic Water Splitting. J. Phys. Chem. C 2018, 122, 21108–21114. [Google Scholar] [CrossRef]
- Jiang, W.; Zhao, Y.; Zong, X.; Nie, H.; Niu, L.; An, L.; Qu, D.; Wang, X.; Kang, Z.; Sun, Z. Photocatalyst for High-Performance H2 Production: Ga-Doped Polymeric Carbon Nitride. Angew. Chem. Int. Ed. 2021, 60, 6124–6129. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Guo, Z.; Zhang, X.; Han, Y.; Xue, Z.; Xie, T.; Yang, W. The effect of indium doping on the hydrogen evolution performance of g-C3N4 based photocatalysts. New J. Chem. 2021, 45, 544–550. [Google Scholar] [CrossRef]
- Aboubakr, A.E.A.; Khan, M.D.; Revaprasadu, N.; Millet, P.; Hung, C.-H.; El Rouby, W.M.A. Sn-doped g-C3N4 as a novel photoelectrocatalyst for water oxidation. J. Phys. Chem. Solids 2023, 176, 111242. [Google Scholar] [CrossRef]
- Valdés, Á.; Qu, Z.-W.; Kroes, G.-J.; Rossmeisl, J.; Nørskov, J.K. Oxidation and Photo-Oxidation of Water on TiO2 Surface. J. Phys. Chem. C 2008, 112, 9872–9879. [Google Scholar] [CrossRef]
- Sun, S.; Shen, G.; Jiang, J.; Mi, W.; Liu, X.; Pan, L.; Zhang, X.; Zou, J.J. Boosting Oxygen Evolution Kinetics by Mn–N–C Motifs with Tunable Spin State for Highly Efficient Solar-Driven Water Splitting. Adv. Energy Mater. 2019, 9, 1901505. [Google Scholar] [CrossRef]
- Nørskov, J.K.; Rossmeisl, J.; Logadottir, A.; Lindqvist, L.; Kitchin, J.R.; Bligaard, T.; Jónsson, H. Origin of the Overpotential for Oxygen Reduction at a Fuel-Cell Cathode. J. Phys. Chem. B 2004, 108, 17886–17892. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, D.; Deng, H.; Li, M.; Chen, J.; Shen, S. Theoretical Insights into the Limitation of Photocatalytic Overall Water Splitting Performance of VIA Group Elements Doped Polymeric Carbon Nitride: A Density Functional Theory Calculation Predicting Solar-to-Hydrogen Efficiency. Solar RRL 2021, 5, 2000630. [Google Scholar] [CrossRef]
- Xu, Z.; Zhang, Y.; Wang, Z.; Chen, D.; You, P.; Li, S.; Guo, H.; Meng, S. Why Does Single-Atom Photocatalysis Work Better Than Conventional Photocatalysis? A Study on Ultrafast Excited Carrier and Structure Dynamics. Nano Lett. 2023, 23, 4023–4031. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Hafner, J. Ab initiomolecular dynamics for liquid metals. Phys. Rev. B 1993, 47, 558–561. [Google Scholar] [CrossRef] [PubMed]
- Blöchl, P.E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953–17979. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed]
- Heyd, J.; Scuseria, G.E.; Ernzerhof, M. Hybrid functionals based on a screened Coulomb potential. J. Chem. Phys. 2003, 118, 8207–8215. [Google Scholar] [CrossRef]
- Setyawan, W.; Curtarolo, S. High-throughput electronic band structure calculations: Challenges and tools. Comput. Mater. Sci. 2010, 49, 299–312. [Google Scholar] [CrossRef]
- Peterson, A.A.; Abild-Pedersen, F.; Studt, F.; Rossmeisl, J.; Nørskov, J.K. How copper catalyzes the electroreduction of carbon dioxide into hydrocarbon fuels. Energy Environ. Sci. 2010, 3, 1311–1315. [Google Scholar] [CrossRef]
- Man, I.C.; Su, H.Y.; Calle-Vallejo, F.; Hansen, H.A.; Martínez, J.I.; Inoglu, N.G.; Kitchin, J.; Jaramillo, T.F.; Nørskov, J.K.; Rossmeisl, J. Universality in Oxygen Evolution Electrocatalysis on Oxide Surfaces. ChemCatChem 2011, 3, 1159–1165. [Google Scholar] [CrossRef]
- Chen, D.; Chen, Z.; Lu, Z.; Zhang, X.; Tang, J.; Singh, C.V. Transition metal–N4 embedded black phosphorus carbide as a high-performance bifunctional electrocatalyst for ORR/OER. Nanoscale 2020, 12, 18721–18732. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Zhang, P.; Xiao, B.-B.; Mi, J.-L. Theoretical study of p-block metal–nitrogen–carbon single-atom catalysts for the oxygen reduction reaction. Catal. Sci. Technol. 2022, 12, 6751–6760. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, M.; Wu, Y.; Wan, Q.; Lin, S. Theoretical Study of p-Block Metal Single-Atom-Loaded Carbon Nitride Catalyst for Photocatalytic Water Splitting. Molecules 2024, 29, 2030. https://doi.org/10.3390/molecules29092030
Chen M, Wu Y, Wan Q, Lin S. Theoretical Study of p-Block Metal Single-Atom-Loaded Carbon Nitride Catalyst for Photocatalytic Water Splitting. Molecules. 2024; 29(9):2030. https://doi.org/10.3390/molecules29092030
Chicago/Turabian StyleChen, Mengning, Yidi Wu, Qiang Wan, and Sen Lin. 2024. "Theoretical Study of p-Block Metal Single-Atom-Loaded Carbon Nitride Catalyst for Photocatalytic Water Splitting" Molecules 29, no. 9: 2030. https://doi.org/10.3390/molecules29092030
APA StyleChen, M., Wu, Y., Wan, Q., & Lin, S. (2024). Theoretical Study of p-Block Metal Single-Atom-Loaded Carbon Nitride Catalyst for Photocatalytic Water Splitting. Molecules, 29(9), 2030. https://doi.org/10.3390/molecules29092030






