HPLC-Based Metabolomic Analysis and Characterization of Amaranthus cruentus Leaf and Inflorescence Extracts for Their Antidiabetic and Antihypertensive Potential
Abstract
1. Introduction
2. Results and Discussion
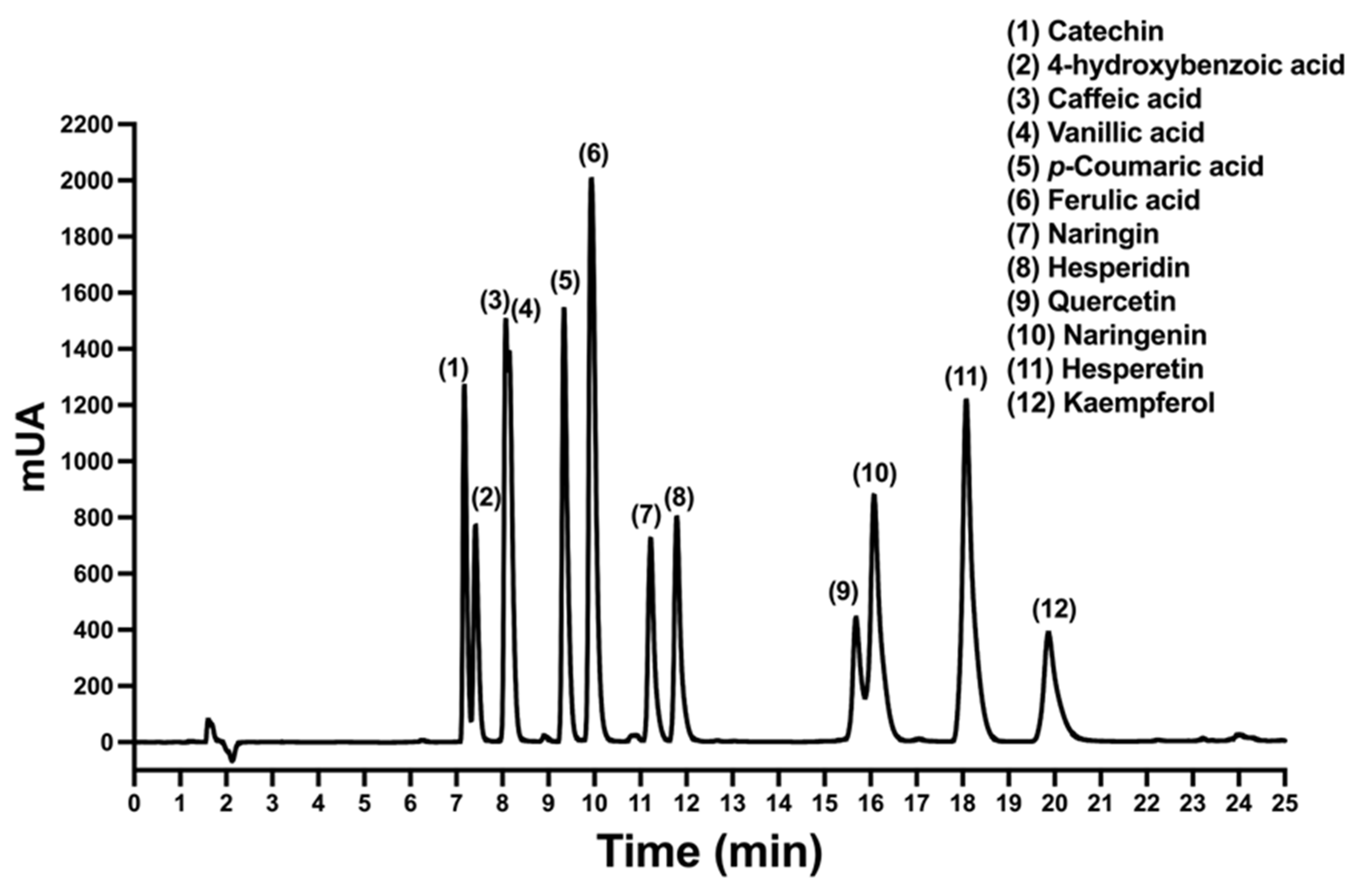
2.1. HPLC Chromatographic Conditions for Polyphenols
2.2. Validation of Developed HPLC Analytical Method
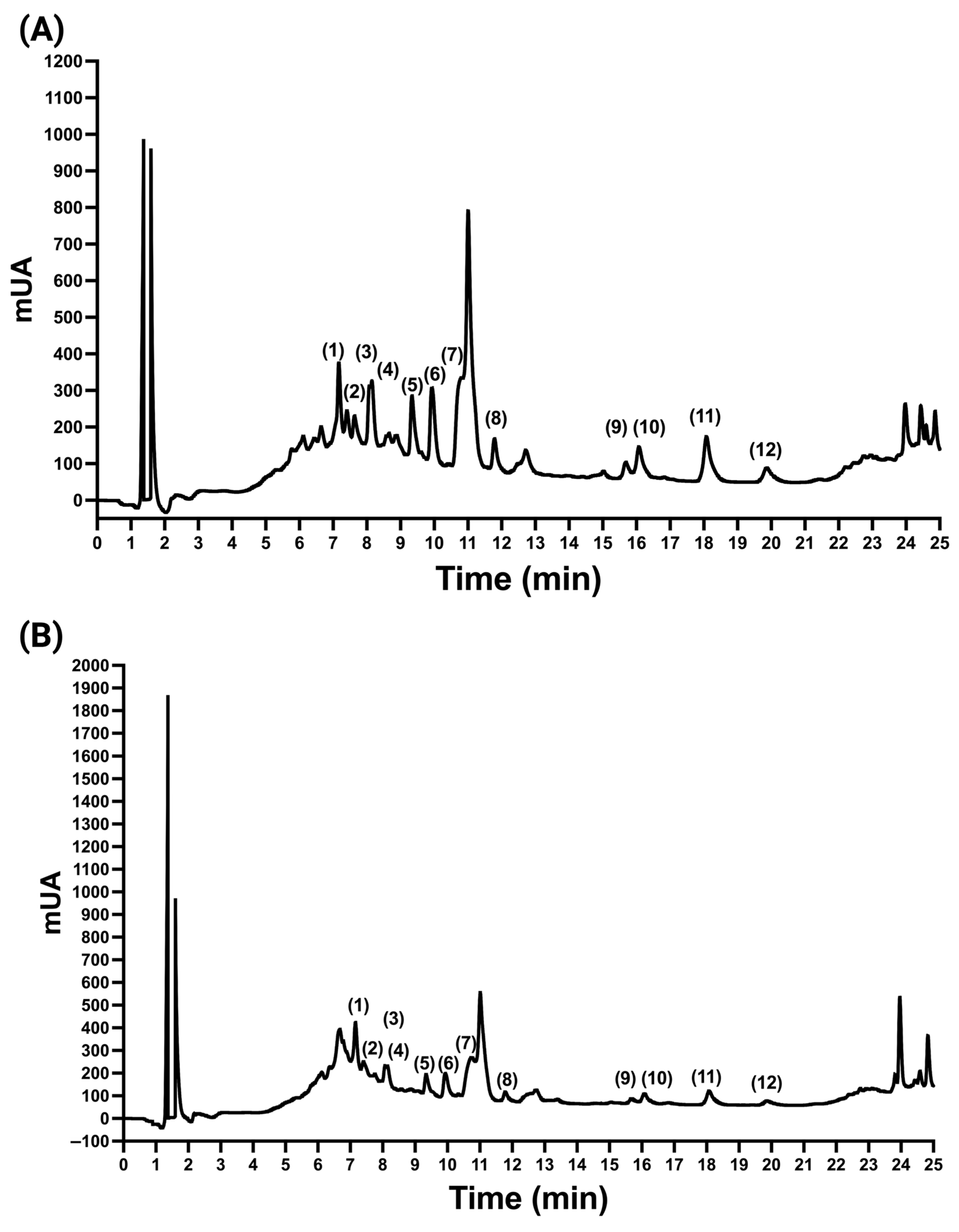
2.3. Quantification of the Polyphenols in A. cruentus
2.4. Quantification of Betalains in A. cruentus
2.5. In Vitro, In Vivo, Ex Vivo, and In Silico Pharmacological Screening of A. cruentus
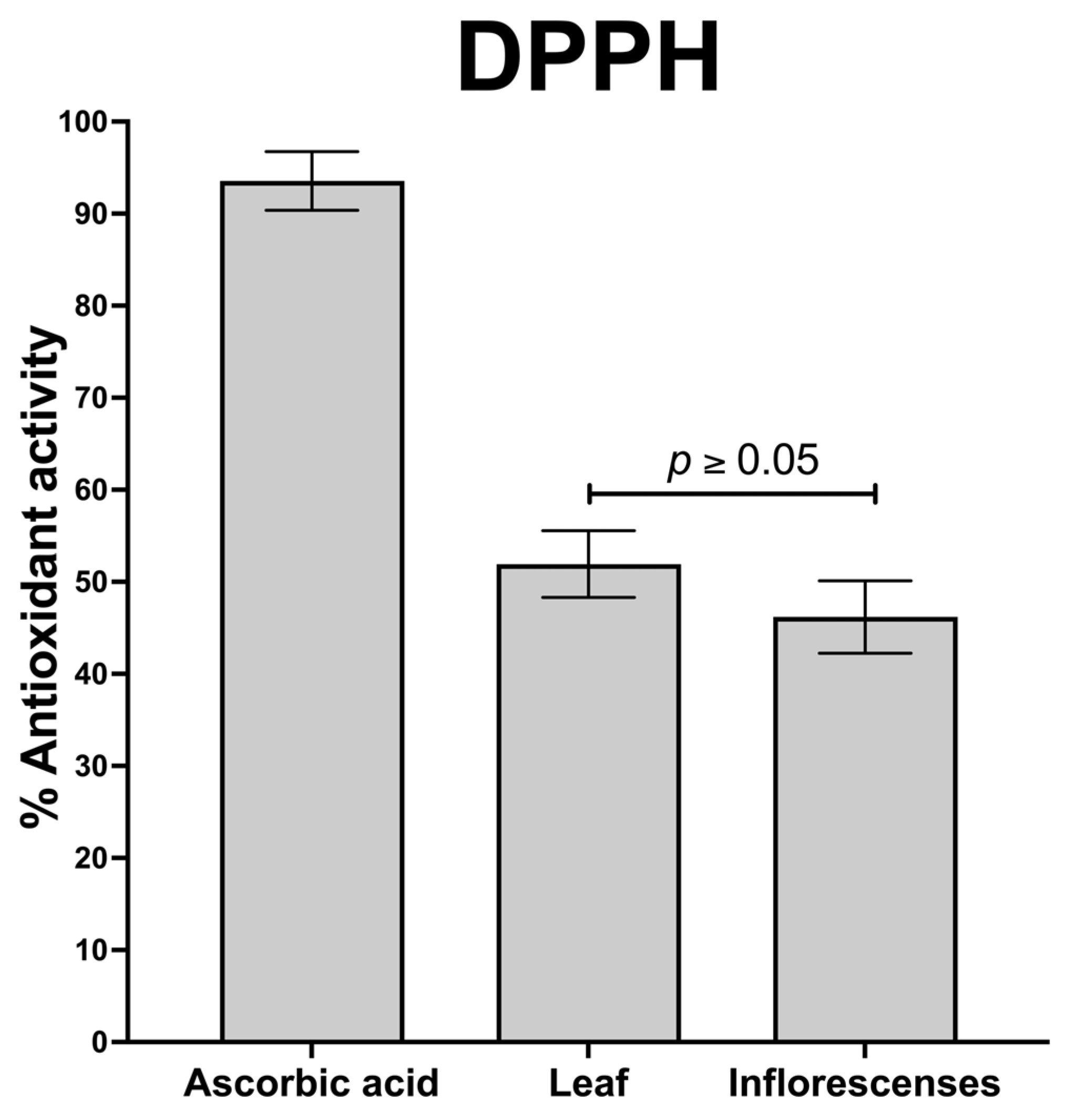
2.5.1. Antioxidant Effect
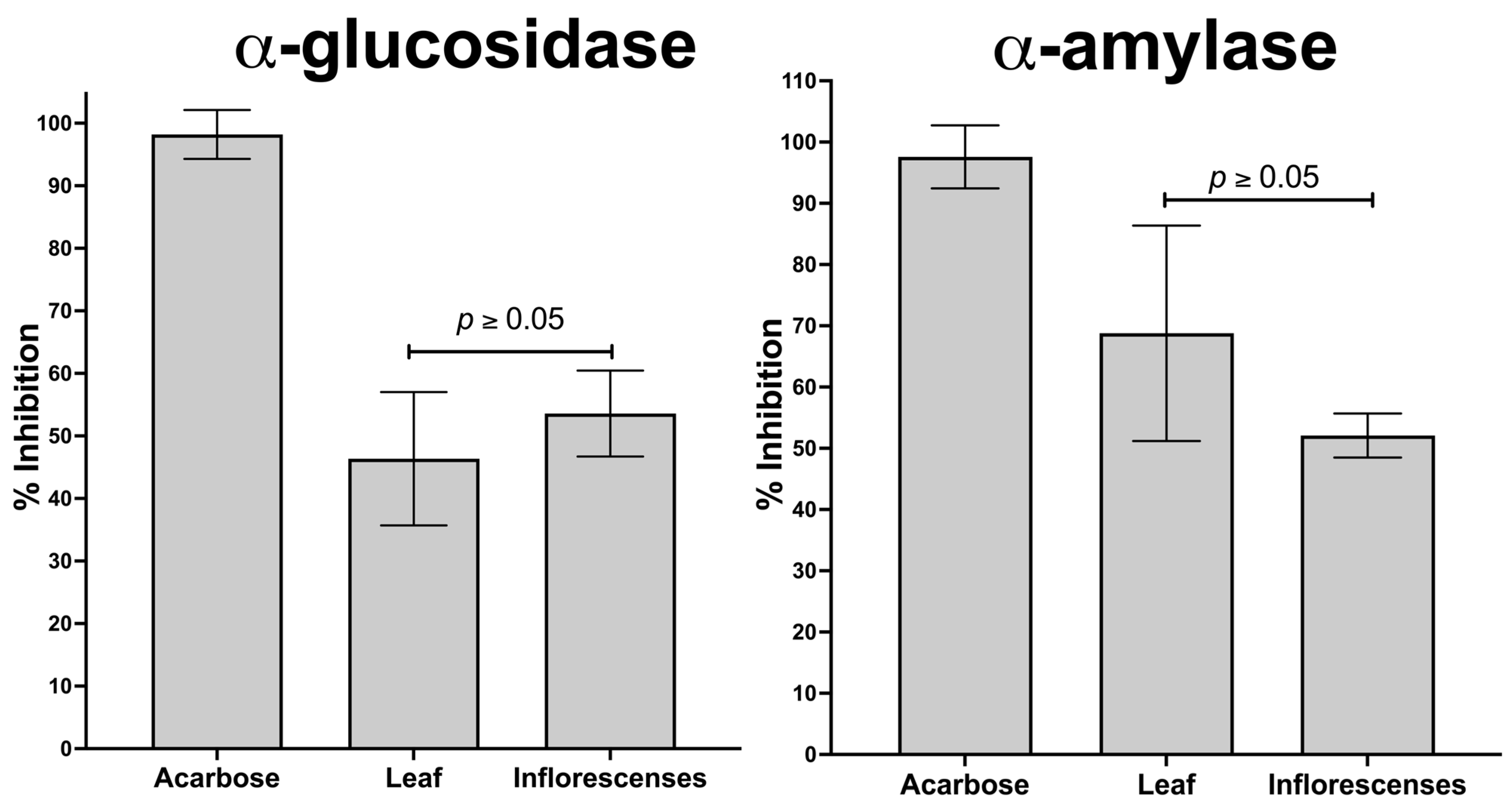
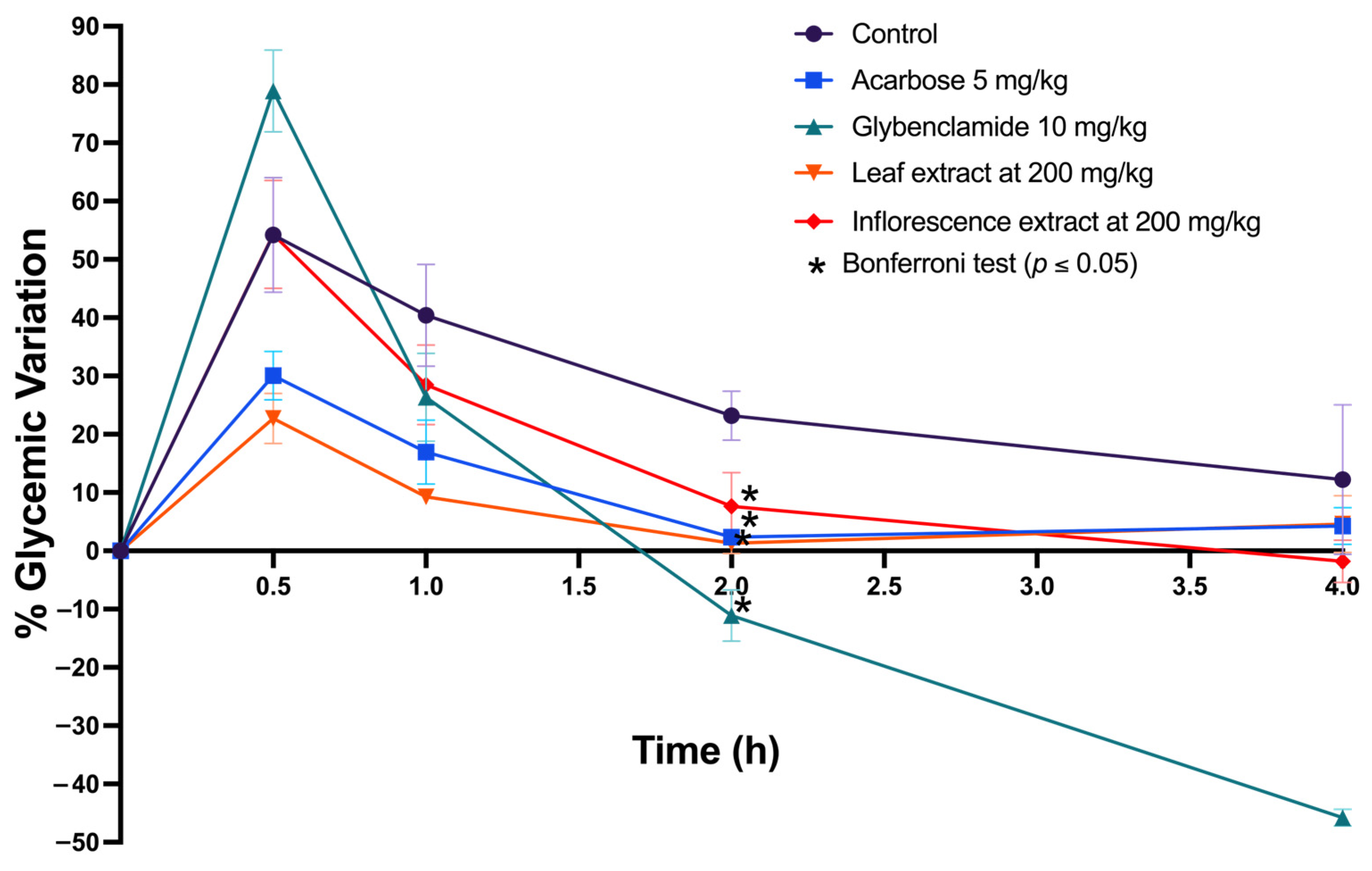
2.5.2. In Vitro and In Silico Inhibition of α-Amylase and α-Glucosidase and In Vivo Oral Sucrose Tolerance Test
2.5.3. In Vitro and In Silico Inhibition of Angiotensin-Converting Enzyme and Ex Vivo Vasorelaxation Experiment
3. Materials and Methods
3.1. Plant Material
3.2. Chemical and Reagents
3.3. Phytochemical Extraction from Leaves and Inflorescences of A. cruentus
3.4. HPLC-UV-DAD Analysis
3.5. HPLC-UV-DAD Method Validation
3.5.1. Linearity
3.5.2. Limits of Detection and Quantification
3.5.3. Precision and Accuracy
3.6. Betalains and Betaxanthins Quantification by Spectrophotometry
3.7. Phenolic Compounds Quantification
3.8. DPPH Assay
3.9. In Vitro α-Amylase Inhibition Assay
3.10. In Vitro α-Glucosidase Inhibition Assay
3.11. In Vitro Angiotensin-Converting Enzyme (ACE) Inhibition Assay
3.12. In Vivo Sucrose Tolerance Test
3.13. Molecular Docking Study
3.13.1. Ligands Preparation
3.13.2. Target Preparation
3.13.3. Target Preparation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Caselato-Sousa, V.M.; Amaya-Farfán, J. State of knowledge on amaranth grain: A comprehensive review. J. Food Sci. 2012, 77, R93–R104. [Google Scholar] [CrossRef] [PubMed]
- Gresta, F.; Meineri, G.; Oteri, M.; Santonoceto, C.; Lo Presti, V.; Costale, A.; Chiofalo, B. Productive and Qualitative Traits of Amaranthus cruentus L.: An Unconventional Healthy Ingredient in Animal Feed. Animals 2020, 10, 1428. [Google Scholar] [CrossRef]
- Araujo-León, J.A.; Sánchez-Del Pino, I.; Aguilar-Hernández, V.; Peraza-Sánchez, S.R.; Ortiz-Andrade, R.; Xingú-López, A.; Brito-Argáez, L.G.; Alcocer Espejel, J.N. El amaranto o huauhtli sagrado del México prehispánico y alimento de alto valor nutricional del México contemporáneo. Desde Herb. CICY 2023, 15, 123–128. [Google Scholar]
- Mlakar, S.G.; Turinek, M.; Jakop, M.; Bavec, M.; Bavec, F. Nutrition value and use of grain amaranth: Potential future application in bread making. Agricultura 2009, 6, 43–53. [Google Scholar]
- Písaříková, B.; Zralý, Z.; Kračmar, S.; Trčková, M.; Herzig, I. Nutritional value of amaranth (genus Amaranthus L.) grain in diets for broiler chickens. Czech J. Anim. Sci. 2005, 50, 568–573. [Google Scholar] [CrossRef]
- Sunil, M.; Hariharan, A.K.; Nayak, S.; Gupta, S.; Nambisan, S.R.; Gupta, R.P.; Panda, B.; Choudhary, B.; Srinivasan, S. The draft genome and transcriptome of Amaranthus hypochondriacus: A C4 dicot producing high-lysine edible pseudo-cereal. DNA Res. 2014, 21, 585–602. [Google Scholar] [CrossRef]
- Joshi, D.C.; Sood, S.; Hosahatti, R.; Kant, L.; Pattanayak, A.; Kumar, A.; Yadav, D.; Stetter, M.G. From zero to hero: The past, present and future of grain amaranth breeding. Theor. Appl. Genet. 2018, 131, 1807–1823. [Google Scholar] [CrossRef] [PubMed]
- Juan, R.; Pastor, J.; Alaiz, M.; Vioque, J. Electrophoretic characterization of Amaranthus L. seed protein and its systematic implication. Bot. J. Linn. Soc. 2007, 155, 57–63. [Google Scholar] [CrossRef]
- Tekieli, A.; Kisiel, A.; Grzegorczyk, A.; Starzak, K.; Wybraniec, S. Antioxidant and Antimicrobial Effects of Baby Leaves of Amaranthus tricolor L. Harvested as Vegetable in Correlation with Their Phytochemical Composition. Molecules 2023, 28, 1463. [Google Scholar] [CrossRef]
- Kumorkiewicz-Jamro, A.; Świergosz, T.; Sutor, K.; Spórna-Kucab, A.; Wybraniec, S. Multi-colored shades of betalains: Recent advances in betacyanin chemistry. Nat. Prod. Rep. 2021, 38, 2315–2346. [Google Scholar] [CrossRef]
- Sarker, U.; Oba, S. Antioxidant constituents of three selected red and green color Amaranthus leafy vegetable. Sci. Rep. 2019, 9, 18233. [Google Scholar] [CrossRef]
- Sarker, U.; Hossain, M.M.; Oba, S. Nutritional and antioxidant components and antioxidant capacity in green morph Amaranthus leafy vegetable. Sci. Rep. 2020, 10, 1336. [Google Scholar] [CrossRef] [PubMed]
- Bang, J.; Lee, K.J.; Jeong, W.T.; Han, S.; Jo, I.; Choi, S.H.; Cho, H.; Hyun, T.K.; Sung, J.; Lee, J.; et al. Antioxidant Activity and Phytochemical Content of Nine Amaranthus Species. Agronomy 2021, 11, 1032. [Google Scholar] [CrossRef]
- Xie, G.R.; Chen, H.J. Comprehensive Betalain Profiling of Djulis (Chenopodium formosanum) Cultivars Using HPLC-Q-Orbitrap High-Resolution Mass Spectrometry. J. Agric. Food Chem. 2021, 69, 15699–15715. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.R.; Valentão, P.; Faria, J.; Ferreres, F.; Sousa, C.; Gil-Izquierdo, A.; Pinho, B.R.; Andrade, P.B. Phytochemical investigations and biological potential screening with cellular and non-cellular models of globe amaranth (Gomphrena globosa L.) inflorescences. Food Chem. 2012, 135, 756–763. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.J.; Wang, Z.J.; Qin, X.J.; Zeng, Q.; Chen, S.; Qin, Y.; Luo, X.D. Phytochemical and Antibacterial Constituents of Edible Globe Amaranth Flower against Pseudomonas aeruginosa. Chem. Biodivers. 2022, 19, e202200139. [Google Scholar] [CrossRef] [PubMed]
- Sauer, J.D. The Grain Amaranths and Their Relatives: A Revised Taxonomic and Geographic Survey. Ann. Missouri Bot. Gard. 1967, 54, 103. [Google Scholar] [CrossRef]
- Espitia, E. Amaranth germplasm development and agronomic studies in Mexico. Food Rev. Int. 1992, 8, 71–86. [Google Scholar] [CrossRef]
- Rastogi, A.; Shukla, S. Amaranth: A New Millennium Crop of Nutraceutical Values. Crit. Rev. Food Sci. Nutr. 2013, 53, 109–125. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Sharma, M.; Tandon, N. Reviews on Indian Medicinal Plants; Indian Council of Medical Research: New Delhi, India, 2004. [Google Scholar]
- Kumar, K.; Goel, A.K. Little known ethno-medicinal plants of Santhal and Paharia tribals in Santhal Paragana, Bihar, India. Ethnobotany 1998, 10, 66–69. [Google Scholar]
- Michael, H. South American Medicinal Plants: Botany, Remedial Properties and General Use; Roth, I., Lindorf, H., Eds.; Springer: Berlin/Heidelberg, Germany, 2002. [Google Scholar]
- Bi Fong, L.; Bor Luen, C.; Jin Yuarn, L. Amaranthus spinosus water extract directly stimulates proliferation of β-lymphocytes in vitro. Int. Immunopharmacol. 2005, 5, 711–722. [Google Scholar]
- Khare, C.P. Indian Herbal Remedies. In Rational Western Therapy, Ayurvedic and Other Raditional Usage, Botany; Springer: Berlin/Heidelberg, Germany, 2004. [Google Scholar]
- Wali, S.; Jan, H.A.; Bussmann, R.W. Quantitative ethnomedicinal study of indigenous medicinal plants used for digestive disorders of Laspur Valley, Chitral, Northern Pakistan. Ethnobot. Res. Appl. 2019, 18, 1–18. [Google Scholar] [CrossRef]
- Tang, Y.; Tsao, R. Phytochemicals in quinoa and amaranth grains and their antioxidant, anti-inflammatory, and potential health beneficial effects: A review. Mol. Nutr. Food Res. 2017, 61, 1600767. [Google Scholar] [CrossRef] [PubMed]
- Soares, R.A.M.; Mendonça, S.; De Castro, L.Í.A.; Menezes, A.C.C.C.C.; Arêas, J.A.G. Major Peptides from Amaranth (Amaranthus cruentus) Protein Inhibit HMG-CoA Reductase Activity. Int. J. Mol. Sci. 2015, 16, 4150–4160. [Google Scholar] [CrossRef]
- Kamal, H.; Mudgil, P.; Bhaskar, B.; Fisayo, A.F.; Gan, C.-Y.; Maqsood, S. Amaranth proteins as potential source of bioactive peptides with enhanced inhibition of enzymatic markers linked with hypertension and diabetes. J. Cereal Sci. 2021, 101, 103308. [Google Scholar] [CrossRef]
- Fisayo Ajayi, F.; Mudgil, P.; Gan, C.Y.; Maqsood, S. Identification and characterization of cholesterol esterase and lipase inhibitory peptides from amaranth protein hydrolysates. Food Chem. X 2021, 12, 100165. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F. Amaranth Proteins and Peptides: Biological Properties and Food Uses. Food Res. Int. 2023, 164, 112405. [Google Scholar] [CrossRef]
- Cai, Y.Z.; Sun, M.; Corke, H. Colorant properties and stability of Amaranthus betacyanin pigments. J. Agric. Food Chem. 1998, 46, 4491–4495. [Google Scholar] [CrossRef]
- Araujo-León, J.A.; Aguilar-Hernández, V.; Sánchez-del Pino, I.; Brito-Argáez, L.; Peraza-Sánchez, S.R.; Xingú-López, A.; Ortiz-Andrade, R. Analysis of Red Amaranth (Amaranthus cruentus L.) Betalains by LC-MS. J. Mex. Chem. Soc. 2023, 67, 227–239. [Google Scholar] [CrossRef]
- Cai, Y.; Sun, M.; Corke, H. Identification and distribution of simple and acylated betacyanins in the Amaranthaceae. J. Agric. Food Chem. 2001, 49, 1971–1978. [Google Scholar] [CrossRef]
- Howard, J.E.; Villamil, M.B.; Riggins, C.W. Amaranth as a natural food colorant source: Survey of germplasm and optimization of extraction methods for betalain pigments. Front. Plant Sci. 2022, 13, 932440. [Google Scholar] [CrossRef] [PubMed]
- Castellanos-Santiago, E.; Yahia, E.M. Identification and quantification of betalains from the fruits of 10 mexican prickly pear cultivars by high-performance liquid chromatography and electrospray ionization mass spectrometry. J. Agric. Food Chem. 2008, 56, 5758–5764. [Google Scholar] [CrossRef] [PubMed]
- Sawicki, T.; Bączek, N.; Wiczkowski, W. Betalain profile, content and antioxidant capacity of red beetroot dependent on the genotype and root part. J. Funct. Foods 2016, 27, 249–261. [Google Scholar] [CrossRef]
- Pari, M.; Lee, W.Q.; Wong, C.K.F.; Teh, C.Y. Induction of callus culture through plant growth regulators supplementation and the effect of elicitors on enhancement of betalain synthesis using Gomphrena globosa. Plant Cell Tiss Organ Cult. 2024, 156, 19. [Google Scholar] [CrossRef]
- Karunanithi, A. Influence of Extraction Techniques on Betalain Yield and Bioactive Phytochemical Analysis of Nopal Fruit Peels. Ind. J. Pharm. Edu. Res. 2023, 57, 1078–1086. [Google Scholar] [CrossRef]
- Kumar, R.; Methven, L.; Oruna-Concha, M.J. A Comparative Study of Ethanol and Citric Acid Solutions for Extracting Betalains and Total Phenolic Content from Freeze-Dried Beetroot Powder. Molecules 2023, 28, 6405. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Félix, F.; Cárdenas-López, J.L.; Montaño-Leyva, B.; Del-Toro-Sánchez, C.L.; Juárez-Onofre, J.E.; Carvajal-Millán, E.; Tapia-Hernández, J.A.; Castro-Enríquez, D.D. Optimization of the Extraction of Betalains from the Pulp of Pitaya (Stenocereus thurberi) and its Antioxidant Capacity. Food Anal. Methods 2023, 16, 1252–1260. [Google Scholar] [CrossRef]
- Khanam, U.K.S.; Oba, S. Bioactive substances in leaves of two amaranth species, Amaranthus tricolor and A. hypochondriacus. Can. J. Plant Sci. 2013, 93, 47–58. [Google Scholar] [CrossRef]
- Li, H.; Deng, Z.; Liu, R.; Zhu, H.; Draves, J.; Marcone, M.; Sun, Y.; Tsao, R. Characterization of phenolics, betacyanins and antioxidant activities of the seed, leaf, sprout, flower and stalk extracts of three Amaranthus species. J. Food Compos. Anal. 2015, 37, 75–81. [Google Scholar] [CrossRef]
- Khanam, U.K.S.; Oba, S.; Yanase, E.; Murakami, Y. Phenolic acids, flavonoids and total antioxidant capacity of selected leafy vegetables. J. Funct. Foods. 2012, 4, 979–987. [Google Scholar] [CrossRef]
- Procopet, O.; Oroian, M. Amaranth Seed Polyphenol, Fatty Acid and Amino Acid Profile. Appl. Sci. 2022, 12, 2181. [Google Scholar] [CrossRef]
- Barba de la Rosa, A.; Fomsgaard, I.S.; Laursen, B.; Mortensen, A.G.; Olvera-Martínez, L.; Silva-Sánchez, C.; Mendoza-Herrera, A.; González-Castañeda, J.; De León-Rodríguez, A. Amaranth (Amaranthus hypochondriacus) as an alternative crop for sustainable food production: Phenolic acids and flavonoids with potential impact on its nutraceutical quality. J. Cereal Sci. 2009, 49, 117–121. [Google Scholar] [CrossRef]
- Karamać, M.; Gai, F.; Longato, E.; Meineri, G.; Janiak, M.A.; Amarowicz, R.; Peiretti, P.G. Antioxidant Activity and Phenolic Composition of Amaranth (Amaranthus caudatus) during Plant Growth. Antioxidants 2019, 8, 173. [Google Scholar] [CrossRef] [PubMed]
- Kalinova, J.; Dadakova, E. Rutin and total quercetin content in amaranth (Amaranthus spp.). Plant Foods Hum. Nutr. 2009, 64, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Mahdavian, K.; Ghorbanli, M.; Kalantari, K.M. The Effects of Ultraviolet Radiation on the Contents of Chlorophyll, Flavonoid, Anthocyanin and Proline in Capsicum annuum L. Turk. J. Bot. 2008, 32, 25–33. [Google Scholar]
- Alqahtani, A.S.; Hidayathulla, S.; Rehman, M.T.; ElGamal, A.A.; Al-Massarani, S.; Razmovski-Naumovski, V.; Alqahtani, M.S.; El Dib, R.A.; AlAjmi, M.F. Alpha-Amylase and Alpha-Glucosidase Enzyme Inhibition and Antioxidant Potential of 3-Oxolupenal and Katononic Acid Isolated from Nuxia oppositifolia. Biomolecules 2020, 10, 61. [Google Scholar] [CrossRef] [PubMed]
- Kajaria, D.; Tiwari, S.; Tripathi, J.; Tripathi, Y.; Ranjana. In-vitro α amylase and glycosidase inhibitory effect of ethanolic extract of antiasthmatic drug-Shirishadi. J. Adv. Pharm. Technol. Res. 2013, 4, 206–209. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.; Patel, R.A.; Kowey, P.R. The clinical use of angiotensin-converting enzyme inhibitors. Prog. Cardiovasc. Dis. 2004, 47, 116–130. [Google Scholar] [CrossRef]
- Liu, J.; Han, X.; Zhang, T.; Tian, K.; Li, Z.; Luo, F. Reactive oxygen species (ROS) scavenging biomaterials for anti-inflammatory diseases: From mechanism to therapy. J. Hematol. Oncol. 2023, 16, 116. [Google Scholar] [CrossRef]
- Adegbola, P.I.; Adetutu, A.; Olaniyi, T.D. Antioxidant activity of Amaranthus species from the Amaranthaceae family—A review. S. Afr. J. Bot. 2020, 133, 111–117. [Google Scholar] [CrossRef]
- Sarker, U.; Oba, S. Color attributes, betacyanin, and carotenoid profiles, bioactive components, and radical quenching capacity in selected Amaranthus gangeticus leafy vegetables. Sci. Rep. 2021, 11, 11559. [Google Scholar] [CrossRef] [PubMed]
- Belhadj Slimen, I.; Najar, T.; Abderrabba, M. Chemical and Antioxidant Properties of Betalains. J. Agric. Food Chem. 2017, 65, 675–689. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Sun, M.; Corke, H. Antioxidant activity of betalains from plants of the Amaranthaceae. J. Agric. Food Chem. 2003, 51, 2288–2294. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Sun, M.; Corke, H. Characterization and application of betalain pigments from plants of the Amaranthaceae. Trends Food Sci. Technol. 2005, 16, 370–376. [Google Scholar] [CrossRef]
- Baranowska, M.; Koziara, Z.; Suliborska, K.; Chrzanowski, W.; Wormstone, M.; Namieśnik, J.; Bartoszek, A. Interactions between polyphenolic antioxidants quercetin and naringenin dictate the distinctive redox-related chemical and biological behavior of their mixtures. Sci. Rep. 2021, 11, 12282. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Biswas, S.; Al-Dayan, N.; Alhegaili, A.S.; Sarwat, M. Antioxidant Role of Kaempferol in Prevention of Hepatocellular Carcinoma. Antioxidants 2021, 10, 1419. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Li, Q.; Bi, K. Bioactive flavonoids in medicinal plants: Structure, activity and biological fate. Asian J. Pharm. Sci. 2018, 13, 12–23. [Google Scholar] [CrossRef]
- Bernatoniene, J.; Kopustinskiene, D.M. The Role of Catechins in Cellular Responses to Oxidative Stress. Molecules 2018, 23, 965. [Google Scholar] [CrossRef] [PubMed]
- Basso Scandolara, T.; Fontana Mezoni, M.; Galvani, M.; Rodrigues Ferreira Seiva, F.; Panis, C.; Miranda-Sapla, M.M.; Pavanelli, W.R. Naringenin and Hesperidin as Promising Alternatives for Prevention and Co-Adjuvant Therapy for Breast Cancer. Antioxidants 2023, 12, 586. [Google Scholar] [CrossRef]
- Krentz, A.J.; Bailey, C.J. Oral antidiabetic agents: Current role in type 2 diabetes mellitus. Drugs 2005, 65, 385–411. [Google Scholar] [CrossRef]
- Lebovitz, H.E. Postprandial hyperglycaemic state: Importance and consequences. Diabetes Res. Clin. Pract. 1998, 40, S27–S28. [Google Scholar] [PubMed]
- Gámez-Valdez, L.C.; Gutiérrez-Dorado, R.; Gómez-Aldapa, C.A.; Perales-Sánchez, J.X.K.; Milán-Carrillo, J.; Cuevas-Rodríguez, E.O.; Mora-Rochín, S.; Reyes-Moreno, C. Effect of the extruded amaranth flour addition on the nutritional, nutraceutical and sensory quality of tortillas produced from extruded creole blue maize flour. Biotecnia 2021, 23, 103–112. [Google Scholar]
- Kunyanga, C.N.; Imungi, J.K.; Okoth, M.W.; Biesalski, H.K.; Vadivel, V. Total phenolic content, antioxidant and antidiabetic properties of methanolic extract of raw and traditionally processed Kenyan indigenous food ingredients. LWT Food Sci. Technol. 2012, 45, 269–276. [Google Scholar] [CrossRef]
- Sultana, R.; Alashi, A.M.; Islam, K.; Saifullah, M.; Haque, C.E.; Aluko, R.E. Inhibitory Activities of Polyphenolic Extracts of Bangladeshi Vegetables against α-Amylase, α-Glucosidase, Pancreatic Lipase, Renin, and Angiotensin-Converting Enzyme. Foods 2020, 9, 844. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.-C.; Mong, M.-C.; Wu, W.-T.; Wang, Z.-H.; Yin, M.-C. Phytochemical profiles and anti-diabetic benefits of two edible Amaranthus species. CyTA J. Food. 2020, 18, 94–101. [Google Scholar] [CrossRef]
- Omoba, O.S.; Olagunju, A.I.; Akinrinlola, F.O.; Oluwajuyitan, T.D. Shallot-enriched amaranth-based extruded snack influences blood glucose levels, hematological parameters, and carbohydrate degrading enzymes in streptozotocin-induced diabetic rats. J. Food Biochem. 2022, 46, e14098. [Google Scholar] [CrossRef] [PubMed]
- Şöhretoğlu, D.; Renda, G.; Arroo, R.; Xiao, J.; Sari, S. Advances in the natural α-glucosidase inhibitors. eFood 2023, 4, e112. [Google Scholar] [CrossRef]
- Madadi, E.; Mazloum-Ravasan, S.; Yu, J.S.; Ha, J.W.; Hamishehkar, H.; Kim, K.H. Therapeutic Application of Betalains: A Review. Plants 2020, 9, 1219. [Google Scholar] [CrossRef]
- Thiyajai, P.; Kawai, D.; Koyama, T. Effective Purification Procedure of Amaranthin from Amaranthus Celosia argentea inflorescence. Int. J. Food Sci. Agric. 2021, 5, 370–375. [Google Scholar] [CrossRef]
- Jimenez-Garcia, S.N.; Garcia-Mier, L.; Ramirez-Gomez, X.S.; Aguirre-Becerra, H.; Escobar-Ortiz, A.; Contreras-Medina, L.M.; Garcia-Trejo, J.F.; Feregrino-Perez, A.A. Pitahaya Peel: A By-Product with Great Phytochemical Potential, Biological Activity, and Functional Application. Molecules 2022, 27, 5339. [Google Scholar] [CrossRef]
- Sawicki, T.; Juśkiewicz, J.; Wiczkowski, W. Using the SPE and Micro-HPLC-MS/MS Method for the Analysis of Betalains in Rat Plasma after Red Beet Administration. Molecules 2017, 22, 2137. [Google Scholar] [CrossRef] [PubMed]
- Allegra, M.; Ianaro, A.; Tersigni, M.; Panza, E.; Tesoriere, L.; Livrea, M.A. Indicaxanthin from Cactus Pear Fruit Exerts Anti-Inflammatory Effects in Carrageenin-Induced Rat Pleurisy. J. Nutr. 2014, 144, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Abedimanesh, N.; Asghari, S.; Mohammadnejad, K.; Daneshvar, Z.; Rahmani, S.; Shokoohi, S.; Farzaneh, A.H.; Hosseini, S.H.; Anarkooli, I.J.; Noubarani, M. The anti-diabetic effects of betanin in streptozotocin-induced diabetic rats through modulating AMPK/SIRT1/NF-κB signaling pathway. Nutr. Metab. 2021, 18, 92. [Google Scholar] [CrossRef]
- Ramesar, S.; Baijnath, H.; Govender, T.; Mackraj, I. Angiotensin I-Converting Enzyme Inhibitor Activity of Nutritive Plants in KwaZulu-Natal. J. Med. Food. 2008, 11, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Vecchi, B.; Añón, M.C. ACE inhibitory tetrapeptides from Amaranthus hypochondriacus 11S globulin. Phytochemistry 2009, 70, 864–870. [Google Scholar] [CrossRef] [PubMed]
- Tovar-Pérez, E.; Guerrero-Legarreta, I.; Farrés-González, A.; Soriano-Santos, J. Angiotensin I-converting enzyme-inhibitory peptide fractions from albumin 1 and globulin as obtained of amaranth grain. Food Chem. 2009, 116, 437–444. [Google Scholar] [CrossRef]
- Quiroga, A.V.; Aphalo, P.; Ventureira, J.L.; Martínez, E.N.; Añón, M.C. Physicochemical, functional and angiotensin converting enzyme inhibitory properties of Amaranth (Amaranthus hypochondriacus) 7S globulin. J. Sci. Food Agric. 2012, 92, 397–403. [Google Scholar] [CrossRef]
- Quiroga, A.V.; Aphalo, P.; Nardo, A.E.; Añón, M.C. In Vitro Modulation of Renin-Angiotensin System Enzymes by Amaranth (Amaranthus hypochondriacus) Protein-Derived Peptides: Alternative Mechanisms Different from ACE Inhibition. J. Agric. Food Chem. 2017, 65, 7415–7423. [Google Scholar] [CrossRef]
- Sánchez-López, F.; Robles-Olvera, V.J.; Hidalgo-Morales, M.; Tsopmo, A. Angiotensin-I converting enzyme inhibitory activity of Amaranthus hypochondriacus seed protein hydrolysates produced with lactic bacteria and their peptidomic profiles. Food Chem. 2021, 363, 130320. [Google Scholar] [CrossRef]
- Sawicki, T.; Martinez-Villaluenga, C.; Frias, J.; Wiczkowski, W.; Peñas, E.; Bączek, N.; Zieliński, H. The effect of processing and in vitro digestion on the betalain profile and ACE inhibition activity of red beetroot products. J. Funct. Foods. 2019, 55, 229–237. [Google Scholar] [CrossRef]
- Wang, C.; Huang, C.; Lu, Y. Changes in Bio-Functional Compounds, ACE Inhibition, and Antioxidant Capacity after Mixed Fermentation of Eight Whole Grains. Fermentation 2023, 9, 209. [Google Scholar] [CrossRef]
- Paiva, L.; Lima, E.; Marcone, M.; Baptista, J. Angiotensin I-converting enzyme (ACE) inhibition and biological activities of green and black tea samples from Azorean Camellia sinensis. J. Funct. Foods. 2023, 107, 105701. [Google Scholar] [CrossRef]
- Moncada, S.; Palmer, R.M.; Higgs, E.A. Nitric oxide: Physiology, pathophysiology, and pharmacology. Pharmacol. Rev. 1991, 43, 109–140. [Google Scholar]
- Raddino, R.; Caretta, G.; Teli, M.; Bonadei, I.; Robba, D.; Zanini, G.; Madureri, A.; Nodari, S.; Dei Cas, L. Nitric oxide and cardiovascular risk factors. Heart Int. 2007, 3, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Shimada, K.; Fujikawa, K.; Yahara, K.; Nakamura, T. Antioxidative properties of xanthan on the autoxidation of soybean oil in cyclodextrin emulsion. J. Agric. Food Chem. 1992, 40, 945–948. [Google Scholar] [CrossRef]
- Tamil, I.G.; Dineshkumar, B.; Nandhakumar, M.; Senthilkumar, M.; Mitra, A. In vitro study on α-amylase inhibitory activity of an Indian medicinal plant, Phyllanthus amarus. Indian J. Pharmacol. 2010, 42, 280–282. [Google Scholar] [PubMed]
- Hayakari, M.; Kondo, Y.; Izumi, H. A rapid and simple spectrophotometric assay of Angiotensin-Converting Enzyme. Anal. Biochem. 1978, 78, 361–369. [Google Scholar] [CrossRef]
- Tehreem, S.; Rahman, S.; Bhatti, M.S.; Uddin, R.; Khan, M.N.; Tauseef, S.; El-Seedi, H.R.; Bin Muhsinah, A.; Uddin, J.; Musharraf, S.G. A UPLC-DAD-Based Bio-Screening Assay for the Evaluation of the Angiotensin Converting Enzyme Inhibitory Potential of Plant Extracts and Compounds: Pyrroquinazoline Alkaloids from Adhatoda vasica as a Case Study. Molecules 2021, 26, 6971. [Google Scholar] [CrossRef] [PubMed]
- Araujo-León, J.A.; Segura-Campos, M.R.; Ortiz-Andrade, R.; Vazquez-Garcia, P.; Carvajal-Sánchez, D.; Cabañas-Wuan, Á.; González-Sánchez, A.A.; Uuh-Narvaez, J.; Sánchez-Salgado, J.C.; Fuentes-Noriega, I.; et al. Hypoglycemic and Antihyperglycemic Potential of Flavonoid Fraction from Citrus sinensis (L.) Osbeck in Normoglycemic and Diabetic Rats. Sci. Pharm. 2023, 91, 46. [Google Scholar] [CrossRef]
- Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, H.; Shindyalov, I.N.; Bourne, P.E. The Protein Data Bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef]
- Eberhardt, J.; Santos-Martins, D.; Tillack, A.F.; Forli, S. AutoDock Vina 1.2.0: New Docking Methods, Expanded Force Field, and Python Bindings. J. Chem. Inf. Model. 2021, 61, 3891–3898. [Google Scholar] [CrossRef] [PubMed]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Temple, N.J. A rational definition for functional foods: A perspective. Front. Nutr. 2022, 9, 957516. [Google Scholar] [CrossRef] [PubMed]



| Phytochemical | tR (min) | Wavelength (nm) | Slope (b) | Intercept (a) | r | Noise (Sy/x) | LOD (µg/mL) | LOQ (µg/mL) | Instrumental Precision at 0.5 µg/mL (% RSD) |
|---|---|---|---|---|---|---|---|---|---|
| Catechin | 7.15 | 280 | 0.45 | −0.05 | 0.9981 | 0.015 | 0.10 | 0.34 | 0.98 |
| 4-HBA | 7.41 | 254 | 6.41 | −0.65 | 0.9932 | 0.030 | 0.01 | 0.05 | 1.34 |
| CA | 8.11 | 320 | 1.29 | −0.35 | 0.9924 | 0.033 | 0.08 | 0.25 | 1.87 |
| VA | 8.16 | 254 | 3.95 | −0.49 | 0.9912 | 0.034 | 0.03 | 0.09 | 1.29 |
| pCA | 9.35 | 254 | 0.83 | −0.07 | 0.9941 | 0.028 | 0.10 | 0.33 | 1.03 |
| FA | 9.93 | 254 | 1.96 | −0.02 | 0.9929 | 0.030 | 0.05 | 0.15 | 1.56 |
| Naringin | 11.26 | 280 | 2.51 | −0.26 | 0.9978 | 0.016 | 0.02 | 0.06 | 0.36 |
| Hesperidin | 11.83 | 280 | 3.22 | −0.31 | 0.9937 | 0.024 | 0.02 | 0.07 | 1.81 |
| Quercetin | 15.75 | 360 | 1.49 | −0.99 | 0.9982 | 0.018 | 0.04 | 0.12 | 1.27 |
| Naringenin | 16.13 | 280 | 5.73 | −0.34 | 0.9975 | 0.012 | 0.01 | 0.02 | 0.71 |
| Hesperetin | 18.11 | 280 | 8.56 | −1.14 | 0.9978 | 0.014 | 0.01 | 0.02 | 1.64 |
| Kaemferol | 19.96 | 360 | 2.52 | −1.38 | 0.9981 | 0.026 | 0.03 | 0.10 | 1.91 |
| Phytochemical | Intraday n = 3 (Precision [% RSD]; Recovery [%]) | Interday n = 9 (Precision [% RSD]; Recovery [%]) | ||||
|---|---|---|---|---|---|---|
| 0.5 µg/mL | 2.5 µg/mL | 5.0 µg/mL | 0.5 µg/mL | 2.5 µg/mL | 5.0 µg/mL | |
| Catechin | 2.77 (88) | 1.95 (91) | 1.84 (98) | 4.65 (84) | 2.68 (88) | 1.67 (94) |
| 4-HBA | 3.74 (84) | 1.83 (89) | 1.57 (97) | 3.91 (86) | 2.32 (87) | 1.62 (95) |
| CA | 2.73 (83) | 2.54 (93) | 1.62 (93) | 2.43 (84) | 3.64 (89) | 1.54 (91) |
| VA | 2.96 (89) | 2.60 (95) | 1.87 (96) | 3.87 (83) | 2.45 (90) | 1.34 (97) |
| pCA | 4.59 (85) | 2.96 (95) | 1.74 (93) | 2.63 (89) | 3.69 (90) | 1.95 (93) |
| FA | 3.91 (89) | 1.64 (86) | 1.64 (92) | 2.86 (83) | 3.45 (87) | 1.63 (95) |
| Naringin | 4.86 (91) | 2.55 (86) | 1.96 (90) | 4.94 (82) | 2.86 (91) | 1.61 (97) |
| Hesperidin | 3.82 (90) | 1.54 (93) | 1.89 (98) | 3.32 (84) | 3.75 (92) | 1.39 (98) |
| Quercetin | 4.98 (88) | 1.74 (91) | 1.53 (91) | 3.43 (81) | 3.89 (88) | 1.54 (97) |
| Naringenin | 3.93 (86) | 1.57 (88) | 1.68 (94) | 3.35 (86) | 2.88 (92) | 1.58 (92) |
| Hesperetin | 4.77 (83) | 1.79 (92) | 1.55 (96) | 2.87 (89) | 3.80 (87) | 1.98 (95) |
| Kaempferol | 3.51 (90) | 1.92 (95) | 1.32 (97) | 4.43 (87) | 3.88 (92) | 1.49 (98) |
| Human Angiotensin- Converting Enzyme | Human Maltase- Glucoamylase | Human Sucrase- Isomaltase | Human Pancreatic Alpha-Amylase | |
|---|---|---|---|---|
| Ligand | ΔG (kcal/mol) | ΔG (kcal/mol) | ΔG (kcal/mol) | ΔG (kcal/mol) |
| Lisinopril | −7.6 | - | - | - |
| Acarbose | - | −7.9 | - | −9.1 |
| Kotalanol | - | - | −5.9 | - |
| Amaranthin | −9.6 | −7.1 | −6.8 | −8.9 |
| Betanin | −9.5 | −8.1 | −8.6 | −9.5 |
| Catechin | −9.9 | −8.6 | −7.6 | −9.5 |
| Gomphrenin-I | −9.4 | −7.8 | −8.8 | −8.6 |
| Hesperetin | −8.1 | −7.0 | −6.5 | −7.7 |
| Isoamaranthin | −6.8 | −9.1 | −8.2 | −11.9 |
| Isobetanin | −9.7 | −8.3 | −8.0 | −9.1 |
| Isogomphrenin-I | −9.50 | −7.4 | −9.0 | −8.4 |
| Kaempferol | −8.0 | −7.4 | −7.7 | −9.0 |
| Naringenin | −8.1 | −6.9 | −6.3 | −7.7 |
| Quercetin | −8.3 | −7.4 | −7.8 | −9.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Araujo-León, J.A.; Sánchez-del Pino, I.; Ortiz-Andrade, R.; Hidalgo-Figueroa, S.; Carrera-Lanestosa, A.; Brito-Argáez, L.G.; González-Sánchez, A.; Giácoman-Vallejos, G.; Hernández-Abreu, O.; Peraza-Sánchez, S.R.; et al. HPLC-Based Metabolomic Analysis and Characterization of Amaranthus cruentus Leaf and Inflorescence Extracts for Their Antidiabetic and Antihypertensive Potential. Molecules 2024, 29, 2003. https://doi.org/10.3390/molecules29092003
Araujo-León JA, Sánchez-del Pino I, Ortiz-Andrade R, Hidalgo-Figueroa S, Carrera-Lanestosa A, Brito-Argáez LG, González-Sánchez A, Giácoman-Vallejos G, Hernández-Abreu O, Peraza-Sánchez SR, et al. HPLC-Based Metabolomic Analysis and Characterization of Amaranthus cruentus Leaf and Inflorescence Extracts for Their Antidiabetic and Antihypertensive Potential. Molecules. 2024; 29(9):2003. https://doi.org/10.3390/molecules29092003
Chicago/Turabian StyleAraujo-León, Jesús Alfredo, Ivonne Sánchez-del Pino, Rolffy Ortiz-Andrade, Sergio Hidalgo-Figueroa, Areli Carrera-Lanestosa, Ligia Guadalupe Brito-Argáez, Avel González-Sánchez, Germán Giácoman-Vallejos, Oswaldo Hernández-Abreu, Sergio R. Peraza-Sánchez, and et al. 2024. "HPLC-Based Metabolomic Analysis and Characterization of Amaranthus cruentus Leaf and Inflorescence Extracts for Their Antidiabetic and Antihypertensive Potential" Molecules 29, no. 9: 2003. https://doi.org/10.3390/molecules29092003
APA StyleAraujo-León, J. A., Sánchez-del Pino, I., Ortiz-Andrade, R., Hidalgo-Figueroa, S., Carrera-Lanestosa, A., Brito-Argáez, L. G., González-Sánchez, A., Giácoman-Vallejos, G., Hernández-Abreu, O., Peraza-Sánchez, S. R., Xingú-López, A., & Aguilar-Hernández, V. (2024). HPLC-Based Metabolomic Analysis and Characterization of Amaranthus cruentus Leaf and Inflorescence Extracts for Their Antidiabetic and Antihypertensive Potential. Molecules, 29(9), 2003. https://doi.org/10.3390/molecules29092003









