Carbon Quantum Dots: Properties, Preparation, and Applications
Abstract
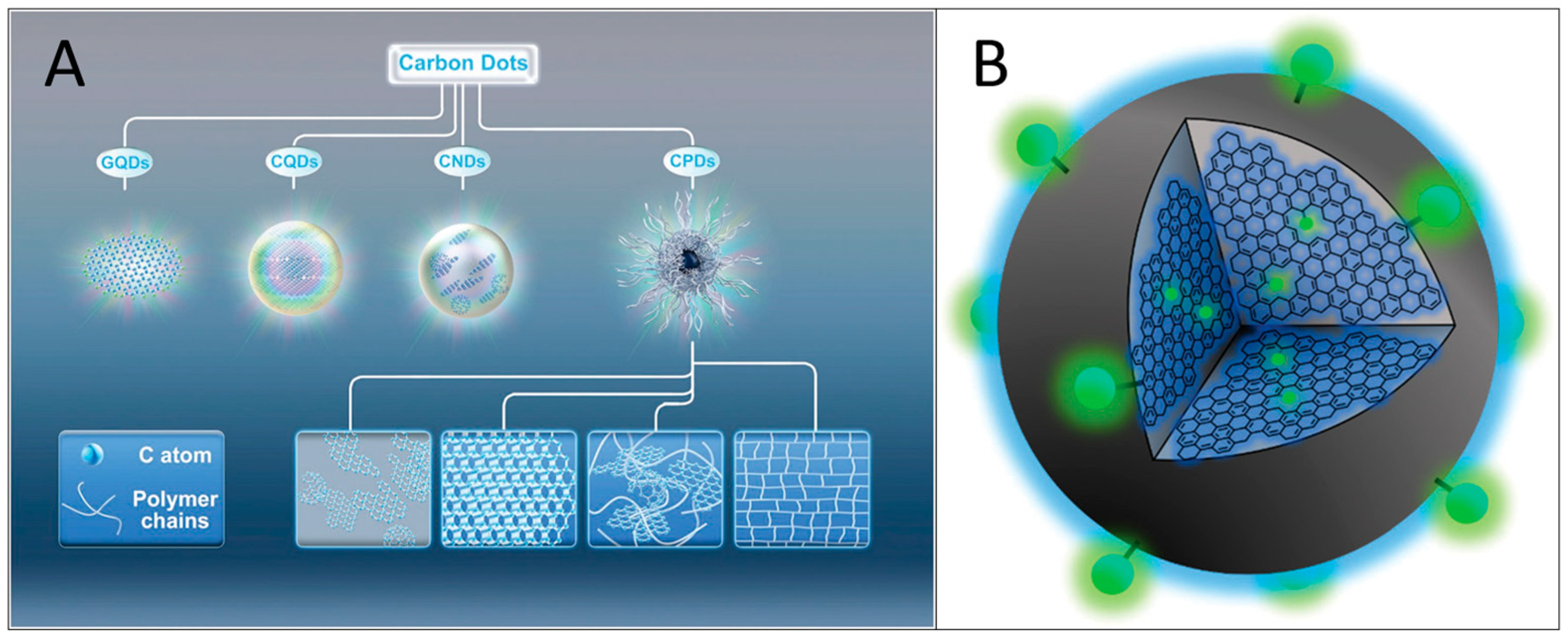
1. Introduction
2. Properties of Carbon Quantum Dots
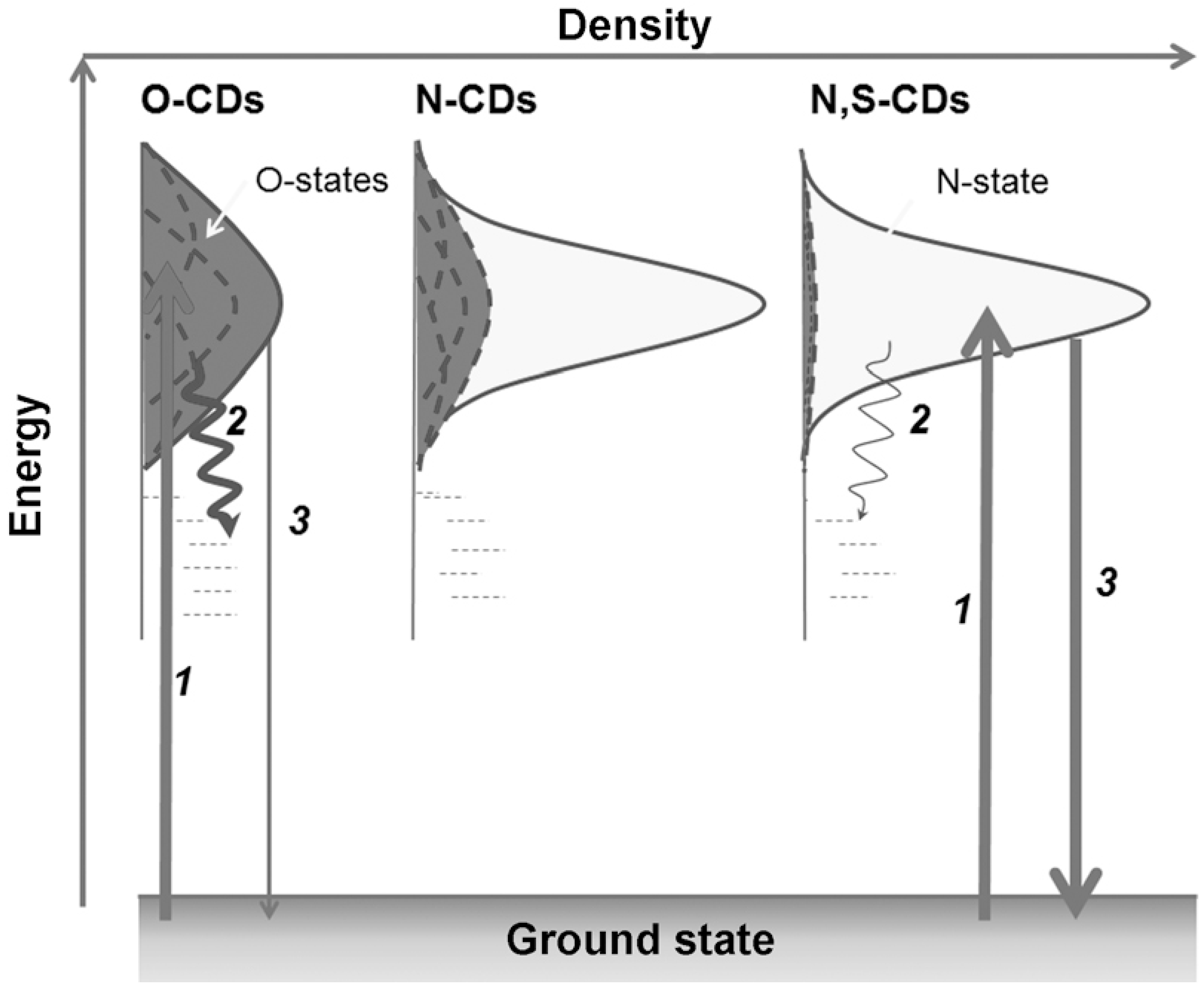
2.1. Optical Properties
2.2. Chemically Inertness
2.3. Biological Performance
2.4. Up-Conversion Photoluminescence
2.5. Adsorption Properties
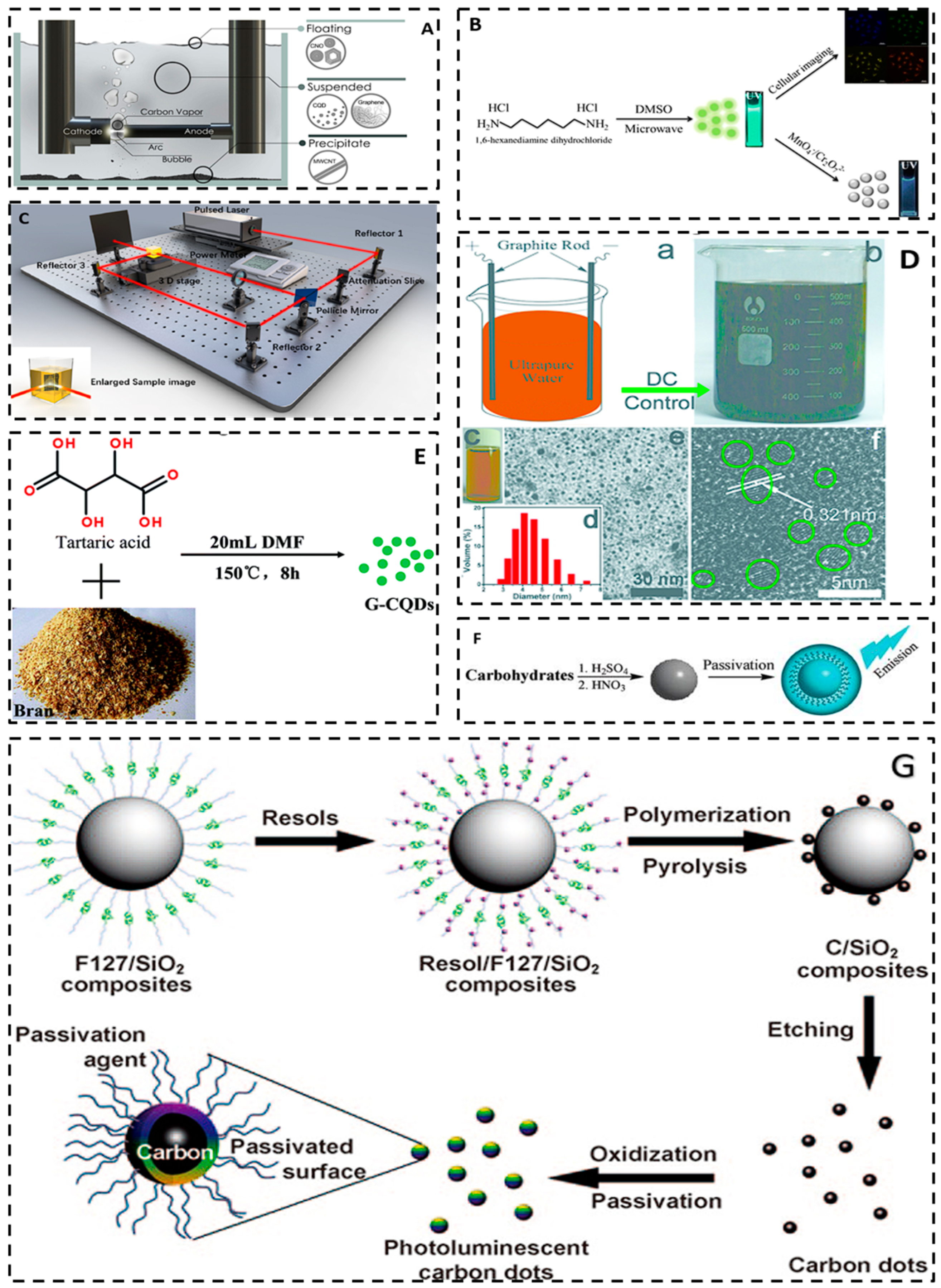
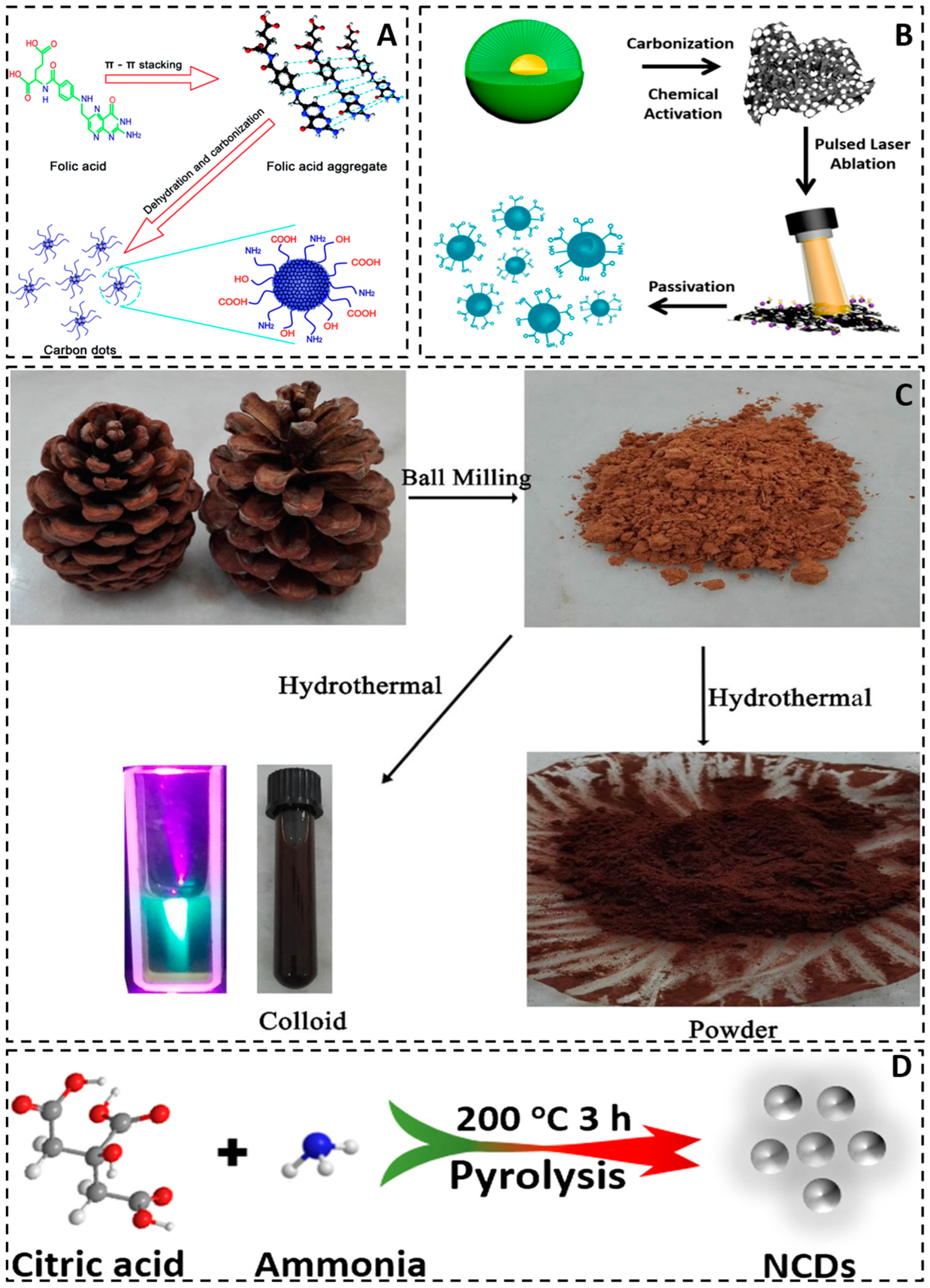
3. Preparation of Carbon Quantum Dots
3.1. Top-Down Approach
3.1.1. Arc Discharge Method
3.1.2. Laser Ablation
3.1.3. Electrochemical Method
3.2. Bottom-Up Approach
3.2.1. Chemical Oxidation
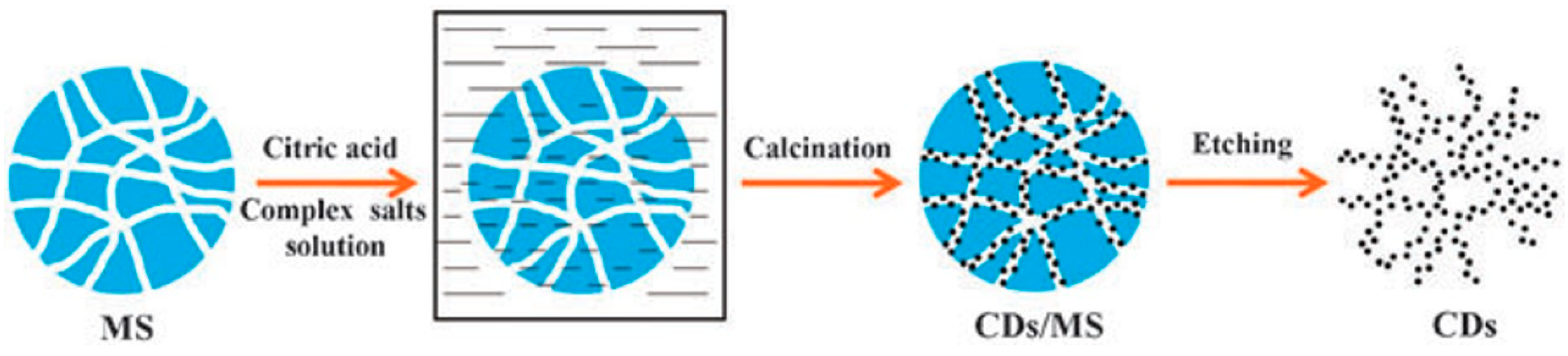
3.2.2. Template Method
3.2.3. Microwave Method
3.2.4. Hydrothermal Method
4. Separation and Purification of Carbon Quantum Dots
5. Materials for the Preparation of Carbon Quantum Dots
5.1. Organic Substance
5.2. Carbon Materials
5.3. Natural Products
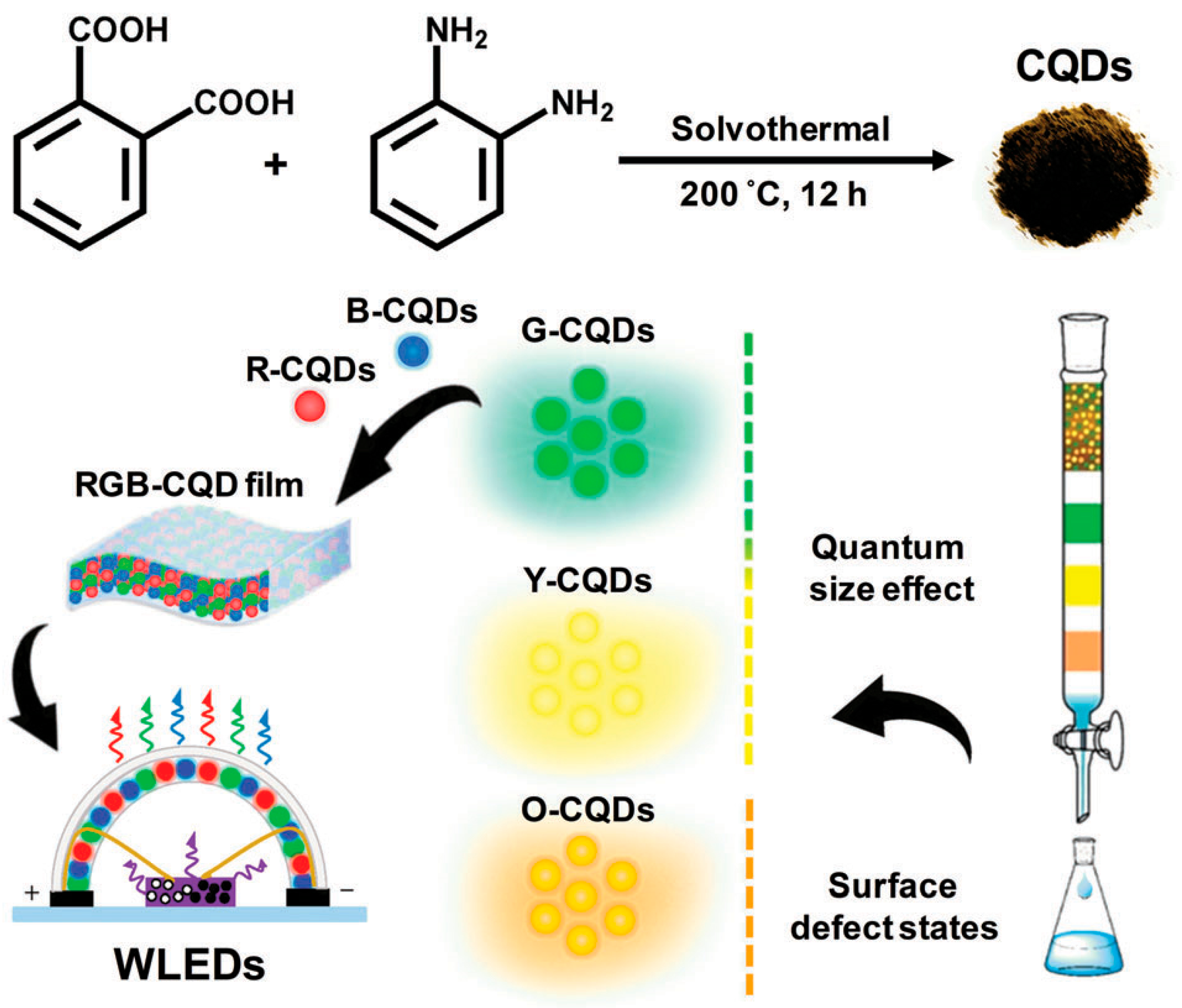
6. Heteroatom Dopants of Carbon Quantum Dots
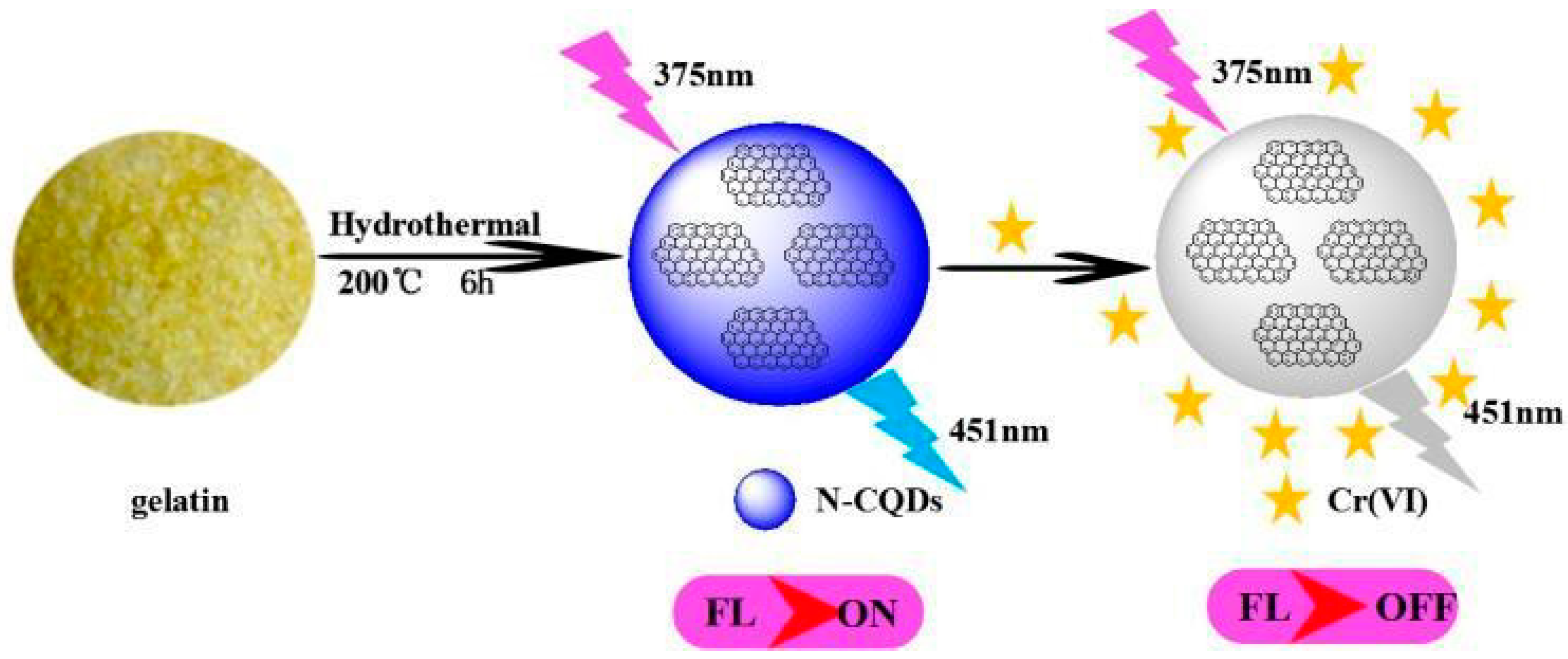
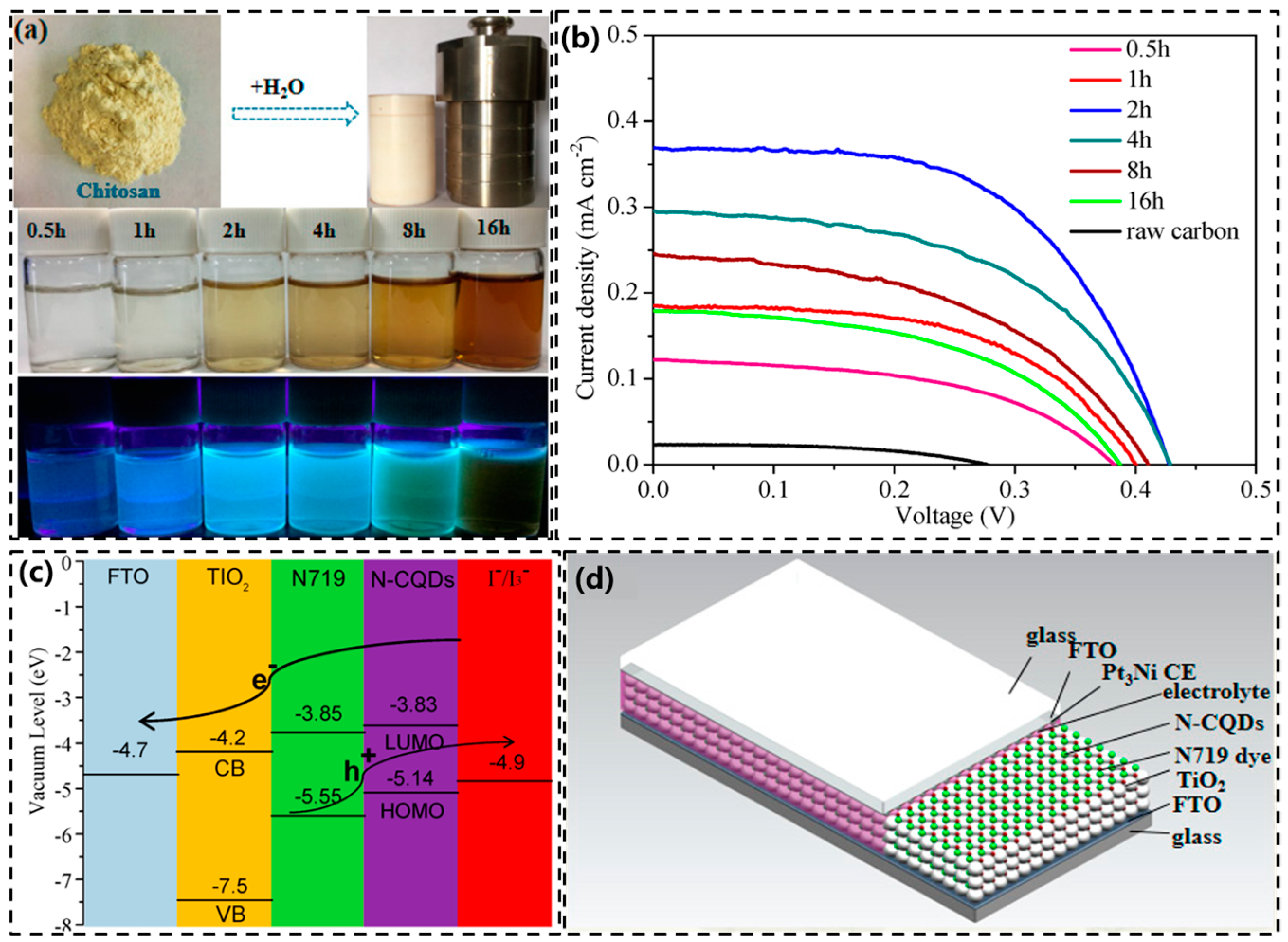
6.1. Nitrogen-Doped Carbon Quantum Dots
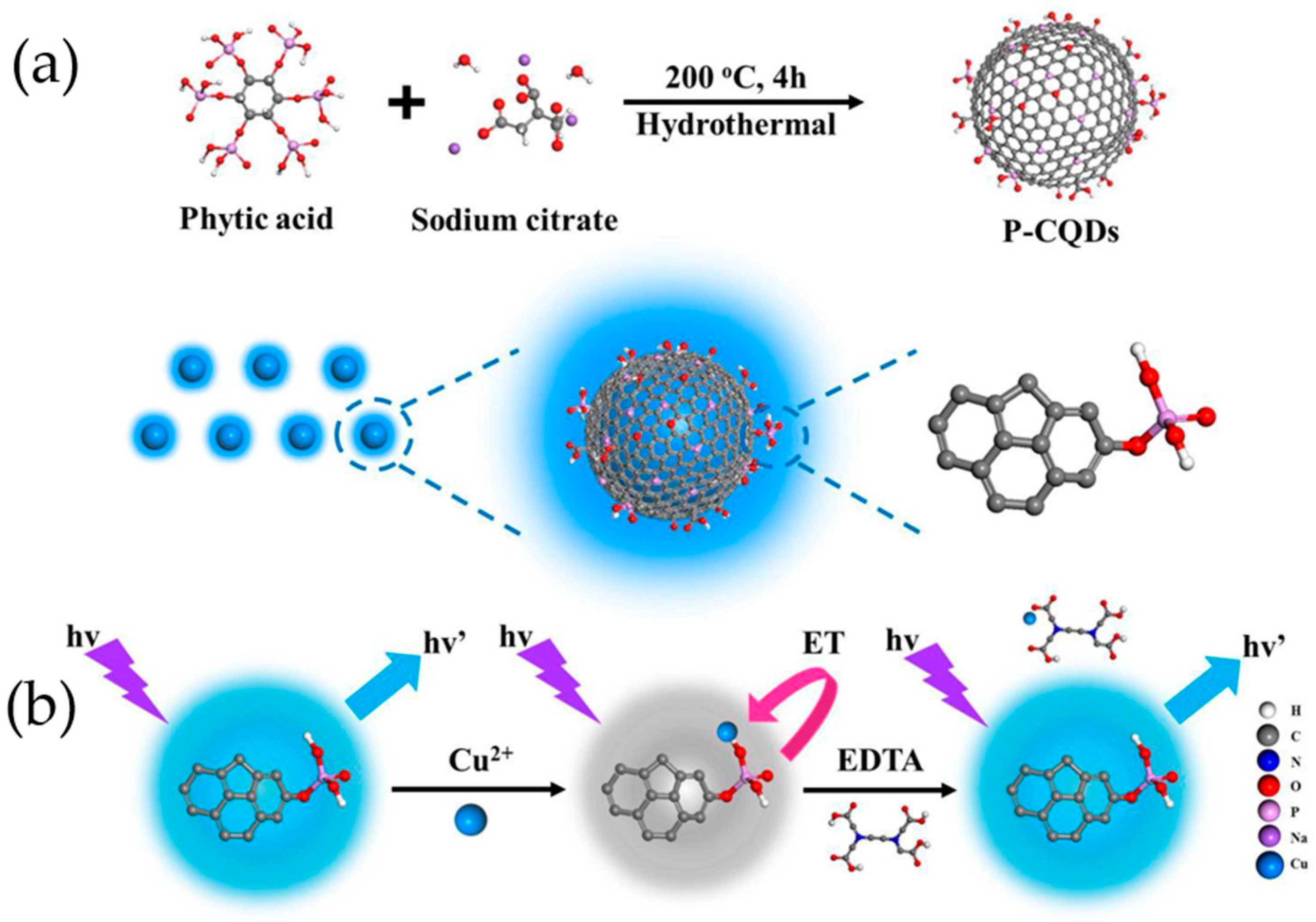
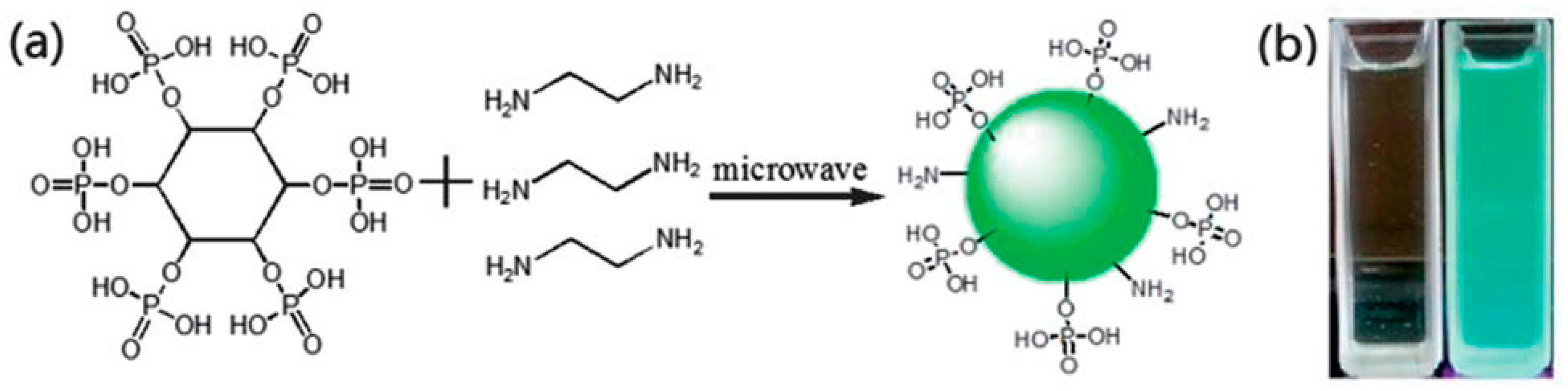
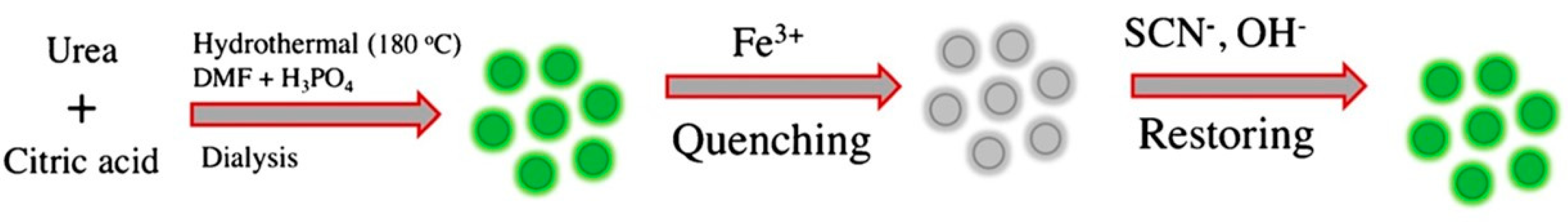
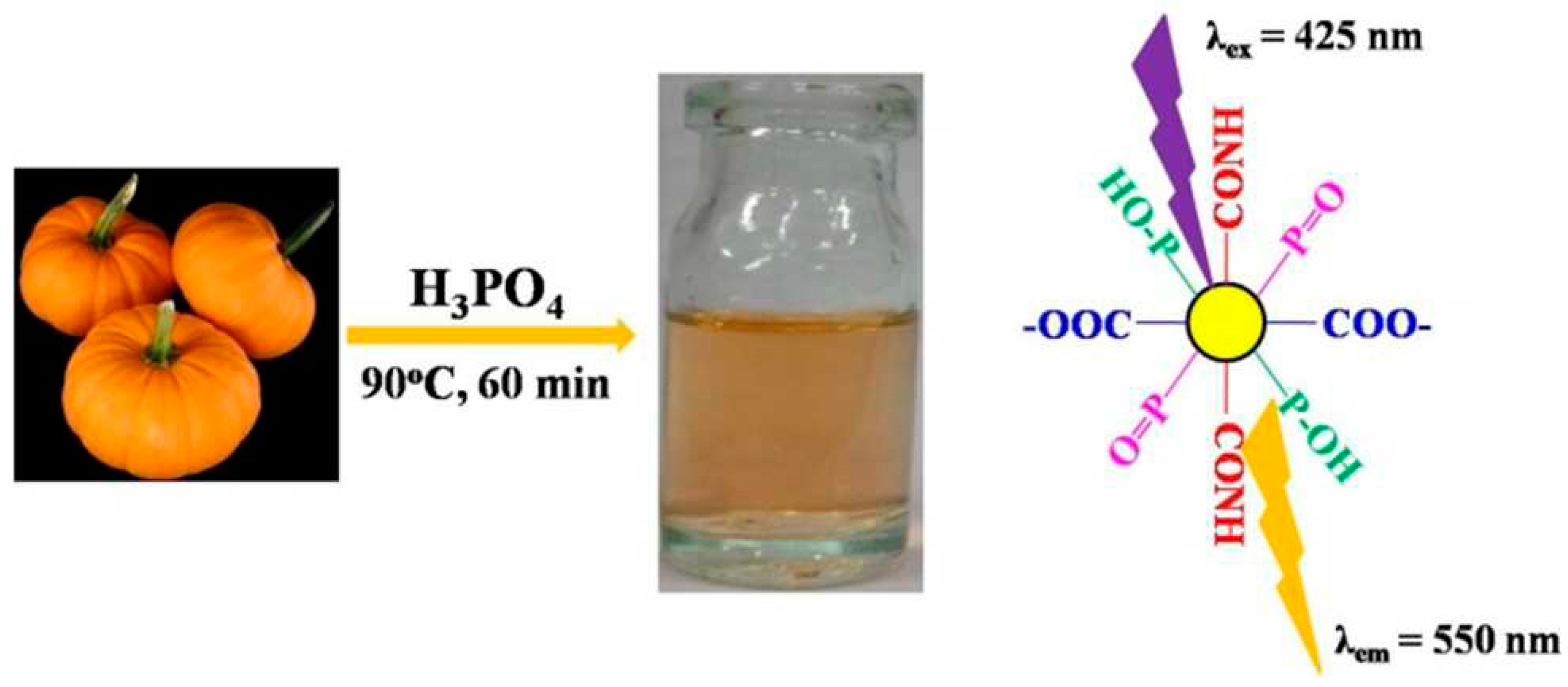
6.2. Phosphorus-Doped Carbon Quantum Dots
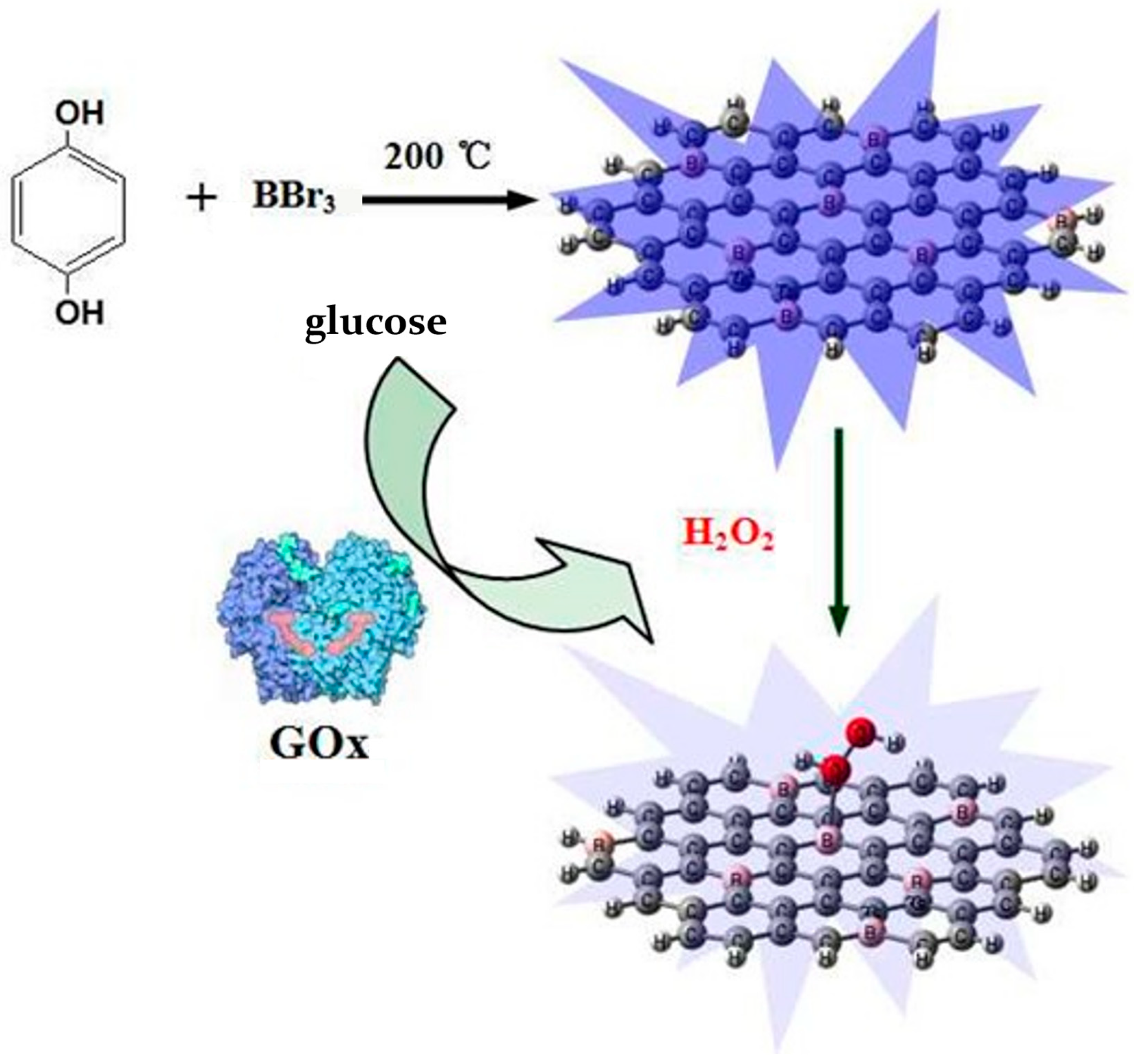
6.3. Boron-Doped Carbon Quantum Dots
6.4. Co-Doped Carbon Quantum Dots
6.5. Mixed-Doped Carbon Quantum Dots
7. Carbon Quantum Dot Applications
7.1. Optoelectronics
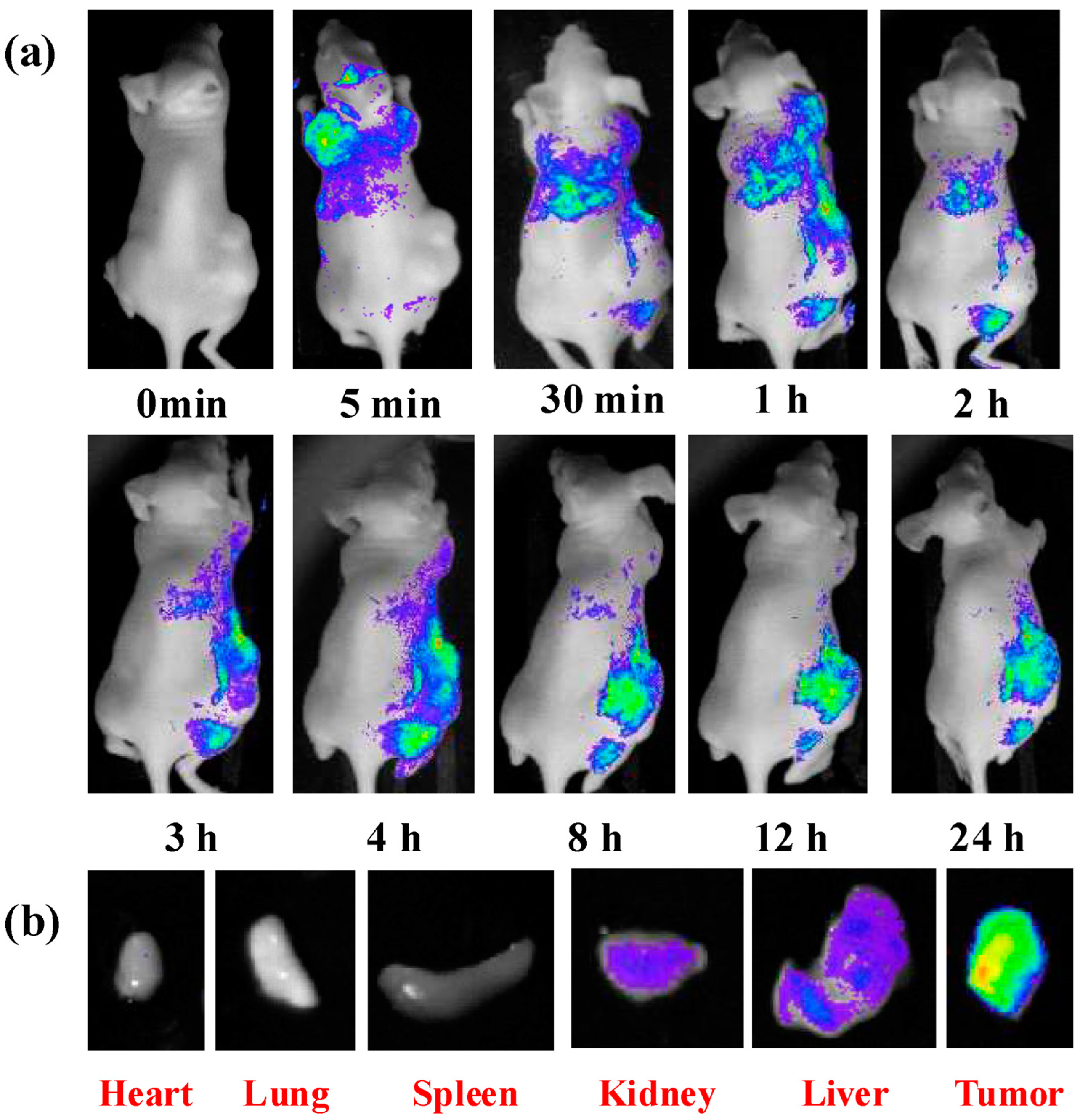
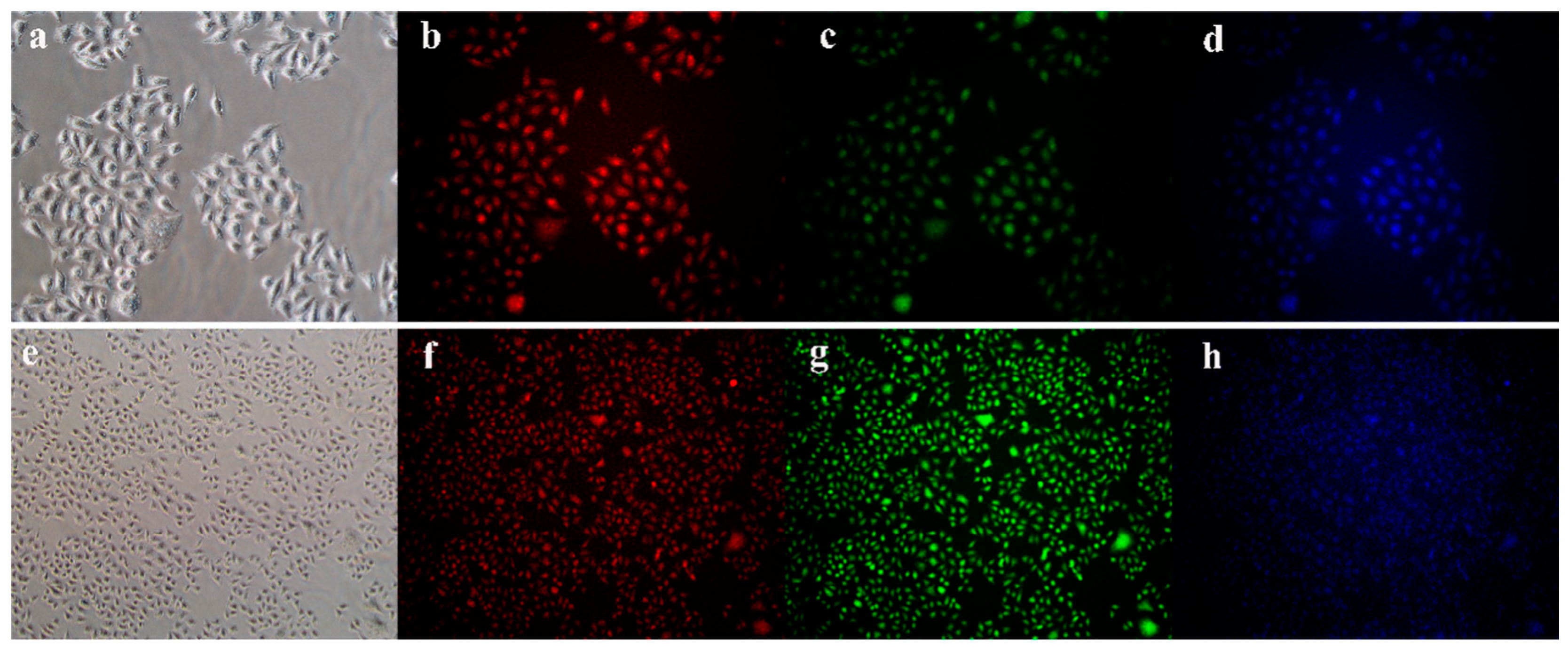
7.2. Bioimaging
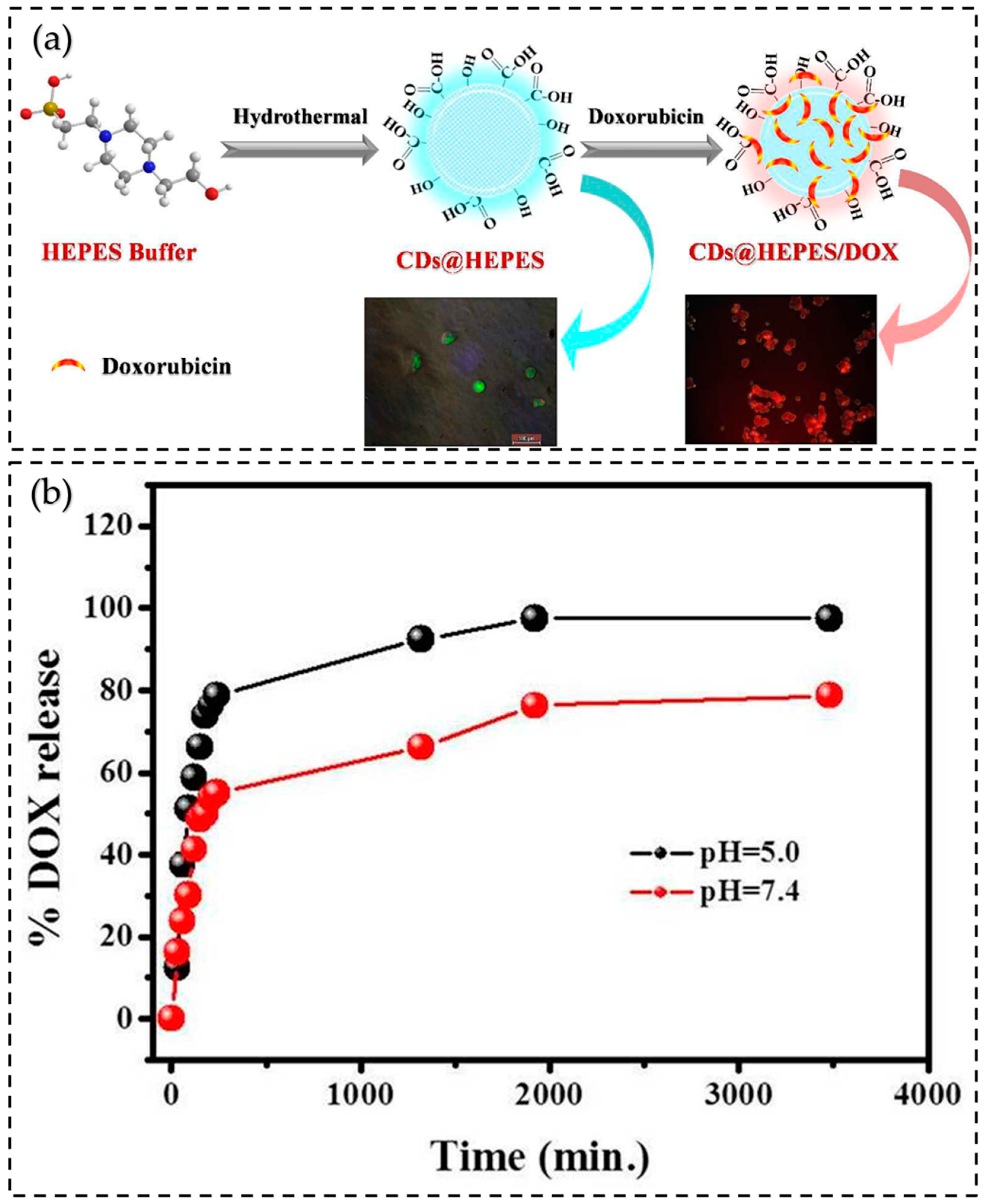
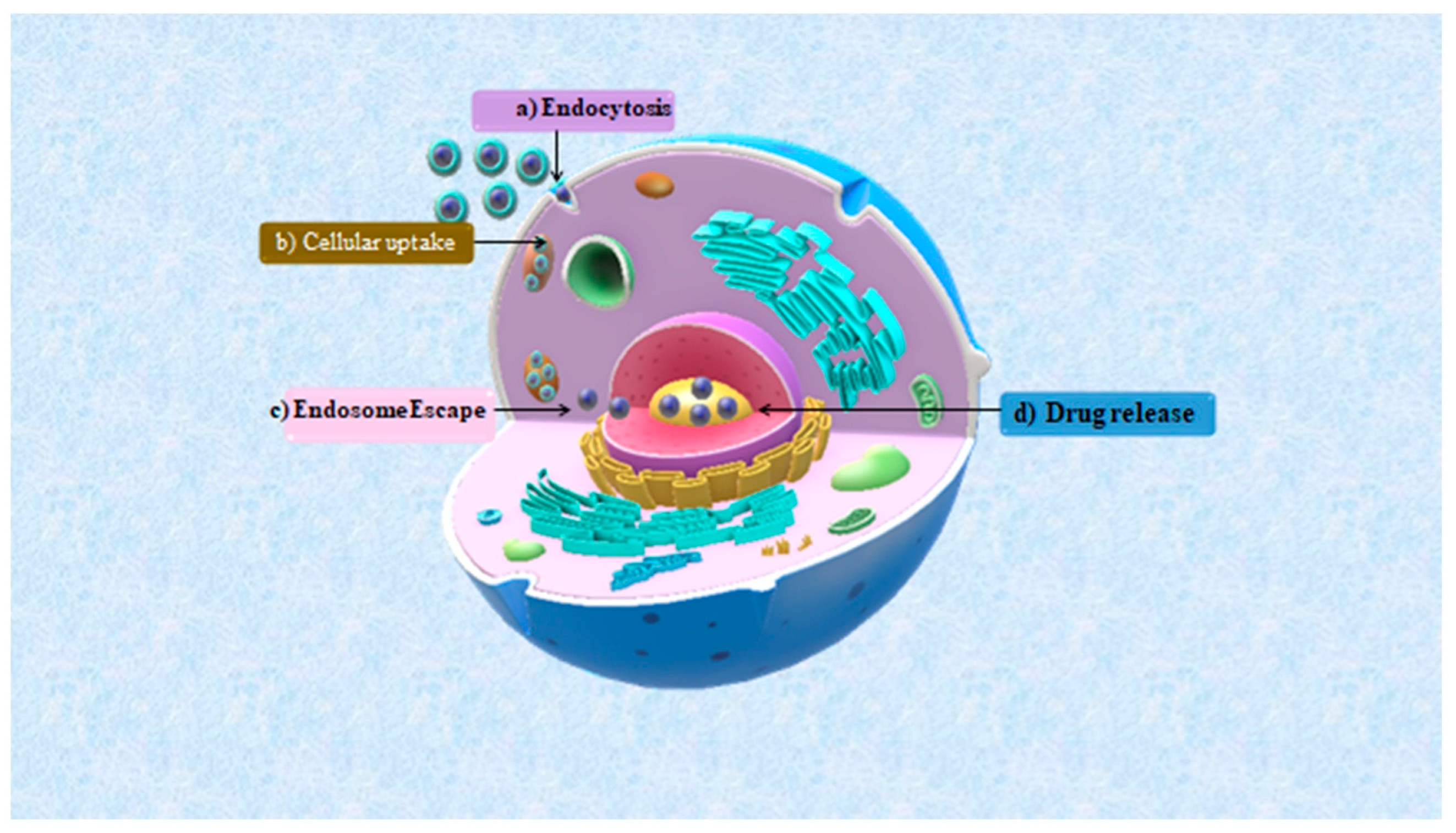
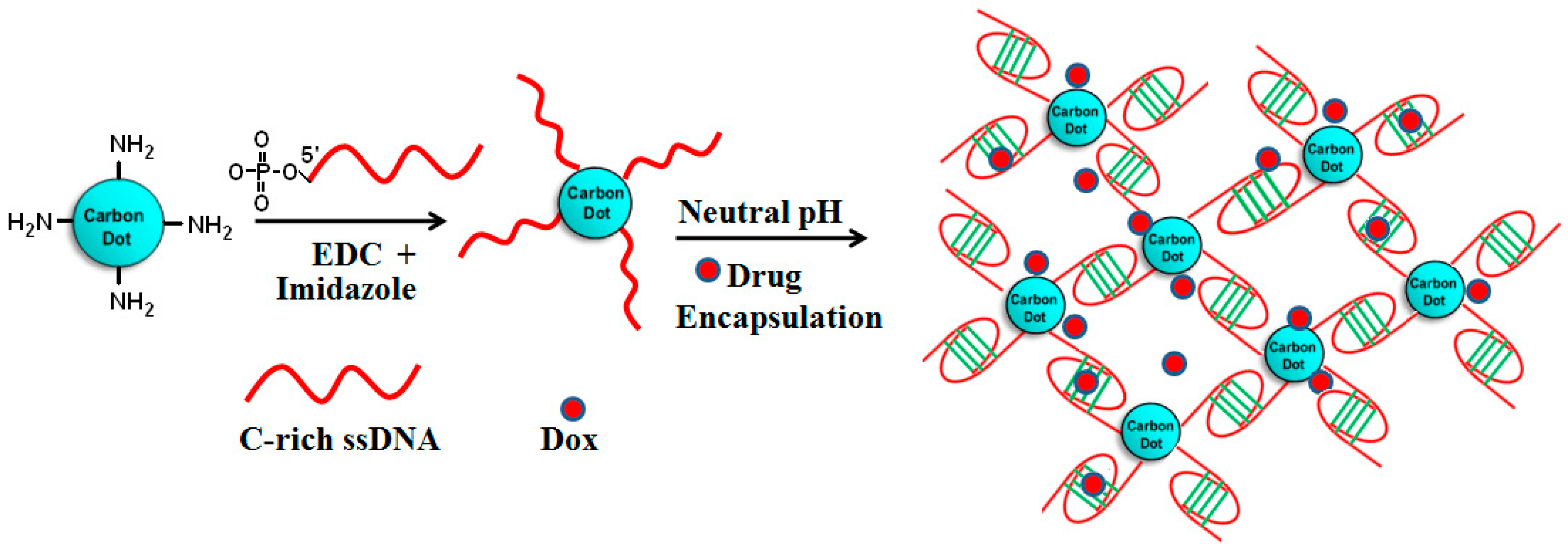
7.3. Drug Delivery
7.4. Cancer Treatment
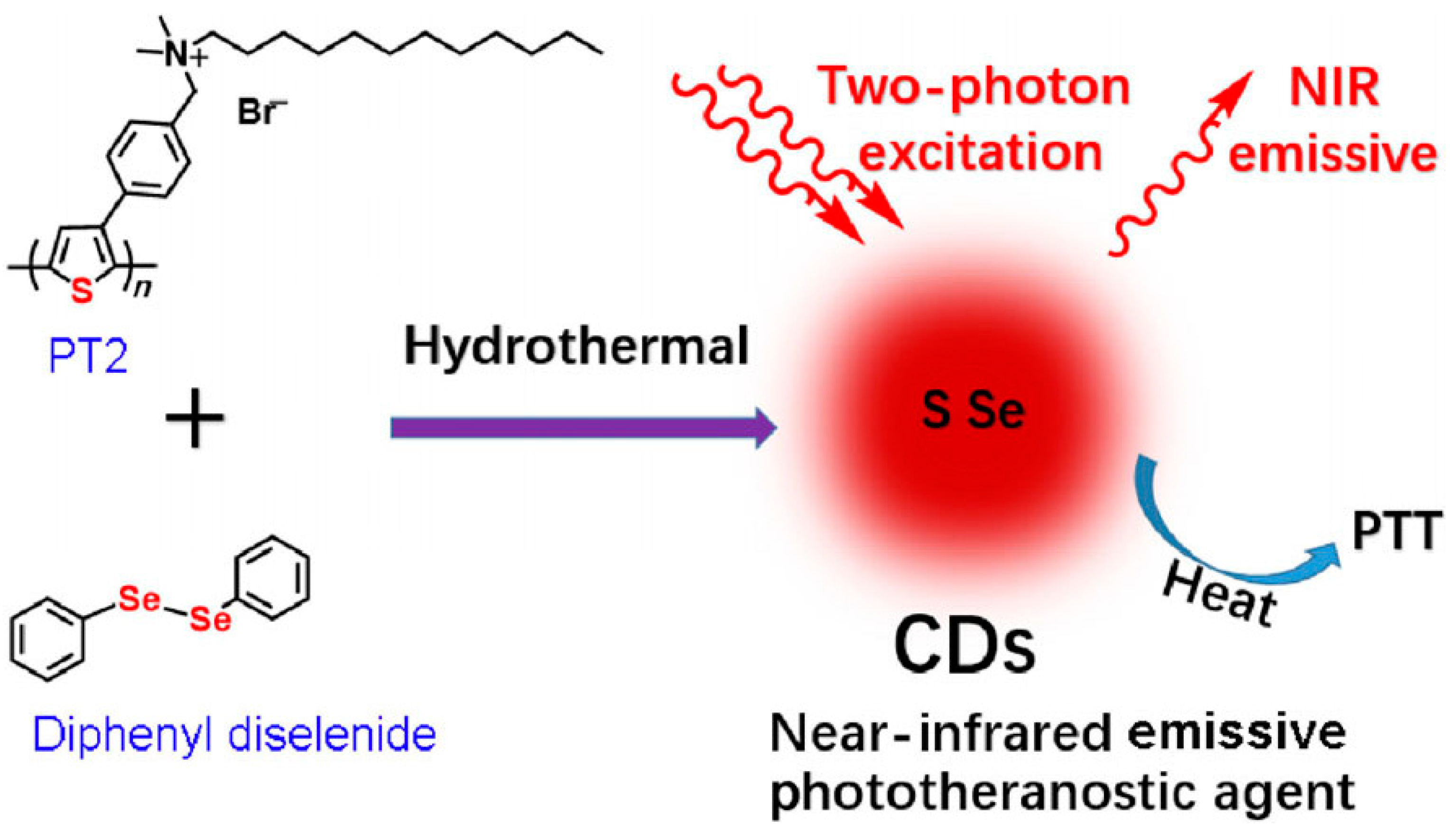
7.4.1. Photothermal Therapy
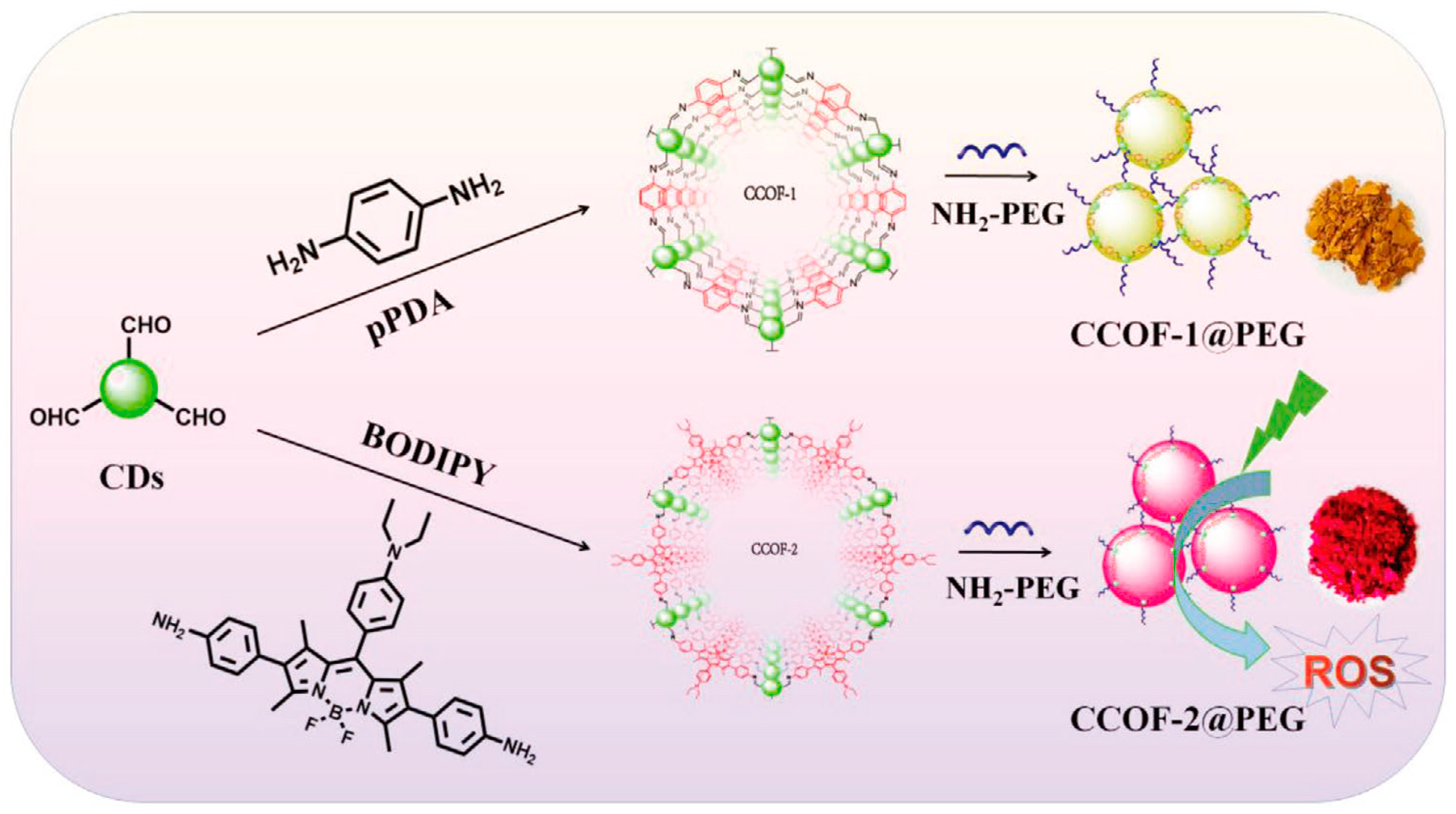
7.4.2. Photodynamic Therapy
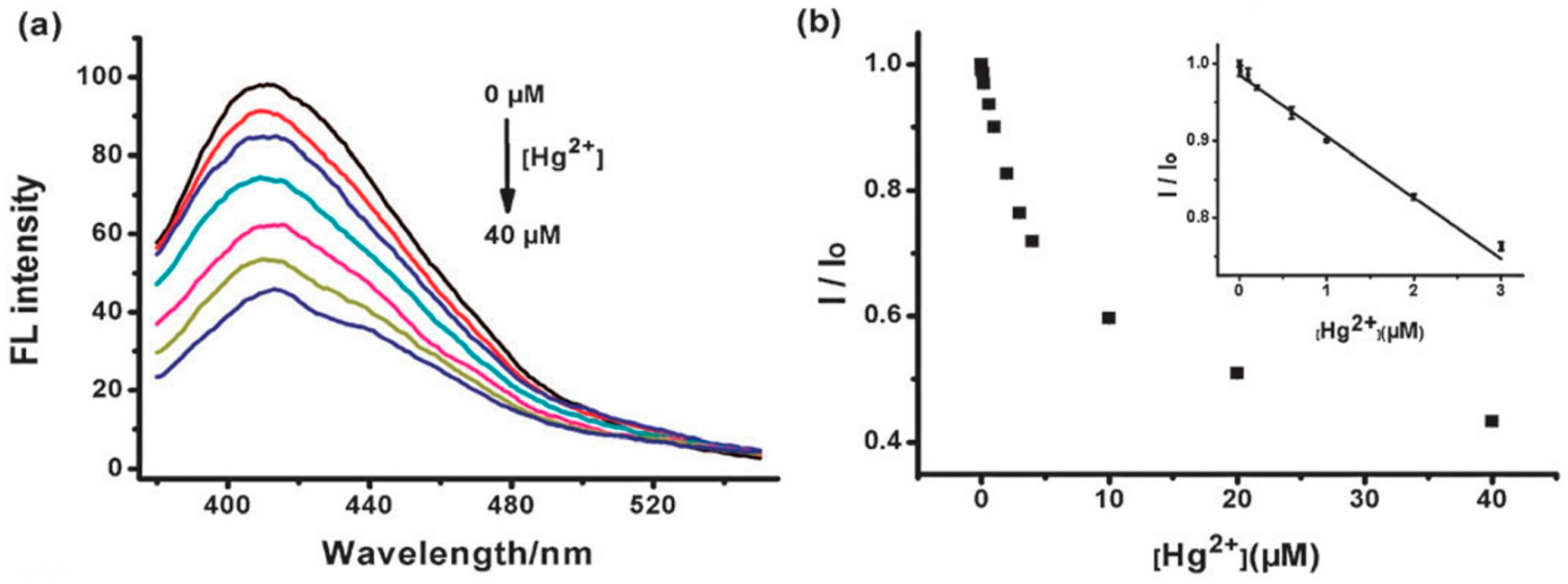
7.5. Sensors
7.6. Environmental Field
8. Summary and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Speranza, G. Carbon nanomaterials: Synthesis, functionalization and sensing applications. Nanomaterials 2021, 11, 967. [Google Scholar] [CrossRef]
- Farmand, M.; Jahanpeyma, F.; Gholaminejad, A.; Azimzadeh, M.; Malaei, F.; Shoaie, N.J.B. Carbon nanostructures: A comprehensive review of potential applications and toxic effects. 3 Biotech 2022, 12, 159. [Google Scholar] [CrossRef]
- Castelletto, S.; Boretti, A. Advantages, limitations, and future suggestions in studying graphene-based desalination membranes. RSC Adv. 2021, 11, 7981–8002. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wu, L.; de Perrot, M.; Zhao, X.J. Carbon nanotubes: A summary of beneficial and dangerous aspects of an increasingly popular group of nanomaterials. Front. Oncol. 2021, 11, 693814. [Google Scholar] [CrossRef]
- Prolongo, S.; Moriche, R.; Jiménez-Suárez, A.; Sánchez, M.; Ureña, A. Advantages and disadvantages of the addition of graphene nanoplatelets to epoxy resins. Eur. Polym. J. 2014, 61, 206–214. [Google Scholar] [CrossRef]
- Xia, C.; Zhu, S.; Feng, T.; Yang, M.; Yang, B. Evolution and synthesis of carbon dots: From carbon dots to carbonized polymer dots. Adv. Sci. 2019, 6, 1901316. [Google Scholar] [CrossRef]
- Manioudakis, J.; Victoria, F.; Thompson, C.A.; Brown, L.; Movsum, M.; Lucifero, R.; Naccache, R. Effects of nitrogen-doping on the photophysical properties of carbon dots. J. Mater. Chem. C 2019, 7, 853–862. [Google Scholar] [CrossRef]
- Bacon, M.; Bradley, S.J.; Nann, T.J.P.; Characterization, P.S. Graphene quantum dots. Part. Part. Syst. Charact. 2014, 31, 415–428. [Google Scholar] [CrossRef]
- Hai, X.; Feng, J.; Chen, X.; Wang, J. Tuning the optical properties of graphene quantum dots for biosensing and bioimaging. J. Mater. Chem. B 2018, 6, 3219–3234. [Google Scholar] [CrossRef]
- Rajakumar, G.; Zhang, X.-H.; Gomathi, T.; Wang, S.-F.; Azam Ansari, M.; Mydhili, G.; Nirmala, G.; Alzohairy, M.A.; Chung, I.-M. Current Use of Carbon-Based Materials for Biomedical Applications—A Prospective and Review. Processes 2020, 8, 355. [Google Scholar] [CrossRef]
- Yadav, P.K.; Chandra, S.; Kumar, V.; Kumar, D.; Hasan, S.H. Carbon Quantum Dots: Synthesis, Structure, Properties, and Catalytic Applications for Organic Synthesis. Catalysts 2023, 13, 422. [Google Scholar] [CrossRef]
- Xu, X.Y.; Ray, R.; Gu, Y.L.; Ploehn, H.J.; Gearheart, L.; Raker, K.; Scrivens, W.A. Electrophoretic analysis and purification of fluorescent single-walled carbon nanotube fragments. J. Am. Chem. Soc. 2004, 126, 12736–12737. [Google Scholar] [CrossRef]
- LeCroy, G.E.; Sonkar, S.K.; Yang, F.; Veca, L.M.; Wang, P.; Tackett, K.N., 2nd; Yu, J.J.; Vasile, E.; Qian, H.; Liu, Y.; et al. Toward structurally defined carbon dots as ultracompact fluorescent probes. ACS Nano 2014, 8, 4522–4529. [Google Scholar] [CrossRef]
- Cui, L.; Ren, X.; Wang, J.; Sun, M. Synthesis of homogeneous carbon quantum dots by ultrafast dual-beam pulsed laser ablation for bioimaging. Mater. Today Nano 2020, 12, 100091. [Google Scholar] [CrossRef]
- Zhou, J.G.; Booker, C.; Li, R.Y.; Zhou, X.T.; Sham, T.K.; Sun, X.L.; Ding, Z.F. An electrochemical avenue to blue luminescent nanocrystals from multiwalled carbon nanotubes (MWCNTs). J. Am. Chem. Soc. 2007, 129, 744–745. [Google Scholar] [CrossRef]
- Peng, H.; Travas-Sejdic, J. Simple Aqueous Solution Route to Luminescent Carbogenic Dots from Carbohydrates. Chem. Mater. 2009, 21, 5563–5565. [Google Scholar] [CrossRef]
- Xu, J.; Wang, C.; Li, H.; Zhao, W. Synthesis of green-emitting carbon quantum dots with double carbon sources and their application as a fluorescent probe for selective detection of Cu2+ ions. RSC Adv. 2020, 10, 2536–2544. [Google Scholar] [CrossRef]
- Ding, C.H.; Deng, Z.Q.; Chen, J.C.; Jin, Y.Z. One-step microwave synthesis of N,S co-doped carbon dots from 1,6-hexanediamine dihydrochloride for cell imaging and ion detection. Colloids Surf. B Biointerfaces 2020, 189, 110838. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.L.; Wu, D.Q.; Liu, S.H.; Koynov, K.; Knoll, W.; Li, Q. An Aqueous Route to Multicolor Photoluminescent Carbon Dots Using Silica Spheres as Carriers. Angew. Chem. Int. Ed. 2009, 48, 4598–4601. [Google Scholar] [CrossRef]
- John, B.K.; Abraham, T.; Mathew, B. A review on characterization techniques for carbon quantum dots and their applications in agrochemical residue detection. J. Fluoresc. 2022, 32, 449–471. [Google Scholar] [CrossRef]
- Zuo, P.; Lu, X.; Sun, Z.; Guo, Y.; He, H. A review on syntheses, properties, characterization and bioanalytical applications of fluorescent carbon dots. Microchim. Acta 2016, 183, 519–542. [Google Scholar] [CrossRef]
- Yang, H.L.; Bai, L.F.; Geng, Z.R.; Chen, H.; Xu, L.T.; Xie, Y.C.; Wang, D.J.; Gu, H.W.; Wang, X.M. Carbon quantum dots: Preparation, optical properties, and biomedical applications. Mater. Today Adv. 2023, 18, 100376. [Google Scholar] [CrossRef]
- Cui, L.; Ren, X.; Sun, M.; Liu, H.; Xia, L. Carbon dots: Synthesis, properties and applications. Nanomaterials 2021, 11, 3419. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.F.; Hu, A.G. Carbon quantum dots: Synthesis, properties and applications. J. Mater. Chem. C 2014, 2, 6921–6939. [Google Scholar] [CrossRef]
- Tan, X.Y.; Li, Y.C.; Li, X.H.; Zhou, S.X.; Fan, L.Z.; Yang, S.H. Electrochemical synthesis of small-sized red fluorescent graphene quantum dots as a bioimaging platform. Chem. Commun. 2015, 51, 2544–2546. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.P.; Zhou, B.; Lin, Y.; Wang, W.; Fernando, K.A.S.; Pathak, P.; Meziani, M.J.; Harruff, B.A.; Wang, X.; Wang, H.F.; et al. Quantum-sized carbon dots for bright and colorful photoluminescence. J. Am. Chem. Soc. 2006, 128, 7756–7757. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.L.; Zhang, Z.L.; Huang, B.H.; Peng, J.; Zhang, M.; Pang, D.W. Facile preparation of low cytotoxicity fluorescent carbon nanocrystals by electrooxidation of graphite. Chem. Commun. 2008, 5116–5118. [Google Scholar] [CrossRef]
- Yang, S.T.; Wang, X.; Wang, H.F.; Lu, F.S.; Luo, P.J.G.; Cao, L.; Meziani, M.J.; Liu, J.H.; Liu, Y.F.; Chen, M.; et al. Carbon Dots as Nontoxic and High-Performance Fluorescence Imaging Agents. J. Phys. Chem. C 2009, 113, 18110–18114. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhang, M.; Bhandari, B.; Yang, C. Recent development of carbon quantum dots: Biological toxicity, antibacterial properties and application in foods. Food Rev. Int. 2022, 38, 1513–1532. [Google Scholar] [CrossRef]
- Ali, H.; Ghosh, S.; Jana, N.R. Fluorescent carbon dots as intracellular imaging probes. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2020, 12, e1617. [Google Scholar] [CrossRef]
- Boakye-Yiadom, K.O.; Kesse, S.; Opoku-Damoah, Y.; Filli, M.S.; Aquib, M.; Joelle, M.M.B.; Farooq, M.A.; Mavlyanova, R.; Raza, F.; Bavi, R.; et al. Carbon dots: Applications in bioimaging and theranostics. Int. J. Pharm. 2019, 564, 308–317. [Google Scholar] [CrossRef] [PubMed]
- Tungare, K.; Bhori, M.; Racherla, K.S.; Sawant, S. Synthesis, characterization and biocompatibility studies of carbon quantum dots from Phoenix dactylifera. 3 Biotech 2020, 10, 540. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.F.; Li, J.; Wang, E.K. One-pot green synthesis of optically pH-sensitive carbon dots with upconversion luminescence. Nanoscale 2012, 4, 5572–5575. [Google Scholar] [CrossRef] [PubMed]
- Isnaeni; Herbani, Y.; Suliyanti, M.M. Concentration effect on optical properties of carbon dots at room temperature. J. Lumin. 2018, 198, 215–219. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, R.; Feng, B.; Zhong, X.; Ostrikov, K. Photoluminescence mechanism of carbon dots: Triggering high-color-purity red fluorescence emission through edge amino protonation. Nat. Commun. 2021, 12, 6856. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.L.; Anilkumar, P.; Cao, L.; Liu, J.H.; Luo, P.J.G.; Tackett, K.N.; Sahu, S.; Wang, P.; Wang, X.; Sun, Y.P. Carbon dots of different composition and surface functionalization: Cytotoxicity issues relevant to fluorescence cell imaging. Exp. Biol. Med. 2011, 236, 1231–1238. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.J.; Li, R.; Yang, B. Carbon Dots: A New Type of Carbon-Based Nanomaterial with Wide Applications. ACS Cent. Sci. 2020, 6, 2179–2195. [Google Scholar] [CrossRef]
- Cruz-Cruz, A.; Gallareta-Olivares, G.; Rivas-Sanchez, A.; González-González, R.B.; Ahmed, I.; Parra-Saldívar, R.; Iqbal, H.M.N. Recent Advances in Carbon Dots Based Biocatalysts for Degrading Organic Pollutants. Curr. Pollut. Rep. 2022, 8, 384–394. [Google Scholar] [CrossRef]
- Li, Z.; Yu, H.J.; Bian, T.; Zhao, Y.F.; Zhou, C.; Shang, L.; Liu, Y.H.; Wu, L.Z.; Tung, C.H.; Zhang, T.R. Highly luminescent nitrogen-doped carbon quantum dots as effective fluorescent probes for mercuric and iodide ions. J. Mater. Chem. C 2015, 3, 1922–1928. [Google Scholar] [CrossRef]
- Liu, J.J.; Lu, S.Y.; Tang, Q.L.; Zhang, K.; Yu, W.X.; Sun, H.C.; Yang, B. One-step hydrothermal synthesis of photoluminescent carbon nanodots with selective antibacterial activity against. Nanoscale 2017, 9, 7135–7142. [Google Scholar] [CrossRef]
- Wang, K.; Ru, Z.L.; Shi, J.W.; Zhu, Y.Z.; Yang, L.G.; Wei, M.X.; Xiao, M.L.; Liu, N.N.; Wang, F. N-doped carbon dots as robust fluorescent probes for the rapid detection of hypochlorite. RSC Adv. 2022, 12, 27170–27178. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Feng, Y.Q.; Dong, P.P.; Huang, J.F. A Mini Review on Carbon Quantum Dots: Preparation, Properties, and Electrocatalytic Application. Front. Chem. 2019, 7, 671. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Song, Y.; Heller, M.J. Seamless aqueous arc discharge process for producing graphitic carbon nanostructures. Carbon 2017, 120, 83–88. [Google Scholar] [CrossRef]
- Li, H.; Zhang, Y.; Ding, J.; Wu, T.; Cai, S.; Zhang, W.; Cai, R.; Chen, C.; Yang, R. Synthesis of carbon quantum dots for application of alleviating amyloid-β mediated neurotoxicity. Colloids Surf. B Biointerfaces 2022, 212, 112373. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.J.; Zhang, Y.; Wang, C.; Wu, X.C.; Yang, Y.Q.; Zheng, B.; Wu, H.X.; Guo, S.W.; Zhang, J.Y. Photo-Fenton Reaction of Graphene Oxide: A New Strategy to Prepare Graphene Quantum Dots for DNA Cleavage. ACS Nano 2012, 6, 6592–6599. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.J.; Sheng, Z.H.; Han, H.Y.; Zou, M.Q.; Li, C.X. Facile synthesis of fluorescent carbon dots using watermelon peel as a carbon source. Mater. Lett. 2012, 66, 222–224. [Google Scholar] [CrossRef]
- Campuzano, S.; Yáñez-Sedeño, P.; Pingarrón, J.M. Carbon Dots and Graphene Quantum Dots in Electrochemical Biosensing. Nanomaterials 2019, 9, 634. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Cui, X.; Li, B.S.; Li, L.S. Large, Solution-Processable Graphene Quantum Dots as Light Absorbers for Photovoltaics. Nano Lett. 2010, 10, 1869–1873. [Google Scholar] [CrossRef]
- Liu, C.J.; Zhang, P.; Zhai, X.Y.; Tian, F.; Li, W.C.; Yang, J.H.; Liu, Y.; Wang, H.B.; Wang, W.; Liu, W.G. Nano-carrier for gene delivery and bioimaging based on carbon dots with PEI-passivation enhanced fluorescence. Biomaterials 2012, 33, 3604–3613. [Google Scholar] [CrossRef]
- Yang, Y.H.; Cui, J.H.; Zheng, M.T.; Hu, C.F.; Tan, S.Z.; Xiao, Y.; Yang, Q.; Liu, Y.L. One-step synthesis of amino-functionalized fluorescent carbon nanoparticles by hydrothermal carbonization of chitosan. Chem. Commun. 2012, 48, 380–382. [Google Scholar] [CrossRef]
- Dey, S.; Govindaraj, A.; Biswas, K.; Rao, C.N.R. Luminescence properties of boron and nitrogen doped graphene quantum dots prepared from arc-discharge-generated doped graphene samples. Chem. Phys. Lett. 2014, 595, 203–208. [Google Scholar] [CrossRef]
- Chao-Mujica, F.J.; Garcia-Hernández, L.; Camacho-López, S.; Camacho-López, M.; Camacho-López, M.A.; Contreras, D.R.; Pérez-Rodríguez, A.; Peña-Caravaca, J.P.; Páez-Rodríguez, A.; Darias-Gonzalez, J.G.; et al. Carbon quantum dots by submerged arc discharge in water: Synthesis, characterization, and mechanism of formation. J. Appl. Phys. 2021, 129, 163301. [Google Scholar] [CrossRef]
- Ge, J.; Lan, M.; Zhou, B.; Liu, W.; Guo, L.; Wang, H.; Jia, Q.; Niu, G.; Huang, X.; Zhou, H. A graphene quantum dot photodynamic therapy agent with high singlet oxygen generation. Nat. Commun. 2014, 5, 4596. [Google Scholar] [CrossRef]
- Luo, P.G.; Yang, F.; Yang, S.-T.; Sonkar, S.K.; Yang, L.; Broglie, J.J.; Liu, Y.; Sun, Y.-P. Carbon-based quantum dots for fluorescence imaging of cells and tissues. RSC Adv. 2014, 4, 10791–10807. [Google Scholar] [CrossRef]
- Ming, H.; Ma, Z.; Liu, Y.; Pan, K.M.; Yu, H.; Wang, F.; Kang, Z.H. Large scale electrochemical synthesis of high quality carbon nanodots and their photocatalytic property. Dalton Trans. 2012, 41, 9526–9531. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.F. Progress of quasi-one-dimension nanomaterials synthesized by laser ablation. Laser Technol. 2005, 29, 4–7. [Google Scholar]
- Liu, M.L.; Chen, B.B.; Li, C.M.; Huang, C.Z. Carbon dots: Synthesis, formation mechanism, fluorescence origin and sensing applications. Green Chem. 2019, 21, 449–471. [Google Scholar] [CrossRef]
- Doñate-Buendia, C.; Torres-Mendieta, R.; Pyatenko, A.; Falomir, E.; Fernández-Alonso, M.; Mínguez-Vega, G. Fabrication by laser irradiation in a continuous flow jet of carbon quantum dots for fluorescence imaging. ACS Omega 2018, 3, 2735–2742. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.L.; Liu, J.; Yang, J.L.; Wang, Y.Z.; Cao, S.R. Laser synthesis and size tailor of carbon quantum dots. J. Nanoparticle Res. 2011, 13, 7247–7252. [Google Scholar] [CrossRef]
- Nguyen, V.; Yan, L.H.; Si, J.H.; Hou, X. Femtosecond laser-induced size reduction of carbon nanodots in solution: Effect of laser fluence, spot size, and irradiation time. J. Appl. Phys. 2015, 117, 084304. [Google Scholar] [CrossRef]
- Nguyen, V.; Zhao, N.; Yan, L.H.; Zhong, P.; Nguyen, V.C.; Le, P.H. Double-pulse femtosecond laser ablation for synthesis of ultrasmall carbon nanodots. Mater. Res. Express 2020, 7, 015606. [Google Scholar] [CrossRef]
- Hou, Y.X.; Lu, Q.J.; Deng, J.H.; Li, H.T.; Zhang, Y.Y. One-pot electrochemical synthesis of functionalized fluorescent carbon dots and their selective sensing for mercury ion. Anal. Chim. Acta 2015, 866, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; He, X.; Kang, Z.; Huang, H.; Liu, Y.; Liu, J.; Lian, S.; Tsang, C.H.A.; Yang, X.; Lee, S.T. Water-soluble fluorescent carbon quantum dots and photocatalyst design. Angew. Chem. Int. Ed. 2010, 49, 4430–4434. [Google Scholar] [CrossRef] [PubMed]
- Rocco, D.; Moldoveanu, V.G.; Feroci, M.; Bortolami, M.; Vetica, F. Electrochemical Synthesis of Carbon Quantum Dots. ChemElectroChem 2023, 10, e202201104. [Google Scholar] [CrossRef] [PubMed]
- Niu, F.; Xu, Y.; Liu, M.; Sun, J.; Guo, P.; Liu, J. Bottom-up electrochemical preparation of solid-state carbon nanodots directly from nitriles/ionic liquids using carbon-free electrodes and the applications in specific ferric ion detection and cell imaging. Nanoscale 2016, 8, 5470–5477. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.S.; Lin, Y.L.; Periasamy, A.P.; Cang, J.S.; Chang, H.T. Parameters affecting the synthesis of carbon dots for quantitation of copper ions. Nanoscale Adv. 2019, 1, 2553–2561. [Google Scholar] [CrossRef] [PubMed]
- Ge, G.; Li, L.; Wang, D.; Chen, M.; Zeng, Z.; Xiong, W.; Wu, X.; Guo, C. Carbon dots: Synthesis, properties and biomedical applications. J. Mater. Chem. B 2021, 9, 6553–6575. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.Y.; Shen, W.; Gao, Z.Q. Carbon quantum dots and their applications. Chem. Soc. Rev. 2015, 44, 362–381. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.W.; Sun, J.; Li, X.B.; Zhou, W.; Wang, Z.Y.; He, P.; Ding, G.Q.; Xie, X.M.; Kang, Z.H.; Jiang, M.H. Large-scale fabrication of heavy doped carbon quantum dots with tunable-photoluminescence and sensitive fluorescence detection. J. Mater. Chem. A 2014, 2, 8660–8667. [Google Scholar] [CrossRef]
- Zong, J.; Zhu, Y.H.; Yang, X.L.; Shen, J.H.; Li, C.Z. Synthesis of photoluminescent carbogenic dots using mesoporous silica spheres as nanoreactors. Chem. Commun. 2011, 47, 764–766. [Google Scholar] [CrossRef]
- Sadhukhan, M.; Bhowmik, T.; Kundu, M.K.; Barman, S. Facile synthesis of carbon quantum dots and thin graphene sheets for non-enzymatic sensing of hydrogen peroxide. RSC Adv. 2014, 4, 4998–5005. [Google Scholar] [CrossRef]
- Kumar, P.; Bhatt, G.; Kaur, R.; Dua, S.; Kapoor, A. Synthesis and modulation of the optical properties of carbon quantum dots using microwave radiation. Fuller. Nanotub. Carbon Nanostruct. 2020, 28, 724–731. [Google Scholar] [CrossRef]
- De Medeiros, T.V.; Manioudakis, J.; Noun, F.; Macairan, J.-R.; Victoria, F.; Naccache, R. Microwave-assisted synthesis of carbon dots and their applications. J. Mater. Chem. C 2019, 7, 7175–7195. [Google Scholar] [CrossRef]
- Sharma, N.; Sharma, I.; Bera, M.K. Microwave-assisted green synthesis of carbon quantum dots derived from calotropis gigantea as a fluorescent probe for bioimaging. J. Fluoresc. 2022, 32, 1039–1049. [Google Scholar] [CrossRef] [PubMed]
- Magdy, G.; Belal, F.; Elmansi, H. Rapid microwave-assisted synthesis of nitrogen-doped carbon quantum dots as fluorescent nanosensors for the spectrofluorimetric determination of palbociclib: Application for cellular imaging and selective probing in living cancer cells. RSC Adv. 2023, 13, 4156–4167. [Google Scholar] [CrossRef] [PubMed]
- Nazibudin, N.A.; Zainuddin, M.F.; Abdullah, C.A.C. Hydrothermal Synthesis of Carbon Quantum Dots: An Updated Review. J. Adv. Res. Fluid Mech. Therm. Sci. 2023, 101, 192–206. [Google Scholar] [CrossRef]
- Hasan, M.R.; Saha, N.; Quaid, T.; Reza, M.T. Formation of carbon quantum dots via hydrothermal carbonization: Investigate the effect of precursors. Energies 2021, 14, 986. [Google Scholar] [CrossRef]
- Zhang, B.; Liu, C.Y.; Liu, Y. A Novel One-Step Approach to Synthesize Fluorescent Carbon Nanoparticles. Eur. J. Inorg. Chem. 2010, 2010, 4411–4414. [Google Scholar] [CrossRef]
- Gomes, M.F.; Gomes, Y.F.; Lopes-Moriyama, A.; Neto, E.L.D.B.; de Souza, C.P. Design of carbon quantum dots via hydrothermal carbonization synthesis from renewable precursors. Biomass Convers. Biorefinery 2019, 9, 689–694. [Google Scholar] [CrossRef]
- Nammahachak, N.; Aup-Ngoen, K.K.; Asanithi, P.; Horpratum, M.; Chuangchote, S.; Ratanaphan, S.; Surareungchai, W. Hydrothermal synthesis of carbon quantum dots with size tunability via heterogeneous nucleation. RSC Adv. 2022, 12, 31729–31733. [Google Scholar] [CrossRef]
- Pang, Y.Q.; Gao, H.; Wu, S.H.; Li, X.L. Facile synthesis the nitrogen and sulfur co-doped carbon dots for selective fluorescence detection of heavy metal ions. Mater. Lett. 2017, 193, 236–239. [Google Scholar] [CrossRef]
- Ren, X.; Zhang, F.; Guo, B.P.; Gao, N.; Zhang, X.L. Synthesis of N-Doped Micropore Carbon Quantum Dots with High Quantum Yield and Dual-Wavelength Photoluminescence Emission from Biomass for Cellular Imaging. Nanomaterials 2019, 9, 495. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Li, X.; Zeng, X.; Lu, Y. Preparation of carbon dots by non-focusing pulsed laser irradiation in toluene. Chem. Commun. 2015, 52, 819–822. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.P.; Wang, X.; Lu, F.S.; Cao, L.; Meziani, M.J.; Luo, P.J.G.; Gu, L.R.; Veca, L.M. Doped Carbon Nanoparticles as a New Platform for Highly Photoluminescent Dots. J. Phys. Chem. C 2008, 112, 18295–18298. [Google Scholar] [CrossRef] [PubMed]
- Hou, H.; Banks, C.E.; Jing, M.; Zhang, Y.; Ji, X. Carbon quantum dots and their derivative 3D porous carbon frameworks for sodium-ion batteries with ultralong cycle life. Adv. Mater. 2015, 27, 7861–7866. [Google Scholar] [CrossRef] [PubMed]
- Zhai, X.Y.; Zhang, P.; Liu, C.J.; Bai, T.; Li, W.C.; Dai, L.M.; Liu, W.G. Highly luminescent carbon nanodots by microwave-assisted pyrolysis. Chem. Commun. 2012, 48, 7955–7957. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.W.; Yang, D.; Wu, X.; Yan, H.R.; Zhao, Y.C.; Feng, B.; Duan, K.; Weng, J.; Wang, J.X. A facile approach for the synthesis of highly luminescent carbon dots using vitamin-based small organic molecules with benzene ring structure as precursors. RSC Adv. 2015, 5, 90245–90254. [Google Scholar] [CrossRef]
- Shahba, H.; Sabet, M. Two-Step and Green Synthesis of Highly Fluorescent Carbon Quantum Dots and Carbon Nanofibers from Pine Fruit. J. Fluoresc. 2020, 30, 927–938. [Google Scholar] [CrossRef]
- Wang, H.; Sun, P.; Cong, S.; Wu, J.; Gao, L.; Wang, Y.; Dai, X.; Yi, Q.; Zou, G. Nitrogen-doped carbon dots for “green” quantum dot solar cells. Nanoscale Res. Lett. 2016, 11, 27. [Google Scholar] [CrossRef]
- Liu, S.; Tian, J.; Wang, L.; Zhang, Y.; Qin, X.; Luo, Y.; Asiri, A.M.; Al-Youbi, A.O.; Sun, X. Hydrothermal treatment of grass: A low-cost, green route to nitrogen-doped, carbon-rich, photoluminescent polymer nanodots as an effective fluorescent sensing platform for label-free detection of Cu (II) ions. Adv. Mater. 2012, 24, 2037–2041. [Google Scholar] [CrossRef]
- Wang, L.; Zhou, H.S. Green Synthesis of Luminescent Nitrogen-Doped Carbon Dots from Milk and Its Imaging Application. Anal. Chem. 2014, 86, 8902–8905. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.T.; Chang, X.Y.; Jing, N.; Zhang, Y. Hydrothermal synthesis of carbon quantum dots as fluorescent probes for the sensitive and rapid detection of picric acid. Anal. Methods 2018, 10, 2775–2784. [Google Scholar] [CrossRef]
- Wang, L.; Bi, Y.D.; Hou, J.; Li, H.Y.; Xu, Y.; Wang, B.; Ding, H.; Ding, L. Facile, green and clean one-step synthesis of carbon dots from wool: Application as a sensor for glyphosate detection based on the inner filter effect. Talanta 2016, 160, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.J.; Yang, Q.; Xu, P.P.; Sun, L.; Sun, D.; Zhuo, K.L. One-Step Synthesis of Acidophilic Highly-Photoluminescent Carbon Dots Modified by Ionic Liquid from Polyethylene Glycol. ACS Omega 2017, 2, 5251–5259. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Tsai, Y.-H.; Chang, C.-W. Evaluation of the dialysis time required for carbon dots by HPLC and the properties of carbon dots after HPLC fractionation. New J. Chem. 2019, 43, 6153–6159. [Google Scholar] [CrossRef]
- Sahu, S.; Behera, B.; Maiti, T.K.; Mohapatra, S. Simple one-step synthesis of highly luminescent carbon dots from orange juice: Application as excellent bio-imaging agents. Chem. Commun. 2012, 48, 8835–8837. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.-M.; Lin, L.-P.; Wang, X.-X.; Lin, S.-Q.; Cai, W.-L.; Zhang, L.-H.; Zheng, Z.-Y. Highly selective and sensitive detection of Cu2+ with lysine enhancing bovine serum albumin modified-carbon dots fluorescent probe. Analyst 2012, 137, 2637–2642. [Google Scholar] [CrossRef] [PubMed]
- Barman, S.; Sadhukhan, M. Facile bulk production of highly blue fluorescent graphitic carbon nitride quantum dots and their application as highly selective and sensitive sensors for the detection of mercuric and iodide ions in aqueous media. J. Mater. Chem. 2012, 22, 21832–21837. [Google Scholar] [CrossRef]
- Wang, L.; Chen, X.; Lu, Y.; Liu, C.; Yang, W. Carbon quantum dots displaying dual-wavelength photoluminescence and electrochemiluminescence prepared by high-energy ball milling. Carbon 2015, 94, 472–478. [Google Scholar] [CrossRef]
- Hinterberger, V.; Damm, C.; Haines, P.; Guldi, D.M.; Peukert, W. Purification and structural elucidation of carbon dots by column chromatography. Nanoscale 2019, 11, 8464–8474. [Google Scholar] [CrossRef]
- Mi, Y.-F.; Huang, Y.-H.; He, S.-H.; Ma, R.; Meng, Y.-D.; Cao, Z.-H. Simultaneous regulation of pore size and surface charge of nanofiltration membrane using carbon quantum dots for improved selective separation. Sep. Purif. Technol. 2023, 317, 123870. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, Y.; Liu, X.; Kong, H.; Wang, Y.; Qin, G.; Cao, P.; Song, X.; Yan, X.; Wang, Q. Novel carbon quantum dots from egg yolk oil and their haemostatic effects. Sci. Rep. 2017, 7, 4452. [Google Scholar] [CrossRef] [PubMed]
- Sridharan, K.; Ilango, V.; Samuel, R.S. Water purification by carbon quantum dots. Inorg. Org. Compos. Water Wastewater Treat. 2022, 2, 113–160. [Google Scholar]
- Zulfajri, M.; Abdelhamid, H.N.; Sudewi, S.; Dayalan, S.; Rasool, A.; Habib, A.; Huang, G.G. Plant Part-Derived Carbon Dots for Biosensing. Biosensors 2020, 10, 68. [Google Scholar] [CrossRef] [PubMed]
- Prakash, A.; Yadav, S.; Yadav, U.; Saxena, P.S.; Srivastava, A. Recent advances on nitrogen-doped carbon quantum dots and their applications in bioimaging: A review. Bull. Mater. Sci. 2023, 46, 7. [Google Scholar] [CrossRef]
- Wang, W.J.; Zeng, Z.T.; Zeng, G.M.; Zhang, C.; Xiao, R.; Zhou, C.Y.; Xiong, W.P.; Yang, Y.; Lei, L.; Liu, Y.; et al. Sulfur doped carbon quantum dots loaded hollow tubular g-C3N4 as novel photocatalyst for destruction of Escherichia coli and tetracycline degradation under visible light. Chem. Eng. J. 2019, 378, 122132. [Google Scholar] [CrossRef]
- Wang, F.X.; Hao, Q.L.; Zhang, Y.H.; Xu, Y.J.; Lei, W. Fluorescence quenchometric method for determination of ferric ion using boron-doped carbon dots. Microchim. Acta 2016, 183, 273–279. [Google Scholar] [CrossRef]
- Omer, K.M.; Hassan, A.Q. Chelation-enhanced fluorescence of phosphorus doped carbon nanodots for multi-ion detection. Microchim. Acta 2017, 184, 2063–2071. [Google Scholar] [CrossRef]
- Liu, Y.; Wei, J.; Yan, X.; Zhao, M.; Guo, C.; Xu, Q. Barium charge transferred doped carbon dots with ultra-high quantum yield photoluminescence of 99.6% and applications. Chin. Chem. Lett. 2021, 32, 861–865. [Google Scholar] [CrossRef]
- Miao, S.H.; Liang, K.; Zhu, J.J.; Yang, B.; Zhao, D.Y.; Kong, B. Hetero-atom-doped carbon dots: Doping strategies, properties and applications. Nano Today 2020, 33, 100879. [Google Scholar] [CrossRef]
- Park, Y.; Yoo, J.; Lim, B.; Kwon, W.; Rhee, S.W. Improving the functionality of carbon nanodots: Doping and surface functionalization. J. Mater. Chem. A 2016, 4, 11582–11603. [Google Scholar] [CrossRef]
- Luo, X.M.; Bai, P.X.; Wang, X.C.; Zhao, G.H.; Feng, J.Y.; Ren, H.J. Preparation of nitrogen-doped carbon quantum dots and its application as a fluorescent probe for Cr(VI) ion detection. New J. Chem. 2019, 43, 5488–5494. [Google Scholar] [CrossRef]
- Zhang, Y.Q.; Ma, D.K.; Zhuang, Y.; Zhang, X.; Chen, W.; Hong, L.L.; Yan, Q.X.; Yu, K.; Huang, S.M. One-pot synthesis of N-doped carbon dots with tunable luminescence properties. J. Mater. Chem. 2012, 22, 16714–16718. [Google Scholar] [CrossRef]
- Pang, Z.Z.; Fu, Y.J.; Yu, H.L.; Liu, S.W.; Yu, S.T.; Liu, Y.X.; Wu, Q.; Liu, Y.; Nie, G.K.; Xu, H.F.; et al. Efficient ethanol solvothermal synthesis of high-performance nitrogen-doped carbon quantum dots from lignin for metal ion nanosensing and cell imaging. Ind. Crops Prod. 2022, 183, 114957. [Google Scholar] [CrossRef]
- Liu, J.; Liu, X.L.; Luo, H.J.; Gao, Y.F. One-step preparation of nitrogen-doped and surface-passivated carbon quantum dots with high quantum yield and excellent optical properties. RSC Adv. 2014, 4, 7648–7654. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Jiang, L.P.; Li, B.J.; Fan, X.Y.; Wang, W.; Liu, P.P.; Xu, S.H.; Luo, X.L. Nitrogen doped carbon dots: Mechanism investigation and their application for label free CA125 analysis. J. Mater. Chem. B 2019, 7, 3053–3058. [Google Scholar] [CrossRef]
- Bi, Z.H.; Huo, L.; Kong, Q.Q.; Li, F.; Chen, J.P.; Ahmad, A.; Wei, X.X.; Xie, L.J.; Chen, C.M. Structural Evolution of Phosphorus Species on Graphene with a Stabilized Electrochemical Interface. ACS Appl. Mater. Interfaces 2019, 11, 11421–11430. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Shan, X.Y.; Ma, J.J.; Gu, Y.M.; Qian, Z.S.; Chen, J.R.; Feng, H. Facile synthesis of P-doped carbon quantum dots with highly efficient photoluminescence. RSC Adv. 2014, 4, 5465–5468. [Google Scholar] [CrossRef]
- Yang, F.; He, X.; Wang, C.X.; Cao, Y.; Li, Y.; Yan, L.N.; Liu, M.M.; Lv, M.Z.; Yang, Y.N.; Zhao, X.; et al. Controllable and eco-friendly synthesis of P-riched carbon quantum dots and its application for copper (II) ion sensing. Appl. Surf. Sci. 2018, 448, 589–598. [Google Scholar] [CrossRef]
- Wang, W.; Li, Y.M.; Cheng, L.; Cao, Z.Q.; Liu, W.G. Water-soluble and phosphorus-containing carbon dots with strong green fluorescence for cell labeling. J. Mater. Chem. B 2014, 2, 46–48. [Google Scholar] [CrossRef]
- Gao, R.; Yi, X.; Liu, X.; Wang, H.; Wang, L.; Zeng, B.; Chen, G.; Xu, Y.; Yuan, C.; Dai, L. Phosphorus-doped carbon dots as an effective flame retardant for transparent PVA composite films with enhanced UV shielding property. React. Funct. Polym. 2024, 197, 105877. [Google Scholar] [CrossRef]
- Ma, Y.; Chen, A.Y.; Huang, Y.Y.; He, X.; Xie, X.F.; He, B.; Yang, J.H.; Wang, X.Y. Off-on fluorescent switching of boron-doped carbon quantum dots for ultrasensitive sensing of catechol and glutathione. Carbon 2020, 162, 234–244. [Google Scholar] [CrossRef]
- Shan, X.Y.; Chai, L.J.; Ma, J.J.; Qian, Z.S.; Chen, J.R.; Feng, H. B-doped carbon quantum dots as a sensitive fluorescence probe for hydrogen peroxide and glucose detection. Analyst 2014, 139, 2322–2325. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Pang, H.; Yang, H.B.; Guo, C.; Shao, J.; Chi, Y.; Li, C.M.; Yu, T. Carbon-based dots co-doped with nitrogen and sulfur for high quantum yield and excitation-independent emission. Angew. Chem. 2013, 125, 7954–7958. [Google Scholar] [CrossRef]
- Sun, D.; Ban, R.; Zhang, P.H.; Wu, G.H.; Zhang, J.R.; Zhu, J.J. Hair fiber as a precursor for synthesizing of sulfur- and nitrogen-co-doped carbon dots with tunable luminescence properties. Carbon 2013, 64, 424–434. [Google Scholar] [CrossRef]
- Omer, K.M.; Tofiq, D.I.; Hassan, A.Q. Solvothermal synthesis of phosphorus and nitrogen doped carbon quantum dots as a fluorescent probe for iron(III). Microchim. Acta 2018, 185, 466. [Google Scholar] [CrossRef] [PubMed]
- Jahan, S.; Mansoor, F.; Naz, S.; Lei, J.P.; Kanwal, S. Oxidative Synthesis of Highly Fluorescent Boron/Nitrogen Co-Doped Carbon Nanodots Enabling Detection of Photosensitizer and Carcinogenic Dye. Anal. Chem. 2013, 85, 10232–10239. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.J.; Lu, W.J.; Liu, Y.; Li, Z.B.; Shuang, S.M.; Dong, C.; Choi, M.M.F. Low temperature synthesis of phosphorous and nitrogen co-doped yellow fluorescent carbon dots for sensing and bioimaging. J. Mater. Chem. B 2015, 3, 6813–6819. [Google Scholar] [CrossRef]
- Akram, Z.; Raza, A.; Mehdi, M.; Arshad, A.; Deng, X.; Sun, S. Recent advancements in metal and non-metal mixed-doped carbon quantum dots: Synthesis and emerging potential applications. Nanomaterials 2023, 13, 2336. [Google Scholar] [CrossRef]
- Sun, S.; Bao, W.; Yang, F.; Yan, X.; Sun, Y.; Zhang, G.; Yang, W.; Li, Y. Electrochemical synthesis of FeNx doped carbon quantum dots for sensitive detection of Cu2+ ion. Green Energy Environ. 2023, 8, 141–150. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, J.; Liu, L.; Xi, X.; Li, Y.; Geng, Z.; Jiang, G.; Zhao, Z. Novel metal doped carbon quantum dots/CdS composites for efficient photocatalytic hydrogen evolution. Nanoscale 2019, 11, 1618–1625. [Google Scholar] [CrossRef] [PubMed]
- Mirtchev, P.; Henderson, E.J.; Soheilnia, N.; Yip, C.M.; Ozin, G.A. Solution phase synthesis of carbon quantum dots as sensitizers for nanocrystalline TiO2 solar cells. J. Mater. Chem. 2012, 22, 1265–1269. [Google Scholar] [CrossRef]
- Zhang, Y.Q.; Ma, D.K.; Zhang, Y.G.; Chen, W.; Huang, S.M. N-doped carbon quantum dots for TiO2-based photocatalysts and dye-sensitized solar cells. Nano Energy 2013, 2, 545–552. [Google Scholar] [CrossRef]
- Xiong, H.Y.; Zhang, X.H.; Dong, B.H.; Lu, H.B.; Zhao, L.; Wan, L.; Dai, G.T.; Wang, S.M. The preparation of carbon dots/ionic liquids-based electrolytes and their applications in quasi-solid-state dye-sensitized solar cells. Electrochim. Acta 2013, 88, 100–106. [Google Scholar] [CrossRef]
- Shen, C.; Wang, J.; Cao, Y.; Lu, Y. Facile access to B-doped solid-state fluorescent carbon dots toward light emitting devices and cell imaging agents. J. Mater. Chem. C 2015, 3, 6668–6675. [Google Scholar] [CrossRef]
- Yang, Q.M.; Yang, W.; Zhang, Y.; Ge, W.; Yang, X.; Yang, P.Z. Precise Surface State Control of Carbon Quantum Dots to Enhance Charge Extraction for Solar Cells. Nanomaterials 2020, 10, 460. [Google Scholar] [CrossRef] [PubMed]
- Han, X.G.; Zhong, S.H.; Pan, W.; Shen, W.Z. A simple strategy for synthesizing highly luminescent carbon nanodots and application as effective down-shifting layers. Nanotechnology 2015, 26, 065402. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Zheng, J.; Wang, J.; Yang, Y.; Liu, X. Direct blending of multicolor carbon quantum dots into fluorescent films for white light emitting diodes with an adjustable correlated color temperature. J. Mater. Chem. C 2019, 7, 1502–1509. [Google Scholar] [CrossRef]
- Sarkar, K.; Devi, P.; Lata, A.; Ghosh, R.; Kumar, P. Engineering carbon quantum dots for enhancing the broadband photoresponse in a silicon process-line compatible photodetector. J. Mater. Chem. C 2019, 7, 13182–13191. [Google Scholar] [CrossRef]
- Su, Y.B.; Yu, B.; Wang, S.; Cong, H.L.; Shen, Y.Q. NIR-II bioimaging of small organic molecule. Biomaterials 2021, 271, 120717. [Google Scholar] [CrossRef]
- Bourlinos, A.B.; Stassinopoulos, A.; Anglos, D.; Zboril, R.; Karakassides, M.; Giannelis, E.P. Surface functionalized carbogenic quantum dots. Small 2008, 4, 455–458. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.J.; Meng, Q.N.; Wang, L.; Zhang, J.H.; Song, Y.B.; Jin, H.; Zhang, K.; Sun, H.C.; Wang, H.Y.; Yang, B. Highly Photoluminescent Carbon Dots for Multicolor Patterning, Sensors, and Bioimaging. Angew. Chem. Int. Ed. 2013, 52, 3953–3957. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.X.; Dong, H.L.; Su, Y.; Wu, Y.; Narron, R.; Yong, Q. Synthesis of Carbon Quantum Dot Nanoparticles Derived from Byproducts in Bio-Refinery Process for Cell Imaging and In Vivo Bioimaging. Nanomaterials 2019, 9, 387. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Wang, X.; Meziani, M.J.; Lu, F.S.; Wang, H.F.; Luo, P.J.G.; Lin, Y.; Harruff, B.A.; Veca, L.M.; Murray, D.; et al. Carbon dots for multiphoton bioimaging. J. Am. Chem. Soc. 2007, 129, 11318–11319. [Google Scholar] [CrossRef]
- Pan, Y.; Yang, J.; Fang, Y.N.; Zheng, J.H.; Song, R.; Yi, C.Q. One-pot synthesis of gadolinium-doped carbon quantum dots for high-performance multimodal bioimaging. J. Mater. Chem. B 2017, 5, 92–101. [Google Scholar] [CrossRef]
- Yang, S.T.; Cao, L.; Luo, P.G.J.; Lu, F.S.; Wang, X.; Wang, H.F.; Meziani, M.J.; Liu, Y.F.; Qi, G.; Sun, Y.P. Carbon Dots for Optical Imaging in Vivo. J. Am. Chem. Soc. 2009, 131, 11308–11309. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Zhou, X.X.; Qin, B.T.; Zhou, Z.Y.; Zhao, Y.P. Highly fluorescent near-infrared emitting carbon dots derived from lemon juice and its bioimaging application. J. Lumin. 2019, 211, 298–304. [Google Scholar] [CrossRef]
- Wei, J.M.; Zhang, X.; Sheng, Y.Z.; Shen, J.M.; Huang, P.; Guo, S.K.; Pan, J.Q.; Liu, B.T.; Feng, B.X. Simple one-step synthesis of water-soluble fluorescent carbon dots from waste paper. New J. Chem. 2014, 38, 906–909. [Google Scholar] [CrossRef]
- Chen, J.C.; Liu, J.H.; Li, J.Z.; Xu, L.Q.; Qiao, Y.J. One-pot synthesis of nitrogen and sulfur co-doped carbon dots and its application for sensor and multicolor cellular imaging. J. Colloid Interface Sci. 2017, 485, 167–174. [Google Scholar] [CrossRef]
- Bandopadhyay, S.; Manchanda, S.; Chandra, A.; Ali, J.; Deb, P.K. Overview of different carrier systems for advanced drug delivery. In Drug Delivery Systems; Elsevier: Amsterdam, The Netherlands, 2020; pp. 179–233. [Google Scholar]
- Probst, C.E.; Zrazhevskiy, P.; Bagalkot, V.; Gao, X.H. Quantum dots as a platform for nanoparticle drug delivery vehicle design. Adv. Drug Deliv. Rev. 2013, 65, 703–718. [Google Scholar] [CrossRef]
- Yao, J.; Li, P.F.; Li, L.; Yang, M. Biochemistry and biomedicine of quantum dots: From biodetection to bioimaging, drug discovery, diagnostics, and therapy. Acta Biomater. 2018, 74, 36–55. [Google Scholar] [CrossRef] [PubMed]
- Samantara, A.K.; Maji, S.; Ghosh, A.; Bag, B.; Dash, R.; Jena, B.K. Good’s buffer derived highly emissive carbon quantum dots: Excellent biocompatible anticancer drug carrier. J. Mater. Chem. B 2016, 4, 2412–2420. [Google Scholar] [CrossRef] [PubMed]
- Nair, A.; Haponiuk, J.T.; Thomas, S.; Gopi, S. Natural carbon-based quantum dots and their applications in drug delivery: A review. Biomed. Pharmacother. 2020, 132, 110834. [Google Scholar] [CrossRef]
- Mathew, S.A.; Praveena, P.; Dhanavel, S.; Manikandan, R.; Senthilkumar, S.; Stephen, A. Luminescent chitosan/carbon dots as an effective nano-drug carrier for neurodegenerative diseases. RSC Adv. 2020, 10, 24386–24396. [Google Scholar] [CrossRef]
- Zheng, M.; Liu, S.; Li, J.; Qu, D.; Zhao, H.F.; Guan, X.G.; Hu, X.L.; Xie, Z.G.; Jing, X.B.; Sun, Z.C. Integrating Oxaliplatin with Highly Luminescent Carbon Dots: An Unprecedented Theranostic Agent for Personalized Medicine. Adv. Mater. 2014, 26, 3554–3560. [Google Scholar] [CrossRef]
- Shu, Y.; Lu, J.; Mao, Q.X.; Song, R.S.; Wang, X.Y.; Chen, X.W.; Wang, J.H. Ionic liquid mediated organophilic carbon dots for drug delivery and bioimaging. Carbon 2017, 114, 324–333. [Google Scholar] [CrossRef]
- Singh, S.; Mishra, A.; Kumari, R.; Sinha, K.K.; Singh, M.K.; Das, P. Carbon dots assisted formation of DNA hydrogel for sustained release of drug. Carbon 2017, 114, 169–176. [Google Scholar] [CrossRef]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Cancer statistics for the year 2020: An overview. Int. J. Cancer 2021, 149, 778–789. [Google Scholar] [CrossRef]
- De Martel, C.; Georges, D.; Bray, F.; Ferlay, J.; Clifford, G.M. Global burden of cancer attributable to infections in 2018: A worldwide incidence analysis. Lancet Glob. Health 2020, 8, e180–e190. [Google Scholar] [CrossRef]
- Ansari, M.A.; Shoaib, S.; Chauhan, W.; Gahtani, R.M.; Hani, U.; Alomary, M.N.; Alasiri, G.; Ahmad, N.; Jahan, R.; Yusuf, N. Nanozymes and carbon-dots based nanoplatforms for cancer imaging, diagnosis and therapeutics: Current trends and challenges. Environ. Res. 2023, 241, 117522. [Google Scholar] [CrossRef]
- Bayda, S.; Amadio, E.; Cailotto, S.; Frión-Herrera, Y.; Perosa, A.; Rizzolio, F. Carbon dots for cancer nanomedicine: A bright future. Nanoscale Adv. 2021, 3, 5183–5221. [Google Scholar] [CrossRef]
- Li, X.S.; Lovell, J.F.; Yoon, J.; Chen, X.Y. Clinical development and potential of photothermal and photodynamic therapies for cancer. Nat. Rev. Clin. Oncol. 2020, 17, 657–674. [Google Scholar] [CrossRef]
- Zheng, M.; Li, Y.; Liu, S.; Wang, W.Q.; Xie, Z.G.; Jing, X.B. One-Pot To Synthesize Multifunctional Carbon Dots for Near Infrared Fluorescence Imaging and Photothermal Cancer Therapy. ACS Appl. Mater. Interfaces 2016, 8, 23533–23541. [Google Scholar] [CrossRef] [PubMed]
- Bao, X.; Yuan, Y.; Chen, J.; Zhang, B.; Li, D.; Zhou, D.; Jing, P.; Xu, G.; Wang, Y.; Holá, K. In vivo theranostics with near-infrared-emitting carbon dots—Highly efficient photothermal therapy based on passive targeting after intravenous administration. Light Sci. Appl. 2018, 7, 91. [Google Scholar] [CrossRef]
- Lan, M.H.; Zhao, S.J.; Zhang, Z.Y.; Yan, L.; Guo, L.; Niu, G.L.; Zhang, J.F.; Zhao, J.F.; Zhang, H.Y.; Wang, P.F.; et al. Two-photon-excited near-infrared emissive carbon dots as multifunctional agents for fluorescence imaging and photothermal therapy. Nano Res. 2017, 10, 3113–3123. [Google Scholar] [CrossRef]
- Li, S.H.; Zhou, S.X.; Li, Y.C.; Li, X.H.; Zhu, J.; Fan, L.Z.; Yang, S.H. Exceptionally High Payload of the IR780 Iodide on Folic Acid-Functionalized Graphene Quantum Dots for Targeted Photothermal Therapy. ACS Appl. Mater. Interfaces 2017, 9, 22332–22341. [Google Scholar] [CrossRef]
- Chen, S.; Sun, T.T.; Zheng, M.; Xie, Z.G. Carbon Dots Based Nanoscale Covalent Organic Frameworks for Photodynamic Therapy. Adv. Funct. Mater. 2020, 30, 2004680. [Google Scholar] [CrossRef]
- Li, Y.; Zheng, X.; Zhang, X.; Liu, S.; Pei, Q.; Zheng, M.; Xie, Z. Porphyrin-based carbon dots for photodynamic therapy of hepatoma. Adv. Healthc. Mater. 2017, 6, 1600924. [Google Scholar] [CrossRef] [PubMed]
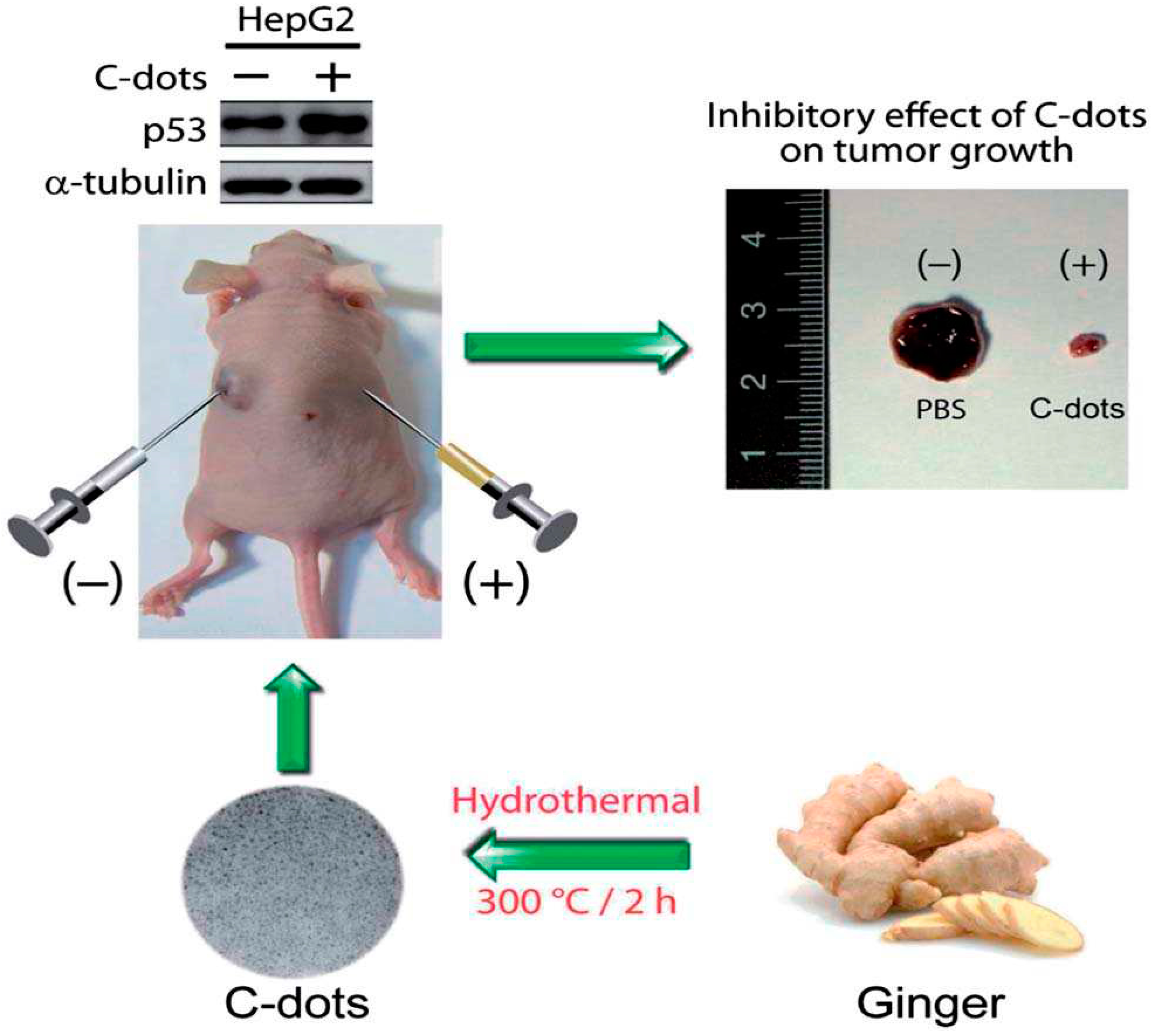
- Li, C.L.; Ou, C.M.; Huang, C.C.; Wu, W.C.; Chen, Y.P.; Lin, T.E.; Ho, L.C.; Wang, C.W.; Shih, C.C.; Zhou, H.C.; et al. Carbon dots prepared from ginger exhibiting efficient inhibition of human hepatocellular carcinoma cells. J. Mater. Chem. B 2014, 2, 4564–4571. [Google Scholar] [CrossRef]
- Dhenadhayalan, N.; Lin, K.C.; Saleh, T.A. Recent advances in functionalized carbon dots toward the design of efficient materials for sensing and catalysis applications. Small 2020, 16, 1905767. [Google Scholar] [CrossRef]
- Costas-Mora, I.; Romero, V.; Lavilla, I.; Bendicho, C. In Situ Building of a Nanoprobe Based on Fluorescent Carbon Dots for Methylmercury Detection. Anal. Chem. 2014, 86, 4536–4543. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Tang, Q.; Meng, Y.; Liu, J.; Feng, T.; Zhao, X.; Zhu, S.; Yu, W.; Yang, B. Reversible “off–on” fluorescence of Zn2+-passivated carbon dots: Mechanism and potential for the detection of EDTA and Zn2+. Langmuir 2018, 34, 7767–7775. [Google Scholar] [CrossRef] [PubMed]
- Qu, K.; Wang, J.; Ren, J.; Qu, X. Carbon dots prepared by hydrothermal treatment of dopamine as an effective fluorescent sensing platform for the label-free detection of iron (III) ions and dopamine. Chem. A Eur. J. 2013, 19, 7243–7249. [Google Scholar] [CrossRef] [PubMed]
- Shen, P.F.; Xia, Y.S. Synthesis-Modification Integration: One-Step Fabrication of Boronic Acid Functionalized Carbon Dots for Fluorescent Blood Sugar Sensing. Anal. Chem. 2014, 86, 5323–5329. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.Q.; Wang, R.X.; Li, G.L.; Chen, C.Q.; Chi, Y.W.; Chen, G.N. Polyamine-Functionalized Carbon Quantum Dots as Fluorescent Probes for Selective and Sensitive Detection of Copper Ions. Anal. Chem. 2012, 84, 6220–6224. [Google Scholar] [CrossRef] [PubMed]
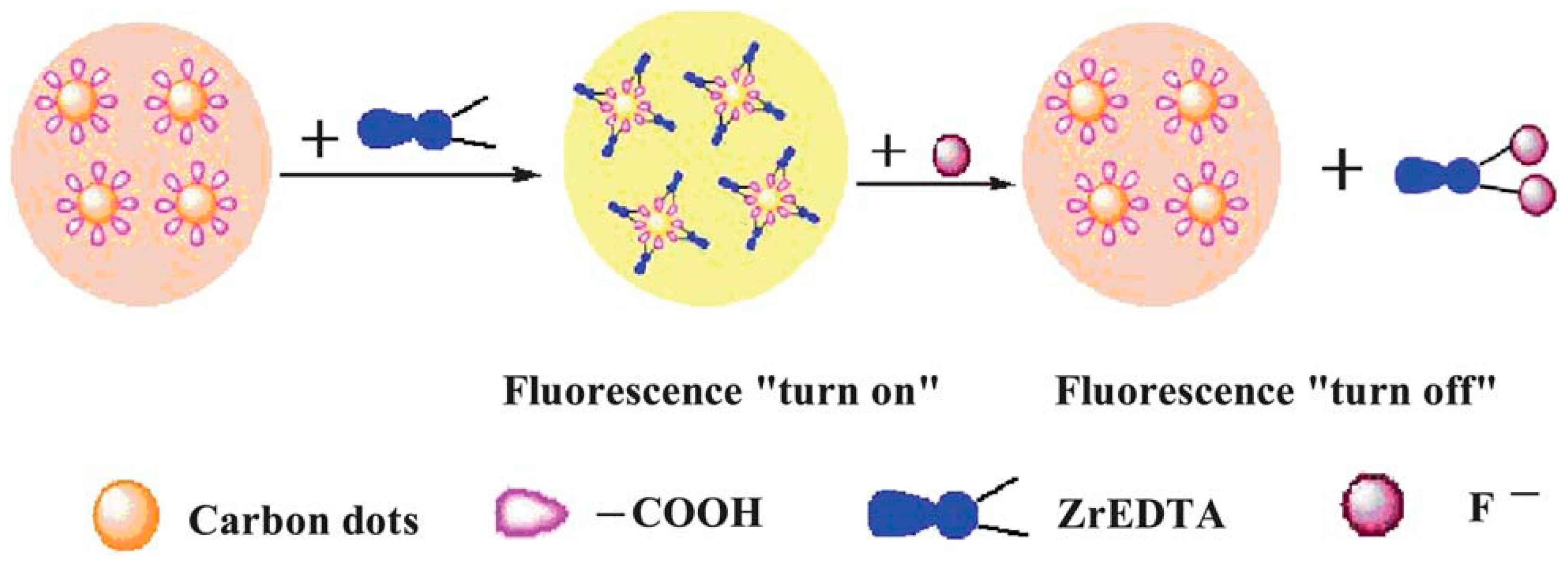
- Liu, J.-M.; Lin, L.-p.; Wang, X.-X.; Jiao, L.; Cui, M.-L.; Jiang, S.-L.; Cai, W.-L.; Zhang, L.-H.; Zheng, Z.-Y. Zr(H2O)2 EDTA modulated luminescent carbon dots as fluorescent probes for fluoride detection. Analyst 2013, 138, 278–283. [Google Scholar] [CrossRef] [PubMed]
- Desa, A.L.; Hairom, N.H.H.; Ng, L.Y.; Ng, C.Y.; Ahmad, M.K.; Mohammad, A.W. Industrial textile wastewater treatment via membrane photocatalytic reactor (MPR) in the presence of ZnO-PEG nanoparticles and tight ultrafiltration. J. Water Process Eng. 2019, 31, 100872. [Google Scholar] [CrossRef]
- Desmond, L.J.; Phan, A.N.; Gentile, P. Critical overview on the green synthesis of carbon quantum dots and their application for cancer therapy. Environ. Sci. Nano 2021, 8, 848–862. [Google Scholar] [CrossRef]
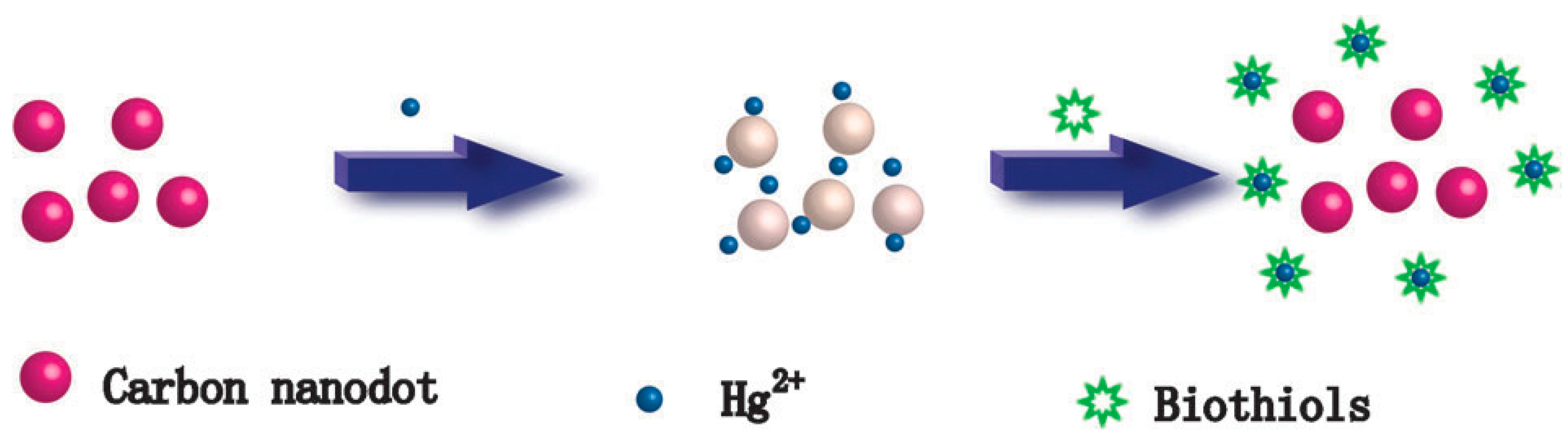
- Zhou, L.; Lin, Y.; Huang, Z.; Ren, J.; Qu, X. Carbon nanodots as fluorescence probes for rapid, sensitive, and label-free detection of Hg2+ and biothiols in complex matrices. Chem. Commun. 2012, 48, 1147–1149. [Google Scholar] [CrossRef]
- Arumugam, N.; Kim, J. Synthesis of carbon quantum dots from Broccoli and their ability to detect silver ions. Mater. Lett. 2018, 219, 37–40. [Google Scholar] [CrossRef]
- Liu, G.H.; Jia, H.S.; Li, N.; Li, X.Y.; Yu, Z.Y.; Wang, J.; Song, Y.T. High-fluorescent carbon dots (CDs) originated from China grass carp scales (CGCS) for effective detection of Hg(II) ions. Microchem. J. 2019, 145, 718–728. [Google Scholar] [CrossRef]
- Pandey, S.C.; Kumar, A.; Sahu, S.K. Single Step Green Synthesis of Carbon Dots from Murraya koenigii leaves; A Unique Turn-off Fluorescent contrivance for Selective Sensing of Cd (II) ion. J. Photochem. Photobiol. A-Chem. 2020, 400, 112620. [Google Scholar] [CrossRef]
- Chen, J.; Shu, J.; Anqi, Z.; Juyuan, H.; Yan, Z.; Chen, J. Synthesis of carbon quantum dots/TiO2 nanocomposite for photo-degradation of Rhodamine B and cefradine. Diam. Relat. Mater. 2016, 70, 137–144. [Google Scholar] [CrossRef]
- Zeng, Y.; Chen, D.; Chen, T.; Cai, M.; Zhang, Q.; Xie, Z.; Li, R.; Xiao, Z.; Liu, G.; Lv, W. Study on heterogeneous photocatalytic ozonation degradation of ciprofloxacin by TiO2/carbon dots: Kinetic, mechanism and pathway investigation. Chemosphere 2019, 227, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Wu, Y.; Wang, Y.; Lin, Z.; Liang, D.; Zheng, X.; Wei, D.; Liu, H.; Lv, W.; Liu, G. Carbon quantum dots-modified reduced ultrathin g-C3N4 with strong photoredox capacity for broad spectrum-driven PPCPs remediation in natural water matrices. Chem. Eng. J. 2021, 420, 129935. [Google Scholar] [CrossRef]
- Zhang, L.-Y.; Han, Y.-L.; Yang, J.-J.; Deng, S.-L.; Wang, B.-Y. Construction and photocatalysis of carbon quantum dots/layered mesoporous titanium dioxide (CQDs/LM-TiO2) composites. Appl. Surf. Sci. 2021, 546, 149089. [Google Scholar] [CrossRef]




| Preparation | Materials | Reaction Condition | QY | Time | Ref. |
|---|---|---|---|---|---|
| Arc discharge method | Pure graphite electrodes | 17 ± 0.5 V, 29.5 ± 0.6 A, 662 ± 19 W, 40–60 min, below 40 °C | 16% | 2021 | [52] |
| Laser ablation | Carbon cloth | 10 Hz, 1064 nm, 6 ns, 20 mJ, 10 min | 35.4% | 2020 | [14] |
| Graphite powders | 1.064 μm, 5 × 106 W/cm2, 4 h | 12.2% | 2011 | [59] | |
| Carbon powder | 800 nm, 150 fs, 1 kHz | 13.6% | 2015 | [60] | |
| Graphite powders | 800 nm, 150 fs, 1 kHz | / | 2020 | [61] | |
| Non-microporous carbon | 1064 nm, 10 Hz, 20 mJ, 3–6 ns, 30 min | 15.5% | 2019 | [82] | |
| Toluene | 10 Hz, 8 ns, 1064 nm | 18% | 2015 | [83] | |
| ZnS/ZnO | / | 50% | 2008 | [84] | |
| Electrochemical method | Graphite rods | 15–60 V, 120 h | 16.5% | 2012 | [55] |
| Histidine hydrochloride | 1–10 V, 1–120 min | 33.8% | 2019 | [66] | |
| Chemical oxidation | Carbohydrates | H2SO4, HNO3 | 0.13 | 2009 | [16] |
| Ink | 5 °C 1 h, 15 °C 5 h | 78% | 2014 | [69] | |
| NaOH, acetone | 1 h | / | 2015 | [85] | |
| Template method | Soluble phenolic resin | 350–400 °C | more than 10% | 2009 | [19] |
| Citric acid | 300 °C, 2 h | 23% | 2011 | [70] | |
| Microwave method | 1,6-hexane-diamine hydrochloride, dimethyl sulfoxide | 180 °C, 35 min | 24% | 2020 | [18] |
| Formic acid | 90 °C, 3 h | 17% (benzene) | 2014 | [71] | |
| Citric acid, urea | 700 W, 165 s | / | 2020 | [72] | |
| 1,2-ethylenediamine | 700 W, 2 min | 30.2% | 2012 | [86] | |
| Hydrothermal method | Tartaric acid, bran | 150 °C, 8 h | 46% | 2020 | [17] |
| L-Ascorbic acid | 180 °C, 4 h | 6.79% | 2010 | [78] | |
| Chitin/chitosan (CH/CS), graphite | 200 °C, 6 h | 17.1% | 2019 | [79] | |
| Sucrose | 180 °C, 2 h | / | 2022 | [80] | |
| Methionine | 180 °C, 6 h | / | 2017 | [81] | |
| Folic acid | 180 °C, 3 h | 31.59% | 2015 | [87] | |
| Pine fruits | 180 °C, 4.5 h | / | 2020 | [88] | |
| Citric acid, ammonia | 200 °C for 3 h with a heating rate of 10 °C/min | 36% | 2016 | [89] | |
| Grass | 180 °C, 3 h | 4.2% | 2012 | [90] | |
| Milk | 180 °C, 2 h | 12% | 2014 | [91] | |
| Mandelic acid, ethylenediamine | 200 °C, 5 h | 41.4% | 2018 | [92] | |
| wool | 200 °C, 1 h | 16.3% | 2016 | [93] | |
| Polyethylene glycol-2000 | 200 °C, 12 h | 43% | 2017 | [94] |
| Doped Type | Preparation Method | Precursors | Reaction Condition | Date | Ref. |
|---|---|---|---|---|---|
| N-CQDs | Hydrothermal method | Gelatin | 200 °C, 6 h | 2019 | [112] |
| Hydrothermal method | CCl4, NaNH2 | 200 °C, 4 h | 2012 | [113] | |
| Hydrothermal method | Diethylenetriamine, lignin | 180 °C, 8 h | 2022 | [114] | |
| Hydrothermal method | Citric acid, linear-structured polyethyleneimine | 150 °C, 5 h | 2014 | [115] | |
| Microwave method | pear juice, ethanediamine | 400 W, 10 min | 2019 | [116] | |
| P-CQDs | Hydrothermal method | Phosphorous tribromide, hydroquinone | 200 °C | 2014 | [118] |
| Hydrothermal method | Phytic acid, sodium citrate | 240 °C, 4 h | 2018 | [119] | |
| Microwave method | phytic acid | 700 W, 8 min | 2014 | [120] | |
| Hydrothermal method | m-Phenylenediamine, phytic acid | 200 °C, 16 h | 2024 | [121] | |
| B-CQDs | Hydrothermal method | NaTPB/borax/boric acid, citric acid | 140–180 °C | 2020 | [122] |
| Hydrothermal method | BBr3, hydroquinone | 200 °C, 2 h | 2014 | [123] | |
| Co-doped | Hydrothermal method | Citric acid, L-cysteine | 200 °C for 3 h with a heating rate of 10 °C/min | 2013 | [124] |
| Hydrothermal method | Human hair fiber, H2SO4 | 24 h at 40, 100 and 140 °C | 2013 | [125] | |
| Hydrothermal method | Citric acid, urea, H3PO4, dimethyl formamide | 180 °C, 24 h | 2018 | [126] | |
| Hydrothermal method | Boric acid, N-(4-hydroxyphenyl) glycine | 150, 200, 250, 300, 350, and 400 °C for 2.5 h | 2013 | [127] | |
| Hydrothermal method | Pumpkin, H3PO4 | 90 °C, 1 h | 2015 | [128] | |
| Mix-doped | Template method | MgO, FeCl3·6H2O, 1,10-phenanthroline | 800 °C under argon for 2 h with a heating rate of 10 °C/min | 2023 | [130] |
| pyrolysis | Citric acid, zinc acetate/cobalt chloride/bismuth nitrate/cadmium nitrate/titanium sulfate | 180 °C, 40 h | 2019 | [131] |
| Preparation Method | Materials | Application | Time | Ref. |
|---|---|---|---|---|
| Chemical oxidation | γ-butyrolactone | As sensitizers for nanocrystalline TiO2 solar cells | 2012 | [132] |
| Hydrothermal method | TiCl3, NaCl, NCQDs | Cationic energy cells | 2013 | [133] |
| Electrochemical method | 1-butyl-3-methylimidazolium hexafluoro-phosphate, 1-butyl-3-methylimidazolium tetrafluoroborate | Production of dye-sensitized solar cells | 2013 | [134] |
| Hydrothermal method | Boric acid and ethylenediamine | B-CQDs-LED | 2015 | [135] |
| Hydrothermal method | Chitosan | Evaluated the performance of the N-CQDs in DSSCs | 2020 | [136] |
| Hydrothermal method | Citric acid, ethylenediamine | Silicon nanowire solar cells | 2015 | [137] |
| Solvothermal method | Phthalic acid, phthalimide | Synthesis of white light-emitting diodes (WLEDs) | 2019 | [138] |
| Pyrolysis | Papaya waste pulp | Photoelectric detector | 2019 | [139] |
| Hydrothermal method | Degradation product of biomass autohydrolysis | Bioimaging | 2019 | [143] |
| Solvothermal method | CA, BPEI and Gd-DTPA | Bioimaging | 2017 | [145] |
| Solvothermal method | Pulp-free lemon juice | Bioimaging | 2019 | [147] |
| Hydrothermal method | Waste paper | Bioimaging | 2014 | [148] |
| Hydrothermal method | Citric acid and cystamine dihydrochloride | Bioimaging | 2017 | [149] |
| Hydrothermal method | HEPES buffer | Drug delivery | 2016 | [153] |
| Hydrothermal method | Chitosan | As an effective nano-drug carrier | 2020 | [155] |
| Solvothermal method | CA and polyene polyamine (PEPA) | Integrating oxaliplatin with carbon Quantum dots | 2014 | [156] |
| Solvothermal method | Cyanine dye (CyOH) and polyethylene glycol (PEG800) | NIR imaging and PTT | 2016 | [164] |
| Hydrothermal method | Sulfur- and nitrogen-containing organics | PTT and optical imaging | 2018 | [165] |
| Hydrothermal method | Polythiophene and diphenyl diselenide | PTT | 2017 | [166] |
| Solvothermal method | Glutaraldehyde | PDT | 2020 | [168] |
| Hydrothermal method | Fresh ginger juice | Induce apoptosis in HepG2 cells | 2014 | [170] |
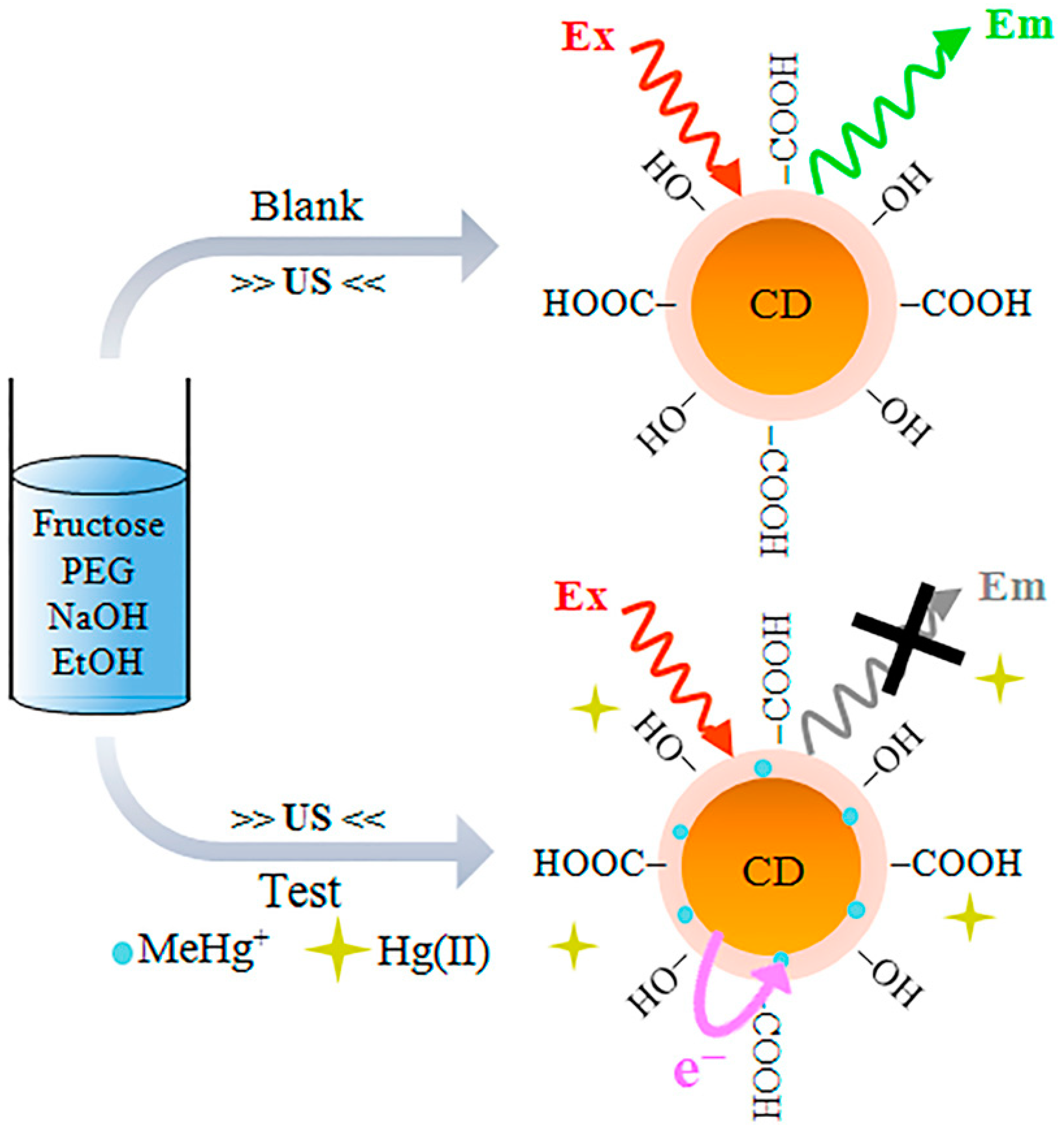
| Ultrasonic oscillation | Fructose | Fluorescent sensors for monitoring CH3Hg+ | 2014 | [172] |
| Solvothermal method | Glucose or zinc gluconate | Fluorescent sensors for the detection of Zn2+ and EDTA | 2018 | [173] |
| Hydrothermal method | Phenylboronic acid | Fluorescent blood sugar sensing | 2014 | [175] |
| Solvothermal method | CA and BPEI | Fluorescent probes for selective and sensitive detection of Cu2+ | 2012 | [176] |
| Microwave method | Glucose and PEG-200 | Fluorescent probes for fluoride detection | 2013 | [177] |
| Pyrolysis at high temperature | EDTA-2Na | Detection of Hg2+ and biothiols in complex matrices | 2012 | [180] |
| Hydrothermal method | Broccoli | Detection of Ag+ | 2018 | [181] |
| Chemical oxidation | Starch | Degradation of rhodamine B and cefradine | 2016 | [184] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kong, J.; Wei, Y.; Zhou, F.; Shi, L.; Zhao, S.; Wan, M.; Zhang, X. Carbon Quantum Dots: Properties, Preparation, and Applications. Molecules 2024, 29, 2002. https://doi.org/10.3390/molecules29092002
Kong J, Wei Y, Zhou F, Shi L, Zhao S, Wan M, Zhang X. Carbon Quantum Dots: Properties, Preparation, and Applications. Molecules. 2024; 29(9):2002. https://doi.org/10.3390/molecules29092002
Chicago/Turabian StyleKong, Jichuan, Yihui Wei, Feng Zhou, Liting Shi, Shuangjie Zhao, Mengyun Wan, and Xiangfeng Zhang. 2024. "Carbon Quantum Dots: Properties, Preparation, and Applications" Molecules 29, no. 9: 2002. https://doi.org/10.3390/molecules29092002
APA StyleKong, J., Wei, Y., Zhou, F., Shi, L., Zhao, S., Wan, M., & Zhang, X. (2024). Carbon Quantum Dots: Properties, Preparation, and Applications. Molecules, 29(9), 2002. https://doi.org/10.3390/molecules29092002






