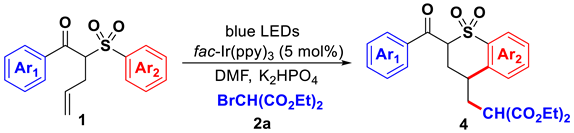
3.4. General Procedure for Gram Scale Reaction of Compound 4a
A 100 mL Schlenk flask was equipped with magnetic stir bar and was charged with compounds 1a (1.256 g, 4.0 mmol, 1.0 equiv.), 2a (3.824g, 8.0 mmol, 2.0 equiv.), K2HPO4 (2.784 g, 8.0 mmol, 2.0 equiv.), dry DMF (40 mL), and fac-Ir(ppy)3 (132 mg, 0.2 mmol). The Schlenk flask was evacuated and backfilled with N2 three times under −78 °C. The mixture was irradiated with blue LEDs for 24 h (monitored using TLC). After the reaction was completed, the reaction mixture was quenched with water (50 mL) and was extracted with EtOAc (50 mL × 4). The organic layer was combined, dried (MgSO4), filtered, and concentrated in vacuo. The resulting residue was purified using column chromatography on silica gel (petroleum ether/EtOAc) to obtain the desired product 4a.
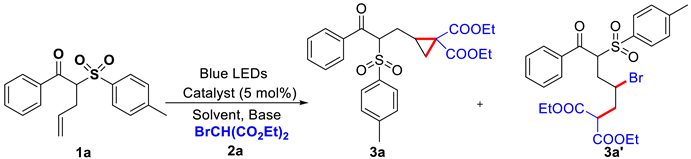
Diethyl 2-(3-oxo-3-phenyl-2-tosylpropyl)cyclopropane-1,1-dicarboxylate (3a): Colorless oil (45.3 mg, 48% yield, dr = 2.0:1). 1H NMR (500 MHz, CDCl3): δH 7.96 (d, J = 7.7 Hz, 2.0 H), 7.59 (t, J = 7.7 Hz, 3.0 H), 7.49–7.44 (m, 2.0 H), 7.28–7.27 (m, 2.0 H), 5.34 (dd, J = 11.5, 3.0 Hz, 0.67 H), 5.19 (dd, J = 8.7, 4.7 Hz, 0.33 H), 4.30–3.97 (m, 4.0 H), 2.41 (s, 3.0 H), 2.39–2.33 (m, 0.67 H), 2.30–2.24 (m, 0.33 H), 2.00–1.94 (m, 1.0 H), 1.87–1.80 (m, 0.33 H), 1.64–1.57 (m, 0.67 H), 1.36 (t, J = 6.7 Hz, 2.0 H), 1.33–1.27 (m, 2.0 H), 1.20 (q, J = 6.7 Hz, 2.0 H), 1.10 (t, J = 6.7 Hz, 2.0 H); 13C NMR (126 MHz, CDCl3): δc 192.5, 192.0, 169.4, 169.4, 167.4, 167.4, 145.5, 145.4, 137.2, 136.7, 134.0, 134.0, 133.4, 133.2, 129.6, 129.5, 129.1, 128.9, 128.7, 69.3, 68.5, 61.9, 61.6, 61.6, 61.5, 34.4, 33.9, 28.1, 27.6, 24.7, 24.4, 21.6, 20.5, 20.1, 14.1, 14.0, 13.9, 13.8; IR: ῡ = 1725, 1678, 1595, 1447, 1147, 748 cm−1; HRMS (ESI-TOF) m/z [M + Na]+ calcd. for C25H28NaO7S+, 495.1448, found 495.1439.
Diethyl 2-(2-bromo-5-oxo-5-phenyl-4-tosylpentyl)malonate (3a′): Colorless oil. 1H NMR (500 MHz, CDCl3): δH 7.97 (d, J = 7.5 Hz, 2.0 H), 7.62–7.57 (m, 3.0 H), 7.46 (t, J = 7.5 Hz, 2.0 H), 7.30–7.27 (m, 2.0 H), 5.56 (dd, J = 11.3, 2.7 Hz, 0.67 H), 5.41 (t, J = 6.0 Hz, 0.33 H), 4.22–4.09 (m, 4.0 H), 3.73–3.67 (m, 1.0 H), 3.63 (dd, J = 10.0, 5.0 Hz, 0.67 H), 2.68–2.63 (m, 1.0 H), 2.56–2.43 (m, 2.0 H), 2.42 (s, 3.0 H), 2.35–2.29 (m, 0.67 H), 2.21–2.15 (m, 0.33 H), 1.27–1.23 (m, 4.33 H), 1.18 (t, J = 7.0 Hz, 2.0 H); 13C NMR (126 MHz, CDCl3): δc 191.9, 191.3, 168.5, 168.5, 168.3, 168.1, 145.7, 145.7, 136.8, 136.3, 134.2, 134.0, 133.2, 132.9, 129.8, 129.7, 129.7, 129.5, 129.3, 129.2, 128.7, 68.8, 68.2, 61.8, 61.7, 51.2, 50.5, 50.2, 50.2, 38.1, 37.7, 37.2, 36.7, 21.7, 14.0, 13.9, 13.9; IR: ῡ = 1728, 1678, 1595, 1447, 1147, 736 cm−1; HRMS (ESI-TOF) m/z [M + Na]+ calcd. for C25H29BrNaO7S+, 575.0710, found 575.0701.
Diethyl 2-(2-((4-(tert-butyl)phenyl)sulfonyl)-3-oxo-3-phenylpropyl)cyclopropane-1,1-dicarboxylate (3b): Colorless oil (52.4 mg, 51% yield, dr = 2.0:1). 1H NMR (500 MHz, CDCl3): δH 7.91 (d, J = 8.0 Hz, 2.0 H), 7.64–7.60 (m, 2.0 H), 7.59–7.55 (m, 1.0 H), 7.46–7.41 (m, 4.0 H), 5.34 (dd, J = 11.5, 3.0 Hz, 0.67 H), 5.18 (dd, J = 9.0, 4.5 Hz, 0.33 H), 4.30–3.97 (m, 4.0 H), 2.46–2.40 (m, 0.67 H), 2.34–2.28 (m, 0.33 H), 2.02–1.94 (m, 1.0 H), 1.88–1.82 (m, 0.33 H), 1.65–1.59 (m, 1.0 H), 1.35 (t, J = 7.3 Hz, 2.0 H), 1.34–1.31(m, 1.67 H), 1.30 (s, 9.0 H), 1.22–1.18 (m, 2.0 H), 1.09 (t, J = 7.3 Hz, 2.0 H); 13C NMR (126 MHz, CDCl3): δc 192.5, 192.0, 169.4, 169.4, 167.5, 167.4, 158.3, 158.2, 137.2, 136.8, 134.0, 134.0, 133.6, 133.3 129.5, 129.4, 129.0, 128.8, 128.7, 125.9, 69.2, 68.4, 61.9, 61.6, 61.5, 35.2, 34.5, 34.0, 31.0, 30.9, 27.8, 27.4, 24.8, 24.5, 20.5, 20.1, 14.1, 14.1, 14.0, 13.9; IR: ῡ = 1723, 1682, 1594, 1447, 1153, 734 cm−1; HRMS (ESI-TOF) m/z [M + Na]+ calcd. for C28H34NaO7S+, 537.1917, found 537.1893.
Diethyl 2-(2-((4-methoxyphenyl)sulfonyl)-3-oxo-3-phenylpropyl)cyclopropane-1,1-dicarboxylate (3c): Colorless oil (51.8 mg, 53% yield, dr = 2.0:1). 1H NMR (500 MHz, CDCl3): δH 7.97 (d, J = 8.0 Hz, 2.0 H), 7.65–7.59 (m, 3.0 H), 7.50–7.45 (m, 2.0 H), 6.94–6.92 (m, 2.0 H), 5.33 (dd, J = 11.5, 3.0 Hz, 0.67 H), 5.19 (dd, J = 9.0, 4.5 Hz, 0.33 H), 4.32–3.98 (m, 4.0 H), 3.85 (s, 3.0 H), 2.35–2.23 (m, 1.0 H), 1.99–1.92 (m, 1.0 H), 1.86–1.79 (m, 0.33 H), 1.63–1.57 (m, 1.0 H), 1.36 (t, J = 7.3 Hz, 2.0 H), 1.33–1.24 (m, 1.67 H), 1.20 (q, J = 7.3 Hz, 2.0 H), 1.10 (t, J = 7.3 Hz, 2.0 H); 13C NMR (126 MHz, CDCl3): δc 192.7, 192.2, 169.4, 169.4, 167.5, 167.4, 164.2, 164.2, 137.2, 136.7, 134.1, 134.1, 131.9, 131.9, 129.2, 129.0, 128.7, 127.6, 127.4, 114.1, 69.3, 68.5, 62.0, 61.6, 61.6, 55.7, 34.4, 33.9, 28.2, 27.7, 24.7, 24.4, 20.5, 20.1, 14.1, 14.0, 14.0, 13.9; IR: ῡ = 1722, 1680, 1594, 1447, 1145, 736 cm−1; HRMS (ESI-TOF) m/z [M + Na]+ calcd. for C25H28NaO8S+, 511.1397, found 511.1398.
Diethyl 2-(2-((4-fluorophenyl)sulfonyl)-3-oxo-3-phenylpropyl)cyclopropane-1,1-dicarboxylate (3d): Colorless oil (20.1 mg, 21% yield, dr = 2.0:1). 1H NMR (500 MHz, CDCl3): δH 7.96 (d, J = 8.0 Hz, 2.0 H), 7.76–7.71 (m, 2.0 H), 7.64–7.60 (m, 1.0 H), 7.50–7.46 (m, 2.0 H), 7.18–7.14 (m, 2.0 H), 5.38 (dd, J = 11.5, 3.0 Hz, 0.67 H), 5.22 (dd, J = 8.7, 4.7 Hz, 0.33 H), 4.33–3.98 (m, 4.0 H), 2.41–2.32 (m, 0.67 H), 2.27–2.20 (m, 0.33 H), 2.04–1.94 (m, 1.0 H), 1.87–1.81 (m, 0.33 H), 1.65–1.60 (m, 0.67 H), 1.37 (t, J = 7.0 Hz, 2.0 H), 1.33–1.25 (m, 2.0 H), 1.21 (m, 2.0 H), 1.10 (t, J = 7.0 Hz, 2.0 H); 13C NMR (126 MHz, CDCl3): δc 192.5, 192.0, 169.4, 169.3, 167.5, 167.2, 166.2 (d, J = 258.2 Hz), 137.0, 136.5, 134.3, 134.3, 132.7, 132.7, 132.6, 132.6, 132.3 (d, J = 3.2 Hz), 132.1 (d, J = 3.2 Hz), 129.1, 128.9, 128.9, 116.2 (d, J = 22.9 Hz), 69.3, 68.4, 62.1, 61.7, 61.6, 34.5, 34.0, 28.2, 27.7, 24.7, 24.3, 20.5, 20.2, 14.1, 14.1, 14.0, 13.9; 19F NMR (470 MHz, CDCl3): δF −102.1 (s) ppm; IR: ῡ = 1722, 1680, 1594, 1447, 1145, 736 cm−1; HRMS (ESI-TOF) m/z [M + Na]+ calcd. for C24H25FNaO7S+, 499.1197, found 499.1189.
Diethyl 2-(2-((3-methoxyphenyl)sulfonyl)-3-oxo-3-phenylpropyl)cyclopropane-1,1-dicarboxylate (3e): Colorless oil (35.1 mg, 36% yield, dr = 2.0:1). 1H NMR (500 MHz, CDCl3): δH 7.93 (d, J = 8.0 Hz, 2.0 H), 7.61–7.57 (m, 1.0 H), 7.48–7.43 (m, 2.0 H), 7.39–7.36 (m, 1.0 H), 7.33–7.31 (m, 1.0 H), 7.18 (d, J = 8.0 Hz, 1.0 H), 7.11 (d, J = 8.0 Hz, 1.0 H), 5.36 (dd, J = 11.3, 2.7 Hz, 0.67 H), 5.20 (dd, J = 9.0, 5.0 Hz, 0.33 H), 4.30–3.97 (m, 4.0 H), 3.80 (s, 2.0 H), 3.79 (s, 1.0 H), 2.47–2.41 (m, 0.67 H), 2.35–2.30 (m, 0.33 H), 2.04–1.95 (m, 1.0 H), 1.87–1.81 (m, 0.33 H), 1.63–1.58 (m, 0.67 H), 1.37–1.25 (m, 5.0 H), 1.22–1.19 (m, 2.0 H), 1.09 (t, J = 7.0 Hz, 2.0 H); 13C NMR (126 MHz, CDCl3): δc 192.2, 191.7, 169.4, 169.4, 167.5, 167.4, 159.7, 137.6, 137.5, 137.1, 136.7, 134.1, 134.1, 130.0, 129.9, 129.0, 128.9, 128.7, 121.7, 121.7, 121.0, 120.8, 113.9, 113.9, 69.3, 68.4, 62.0, 61.7, 61.6, 61.6, 55.7, 55.6, 34.5, 33.9, 27.9, 27.5, 24.8, 24.4, 20.5, 20.2, 14.1, 14.0, 14.0, 13.9; IR: ῡ = 1722, 1680, 1594, 1447, 1145, 736 cm−1; HRMS (ESI-TOF) m/z [M + Na]+ calcd. for C24H25FNaO7S+, 499.1197, found 499.1193.
Diethyl 2-(2-((2-methoxyphenyl)sulfonyl)-3-oxo-3-phenylpropyl)cyclopropane-1,1-dicarboxylate (3f): Colorless oil (27.4 mg, 28% yield, dr = 2.0:1). 1H NMR (500 MHz, CDCl3): δH 7.93 (d, J = 8.0 Hz, 2.0 H), 7.80 (dd, J = 8.0, 1.3 Hz, 1.0 H), 7.57–7.53 (m, 1.0 H), 7.52–7.47 (m, 1.0 H), 7.42 (q, J = 8.0 Hz, 2.0 H), 7.04–6.99 (m, 1.0 H), 6.92 (d, J = 8.0 Hz, 0.33 H), 6.86 (d, J = 8.0 Hz, 0.67 H), 5.77 (dd, J = 11.5, 3.0 Hz, 0.67 H), 5.64 (dd, J = 8.0, 5.5 Hz, 0.33 H), 4.19–3.97 (m, 4.0 H), 3.93 (s, 1.0 H), 3.91 (s, 2.0 H), 2.71–2.65 (m, 0.67 H), 2.48–2.42 (m, 0.33 H), 2.04–1.99 (m, 0.33 H), 1.93–1.81 (m, 1.0 H), 1.71–1.64 (m, 0.67 H), 1.38–1.25 (m, 5.0 H), 1.20 (t, J = 7.3 Hz, 1.0 H), 1.16 (t, J = 7.3 Hz, 1.0 H), 1.09 (t, J = 7.3 Hz, 2.0 H); 13C NMR (126 MHz, CDCl3): δc 192.3, 191.6, 169.5, 169.5, 167.4, 167.4, 157.5, 157.3, 137.6, 137.1, 136.1, 136.0, 133.7, 133.7, 131.6, 131.3, 128.9, 128.7, 128.5, 126.1, 125.7, 120.8, 120.6, 112.3, 112.1, 67.6, 66.3, 61.7, 61.6, 61.6, 61.5, 56.2, 56.1, 34.6, 33.9, 27.2, 27.0, 25.1, 24.7, 20.7, 20.3, 14.0, 14.0, 14.0, 13.9; IR: ῡ = 1722, 1680, 1594, 1447, 1145, 736 cm−1; HRMS (ESI-TOF) m/z [M + Na]+ calcd. for C24H25FNaO7S+, 499.1197, found 499.1192.
Diethyl 2-(3-oxo-3-phenyl-2-(phenylsulfonyl)propyl)cyclopropane-1,1-dicarboxylate (3g): Colorless oil (24.8 mg, 27% yield, dr = 2.0:1). 1H NMR (500 MHz, CDCl3): δH 7.94 (d, J = 7.5 Hz, 2.0 H), 7.72 (t, J = 7.5 Hz, 2.0 H), 7.62–7.57 (m, 2.0 H), 7.50–7.43 (m, 4.0 H), 5.37 (dd, J = 11.5, 3.0 Hz, 0.67 H), 5.21 (dd, J = 9.0, 5.0 Hz, 0.33 H), 4.29–3.98 (m, 4.0 H), 2.42–2.36 (m, 0.67 H), 2.34–2.25 (m, 0.33 H), 2.02–1.94 (m, 1.0 H), 1.86–1.80 (m, 0.33 H), 1.63–1.57 (m, 0.67 H), 1.37–1.29 (m, 4.0 H), 1.20 (q, J = 7.3 Hz, 2.0 H), 1.09 (t, J = 7.3 Hz, 2.0 H); 13C NMR (126 MHz, CDCl3): δc 192.3, 191.9, 169.4, 169.3, 167.4, 167.4, 137.1, 136.7, 136.4, 136.3, 134.3, 134.2, 134.1, 134.1, 129.6, 129.6, 129.1, 128.9, 128.7, 69.3, 68.4, 61.9, 61.6, 61.6, 61.5, 34.4, 33.9, 28.0, 27.6, 24.7, 24.4, 20.5, 20.1, 14.1, 14.0, 13.9, 13.8; IR: ῡ = 1720, 1681, 1447, 1137, 911, 731 cm−1; HRMS (ESI-TOF) m/z [M + Na]+ calcd. for C24H26NaO7S+, 481.1291, found 481.1272.
Diethyl 2-(3-(4-fluorophenyl)-2-((4-methoxyphenyl)sulfonyl)-3-oxopropyl)cyclopropane-1,1-dicarboxylate (3h): Colorless oil (48.6 mg, 48% yield, dr = 2.0:1). 1H NMR (500 MHz, CDCl3): δH 8.02–8.00 (m, 2.0 H), 7.61 (t, J = 8.5 Hz, 2.0 H), 7.16–7.12 (m, 2.0 H), 6.94–6.92 (m, 2.0 H), 5.27 (dd, J = 11.5, 2.7 Hz, 0.67 H), 5.13 (dd, J = 9.0, 4.5 Hz, 0.33 H), 4.30–3.98 (m, 4.0 H), 3.85 (s, 3.0 H), 2.36–2.30 (m, 0.67 H), 2.24–2.18 (m, 0.33 H), 1.98–1.89 (m, 1.0 H), 1.84–1.78 (m, 0.33 H), 1.61–1.54 (m, 0.67 H), 1.35 (t, J = 7.3 Hz, 2.0 H), 1.32–1.24 (m, 2.0 H), 1.22–1.18 (m, 2.0 H), 1.10 (t, J = 7.3 Hz, 2.0 H); 13C NMR (126 MHz, CDCl3): δc 191.0, 190.6, 169.4, 169.3, 167.4, 166.3 (d, J = 257.5 Hz), 164.3, 164.2, 133.6 (d, J = 2.8 Hz), 133.2 (d, J = 2.8 Hz), 132.0, 132.0, 131.8, 131.8, 131.8, 131.8, 127.5, 127.3, 115.9 (d, J = 22.2 Hz), 115.9 (d, J = 22.2 Hz), 114.1, 114.1, 69.4, 68.5, 62.0, 61.6, 61.6, 61.6, 55.6, 34.4, 33.9, 28.1, 27.7, 24.7, 24.3, 20.5, 20.1, 14.1, 14.0, 13.9, 13.8; 19F NMR (470 MHz, CDCl3): δF −103.0 (s) ppm; IR: ῡ = 1720, 1675, 1590, 1492, 1148, 733 cm−1; HRMS (ESI-TOF) m/z [M + Na]+ calcd. for C25H27FNaO7S+, 529.1303, found 529.1297.
Diethyl 2-(3-(4-bromophenyl)-2-((4-methoxyphenyl)sulfonyl)-3-oxopropyl)cyclopropane-1,1-dicarboxylate (3i): Colorless oil (57.8 mg, 51% yield, dr = 2.0:1). 1H NMR (500 MHz, CDCl3): δH 7.83 (d, J = 8.5 Hz, 2.0 H), 7.63–7.59 (m, 4.0 H), 6.95–6.92 (m, 2.0 H), 5.26 (dd, J = 11.5, 3.0 Hz, 0.67 H), 5.12 (dd, J = 8.7, 4.7 Hz, 0.33 H), 4.31–3.99 (m, 4.0 H), 3.86 (s, 3.0 H), 2.39–2.31 (m, 0.67 H), 2.23–2.17 (m, 0.33 H), 2.01–1.89 (m, 1.0 H), 1.85–1.79 (m, 0.33 H), 1.59–1.54 (m, 0.67 H), 1.36 (t, J = 7.0 Hz, 2.0 H), 1.34–1.28 (m, 2.0 H), 1.23–1.19 (m, 2.0 H), 1.12 (t, J = 7.0 Hz, 2.0 H); 13C NMR (126 MHz, CDCl3): δc 191.8, 191.3, 169.4, 169.4, 167.4, 164.4, 164.3, 135.9, 135.5, 132.1, 132.1, 131.8, 130.6, 130.4, 129.6, 127.5, 127.3, 114.2, 69.6, 68.6, 62.0, 61.7, 61.7, 61.6, 55.7, 34.4, 33.9, 28.1, 27.7, 24.7, 24.3, 20.5, 20.1, 14.1, 14.0, 14.0, 13.9; IR: ῡ = 1720, 1680, 1593, 1496, 1136, 731 cm−1; HRMS (ESI-TOF) m/z [M + Na]+ calcd. for C25H27BrNaO8S+, 589.0502, found 589.0512.
Diethyl 2-(2-((4-methoxyphenyl)sulfonyl)-3-oxo-3-(p-tolyl)propyl)cyclopropane-1,1-dicarboxylate (3j): Colorless oil (63.3 mg, 63% yield, dr = 2.0:1). 1H NMR (500 MHz, CDCl3): δH 7.87 (d, J = 8.7 Hz, 2.0 H), 7.62 (t, J = 8.7 Hz, 2.0 H), 7.28–7.25 (m, 2.0 H), 6.94–6.91 (m, 2.0 H), 5.29 (dd, J = 11.5, 3.0 Hz, 0.67 H), 5.15 (dd, J = 9.0, 4.5 Hz, 0.33 H), 4.32–3.98 (m, 4.0 H), 3.85 (s, 3.0 H), 2.42 (s, 1.0 H), 2.41 (s, 2.0 H), 2.32–2.22 (m, 1.0 H), 1.98–1.89 (m, 1.0 H), 1.85–1.78 (m, 0.33 H), 1.62–1.57 (m, 0.67 H), 1.36 (t, J = 7.3 Hz, 2.0 H), 1.34–1.24 (m, 2.0 H), 1.23–1.18 (m, 2.0 H), 1.10 (t, J = 7.3 Hz, 2.0 H); 13C NMR (126 MHz, CDCl3): δc 192.0, 191.6, 169.4, 169.4, 167.5, 167.4, 164.2, 164.1, 145.3, 145.2, 134.8, 134.3, 131.9, 129.4, 129.3, 129.1, 127.7, 127.5, 114.0, 114.0, 69.2, 68.4, 62.0, 61.6, 61.6, 61.5, 55.6, 34.4, 33.9, 28.2, 27.7, 24.7, 24.5, 21.7, 21.7, 20.5, 20.1, 14.1, 14.1, 14.0, 13.8; IR: ῡ = 1720, 1675, 1590, 1492, 1148, 733 cm−1; HRMS (ESI-TOF) m/z [M + Na]+ calcd. for C26H30NaO8S+, 525.1554, found 525.1542.
Diethyl 2-(3-(4-methoxyphenyl)-2-((4-methoxyphenyl)sulfonyl)-3-oxopropyl)cyclopropane-1,1-dicarboxylate (3k): Colorless oil (87.1 mg, 84% yield, dr = 2.0:1). 1H NMR (500 MHz, CDCl3): δH 7.96 (d, J = 9.3 Hz, 2.0 H), 7.62 (t, J = 9.3 Hz, 2.0 H), 6.94–6.91 (m, 4.0 H), 5.25 (dd, J = 11.3, 2.7 Hz, 0.67 H), 5.11 (dd, J = 9.3, 4.7 Hz, 0.33 H), 4.30–3.97 (m, 4.0 H), 3.88 (s, 1.0 H), 3.87 (s, 2.0 H), 3.84 (s, 3.0 H), 2.33–2.22 (m, 1.0 H), 1.95–1.86 (m, 1.0 H), 1.84–1.78 (m, 0.33 H), 1.63–1.57 (m, 0.67 H), 1.35 (t, J = 7.3 Hz, 2.0 H), 1.34–1.24 (m, 2.0 H), 1.23–1.18 (m, 2.0 H), 1.10 (t, J = 7.3 Hz, 2.0 H); 13C NMR (126 MHz, CDCl3): δc 190.5, 190.1, 169.4, 169.4, 167.4, 167.4, 164.3, 164.1, 164.1, 131.8, 131.7, 131.5, 130.2, 129.8, 127.7, 114.0, 114.0, 113.9, 69.0, 68.2, 63.1, 62.0, 61.9, 61.6, 61.5, 56.4, 55.6, 55.6, 55.6, 34.4, 33.9, 28.1, 27.7, 24.7, 24.5, 20.5, 20.1, 14.1, 14.0, 13.9, 13.9; IR: ῡ = 1720, 1679, 1583, 1318, 1135, 804 cm−1; HRMS (ESI-TOF) m/z [M + Na]+ calcd. for C26H30NaO9S+, 541.1503, found 541.1496.
Diethyl 2-(3-(3-methoxyphenyl)-2-((4-methoxyphenyl)sulfonyl)-3-oxopropyl)cyclopropane-1,1-dicarboxylate (3l): Colorless oil (45.7 mg, 44% yield, dr = 2.0:1). 1H NMR (500 MHz, CDCl3): δH 7.63 (t, J = 8.0 Hz, 2.0 H), 7.54 (d, J = 8.0 Hz, 1.0 H), 7.44 (s, 1.0 H), 7.39–7.34 (m, 1.0 H), 7.15–7.12 (m, 1.0 H), 6.94–6.91 (m, 2.0 H), 5.31 (dd, J = 11.5, 3.0 Hz, 0.67 H), 5.15 (dd, J = 9.3, 4.7 Hz, 0.33 H), 4.31–3.98 (m, 4.0 H), 3.85 (s, 6.0 H), 2.37–2.23 (m, 1.0 H), 1.97–1.92 (m, 1.0 H), 1.85–1.78 (m, 0.33 H), 1.63–1.57 (m, 0.67 H), 1.36 (t, J = 7.3 Hz, 3.0 H), 1.32–1.24 (m, 2.0 H), 1.23–1.18 (m, 2.0 H), 1.11 (t, J = 7.3 Hz, 3.0 H); 13C NMR (126 MHz, CDCl3): δc 192.6, 192.1, 169.4, 169.4, 167.4, 167.4, 164.2, 164.2, 159.8, 159.8, 138.5, 138.1, 131.9, 129.7, 129.7, 127.7, 127.6, 121.9, 121.7, 120.8, 120.7, 114.1, 114.1, 113.0, 112.7, 69.5, 68.6, 62.0, 61.6, 61.6, 61.5, 55.7, 55.6, 55.5, 55.4, 34.4, 33.9, 28.3, 27.8, 24.7, 24.4, 20.5, 20.1, 14.1, 14.0, 13.9, 13.8; IR: ῡ = 1720, 1679, 1583, 1318, 1135, 804 cm−1; HRMS (ESI-TOF) m/z [M + Na]+ calcd. for C26H30NaO9S+, 541.1503, found 541.1495.
Diethyl 2-(3-(2-methoxyphenyl)-2-((4-methoxyphenyl)sulfonyl)-3-oxopropyl)cyclopropane-1,1-dicarboxylate (3m): Colorless oil (44.5 mg, 43% yield, dr = 2.0:1). 1H NMR (500 MHz, CDCl3): δH 7.60–7.55 (m, 3.0 H), 7.47–7.42 (m, 1.0 H), 6.97 (t, J = 7.5 Hz, 1.0 H), 6.89–6.85 (m, 3.0 H), 5.82 (dd, J = 11.5, 3.0 Hz, 0.67 H), 5.67 (dd, J = 9.7, 3.7 Hz, 0.33 H), 4.34–4.07 (m, 4.0 H), 3.89 (s, 1.0 H), 3.88 (s, 2.0 H), 3.83 (s, 3.0 H), 2.47–2.41 (m, 0.33 H), 2.34–2.28 (m, 1.0 H), 1.98–1.87 (m, 1.0 H), 1.84–1.78 (m, 1.0 H), 1.42–1.37 (m, 1.67 H), 1.34 (t, J = 7.3 Hz, 3.0 H), 1.19 (t, J = 7.3 Hz, 3.0 H); 13C NMR (126 MHz, CDCl3): δc 194.0, 193.6, 169.9, 169.7, 167.5, 167.4, 163.9, 163.8, 158.5, 158.4, 134.7, 134.6, 131.5, 131.5, 131.3, 131.2, 128.5, 128.4, 127.9, 127.7, 120.9, 120.8, 113.8, 111.6, 111.6, 73.0, 72.5, 61.8, 61.5, 61.5, 61.4, 55.6, 55.4, 34.5, 34.2, 27.2, 27.0, 25.1, 25.0, 20.9, 20.5, 14.1, 14.1, 14.0, 14.0; IR: ῡ = 1720, 1679, 1583, 1318, 1135, 804 cm−1; HRMS (ESI-TOF) m/z [M + Na]+ calcd. for C26H30NaO9S+, 541.1503, found 541.1501.
Diethyl 2-((2-benzoyl-6-methyl-1,1-dioxidothiochroman-4-yl)methyl)malonate (4a): Colorless oil (63.4 mg, 67% yield, dr = 2.0:1; 4.5 mmol scale, 1.413 g, 64% yield). 1H NMR (500 MHz, CDCl3): δH 8.14 (d, J = 7.5 Hz, 1.34 H), 8.07 (d, J = 7.5 Hz, 0.66 H), 7.81 (d, J = 8.5 Hz, 0.33 H), 7.76 (d, J = 8.5 Hz, 0.67 H), 7.65–7.59 (m, 1.0 H), 7.54–7.48 (m, 2.0 H), 7.33 (s, 0.33 H), 7.26–7.23 (m, 1.0 H), 7.18 (s, 0.67 H), 5.41 (dd, J = 10.5, 3.5 Hz, 0.67 H), 5.19 (dd, J = 11.0, 4.5 Hz, 0.33 H), 4.30–4.10 (m, 4.0 H), 3.61 (dd, J = 11.0, 5.0 Hz, 0.33 H), 3.46 (t, J = 7.3 Hz, 0.67 H), 3.25–3.20 (m, 1.0 H), 3.07–3.00 (m, 0.67 H), 2.79–2.70 (m, 0.67 H), 2.59–2.44 (m, 1.33 H), 2.41 (s, 3.0 H), 2.39–2.23 (m, 1.67 H), 1.31–1.23 (m, 6.0 H); 13C NMR (126 MHz, CDCl3): δc 189.9, 189.8, 169.0, 168.9, 168.6, 144.1, 143.6, 139.7, 139.7, 136.4, 136.3, 135.1, 135.0, 134.3, 134.3, 129.7, 129.6, 129.5, 129.0, 128.7, 128.7, 128.4, 128.3, 124.6, 124.2, 63.6, 61.9, 61.8, 61.4, 49.9, 49.2, 34.7, 34.5, 34.5, 33.7, 30.3, 28.7, 21.7, 21.5, 14.1, 14.0, 14.0, 14.0; IR: ῡ = 1726, 1682, 1596, 1447, 1137, 735 cm−1; HRMS (ESI-TOF) m/z [M + Na]+ calcd. for C24H26NaO7S+, 495.1448, found 495.1436.
Diethyl 2-((2-benzoyl-6-(tert-butyl)-1,1-dioxidothiochroman-4-yl)methyl)malonate (4b): Colorless oil (59.6 mg, 61% yield, dr = 2.0:1). 1H NMR (500 MHz, CDCl3): δH 8.16 (d, J = 7.3 Hz, 1.34 H), 8.08 (d, J = 7.3 Hz, 0.66 H), 7.86 (d, J = 7.5 Hz, 0.33 H), 7.80 (d, J = 7.5 Hz, 0.67 H), 7.65–7.60 (m, 1.0 H), 7.52 (t, J = 7.7 Hz, 2.0 H), 7.49–7.46 (m, 1.33 H), 7.34 (d, J = 2.5 Hz, 0.67 H), 5.44 (dd, J = 11.3, 4.0 Hz, 0.67 H), 5.19 (dd, J = 11.3, 4.0 Hz, 0.33 H), 4.29–4.12 (m, 4.0 H), 3.59 (dd, J = 9.3, 5.3 Hz, 0.33 H), 3.44 (t, J = 7.3 Hz, 0.67 H), 3.27–3.21 (m, 1.0 H), 3.10–3.04 (m, 0.67 H), 2.83–2.68 (m, 0.67 H), 2.60–2.54 (m, 0.33 H), 2.47–2.42 (m, 0.67 H), 2.40–2.33 (m, 1.67 H), 1.34 (s, 9.0 H), 1.29–1.24 (m, 6.0 H); 13C NMR (126 MHz, CDCl3): δc 189.8, 189.7, 169.0, 169.0, 168.9, 168.6, 157.0, 156.6, 139.3, 139.1, 136.4, 135.3, 135.2, 134.3, 134.2, 129.7, 129.5, 128.7, 128.7, 126.1, 125.6, 124.9, 124.8, 124.4, 124.0, 63.8, 61.9, 61.8, 61.8, 61.6, 50.0, 49.2, 35.3, 35.1, 34.9, 34.8, 34.3, 31.0, 30.9, 30.5, 29.1, 14.0, 14.0, 14.0; IR: ῡ = 1725, 1596, 1448, 1304, 1143, 732 cm−1; HRMS (ESI-TOF) m/z [M + Na]+ calcd. for C28H34NaO7S+, 537.1917, found 537.1929.
Diethyl 2-((2-benzoyl-6-methoxy-1,1-dioxidothiochroman-4-yl)methyl)malonate (4c): Colorless oil (59.6 mg, 61% yield, dr = 2.0:1). 1H NMR (500 MHz, CDCl3): δH 8.14 (d, J = 7.7 Hz, 1.34 H), 8.06 (d, J = 7.7 Hz, 0.66 H), 7.84 (d, J = 9.0 Hz, 0.33 H), 7.80 (d, J = 9.0 Hz, 0.67 H), 7.65–7.60 (m, 1.0 H), 7.53–7.48 (m, 2.0 H), 7.00 (d, J = 2.5 Hz, 0.33 H), 6.93 (dt, J = 7.7, 2.3 Hz, 1.0 H), 6.86 (d, J = 2.5 Hz, 0.67 H), 5.40 (dd, J = 10.3, 4.5 Hz, 0.67 H), 5.21 (dd, J = 10.3, 4.5 Hz, 0.33 H), 4.27–4.13 (m, 4.0 H), 3.88 (s, 1.0 H), 3.86 (s, 2.0 H), 3.60 (dd, J = 9.5, 5.5 Hz, 0.33 H), 3.47 (t, J = 7.3 Hz, 0.67 H), 3.28–3.21 (m, 1.0 H), 3.07–3.01 (m, 0.67 H), 2.74–2.67 (m, 0.67 H), 2.58–2.45 (m, 1.0 H), 2.38–2.28 (m, 1.67 H), 1.29–1.23 (m, 6.0 H); 13C NMR (126 MHz, CDCl3): δc 190.1, 189.9, 169.0, 168.9, 168.9, 168.6, 163.3, 162.7, 142.3, 142.1, 136.4, 136.4, 134.3, 134.2, 129.7, 129.6, 129.5, 128.7, 127.0, 126.4, 114.1, 113.9, 112.9, 112.9, 63.8, 61.9, 61.8, 61.6, 55.6, 55.6, 49.9, 49.2, 34.7, 34.4, 34.1, 33.9, 30.7, 29.2, 14.0, 14.0, 14.0; IR: ῡ = 1684, 1595, 1448, 1294, 1134, 742 cm−1; HRMS (ESI-TOF) m/z [M + Na]+ calcd. for C25H28NaO8S+, 511.1397, found 511.1389.
Diethyl 2-((2-benzoyl-6-bromo-1,1-dioxidothiochroman-4-yl)methyl)malonate (4d): Colorless oil (35.4 mg, 33% yield, dr = 2.0:1). 1H NMR (500 MHz, CDCl3): δH 8.12 (d, J = 8.5 Hz, 1.34 H), 8.05 (d, J = 8.5 Hz, 0.66 H), 7.78 (d, J = 8.5 Hz, 0.33 H), 7.74 (d, J = 8.5 Hz, 0.67 H), 7.71 (s, 0.33 H), 7.67–7.62 (m, 1.0 H), 7.60–7.56 (m, 1.67 H), 7.54–7.49 (m, 2.0 H), 5.41 (dd, J = 10.7, 4.0 Hz, 0.67 H), 5.21 (dd, J = 10.7, 4.0 Hz, 0.33 H), 4.29–4.13 (m, 4.0 H), 3.59 (dd, J = 9.7, 5.3 Hz, 0.33 H), 3.45 (t, J = 7.3 Hz, 0.67 H), 3.29–3.23 (m, 1.0 H), 3.59 (dd, J = 9.7, 5.3 Hz, 0.33 H), 3.45 (t, J = 7.3 Hz, 0.67 H), 3.29–3.23 (m, 1.0 H), 3.05–2.99 (m, 0.67 H), 2.75–2.68 (m, 0.67 H), 2.61–2.56 (m, 0.33 H), 2.50–2.44 (m, 0.67 H), 2.41–2.24 (m, 1.67 H), 1.31–1.23 (m, 6.0 H); 13C NMR (126 MHz, CDCl3): δc 189.5, 189.5, 168.8, 168.7, 168.5, 141.9, 141.8, 136.7, 136.1, 136.1, 134.5, 134.5, 132.2, 131.5, 131.0, 130.9, 129.6, 129.5, 128.8, 128.4, 127.7, 126.3, 125.9, 63.5, 62.0, 62.0, 61.3, 49.7, 49.0, 34.4, 34.3, 33.7, 30.3, 28.7, 14.1, 14.0, 14.0,14.0; IR: ῡ = 1723, 1682, 1581, 1447, 1148, 742 cm−1; HRMS (ESI-TOF) m/z [M + Na]+ calcd. for C24H25BrNaO7S+, 559.0397, found 559.0378.
Diethyl 2-((2-benzoyl-1,1-dioxidothiochroman-4-yl)methyl)malonate (4e): Colorless oil (36.7 mg, 40% yield, dr = 2.0:1). 1H NMR (500 MHz, CDCl3): δH 8.15 (d, J = 7.5 Hz, 1.34 H), 8.07 (d, J = 7.5 Hz, 0.66 H), 7.93 (d, J = 8.0 Hz, 0.33 H), 7.88 (d, J = 8.0 Hz, 0.67 H), 7.66–7.59 (m, 1.33 H), 7.57–7.48 (m, 3.0 H), 7.47–7.43 (m, 1.0 H), 7.40 (d, J = 8.0 Hz, 0.67 H), 5.44 (dd, J = 11.0, 4.0 Hz, 0.67 H), 5.21 (dd, J = 11.0, 4.0 Hz, 0.33 H), 4.28–4.12 (m, 4.0 H), 3.60 (dd, J = 9.7, 5.3 Hz, 0.33 H), 3.47 (t, J = 7.3 Hz, 0.67 H), 3.30–3.25 (m, 1.0 H), 3.09–3.03 (m, 0.67 H), 2.80–2.72 (m, 0.67 H), 2.61–2.56 (m, 0.33 H), 2.49–2.26 (m, 2.33 H), 1.30–1.22 (m, 6.0 H); 13C NMR (126 MHz, CDCl3): δc 189.7, 189.7, 168.9, 168.9, 168.9, 168.6, 139.8, 139.7, 138.0, 138.0, 136.3, 134.4, 134.3, 133.3, 132.9, 129.7, 129.5, 129.5, 129.4, 128.8, 128.2, 128.0, 127.6, 124.6, 124.2, 63.6, 61.9, 61.8, 61.4, 49.9, 49.2, 34.7, 34.6, 34.4, 33.9, 30.3, 28.7, 14.0, 14.0, 14.0; IR: ῡ = 1724, 1683, 1595, 1448, 1294, 741 cm−1; HRMS (ESI-TOF) m/z [M + Na]+ calcd. for C24H26NaO7S+, 481.1291, found 481.1277.
Diethyl 2-((2-benzoyl-8-methyl-1,1-dioxidothiochroman-4-yl)methyl)malonate (4f): Colorless oil (32.2 mg, 34% yield, dr = 4.0:1). 1H NMR (500 MHz, CDCl3): δH 8.21 (d, J = 8.0 Hz, 1.6 H), 8.12 (d, J = 8.0 Hz, 0.4 H), 7.67–7.62 (m, 1.0 H), 7.57–7.50 (m, 2.2 H), 7.37 (t, J = 8.0 Hz, 1.0 H), 7.21–7.17 (m, 1.8 H), 5.53 (dd, J = 12.7, 2.7 Hz, 0.8 H), 5.17 (dd, J = 12.7, 2.7 Hz, 0.2 H), 4.28–4.11 (m, 4.0 H), 3.58–3.55 (m, 0.2 H), 3.43 (t, J = 7.5 Hz, 0.8 H), 3.21–3.16 (m, 1.0 H), 3.10–3.03 (m, 1.0 H), 2.70 (s, 0.6 H), 2.68 (s, 2.4 H), 2.41 (t, J = 7.5 Hz, 1.6 H), 2.30–2.26 (m, 1.0 H), 2.24–2.18 (m, 0.4 H), 1.31–1.23 (m, 6.0 H); 13C NMR (126 MHz, CDCl3): δc 189.4, 169.0, 169.0, 168.6, 139.6, 137.5, 137.0, 136.5, 134.4, 132.1, 132.0, 131.9, 129.9, 129.8, 129.7, 128.7, 128.7, 127.6, 65.0, 62.8, 62.0, 61.9, 61.9, 61.8, 50.1, 49.9, 35.8, 35.4, 34.5, 34.1, 27.5, 20.2, 14.1, 14.0, 14.0; HRMS (ESI-TOF) m/z [M + Na]+ calcd. for C25H28NaO7S+, 495.1448, found 495.1427.
Diethyl 2-((2-benzoyl-8-methyl-1,1-dioxidothiochroman-4-yl)methyl)malonate (4g): Colorless oil (40.7 mg, 43% yield, dr = 2.0:1). 1H NMR (500 MHz, CDCl3): δH 8.20 (d, J = 7.7 Hz, 1.34 H), 8.15 (d, J = 7.7 Hz, 0.66 H), 7.75 (d, J = 7.7 Hz, 0.66 H), 7.68–7.62 (m, 1.34 H), 7.56–7.49 (m, 2.0 H), 7.42–7.39 (m, 0.67 H), 7.34 (d, J = 7.7 Hz, 1.0 H), 7.28 (d, J = 7.7 Hz, 0.33 H), 5.55 (dd, J = 12.3, 3.3 Hz, 0.67 H), 5.42 (dd, J = 10.7, 3.3 Hz, 0.33 H), 4.26–4.09 (m, 4.0 H), 3.47–3.44 (m, 1.0 H), 3.37–3.34 (m, 0.67 H), 3.24–3.20 (m, 0.33 H), 3.08–2.97 (m, 1.0 H), 2.78–2.71 (m, 0.33 H), 2.44 (s, 2.0 H), 2.41–2.31 (m, 3.67 H), 1.30–1.20 (m, 6.0 H); 13C NMR (126 MHz, CDCl3): δc 189.8, 189.5, 169.0, 168.9, 168.7, 168.5, 138.8, 138.3, 136.5, 136.5, 135.0, 134.4, 133.8, 129.7, 129.7, 129.6, 129.3, 128.8, 128.7, 128.0, 124.2, 122.2, 62.0, 61.9, 61.9, 61.8, 61.3, 61.2, 50.0, 49.9, 34.5, 34.2, 33.7, 32.1, 31.0, 28.9, 26.9, 21.0, 19.1, 14.0, 14.0, 14.0, 13.9; HRMS (ESI-TOF) m/z [M + Na]+ calcd. for C25H28NaO7S+, 495.1448, found 495.1420.
Diethyl 2-((6-methyl-2-(4-methylbenzoyl)-1,1-dioxidothiochroman-4-yl)methyl)malonate (4h): Colorless oil (66.2 mg, 68% yield, dr = 2.0:1). 1H NMR (500 MHz, CDCl3): δH 8.04 (d, J = 8.3 Hz, 1.34 H), 7.97 (d, J = 8.3 Hz, 0.66 H), 7.81(d, J = 8.3 Hz, 0.33 H), 7.76 (d, J = 8.3 Hz, 0.67 H), 7.32–7.28 (m, 2.33 H), 7.25–7.22 (m, 1.0 H), 7.18 (s, 0.67 H), 5.38 (dd, J = 10.7, 4.0 Hz, 0.67 H), 5.16 (dd, J = 10.7, 4.0 Hz, 0.33 H), 4.29–4.12 (m, 4.0 H), 3.61 (dd, J = 10.0, 5.0 Hz, 0.33 H), 3.46 (t, J = 7.5 Hz, 0.67 H), 3.24–3.20 (m, 1.0 H), 3.06–3.00 (m, 0.67 H), 2.79–2.69 (m, 0.67 H), 2.58–2.53 (m, 0.33 H), 2.49–2.44 (m, 0.67 H), 2.43 (s, 3.0 H), 2.42 (s, 1.0 H), 2.41 (s, 2.0 H), 2.38–2.30 (m, 1.67 H), 1.31–1.23 (m, 6.0 H); 13C NMR (126 MHz, CDCl3): δc 189.2, 169.0, 168.9, 168.7, 145.5, 145.5, 144.0, 143.6, 139.7, 135.2, 135.1, 133.9, 133.9, 129.8, 129.7, 129.7, 129.4, 129.4, 128.9, 128.4, 128.3, 124.6, 124.2, 63.4, 61.9, 61.8, 61.2, 49.9, 49.1, 34.7, 34.5, 34.5, 33.8, 30.2, 28.7, 21.8, 21.7, 21.6, 14.1, 14.0, 14.0, 14.0; IR: ῡ = 1725, 1678, 1603, 1447, 1136, 729 cm−1; HRMS (ESI-TOF) m/z [M + Na]+ calcd. for C26H30NaO7S+, 509.1604, found 509.1606.
Diethyl 2-((2-(4-methoxybenzoyl)-6-methyl-1,1-dioxidothiochroman-4-yl)methyl)malonate (4i): Colorless oil (62.5 mg, 62% yield, dr = 2.0:1). 1H NMR (500 MHz, CDCl3): δH 8.13 (d, J = 9.0 Hz, 1.34 H), 8.05 (d, J = 8.7 Hz, 0.66 H), 7.80 (d, J = 8.0 Hz, 0.33 H), 7.75 (d, J = 8.0 Hz, 0.67 H), 7.32 (s, 0.33 H), 7.23 (d, J = 8.0 Hz, 1.0 H), 7.17 (s, 0.67 H), 6.99–6.94 (m, 2.0 H), 5.34 (dd, J = 10.7, 3.3 Hz, 0.67 H), 5.12 (dd, J = 11.3, 4.3 Hz, 0.33 H), 4.26–4.11 (m, 4.0 H), 3.88 (s, 2.0 H), 3.86 (s, 1.0 H), 3.61 (dd, J = 10.0, 5.0 Hz, 0.33 H), 3.46 (t, J = 7.3 Hz, 0.67 H), 3.23–3.17 (m, 1.0 H), 3.05–2.99 (m, 0.67 H), 2.78–2.69 (m, 0.67 H), 2.58–2.53 (m, 0.33 H), 2.48–2.44 (m, 0.67 H), 2.43 (s, 1.0 H), 2.41 (s, 2.0 H), 2.37–2.22 (m, 1.67 H), 1.29–1.22 (m, 6.0 H); 13C NMR (126 MHz, CDCl3): δc 187.8, 187.7, 169.0, 168.9, 168.7, 168.7, 164.5, 164.5, 143.9, 143.5, 139.7, 139.7, 135.3, 135.1, 132.2, 132.1, 129.7, 129.5, 129.4, 128.9, 128.4, 128.2, 124.6, 124.2, 113.9, 113.9, 63.3, 61.8, 61.8, 61.0, 55.5, 49.9, 49.1, 34.5, 33.8, 30.2, 28.6, 21.7, 21.5, 14.0, 14.0, 14.0, 14.0; IR: ῡ = 1743, 1721, 1598, 1134, 1023, 687 cm−1; HRMS (ESI-TOF) m/z [M + Na]+ calcd. for C26H30NaO8S+, 525.1554, found 525.1534.
Diethyl 2-((2-(4-bromobenzoyl)-6-methyl-1,1-dioxidothiochroman-4-yl)methyl)malonate (4j): Colorless oil (36.4 mg, 33% yield, dr = 2.0:1). 1H NMR (500 MHz, CDCl3): δH 8.01 (d, J = 8.7 Hz, 1.34 H), 7.94 (d, J = 8.5 Hz, 0.66 H), 7.80 (d, J = 8.0 Hz, 0.33 H), 7.75 (d, J = 8.0 Hz, 0.67 H), 7.66 (d, J = 8.7 Hz, 1.34 H), 7.64 (d, J = 8.7 Hz, 0.66 H), 7.33 (s, 0.33 H), 7.24 (d, J = 8.0 Hz, 1.0 H), 7.17 (s, 0.67 H), 5.36 (dd, J = 10.7, 3.3 Hz, 0.67 H), 5.12 (dd, J = 11.0, 4.5 Hz, 0.33 H), 4.28–4.12 (m, 4.0 H), 3.60 (dd, J = 10.0, 5.0 Hz, 0.33 H), 3.46 (t, J = 7.3 Hz, 0.67 H), 3.25–3.19 (m, 1.0 H), 3.05–2.99 (m, 0.67 H), 2.79–2.69 (m, 0.67 H), 2.58–2.53 (m, 0.33 H), 2.47–2.41 (m, 3.67 H), 2.36–2.23 (m, 1.67 H), 1.30–1.23 (m, 6.0 H); 13C NMR (126 MHz, CDCl3): δc 189.0, 188.9, 169.0, 168.9, 168.6, 144.2, 143.8, 139.7, 139.6, 135.1, 135.0, 134.9, 132.1, 131.1, 131.0, 130.0, 129.9, 129.8, 129.1, 128.4, 128.4, 124.7, 124.2, 63.8, 61.9, 61.8, 61.5, 50.0, 49.2, 34.7, 34.4, 33.7, 30.1, 28.5, 21.8, 21.6, 14.1, 14.0, 14.0, 14.0; IR: ῡ = 1724, 1683, 1583, 1447, 1137, 732 cm−1; HRMS (ESI-TOF) m/z [M + Na]+ calcd. for C25H27BrNaO7S+, 573.0553, found 573.0548.
Diethyl 2-((6-fluoro-2-(4-methylbenzoyl)-1,1-dioxidothiochroman-4-yl)methyl)malonate (4k): Colorless oil (57.1 mg, 58% yield, dr = 2.0:1). 1H NMR (500 MHz, CDCl3): δH 8.01 (d, J = 8.3 Hz, 1.34 H), 7.95 (d, J = 8.3 Hz, 0.66 H), 7.93–7.87 (m, 1.0 H), 7.32–7.28 (m, 2.0 H), 7.27–7.24 (m, 0.33 H), 7.15–7.11 (m, 1.67 H), 5.37 (dd, J = 10.0, 4.0 Hz, 0.67 H), 5.20 (dd, J = 10.0, 4.0 Hz, 0.33 H), 4.29–4.13 (m, 4.0 H), 3.59 (dd, J = 9.5, 5.0 Hz, 0.33 H), 3.46 (t, J = 7.3 Hz, 0.67 H), 3.31–3.23 (m, 1.0 H), 3.05–2.99 (m, 0.67 H), 2.74–2.67 (m, 0.67 H), 2.61–2.56 (m, 0.33 H), 2.49–2.24 (m, 5.33 H), 1.31–1.23 (m, 6.0 H); 13C NMR (126 MHz, CDCl3): δc 189.0, 189.0, 168.8, 168.7, 168.7, 168.5, 165.3 (d, J = 255.8 Hz), 164.7 (d, J = 255.8 Hz), 145.7, 145.7, 143.5 (d, J = 8.2 Hz), 143.4 (d, J = 8.2 Hz), 133.8 (d, J = 3.3 Hz), 133.8, 133.7, 129.8, 129.7, 129.5, 127.7 (d, J = 9.5 Hz), 127.2 (d, J = 9.5 Hz), 116.1 (d, J = 22.7 Hz), 115.8 (d, J = 22.7 Hz), 115.1 (d, J = 23.5 Hz), 114.9 (d, J = 23.5 Hz), 63.4, 62.0, 61.9, 61.2, 49.7, 49.1, 34.5, 34.5, 34.1, 33.9, 30.4, 28.9, 21.8, 21.7, 14.0, 14.0, 14.0, 14.0; IR: ῡ = 1725, 1678, 1605, 1305, 1148, 732 cm−1; 19F NMR (470 MHz, CDCl3): δF −103.4, −104.2 (s) ppm; HRMS (ESI-TOF) m/z [M + Na]+ calcd. for C25H27FNaO7S+, 513.1354, found 513.1349.
Diethyl 2-((6-fluoro-2-(4-methoxybenzoyl)-1,1-dioxidothiochroman-4-yl)methyl)malonate (4l): Colorless oil (56.8 mg, 56% yield, dr = 2.0:1). 1H NMR (500 MHz, CDCl3): δH 8.09 (d, J = 8.7 Hz, 1.34 H), 8.02 (d, J = 8.7 Hz, 0.66 H), 7.93–7.86 (m, 1.0 H), 7.26–7.23 (m, 0.33 H), 7.14–7.10 (m, 1.67 H), 6.97–6.93 (m, 2.0 H), 5.33 (dd, J = 10.3, 4.3 Hz, 0.66 H), 5.16 (dd, J = 10.3, 4.3 Hz, 0.34 H), 4.26–4.12 (m, 4.0 H), 3.87 (s, 2.0 H), 3.86 (s, 1.0 H), 3.58 (dd, J = 9.7, 5.3 Hz, 0.33 H), 3.47–3.44 (m, 0.67 H), 3.30–3.21 (m, 1.0 H), 3.04–2.98 (m, 0.67 H), 2.73–2.66 (m, 0.67 H), 2.59–2.54 (m, 0.33 H), 2.48–2.22 (m, 2.33 H), 1.29–1.22 (m, 6.0 H); 13C NMR (126 MHz, CDCl3): δc 187.6, 187.5, 168.8, 168.7, 168.7, 168.5, 164.7, 164.6, 165.2 (d, J = 255.7 Hz), 164.7 (d, J = 255.4 Hz), 143.5 (d, J = 8.3 Hz), 143.4 (d, J = 9.5 Hz), 134.0 (d, J = 3.0 Hz), 133.9 (d, J = 2.9 Hz), 132.1, 132.0, 129.3, 129.2, 127.6 (d, J = 9.5 Hz), 127.2 (d, J = 9.5 Hz), 116.0 (d, J = 22.8 Hz), 115.7 (d, J = 22.9 Hz), 115.2, 114.8 (d, J = 22.9 Hz), 114.0, 114.0, 63.2, 61.9, 61.9, 60.9, 55.5, 49.7, 49.1, 34.6, 34.5, 34.2, 33.9, 30.3, 28.8, 14.0, 14.0, 13.9, 13.9; IR: ῡ = 1724, 1672, 1597, 1172, 1026, 731 cm−1; 19F NMR (470 MHz, CDCl3): δF −103.5, −104.3 (s) ppm; HRMS (ESI-TOF) m/z [M + Na]+ calcd. for C25H27FNaO8S+, 529.1303, found 529.1295.
Diethyl 2-((6-bromo-2-(4-methoxybenzoyl)-1,1-dioxidothiochroman-4-yl)methyl)malonate (4m): Colorless oil (62.3 mg, 55% yield, dr = 2.0:1). 1H NMR (500 MHz, CDCl3): δH 8.08 (d, J = 8.7 Hz, 1.34 H), 8.01 (d, J = 8.7 Hz, 0.66 H), 7.75 (d, J = 8.7 Hz, 0.33 H), 7.71 (d, J = 8.7 Hz, 0.67 H), 7.68 (s, 0.33 H), 7.57–7.54 (m, 1.67 H), 6.97–6.93 (m, 2.0 H), 5.34 (dd, J = 10.0, 3.5 Hz, 0.67 H), 5.14 (dd, J = 10.7, 4.7 Hz, 0.33 H), 4.28–4.12 (m, 4.0 H), 3.87 (s, 2.0 H), 3.86 (s, 1.0 H), 3.58 (dd, J = 9.7, 4.7 Hz, 0.33 H), 3.45 (t, J = 7.5 Hz, 0.67 H), 3.27–3.20 (m, 1.0 H), 3.02–2.96 (m, 0.67 H), 2.73–2.66 (m, 0.67 H), 2.58–2.53 (m, 0.33 H), 2.47–2.41 (m, 0.67 H), 2.38–2.22 (m, 1.67 H), 1.28–1.22 (m, 6.0 H); 13C NMR (126 MHz, CDCl3): δc 187.4, 168.8, 168.7, 168.7, 168.4, 164.6, 164.6, 141.9, 141.9, 136.9, 136.8, 132.2, 132.1, 132.0, 131.3, 130.9, 130.8, 129.2, 129.1, 128.2, 127.5, 126.2, 125.8, 114.0, 114.0, 63.1, 61.9, 61.9, 60.8, 55.5, 49.7, 49.0, 34.4, 34.3, 34.3, 33.8, 30.1, 28.6, 14.0, 14.0, 13.9, 13.9; IR: ῡ = 1737, 1674, 1599, 1258, 1138, 840 cm−1; HRMS (ESI-TOF) m/z [M + Na]+ calcd. for C25H27BrNaO8S+, 589.0502, found 589.0521.
Diethyl 2-((2-(4-fluorobenzoyl)-6-methoxy-1,1-dioxidothiochroman-4-yl)methyl)malonate (4n): Colorless oil (58.9 mg, 58% yield, dr = 2.0:1). 1H NMR (500 MHz, CDCl3): δH 8.18 (dd, J = 8.7, 5.3 Hz, 1.34 H), 8.10 (dd, J = 8.7, 5.3 Hz, 0.66 H), 7.82 (d, J = 8.7 Hz, 0.33 H), 7.78 (d, J = 8.7 Hz, 0.67 H), 7.20–7.14 (m, 2.0 H), 7.00 (d, J = 1.7 Hz, 0.33H), 6.94–6.91 (m, 1.0 H), 6.85 (d, J = 1.7 Hz, 0.67 H), 5.35 (dd, J = 10.5, 4.3 Hz, 0.67 H), 5.15 (dd, J = 10.5, 4.3 Hz, 0.33 H), 4.27–4.12 (m, 4.0 H), 3.88 (s, 1.0 H), 3.86 (s, 2.0 H), 3.59 (dd, J = 9.5, 5.5 Hz, 0.33 H), 3.47 (t, J = 7.3 Hz, 0.67 H), 3.24–3.22 (m, 1.0 H), 3.05–2.99 (m, 0.67 H), 2.73–2.66 (m, 0.67 H), 2.57–2.52 (m, 0.33 H), 2.48–2.42 (m, 0.67 H), 2.37–2.27 (m, 1.67 H), 1.30–1.22 (m, 6.0 H); 13C NMR (126 MHz, CDCl3): δc 188.4, 188.3, 169.0, 168.9, 168.9, 168.6, 166.5 (d, J = 258.0 Hz), 163.4 (d, J = 258.0 Hz), 163.3, 162.8, 142.2, 142.1, 132.9 (d, J = 2.9 Hz), 132.8 (d, J = 2.9 Hz), 132.5 (d, J = 9.6 Hz), 132.4 (d, J = 9.6 Hz), 129.6, 129.5, 127.0, 126.4, 115.9 (d, J = 22.1 Hz), 114.0 (d, J = 30.9 Hz), 112.9 (d, J = 5.3 Hz), 63.8, 61.9, 61.9, 61.8, 61.6, 55.6, 55.6, 49.9, 49.2, 34.8, 34.3, 34.2, 33.9, 30.5, 29.0, 14.0, 14.0, 14.0; IR: ῡ = 1682,1595, 1448, 1294, 1134, 730 cm−1; 19F NMR (470 MHz, CDCl3): δF −102.8 (s) ppm; HRMS (ESI-TOF) m/z [M + Na]+ calcd. for C25H27FNaO8S+, 529.1303, found 529.1306.
Diethyl 2-((2-(4-bromobenzoyl)-6-methoxy-1,1-dioxidothiochroman-4-yl)methyl)malonate (4o): Colorless oil (33.0 mg, 29% yield, dr = 2.0:1). 1H NMR (500 MHz, CDCl3): δH 8.01 (d, J = 8.7 Hz, 1.34 H), 7.93 (d, J = 8.7 Hz, 0.66 H), 7.83 (d, J = 8.7 Hz, 0.33 H), 7.79 (d, J = 8.7 Hz, 0.67 H), 7.67–7.63 (m, 2.0 H), 7.01 (s, 0.33 H), 6.93 (dd, J = 8.7, 2.3 Hz, 1.0 H), 6.85 (d, J = 2.3 Hz, 0.67 H), 5.34 (dd, J = 10.0, 3.5 Hz, 0.67 H), 5.14 (dd, J = 10.5, 5.0 Hz, 0.33 H), 4.28–4.11 (m, 4.0 H), 3.89 (s, 1.0 H), 3.87 (s, 2.0 H), 3.60 (dd, J = 9.0, 5.5 Hz, 0.33 H), 3.47 (t, J = 7.3 Hz, 0.67 H), 3.25–3.23 (m, 1.0 H), 3.06–3.00 (m, 0.67 H), 2.74–2.66 (m, 0.67 H), 2.57–2.52 (m, 0.33 H), 2.49–2.43 (m, 0.67 H), 2.37–2.28 (m, 1.67 H), 1.31–1.22 (m, 6.0 H); 13C NMR (126 MHz, CDCl3): δc 189.1, 189.1, 169.0, 168.9, 168.6, 163.4, 162.8, 142.3, 142.1, 135.1, 135.1, 132.1, 131.1, 131.0, 129.9, 129.6, 129.4, 127.1, 126.5, 114.2, 113.9, 113.0, 112.9, 64.0, 61.9, 61.9, 61.7, 55.7, 55.6, 50.0, 49.2, 34.9, 34.4, 34.1, 33.9, 30.5, 29.0, 14.1, 14.0, 14.0; IR: ῡ = 1732, 1664, 1592, 1248, 1131, 848 cm−1; HRMS (ESI-TOF) m/z [M + Na]+ calcd. for C25H27BrNaO8S+, 589.0502, found 589.0515.
Diethyl 2-((6-fluoro-2-(3-methoxybenzoyl)-1,1-dioxidothiochroman-4-yl)methyl)malonate (4p): Colorless oil (29.6 mg, 29% yield, dr = 2.0:1). 1H NMR (500 MHz, CDCl3): δH 7.95–7.88 (m, 1.0 H), 7.73 (d, J = 8.0 Hz, 0.67 H), 7.64 (d, J = 8.0 Hz, 0.33 H), 7.60 (s, 0.67 H), 7.55 (s, 0.33 H), 7.45–7.40 (m, 1.0 H), 7.27–7.25 (m, 0.67 H), 7.19 (dd, J = 8.5, 2.5 Hz, 0.67 H), 7.17–7.12 (m, 1.67 H), 5.37 (dd, J = 9.7, 3.7 Hz, 0.67 H), 5.21 (dd, J = 10.5, 5.0 Hz, 0.33 H), 4.29–4.13 (m, 4.0 H), 3.86 (s, 2.0 H), 3.85 (s, 1.0 H), 3.59 (dd, J = 9.7, 5.3 Hz, 0.33 H), 3.46 (t, J = 7.3 Hz, 0.67 H), 3.31–3.25 (m, 1.0 H), 3.06–3.00 (m, 0.67 H), 2.74–2.66 (m, 0.67 H), 2.62–2.57 (m, 0.33 H), 2.50–2.24 (m, 2.33 H), 1.31–1.23 (m, 6.0 H); 13C NMR (126 MHz, CDCl3): δc 189.6, 189.5, 168.8, 168.7, 168.7, 168.5, 165.4 (d, J = 255.9 Hz), 164.8 (d, J = 255.9 Hz), 159.9, 143.5 (d, J = 8.2 Hz), 143.3 (d, J = 8.2 Hz), 137.5, 137.4, 133.8 (d, J = 3.2 Hz), 133.8 (d, J = 3.2 Hz), 129.8, 129.8, 127.8 (d, J = 9.6 Hz), 127.3 (d, J = 9.6 Hz), 122.5, 122.4, 121.4, 121.2, 116.1 (d, J = 22.7 Hz), 115.8 (d, J = 22.7 Hz), 115.1 (d, J = 23.1 Hz), 114.9 (d, J = 23.1 Hz), 113.2, 113.2, 63.7, 62.0, 61.9, 61.6, 55.5, 49.7, 49.1, 34.5, 34.4, 34.0, 33.8, 30.6, 29.1, 14.0, 14.0, 14.0, 14.0; 19F NMR (470 MHz, CDCl3): δF −103.2, −104.1 (s) ppm; IR: ῡ = 1724, 1682, 1580, 1146, 1026, 730 cm−1; HRMS (ESI-TOF) m/z [M + Na]+ calcd. for C25H27FNaO8S+, 529.1303, found 529.1291.
Diethyl 2-((6-fluoro-2-(2-methoxybenzoyl)-1,1-dioxidothiochroman-4-yl)methyl)malonate (4q): Colorless oil (54.8 mg, 54% yield, dr = 2.0:1). 1H NMR (500 MHz, CDCl3): δH 7.90–7.83 (m, 1.0 H), 7.68 (dd, J = 7.7, 1.7 Hz, 0.33 H), 7.60 (dd, J = 7.7, 1.7 Hz, 0.67 H), 7.52 (td, J = 7.7, 1.7 Hz, 1.0 H), 7.23 (dd, J = 10.0, 1.5 Hz, 0.33 H), 7.14–7.08 (m, 1.67 H), 7.04–6.99 (m, 2.0 H), 5.77 (dd, J = 9.0, 3.5 Hz, 0.67 H), 5.60 (dd, J = 10.0, 6.0 Hz, 0.33 H), 4.29–4.17 (m, 4.0 H), 3.96 (s, 2.0 H), 3.93 (s, 1.0 H), 3.59 (dd, J = 9.3, 5.7 Hz, 0.33 H), 3.49 (dd, J = 9.0, 6.0 Hz, 0.67 H), 3.31–3.20 (m, 1.0 H), 2.96–2.90 (m, 0.67 H), 2.72–2.66 (m, 0.67 H), 2.54–2.47 (m, 1.67 H), 2.33–2.22 (m, 1.0 H), 1.32–1.25 (m, 6.0 H); 13C NMR (126 MHz, CDCl3): δc 192.1, 192.1, 168.8, 168.8, 168.6, 165.3 (d, J = 255.5 Hz), 164.7 (d, J = 255.5 Hz), 158.8, 158.7, 143.9 (d, J = 8.4 Hz), 143.3 (d, J = 8.4 Hz), 135.1, 134.9, 134.2 (d, J = 3.2 Hz), 133.7 (d, J = 3.2 Hz), 131.1, 130.9, 127.7 (d, J = 9.6 Hz), 127.5, 127.2, 126.9 (d, J = 9.6 Hz), 121.2, 116.1 (d, J = 22.7 Hz), 115.5 (d, J = 22.7 Hz), 114.8 (d, J = 22.8 Hz), 114.3 (d, J = 22.8 Hz), 111.7, 111.7, 68.0, 65.1, 61.9, 61.9, 55.9, 55.8, 49.5, 49.2, 34.9, 34.0, 33.4, 33.3, 30.8, 28.5, 14.0, 14.0, 14.0, 14.0; 19F NMR (470 MHz, CDCl3): δF −103.7, −104.7 (s) ppm; IR: ῡ = 1726, 1597, 1299, 1147, 1020, 730 cm−1; HRMS (ESI-TOF) m/z [M + Na]+ calcd. for C25H27FNaO8S+, 529.1303, found 529.1319.
Diethyl 2-((2-benzoyl-6-methyl-1,1-dioxidothiochroman-4-yl)methyl)-2-methylmalonate (5ab): Colorless oil (43.7 mg, 45% yield, dr = 2.0:1). 1H NMR (500 MHz, CDCl3): δH 8.18 (dd, J = 8.3, 1.3 Hz, 1.34 H), 8.06 (dd, J = 8.3, 1.3 Hz, 0.66 H), 7.79 (d, J = 8.3 Hz, 0.33 H), 7.75 (d, J = 8.3 Hz, 0.67 H), 7.65–7.60 (m, 1.0 H), 7.55–7.48 (m, 2.0 H), 7.33 (s, 0.33 H), 7.23 (d, J = 8.3 Hz, 1.0 H), 7.12 (s, 0.67 H), 5.59 (dd, J = 11.5, 3.7 Hz, 0.67 H), 5.17 (dd, J = 11.5, 3.7 Hz, 0.33 H), 4.26–4.00 (m, 4.0 H), 3.38–3.31 (m, 1.0 H), 2.99–2.93 (m, 0.67 H), 2.74–2.67 (m, 0.33 H), 2.60–2.49 (m, 1.0 H), 2.44 (s, 1.0 H), 2.40 (s, 2.0 H), 2.36–2.21 (m, 2.0 H), 1.57 (s, 1.0 H), 1.56 (s, 2.0 H), 1.30–1.24 (m, 3.0 H), 1.23–1.16 (m, 3.0 H); 13C NMR (126 MHz, CDCl3): δc190.0, 189.9, 172.2, 172.1, 172.1, 171.9, 144.0, 143.5, 141.3, 140.6, 136.7, 136.4, 135.7, 135.0, 134.2, 133.6, 129.8, 129.7, 129.5, 129.0, 128.7, 128.7, 128.3, 128.1, 124.7, 124.1, 63.9, 61.8, 61.7, 61.5, 53.3, 53.1, 41.7, 40.4, 33.4, 32.7, 31.9, 29.7, 28.3, 21.8, 21.5, 21.1, 20.5, 14.0, 13.9, 13.9, 13.8; IR: ῡ = 1723, 1682, 1448, 1108, 911, 728 cm−1; HRMS (ESI-TOF) m/z [M + Na]+ calcd. for C26H30NaO7S+, 509.1604, found 509.1601.
Dmethyl 2-((2-benzoyl-6-methyl-1,1-dioxidothiochroman-4-yl)methyl)malonate (5ac): Colorless oil (41.9 mg, 47% yield, dr = 2.0:1). 1H NMR (500 MHz, CDCl3): δH 8.15 (d, J = 7.7 Hz, 1.34 H), 8.08 (d, J = 7.7 Hz, 0.66 H), 7.82 (d, J = 7.7 Hz, 0.33 H), 7.77 (d, J = 7.7 Hz, 0.67 H), 7.67–7.61 (m, 1.0 H), 7.55–7.49 (m, 2.0 H), 7.32 (s, 0.33 H), 7.26–7.24 (m, 1.0 H), 7.17 (s, 0.67 H), 5.40 (dd, J = 10.7, 4.0 Hz, 0.67 H), 5.19 (dd, J = 10.7, 4.0 Hz, 0.33 H), 3.80 (s, 1.0 H), 3.76 (s, 2.0 H), 3.74 (s, 1.0 H), 3.71 (s, 2.0 H), 3.68–3.65 (m, 0.33 H), 3.52 (t, J = 7.3 Hz, 0.67 H), 3.25–3.18 (m, 1.0 H), 3.07–2.99 (m, 0.67 H), 2.80–2.73 (m, 0.67 H), 2.58–2.53 (m, 0.33 H), 2.52–2.47 (m, 0.67 H), 2.45 (s, 1.0 H), 2.42 (s, 2.0 H), 2.38–2.28 (m, 1.67 H); 13C NMR (126 MHz, CDCl3): δc 189.9 189.8, 169.3, 169.3, 169.0, 144.2, 143.7, 139.6, 136.4, 136.3, 135.1, 135.0, 134.4, 134.3, 129.7, 129.7, 129.6, 129.1, 128.8, 128.8, 128.4, 128.4, 124.7, 124.3, 63.6, 61.5, 52.9, 52.9, 52.9, 52.8, 49.6, 48.8, 34.8, 34.6, 34.5, 33.7, 30.3, 28.8, 21.8, 21.6; HRMS (ESI-TOF) m/z [M + Na]+ calcd. for C23H24NaO7S+, 467.1135, found 467.1119.
3-(2-Benzoyl-6-methyl-1,1-dioxidothiochroman-4-yl)-1-phenylpropan-1-one (5af): Colorless oil (49.3 mg, 57% yield, dr = 1.5:1). 1H NMR (500 MHz, CDCl3): δH 8.14 (d, J = 7.7 Hz, 1.2 H), 8.08 (d, J = 7.7 Hz, 0.8 H), 7.94 (d, J = 7.7 Hz, 0.8 H), 7.90 (d, J = 7.7 Hz, 1.2 H), 7.82 (d, J = 7.7 Hz, 0.4 H), 7.76 (d, J = 7.7 Hz, 0.6 H), 7.64–7.59 (m, 1.0 H), 7.55 (t, J = 7.7 Hz, 1.0 H), 7.52–7.48 (m, 2.0 H), 7.46–7.42 (m, 2.0 H), 7.32 (s, 0.4 H), 7.23–7.20 (m, 1.6 H), 5.45 (dd, J = 9.7, 3.7 Hz, 0.6 H), 5.24 (dd, J = 11.5, 3.7 Hz, 0.4 H), 3.40–3.31 (m, 1.0 H), 3.22–2.97 (m, 3.0 H), 2.56–2.43 (m, 1.2 H), 2.41 (s, 1.2 H), 2.38 (s, 1.8 H), 2.33–2.20 (m, 1.8 H); 13C NMR (126 MHz, CDCl3): δc 199.3, 199.1, 190.2, 190.0, 144.0, 143.6, 140.7, 139.9, 136.5, 136.5, 136.4, 136.3, 135.6, 134.8, 134.2, 133.3, 133.2, 129.6, 129.5, 129.5, 128.7, 128.7, 128.6, 128.6, 128.3, 128.2, 127.9, 127.9, 124.4, 124.1, 63.9, 61.6, 35.5, 35.3, 35.0, 34.2, 30.3, 29.1, 28.9, 28.7, 21.7, 21.5; IR: ῡ = 1679, 1596, 1447, 1298, 1133, 729 cm−1; HRMS (ESI-TOF) m/z [M + Na]+ calcd. for C26H24NaO4S+, 455.1288, found 455.1264.
3-(2-Benzoyl-6-methyl-1,1-dioxidothiochroman-4-yl)-1-(4-methoxyphenyl)propan-1-one (5ag): Colorless oil (58.3 mg, 63% yield, dr = 1.5:1). 1H NMR (500 MHz, CDCl3): δH 8.12 (d, J = 8.0 Hz, 1.2 H), 8.06 (d, J = 8.0 Hz, 0.8 H), 7.89 (d, J = 8.0 Hz, 0.8 H), 7.86 (d, J = 8.0 Hz, 1.2 H), 7.78 (d, J = 8.0 Hz, 0.4 H), 7.72 (d, J = 8.0 Hz, 0.6 H), 7.58 (q, J = 8.0 Hz, 1.0 H), 7.48–7.42 (m, 2.0 H), 7.30 (s, 0.6 H), 7.20–7.17 (m, 1.4 H), 6.90–6.86 (m, 2.0 H), 5.46 (dd, J = 10.0, 3.7 Hz, 0.6 H), 5.25 (dd, J = 11.5, 3.7 Hz, 0.4 H), 3.81 (s, 1.2 H), 3.81 (s, 1.8 H), 3.36–3.26 (m, 1.0 H), 3.12–2.91 (m, 2.6 H), 2.84–2.77 (m, 0.4 H), 2.55–2.40 (m, 1.4 H), 2.38 (s, 1.2 H), 2.36 (s, 1.8 H), 2.28–2.17 (m, 1.6 H); 13C NMR (126 MHz, CDCl3): δc 197.9, 197.7, 190.3, 190.2, 163.7, 163.6, 144.0, 143.6, 140.9, 140.1, 136.5, 135.7, 135.0, 134.3, 130.3, 130.3, 129.7, 129.6, 128.8, 128.7, 128.7, 128.5, 128.2, 124.4, 124.0, 113.8, 113.8, 64.0, 61.6, 55.5, 35.6, 35.3, 35.1, 33.9, 30.4, 29.4, 29.0, 28.9, 21.8, 21.6; IR: ῡ = 1652, 1595, 1571, 1294, 1134, 741 cm−1; HRMS (ESI-TOF) m/z [M + Na]+ calcd. for C27H26NaO5S+, 485.1393, found 485.1387.
3-(2-Benzoyl-6-methyl-1,1-dioxidothiochroman-4-yl)-1-(p-tolyl)propan-1-one (5ah): Colorless oil (52.8 mg, 59% yield, dr = 1.5:1). 1H NMR (500 MHz, CDCl3): δH 8.14 (d, J = 7.7 Hz, 1.2 H), 8.08 (d, J = 7.7 Hz, 0.8 H), 7.84–7.75 (m, 3.0 H), 7.64–7.59 (m, 1.0 H), 7.50 (t, J = 7.7 Hz, 1.0 H), 7.48 (t, J = 7.7 Hz, 1.0 H), 7.32 (s, 0.4 H), 7.24–7.20 (m, 3.6 H), 5.45 (dd, J = 9.7, 3.7 Hz, 0.6 H), 5.24 (dd, J = 11.5, 3.5 Hz, 0.4 H), 3.39–3.30 (m, 1.0 H), 3.18–2.95 (m, 2.6 H), 2.85–2.80 (m, 0.4 H), 2.56–2.43 (m, 1.4 H), 2.39 (s, 6.0 H), 2.30–2.19 (m, 1.6 H); 13C NMR (126 MHz, CDCl3): δc 198.9, 198.7, 190.2, 190.0, 144.1, 144.0, 144.0, 143.5, 140.7, 139.9, 136.4, 136.3, 135.6, 134.9, 134.2, 134.1, 134.1, 129.6, 129.5, 129.3, 129.3, 128.7, 128.7, 128.6, 128.3, 128.1, 128.1, 128.0, 124.4, 124.0, 63.9, 61.5, 35.4, 35.4, 35.0, 34.0, 30.3, 29.2, 28.9, 28.8, 21.7, 21.6, 21.5; IR: ῡ = 1674, 1604, 1448, 1299, 1134, 728 cm−1; HRMS (ESI-TOF) m/z [M + Na]+ calcd. for C27H26NaO4S+, 469.1444, found 469.1420.
3-(2-Benzoyl-6-methyl-1,1-dioxidothiochroman-4-yl)-1-(3-methoxyphenyl)propan-1-one (5ai): Colorless oil (53.8 mg, 58% yield, dr = 1.5:1). 1H NMR (500 MHz, CDCl3): δH 8.14 (d, J = 7.7 Hz, 1.2 H), 8.08 (d, J = 7.7 Hz, 0.8 H), 7.81 (d, J = 7.7 Hz, 0.4 H), 7.76 (d, J = 7.7 Hz, 0.6 H), 7.64–7.59 (m, 1.0 H), 7.52–7.47 (m, 3.0 H), 7.42 (s, 0.8 H), 7.36–7.32 (m, 1.6 H), 7.24–7.20 (m, 1.6 H), 7.10 (d, J = 7.7 Hz, 1.0 H), 5.44 (dd, J = 10.0, 4.0 Hz, 0.6 H), 5.24 (dd, J = 11.5, 4.0 Hz, 0.4 H), 3.84 (s, 1.2 H), 3.81 (s, 1.8 H), 3.39–3.30 (m, 1.0 H), 3.20–2.97 (m, 2.6 H), 2.85–2.80 (m, 0.4 H), 2.56–2.43 (m, 1.4 H), 2.41 (s, 1.2 H), 2.39 (s, 1.8 H), 2.31–2.21 (m, 1.6 H); 13C NMR (126 MHz, CDCl3): δc 199.1, 198.9, 190.2, 190.0, 159.8, 144.0, 143.6, 140.7, 139.9, 137.9, 137.9, 136.4, 136.3, 135.6, 134.9, 134.2, 129.6, 129.6, 129.5, 129.5, 128.7, 128.7, 128.6, 128.3, 128.2, 124.5, 124.1, 120.6, 120.5, 119.7, 119.6, 112.3, 112.2, 63.9, 61.6, 55.4, 55.4, 35.7, 35.4, 35.0, 34.4, 30.3, 29.3, 28.9, 28.8, 21.7, 21.5; IR: ῡ = 1679, 1596, 1448, 1260, 1135, 731 cm−1; HRMS (ESI-TOF) m/z [M + Na]+ calcd. for C27H26NaO5S+, 485.1393, found 485.1388.
3-(2-Benzoyl-6-methyl-1,1-dioxidothiochroman-4-yl)-1-(3-chlorophenyl)propan-1-one (5aj): Colorless oil (61.8 mg, 66% yield, dr = 1.5:1). 1H NMR (500 MHz, CDCl3): δH 8.13 (d, J = 8.0 Hz, 1.2 H), 8.08 (d, J = 8.0 Hz, 0.8 H), 7.91 (t, J = 1.7 Hz, 0.4 H), 7.87 (t, J = 1.7 Hz, 0.6 H), 7.83–7.76 (m, 2.0 H), 7.65–7.60 (m, 1.0 H), 7.53–7.47 (m, 3.0 H), 7.40–7.37 (m, 1.0 H), 7.30 (s, 0.4 H), 7.25–7.20 (m, 1.6 H), 5.43 (dd, J = 9.5, 4.0 Hz, 0.6 H), 5.24 (dd, J = 11.5, 4.0 Hz, 0.4 H), 3.40–3.32 (m, 1.0 H), 3.18–2.95 (m, 2.6 H), 2.88–2.80 (m, 0.4 H), 2.56–2.43 (m, 1.4 H), 2.42 (s, 1.2 H), 2.40 (s, 1.8 H), 2.34–2.21(m, 1.6 H); 13C NMR (126 MHz, CDCl3): δc 198.0, 197.8, 190.2, 189.9, 144.1, 143.7, 140.6, 139.8, 138.1, 138.1, 136.4, 136.3, 135.6, 135.0, 134.8, 134.3, 133.2, 133.1, 130.0, 130.0, 129.6, 129.5, 129.4, 128.7, 128.7, 128.7, 128.2, 128.2, 128.0, 128.0, 126.1, 126.0, 124.5, 124.2, 63.9, 61.7, 35.6, 35.2, 34.9, 34.3, 30.3, 29.1, 29.0, 28.6, 21.8, 21.6; IR: ῡ = 1682, 1596, 1448, 1298, 1134, 729 cm−1; HRMS (ESI-TOF) m/z [M + Na]+ calcd. for C26H23ClNaO4S+, 489.0898, found 489.0885.
3-(2-Benzoyl-6-methyl-1,1-dioxidothiochroman-4-yl)-1-(2-methoxyphenyl)propan-1-one (5ak): Colorless oil (35.3 mg, 38% yield, dr = 1.5:1). 1H NMR (500 MHz, CDCl3): δH 8.17 (d, J = 8.0 Hz, 1.2 H), 8.09 (d, J = 8.0 Hz, 0.8 H), 7.81 (d, J = 8.0 Hz, 0.4 H), 7.76 (d, J = 8.0 Hz, 0.6 H), 7.70 (dd, J = 8.0, 2.0 Hz, 0.4 H), 7.66–7.60 (m, 1.6 H), 7.54–7.44 (m, 3.0 H), 7.34 (s, 0.4 H), 7.24–7.20 (m, 1.6 H), 7.01–6.93 (m, 2.0 H), 5.45 (dd, J = 10.3, 3.7 Hz, 0.6 H), 5.22 (dd, J = 11.5, 4.0 Hz, 0.4 H), 3.90 (s, 1.2 H), 3.83 (s, 1.8 H), 3.36–3.25 (m, 1.0 H), 3.24–3.14 (m, 1.0 H), 3.10–2.99 (m, 1.6 H), 2.87–2.80 (m, 0.4 H), 2.57–2.43 (m, 1.4 H), 2.42 (s, 1.2 H), 2.40 (s, 1.8 H), 2.26–2.14 (m, 1.6 H); 13C NMR (126 MHz, CDCl3): δc 201.7, 201.4, 190.1, 190.1, 158.6, 158.5, 143.4, 140.8, 140.4, 136.4, 135.5, 135.0, 134.3, 134.2, 133.7, 133.7, 130.2, 129.7, 129.7, 129.5, 129.5, 128.7, 128.7, 128.6, 128.5, 128.4, 128.0, 127.8, 127.8, 124.3, 124.0, 120.7, 120.6, 111.6, 63.9, 61.5, 55.5, 55.5, 41.2, 39.7, 35.8, 30.3, 29.7, 29.5, 28.7, 21.8, 21.6; IR: ῡ = 1678, 1595, 1484, 1298, 1134, 729 cm−1; HRMS (ESI-TOF) m/z [M + Na]+ calcd. for C27H26NaO5S+, 485.1393, found 485.1370.
3-(2-Benzoyl-6-methyl-1,1-dioxidothiochroman-4-yl)-1-(3,4-dichlorophenyl)propan-1-one (5al): Colorless oil (55.3 mg, 55% yield, dr = 1.5:1). 1H NMR (500 MHz, CDCl3): δH 8.13 (d, J = 7.3 Hz, 1.2 H), 8.08 (d, J = 7.3 Hz, 0.8 H), 8.00 (d J = 2.0 Hz, 0.4 H), 7.97 (d, J = 2.0 Hz, 0.6 H), 7.82 (d, J = 8.0 Hz, 0.4 H), 7.76 (d, J = 8.0 Hz, 0.6 H), 7.74–7.71 (m, 1.0 H), 7.65–7.59 (m, 1.0 H), 7.53–7.47 (m, 3.0 H), 7.29 (s, 0.4 H), 7.23 (t, J = 8.0 Hz, 1.0 H), 7.19 (s, 0.6 H), 5.41 (dd, J = 9.0, 4.0 Hz, 0.6 H), 5.23 (dd, J = 11.5, 4.0 Hz, 0.4 H), 3.40–3.33 (m, 1.0 H), 3.14–2.94 (m, 2.6 H), 2.88–2.81 (m, 0.4 H), 2.55–2.44 (m, 1.4 H), 2.42 (s, 1.2 H), 2.40 (s, 1.8 H), 2.33–2.21(m, 1.6 H); 13C NMR (126 MHz, CDCl3): δc 197.0, 196.9, 190.2, 189.9, 144.1, 143.7, 140.5, 139.7, 137.9, 137.8, 136.4, 136.3, 136.1, 136.1, 135.7, 134.7, 134.3, 133.4, 133.4, 130.8, 130.7, 129.9, 129.6, 129.6, 129.4, 128.7, 128.7, 128.7, 128.3, 128.2, 127.1, 127.0, 124.6, 124.2, 63.8, 61.7, 35.5, 35.1, 34.9, 34.2, 30.2, 29.1, 28.9, 28.5, 21.8, 21.6; IR: ῡ = 1682, 1596, 1448, 1298, 1133, 726 cm−1; HRMS (ESI-TOF) m/z [M + Na]+ calcd. for C26H22Cl2NaO4S+, 523.0508, found 523.0516.
3-(2-Benzoyl-6-methyl-1,1-dioxidothiochroman-4-yl)-1-(thiophen-2-yl)propan-1-one (5am): Colorless oil (36.9 mg, 42% yield, dr = 1.5:1). 1H NMR (500 MHz, CDCl3): δH 8.15 (d, J = 8.0 Hz, 1.2 H), 8.10 (d, J = 8.0 Hz, 0.8 H), 7.83 (d, J = 8.0 Hz, 0.4 H), 7.78 (d, J = 8.0 Hz, 0.6 H), 7.70 (dd, J = 10.3, 3.7 Hz, 1.0 H), 7.66–7.60 (m, 2.0 H), 7.54–7.49 (m, 2.0 H), 7.33 (s, 0.4 H), 7.25–7.22 (m, 1.0 H), 7.21 (s, 0.6 H), 7.12 (t, J = 4.3 Hz, 1.0 H), 5.42 (dd, J = 10.5, 4.0 Hz, 0.6 H), 5.23 (dd, J = 10.5, 4.0 Hz, 0.4 H), 3.41–3.34 (m, 1.0 H), 3.15–2.95 (m, 2.6 H), 2.90–2.83 (m, 0.4 H), 2.58–2.45 (m, 1.4 H), 2.43 (s, 1.2 H), 2.40 (s, 1.8 H), 2.35–2.23 (m, 1.6 H); 13C NMR (126 MHz, CDCl3): δc 192.2, 191.9, 190.2, 190.0, 144.1, 143.9, 143.8, 143.7, 140.6, 139.8, 136.4, 136.4, 135.6, 134.8, 134.3, 133.9, 133.8, 132.1, 132.0, 129.6, 129.6, 129.5, 128.8, 128.8, 128.7, 128.4, 128.3, 128.2, 124.6, 124.2, 63.9, 61.7, 36.2, 35.3, 35.0, 34.9, 30.3, 29.7, 29.5, 29.1, 21.8, 21.6; IR: ῡ = 1658, 1595, 1415, 1297, 1129, 725 cm−1; HRMS (ESI-TOF) m/z [M + Na]+ calcd. for C24H22NaO4S2+, 461.0852, found 461.0874.
3-(2-Benzoyl-6-methyl-1,1-dioxidothiochroman-4-yl)-1-cyclopropylpropan-1-one (5an): Colorless oil (24.7 mg, 31% yield, dr = 1.5:1). 1H NMR (500 MHz, CDCl3): δH 8.15 (d, J = 7.3 Hz, 1.2 H), 8.09 (d, J = 7.3 Hz, 0.8 H), 7.82 (d, J = 8.0 Hz, 0.4 H), 7.76 (d, J = 8.0 Hz, 0.6 H), 7.66–7.61 (m, 1.0 H), 7.54–7.49 (m, 2.0 H), 7.28 (s, 0.4 H), 7.25–7.21 (m, 1.0 H), 7.17 (s, 0.6 H), 5.40 (dd, J = 10.0, 4.0 Hz, 0.6 H), 5.21 (dd, J = 11.5, 4.0 Hz, 0.4 H), 3.30–3.21 (m, 1.0 H), 3.00–2.95 (m, 0.4 H), 2.81–2.62 (m, 2.6 H), 2.51–2.45 (m, 0.4 H), 2.43 (s, 1.2 H), 2.41 (s, 1.8 H), 2.39–2.32 (m, 1.6 H), 2.17–2.07 (m, 1.0 H), 1.94–1.89 (m, 1.0 H), 1.03–0.95 (m, 2.0 H), 0.90–0.85 (m, 2.0 H); 13C NMR (126 MHz, CDCl3): δc 210.0, 209.8, 190.2, 190.0, 144.0, 143.5, 140.6, 139.9, 136.4, 136.4, 135.6, 134.9, 134.3, 129.6, 129.6, 129.5, 128.7, 128.7, 128.7, 128.3, 128.2, 124.5, 124.1, 63.9, 61.6, 40.6, 39.2, 35.4, 35.0, 30.3, 29.7, 28.9, 28.5, 21.8, 21.6, 20.7, 20.7, 11.1, 11.0, 11.0, 10.9; IR: ῡ = 1682, 1596, 1448, 1300, 1134, 728 cm−1; HRMS (ESI-TOF) m/z [M + Na]+ calcd. for C23H24NaO4S+, 419.1288, found 419.1268.