1. Introduction
The human skin, serving as the initial shield against environmental factors, has evolved a comprehensive immune system to shield its internal organs and tissues from chemical, physical, or microbial damage (Georgetti et al., 2013; Balupillai et al., 2018) [
1,
2]. The aging of skin is a multifaceted biological phenomenon, shaped by a combination of genetic and environmental elements. Such elements result in a gradual accumulation of physiological and structural alterations in the skin’s appearance. Ultraviolet radiation (UV) significantly accelerates the aging of the skin, with prolonged ultraviolet radiation-B (UVB) exposure frequently leading to the generation of reactive oxygen species (ROS) and harm to biomolecules like proteins and nucleic acids in the skin (Kammeyer and Luiten, 2015) [
3]. The excessive exposure to UVB radiation also leads to the release of inflammatory cytokines such as Interleukin-6 (IL-6), Interleukin-1β (IL-1β), and Tumor Necrosis Factor-α (TNF-α) (Kim et al., 2012; Zhang, C et al., 2021; Jung et al., 2022) [
4,
5,
6]. Melanocytes generate melanin as a protective response when subjected to UV rays. UV-induced damage is also involved in the formation of melanoma, and exposure to UVB accelerates skin aging and pigmentation (Baldea et al., 2009; Ma, L.-P et al., 2023) [
7,
8]. Additionally, these pro-inflammatory cytokines activate inflammatory cells to generate NADPH oxidase (Liu-Smith et al., 2015) [
9], leading to the generation of ROS and oxidative stress in skin tissues. Consequently, this results in weakened immune systems, swelling, oxidative harm, aging, and a heightened likelihood of skin cancer. UVB radiation primarily induces premature skin aging and is considered a significant factor in the development of skin cancer. Recent studies have shown that several substances with anti-inflammatory biological activities possess protective effects against UVB-induced cell senescence (Choi E et al., 2019; Lu W et al., 2023) [
10,
11]. These findings suggest that inhibiting cellular processes or signaling pathways associated with UVB-induced cellular senescence may be a potential strategy for treating cellular senescence diseases resulting from excessive UVB exposure (Lim et al., 2023) [
12].
Although there are a variety of sunscreen strategies available, including protective clothing, finding shade or staying indoors, applying sunscreen, etc., sunscreen is still the first choice (Koch, S. et al., 2017) [
13]. At present, sunscreen clothing is usually evaluated by the transmittance of ultraviolet light through the fabric (Ultraviolet Protection Factor; UPF), and UPF > 50 is regarded as effective protection. There are two more common types of sunscreen: physical sunscreen and chemical sunscreen. Physical sunscreen is applied to the skin to form a physical sunscreen film, which directly reflects the UV rays, thus achieving the effect of sunscreen. Physical sunscreen is generally zinc oxide and titanium dioxide, which are generally not absorbed by the human body, so it is milder and safer to use, but its texture is thicker than chemical sunscreen, and it has obvious whitening after use and a slightly greasy and bad skin feeling. Chemical sunscreen is also known as an ultraviolet absorber, which is achieved by absorbing harmful ultraviolet rays and comprises ingredients such as benzophenone and ethyl hexyl salicylate. Because the molecules of chemical sunscreen are absorbed by the skin, the process of absorbing ultraviolet rays takes place inside the skin and is metabolized by the human body. At the same time, it will also cause some irritation to the skin (Dromgoole S H et al., 1990) [
14]. Because of the huge heterogeneity of sunscreen products on the market in 2012, the European Commission determined several protection categories to classify the Sun Protection Factor (SPF) into ‘Low Protection’ (SPF 6, 10), ‘Medium Protection’ (SPF 15, 20, 25), ‘High Protection’ (SPF 30, 50) and ‘Very High Protection’ (SPF 50+). To label sunscreen, the product must provide at least 6 SPF (Sebastian Singer et al., 2019) [
15]. It is worth noting that fps only represents the category of protection for UVB. In addition, PA is an acronym for Protection Grade of UVA, with “+” indicating the product’s ability to defend against long-wave ultraviolet rays. The PA grade is determined according to the long-wave ultraviolet protection index (Protection Factor of UVA, PFA) of sunscreen cosmetics, which reflects the protective effect of long-wave ultraviolet tanning and is a protective index to evaluate the ability of sunscreen cosmetics to prevent sunburn. The higher the PA level, the better the effect of preventing skin from tanning. China’s laws and regulations require the PA logo to be based on the actual PFA value of the product, and the range can be marked as PA+~PA++++ according to the sunscreen ability. Most sunscreens have good adsorption effects, but their anti-sweat and anti-sebum effects are poor. On the one hand, sweat directly washes off sunscreen, resulting in a decrease in film thickness; on the other hand, sunscreen is redistributed with the flow of sweat, resulting in reduced uniformity. Both of these mechanisms will have a negative impact on the activity of sunscreen and lead to a decrease in UV protection. The experimental studies show that after sweating for six hours, the SPF effect of sunscreen decreased from 50 to 30 (Keshavarzi F et al., 2021; Ruvolo E et al., 2020) [
16,
17].
Nowadays, there has been growing interest in plant-based ingredients as potential sunscreens due to their capacity to absorb UVB and strong antioxidant properties (Burns et al., 2013) [
18]. Relative to “biological sunscreen,” “plant-derived sunscreen” research is more in-depth. The principle of “plant sunscreen” is similar to chemical sunscreen and physical sunscreen, mainly through flavonoids, anthraquinones and their derivatives, plant polyphenols and other components in plant extracts to absorb ultraviolet rays (Cooman, L et al., 1998) [
19]. Therefore, natural medicines showing absorption spectra within the UVB range receive a lot of attention for their potential in preventing UVB-induced skin damage (Duan et al., 2019; Ng S Y et al., 2012; Saraf S et al., 2010) [
20,
21,
22].
Arbutin is a hydroquinone glycoside (497-76-7). Because of its strong inhibitory effect on human tyrosinase activity, arbutin is used as a powerful whitening agent in the cosmetic industry. It is a natural compound found in many plants in the form of the isomerization of glycoside bonds between glucose and hydroquinone. α-arbutin (84380-01-8) is an isomer of natural arbutin. α-arbutin is usually produced by transglucylation of hydroquinone by microbial glycosyltransferase (Zhu X et al., 2018) [
23]. Studies have indicated that arbutin can prevent tyrosinase activity, thereby preventing melanin production and reducing skin pigmentation (Won et al., 2012; Chunhakant and Chaicharoenpong, 2019; Ye et al., 2019; Zhang et al., 2022) [
24,
25,
26,
27]. Recent studies have shown that α-Arbutin shows potential anti-photoaging effects, likely linked to diminished cellular inflammation from IL-6 and TNF-α (Hazman Ö et al., 2021; Lee et al., 2012) [
28,
29]. The increase in MMP expression is one of the main effects of ultraviolet radiation on the skin. MMP is responsible for the degradation of ECM proteins such as collagen, fibronectin, elastin and proteoglycan. They also play an important role in tissue remodeling and repair. The excessive degradation of these proteins caused by excessive production of MMP-1, MMP-3, and MMP-9 will lead to photoaging of the skin, resulting in thick wrinkles and skin relaxation through the oxidation, decomposition, or destruction of collagen and elastin (Krystyna J et al., 2021) [
30]. However, due to the complex and multi-target actions of the active components of natural plants, the pharmacological mechanism cannot be fully revealed by conventional means. Thus, exploring a plant’s manifold potential mechanisms warrants more efficient methods.
In this study, we aimed to investigate whether α-arbutin can protect mice from UVB-induced UV damage. KM mice were irradiated with UVB, and the mice in different groups were smeared with corresponding drugs before irradiation. The effect of α-arbutin on UVB damage was studied by (Hematoxylin and eosin) HE staining, (Toluidine Blue) TB staining, MASSON staining, biochemical tests, Network pharmacology and metabonomics. The results show that the intrinsic protective mechanism of α-arbutin against UVB-mediated UV injury may be regulated by the inflammatory response, immune regulation pathway and downstream factors such as COL-I, IL-1 β, TNF- α and IL-6. This study provides a new insight into the protective mechanism of α-arbutin against UV damage.
3. Discussion
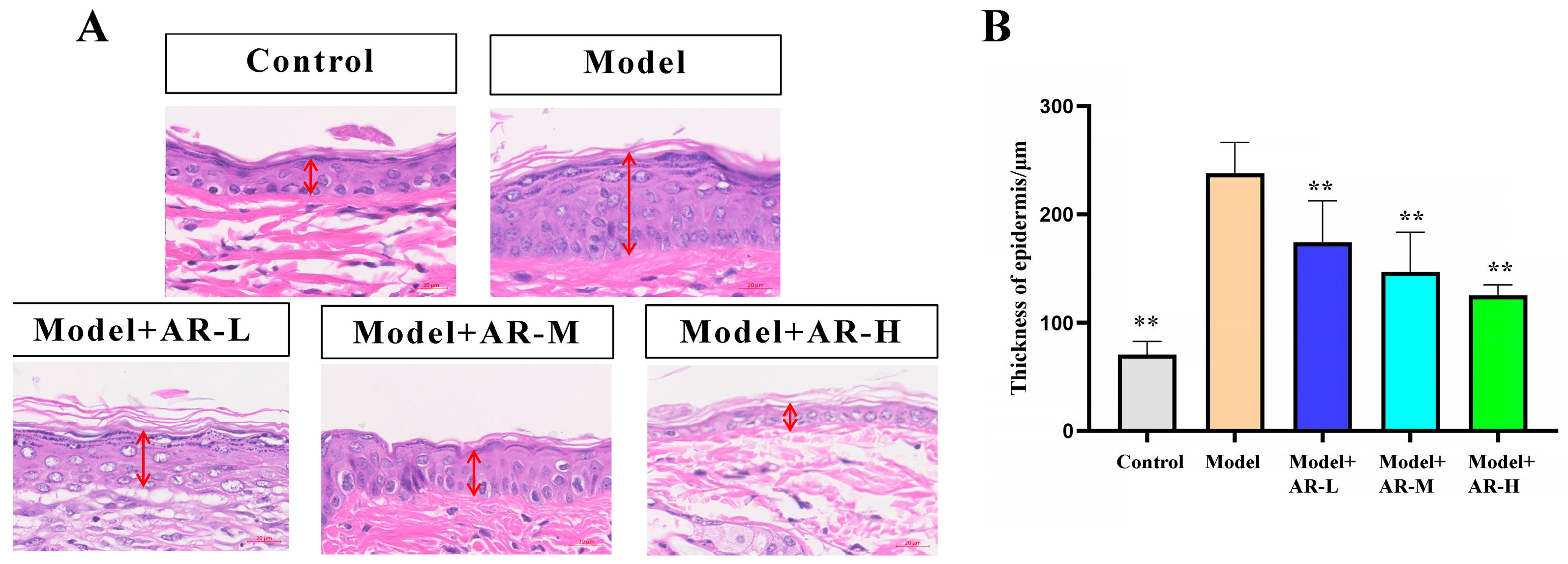
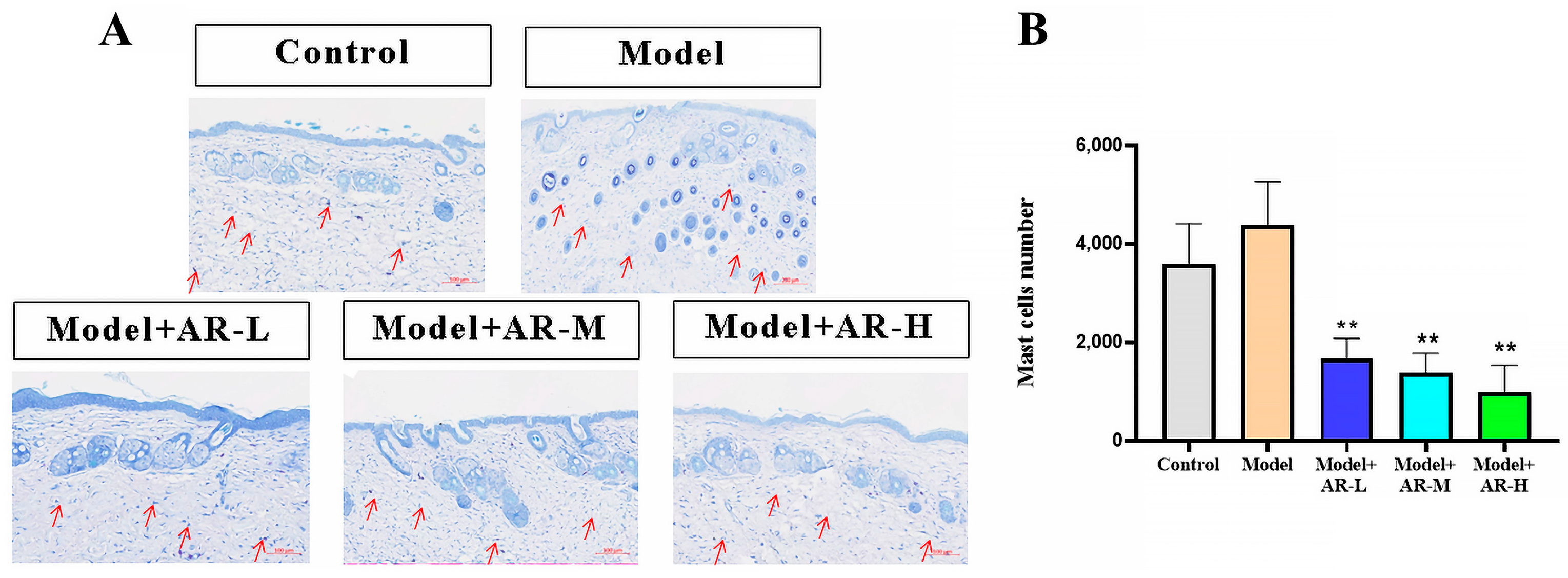
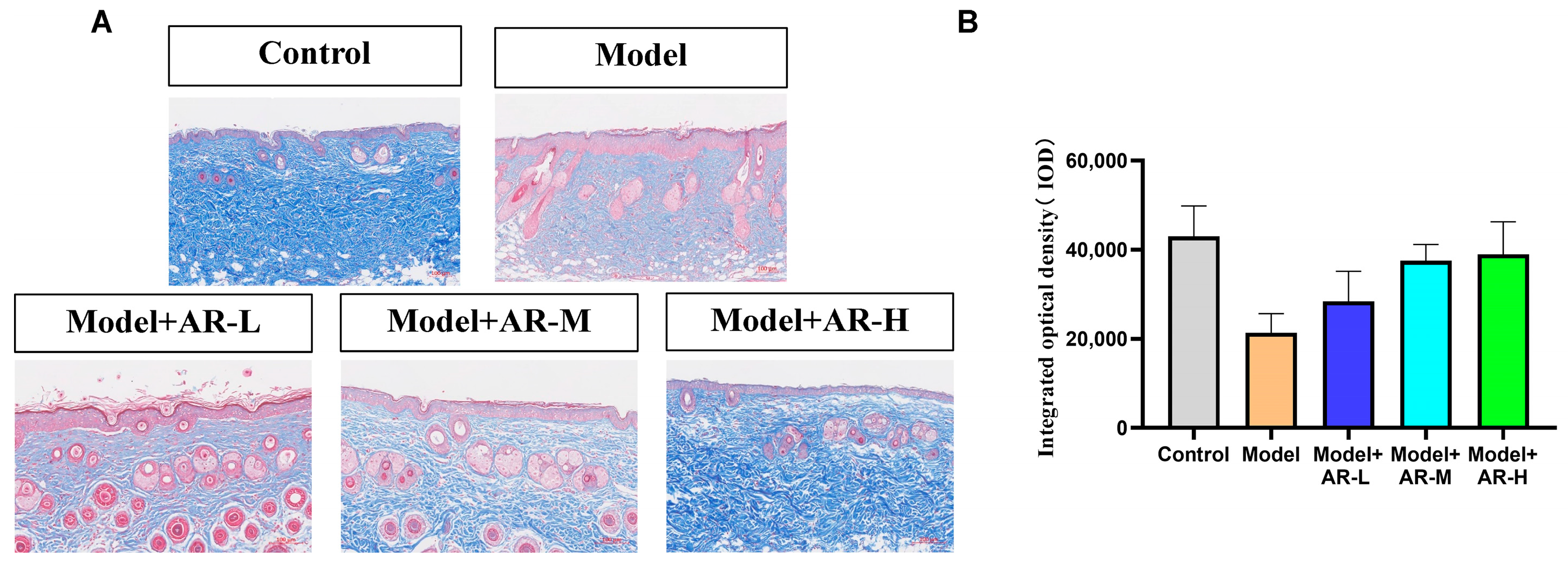
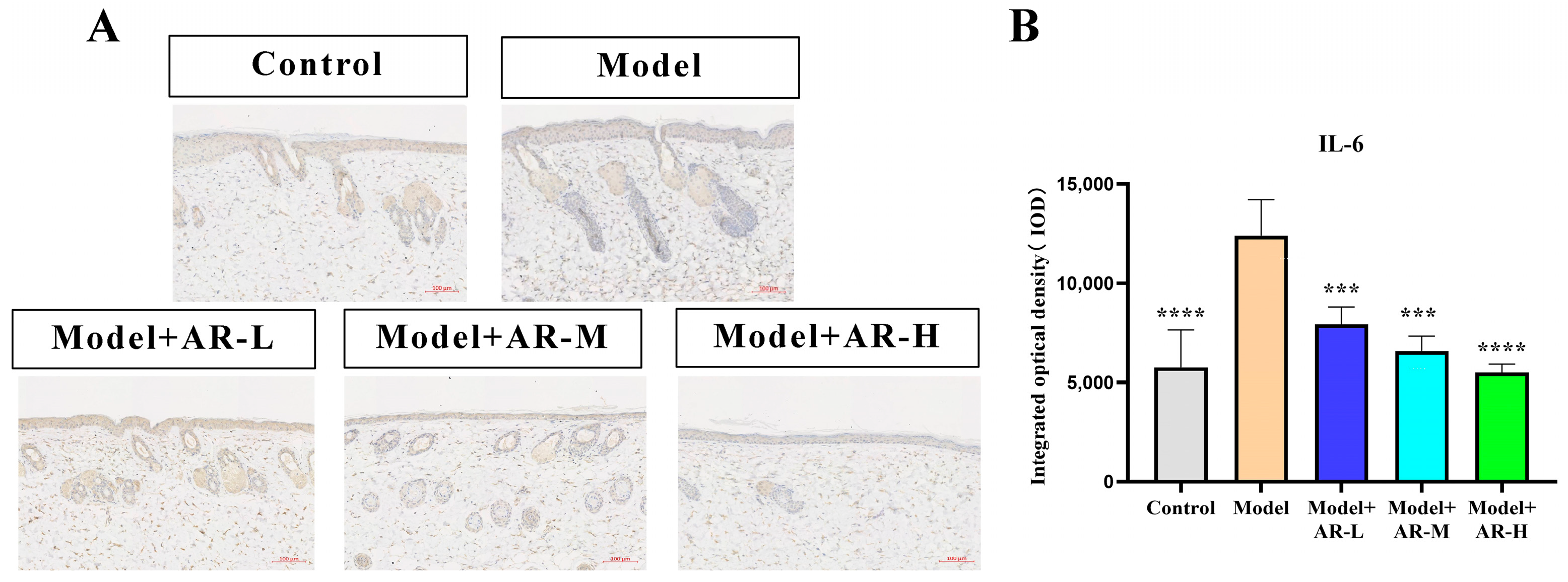
The effect of α-arbutin on skin pigmentation has long been verified (Saeedi M et al., 2021) [
32], but the protective effect and mechanism of α-arbutin on skin induced by UVB are not comprehensive. In addition, (Wang, Y et al., 2020) [
33] showed that the arbutin in Rhodiola crenulatahas has antioxidant and anti-photoaging effects, but this study has some shortcomings. On the one hand, according to the structural formula given by the study, it is found that the object of their study is β-arbutin, which is different from α-arbutin in this study. Secondly, they only demonstrated that β-arbutin could effectively improve the apoptosis induced by UVB irradiation and regulate the production of inflammatory cytokines IL-6 and TNF- α, but there was no experimental study on arbutin in vivo. In this study, α-arbutin was utilized to investigate its anti aging effect on a mouse model of photoaging. The results of animal experiments showed that α-arbutin can significantly reduce the symptoms of erythema and wrinkles on the back of mouse skin caused by ultraviolet light. The results of HE staining and Masson staining showed that α-arbutin effectively improved UV-induced epidermal thickening and reduced dermal collagen fiber breakage. However, the results of immunohistochemistry and TB staining showed that α-arbutin could significantly reduce the aggregation of mast cells and down-regulate the expression of TNF-α, IL-6, and IL-1β, mitigating the occurrence of cellular inflammation. Collagen is the main frame structure of the extracellular matrix. Human skin collagen is mainly composed of type I collagen, and fibroblasts are the main cells that synthesize collagen. Type I collagen is the most common collagen, accounting for more than 70% of normal skin collagen. Collagen plays an important role in the normal physiological processes of the skin and the occurrence of diseases, such as wound healing and skin aging. In recent years, promoting collagen synthesis in fibroblasts for medical and cosmetic purposes has become a research hotspot. In this study, the immunohistochemistry analysis of mouse back skin tissue not only reduced the expression of inflammatory factors but also increased the expression level of COL-1, thereby supplementing the skin’s collagen content. Through network pharmacology studies, it has been found that α-arbutin and the cross-targets of photoaging and whitening are related to signaling pathways such as TYR. Through transcriptomic and metabolomic analysis, it is suggested that α-arbutin may regulate a variety of signaling pathways through multiple target proteins such as COL-1 and IL-6, IL-1β, IL-17, AMPK, and TNF-α, thereby affecting the anti aging response of the body. α-Arbutin can inhibit the activity of human tyrosinase and reduce the production of melanin. However, the high hydrophilicity and hygroscopic properties of alpha-arbutin result in inadequate absorption from the skin layer, thus reducing the therapeutic effectiveness of alpha-arbutin topical products (Zhu X et al., 2018) [
23]. The results only discussed the therapeutic effect of α-arbutin on UVB-induced skin photoaging at a certain safe dose, and the specific action process of α-arbutin after entering the human body needs further study.
4. Materials and Methods
4.1. MTT Cytotoxic Assay
Hakata cells were used in the experiment, and the cells were extracted from liquid nitrogen for resuscitation. When the cells were in good viability, they were inoculated in 96-well plates at a density of 50,000 cells/mL, and 100 μL of cell suspension was added to each well. After 24 h, the cells adhered to the wall, discarded the former medium, and were supplemented with 100 μL of complete medium amended with 2 μg/mL, 1 μg/mL, and 0.5 μg/mL of α-arbutin, respectively. These cultures were incubated for another 24 h at a constant 37 °C and 5% CO2 in an environment-controlled incubator. Following this, 100 μL of MTT (0.5 mg/mL, base medium dilution) was added to a 96-well plate. With the aid of tin foil, it was placed in a culture box for 4 h before discarding the initial solution and adding 100 μL of DMSO to each well, which was then shaken for 10 min. The absorbance reading was acquired at 570 nm, calculating the survival rate of the cells.
4.2. Animals and Treatment
SPF-grade KM (Kunming) mice (5 weeks old, male, 20–25 g) were acquired from Guangdong Experimental Animal Center. Ethical approval for all experiments was granted by the Animal Ethics Committee, the Guangdong University of Technology. The α-arbutin (C12H16O7, purity ≥ 98%) used in the experiment was purchased from Aladdin, Shanghai. Mice were maintained under standard conditions (24 ± 1 °C temperature, 70–75% humidity, 12 h light/dark cycle) with free access to food and water. The day before the experiment, after 4 × 4 cm of hair removal on the backs of the mice. Fifty mice were randomly divided into 5 groups: control group, model group, 0.5% α-arbutin (model + AR − L), 1% α-arbutin (model + AR-M), and 2% α-arbutin (model + AR − H).
Among them, the mice in the control group were fed normally without any treatment, while the mice in the model group were irradiated with a UVB lamp for a certain period of time and smeared with a certain dose of normal saline (irradiation intensity 300 mj/days, irradiation every other day for 28 days, and saline 200 μL/day for 28 days). The mice in the AR − L group were irradiated with a certain dose of UVB lamp and smeared with a certain dose of drugs after irradiation (radiation intensity: 300 mj/days, every other day for 28 days; saline containing 0.5% α-arbutin 200 μL/each for 28 days), and mice in the AR m group were irradiated with a certain dose of UVB lamp and smeared with a certain dose of drugs after irradiation (radiation intensity 300 mj/day, every other day for 28 days; saline containing 1% α-arbutin 200 μL/each for 28 days). The mice in the AR − H group were irradiated with a certain dose of UVB lamp and smeared with a certain dose of drugs after irradiation (radiation intensity 300 mj/days, irradiation every other day for 28 days; smearing 200 μL of saline containing 2% α-arbutin per mouse/day for 28 days). On the 28th day after administration, the back skin of the mice was photographed and scored according to the visual scoring standard. Subsequently, the mice were euthanized by cervical dislocation, and the skin tissues from their backs were fixed and frozen using 4% paraformaldehyde.
4.3. Hematoxylin and Eosin Staining (H&E)
The skin tissue to be fixed was made into sections of paraffin and stained with hematoxylin and eosin. It was then examined under a microscope, and images were collected for histological analysis.
4.4. Toluidine Blue Staining
Paraffin sections underwent water dewaxing. Tissue sections were immersed in a dye solution for 2–5 min, followed by H2O washes, differentiation with 0.1% glacial acetic acid, drying, and transparent sealing. Observation under microscopy, image capture, and analysis.
4.5. Masson Staining
After paraffin sectioning of the tissue, soak in xylene and dehydrate with a gradient of anhydrous ethanol. They were then stained with Weigert-iron-hematoxylin for 10 min, followed by rinsing with distilled water. One percent hydrochloric acid alcohol was used for differentiation and then the tissue washed with distilled water. Stain with Ponceau S Staining Solution for 5–10 min. Treatment with 1% Phosphomolybdic acid for 1 min, then re-dye with Water blue for 5 min without water washing. Using 1% acetic acid treatment for 1 min and dehydrating with 95% anhydrous ethanol multiple times. Finally, they were transparentized with xylene and sealed with a neutral adhesive. Following the staining, collagen fibers will appear blue, cytoplasm and red blood cells will appear red, and the nucleus will appear blue-brown.
4.6. Immunohistochemistry
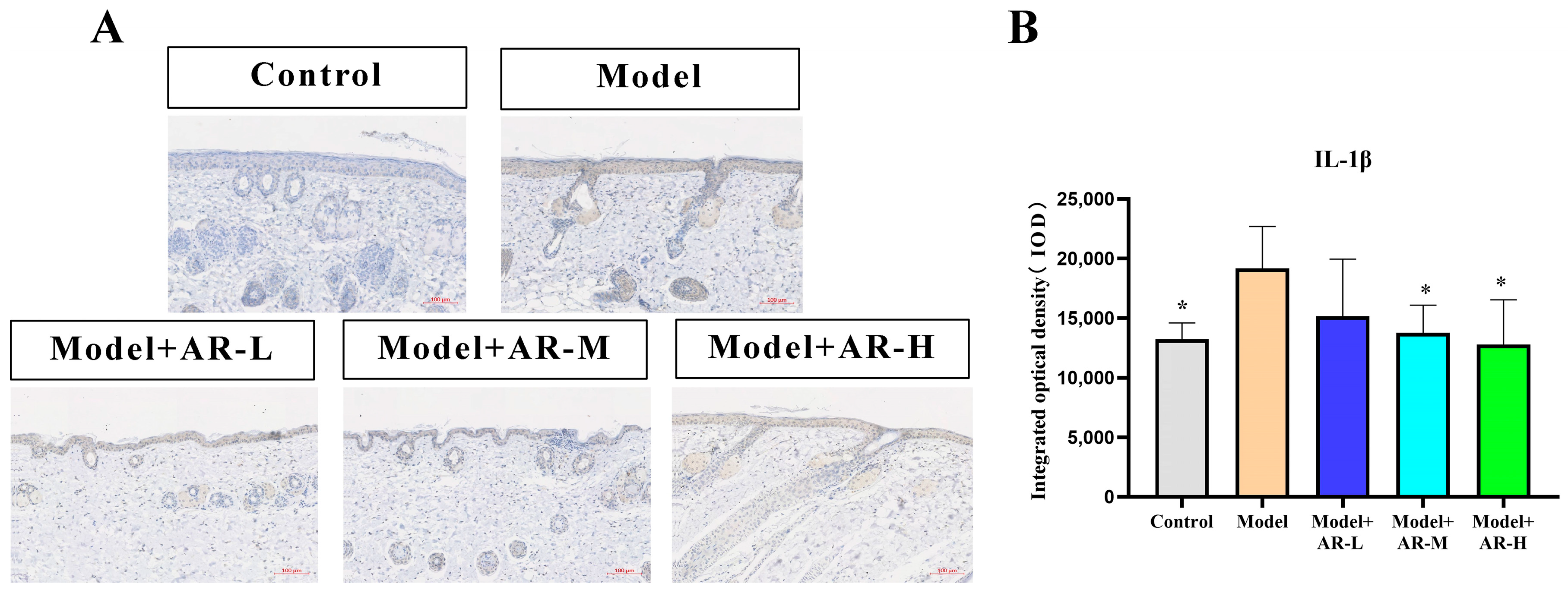
Paraffin sections at a concentration of 4 μM were dewaxed with xylene, gradually dehydrated with anhydrous ethanol, and then treated with 3% hydrogen peroxide for 10 min. Afterwards, they were washed with a phosphate-buffered solution. Following serum blockade, antibodies for COL-1, TNF-α, IL-6, and IL-1β (diluted with distilled water in a 1:100 volume ratio) were incubated overnight at 4 °C. The second antibody, a rabbit IgG enzyme-linked antibody, was then added, and the samples were incubated at 37 °C. After incubation with Horseradish peroxidase, DAB staining and Haematoxylin restaining were performed. After the production is completed, randomly select the shooting site under a 200× microscope. Apply the software Image-Pro Plus to measure the IOD value of COL-1, TNF-α, IL-6, and IL-1β. Finally, data were collected and plotted with GraphPad Prism (8.0.2).
4.7. Metabolomics Study
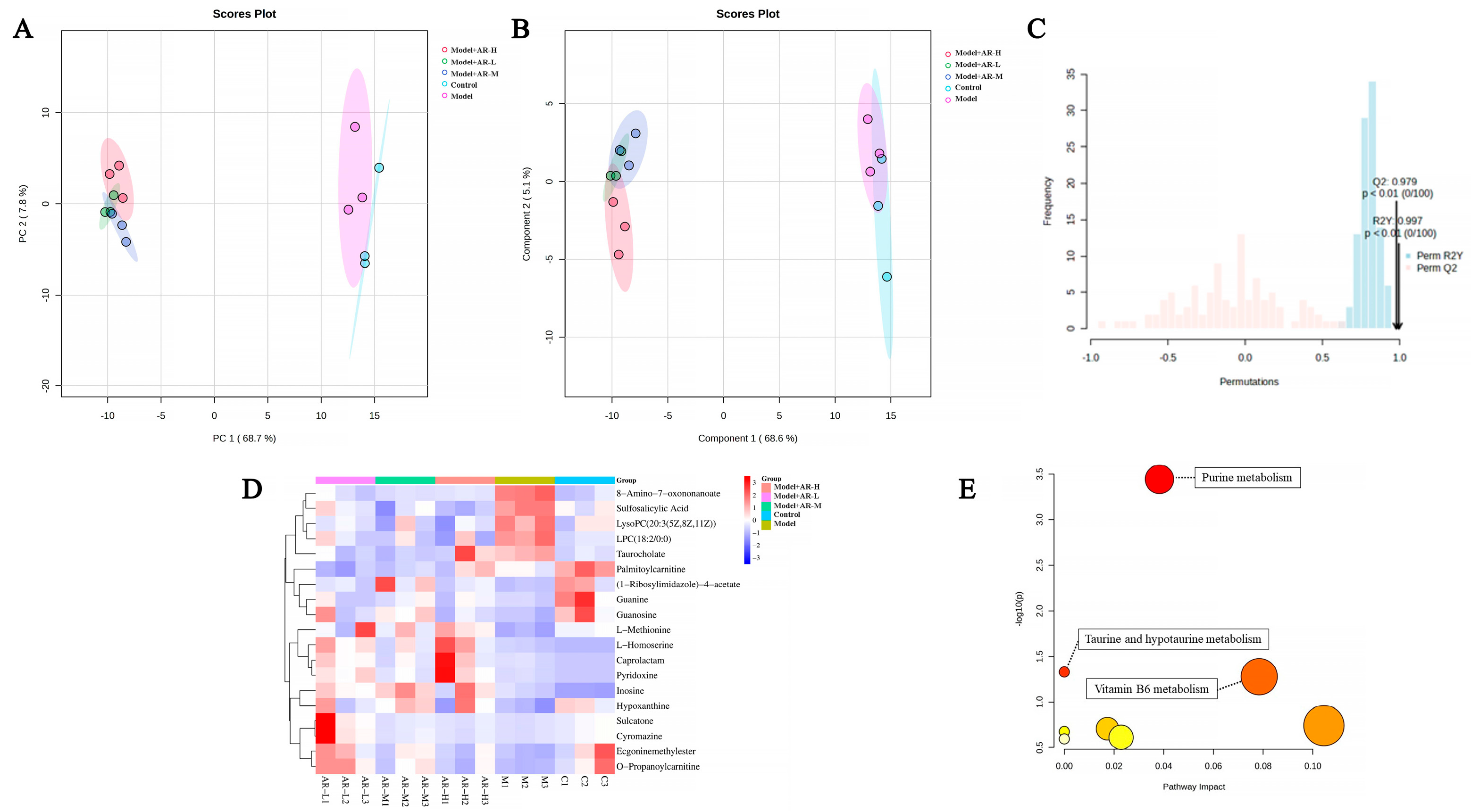
The chosen experimental approach for metabolomics was informed by earlier studies, albeit with minor alterations. To prepare the sample, the homogenate was prepared by mixing 0.1 g of skin tissue with 1 mL of chromatographic-grade methanol and spun at 13,000 g for 15 min at 4 °C. Subsequently, the supernatant underwent filtration with a 0.22 μm filter (NEST Biotechnology Wuxi Nest Life Technology Co., Ltd., Wuxi, China), followed by the addition of 100 μL of this supernatant to the vial for further analysis. Using an ultrahigh-performance liquid chromatography system to perform chromatographic separation of the target compound through a liquid chromatography column. Chromatography settings comprise the Agilent 1290 Infinity LC ultrahigh-performance liquid chromatography system (UPLC), a hydrophilic interaction liquid chromatographic (HILIC) column for separation at 25 °C, a flow rate of 0.5 mL/min and a 2 µL injection volume. The composition of the mobile phase ought to include A: water mixed with 25 mmol/L ammonium acetate and 25 mmol/L ammonia water, and B: acetonitrile. Gradual elution conditions should be maintained as follows: 0 → 0.5 min, 95% B; 0.5 min → 7 min, 95% → 65% B; 7 min → 8 min, 65% → 40% B; 8 min → 9 min, 40% B; 9 min → 9.1 min, 40% → 95% B; and 9.1 min → 12 min, 95% B. The AB Triple TOF 6600 mass spectrometer was employed for gathering both primary and secondary spectra of the specimen. Conditions for ESI source: Gas from Ion Source at a ratio of 1:60, Gas from Ion Source at 2:60, Current gas at 30, Voltage of IonSapary Floating: ±5500 V for both positive and negative modes; Mass Spectrometry (TOF) scan in mass-to-charge (m/z) range: 60–1000 Da, and ion scan of the product in mass-to-charge (m/z) range: 25–1000 Da; Secondary mass spectrometry was conducted using data-dependent acquisition and a high-sensitivity mode, featuring a clustering potential of ±60 V for both positive and negative modes, and a Collision Energy of (35 ± 15) eV.
4.8. Transcriptome Sequencing and Analysis
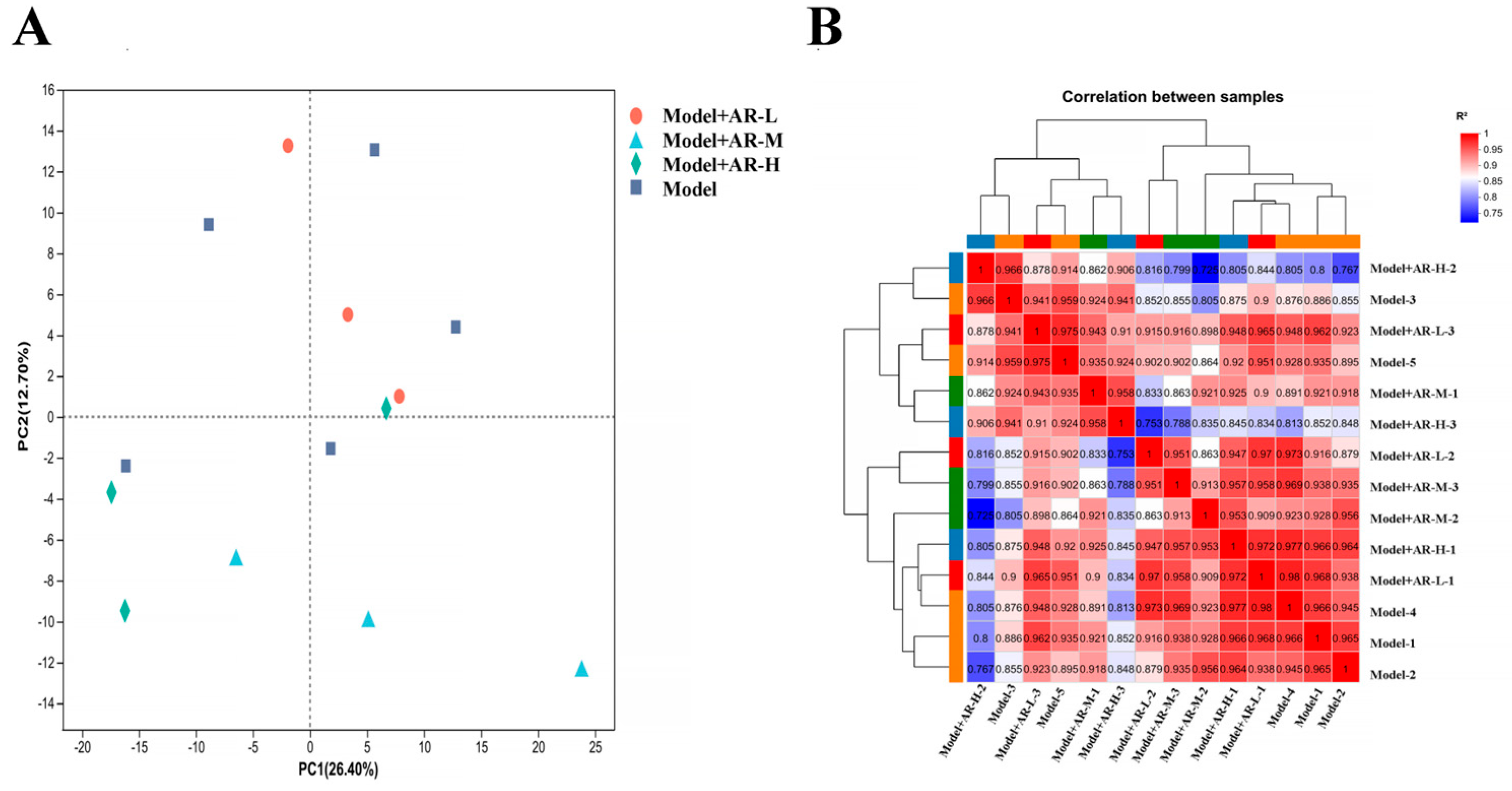
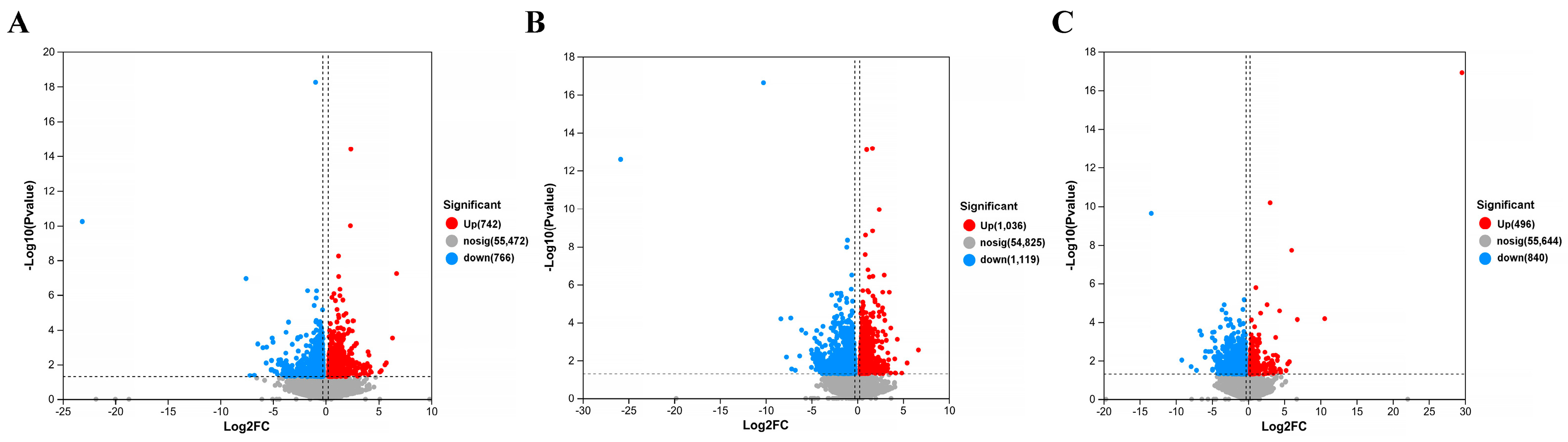
Transcriptome sequencing of library data was performed using the Illumina Hiseq 4000 platform. In order to ensure data quality, fast quality control is used to filter out low-quality data for offline data raw reads and remove sequences containing connectors, sequences containing more than 10% N (uncertain base), sequences containing base A, and sequences containing more than 50% of low-quality base content (Q ≤ 20). The Bowtie2 short sequence alignment tool was used for ribosome alignment, while HISAT2 v2. software (
http://ccb.jhu.edu/software/hisat2/index.shtml accessed on 11 April 2024) was used for sequencing sequence alignment and reference genome alignment. Stringtie software (accessed on 11 April 2024) was used for transcript reconstruction, and the expression amount of all genes in each sample was calculated. Calculate the correlation of samples using the R language. Using DESeq software 1.26.0 (
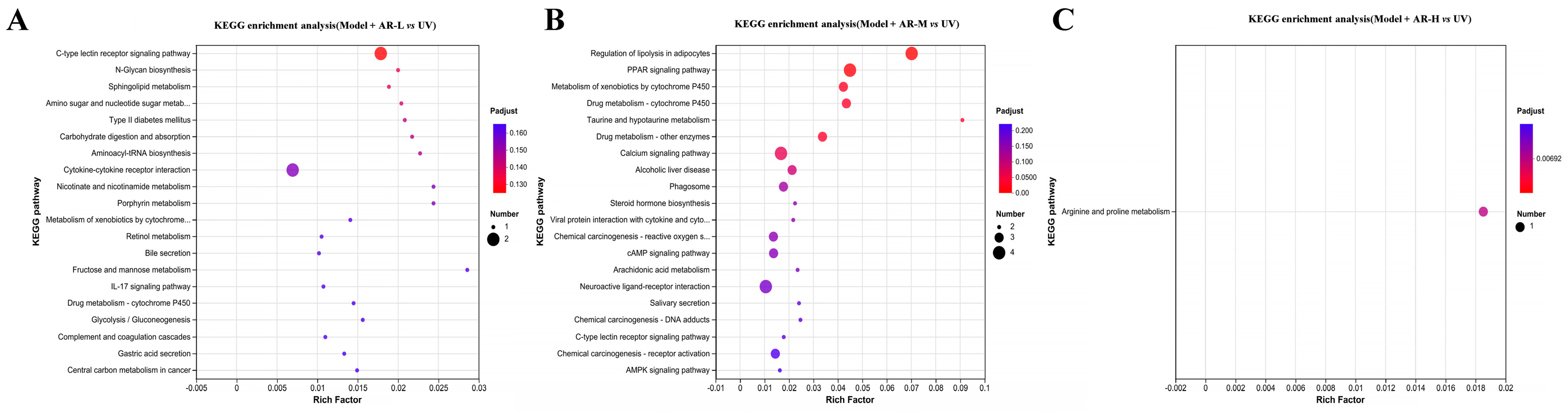
https://www.rdocumentation.org/packages/DEGseq/versions/1.26.0 (accessed on 11 April 2024)) to analyze differentially expressed genes, FDR < 0 Genes with 0.05 and |log2(FC)| > 1 were labeled as significantly different genes. Map differentially expressed proteins to various terms in the GO database, perform comparative tests, and perform GO enrichment analysis on differentially expressed genes. Compare sequencing results with the KEGG database to analyze their pathways and perform functional annotation and classification of differentially expressed genes.
4.9. Network Pharmacology Analysis
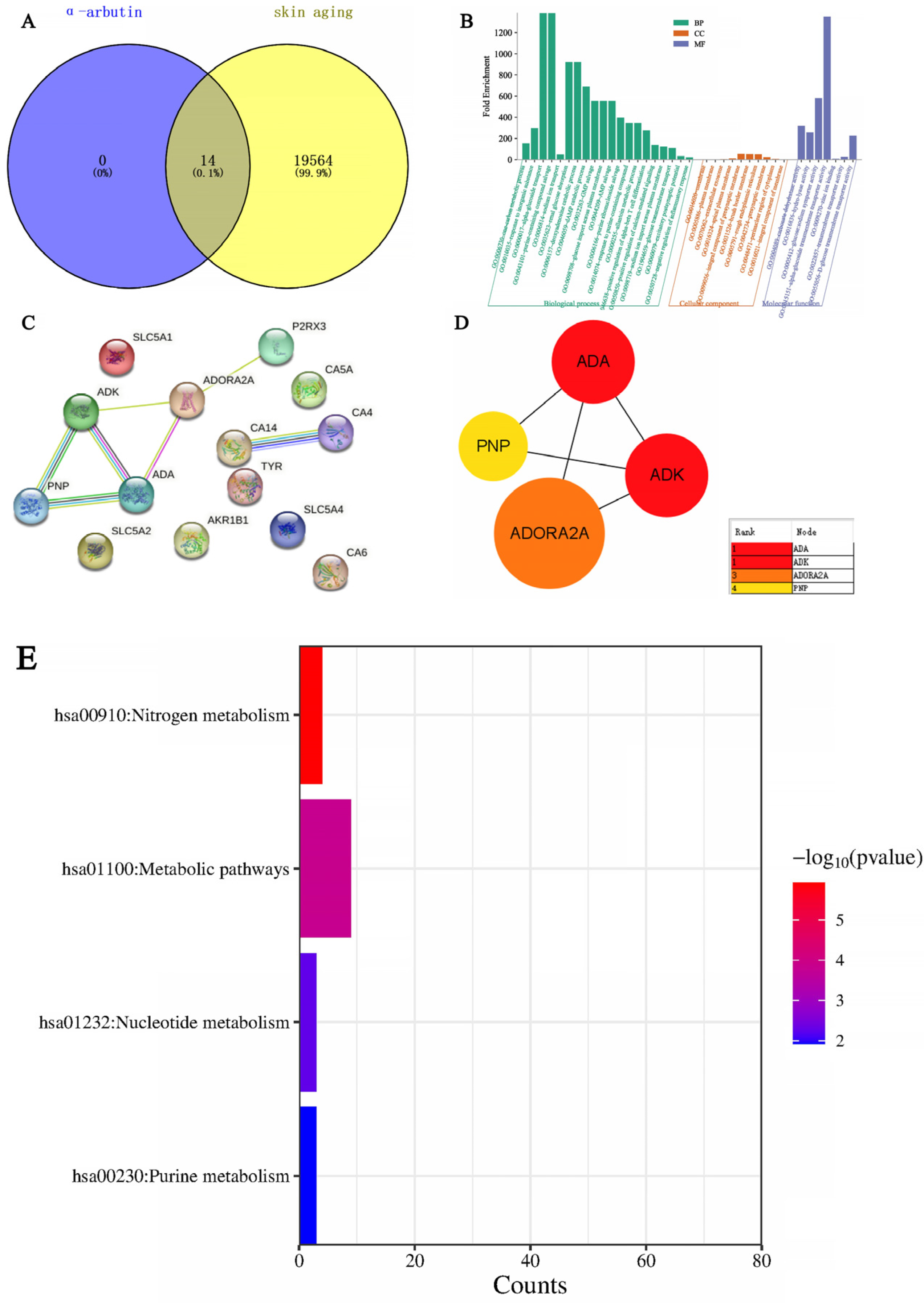
The search for the “senescence” keyword in the GeneCards database (
www.genecards.org/ (accessed on 11 April 2024)) generated targets related to senescence. Potential targets of differential metabolites (DMs) were sourced from the SEA (
https://sea.bkslab.org/ (accessed on 11 April 2024)) and TCMSP databases (
http://tcmspw.com/tcmsp.php (accessed on 11 April 2024)) and their intersections with senescence were determined through Venny (
https://bioinfogp.cnb.csic.es/tools/venny/index.html (accessed on 11 April 2024)). The tool STRING (
www.string-db.org/ (accessed on 11 April 2024)) was used to examine both the direct and indirect interplays among these targets. Subsequently, a PPI network map was created, visualizing the top 20 targets of the highest degree. DAVID (
https://david.ncifcrf.gov (accessed on 11 April 2024)) served the purpose of categorizing Gene Ontology (GO) and enriching pathways in the Kyoto Encyclopedia of Genes and Genomes (KEGG). Visualization of GO terms and KEGG pathways was achieved through the online tool Weishengxin (
http://www.bio-informatics.com.cn/ (accessed on 11 April 2024)).