Study on the Compositional Analysis, Extraction Process, and Hemostatic and Anti-Inflammatory Activities of Cirsium japonicum Fisch. ex DC.–Cirsium setosum (Willd.) MB Extracts
Abstract
1. Introduction
2. Results and Discussion
2.1. Chemical Composition Analysis of CF-CS
2.2. The results of the Single-Factor Experiments
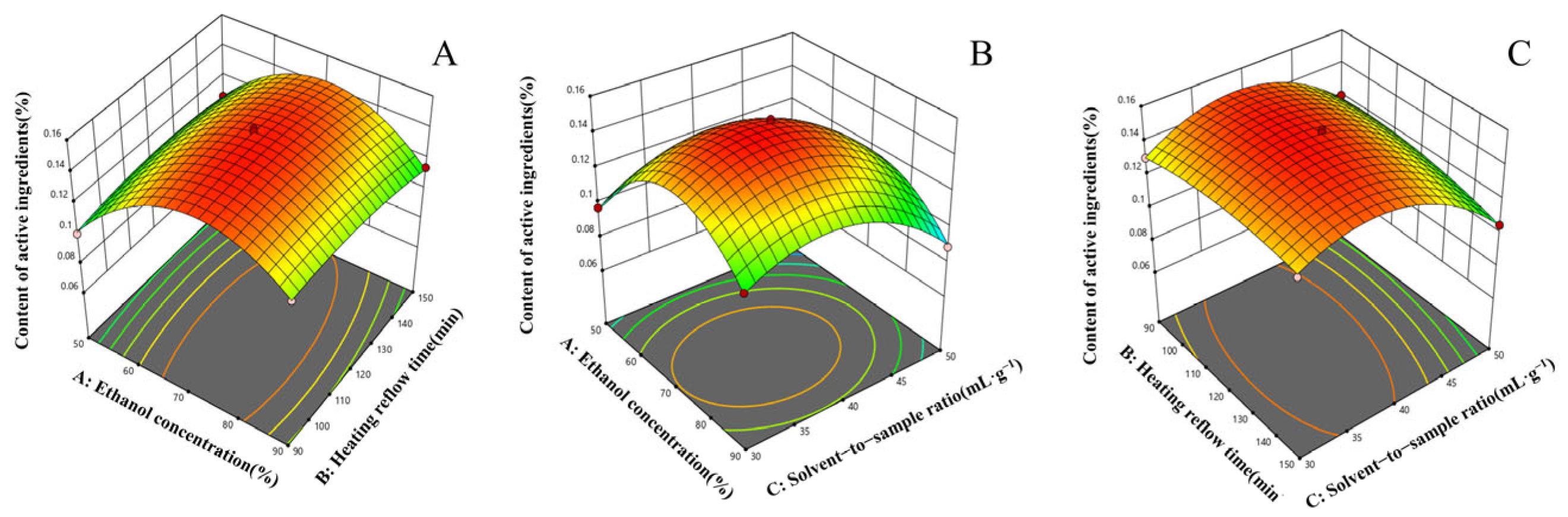
2.3. Response Surface Experiment Results and Analysis
2.3.1. The Results of the Response Surface Experiments
2.3.2. Model Fitting and Statistical Analysis
2.3.3. Graphical Interpretation and Optimization of Procedure
2.4. Validation Results of the Quantitative Method of Linarin
2.5. Results of Linarin Content Determination
2.6. Results of Coagulation and Hemostasis Tests
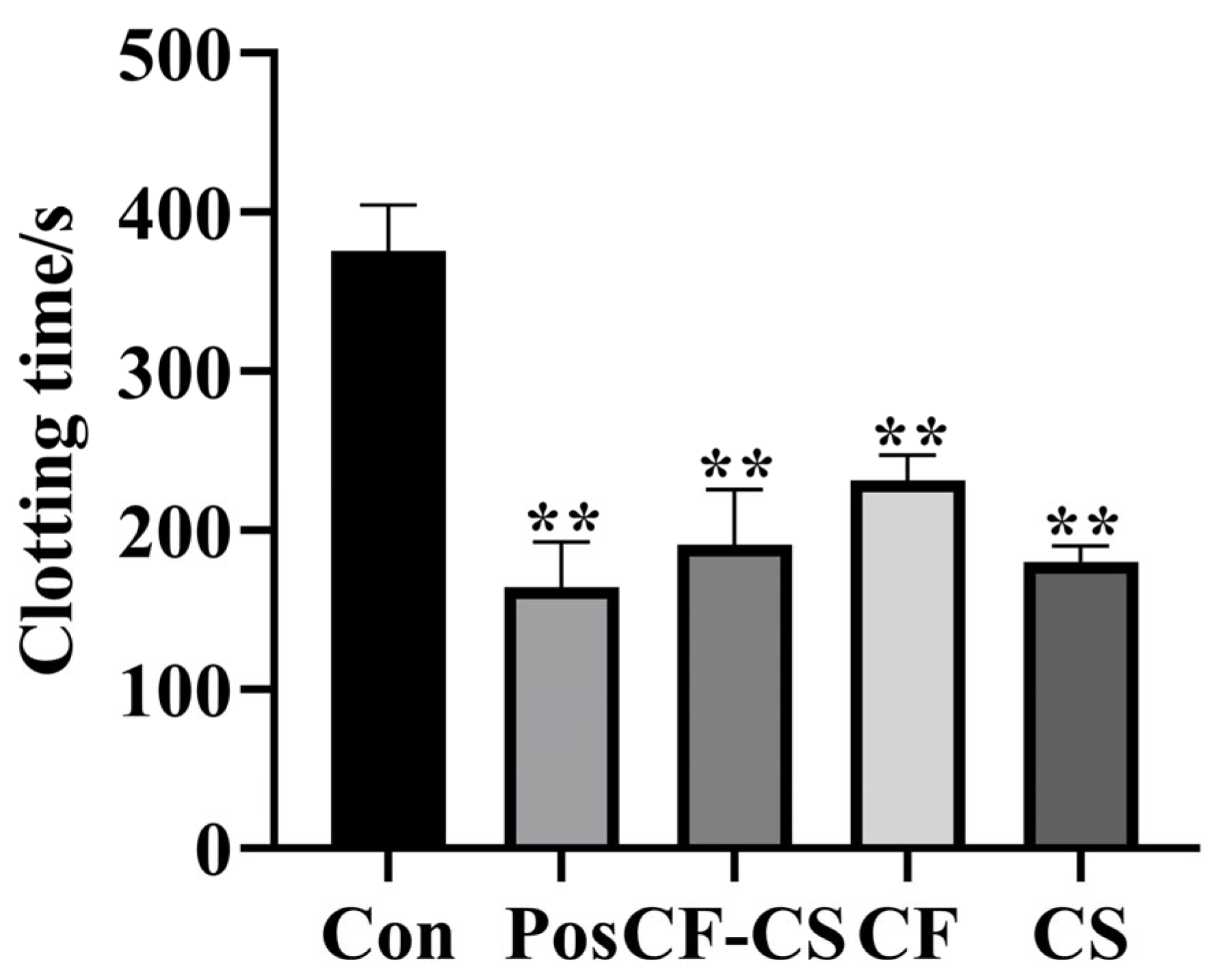
2.6.1. Results of In Vitro Coagulation Tests
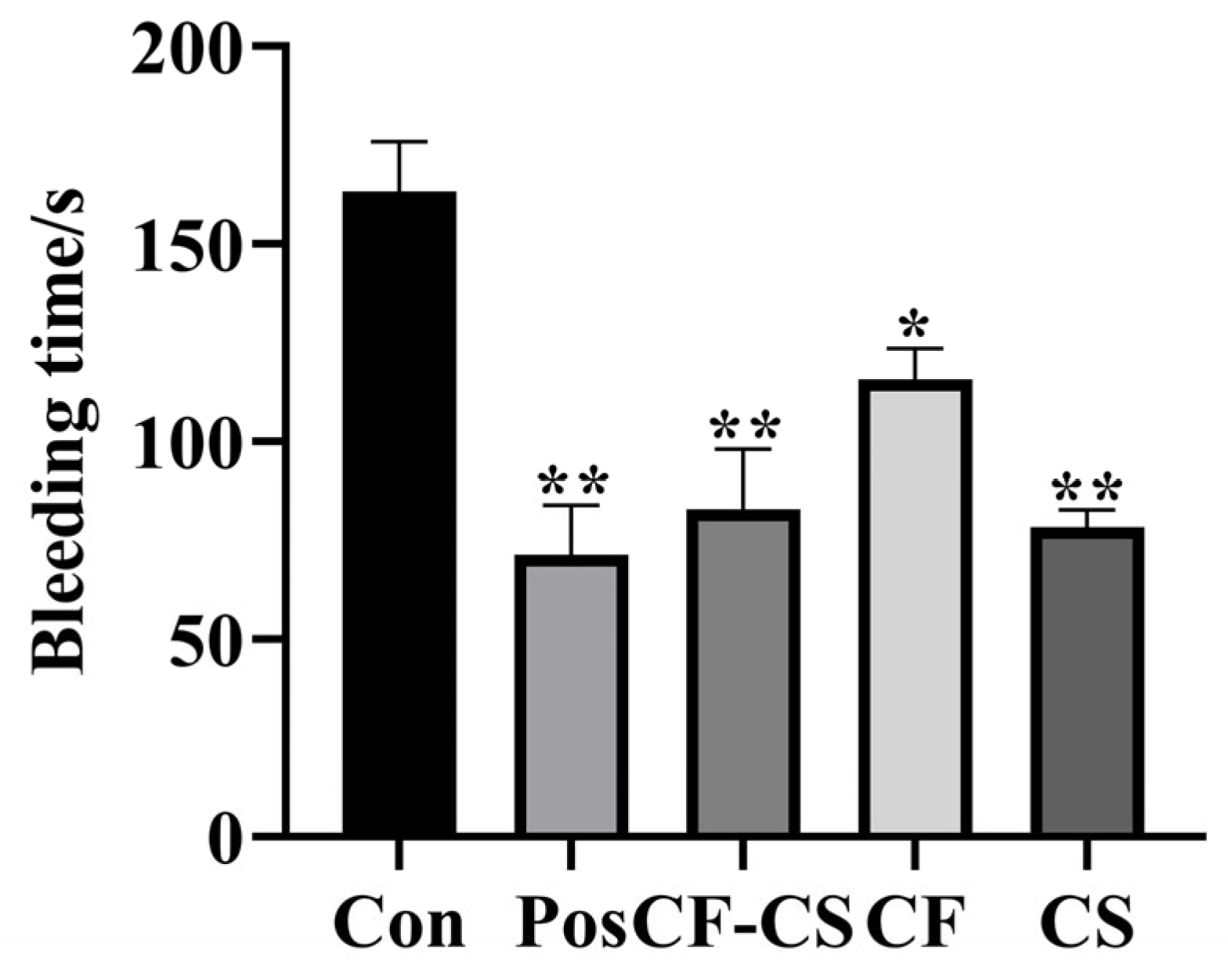
2.6.2. Results of Experiments on Traumatic Hemorrhage in Rabbits
2.7. Results of Anti-Inflammatory Experiments
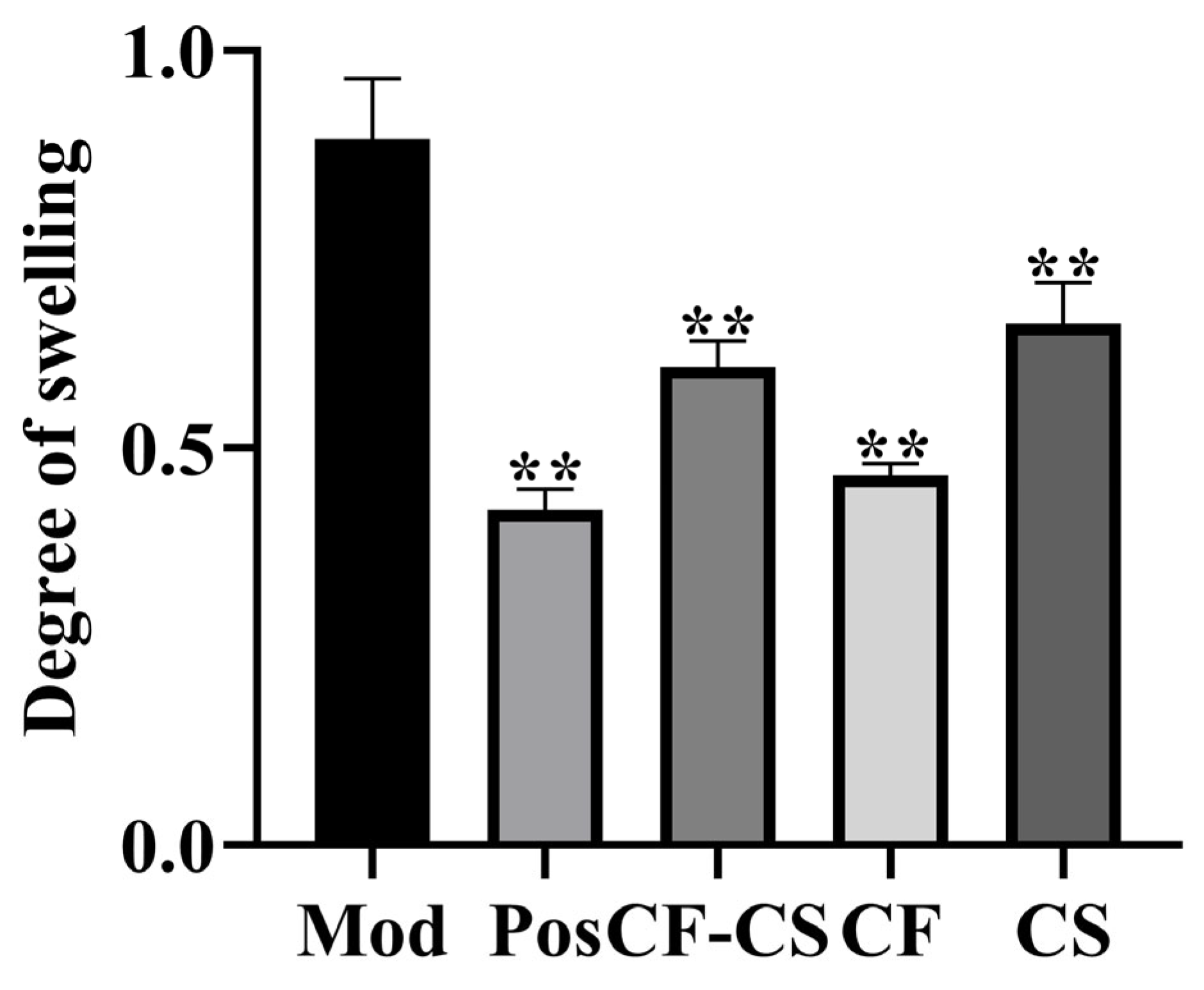
2.7.1. Experimental Results of Xylene-Induced Ear Swelling in Rabbits
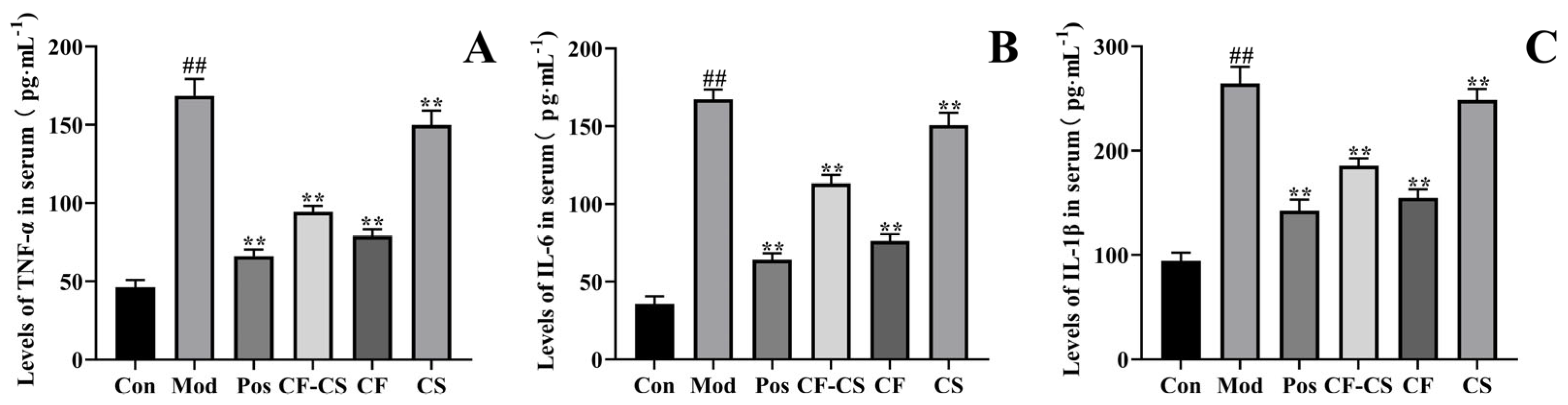
2.7.2. Results of the LPS-Induced Inflammation Experiment in Rabbits In Vivo
2.8. Signal Pathway Analysis of CF and CS
3. Materials and Methods
3.1. Instruments, Reagents, and Drugs
3.2. Samples and Processing
3.3. Preparation of Test Solution
3.4. UHPLC-MS Detection Conditions
3.4.1. Chromatographic Conditions
3.4.2. Mass Spectrometry Conditions
3.5. Analysis of Chemical Constituents in CF-CS Extracts
3.6. Experimental Design of the Extraction Process
3.6.1. Single-Factor Experiments
3.6.2. Box–Behnken Design Optimization
3.7. Validation of Quantitative Method for Linarin
3.8. Determination of Linarin Content
3.9. Study on the Role of Coagulation and Hemostasis
3.9.1. In Vitro Coagulation Assay in Rabbits
3.9.2. Bleeding Test of the Marginal Artery of the Ear in Rabbits
3.9.3. Data Processing
3.10. Study of Anti-Inflammatory Effects
3.10.1. Xylene-Induced Ear Swelling Experiment in Rabbits
3.10.2. LPS-Induced Inflammation in Rabbits
3.10.3. Data Processing
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chinese Pharmacopoeia Commission. Pharmacopoeia of the People’s Republic of China (One Part); China Medical Science Press: Beijing, China, 2020; Volume 50–51, pp. 26–27. [Google Scholar]
- Zhang, X.; Liao, M.; Cheng, X.; Liang, C.; Diao, X.; Zhang, L. Ultrahigh-performance liquid chromatography coupled with triple quadrupole and time-of-flight mass spectrometry for the screening and identification of the main flavonoids and their metabolites in rats after oral administration of Cirsium japonicum DC. extract. Rapid Commun. Mass Spectrom. 2018, 32, 1451–1461. [Google Scholar] [PubMed]
- Wang, H.C.; Bao, Y.R.; Wang, S.; Li, T.J.; Meng, X.S. Simultaneous determination of eight bioactive components of Cirsium setosum flavonoids in rat plasma using triple quadrupole LC/MS and its application to a pharmacokinetic study. Biomed. Chromatogr. 2019, 33, e4632. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.X. Clinical Research of National Medical Masters: Identification and Clinical Application of Liu Shangyi’s Commonly Used Drug Pairs; Science Press: Beijing, China, 2016; p. 62. [Google Scholar]
- Ye, Y.; Chen, Z.; Wu, Y.; Gao, M.; Zhu, A.; Kuai, X.; Luo, D.; Chen, Y.; Li, K. Purification Process and In Vitro and In Vivo Bioactivity Evaluation of Pectolinarin and Linarin from Cirsium japonicum. Molecules 2022, 27, 8695. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.C.; Ji, L.L.; Zhong, X.J.; Li, J.J.; Wang, X.; Shang, X.Y.; Lin, S. Taraxastane-type triterpenoids from the medicinal and edible plant Cirsium setosum. Chin. J. Nat. Med. 2019, 17, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Mu, K.; Liu, Y.; Liu, G.; Ran, F.; Zhou, L.; Wu, Y.; Peng, L.; Shao, M.; Li, C.; Zhang, Y. A review of hemostatic chemical components and their mechanisms in traditional Chinese medicine and ethnic medicine. J. Ethnopharmacol. 2023, 307, 116200. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Hong, R.; Li, B.; Wang, A.; Chen, Y.; Wang, Y.; Mo, F.; Liu, M.; Tian, C. Extraction technology, components analysis and anti-inflammatory activity in vitro of total flavonoids extract from Artemisia anomala S. Moore. Fitoterapia 2023, 170, 105630. [Google Scholar] [CrossRef]
- Xiao, G.L.; Jiang, J.Y.; Cheng, P.Y.; Zhang, J.N.; Tang, R.Y.; Li, D.M.; Li, Y.X. Analysis of Chemical Constituents in the Leaves of Pluchea lndica (L.) Less. by UPLC-O-TOF-MS/MS. J. Instrum. Anal. 2023, 42, 1424–1433. [Google Scholar]
- Jiang, Q.Y.; Li, C.C.; Chen, H.L.; Huang, Z.F.; Zhao, W.; Liang, Y.; Pan, H.F.; Zhuo, Y. Qualitative and Quantitative Analysis of Chemical Constituents in Liu Junzitang by UPLC-O-TOF-MS/MS and UPLC-UV. Chin. J. Exp. Tradit. Med. Form. 2023, 1–14. [Google Scholar]
- Gou, X.L.; Ding, Y.; Lu, Y.T.; Yi, H.; Xie, Y.C.; Zeng, Y.J.; Fan, G. Chemical Composition Analysis of Tibetan Medicine Sabinae strobilus Based on UPLC-Q-Exacutive Orbitrap MS Technology. J. Chengdu Univ. Tradit. Chin. Med. 2023, 46, 22–30. [Google Scholar]
- Ma, D.Y.; Gao, X.Y.; Peng, L.F.; Wu, S.F.; Wang, Q.T.; Hao, Z.H. Composition Analvsis of Seed of Areca catechu L. in Deep Processing Based on UHPLC-QE-Orbitrap-Ms Technology. Acta Vet. Zootech. Sinica. 2023, 54, 5275–5292. [Google Scholar]
- Wang, L.L.; Li, Y.C.; Ma, Z.; Yang, C.L. Rapid analysis of chemical constituents of Bidens by ultra-performance liquid chromatography-quadrupole/exactive orbitrap mass spectrometer. Chem. Ana. Meterage 2023, 32, 11–17. [Google Scholar]
- Tao, X.; Zhang, J.X.; Hu, Q.; Sun, J.; Dong, Y.; Ding, J.G.; Yu, H.; Shen, Y.Y.; Mao, X.H.; Ji, S. Simultaneously quantitative analysis of 35 components in gualoupi injection using hydrophilic interaction liquid chromatography tandem mass spectrometry. Acta Pharm. Sin. 2023, 58, 1293–1300. [Google Scholar]
- Hong, L.L.; Zhao, Y.; Chen, W.D.; Yang, C.Y.; Li, G.Z.; Wang, H.S.; Cheng, X.Y. Tentative exploration of pharmacodynamic substances: Pharmacological effects, chemical compositions, and multi-components pharmacokinetic characteristics of ESZWD in CHF-HKYd rats. Front. Cardiovasc. Med. 2022, 9, 913661. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yang, H.; Liao, H.; Fan, H.; Liang, C.; Deng, L.; Jin, S. Simultaneous determination of ten biogenic amines in a thymopolypeptides injection using ultra-performance liquid chromatography coupled with electrospray ionization tandem quadrupole mass spectrometry. J. Chromatogr. B Analyt Technol. Biomed. Life Sci. 2013, 929, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Zhao, Y.J.; Xue, Z.P.; Wang, N.; Bai, J.Q.; Wang, X.P. Analysis of chemical constituents in Rhamnus erythroxylum Pallas by HPLC-Q-TOF-MS. Cent. South Pharm. 2024, 22, 78–85. [Google Scholar]
- Lv, W.S.; Wei, C.J.; Pan, X.J.; Yang, W.H.; He, M.Y.; Chen, X.D.; Sun, D.M.; Wei, M.; Li, Z.Y. Variations of Chemical Components in lnula japonica by UPLC-MS/MS before and after Honey-frying. J. China Pharm. 2021, 32, 2478–2484. [Google Scholar]
- Xie, Y.; Ye, K.W.; Li, B.; Hou, X.T. Analysis of chemical constituents in Kanglao Capsule by UHPLC-Q-TOF-MS/MS. Chin. Tradit. Patent Med. 2023, pp. 1–9. Available online: http://kns.cnki.net/kcms/detail/31.1368.R.20231024.1512.004.html (accessed on 19 March 2024).
- Li, Y.S. Study on chemical composition and extraction technology of Xinhui tangerine peel. FOSU 2022. [Google Scholar]
- Zhao, Y.M.; Zhang, L.X.; Yang, S.Y.; Wang, Z.K.; Li, C.Y.; Shu, Y.C. Characterization of chemical constituents from traditional Chinese medicine Schizonepetae Spica based on UHPIC-Q-TOF-MS/MS technique. China J. Chin. Mater. Med. 2024, 49, 420–430. [Google Scholar]
- Chen, X.; Zhang, X.R.; Mu, L.T.; Ren, H.X.; Zhang, Y.; Wang, L.H.; Sun, C.H. Characterization of chemical constituents and identification of absorbed prototypes components in rat serum of Scutellaria baicalensis by UHPLC-Q-Orbitrap-MS. Chin. Tradit. Herb. Durg. 2023, 54, 2722–2732. [Google Scholar]
- Su, K.X.; Zhao, Y.H. Bioactivity Evaluation and UPLC-MS Analysis of Different Solvent Extracts from Flat-European Hazelnut By-products. Food Sci. 2023, pp. 1–15. Available online: http://kns.cnki.net/kcms/detail/11.2206.TS.20230530.0925.014.html (accessed on 19 March 2024).
- Min, S.; Liu, R.; Wang, Y.; Wang, D.Y.; Wang, S. Determination of capsaicin in edible oils by ultra performance liquid chromatography-tandem mass spectrometry. J. Food Saf. Qual. 2021, 12, 5707–5712. [Google Scholar]
- Jing, L.J.; Xiao, W.K.; Gan, Y.X.; Meng, X.L.; Chen, X.R.; Zheng, S.C. Study on the chemical constituents of Prepared Chuanwu-White Paeonia lactiflora pairs based on UPLC-Q-Orbitrap HRMS technique. Chin. Med. Mat. 2023, 4, 911–918. [Google Scholar]
- Ma, B.J.; Xiao, Y.; Chen, Z.D.; Shu, R.G.; Li, T.; Jiang, L.; Xu, G.L.; Zhang, Q.Y. Analysis of Chemical Constituents in Percolate the Extract of Cyclocarya paliurus Tender Leaves by UHPLC-Q-TOF-MS/MS. Sci. Technol. Food Ind. 2023, 44, 281–291. [Google Scholar]
- Zhang, J.W.; Liu, W.; Shen, Q.; Li, L. Analysis of Chemical Components and Tissue Distribution of Lujiao Formula Based on UPLC-Q-Orbitrap-MS. Chin. J. Exp. Tradit. Med. Form. 2024, 30, 148–156. [Google Scholar]
- Li, J.F.; Zhao, L.J.; Zhang, J.Q.; Wang, J.Y.; Zhang, Y.; Wang, Y.L.; Yin, H.Q.; Han, R.; Yang, Z.; Song, L.L.; et al. Analysis of in vivo and in vitro components of Dachaihu Decoction by UPLC-QTOF/MS. Chin. Tradit. Patent Med. 2023, 45, 2124–2130. [Google Scholar]
- Park, J.Y.; Jo, S.G.; Lee, H.N.; Choi, J.H.; Lee, Y.J.; Kim, Y.M.; Cho, J.Y.; Lee, S.K.; Park, J.H. Tendril extract of Cucurbita moschata suppresses NLRP3 inflammasome activation in murine macrophages and human trophoblast cells. Int. J. Med. Sci. 2020, 7, 1006–1014. [Google Scholar] [CrossRef] [PubMed]
- Mohany, M.; Ahmed, M.M.; Al-Rejaie, S.S. Molecular Mechanistic Pathways Targeted by Natural Antioxidants in the Prevention and Treatment of Chronic Kidney Disease. Antioxidants 2021, 11, 15. [Google Scholar] [CrossRef] [PubMed]
- Shan, S.N.; Xie, M.Y.; Wang, L.; Wei, M.L.; Liu, X.Q.; Cheng, W.X. ldentification and quality study of Cirsium japonicum and itsadulterants by FT-IR. Cent. South Pharm. 2022, 20, 2076–2081. [Google Scholar]
- Xie, M.Y.; Zhang, Z.; Huang, Y.; Zhang, Z.P.; Hu, Y.; Cheng, X.R. Study on HPLC Characteristic Chromatogram and Chemical Pattern Recognition of Different Medicinal Parts of Cirsium japonicum. J. China Pharm. 2020, 31, 820–825. [Google Scholar]

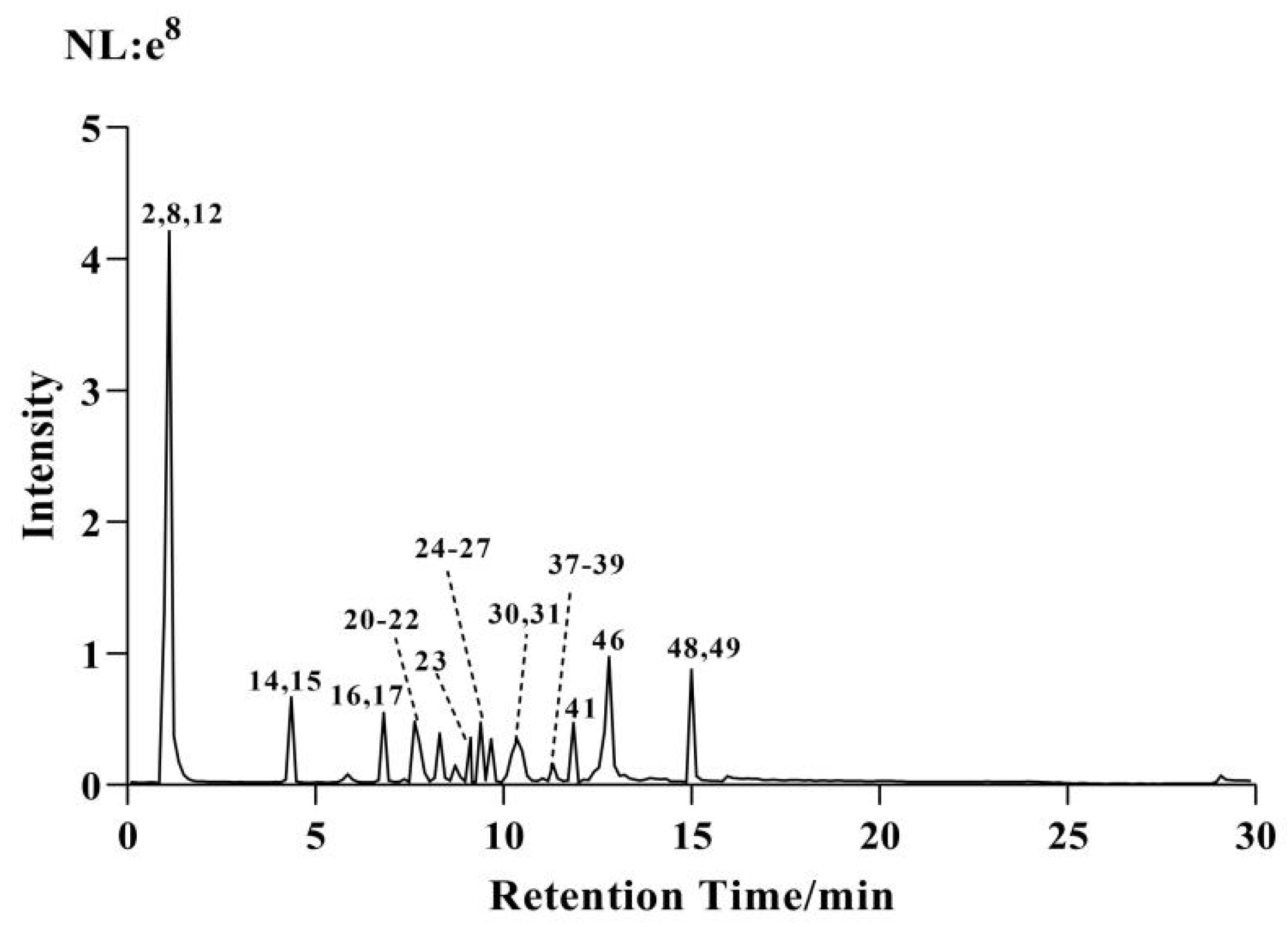


| No. | tR (min) | Compound | [M+H]+ (m/z) | [M−H]− (m/z) | Formula | Error (ppm) | MS2/m/z | Compound Class | Reference |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 0.91 | DL-Arginine | 175.11871 | C6H15O2N4 | −1.383 | 130.09734 | amino acids | [9] | |
| 2 | 0.98 | D-(-)-Quinic acid | 191.0553 | C7H11O6 | 1.494 | 173.04468, 155.03383, 137.02299, 127.03873 | organic acids | [9] | |
| 3 | 1.05 | Guanine | 152.05647 | C5H6ON5 | −1.423 | 135.02994, 110.03501 | alkaloids | [10] | |
| 4 | 1.05 | DL-Stachydrine | 144.10178 | C7H14O2N | −0.869 | 102.05520, 98.09674 | alkaloids | [11] | |
| 5 | 1.05 | Trigonelline | 138.05487 | C7H8O2N | −0.616 | 121.06477, 110.06025, 94.06547 | alkaloids | [12] | |
| 6 | 1.05 | D-(+)-Proline | 116.07080 | C5H10O2N | 1.679 | 98.06027, 70.06575 | amino acids | [13] | |
| 7 | 1.05 | Betaine | 118.08638 | C5H12O2N | 1.057 | 100.07584, 72.08136 | alkaloids | [9] | |
| 8 | 1.10 | Malic acid | 133.01288 | C4H5O5 | −2.028 | 71.01218, 115.00221 | organic acids | [9] | |
| 9 | 1.17 | 4-Guanidinobutyric acid | 146.09221 | C5H12O2N3 | −1.322 | 128.08156, 86.06042, 69.09171 | organic acids | [14] | |
| 10 | 1.17 | Salsolinol | 180.10158 | C10H14O2N | −1.806 | 163.07486, 151.07474, 137.05939 | others | [15] | |
| 11 | 1.17 | L-Phenylalanine | 166.08600 | C9H12O2N | −1.536 | 121.06471, 119.04895, 103.05429 | amino acids | [9] | |
| 12 | 1.36 | L-Pyroglutamic acid | 128.03394 | C5H6O3N | −2.184 | 82.02816 | amino acids | [12] | |
| 13 | 1.43 | Tyramine | 138.09119 | C8H12ON | −1.09 | 121.06479, 103.05445, 93.07022 | others | [16] | |
| 14 | 4.35 | Gentisic acid | 153.01811 | C7H5O4 | −0.818 | 109.02798, 91.01743 | organic acids | [17] | |
| 15 | 4.35 | Protocatechuic acid | 153.01811 | C7H5O4 | −0.818 | 109.02798, 91.02014 | organic acids | [9] | |
| 16 | 6.81 | Protocatechualdehyde | 137.02312 | C7H5O3 | −1.464 | 119.01241, 123.00711 | phenols | [12] | |
| 17 | 6.81 | Salicylic acid | 137.02312 | C7H5O3 | −1.829 | 119.01241, 109.02798 | organic acids | [12] | |
| 18 | 7.63 | Neochlorogenic acid | 353.08817 | C16H17O9 | 4.139 | 191.05528, 173.04469, 161.02319 | phenylpropanoids | [9] | |
| 19 | 7.7 | Chlorogenic acid | 355.10144 | C16H19O9 | −2.587 | 179.05412, 191.05495, 173.04431, 161.02290, 135.04384 | phenylpropanoids | [9] | |
| 20 | 8.03 | Daphnetin | 177.01843 | C9H5O4 | 1.101 | 121.02822, 133.02815, | phenylpropanoids | [18] | |
| 21 | 8.03 | Bergenin | 327.07251 | C14H15O9 | 1.085 | 312.03429, 234.02808, 192.02785 | phenylpropanoids | [19] | |
| 22 | 8.30 | Caffeic acid | 179.03409 | C9H7O4 | 1.144 | 135.04379, 117.03312, 107.04871 | organic acids | [9] | |
| 23 | 9.12 | 3-O-Famprofazone | 367.10391 | C17H19O9 | 4.226 | 193.05006, 173.04462, 191.05490 | organic acids | [9] | |
| 24 | 9.39 | Rutin | 609.14691 | C27H29O16 | 3.117 | 301.03467, 285.04059, 271.02509, 227.03450, 151.00270 | flavonoids | [9] | |
| 25 | 9.39 | Kaempferol-7-O-neohesperidoside | 593.15295 | C27H29O15 | 2.962 | 577.97946, 285.04056 | flavonoids | [20] | |
| 26 | 9.53 | Hyperoside | 463.08871 | C21H19O12 | 3.472 | 316.02271, 301.03601, 287.02005, 271.02515 | flavonoids | [9] | |
| 27 | 9.66 | Luteolin-7-O-glucoside | 447.09402 | C21H19O11 | 4.098 | 285.04047, 151.00221, 133.02782 | flavonoids | [21] | |
| 28 | 9.74 | Scutellarin | 463.08572 | C21H19O12 | −2.985 | 287.05402, 269.04480, 153.01784, 135.04370 | flavonoids | [22] | |
| 29 | 10.42 | Apigenin 7-O-glucuronide | 447.09125 | C21H19O11 | −2.097 | 269.05927, 187.03818, 153.01785, 119.04910 | flavonoids | [21] | |
| 30 | 10.49 | 4,5-Dicaffeoylquinic acid | 515.12006 | C25H23O12 | 3.218 | 353.08817, 191.05525, 179.03404, 173.04459, 135.04379 | phenylpropanoids | [9] | |
| 31 | 10.49 | 3,5-Dicaffeoylquinic acid | 515.11981 | C25H23O12 | 0.603 | 353.08817, 179.03404, 173.04459 | phenylpropanoids | [9] | |
| 32 | 10.56 | 6-O-Methylscutellarin | 477.10190 | C22H21O12 | −1.787 | 299.06982, 284.04636, 272.11987, 186.01543, 168.00481, 137.05907, 121.02834 | flavonoids | [22] | |
| 33 | 11.37 | Pectolinarin | 623.19562 | C29H35O15 | −2.289 | 477.13788, 315.08527, 300.06201 | flavonoids | [2] | |
| 34 | 11.37 | Linarin | 593.18604 | C28H33O14 | −0.745 | 447.12650, 285.07465, 270.05136, 242.05632, 153.01770 | flavonoids | [2] | |
| 35 | 11.79 | Isorhamnetin | 317.06442 | C16H13O7 | −3.656 | 168.00479, 153.01802 | flavonoids | [17] | |
| 36 | 11.79 | Fisetin | 287.05423 | C15H11O6 | −2.733 | 269.04388, 165.04852, 157.05414, 153.01796, 135.04391 | flavonoids | [23] | |
| 37 | 11.85 | Nepetin | 315.05148 | C16H11O7 | 4.923 | 300.02765, 243.02972, 228.04236, 201.01862, 188.04700, 165.98959, 136.98665 | flavonoids | [17] | |
| 38 | 11.85 | Luteolin | 285.04065 | C15H9O6 | 4.51 | 241.05040, 171.05032, 153.02318, 135.02812 | flavonoids | [17] | |
| 39 | 11.85 | Quercetin | 301.03561 | C15H9O7 | 4.421 | 201.03961, 153.00240, 137.03880, 121.02810 | flavonoids | [23] | |
| 40 | 12.06 | Dihydrocapsaicin | 308.22092 | C18H30O3N | −3.57 | 290.21048, 262.21564, 184.13004, 137.07539, 122.05996 | others | [24] | |
| 41 | 12.54 | Corchorifatty acid F | 327.21802 | C18H31O5 | 4.338 | 309.12856, 291.19626 | terpenes | [25] | |
| 42 | 12.68 | Tricin | 329.06696 | C17H13O7 | 4.196 | 313.04358, 300.01981, 272.02499, 161.02321 | flavonoids | [26] | |
| 43 | 12.74 | Hispidulin | 301.06982 | C16H13O6 | −2.805 | 286.04633, 168.00494, 153.99683, 119.01024, 107.02839 | flavonoids | [17] | |
| 44 | 12.74 | Apigenin | 271.05939 | C15H11O5 | −2.619 | 243.06456, 229.04869, 197.05914, 163.03847, 153.01791 | flavonoids | [2] | |
| 45 | 12.74 | Genistein | 271.05936 | C15H11O5 | −2.73 | 253.04716, 225.05359, 197.05914, 137.02843, 153.01791 | flavonoids | [27] | |
| 46 | 12.81 | Diosmetin | 299.05624 | C16H11O6 | 4.098 | 284.03287, 164.01042, 136.98671 | flavonoids | [2] | |
| 47 | 14.93 | Physcion | 285.07495 | C16H13O5 | −2.806 | 257.07962, 242.05663, 213.05420, 153.01784 | anthraquinones | [28] | |
| 48 | 15.00 | Glycitein | 283.06137 | C16H11O5 | 4.487 | 240.04251, 223.03918, 211.03937 | flavonoids | [17] | |
| 49 | 15.00 | Acacetin | 283.06137 | C16H11O5 | 4.487 | 268.03796, 240.04251, 151.00246 | flavonoids | [2] | |
| 50 | 15.20 | Pectolinarigenin | 315.08514 | C17H15O6 | −3.728 | 300.06189, 257.04364, 154.99695, 135.04384 | flavonoids | [2] | |
| 51 | 15.34 | Scopoletin | 193.04913 | C10H9O4 | −2.099 | 178.02556, 133.02820, 105.03364 | phenylpropanoids | [17] |
| No. | Ethanol Concentration (A)/% | Heating Reflow Time (B)/min | Solvent-to-Sample Ratio (C)/mL·g−1 | Extraction Rate/% |
|---|---|---|---|---|
| 1 | −1 | 0 | −1 | 0.1771 |
| 2 | 1 | 0 | −1 | 0.2214 |
| 3 | −1 | 0 | 1 | 0.1878 |
| 4 | 1 | 0 | 1 | 0.2020 |
| 5 | −1 | −1 | 0 | 0.1716 |
| 6 | 1 | −1 | 0 | 0.2005 |
| 7 | −1 | 1 | 0 | 0.1336 |
| 8 | 1 | 1 | 0 | 0.1568 |
| 9 | 0 | −1 | −1 | 0.2295 |
| 10 | 0 | −1 | 1 | 0.2362 |
| 11 | 0 | 1 | −1 | 0.2117 |
| 12 | 0 | 1 | 1 | 0.1855 |
| 13 | 0 | 0 | 0 | 0.2698 |
| 14 | 0 | 0 | 0 | 0.2651 |
| 15 | 0 | 0 | 0 | 0.2600 |
| 16 | 0 | 0 | 0 | 0.2668 |
| 17 | 0 | 0 | 0 | 0.2668 |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value | Significant |
|---|---|---|---|---|---|---|
| Model | 0.0288 | 9 | 0.0032 | 239.71 | <0.0001 | significant |
| A | 0.0015 | 1 | 0.0015 | 114.40 | <0.0001 | |
| B | 0.0028 | 1 | 0.0028 | 210.99 | <0.0001 | |
| C | 0.0001 | 1 | 0.0001 | 7.44 | 0.0295 | |
| AB | 8.122 × 10−6 | 1 | 8.122 × 10 −6 | 0.6077 | 0.4612 | |
| AC | 0.0002 | 1 | 0.0002 | 16.95 | 0.0045 | |
| BC | 0.0003 | 1 | 0.0003 | 20.25 | 0.0028 | |
| A2 | 0.0148 | 1 | 0.0148 | 1110.12 | <0.0001 | |
| B2 | 0.0070 | 1 | 0.0070 | 522.16 | <0.0001 | |
| C2 | 0.0004 | 1 | 0.0004 | 27.03 | 0.0013 | |
| Residual | 0.0001 | 7 | 0.0000 | |||
| Lack of Fit | 0.0000 | 3 | 0.0000 | 1.06 | 0.4583 | not significant |
| Pure Error | 0.0001 | 4 | 0.0000 | |||
| Cor Total | 0.0289 | 16 | ||||
| R2 | 0.9968 | |||||
| Adjusted R2 | 0.9926 | |||||
| Predicted R2 | 0.9742 | |||||
| Adeq Precision | 46.8088 |
| Compound | Original (µg) | Added (µg) | Found (µg) | Recovery Yield (%) | RSD (%) |
|---|---|---|---|---|---|
| Linarin | 100.3307 | 101.0880 | 201.0308 | 99.6 | 1.64 |
| 97.2304 | 200.8688 | 102.5 | |||
| 98.4671 | 200.2837 | 100.7 | |||
| 100.7545 | 200.0487 | 98.2 | |||
| 97.7450 | 200.0459 | 101.2 | |||
| 97.1247 | 200.6140 | 102.4 |
| Compound | No. | Content (mg·g−1) | Average Content (mg·g−1) | RSD% |
|---|---|---|---|---|
| Linarin | 1 | 2.98 | 2.92 | 1.43 |
| 2 | 2.92 | |||
| 3 | 2.88 | |||
| 4 | 2.91 | |||
| 5 | 2.87 | |||
| 6 | 2.95 |
| Level | Ethanol Concentration (A)/% | Heating Reflow Time (B)/min | Solvent-to-Sample Ratio (C)/mL·g−1 |
|---|---|---|---|
| −1 | 50 | 90 | 30 |
| 0 | 70 | 120 | 40 |
| 1 | 90 | 150 | 50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kong, F.; Fang, Z.; Cui, B.; Gao, J.; Sun, C.; Zhang, S. Study on the Compositional Analysis, Extraction Process, and Hemostatic and Anti-Inflammatory Activities of Cirsium japonicum Fisch. ex DC.–Cirsium setosum (Willd.) MB Extracts. Molecules 2024, 29, 1918. https://doi.org/10.3390/molecules29091918
Kong F, Fang Z, Cui B, Gao J, Sun C, Zhang S. Study on the Compositional Analysis, Extraction Process, and Hemostatic and Anti-Inflammatory Activities of Cirsium japonicum Fisch. ex DC.–Cirsium setosum (Willd.) MB Extracts. Molecules. 2024; 29(9):1918. https://doi.org/10.3390/molecules29091918
Chicago/Turabian StyleKong, Fanyu, Zhongxue Fang, Biyue Cui, Jinshuang Gao, Changhai Sun, and Shuting Zhang. 2024. "Study on the Compositional Analysis, Extraction Process, and Hemostatic and Anti-Inflammatory Activities of Cirsium japonicum Fisch. ex DC.–Cirsium setosum (Willd.) MB Extracts" Molecules 29, no. 9: 1918. https://doi.org/10.3390/molecules29091918
APA StyleKong, F., Fang, Z., Cui, B., Gao, J., Sun, C., & Zhang, S. (2024). Study on the Compositional Analysis, Extraction Process, and Hemostatic and Anti-Inflammatory Activities of Cirsium japonicum Fisch. ex DC.–Cirsium setosum (Willd.) MB Extracts. Molecules, 29(9), 1918. https://doi.org/10.3390/molecules29091918






