Anti-Tumor Effects and Toxicity Reduction Mechanisms of Prunella vulgaris: A Comprehensive Review
Abstract
1. Introduction
2. Methodology
3. History of Medicine and Food
4. Active Ingredient Related Target Pathway Network Diagram
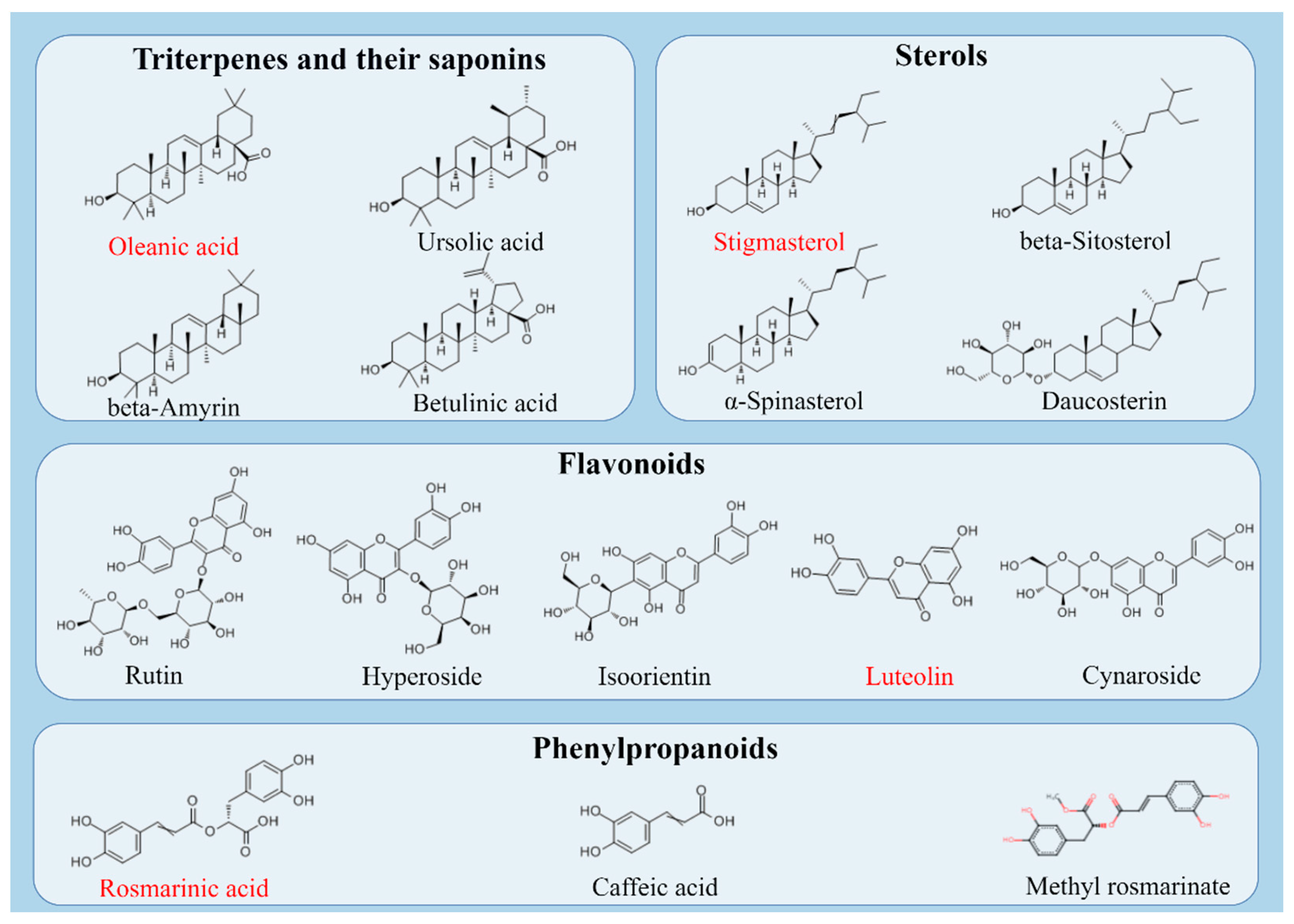
5. Active Ingredient
5.1. Triterpenes and Their Saponins
5.2. Sterols
5.3. Flavonoids
5.4. Phenylpropanoids
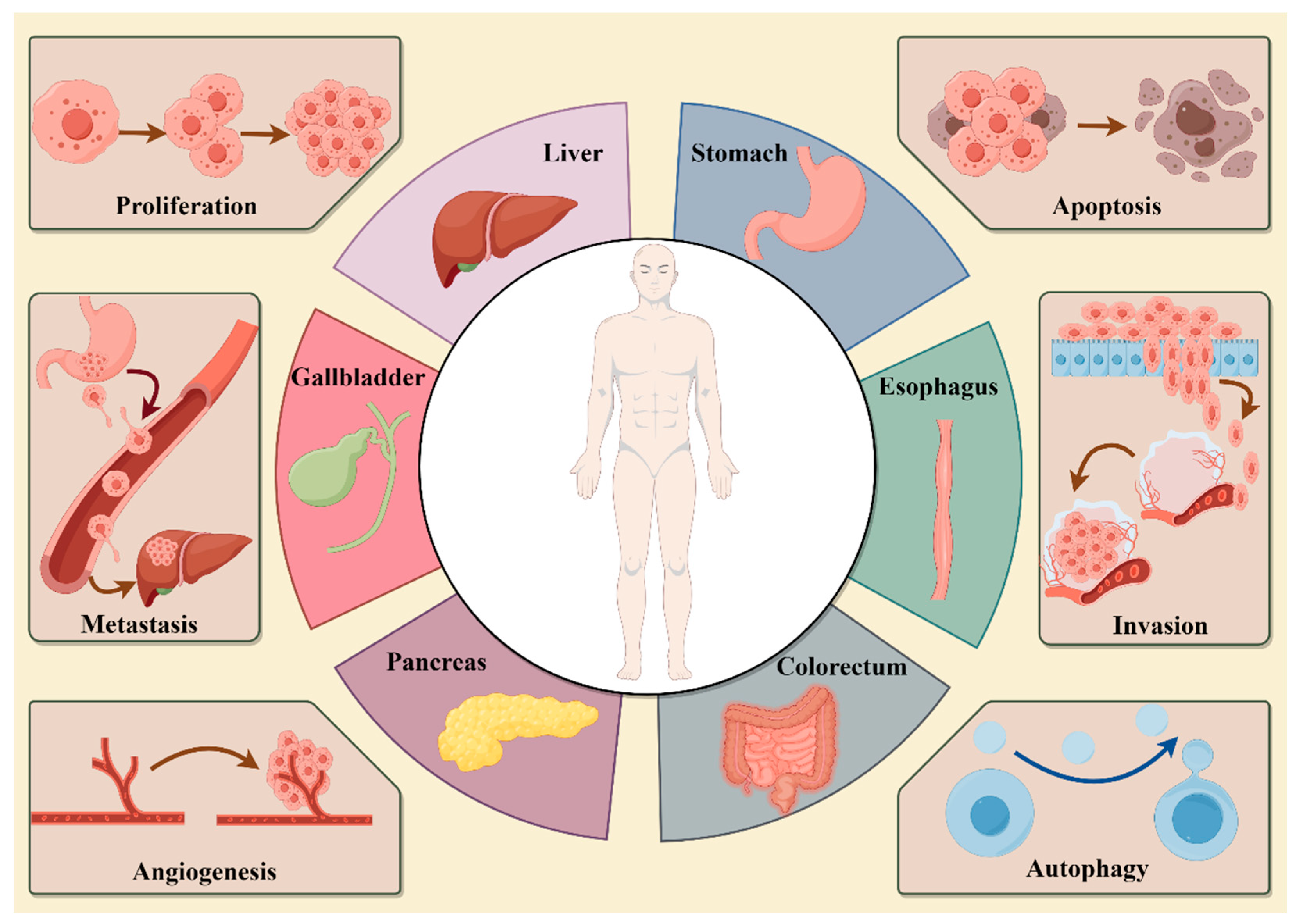
6. Pharmacological Actions
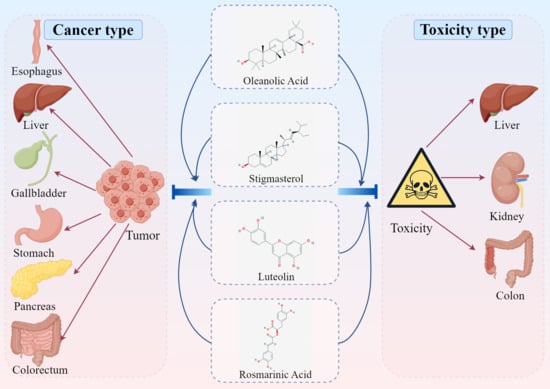
7. Anti-Tumor Effect of Active Ingredients
7.1. Oleanolic Acid
7.2. Stigmasterol
7.3. Luteolin
7.4. Rosmarinic Acid
8. The Attenuated Effect of Active Ingredients
9. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| OB | Oral Availability |
| DL | Drug-like properties |
| KEGG | Kyoto Encyclopedia of Traditional Chinese Medicine |
| TC | Total Cholestero |
| LDL-C | Low-density lipoprotein cholesterol |
| HDL-C | High-density lipoprotein cholesterol |
| BAX | Bcl-2-associated X |
| Bcl-2 | B-cell lymphoma-2 |
| VEGF | Vascular endothelial growth factor |
| NF-κB | Nuclear factor kappa-B |
| PI3K | Phosphatidylinositol 3-kinase |
| AKT | Protein kinase-B |
| JAK | Janus Kinase |
| STAT | Signal transducer and activator of transcription |
| ROS | Reactive oxygen species |
| PERK | PKR-like endoplasmic reticulum kinase |
| ATF4 | Recombinant activating transcription factor 4 |
| APAF-1 | Apoptotic protease activating factor-1 |
| NF-κB | Nuclear factor kappa-beta |
| MEK | Methyl ethyl ketone |
| ERK | Extracellular regulated protein kinases |
| JNK | C-Jun N-terminal kinase |
| VEGFR2 | Vascular endothelial growth factor receptor 2 |
| PCNA | Proliferating cell nuclear antigen |
| PARP | Poly-ADP-ribose polymerase |
| mTOR | Mechanistic target of rapamycin |
| LC3-II | Iight chain 3-II |
| XIAP | X-linked inhibitor of apoptosis protein |
| Jab1 | Jun activation domain-binding protein 1 |
| cMet | C-mesenchymal-epithelial transition |
| PARP-1 | Poly-ADP-ribose polymerase 1 |
| FOXO3a | Forkhead box O3a |
| Nrf2 | Nuclear factor erythroid-2 related factor 2 |
| HO-1 | Heme oxygenase-1 |
| MMP | Matrix metalloproteinase |
| CYT-c | Cytochrome C |
| MAPK | Mitogen-activated protein kinase |
| ERK | Extracellular signal-regulated kinase |
| MMP2 | Matrix metallopeptidase 2 |
| MMP16 | Matrix metallopeptidase 16 |
| MMP9 | Matrix metallopeptidase 9 |
| AMPK | AMP-activated protein kinase |
| IL-6 | Interleukin 6 |
| IL-8 | Interleukin 8 |
| IL-10 | Interleukin 10 |
| TNF-α | Tumor necrosis factorα |
| COX-2 | Cyclo-oxygenase-2 |
| TGF-β1 | Transforming growth factor-β1 |
| α-SMA | α-Smooth muscle actin |
| NLRP3 | NOD-like receptor thermal protein domain associated protein 3 |
| SIRT1 | Recombinant Sirtuin 1 |
| GSH | Glutathione |
| SOD | Superoxide dismutase |
| MDA | Malondialdehyde |
| ALT | Alanine transaminase |
| AST | Aspartate transaminase |
| CSF-1 | Macrophage colony-stimulating factor 1 |
| CAT | Catalase |
| IL-1β | Interleukin—1β |
| ALP | Alkaline phosphatase |
| TG | Triglyceride |
References
- Nagaraju, G.P.; Kasa, P.; Dariya, B.; Surepalli, N.; Peela, S.; Ahmad, S. Epigenetics and therapeutic targets in gastrointestinal malignancies. Drug Discov. Today 2021, 26, 2303–2314. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Laversanne, M.; Weiderpass, E.; Soerjomataram, I. The ever-increasing importance of cancer as a leading cause of premature death worldwide. Cancer 2021, 127, 3029–3030. [Google Scholar] [CrossRef] [PubMed]
- Ying, J.; Zhang, M.; Qiu, X.; Lu, Y. The potential of herb medicines in the treatment of esophageal cancer. Biomed. Pharmacother. 2018, 103, 381–390. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Sun, J.; Zeng, Z.; Liu, Z.; Ma, M.; Zheng, Z.; He, Y.; Kang, W. Tumour-associated macrophages in gastric cancer: From function and mechanism to application. Clin. Transl. Med. 2023, 13, e1386. [Google Scholar] [CrossRef] [PubMed]
- Johnston, F.M.; Beckman, M. Updates on Management of Gastric Cancer. Curr. Oncol. Rep. 2019, 21, 67. [Google Scholar] [CrossRef] [PubMed]
- Gravitz, L. Liver cancer. Nature 2014, 516, S1. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [PubMed]
- Anwanwan, D.; Singh, S.K.; Singh, S.; Saikam, V.; Singh, R. Challenges in liver cancer and possible treatment approaches. Biochim. Biophys. Acta Rev. Cancer 2020, 1873, 188314. [Google Scholar] [CrossRef]
- Haraldsdottir, S.; Einarsdottir, H.M.; Smaradottir, A.; Gunnlaugsson, A.; Halfdanarson, T.R. Colorectal cancer—Review. Laeknabladid 2014, 100, 75–82. [Google Scholar] [CrossRef]
- Baiu, I.; Visser, B. Gallbladder Cancer. JAMA 2018, 320, 1294. [Google Scholar] [CrossRef]
- Ansari, D.; Tingstedt, B.; Andersson, B.; Holmquist, F.; Sturesson, C.; Williamsson, C.; Sasor, A.; Borg, D.; Bauden, M.; Andersson, R. Pancreatic cancer: Yesterday, today and tomorrow. Future Oncol. 2016, 12, 1929–1946. [Google Scholar] [CrossRef] [PubMed]
- Kawakubo, H.; Ozawa, S.; Ando, N.; Kitagawa, Y.; Mukai, M.; Ueda, M.; Kitajima, M. Alterations of p53, cyclin D1 and pRB expression in the carcinogenesis of esophageal squamous cell carcinoma. Oncol. Rep. 2005, 14, 1453–1459. [Google Scholar] [CrossRef]
- Li, F.S.; Weng, J.K. Demystifying traditional herbal medicine with modern approach. Nat. Plants 2017, 3, 17109. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.Y.; Bai, X.Y.; Wang, C.H. Traditional Chinese medicine: A treasured natural resource of anticancer drug research and development. Am. J. Chin. Med. 2014, 42, 543–559. [Google Scholar] [CrossRef]
- Guo, P.; Zhang, B.; Zhao, J.; Wang, C.; Wang, Z.; Liu, A.; Du, G. Medicine-Food Herbs against Alzheimer’s Disease: A Review of Their Traditional Functional Features, Substance Basis, Clinical Practices and Mechanisms of Action. Molecules 2022, 27, 901. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Jiang, J.G. Origin and concept of medicine food homology and its application in modern functional foods. Food Funct. 2013, 4, 1727–1741. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Lv, G.P.; Chen, Y.W.; Li, S.P. Advanced development in analysis of phytochemicals from medicine and food dual purposes plants used in China. J. Chromatogr. A 2011, 1218, 7453–7475. [Google Scholar] [CrossRef] [PubMed]
- Psotová, J.; Kolár, M.; Sousek, J.; Svagera, Z.; Vicar, J.; Ulrichová, J. Biological activities of Prunella vulgaris extract. Phytother. Res. 2003, 17, 1082–1087. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Qi, H.; Ni, J. Extracts of endophytic fungus xkc-s03 from Prunella vulgaris L. spica inhibit gastric cancer in vitro and in vivo. Oncol. Lett. 2015, 9, 945–949. [Google Scholar] [CrossRef]
- Kim, S.H.; Huang, C.Y.; Tsai, C.Y.; Lu, S.Y.; Chiu, C.C.; Fang, K. The aqueous extract of Prunella vulgaris suppresses cell invasion and migration in human liver cancer cells by attenuating matrix metalloproteinases. Am. J. Chin. Med. 2012, 40, 643–656. [Google Scholar] [CrossRef]
- Fang, Y.; Zhang, L.; Feng, J.; Lin, W.; Cai, Q.; Peng, J. Spica Prunellae extract suppresses the growth of human colon carcinoma cells by targeting multiple oncogenes via activating miR-34a. Oncol. Rep. 2017, 38, 1895–1901. [Google Scholar] [CrossRef]
- Pan, J.; Wang, H.; Chen, Y. Prunella vulgaris L.—A Review of its Ethnopharmacology, Phytochemistry, Quality Control and Pharmacological Effects. Front. Pharmacol. 2022, 13, 903171. [Google Scholar] [CrossRef]
- Luo, H.; Zhao, L.; Li, Y.; Xia, B.; Lin, Y.; Xie, J.; Wu, P.; Liao, D.; Zhang, Z.; Lin, L. An in vivo and in vitro assessment of the anti-breast cancer activity of crude extract and fractions from Prunella vulgaris L. Heliyon 2022, 8, e11183. [Google Scholar] [CrossRef]
- Guo, Q.; Qu, H.; Zhang, H.; Zhong, X. Prunella vulgaris L. Attenuates Experimental Autoimmune Thyroiditis by Inhibiting HMGB1/TLR9 Signaling. Drug Des. Devel Ther. 2021, 15, 4559–4574. [Google Scholar] [CrossRef]
- Han, E.H.; Choi, J.H.; Hwang, Y.P.; Park, H.J.; Choi, C.Y.; Chung, Y.C.; Seo, J.K.; Jeong, H.G. Immunostimulatory activity of aqueous extract isolated from Prunella vulgaris. Food Chem. Toxicol. 2009, 47, 62–69. [Google Scholar] [CrossRef]
- Hwang, Y.J.; Lee, E.J.; Kim, H.R.; Hwang, K.A. NF-κB-targeted anti-inflammatory activity of Prunella vulgaris var. lilacina in macrophages RAW 264.7. Int. J. Mol. Sci. 2013, 14, 21489–21503. [Google Scholar] [CrossRef]
- Li, Z.; He, Q.; Xu, F.; Yin, X.; Guan, Z.; Song, J.; He, Z.; Yang, X.; Situ, C. Exploring the Antibacterial Potential and Underlying Mechanisms of Prunella vulgaris L. on Methicillin-Resistant Staphylococcus aureus. Foods 2024, 13, 660. [Google Scholar] [CrossRef]
- Chen, Y.; Guo, Q.; Wang, C. Research on the Changes of Prunella vulgaris and Its Medicinal Parts. Chin. J. Chin. Mater. Med. 2010, 35, 242–246. [Google Scholar]
- Guo, Q.; Chen, Y. Research on the Original Plants of Prunella vulgaris and Their Dietary Therapy History. Chin. J. Chin. Mater. Med. 2011, 36, 3057–3062. [Google Scholar]
- Wang, X.; Ma, M.; Zhang, J.; Dai, Y.; Ji, Q.; Gu, X.; Nian, H. Overview of Traditional Chinese Medicine Prunella vulgaris for Medicinal Use. Chin. J. Mod. Appl. Pharm. 2019, 36, 625–632. [Google Scholar] [CrossRef]
- Jin, Z.; He, J.; Hu, Y.; Bu, Z.; Ma, N. Progress in Clinical Application of Prunella vulgaris Preparations. China Pharm. 2016, 27, 5034–5037. [Google Scholar]
- Brindley, M.A.; Widrlechner, M.P.; McCoy, J.A.; Murphy, P.; Hauck, C.; Rizshsky, L.; Nikolau, B.; Maury, W. Inhibition of lentivirus replication by aqueous extracts of Prunella vulgaris. Virol. J. 2009, 6, 8. [Google Scholar] [CrossRef]
- Ryu, S.Y.; Oak, M.H.; Yoon, S.K.; Cho, D.I.; Yoo, G.S.; Kim, T.S.; Kim, K.M. Anti-allergic and anti-inflammatory triterpenes from the herb of Prunella vulgaris. Planta Med. 2000, 66, 358–360. [Google Scholar] [CrossRef]
- Rasool, R.; Ganai, B.A.; Akbar, S.; Kamili, A.N.; Masood, A. Phytochemical screening of Prunella vulgaris l. —An important medicinal plant of Kashmir. Pak. J. Pharm. Sci. 2010, 23, 399–402. [Google Scholar]
- Xia, B.H.; Xiong, S.H.; Tang, J.; Zhang, Z.M.; Li, Y.M.; Li, M.J.; Lin, L.M. Extraction of flavonoids in Prunella vulgaris based on deep eutectic solvent method: Application of new green solvent. Zhongguo Zhong Yao Za Zhi 2018, 43, 3484–3492. [Google Scholar] [CrossRef]
- Fang, X.; Yu, M.M.; Yuen, W.H.; Zee, S.Y.; Chang, R.C. Immune modulatory effects of Prunella vulgaris L. on monocytes/macrophages. Int. J. Mol. Med. 2005, 16, 1109–1116. [Google Scholar]
- Wang, Z.J.; Zhao, Y.Y.; Chen, Y.Y.; Ma, B.N. Triterpenoid compounds of Prunella genus and their features of 13C NMR spectroscopy. Zhongguo Zhong Yao Za Zhi 2000, 25, 583–588. [Google Scholar]
- Pollier, J.; Goossens, A. Oleanolic acid. Phytochemistry 2012, 77, 10–15. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, H.; Zhang, L.; Guo, T.; Wang, P.; Geng, M.; Li, Y. Synthesis and antitumor activities of naturally occurring oleanolic acid triterpenoid saponins and their derivatives. Eur. J. Med. Chem. 2013, 64, 1–15. [Google Scholar] [CrossRef]
- Kim, Y.S.; Li, X.F.; Kang, K.H.; Ryu, B.; Kim, S.K. Stigmasterol isolated from marine microalgae Navicula incerta induces apoptosis in human hepatoma HepG2 cells. BMB Rep. 2014, 47, 433–438. [Google Scholar] [CrossRef]
- Singh Tuli, H.; Rath, P.; Chauhan, A.; Sak, K.; Aggarwal, D.; Choudhary, R.; Sharma, U.; Vashishth, K.; Sharma, S.; Kumar, M.; et al. Luteolin, a Potent Anticancer Compound: From Chemistry to Cellular Interactions and Synergetic Perspectives. Cancers 2022, 14, 5373. [Google Scholar] [CrossRef]
- Psotova, J.; Svobodova, A.; Kolarova, H.; Walterova, D. Photoprotective properties of Prunella vulgaris and rosmarinic acid on human keratinocytes. J. Photochem. Photobiol. B 2006, 84, 167–174. [Google Scholar] [CrossRef]
- Swamy, M.K.; Sinniah, U.R.; Ghasemzadeh, A. Anticancer potential of rosmarinic acid and its improved production through biotechnological interventions and functional genomics. Appl. Microbiol. Biotechnol. 2018, 102, 7775–7793. [Google Scholar] [CrossRef] [PubMed]
- González-Vallinas, M.; Reglero, G.; Ramírez de Molina, A. Rosemary (Rosmarinus officinalis L.) Extract as a Potential Complementary Agent in Anticancer Therapy. Nutr. Cancer 2015, 67, 1221–1229. [Google Scholar] [CrossRef]
- Gu, X.; Li, Y.; Mu, J.; Zhang, Y. Chemical constituents of Prunella vulgaris. J. Environ. Sci. 2013, 25 (Suppl. S1), S161–S163. [Google Scholar] [CrossRef]
- Küpeli Akkol, E.; Renda, G.; İlhan, M.; Bektaş, N.Y. Wound healing acceleration and anti-inflammatory potential of Prunella vulgaris L.: From conventional use to preclinical scientific verification. J. Ethnopharmacol. 2022, 295, 115411. [Google Scholar] [CrossRef]
- Komal, S.; Kazmi, S.A.J.; Khan, J.A.; Gilani, M.M. Antimicrobial activity of Prunella vulgaris extracts against multi-drug resistant Escherichia Coli from patients of urinary tract infection. Pak. J. Med. Sci. 2018, 34, 616–620. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Lin, L.M.; Deng, J.; Lin, X.L.; Li, Y.M.; Xia, B.H. The therapeutic effects of Prunella vulgaris against fluoride-induced oxidative damage by using the metabolomics method. Environ. Toxicol. 2021, 36, 1802–1816. [Google Scholar] [CrossRef] [PubMed]
- Raafat, K.; Wurglics, M.; Schubert-Zsilavecz, M. Prunella vulgaris L. active components and their hypoglycemic and antinociceptive effects in alloxan-induced diabetic mice. Biomed. Pharmacother. 2016, 84, 1008–1018. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhou, Y.; Lin, Y.; Li, Y.; Xia, B.; Lin, L.; Liao, D. GC-MS-based metabolomics research on the anti-hyperlipidaemic activity of Prunella vulgaris L. polysaccharides. Int. J. Biol. Macromol. 2020, 159, 461–473. [Google Scholar] [CrossRef]
- Li, C.; You, L.; Fu, X.; Huang, Q.; Yu, S.; Liu, R.H. Structural characterization and immunomodulatory activity of a new heteropolysaccharide from Prunella vulgaris. Food Funct. 2015, 6, 1557–1567. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Ao, Z.; Bello, A.; Ran, X.; Liu, S.; Wigle, J.; Kobinger, G.; Yao, X. Characterization of the inhibitory effect of an extract of Prunella vulgaris on Ebola virus glycoprotein (GP)-mediated virus entry and infection. Antiviral Res. 2016, 127, 20–31. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.J.; Wang, X.H.; Dai, Y.Y.; Ma, M.H.; Rahman, K.; Nian, H.; Zhang, H. Prunella vulgaris: A Comprehensive Review of Chemical Constituents, Pharmacological Effects and Clinical Applications. Curr. Pharm. Des. 2019, 25, 359–369. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Huang, Q.; Xiao, J.; Fu, X.; You, L.; Liu, R.H. Preparation of Prunella vulgaris polysaccharide-zinc complex and its antiproliferative activity in HepG2 cells. Int. J. Biol. Macromol. 2016, 91, 671–679. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.C.; Lin, I.H.; Siao, Y.M.; Liu, C.J.; Yeh, C.C. Modulation of the Tumor Metastatic Microenvironment and Multiple Signal Pathways by Prunella vulgaris in Human Hepatocellular Carcinoma. Am. J. Chin. Med. 2016, 44, 835–849. [Google Scholar] [CrossRef]
- Zhang, X.; Shen, T.; Zhou, X.; Tang, X.; Gao, R.; Xu, L.; Wang, L.; Zhou, Z.; Lin, J.; Hu, Y. Network pharmacology based virtual screening of active constituents of Prunella vulgaris L. and the molecular mechanism against breast cancer. Sci. Rep. 2020, 10, 15730. [Google Scholar] [CrossRef]
- Baer-Dubowska, W.; Narożna, M.; Krajka-Kuźniak, V. Anti-Cancer Potential of Synthetic Oleanolic Acid Derivatives and Their Conjugates with NSAIDs. Molecules 2021, 26, 4957. [Google Scholar] [CrossRef]
- Shanmugam, M.K.; Dai, X.; Kumar, A.P.; Tan, B.K.; Sethi, G.; Bishayee, A. Oleanolic acid and its synthetic derivatives for the prevention and therapy of cancer: Preclinical and clinical evidence. Cancer Lett. 2014, 346, 206–216. [Google Scholar] [CrossRef]
- Gao, L.; Xu, Z.; Wang, Y.; Sun, B.; Song, Z.; Yang, B.; Liu, X.; Lin, Y.; Peng, J.; Han, G.; et al. Anticancer effect of SZC017, a novel derivative of oleanolic acid, on human gastric cancer cells. Oncol. Rep. 2016, 35, 1101–1108. [Google Scholar] [CrossRef]
- Liu, L.; Zhao, J.L.; Wang, J.G. [Oleanolic acid induces G₂/M phase arrest and apoptosis in human hepatocellular carcinoma Bel-7402 cells]. Zhongguo Zhong Yao Za Zhi 2015, 40, 4897–4902. [Google Scholar]
- Wei, J.; Liu, H.; Liu, M.; Wu, N.; Zhao, J.; Xiao, L.; Han, L.; Chu, E.; Lin, X. Oleanolic acid potentiates the antitumor activity of 5-fluorouracil in pancreatic cancer cells. Oncol. Rep. 2012, 28, 1339–1345. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, J.; Zhu, L.; Wang, X.; Meng, F.; Xia, L.; Zhang, H. Advances in Stigmasterol on its anti-tumor effect and mechanism of action. Front. Oncol. 2022, 12, 1101289. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Yuan, D.; Yan, R.; Meng, L.; Zhang, Y.; Zhu, K. Stigmasterol exhibits potent antitumor effects in human gastric cancer cells mediated via inhibition of cell migration, cell cycle arrest, mitochondrial mediated apoptosis and inhibition of JAK/STAT signalling pathway. JBUON 2018, 23, 1420–1425. [Google Scholar] [PubMed]
- Dong, Y.; Chen, C.; Chen, C.; Zhang, C.; Zhang, L.; Zhang, Y.; Li, Y.; Dong, Z. Stigmasterol inhibits the progression of lung cancer by regulating retinoic acid-related orphan receptor C. Histol. Histopathol. 2021, 36, 1285–1299. [Google Scholar] [CrossRef]
- Imran, M.; Rauf, A.; Abu-Izneid, T.; Nadeem, M.; Shariati, M.A.; Khan, I.A.; Imran, A.; Orhan, I.E.; Rizwan, M.; Atif, M.; et al. Luteolin, a flavonoid, as an anticancer agent: A review. Biomed. Pharmacother. 2019, 112, 108612. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, B.; Gao, F.; Shi, R. Modulation of G(2)/M cell cycle arrest and apoptosis by luteolin in human colon cancer cells and xenografts. Oncol. Lett. 2018, 15, 1559–1565. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, X.; Tang, H.; Pan, Y.; Hu, B.; Huang, G. Rosmarinic acid inhibits cell proliferation, migration, and invasion and induces apoptosis in human glioma cells. Int. J. Mol. Med. 2021, 47, 67. [Google Scholar] [CrossRef]
- Jin, B.R.; Chung, K.S.; Hwang, S.; Hwang, S.N.; Rhee, K.J.; Lee, M.; An, H.J. Rosmarinic acid represses colitis-associated colon cancer: A pivotal involvement of the TLR4-mediated NF-κB-STAT3 axis. Neoplasia 2021, 23, 561–573. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Hu, C.; Wu, L.; Xu, L.; Jiang, W. Rosmarinic acid inhibits inflammation and angiogenesis of hepatocellular carcinoma by suppression of NF-κB signaling in H22 tumor-bearing mice. J. Pharmacol. Sci. 2016, 132, 131–137. [Google Scholar] [CrossRef]
- Radziejewska, I.; Supruniuk, K.; Bielawska, A. Anti-cancer effect of combined action of anti-MUC1 and rosmarinic acid in AGS gastric cancer cells. Eur. J. Pharmacol. 2021, 902, 174119. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, W.; Li, Z.; Chen, L.; Wen, C.; Ruan, Q.; Xu, Z.; Liu, R.; Xu, J.; Bai, Y.; et al. Rosmarinic Acid Decreases the Malignancy of Pancreatic Cancer Through Inhibiting Gli1 Signaling. Phytomedicine 2022, 95, 153861. [Google Scholar] [CrossRef] [PubMed]
- Rui, L.X.; Shu, S.Y.; Jun, W.J.; Mo, C.Z.; Wu, S.Z.; Min, L.S.; Yuan, L.; Yong, P.J.; Cheng, S.Z.; Sheng, W.S.; et al. The dual induction of apoptosis and autophagy by SZC014, a synthetic oleanolic acid derivative, in gastric cancer cells via NF-κB pathway. Tumour Biol. 2016, 37, 5133–5144. [Google Scholar] [CrossRef]
- Niu, G.; Sun, L.; Pei, Y.; Wang, D. Oleanolic Acid Inhibits Colorectal Cancer Angiogenesis by Blocking the VEGFR2 Signaling Pathway. Anticancer. Agents Med. Chem. 2018, 18, 583–590. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Bai, H.; Zhang, X.; Liu, J.; Cao, P.; Liao, N.; Zhang, W.; Wang, Z.; Hai, C. Inhibitory effect of oleanolic acid on hepatocellular carcinoma via ERK-p53-mediated cell cycle arrest and mitochondrial-dependent apoptosis. Carcinogenesis 2013, 34, 1323–1330. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Caldwell, T.S.; Rivera, M.N.; Gullapalli, R.R. Cadmium exposure activates Akt/ERK Signaling and pro-inflammatory COX-2 expression in human gallbladder epithelial cells via a ROS dependent mechanism. Toxicol. In Vitro 2020, 67, 104912. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Liu, M.; Liu, H.; Wang, H.; Wang, F.; Zhang, Y.; Han, L.; Lin, X. Oleanolic acid arrests cell cycle and induces apoptosis via ROS-mediated mitochondrial depolarization and lysosomal membrane permeabilization in human pancreatic cancer cells. J. Appl. Toxicol. 2013, 33, 756–765. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Zhang, X.; Wang, M.; Lin, Y.; Zhou, S. Stigmasterol Simultaneously Induces Apoptosis and Protective Autophagy by Inhibiting Akt/mTOR Pathway in Gastric Cancer Cells. Front. Oncol. 2021, 11, 629008. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.H.; Kong, K.R.; Kim, Y.A.; Jung, K.O.; Kil, J.H.; Rhee, S.H.; Park, K.Y. Induction of BAX and activation of caspases during beta-sitosterol-mediated apoptosis in human colon cancer cells. Int. J. Oncol. 2003, 23, 1657–1662. [Google Scholar]
- Pandey, P.; Bajpai, P.; Siddiqui, M.H.; Sayyed, U.; Tiwari, R.; Shekh, R.; Mishra, K.; Kapoor, V.K. Elucidation of the Chemopreventive Role of Stigmasterol Against Jab1 in Gall Bladder Carcinoma. Endocr. Metab. Immune Disord. Drug Targets 2019, 19, 826–837. [Google Scholar] [CrossRef]
- Lu, J.; Li, G.; He, K.; Jiang, W.; Xu, C.; Li, Z.; Wang, H.; Wang, W.; Wang, H.; Teng, X.; et al. Luteolin exerts a marked antitumor effect in cMet-overexpressing patient-derived tumor xenograft models of gastric cancer. J. Transl. Med. 2015, 13, 42. [Google Scholar] [CrossRef]
- Potočnjak, I.; Šimić, L.; Gobin, I.; Vukelić, I.; Domitrović, R. Antitumor activity of luteolin in human colon cancer SW620 cells is mediated by the ERK/FOXO3a signaling pathway. Toxicol. In Vitro 2020, 66, 104852. [Google Scholar] [CrossRef] [PubMed]
- Jang, C.H.; Moon, N.; Lee, J.; Kwon, M.J.; Oh, J.; Kim, J.S. Luteolin Synergistically Enhances Antitumor Activity of Oxaliplatin in Colorectal Carcinoma via AMPK Inhibition. Antioxidants 2022, 11, 626. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Yang, T.; Liu, X.; Tian, Y.; Chen, X.; Yuan, R.; Su, S.; Lin, X.; Du, G. Luteolin synergizes the antitumor effects of 5-fluorouracil against human hepatocellular carcinoma cells through apoptosis induction and metabolism. Life Sci. 2016, 144, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Huang, M.; Liu, Y.; Shu, Y.; Liu, P. Luteolin Induces Apoptosis by Up-regulating miR-34a in Human Gastric Cancer Cells. Technol. Cancer Res. Treat. 2015, 14, 747–755. [Google Scholar] [CrossRef] [PubMed]
- Xavier, C.P.; Lima, C.F.; Fernandes-Ferreira, M.; Pereira-Wilson, C. Salvia fruticosa, Salvia officinalis, and rosmarinic acid induce apoptosis and inhibit proliferation of human colorectal cell lines: The role in MAPK/ERK pathway. Nutr. Cancer 2009, 61, 564–571. [Google Scholar] [CrossRef] [PubMed]
- Yehya, A.H.S.; Asif, M.; Abdul Majid, A.M.S.; Oon, C.E. Complementary effects of Orthosiphon stamineus standardized ethanolic extract and rosmarinic acid in combination with gemcitabine on pancreatic cancer. Biomed. J. 2021, 44, 694–708. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Ma, L.; Zhao, L.; Feng, W.; Zheng, X. Rosmarinic inhibits cell proliferation, invasion and migration via up-regulating miR-506 and suppressing MMP2/16 expression in pancreatic cancer. Biomed. Pharmacother. 2019, 115, 108878. [Google Scholar] [CrossRef]
- An, Y.; Zhao, J.; Zhang, Y.; Wu, W.; Hu, J.; Hao, H.; Qiao, Y.; Tao, Y.; An, L. Rosmarinic Acid Induces Proliferation Suppression of Hepatoma Cells Associated with NF-κB Signaling Pathway. Asian Pac. J. Cancer Prev. 2021, 22, 1623–1632. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Li, Q.; Wei, L.; Pan, X.; Huang, D.; Gan, J.; Tang, S. Rosmarinic Acid Analogue-11 Induces Apoptosis of Human Gastric Cancer SGC-7901 Cells via the Epidermal Growth Factor Receptor (EGFR)/Akt/Nuclear Factor kappa B (NF-κB) Pathway. Med. Sci. Monit. Basic. Res. 2019, 25, 63–75. [Google Scholar] [CrossRef]
- Hao, B.B.; Pan, X.X.; Fan, Y.; Lu, L.; Qian, X.F.; Wang, X.H.; Zhang, F.; Rao, J.H. Oleanolic acid attenuates liver ischemia reperfusion injury by HO-1/Sesn2 signaling pathway. Hepatobiliary Pancreat. Dis. Int. 2016, 15, 519–524. [Google Scholar] [CrossRef]
- Jie, F.; Yang, X.; Yang, B.; Liu, Y.; Wu, L.; Lu, B. Stigmasterol attenuates inflammatory response of microglia via NF-κB and NLRP3 signaling by AMPK activation. Biomed. Pharmacother. 2022, 153, 113317. [Google Scholar] [CrossRef]
- Mahdiani, S.; Omidkhoda, N.; Heidari, S.; Hayes, A.W.; Karimi, G. Protective effect of luteolin against chemical and natural toxicants by targeting NF-κB pathway. Biofactors 2022, 48, 744–762. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Luo, H.; Zhong, Z.; Ai, Y.; Zhao, Y.; Liang, Q.; Wang, Y. Phytochemistry, Pharmacology and Quality Control of Xiasangju: A Traditional Chinese Medicine Formula. Front. Pharmacol. 2022, 13, 930813. [Google Scholar] [CrossRef]
- Peng, X.P.; Li, X.H.; Li, Y.; Huang, X.T.; Luo, Z.Q. The protective effect of oleanolic acid on NMDA-induced MLE-12 cells apoptosis and lung injury in mice by activating SIRT1 and reducing NF-κB acetylation. Int. Immunopharmacol. 2019, 70, 520–529. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.Y.; Lee, M.K.; Park, M.J.; Kim, S.J.; Park, H.J.; Choi, J.W.; Kim, S.H.; Cho, S.Y.; Lee, J.S. Momordin Ic and oleanolic acid from Kochiae Fructus reduce carbon tetrachloride-induced hepatotoxicity in rats. J. Med. Food 2005, 8, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Qiu, A.; Guan, J.; Shi, Z. Antioxidant and protective effect of an oleanolic acid-enriched extract of A. deliciosa root on carbon tetrachloride induced rat liver injury. Asia Pac. J. Clin. Nutr. 2007, 16 (Suppl. 1), 169–173. [Google Scholar]
- Feng, S.; Sui, M.; Wang, D.; Ritzoulis, C.; Farag, M.A.; Shao, P. Pectin-zein based stigmasterol nanodispersions ameliorate dextran sulfate sodium-induced colitis in mice. Food Funct. 2021, 12, 11656–11670. [Google Scholar] [CrossRef] [PubMed]
- Owumi, S.E.; Lewu, D.O.; Arunsi, U.O.; Oyelere, A.K. Luteolin attenuates doxorubicin-induced derangements of liver and kidney by reducing oxidative and inflammatory stress to suppress apoptosis. Hum. Exp. Toxicol. 2021, 40, 1656–1672. [Google Scholar] [CrossRef]
- Dar, A.A.; Fehaid, A.; Alkhatani, S.; Alarifi, S.; Alqahtani, W.S.; Albasher, G.; Almeer, R.; Alfarraj, S.; Moneim, A.A. The protective role of luteolin against the methotrexate-induced hepato-renal toxicity via its antioxidative, anti-inflammatory, and anti-apoptotic effects in rats. Hum. Exp. Toxicol. 2021, 40, 1194–1207. [Google Scholar] [CrossRef]
- Yan, Y.; Jun, C.; Lu, Y.; Jiangmei, S. Combination of metformin and luteolin synergistically protects carbon tetrachloride-induced hepatotoxicity: Mechanism involves antioxidant, anti-inflammatory, antiapoptotic, and Nrf2/HO-1 signaling pathway. Biofactors 2019, 45, 598–606. [Google Scholar] [CrossRef]
- Jafaripour, L.; Naserzadeh, R.; Alizamani, E.; Javad Mashhadi, S.M.; Moghadam, E.R.; Nouryazdan, N.; Ahmadvand, H. Effects of Rosmarinic Acid on Methotrexate-induced Nephrotoxicity and Hepatotoxicity in Wistar Rats. Indian. J. Nephrol. 2021, 31, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.; Ji, M.; Wu, L.; Lv, L.; Liang, Q.; Deng, R.; Deng, Z.; Liu, X.; Ren, L.; Feng, X.; et al. Rosmarinic Acid Prevents Cisplatin-Induced Liver and Kidney Injury by Inhibiting Inflammatory Responses and Enhancing Total Antioxidant Capacity, Thereby Activating the Nrf2 Signaling Pathway. Molecules 2022, 27, 7815. [Google Scholar] [CrossRef]
- Lu, Y.H.; Hong, Y.; Zhang, T.Y.; Chen, Y.X.; Wei, Z.J.; Gao, C.Y. Rosmarinic acid exerts anti-inflammatory effect and relieves oxidative stress via Nrf2 activation in carbon tetrachloride-induced liver damage. Food Nutr. Res. 2022, 66, 8359. [Google Scholar] [CrossRef] [PubMed]
- Hasanein, P.; Sharifi, M. Effects of rosmarinic acid on acetaminophen-induced hepatotoxicity in male Wistar rats. Pharm. Biol. 2017, 55, 1809–1816. [Google Scholar] [CrossRef] [PubMed]
- Ji, M.Y.; Bo, A.; Yang, M.; Xu, J.F.; Jiang, L.L.; Zhou, B.C.; Li, M.H. The Pharmacological Effects and Health Benefits of Platycodon grandiflorus-A Medicine Food Homology Species. Foods 2020, 9, 142. [Google Scholar] [CrossRef] [PubMed]
- Taoerdahong, H.; Kadeer, G.; Chang, J.; Kang, J.; Ma, X.; Yang, F. A Review Concerning the Polysaccharides Found in Edible and Medicinal Plants in Xinjiang. Molecules 2023, 28, 2054. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yan, Q.; Fan, C.; Mo, Y.; Wang, Y.; Li, X.; Liao, Q.; Guo, C.; Li, G.; Zeng, Z.; et al. Overview and countermeasures of cancer burden in China. Sci. China Life Sci. 2023, 66, 2515–2526. [Google Scholar] [CrossRef]
- Lu, Q.; Li, R.; Yang, Y.; Zhang, Y.; Zhao, Q.; Li, J. Ingredients with anti-inflammatory effect from medicine food homology plants. Food Chem. 2022, 368, 130610. [Google Scholar] [CrossRef]



| Component | Tumor | Experimental Model | Mechanism | Action Target | Phenotype | References |
|---|---|---|---|---|---|---|
| Oleanolic Acid | Gastric cancer | Gastric cancer cell lines SGC-7901 and MGC803 | NF-κB axis | Blocked G2/M phase, upregulated Bcl-2 and Beclin 1 and downregulated the reflection of BAX and ATG 5, inducing apoptosis and autophagy. | Apoptosis Autophagy | [72] |
| Colorectal cancer | Human Colorectal carcinoma cell line HCT-116 | MEK/ERK/JNK axis | Inhibited VEGFR2 phosphorylation and tumor angiogenesis. | Angiogenesis | [73] | |
| Liver cancer | Human Liver cancer cell HepG2 | Mitochondrial apoptosis pathway | Blocked the G2/M phase; upregulated Caspase3, BAX; and Cyt. C; and downregulated Bcl-2 expression, inducing tumor cell apoptosis. | Apoptosis | [74] | |
| Gallbladder cancer | Human Gallbladder cancer cell lines GBC-SD and NOZ | AKT/ERK axis | Blocked G0 phase and downregulated PCNA, ICAM-1 and RhoA to inhibit tumor development. | Proliferation | [75] | |
| Pancreatic cancer | Human Pancreatic cancer cell line PANC-28 | Mitochondrial apoptosis pathway | Blocked G1 phase and G2/M phases, inducing ROS production and Cyt. C release, and activated the lysis of caspases-3/9 and PARP, inducing cell apoptosis. | Apoptosis | [76] | |
| Stigmasterol | Gastric cancer | Gastric cancer cell lines SGC-7901 and MGC803 | AKT/mTOR axis | The expressions of BAX, Caspase-3, PARP and LC3-II were upregulated, while the expression of Bcl-2 was downregulated, inducing apoptosis and autophagy. | Apoptosis Autophagy | [77] |
| Liver cancer | Human Liver cancer cell HepG2 | Mitochondrial apoptosis pathway | Blocked G2/M phase, BAX and p53 were upregulated, Bcl-2 and XIAP were downregulated and aspase-8/9 was activated, inducing apoptosis. | Apoptosis | [40] | |
| Colorectal cancer | Human Colorectal cancer cell line HCT-116 | - | Decline in the expressions of Bcl-2, cIAP-1 and mRNA; elevation in BAX and Mrn;, and promotion of the release of Cyt.C, inducing cell apoptosis. | Apoptosis | [78] | |
| Gallbladder cancer | Mitochondrial apoptosis pathway | Upregulation of p27 expression, downregulation of Jab1 gene and activation of Caspase-3, inducing cell apoptosis. | Apoptosis | [79] | ||
| Luteolin | Gastric cancer | Gastric cancer cell line SGC-7901 | cMet/Akt/ERK axis | Reduction in the reflection and phosphorylation of MMP9 and cMet and increase in the reflection of Caspase-3 and PARP-1, inducing apoptosis and reduce invasion. | Apoptosis Invasion | [80] |
| Colon cancer | Human Colon cancer SW620 cells | ERK/FOXO3a axis | Decrease BAX, Caspase-3, PARP, and FOXO3a, increase Beclin-1, Atg5, and LC3B-I/II; Induce apoptosis and inhibit autophagy. | Apoptosis Autophagy | [81] | |
| Rosmarinic Acid | Colorectal cancer | Human Colorectal cancer cell line HCT-116 | Nrf2/ARE/HO-1 axis | Upregulation of Nrf2 transcription activity, enhancement of HO-1 expression and suppression of proliferation. | Proliferation | [82] |
| Liver cancer | Human Liver carcinoma cell HepG2 | - | Upregulation of BAX and p53, downregulation of Bcl-2 expression and induction of PARP lysis, inducing apoptosis. | Apoptosis | [83] | |
| Esophageal cancer | Esophageal carcinoma cell line EC1 | - | Downregulation of MMP; upregulation of p21, p53, CYT-c, Bim, and cPARP levels; and activation of Caspase-3 expression, suppressing tumor growth. | Apoptosis | [84] | |
| Colon cancer | Human Colon carcinoma cell line HCT15 | MAPK/ERK axis | The reflection of BAX and Caspase-3 was decreased, while the reflection of Bcl-2 was increased, inducing apoptosis of cancer cells. | Apoptosis Proliferation | [85] | |
| Pancreatic cancer | Human Pancreatic carcinoma cell line Panc-1 | - | Blocked S stage stagnation, activated the cleavage of Caspase-3/9 and PARP and facilitated cell apoptosis. | Apoptosis | [86] | |
| Pancreatic cancer | Human Pancreatic carcinoma cell line Panc-1 | MMP-2/16 axis | Downregulated MMP2 and MMP16 and inhibited cell invasion and migration. | Invasion Migration | [87] | |
| Liver cancer | Human Liver carcinoma cell HepG2 | NF-κB axis | Downregulated Bcl-2 and upregulated BAX, Caspase-3, MMP2 and MMP9. | Migration Invasion Proliferation | [88] | |
| Gastric cancer | Gastric carcinoma cell line SGC-7901 | - | Downregulated Bcl-2, EGFR, Akt, p-Akt and NF-κ, and upregulated BAX and Caspase-3, inducing apoptosis of cancer cells. | Apoptosis | [89] |
| Active Ingredient | Disease | Experimental Model | Mechanism | Effect | References |
|---|---|---|---|---|---|
| Oleanolic Acid | Lung injury | NMDA-induced acute lung injury in mice. | Lower NF-κB, NLRP3 and BAX upregulate the levels of SIRT1, Nrf2 and Bcl-2 proteins and reduce lung injury. | Inflammation Oxidative stress Apoptosis | [94] |
| Liver injury | CCl4-induced liver injury in rats | The levels of GSH and SOD in mice with liver injury were upregulated, and the antioxidant defense system of liver was enhanced to reduce liver injury. | - | [95] | |
| Liver injury | CCl4-induced liver injury in rats | Downregulate MDA, ALT and AST and upregulate GSH to alleviate liver toxicity. | Inflammation | [96] | |
| Stigmasterol | Colitis | DSS-induced colitis mouse model | Downregulated levels of TNF-α, IL-6, IL-1β, CSF-1 and COX-2; inhibited NF-κB pathway; and alleviated colitis. | Inflammation | [97] |
| Luteolin | Liver and kidney injury | Doxorubicin-induced hepatic and renal disorders in rats | The antioxidant capacity and IL-10 level were improved, the activity of caspase-3/9 was reduced and hepatorenal toxicity was alleviated. | Inflammation Apoptosis | [98] |
| Liver and kidney injury | Methotrexate-induced hepatorenal toxicity in rats | The expressions of Nrf2, GSH, CAT and Bcl-2 were upregulated, and the expressions of ROS, NF-κB and BAX were downregulated to reduce hepatorenal toxicity. | Inflammation Apoptosis | [99] | |
| Liver injury | CCl4-induced liver injury in mice | TNF-α, IL-6, IL-1β, Caspase-3 and BAX were downregulated, and Bcl-2 was upregulated, reduce toxicity and protect ing against liver damage. | Inflammation Apoptosis | [100] | |
| Rosmarinic Acid | Liver and kidney injury | Methotrexate-induced hepatorenal toxicity in rats | Upregulation of GSH and CAT, downregulation of MDA, reduction in degeneration and cell vacuolization in liver tissue and reduction in hepatorenal toxicity. | Inflammation | [101] |
| Liver and kidney injury | Cisplatin-induced liver and kidney injury in mouse model | Downregulation of ALT, AST, BUN and CRE levels and inflammatory factor IL-1 β, IL-6 and TNF-α, activating the Nrf2 signaling pathway to prevent cisplatin induced liver and kidney damage. | Inflammation | [102] | |
| Liver and kidney injury | CCl4-induced liver injury in mice | Downregulated ALT, ALP, Caspase-3, TG, TC, MDA, TNF-α, IL-6 and IL-8 and upregulated GSH, SOD, CAT and Nrf2 levels to alleviate liver and kidney damage. | Inflammation Apoptosis | [103] | |
| Liver injury | Paracetamol-induced hepatotoxicity in rats | MDA, ALT and AST were decreased, while TAC, GSH and GST were increased, reducing liver damage. | Inflammation | [104] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ning, N.; Nan, Y.; Chen, G.; Huang, S.; Lu, D.; Yang, Y.; Meng, F.; Yuan, L. Anti-Tumor Effects and Toxicity Reduction Mechanisms of Prunella vulgaris: A Comprehensive Review. Molecules 2024, 29, 1843. https://doi.org/10.3390/molecules29081843
Ning N, Nan Y, Chen G, Huang S, Lu D, Yang Y, Meng F, Yuan L. Anti-Tumor Effects and Toxicity Reduction Mechanisms of Prunella vulgaris: A Comprehensive Review. Molecules. 2024; 29(8):1843. https://doi.org/10.3390/molecules29081843
Chicago/Turabian StyleNing, Na, Yi Nan, Guoqing Chen, Shicong Huang, Doudou Lu, Yating Yang, Fandi Meng, and Ling Yuan. 2024. "Anti-Tumor Effects and Toxicity Reduction Mechanisms of Prunella vulgaris: A Comprehensive Review" Molecules 29, no. 8: 1843. https://doi.org/10.3390/molecules29081843
APA StyleNing, N., Nan, Y., Chen, G., Huang, S., Lu, D., Yang, Y., Meng, F., & Yuan, L. (2024). Anti-Tumor Effects and Toxicity Reduction Mechanisms of Prunella vulgaris: A Comprehensive Review. Molecules, 29(8), 1843. https://doi.org/10.3390/molecules29081843