The Synthesis of 2′-Hydroxychalcones under Ball Mill Conditions and Their Biological Activities
Abstract
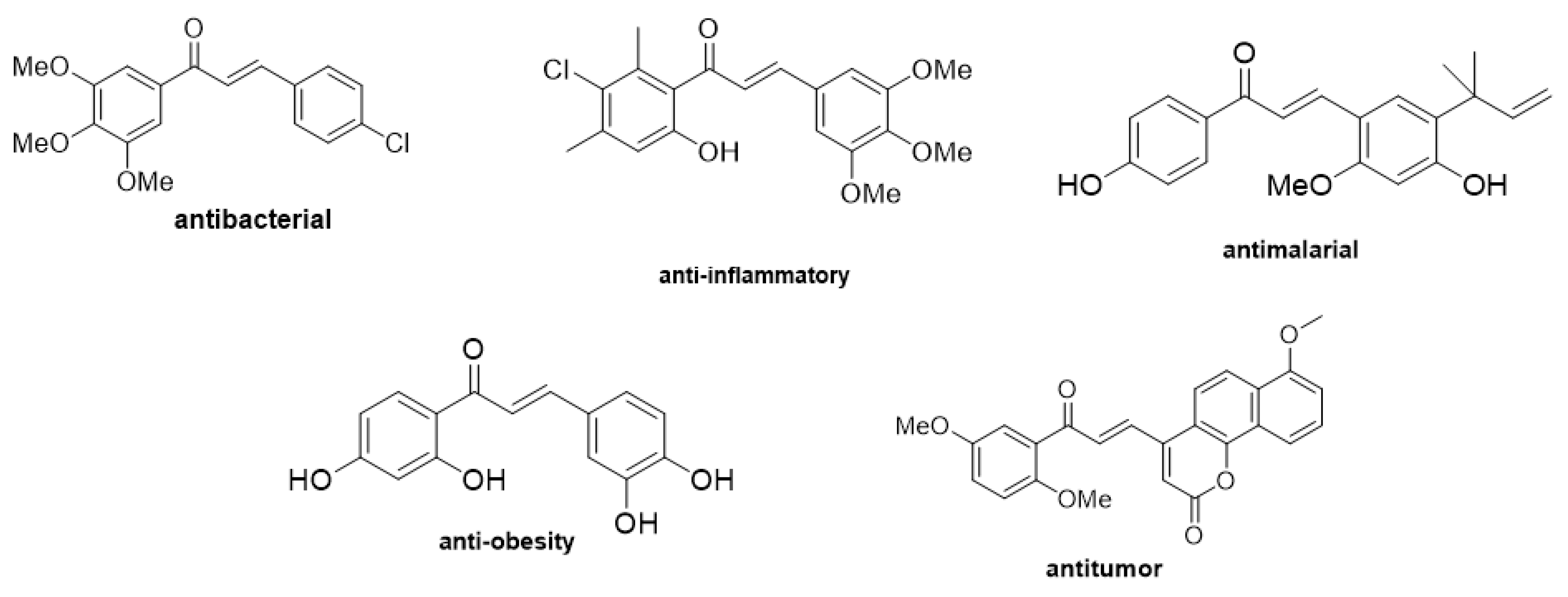
1. Introduction
2. Results and Discussion
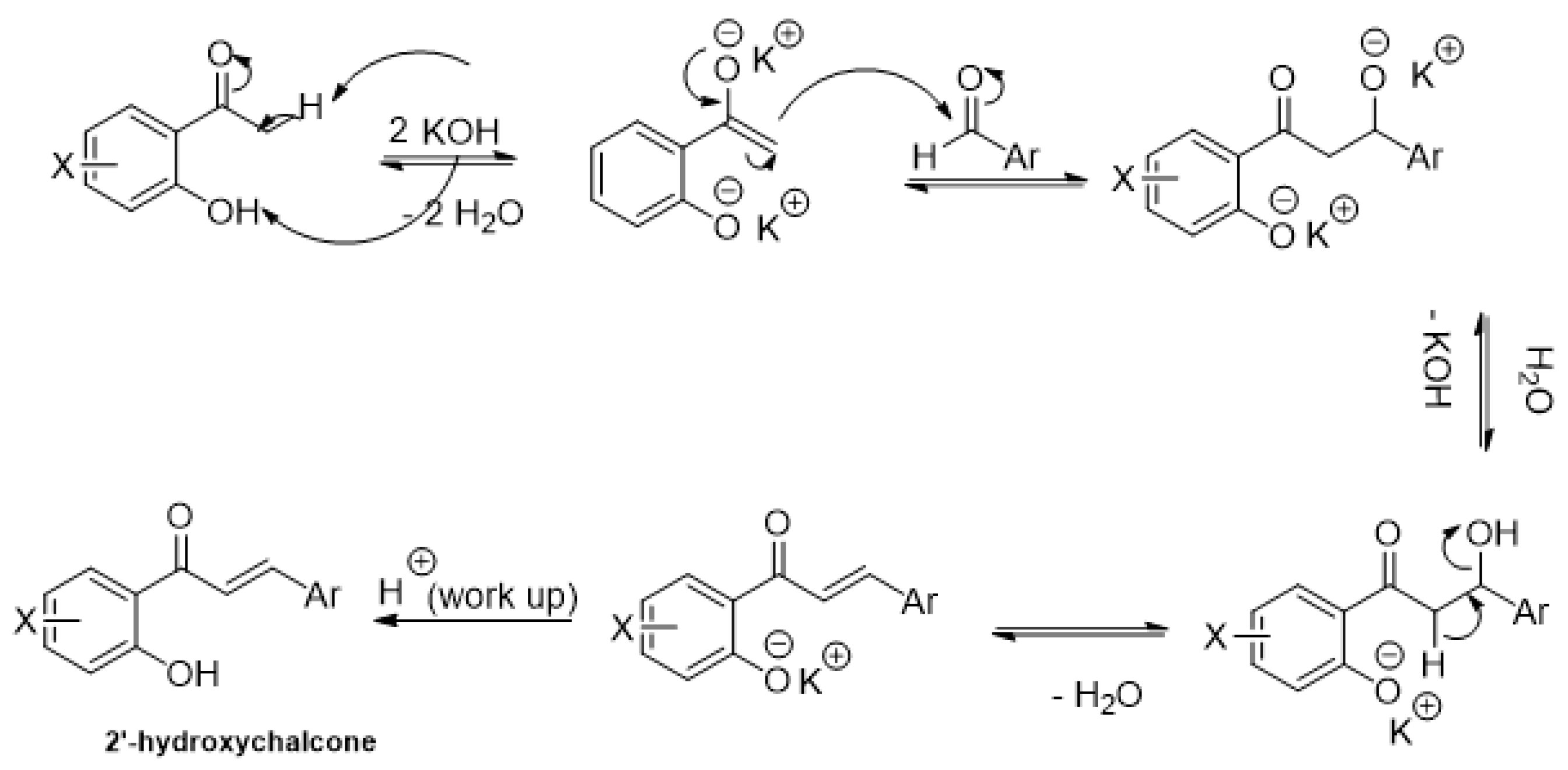
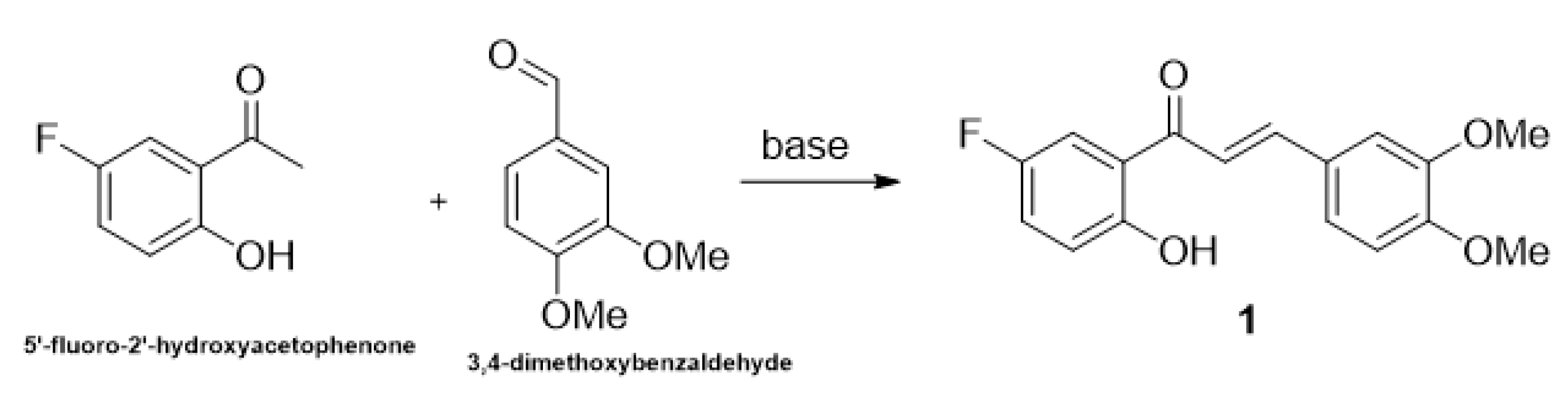
2.1. Research of Optimal Conditions in a Model Reaction
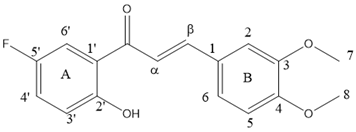
2.2. Reactions of 5′-Halogenated-2′-Hydroxychalcones and Methoxylated Benzaldehydes
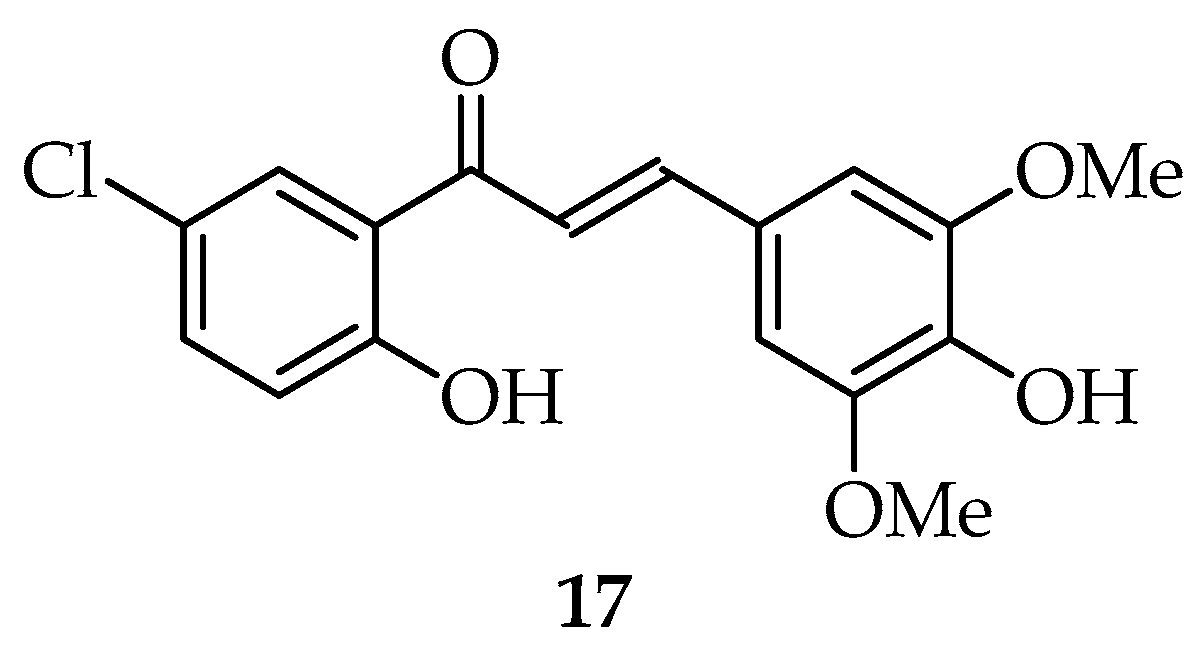
2.3. Miscellaneous Synthesized Chalcones
2.4. Green Chemistry Parameters
3. Biological Activities
3.1. Antileishmanial Activities
3.1.1. Effect of 2′-Hydroxychalcones on RAW 264.7 Cells
3.1.2. Effect of 2′-Hydrochalcones on L. donovani LV9
3.2. Antiplasmodial Activities
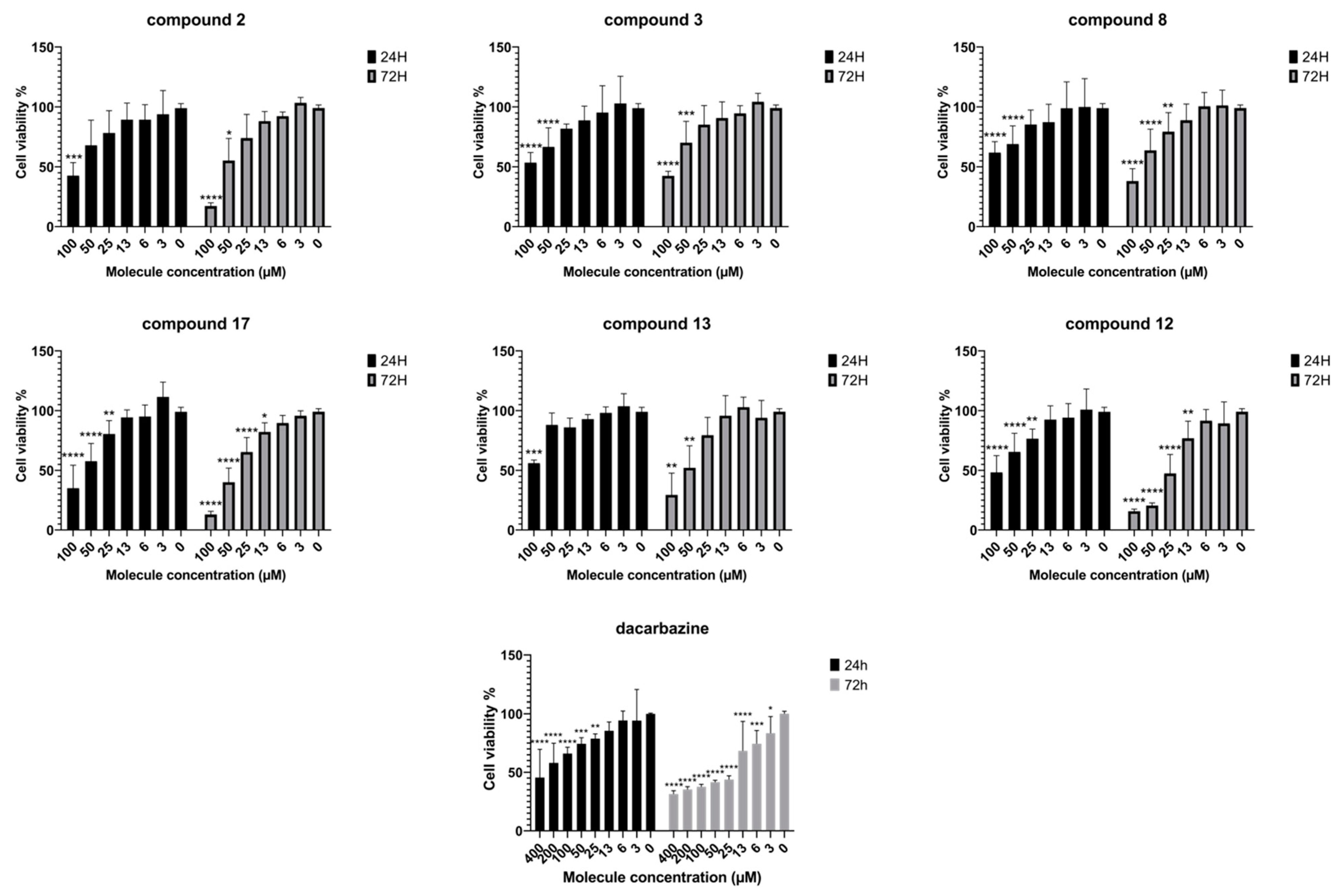
3.3. Antimelanoma Activities
4. Conclusions
5. Materials and Methods
5.1. Synthesis and Characterization
5.2. Biological Tests
5.2.1. Evaluation of the Antileishmanial Activity
5.2.2. Evaluation of the Antimalarial Activity on P. 3D7 Strain
5.2.3. Antimelanoma Tests against IGR-39 Cell Culture
5.3. Experimental Results in Chemistry
5.3.1. General Procedure for the 2′-Hydroxychalcone Derivatives Synthesis
5.3.2. General Procedure for the Synthesis of Compound 12 in the Conventional Method
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cory, H.; Passarelli, S.; Szeto, J.; Tamez, M.; Mattei, J. The Role of Polyphenols in Human Health and Food Systems: A Mini-Review. Front. Nutr. 2018, 5, 87. [Google Scholar] [CrossRef] [PubMed]
- Dao, T.T.; Linthorst, H.J.; Verpoorte, R. Chalcone synthase and its functions in plant resistance. Phytochem. Rev. Proc. Phytochem. Soc. Eur. 2011, 10, 397–412. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Wu, P.; Shen, F.; Ji, J.; Rakesh, K.P. Chalcone derivatives and their antibacterial activities: Current development. Bioorg. Chem. 2019, 91, 103133. [Google Scholar] [CrossRef]
- Qing, Z.; Xuemei, T.; Shuai, C.; Wenliang, Z.; Die, H.; Ran, Z.; Nan, S.; Wu, Y.J.; Wei, X. Design, Synthesis, and Antifungal Activity of Novel Chalcone Derivatives Containing a Piperazine Fragment. J. Agric. Food Chem. 2022, 70, 1029–1036. [Google Scholar] [CrossRef]
- Sinha, S.; Medhi, B.; Radotra, B.D.; Batovska, D.I.; Markova, N.; Bhalla, A.; Sehgal, R. Antimalarial and immunomodulatory potential of chalcone derivatives in experimental model of malaria. BMC Complement. Med. Ther. 2022, 22, 330. [Google Scholar] [CrossRef]
- Haroon, R.; Yiming, X.; Nasir, A.; Yaseen, M.; Lisheng, W. Promising anti-inflammatory effects of chalcones via inhibition of cyclooxygenase, prostaglantin E2, Inducible NO synthase and nuclear factor kb activities. Bioorg. Chem. 2019, 87, 335–365. [Google Scholar] [CrossRef]
- Maisto, M.; Marzocchi, A.; Keivani, N.; Piccolo, V.; Summa, V.; Tenore, G.C. Natural Chalcones for the Management of Obesity Disease. Int. J. Mol. Sci. 2023, 24, 15929. [Google Scholar] [CrossRef]
- Leite, F.F.; de Sousa, N.F.; de Oliveira, B.H.M.; Duarte, G.D.; Ferreira, M.D.L.; Scotti, M.T.; Filho, J.M.B.; Rodrigues, L.C.; de Moura, R.O.; Mendonça-Junior, F.J.B.; et al. Anticancer Activity of Chalcones and Its Derivatives: Review and In Silico Studies. Molecules 2023, 28, 4009. [Google Scholar] [CrossRef]
- Tom, A.; Jacob, J.; Mathews, M.; Rajagopal, R.; Alfarhan, A.; Barcelo, D.; Narayanan Kutty, A. Synthesis of Bis-Chalcones and Evaluation of Its Effect on Peroxide-Induced Cell Death and Lipopolysaccharide-Induced Cytokine Production. Molecules 2023, 28, 6354. [Google Scholar] [CrossRef]
- Akhtar, M.S.; Rehman, A.-U.; Arshad, H.; Malik, A.; Fatima, M.; Tabassum, T.; Raza, A.R.; Bukhsh, M.; Murtaza, M.A.; Mehmood, M.H.; et al. In Vitro Antioxidant Activities and the Therapeutic Potential of Some Newly Synthesized Chalcones Against 4-Acetaminophenol Induced Hepatotoxicity in Rats. Dose-Response 2021, 19, 1559325821996955. [Google Scholar] [CrossRef]
- Karimi-Sales, E.; Mohaddes, G.; Alipour, M.R. Chalcones as putative hepatoprotective agents: Preclinical evidence and molecular mechanisms. Pharm. Res. 2018, 129, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Sha, F.; Zhao, C.; Zheng, Z.; Zhao, S.; Zhu, Z.; Zhu, H.; Chen, J.; Chen, Y. Synthesis and anti-inflammatory activity of novel steroidal chalcones with 3β-pregnenolone ester derivatives in RAW 264.7 cells in vitro. Steroids 2021, 171, 108830. [Google Scholar] [CrossRef] [PubMed]
- Rudrapal, M.; Khan, J.; Dukhyil, A.A.B.; Alarousy, R.; Attah, E.I.; Sharma, T.; Khairnar, S.J.; Bendale, A.R. Chalcone Scaffolds, Bioprecursors of Flavonoids: Chemistry, Bioactivities, and Pharmacokinetics. Molecules 2021, 26, 7177. [Google Scholar] [CrossRef] [PubMed]
- Marotta, L.; Rossi, S.; Ibba, R.; Brogi, S.; Calderone, V.; Butini, S.; Campiani, G.; Gemma, S. The green chemistry of chalcones: Valuable sources of privileged core structures for drug discovery. Front. Chem. 2022, 10, 988376. [Google Scholar] [CrossRef] [PubMed]
- Pozzetti, L.; Ibba, R.; Rossi, S.; Taglialatela-Scafati, O.; Taramelli, D.; Basilico, N.; D’Alessandro, S.; Parapini, S.; Butini, S.; Campiani, G.; et al. Total Synthesis of the Natural Chalcone Lophirone E, Synthetic Studies toward Benzofuran and Indole-Based Analogues, and Investigation of Anti-Leishmanial Activity. Molecules 2022, 27, 463. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, C.; Zhang, W.; Sheng, C.; Zhang, W.; Xing, C.; Miao, Z. Chalcone: A Privileged Structure in Medicinal Chemistry. Chem. Rev. 2017, 117, 7762–7810. [Google Scholar] [CrossRef] [PubMed]
- Jasim, H.A.; Nahar, L.; Jasim, M.A.; Moore, S.A.; Ritchie, K.J.; Sarker, S.D. Chalcones: Synthetic Chemistry Follows Where Nature Leads. Biomolecules 2021, 11, 1203. [Google Scholar] [CrossRef] [PubMed]
- Anastas, P.T.; Warner, J.C. Green Chemistry: Theory and Practice; Oxford University Press: Oxford, UK, 1998; ISBN 0198502346, 9780198502340. [Google Scholar]
- Baig, R.B.N.; Varma, R.S. Alternative energy input: Mechanochemical, microwave and ultrasound-assisted organic synthesis. Chem. Soc. Rev. 2012, 41, 1559–1584. [Google Scholar] [CrossRef]
- Toda, F.; Tanaka, K.; Iwata, S. Oxidative coupling reactions of phenols with iron (III) chloride in the solid state. J. Org. Chem. 1989, 54, 3007–3009. [Google Scholar] [CrossRef]
- Kaupp, G. Mechanochemistry: The Varied Applications of Mechanical Bond-Breaking. CrystEngComm 2009, 11, 388–403. [Google Scholar] [CrossRef]
- Tan, D.; García, F. Main group mechanochemistry: From curiosity to established protocols. Chem. Soc. Rev. 2019, 48, 2274–2292. [Google Scholar] [CrossRef] [PubMed]
- Klok, H.A.; Genzer, J. Expanding the Polymer Mechanochemistry Toolbox through Surface-Initiated Polymerization. ACS Macro Lett. 2015, 4, 636–639. [Google Scholar] [CrossRef] [PubMed]
- Do, J.L.; Friscic, T. Mechanochemistry: A Force of Synthesis. ACS Cent. Sci. 2017, 3, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Achar, T.K.; Bose, A.; Mal, P. Mechanochemical synthesis of small organic molecules. Beilstein J. Org. Chem. 2017, 13, 1907–1931. [Google Scholar] [CrossRef] [PubMed]
- Gonnet, L.; André-Barrès, C.; Guidetti, B.; Chamayou, A.; Menendez, C.; Baron, M.; Calvet, R.; Baltas, M. Study of the two steps and one-pot two-step mechanochemical synthesis of annulated 1,2,4-triazoles. ACS Sustain. Chem. Eng. 2020, 8, 3114–3125. [Google Scholar] [CrossRef]
- Sharma, P.; Vetter, C.; Ponnusamy, E.; Colacino, E. Assessing the greenness of mechanochemical processes with the DOZN 2.0 tool. ACS Sustain. Chem. Eng. 2022, 10, 5110–5116. [Google Scholar] [CrossRef]
- Kudličková, Z.; Stahorsky, M.; Michalková, R.; Vilková, M.; Matej Baláž, M. Mechanochemical synthesis of indolyl chalcones with antiproliferative activity. Green Chem. Lett. Rev. 2022, 15, 474–482. [Google Scholar] [CrossRef]
- Kapusterynska, A.; Bijani, C.; Paliwoda, D.; Vendier, L.; Bourdon, V.; Imbert, N.; Cojean, S.; Loiseau, P.M.; Recchia, D.; Scoffone, V.C.; et al. Mechanochemical Studies on Coupling of Hydrazines and Hydrazine Amides with Phenolic and Furanyl Aldehydes-Hydrazones with Antileishmanial and Antibacterial Activities. Molecules 2023, 28, 5284. [Google Scholar] [CrossRef] [PubMed]
- Kakati, D.; Sharma, C. Microwave assisted solvent free synthesis of 1,3-diphenylpropenones. Chem. Central J. 2011, 5, 8. [Google Scholar] [CrossRef]
- Kamble, V.M.; Hatnapure, G.D.; Keche, A.P.; Birajdar, S.; Patil, S.G.; Tale, R.H.; Rodge, A.H.; Turkar, S.S.; Gour, K. Synthesis and biological evaluation of a novel series of methoxylated chalcones as antioxidant and anti-microbial agents. J. Chem. Pharm. Res. 2011, 3, 639–648. [Google Scholar]
- Albogami, A.S.; Karama, U.; Mousa, A.A.; Khan, M.; Abdullah, S.; Alkhathlan, H.Z. Simple and efficient one step synthesis of functionalized flavanones and chalcones. Orient. J. Chem. 2012, 28, 619–626. [Google Scholar] [CrossRef]
- Detsi, A.; Kotopoulou, I.; Tzani, A.; Polyzos, N.O.; Karadendrou, M.A.; Kritsi, E.; Pontiki, E.; Liargkova, T.; Zoumpoulakis, P.; Hadji pavlou-Litina, D. Exploring the 2′-hydroxy-chalcone Framework for the Development of Dual Antioxidant and Soybean Lipoxygenase Inhibitory Agents. Molecules 2021, 26, 2777. [Google Scholar] [CrossRef] [PubMed]
- Szabados-Furjesi, P.; Patjas, D.; Barta, A.; Csepanyi, E.; Kiss-Szikszai, A.; Tosaki, A.; Bak, I. Synthesis, in vitro biological evaluation and oxidative transformation of new flavonol derivatives: The possible role of the phenyl-N,N-dimethylamino group. Molecules 2018, 23, 3161. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, R.A. The E-factor: Fifteen Years On. Green Chem. 2007, 9, 1273–1283. [Google Scholar] [CrossRef]
- Harshitha, K.R.; Sarojini, B.K.; Narayana, B.; Lobo, A.G.; Kalal, B.S. Molecular Docking of 4-Ethoxychalcones on Oxidoreductase/Pirin Inhibitors and Cytotoxic Evaluation on Breast/Skin Cancer Cell Lines. Lett. Drug Des. Discov. 2020, 17, 1245–1260. [Google Scholar] [CrossRef]
- Castaño, L.F.; Quiroga, J.; Abonia, R.; Insuasty, D.; Vidal, O.M.; Seña, R.; Rubio, V.; Puerto, G.; Nogueras, M.; Cobo, J.; et al. Synthesis, Anticancer and Antitubercular Properties of New Chalcones and Their Nitrogen-Containing Five-Membered Heterocyclic Hybrids Bearing Sulfonamide Moiety. Int. J. Mol. Sci. 2022, 23, 12589. [Google Scholar] [CrossRef] [PubMed]
- Gazdova, M.; Michalkova, R.; Kello, M.; Vilkova, M.; Kudlickova, Z.; Baloghova, J.; Mirossay, L.; Mojzis, J. Chalcone-Acridine Hybrid Suppresses Melanoma Cell Progression via G2/M Cell Cycle Arrest, DNA Damage, Apoptosis, and Modulation of MAP Kinases Activity. Int. J. Mol. Sci. 2022, 23, 12266. [Google Scholar] [CrossRef] [PubMed]
- Bazin, M.-A.; Cojean, S.; Pagniez, F.; Bernadat, G.; Cavé, C.; Ourliac-Garnier, I.; Nourrisson, M.-R.; Cathy Morgado, C.; Picot, C.; Leclercq, O.; et al. In vitro identification of imidazo [1,2-a] pyrazine-based antileishmanial agents and evaluation of L. major casein kinase 1 inhibition. Eur. J. Med. Chem. 2021, 210, 112956. [Google Scholar] [CrossRef]
- Seck, R.; Gassama, A.; Cojean, S.; Cavé, C. Synthesis and Antimalarial Activity of 1,4-Disubstituted Piperidine Derivatives. Molecules 2020, 25, 299. [Google Scholar] [CrossRef]


| Entry | Base | Eq.: Ketone: Aldehyde: Base | Time Cycle × min | Additive (1 or 3 eq.) | Yields (%) |
|---|---|---|---|---|---|
| 1 | NaOH | 1:1:1 | 2 × 15 | - | No reaction |
| 2 | NaOH | 1:1:2 | 2 × 15 | - | 20 |
| 3 | NaOH | 1:1:3 | 2 × 15 | - | 23 |
| 4 | LiOH | 1:1:3 | 2 × 15 | - | No reaction |
| 5 | KOH | 1:1:2 | 2 × 15 | - | 40 |
| 6 | KOH | 1:1:3 | 2 × 15 | - | 43 |
| 7 | KOH | 1:1:2 | 2 × 30 | - | 78 |
| 8 | KOH | 1:1 + 1:2 | 2 × 30 | - | 96 |
| 9 | KOH | 1:1:2 | 2 × 15 | K2CO3 | 54 |
| 10 | KOH | 1:1:2 | 1 × 60 | 1 eq. K2CO3 3 eq. K2CO3 | 74 76 |
| 11 | KOH | 1:2:2 | 1 × 60 | K2CO3 | 95 |
| 12 | KOH | 1:1:2 | 2 × 15 | 1 eq. Alumina 3 eq. Alumina | - |
| 1H and/or 13C | 1H Chemical Shift | 13C Chemical Shift |
|---|---|---|
| C=O | 192.69 | |
| CH-α | 7.40 | 117.16 |
| CH-β | 7.91 | 146.60 |
| C-1 | 127.35 | |
| CH-2 | 7.18 | 110.23 |
| C-3 | 149.40 | |
| C-4 | 152.10 | |
| CH-5 | 6.93 | 111.2 |
| CH-6 | 7.28 | 124.0 |
| C-1′ | 119.62 | |
| C-2′ | 159.7 | |
| CH-3′ | 7.00 | 119.83 |
| CH-4′ | 7.23 | 123.66 |
| C-5′ | 154.85 | |
| CH-6′ | 7.60 | 114.48 |
| CH3-7 | 3.98 | 56.07 |
| CH3-8 | 3.95 | 56.09 |
| Entry | Ketone X = F, Cl, Br | Benzaldehyde | Chalcone | Yield (%) |
|---|---|---|---|---|
| 1 | 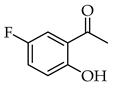 | R3, R4 = OMe; R2, R5 = H | 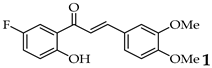 | 96 |
| 2 | R2, R3 = OMe R4, R5 = H | 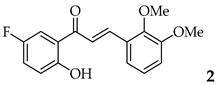 | 74 | |
| 3 | R3, R4, R5 = OMe R2 = H | 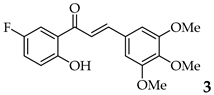 | 84 | |
| 4 | R2, R4, R5 = OMe R3 = H | 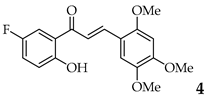 | 72 | |
| 5 | R3, R5 = OMe R2, R4 = H | 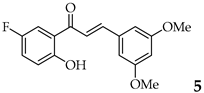 | 88 | |
| 6 | 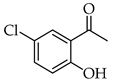 | R3, R4 = OMe R2, R5 = H | 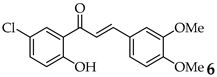 | 92 |
| 7 | R2, R3 = OMe R4, R5 = H | 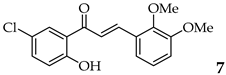 | 95 | |
| 8 | R3, R4, R5 = OMe R2 = H | 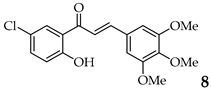 | 88 | |
| 9 | R2, R4, R5 = OMe | -- | No reaction | |
| 10 | R3, R5 = OMe R2, R4 = H | 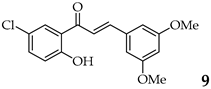 | 94 | |
| 11 |  | R3, R4 = OMe R2, R5 = H | 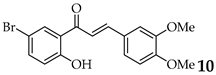 | 85 |
| 12 | R2, R3 = OMe R4, R5 = H | 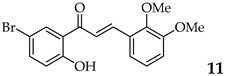 | 79 | |
| 13 | R3, R4, R5 = OMe R2 = H | 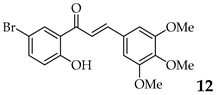 | 86 | |
| 14 | R2, R4, R5 = OMe | -- | No reaction | |
| 15 | R3, R5 = OMe R2, R4 = H |  | 94 |
| Settings | In Solution | Ball Mill |
|---|---|---|
| Yield (%) | 47 | 86 |
| Reaction time (min) | 120 | 60 |
| Energy consumption (W × h) | 38 | 30 |
| E-factor | 560 | 293 |
| Compound | L. donovani Axenic Amastigote | L. donovani Intramacrophage Amastigote | SI * (Selectivity Index) | 3D7 P. falciparum | IGR-39 Cells Viability (24 h) | IGR-39 Cells Viability (72 h) |
|---|---|---|---|---|---|---|
| IC50 ± SD (µM) | IC50 ± SD (µM) | IC50 ± SD (µM) | IC50 (µM) | IC50 (µM) | ||
| 1 | 17.37 ± 2.64 | 5.42 ± 1.48 | 10.7 | >100 | >>100 | >>100 |
| 2 | 14.24 ± 2.96 | 12.80 ± 1.61 | >7.81 | 7.59 ± 1.46 | 82.7 | 54.1 |
| 3 | 5.59 ± 1.12 | 5.18 ± 1.68 | >19.3 | 14.12 ± 1.99 | >100 | 83.4 |
| 4 | 6.67 ± 1.52 | 1.59 ± 0.09 | 39 | 15.01± 0.08 | >>100 | >>100 |
| 5 | 8.68 ± 0.67 | ND | ND | >100 | >>100 | >>100 |
| 6 | 2.33 ± 0.53 | 1.36 ± 0.51 | >73.5 | 5.26 ± 0.05 | >>100 | >>100 |
| 7 | 4.29 ± 0.82 | 2.48 ± 0.42 | >40.3 | 8.05 ± 0.03 | >>100 | >>100 |
| 8 | 9.82 ± 2.47 | 9.82 ± 2.47 | >10.2 | 10.21 ± 2.56 | >100 | 70.5 |
| 9 | 8.59 ± 1.08 | ND | ND | 13.41 ± 1.54 | >>100 | >>100 |
| 10 | 5.87 ± 2.11 | 10.03 ± 2.14 | >9.97 | 36.84 ± 2.54 | >>100 | >>100 |
| 11 | 2.82 ± 0.77 | 3.29 ± 1.99 | >30.4 | 51.35 ± 2.54 | >>100 | >>100 |
| 12 | >100 | >100 | ND | 17.51 ± 2.18 | 91 | 24.7 |
| 13 | 11.52 ± 0.47 | 15.22 ± 1.43 | >6.57 | 15.11 ± 1.29 | >100 | 51.5 |
| 14 | 11.38 ± 2.52 | 22.91 ± 3.91 | >4.36 | >100 | >>100 | >>100 |
| 15 | 9.44 ± 2.20 | 2.87 ± 0.36 | >34.8 | 3.21 | >>100 | >>100 |
| 16 | 11.23 ± 2.06 | 13.49 ± 2.99 | >7.41 | 53.43 ± 4.16 | >>100 | >>100 |
| 17 | 24.24 ± 2.56 | 18.41 ± 2.33 | 2.62 | 6.13 ± 0.51 | 66.3 | 38.6 |
| Reference | 3.66 ± 0.73 a | 5.78 ± 1.02 a | 9 a | 0.012 ± 0.002 b | 372.0 c | 25.0 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abid, I.; Moslah, W.; Cojean, S.; Imbert, N.; Loiseau, P.M.; Chamayou, A.; Srairi-Abid, N.; Calvet, R.; Baltas, M. The Synthesis of 2′-Hydroxychalcones under Ball Mill Conditions and Their Biological Activities. Molecules 2024, 29, 1819. https://doi.org/10.3390/molecules29081819
Abid I, Moslah W, Cojean S, Imbert N, Loiseau PM, Chamayou A, Srairi-Abid N, Calvet R, Baltas M. The Synthesis of 2′-Hydroxychalcones under Ball Mill Conditions and Their Biological Activities. Molecules. 2024; 29(8):1819. https://doi.org/10.3390/molecules29081819
Chicago/Turabian StyleAbid, Imen, Wassim Moslah, Sandrine Cojean, Nicolas Imbert, Philippe M. Loiseau, Alain Chamayou, Najet Srairi-Abid, Rachel Calvet, and Michel Baltas. 2024. "The Synthesis of 2′-Hydroxychalcones under Ball Mill Conditions and Their Biological Activities" Molecules 29, no. 8: 1819. https://doi.org/10.3390/molecules29081819
APA StyleAbid, I., Moslah, W., Cojean, S., Imbert, N., Loiseau, P. M., Chamayou, A., Srairi-Abid, N., Calvet, R., & Baltas, M. (2024). The Synthesis of 2′-Hydroxychalcones under Ball Mill Conditions and Their Biological Activities. Molecules, 29(8), 1819. https://doi.org/10.3390/molecules29081819







