Dendritic Pyridine–Imine Copper Complexes as Metallo-Drugs
Abstract
1. Introduction
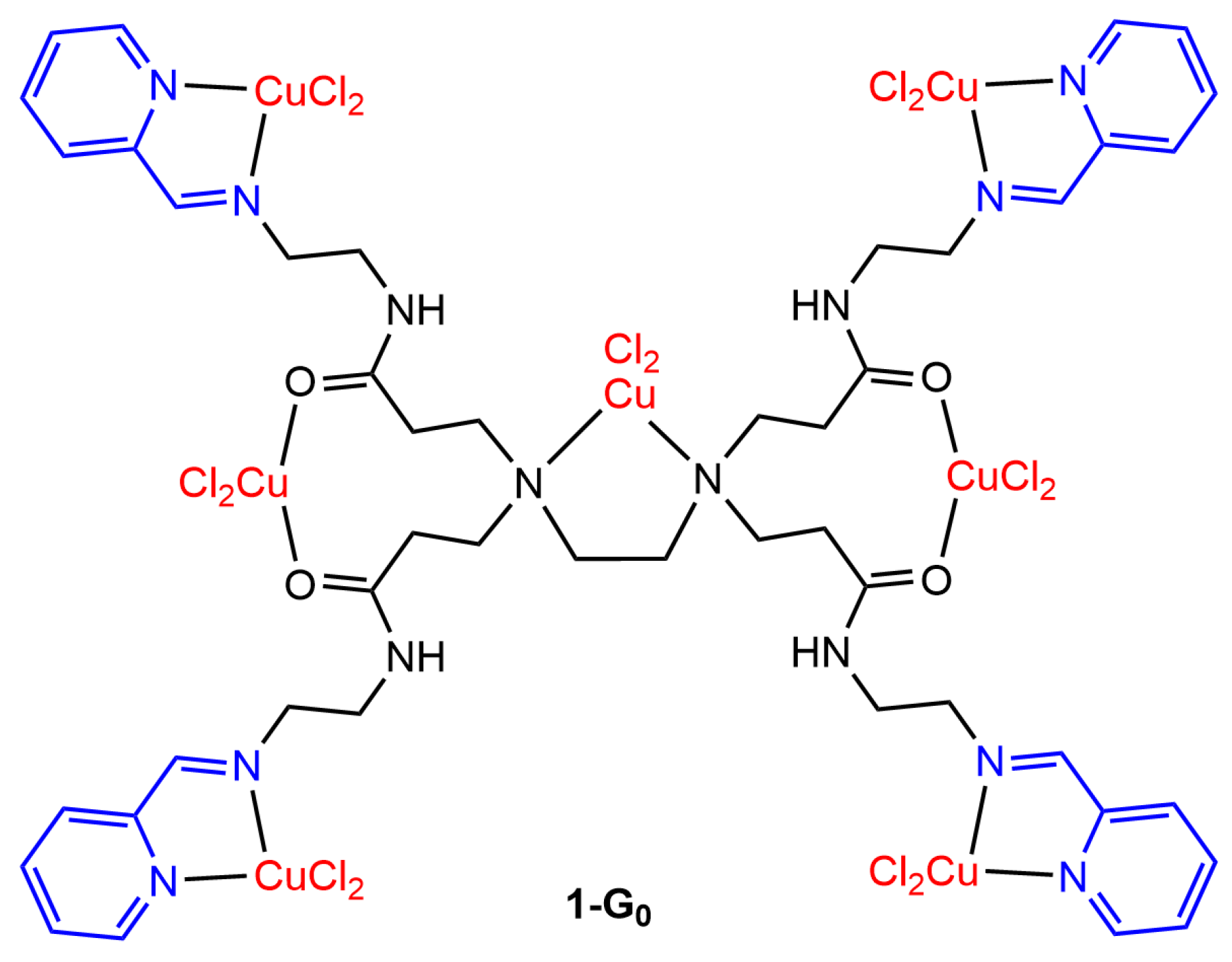
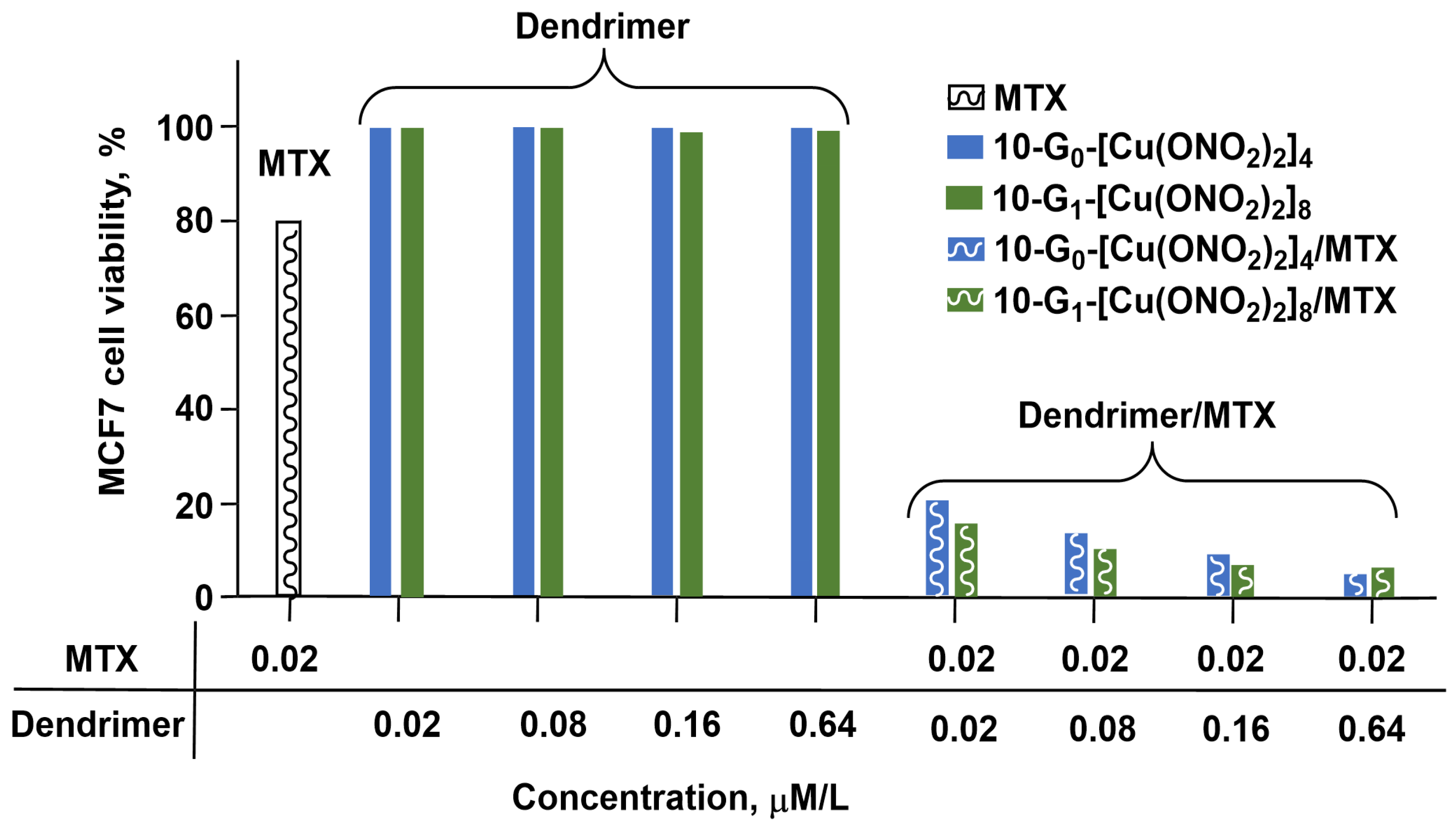
2. PAMAM Dendrimer Cu Complexes
3. Phosphorus Dendrimers and Dendrons
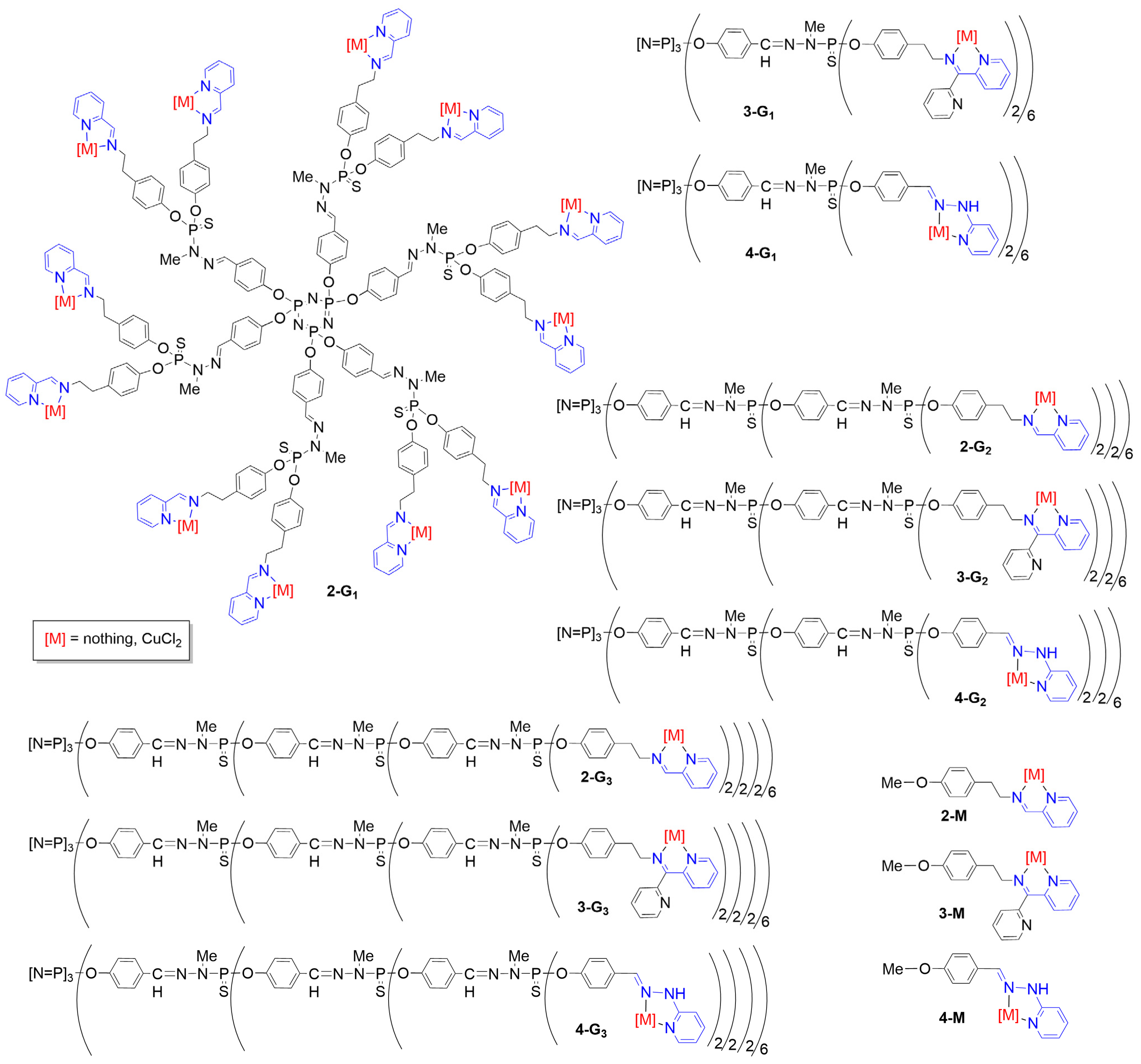
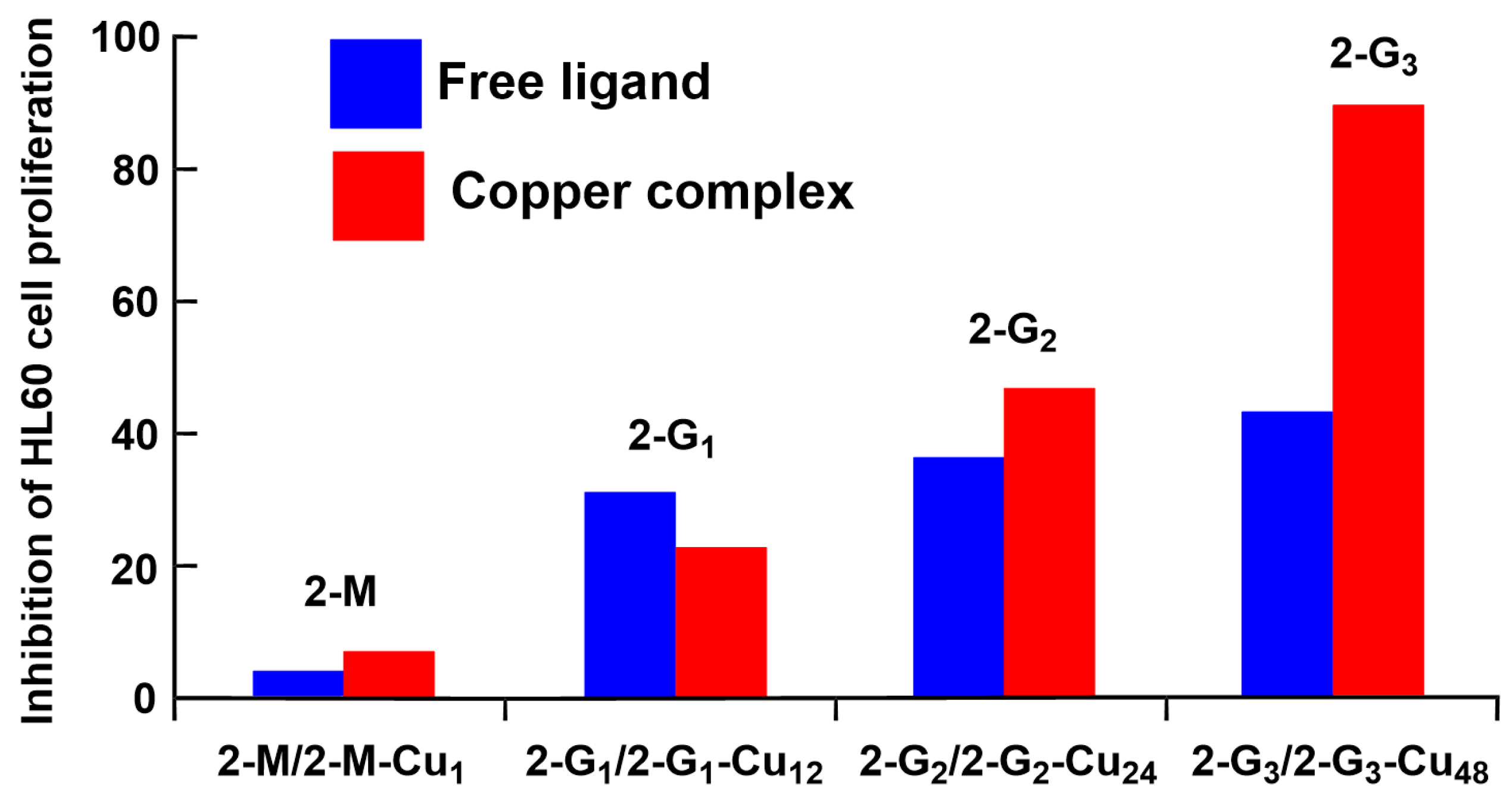
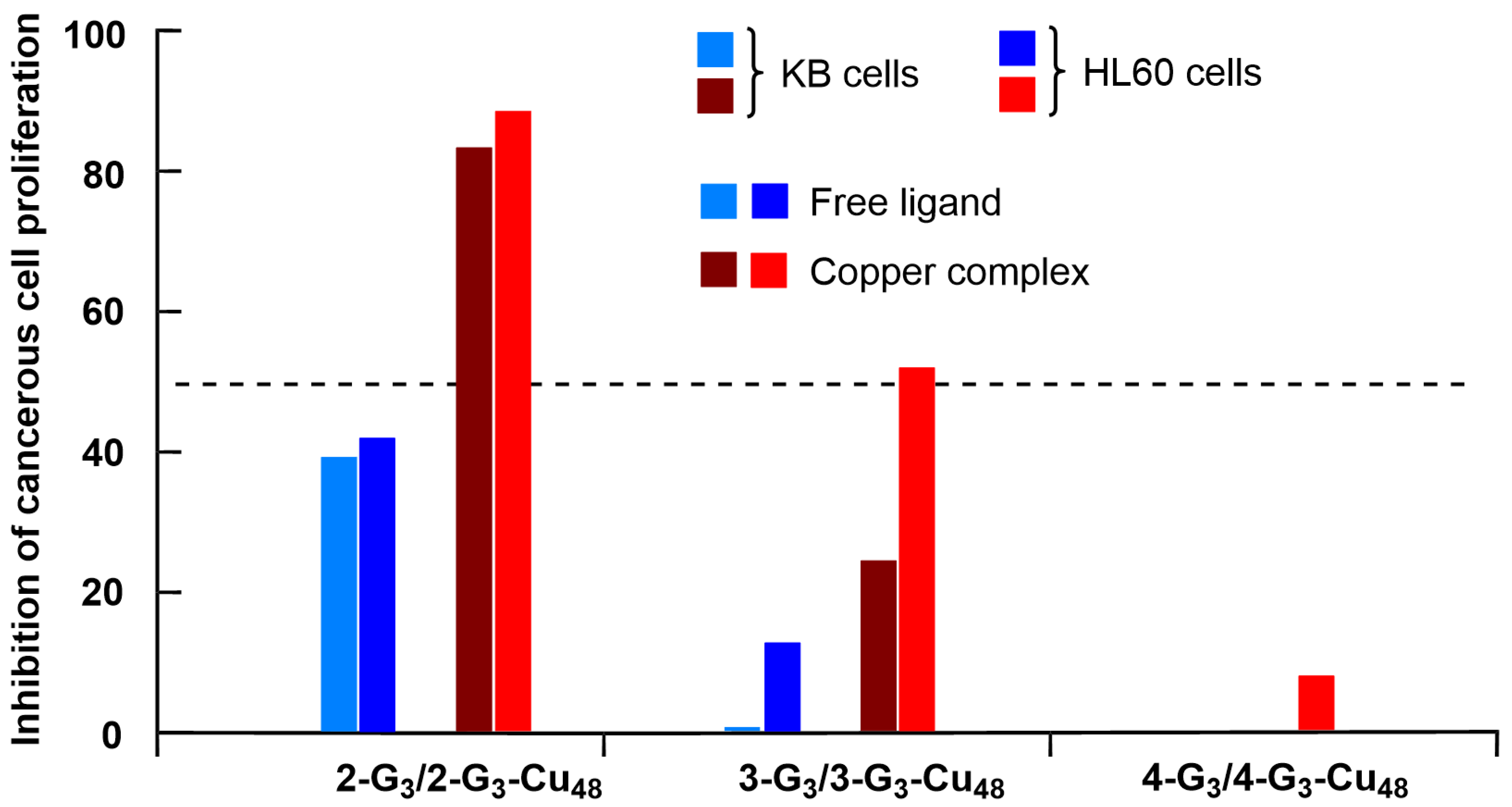
3.1. Phosphorus Dendrimers
3.2. Phosphorus Dendrons
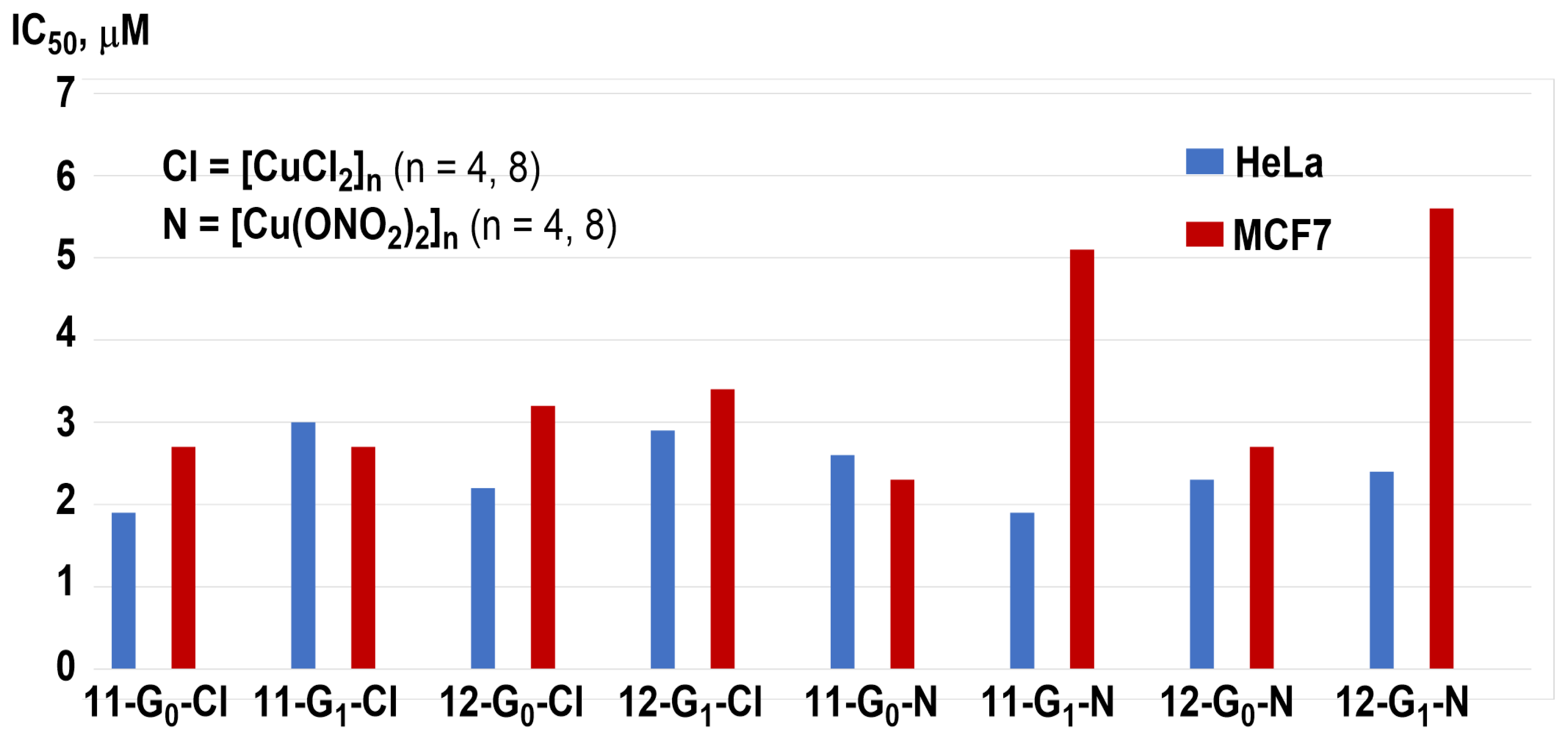
4. Carbosilane Dendrimers
4.1. Anticancer Properties of Carbosilane Dendrimers
4.2. Antibacterial Properties of Carbosilane Dendrimers
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rosenberg, B.; Vancamp, L.; Trosko, J.E.; Mansour, V.H. Platinum compounds: A new class of potent antitumour agents. Nature 1969, 222, 385–386. [Google Scholar] [CrossRef]
- Kelland, L. The resurgence of platinum-based cancer chemotherapy. Nat. Rev. Cancer 2007, 7, 573–584. [Google Scholar] [CrossRef]
- Adhikari, S.; Nath, P.; Das, A.; Datta, A.; Baildya, N.; Duttaroy, A.K.; Pathak, S. A review on metal complexes and its anti-cancer activities: Recent updates from in vivo studies. Biomed. Pharmacother. 2024, 171, 116211. [Google Scholar] [CrossRef]
- Temesgen, A.; Ananda Murthy, H.C.; Zereffa Enyew, A.; Revathi, R.; Venkatesha Perumal, R. Emerging trends in metal-based anticancer agents: Drug design to clinical trials and their mechanism of action. Chem. Select 2023, 8, e202302113. [Google Scholar] [CrossRef]
- Lucaciu, R.L.; Hangan, A.C.; Sevastre, B.; Oprean, L.S. Metallo-drugs in Cancer Therapy: Past, Present and Future. Molecules 2022, 27, 6485. [Google Scholar] [CrossRef]
- Marzano, C.; Pellei, M.; Tisato, F.; Santini, C. Copper complexes as anticancer agents. Anti-Cancer Agents Med. Chem. 2009, 9, 185–211. [Google Scholar] [CrossRef]
- Santini, C.; Pellei, M.; Gandin, V.; Porchia, M.; Tisato, F.; Marzano, C. Advances in copper complexes as anticancer agents. Chem. Rev. 2014, 114, 815–862. [Google Scholar] [CrossRef]
- Feng, Q.; Huo, C.; Wang, M.; Huang, H.; Zheng, X.; Xie, M. Research progress on cuproptosis in cancer. Front. Pharmacol. 2024, 15, 1290592. [Google Scholar] [CrossRef]
- Chen, L.; Min, J.; Wang, F. Copper homeostasis and cuproptosis in health and disease. Signal Transduct. Target. Ther. 2022, 7, 378. [Google Scholar] [CrossRef]
- Wang, Z.; Jin, D.; Zhou, S.; Dong, N.; Ji, Y.; An, P.; Wang, J.; Luo, Y.; Luo, J. Regulatory roles of copper metabolism and cuproptosis in human cancers. Front. Oncol. 2023, 13, 1123420. [Google Scholar] [CrossRef]
- Xie, J.; Yang, Y.; Gao, Y.; He, J. Cuproptosis: Mechanisms and links with cancers. Mol. Cancer 2023, 22, 46. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, Y.; Zhang, J.; Yang, Y.; Fleishman, J.S.; Wang, Y.; Wang, J.; Chen, J.; Li, Y.; Wang, H. Cuproptosis: A novel therapeutic target for overcoming cancer drug resistance. Drug Resist. Updates 2024, 72, 101018. [Google Scholar] [CrossRef]
- De Luca, A.; Barile, A.; Arciello, M.; Rossi, L. Copper homeostasis as target of both consolidated and innovative strategies of anti-tumor therapy. J. Trace Elem. Med. Biol. 2019, 55, 204–213. [Google Scholar] [CrossRef]
- Lowndes, S.A.; Harris, A.L. The role of copper in tumour angiogenesis. J. Mammary Gland. Biol. Neoplasia 2005, 10, 299–310. [Google Scholar] [CrossRef]
- Nasulewiez, A.; Mazur, A.; Opolski, A. Role of copper in tumour angiogenesis–clinical implications. J. Trace Elem. Med. Biol. 2004, 18, 1–8. [Google Scholar] [CrossRef]
- Yang, Y.; Li, M.; Chen, G.; Liu, S.; Guo, H.; Dong, X.; Wang, K.; Geng, H.; Jiang, J.; Li, X. Dissecting copper biology and cancer treatment: ‘Activating Cuproptosis or suppressing Cuproplasia’. Coord. Chem. Rev. 2023, 495, 215395. [Google Scholar] [CrossRef]
- Lelievre, P.; Sancey, L.; Coll, J.-L.; Deniaud, A.; Busser, B. The Multifaceted Roles of Copper in Cancer: A Trace Metal Element with Dysregulated Metabolism, but Also a Target or a Bullet for Therapy. Cancers 2020, 12, 3594. [Google Scholar] [CrossRef]
- Merlot, A.M.; Kalinowski, D.S.; Richardson, D.R. Novel Chelators for Cancer Treatment: Where Are We Now? Antioxid. Redox Signal. 2013, 18, 973–1006. [Google Scholar] [CrossRef]
- Wang, W.; Mo, W.; Hang, Z.; Huang, Y.; Yi, H.; Sun, Z.; Lei, A. Cuproptosis: Harnessing Transition Metal for Cancer Therapy. ACS Nano 2023, 17, 19581–19599. [Google Scholar] [CrossRef]
- Denoyer, D.; Masaldan, S.; La Fontaine, S.; Cater, M.A. Targeting copper in cancer therapy: ‘Copper That Cancer’. Metallomics 2015, 7, 1459–1476. [Google Scholar] [CrossRef]
- Richa; Kumar, V.; Kataria, R. Phenanthroline and Schiff Base associated Cu(II)-coordinated compounds containing N, O as donor atoms for potent anticancer activity. J. Inorg. Biochem. 2024, 251, 112440. [Google Scholar] [CrossRef]
- More, M.S.; Joshi, P.G.; Mishra, Y.K.; Khanna, P.K. Metal complexes driven from Schiff bases and semicarbazones for biomedical and allied applications: A review. Mater. Today Chem. 2019, 14, 100195. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H. Toward a full understanding of the EPR effect in primary and metastatic tumors as well as issues related to its heterogeneity. Adv. Drug Deliv. Rev. 2015, 91, 3–6. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.; Xiang, J.; Zhou, Q.; Piao, Y.; Tang, J.; Shao, S.; Zhou, Z.; Bae, Y.H.; Shen, Y. The tumor EPR effect for cancer drug delivery: Current status, limitations, and alternatives. Adv. Drug Deliv. Rev. 2022, 191, 114614. [Google Scholar] [CrossRef]
- Svenson, S. Dendrimers as versatile platform in drug delivery applications. Eur. J. Pharm. Biopharm. 2009, 71, 445–462. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Zhao, L.; Li, Y.; Xu, T. Design of biocompatible dendrimers for cancer diagnosis and therapy: Current status and future perspectives. Chem. Soc. Rev. 2011, 40, 2673–2703. [Google Scholar] [CrossRef] [PubMed]
- Tomalia, D.A.; Naylor, A.M.; Goddard, W.A. Starburst dendrimers–Molecular level control of size, shape, surface chemistry, topology, and flexibility from atoms to macroscopic matter. Angew. Chem.-Int. Edit. Engl. 1990, 29, 138–175. [Google Scholar] [CrossRef]
- Caminade, A.M.; Turrin, C.O.; Laurent, R.; Ouali, A.; Delavaux-Nicot, B. Dendrimers: Towards Catalytic, Material and Biomedical Uses; Wiley: Hoboken, NJ, USA, 2011; pp. 1–538. Available online: https://www.wiley.com/en-us/Dendrimers%3A+Towards+Catalytic%2C+Material+and+Biomedical+Uses-p-9781119977575 (accessed on 6 March 2024).
- Astruc, D.; Boisselier, E.; Ornelas, C. Dendrimers Designed for Functions: From Physical, Photophysical, and Supramolecular Properties to Applications in Sensing, Catalysis, Molecular Electronics, Photonics, and Nanomedicine. Chem. Rev. 2010, 110, 1857–1959. [Google Scholar] [CrossRef]
- Tomalia, D.A.; Baker, H.; Dewald, J.; Hall, M.; Kallos, G.; Martin, S.; Roeck, J.; Ryder, J.; Smith, P. A new class of polymers–Starburst-dendritic macromolecules. Polymer J. 1985, 17, 117–132. [Google Scholar] [CrossRef]
- Majoral, J.P.; Caminade, A.M. Dendrimers containing heteroatoms (Si, P, B, Ge, or Bi). Chem. Rev. 1999, 99, 845–880. [Google Scholar] [CrossRef]
- Caminade, A.M. Inorganic dendrimers: Recent advances for catalysis, nanomaterials, and nanomedicine. Chem. Soc. Rev. 2016, 45, 5174–5186. [Google Scholar] [CrossRef] [PubMed]
- Apartsin, E.K.; Knauer, N.; Kahlert, U.D.; Caminade, A.-M. Amphiphilic Triazine-Phosphorus Metallodendrons Possessing Anti-Cancer Stem Cell Activity. Pharmaceutics 2022, 14, 393. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.X.; Loo, S.C.J.; Lee, P.P.F.; Tan, T.T.Y.; Chu, C.K. Synthesis and cytotoxic activities of chloropyridylimineplatinum(II) and chloropyridyliminecopper(II) surface-functionalized poly(amidoamine) dendrimers. J. Inorg. Biochem. 2010, 104, 105–110. [Google Scholar] [CrossRef] [PubMed]
- El Brahmi, N.; El Kazzouli, S.; Mignani, S.M.; Essassi, E.; Aubert, G.; Laurent, R.; Caminade, A.M.; Bousmina, M.M.; Cresteil, T.; Majoral, J.P. Original Multivalent Copper(II)-Conjugated Phosphorus Dendrimers and Corresponding Mononuclear Copper(II) Complexes with Antitumoral Activities. Mol. Pharm. 2013, 10, 1459–1464. [Google Scholar] [CrossRef]
- Mignani, S.M.; El Brahmi, N.; El Kazzouli, S.; Laurent, R.; Ladeira, S.; Caminade, A.-M.; Pedziwiatr-Werbicka, E.; Szewczyk, E.M.; Bryszewska, M.; Bousmina, M.M.; et al. Original Multivalent Gold(III) and Dual Gold(III)-Copper(II) Conjugated Phosphorus Dendrimers as Potent Antitumoral and Antimicrobial Agents. Mol. Pharm. 2017, 14, 4087–4097. [Google Scholar] [CrossRef] [PubMed]
- Sanz del Olmo, N.; Carloni, R.; Bajo, A.M.; Ortega, P.; Fattori, A.; Gomez, R.; Ottaviani, M.F.; Garcia-Gallego, S.; Cangiotti, M.; Javier de la Mata, F. Insight into the antitumor activity of carbosilane Cu(ii)-metallodendrimers through their interaction with biological membrane models. Nanoscale 2019, 11, 13330–13342. [Google Scholar] [CrossRef] [PubMed]
- Mignani, S.; El Brahmi, N.; Eloy, L.; Poupon, J.; Nicolas, V.; Steinmetz, A.; El Kazzouli, S.; Bousmina, M.M.; Blanchard-Desce, M.; Caminade, A.M.; et al. Anticancer copper(II) phosphorus dendrimers are potent proapoptotic Bax activators. Eur. J. Med. Chem. 2017, 132, 142–156. [Google Scholar] [CrossRef] [PubMed]
- Ottaviani, M.F.; El Brahmi, N.; Cangiotti, M.; Coppola, C.; Buccella, F.; Cresteil, T.; Mignani, S.; Caminade, A.M.; Costes, J.P.; Majoral, J.P. Comparative EPR studies of Cu(II)-conjugated phosphorous-dendrimers in the absence and presence of normal and cancer cells. RSC Adv. 2014, 4, 36573–36583. [Google Scholar] [CrossRef]
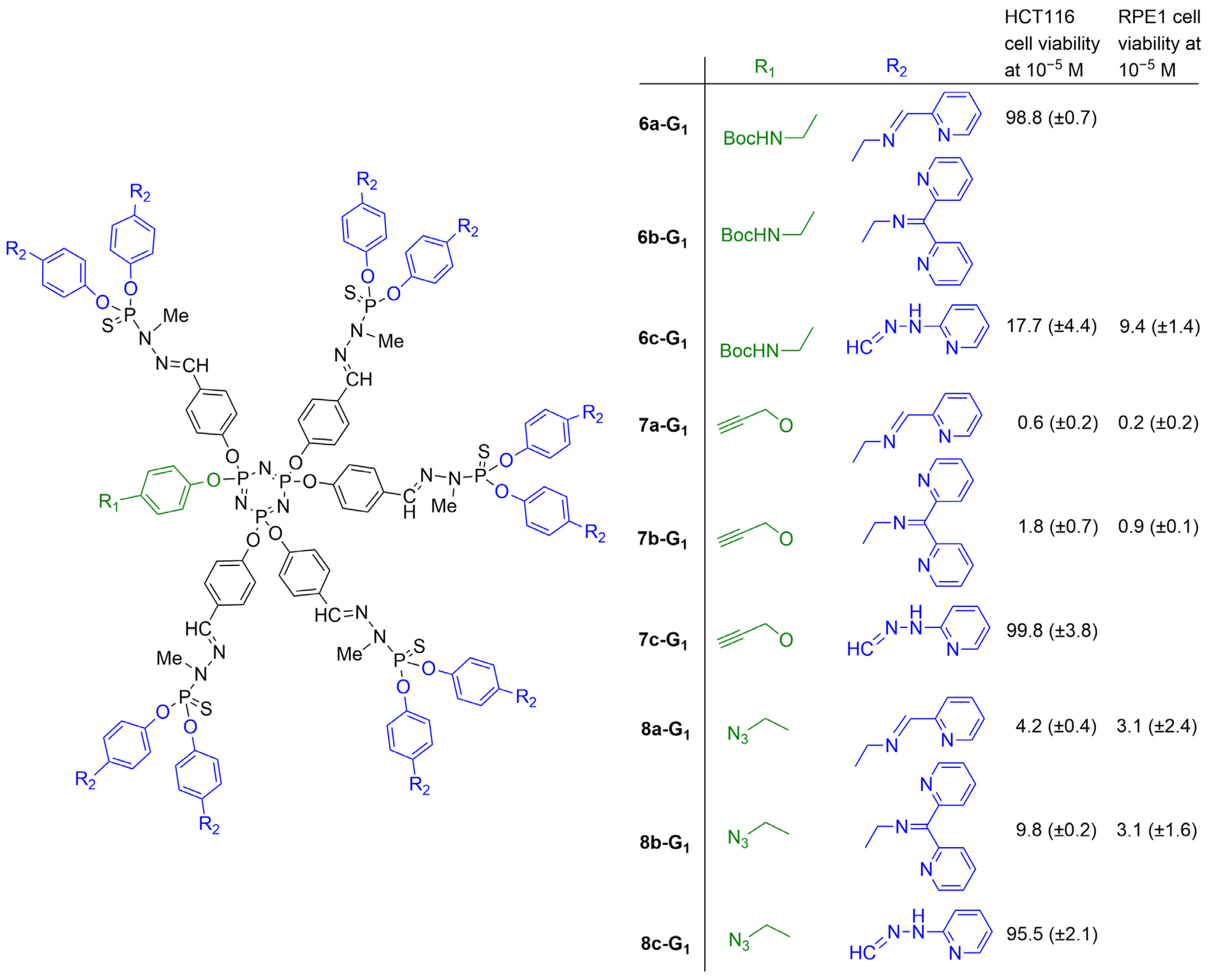
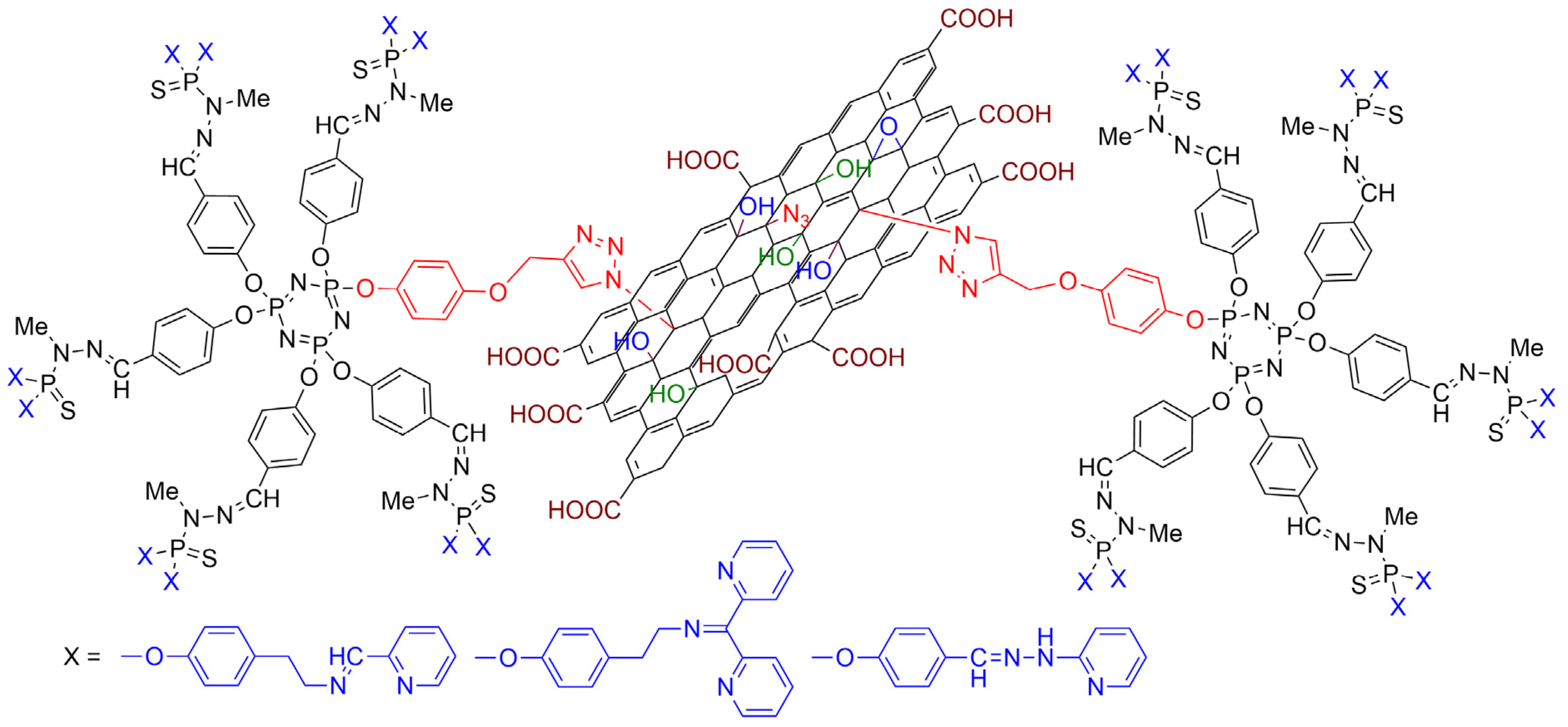
- Alami, O.; Laurent, R.; Tasse, M.; Coppel, Y.; Colliere, V.; Bignon, J.; Majoral, J.-P.; El Kazzouli, S.; El Brahmi, N.; Caminade, A.-M. Functionalization of graphene oxide surfaces with phosphorus dendrimer and dendron. FlatChem 2023, 42, 100564. [Google Scholar] [CrossRef]
- Alami, O.; Laurent, R.; Tasse, M.; Coppel, Y.; Bignon, J.; El Kazzouli, S.; Majoral, J.-P.; El Brahmi, N.; Caminade, A.-M. “Click” Chemistry for the Functionalization of Graphene Oxide with Phosphorus Dendrons: Synthesis, Characterization and Preliminary Biological Properties. Chem.-Eur. J. 2023, 29, e202302198. [Google Scholar] [CrossRef]
- Sanz del Olmo, N.; Maroto-Diaz, M.; Gomez, R.; Ortega, P.; Cangiotti, M.; Ottaviani, M.F.; Javier de la Mata, F. Carbosilane metallodendrimers based on copper (II) complexes: Synthesis, EPR characterization and anticancer activity. J. Inorg. Biochem. 2017, 177, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Carloni, R.; del Olmo, N.S.; Canonico, B.; Montanari, M.; Ciacci, C.; Ambrosi, G.; de la Mata, F.J.; Ottaviani, M.F.; Garcia-Gallego, S. Elaborated study of Cu(II) carbosilane metallodendrimers bearing substituted iminopyridine moieties as antitumor agents. Eur. J. Med. Chem. 2021, 215, 113292. [Google Scholar] [CrossRef] [PubMed]
- Holota, M.; Michlewska, S.; Garcia-Gallego, S.; del Olmo, N.S.; Ortega, P.; Bryszewska, M.; de la Mata, F.J.; Ionov, M. Combination of Copper Metallodendrimers with Conventional Antitumor Drugs to Combat Cancer in In Vitro Models. Int. J. Mol. Sci. 2023, 24, 4076. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Mignani, S.; Caminade, A.-M.; Majoral, J.-P. Metal-based phosphorus dendrimers as novel nanotherapeutic strategies to tackle cancers: A concise overview. Wiley Interdiscip. Rev.-Nanomed. Nanobiotechnol. 2019, 11, e1577. [Google Scholar] [CrossRef] [PubMed]
- Holota, M.; Magiera, J.; Michlewska, S.; Kubczak, M.; Sanz del Olmo, N.; Garcia-Gallego, S.; Ortega, P.; Javier de la Mata, F.; Ionov, M.; Bryszewska, M. In Vitro Anticancer Properties of Copper Metallodendrimers. Biomolecules 2019, 9, 155. [Google Scholar] [CrossRef] [PubMed]
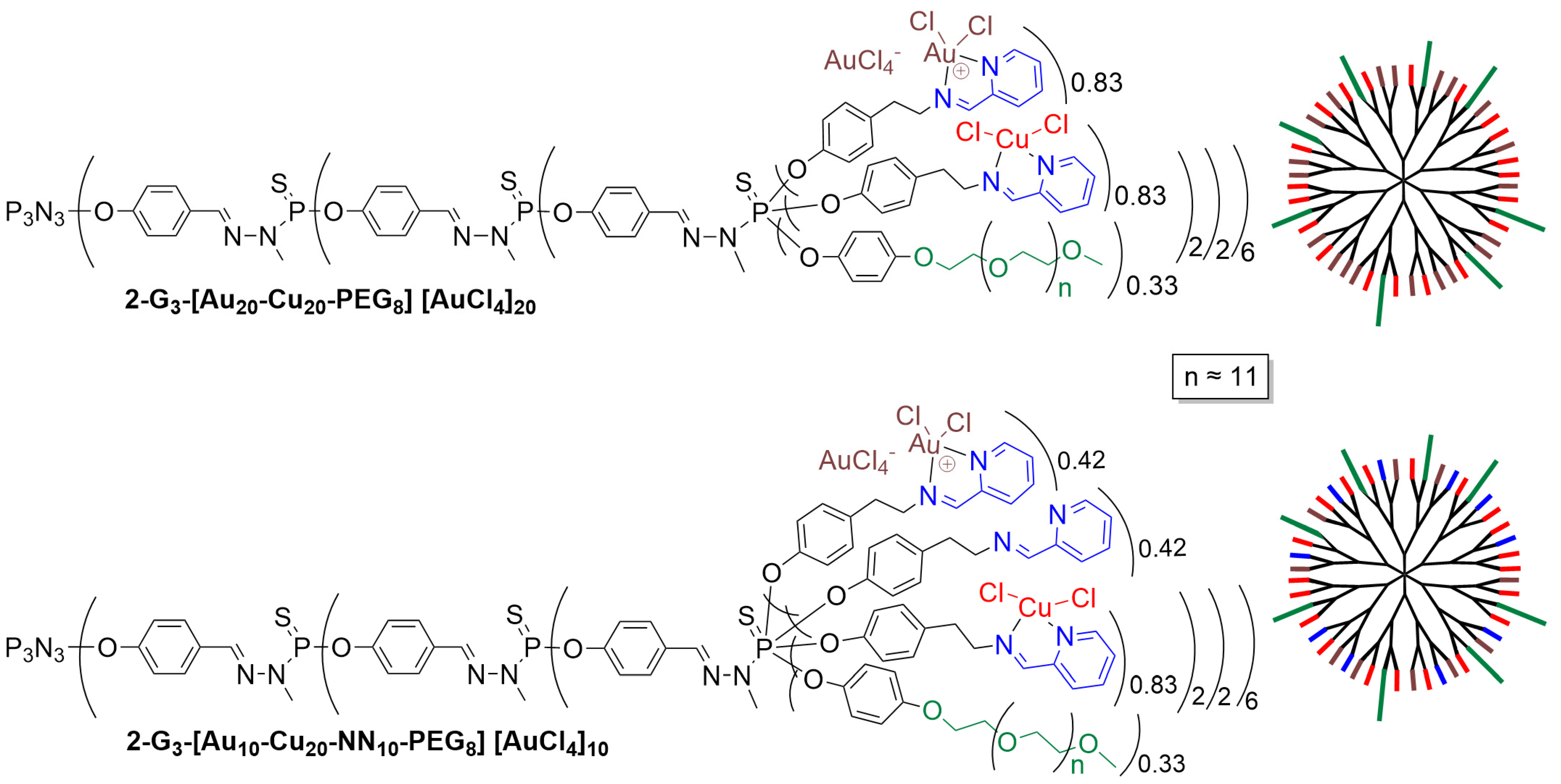
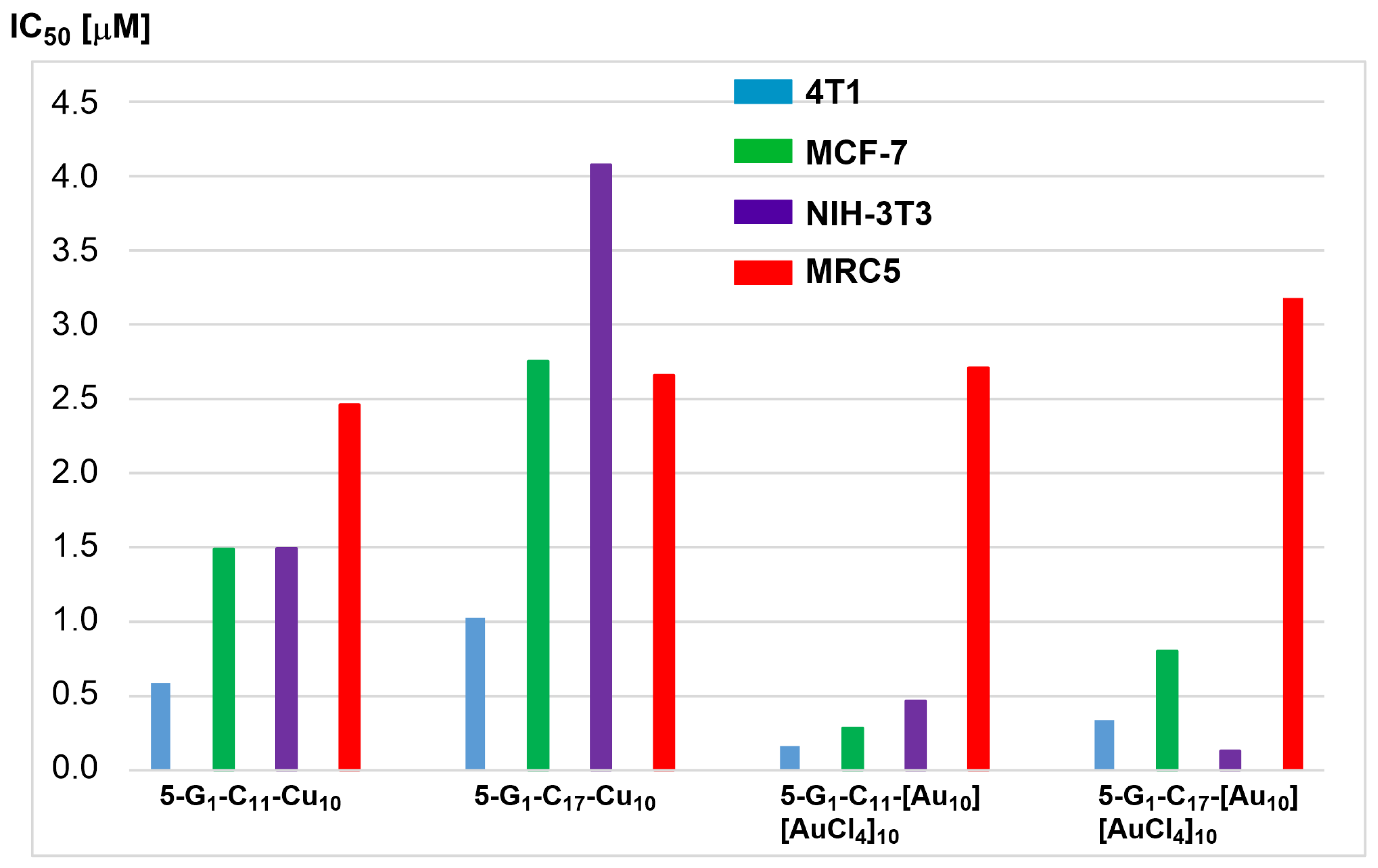
- Chen, L.; Fan, Y.; Qiu, J.; Laurent, R.; Li, J.; Bignon, J.; Mignani, S.; Caminade, A.-M.; Shi, X.; Majoral, J.-P. Potent Anticancer Efficacy of First-In-Class Cu-II and Au-III Metaled Phosphorus Dendrons with Distinct Cell Death Pathways. Chem.-Eur. J. 2020, 26, 5903–5910. [Google Scholar] [CrossRef]
- Sanz Del Olmo, N.; Holota, M.; Michlewska, S.; Gomez, R.; Ortega, P.; Ionov, M.; de la Mata, F.J.; Bryszewska, M. Copper (II) Metallodendrimers Combined with Pro-Apoptotic siRNAs as a Promising Strategy Against Breast Cancer Cells. Pharmaceutics 2020, 12, 727. [Google Scholar] [CrossRef]
- Canonico, B.; Carloni, R.; Sanz del Olmo, N.; Papa, S.; Nasoni, M.G.; Fattori, A.; Cangiotti, M.; Javier de la Mata, F.; Ottaviani, M.F.; Garcia-Gallego, S. Fine-Tuning the Interaction and Therapeutic Effect of Cu(II) Carbosilane Metallodendrimers in Cancer Cells: An In Vitro Electron Paramagnetic Resonance Study. Mol. Pharm. 2020, 17, 2691–2702. [Google Scholar] [CrossRef] [PubMed]
- Launay, N.; Caminade, A.M.; Lahana, R.; Majoral, J.P. A general synthetic strategy for neutral phosphorus-containing dendrimers. Angew. Chem.-Int. Edit. Engl. 1994, 33, 1589–1592. [Google Scholar] [CrossRef]
- Slany, M.; Bardaji, M.; Casanove, M.J.; Caminade, A.M.; Majoral, J.P.; Chaudret, B. Dendrimer surface-chemistry–Facile route to polyphosphines and their gold complexes. J. Am. Chem. Soc. 1995, 117, 9764–9765. [Google Scholar] [CrossRef]
- Ouali, A.; Laurent, R.; Caminade, A.M.; Majoral, J.P.; Taillefer, M. Enhanced catalytic properties of copper in O- and N-arylation and vinylation reactions, using phosphorus dendrimers as ligands. J. Am. Chem. Soc. 2006, 128, 15990–15991. [Google Scholar] [CrossRef] [PubMed]
- Caminade, A.-M.; Hameau, A.; Majoral, J.-P. The specific functionalization of cyclotriphosphazene for the synthesis of smart dendrimers. Dalton Trans. 2016, 45, 1810–1822. [Google Scholar] [CrossRef] [PubMed]
- Huisgen, R.; Szeimies, G.; Moebius, L. 1.3-Dipolare Cycloadditionen XXXII. Kinetik der Additionen organischer Azide an CC-Mehrfachbindungen. Chem. Ber. 1967, 100, 2494–2507. [Google Scholar] [CrossRef]
- Kolb, H.C.; Finn, M.G.; Sharpless, K.B. Click chemistry: Diverse chemical function from a few good reactions. Angew. Chem. Int. Ed. 2001, 40, 2004–2021. [Google Scholar] [CrossRef]
- Zhou, L.L.; Roovers, J. Synthesis of novel carbosilane dendritic macromolecules. Macromolecules 1993, 26, 963–968. [Google Scholar] [CrossRef]
- Llamazares, C.; del Olmo, N.S.; Ortega, P.; Gomez, R.; Soliveri, J.; de la Mata, F.J.; Garcia-Gallego, S.; Copa-Patino, J.L. Antibacterial Effect of Carbosilane Metallodendrimers in Planktonic Cells of Gram-Positive and Gram-Negative Bacteria and Staphylococcus aureus Biofilm. Biomolecules 2019, 9, 405. [Google Scholar] [CrossRef] [PubMed]
- Llamazares, C.; Sanz del Olmo, N.; Soliveri, J.; de la Mata, F.J.; Copa-Patino, J.L.; Garcia-Gallego, S. Insight on the Structure-to-Activity of Carbosilane Metallodendrimers in the Fight against Staphylococcus aureus Biofilms. Antibiotics 2021, 10, 589. [Google Scholar] [CrossRef] [PubMed]
- Lemaire, V.; Shemesh, C.S.; Rotte, A. Pharmacology-based ranking of anti-cancer drugs to guide clinical development of cancer immunotherapy combinations. J. Exp. Clin. Cancer Res. 2021, 40, 311. [Google Scholar] [CrossRef] [PubMed]
- Debela, D.T.; Muzazu, S.G.Y.; Heraro, K.D.; Ndalama, M.T.; Mesele, B.W.; Haile, D.C.; Kitui, S.K.; Manyazewal, T. New approaches and procedures for cancer treatment: Current perspectives. SAGE Open Medicine 2021, 9, 1–10. [Google Scholar] [CrossRef]
- Caminade, A.-M. Dendrimers, an Emerging Opportunity in Personalized Medicine? J. Pers. Med. 2022, 12, 1334. [Google Scholar] [CrossRef]



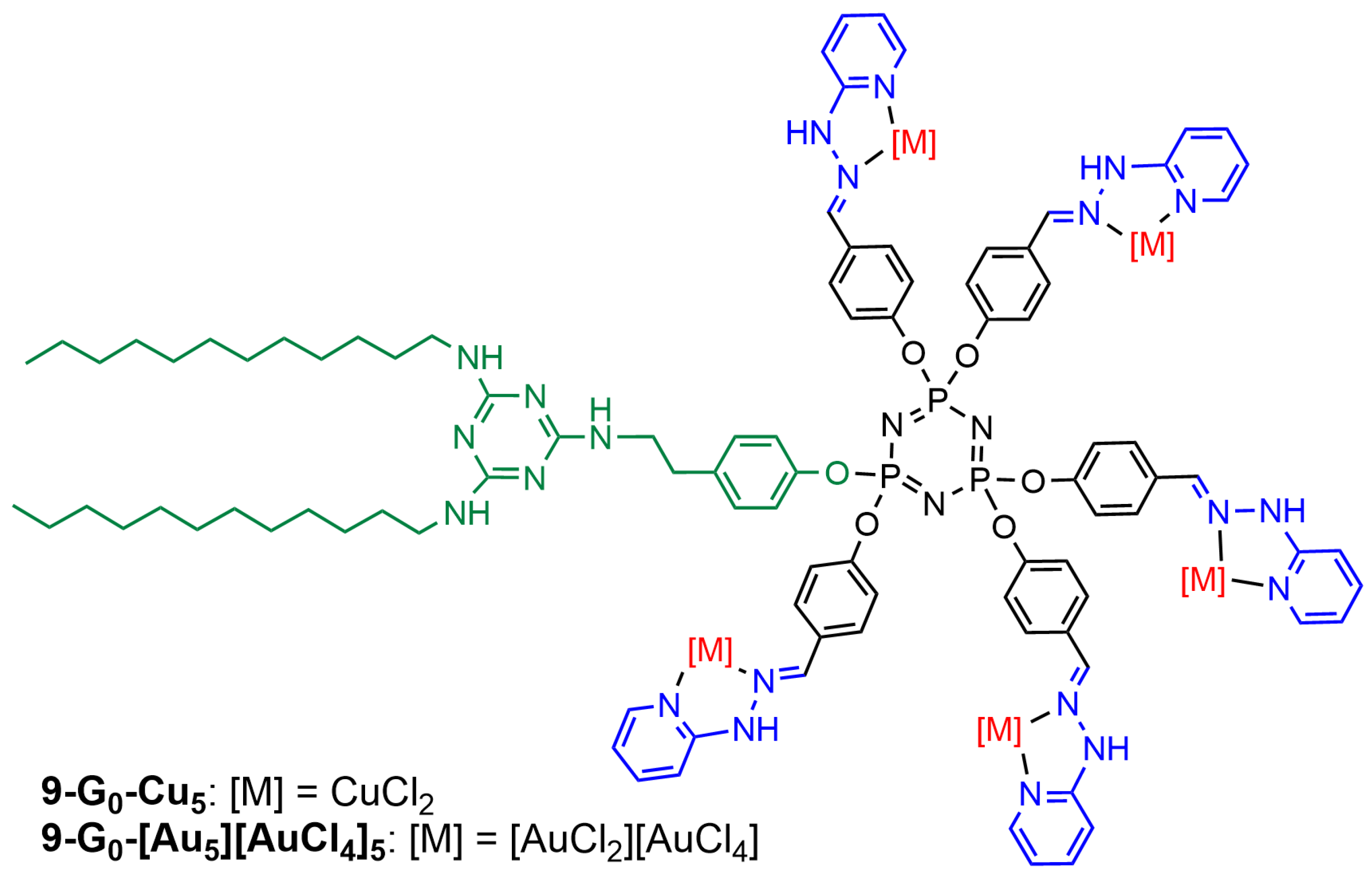
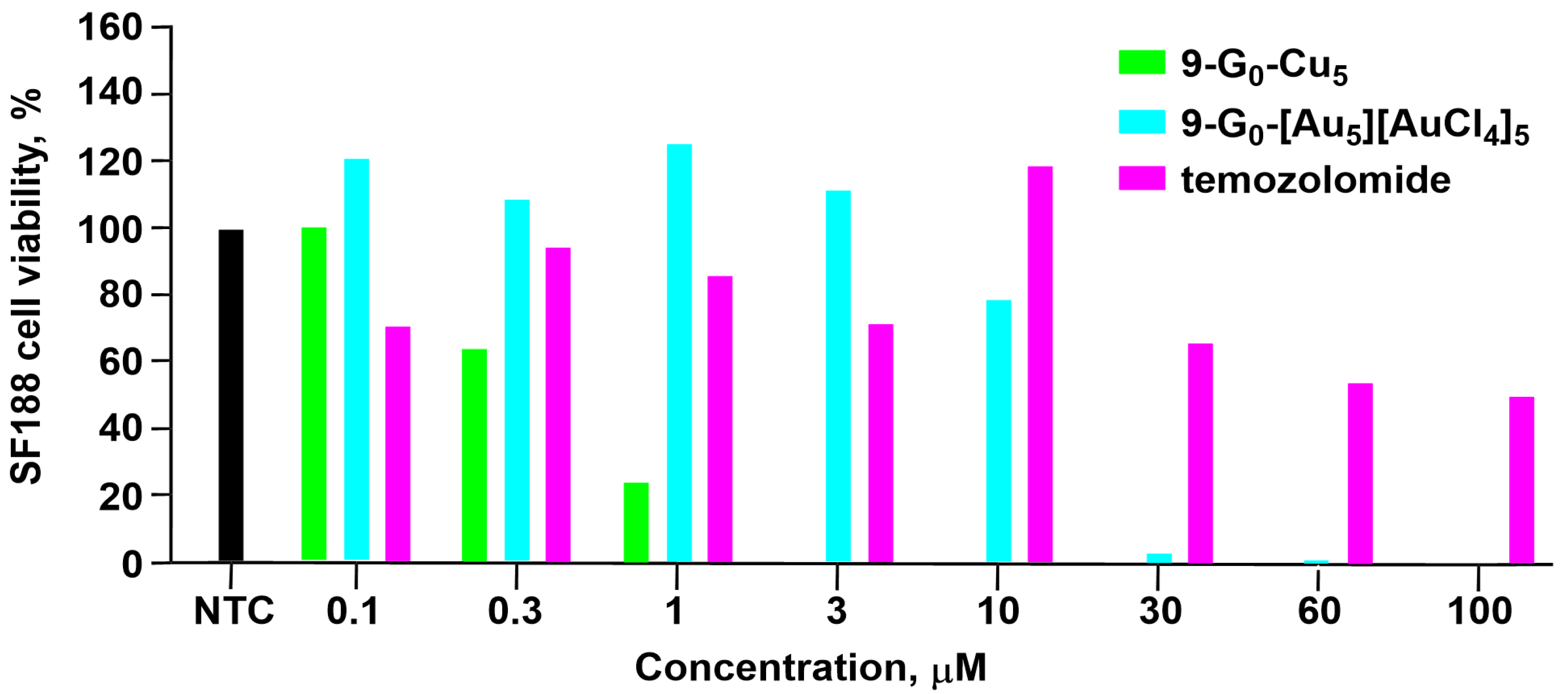
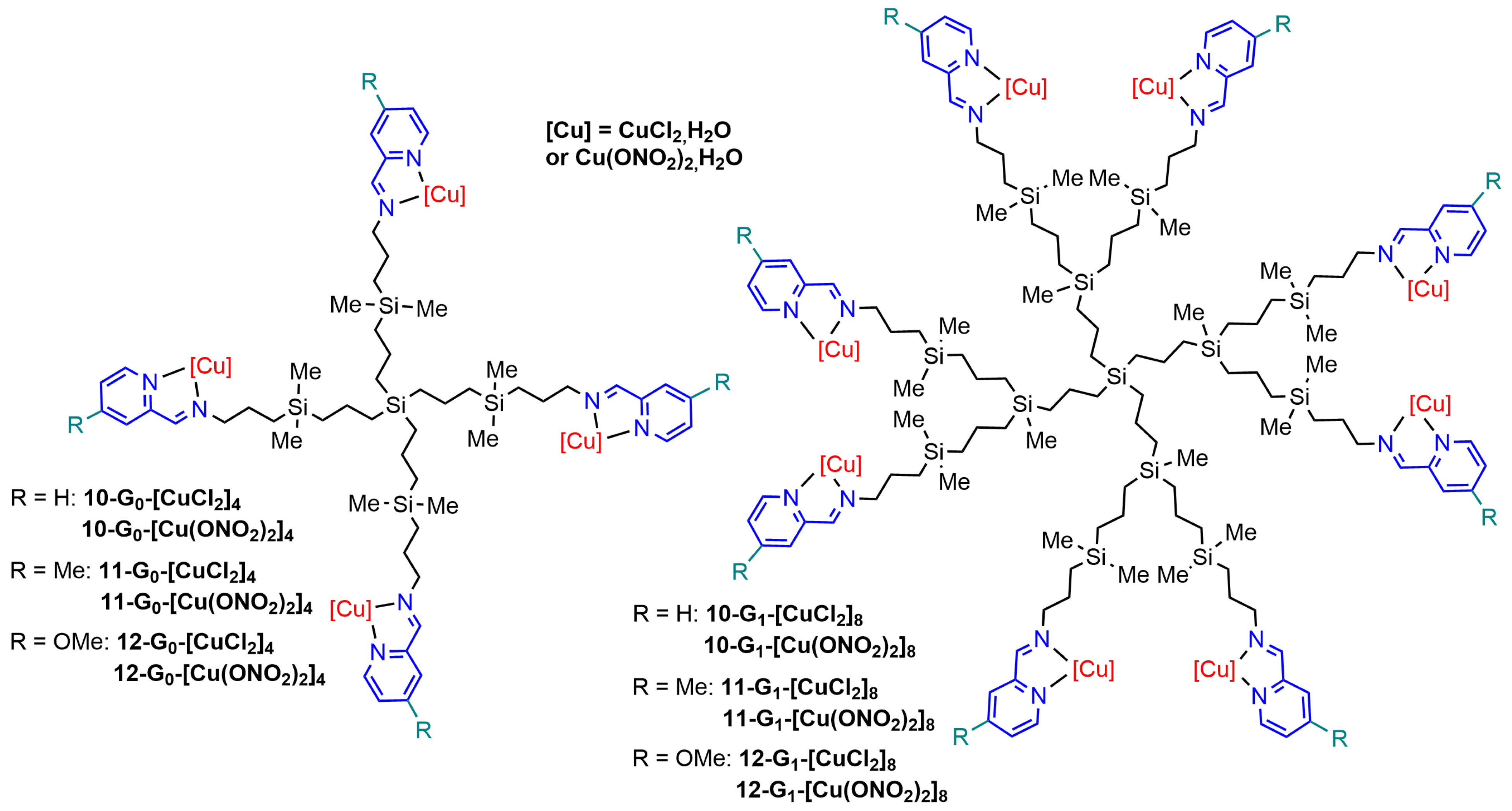
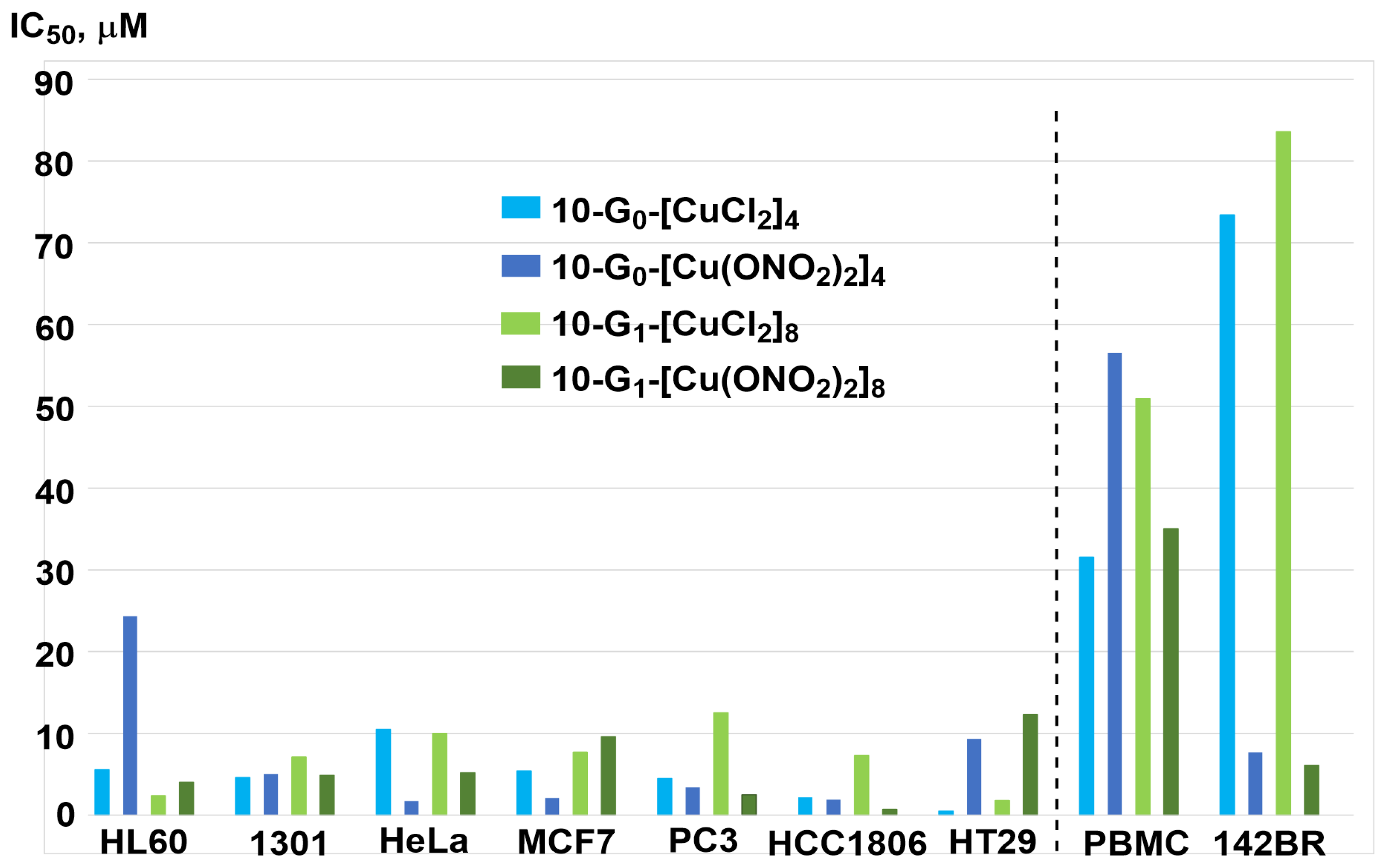
| Cell Names | Cell Lines 1 | References |
|---|---|---|
| BTSC233 | Glioblastoma stem cells | [33] |
| Chang liver | Liver cells, non-cancerous | [34] |
| EPC | Endothelial progenitor, Cyprinus carpio, non-cancerous | [35,36] |
| HCC1806 | Resistant breast cancer cells | [37] |
| HCT15 | Colon cancer | [38] |
| HCT116 | Colon cancer | [35,38,39,40,41] |
| HeLa | Cervical cancer cells | [37,42,43] |
| HepG2 | Liver carcinoma | [44] |
| HL60 | Leukemia | [35,36,38,45,46] |
| HT29 | Colorectal tumor | [37] |
| JHH520 | Glioblastoma stem cells | [33] |
| KB | Epidermal carcinoma | [35,36,45] |
| MCF-7 | Breast cancer cells, cisplatin resistant | [34,35,36,37,38,43,44,47,48] |
| MOLT-4 | Leukemia | [34] |
| MRC5 | Proliferative lung fibroblasts, non-cancerous | [35,36,39,47] |
| NCH644 | Glioblastoma stem cells | [33] |
| NIH-3T3 | Normal fibroblasts, non-cancerous | [47] |
| OVCAR8 | Ovarian carcinoma | [35] |
| PBMC | Peripheral blood mononuclear cells, non-cancerous | [43,46] |
| PC3 | Prostatic small cell carcinoma | [36,37,42] |
| RPE1 | Retinal pigment epithelial-1, non-cancerous | [40,41] |
| SF188 | Pediatric glioma cells | [33] |
| U87 | Glioblastoma-astrocytoma, epithelial-like | [33,35] |
| U937 | Myeloid tumor cells | [43,49] |
| 1301 | Leukemia | [46] |
| 142BR | Fibroblasts, non-cancerous | [37] |
| 4T1 | Mouse breast adenocarcinoma cells | [47] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laurent, R.; Maraval, V.; Bernardes-Génisson, V.; Caminade, A.-M. Dendritic Pyridine–Imine Copper Complexes as Metallo-Drugs. Molecules 2024, 29, 1800. https://doi.org/10.3390/molecules29081800
Laurent R, Maraval V, Bernardes-Génisson V, Caminade A-M. Dendritic Pyridine–Imine Copper Complexes as Metallo-Drugs. Molecules. 2024; 29(8):1800. https://doi.org/10.3390/molecules29081800
Chicago/Turabian StyleLaurent, Régis, Valérie Maraval, Vania Bernardes-Génisson, and Anne-Marie Caminade. 2024. "Dendritic Pyridine–Imine Copper Complexes as Metallo-Drugs" Molecules 29, no. 8: 1800. https://doi.org/10.3390/molecules29081800
APA StyleLaurent, R., Maraval, V., Bernardes-Génisson, V., & Caminade, A.-M. (2024). Dendritic Pyridine–Imine Copper Complexes as Metallo-Drugs. Molecules, 29(8), 1800. https://doi.org/10.3390/molecules29081800









