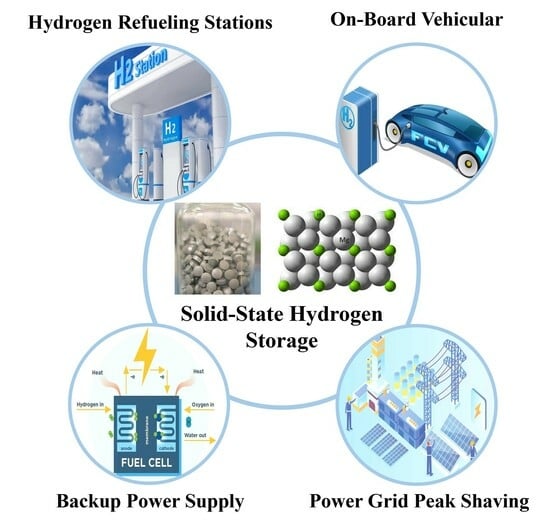
Research Progress and Application Prospects of Solid-State Hydrogen Storage Technology
Abstract
1. Introductions
2. Hydrogen Storage Technologies
2.1. Compressed Gaseous Hydrogen Storage
2.2. Cryogenic Liquid Hydrogen Storage
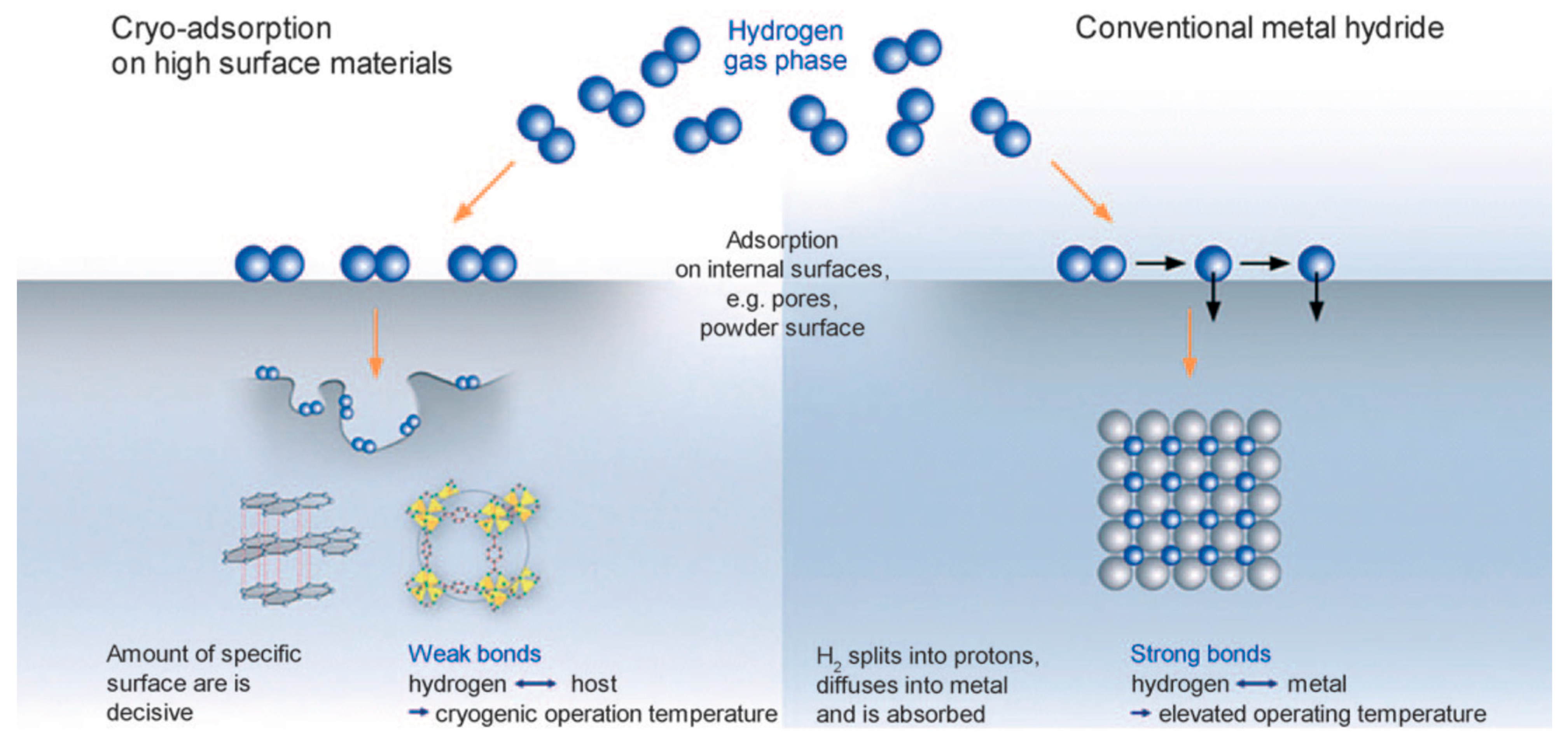
2.3. Solid-State Hydrogen Storage
2.3.1. Hydrogen Storage Principles
2.3.2. Hydrogen Storage Materials
- (1)
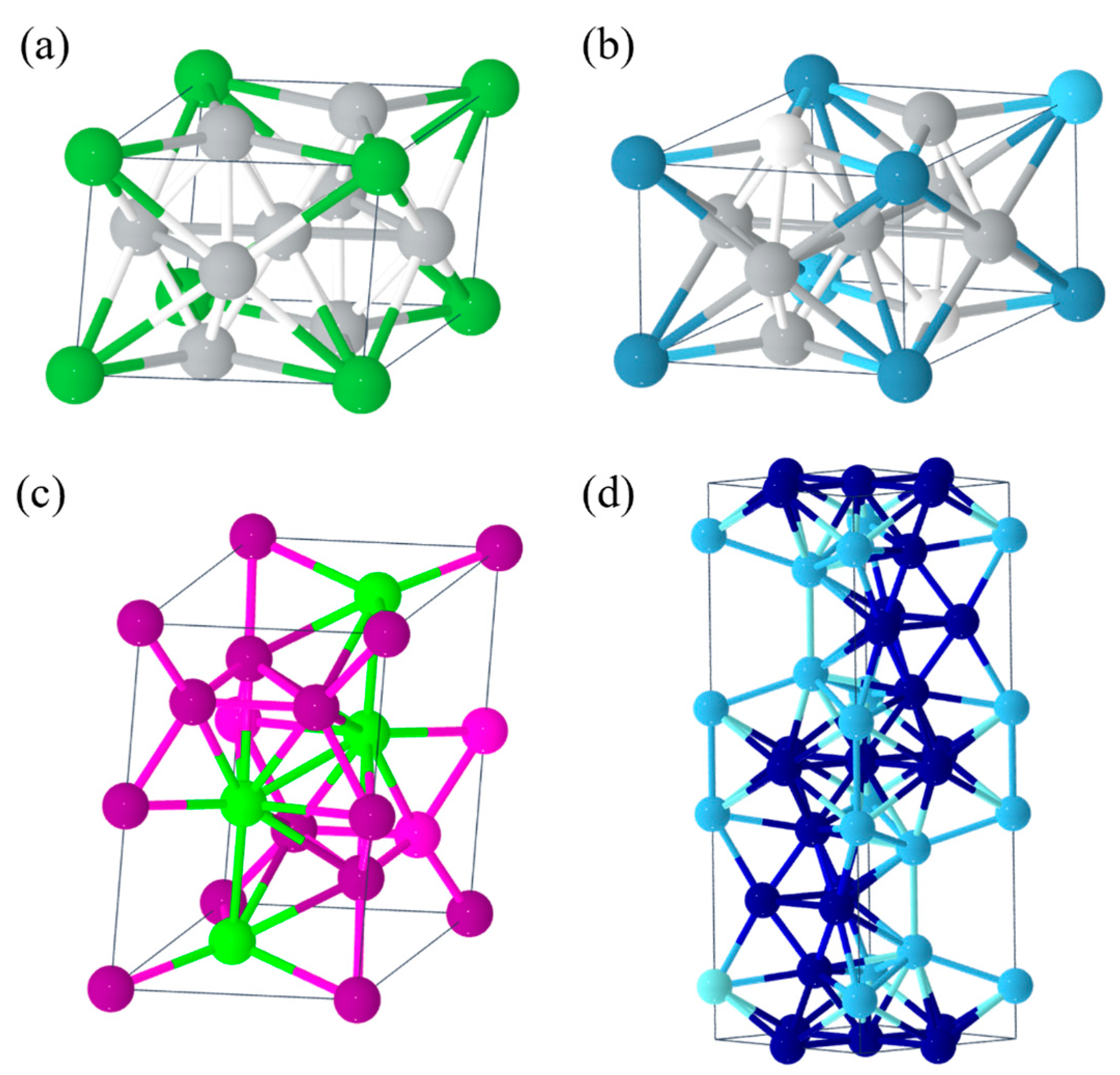
- AB5-type metal hydrides such as LaNi5 [51], CaNi5 [52] (Figure 2a,b), etc., with a mass hydrogen storage density of 1.4–1.8 wt.% and a volumetric hydrogen storage density of up to 115 kg/m3. The dehydrogenation plateau pressure is 0.1–0.3 MPa, and the dehydrogenation temperature is 25–100 °C. These materials have good activation properties and moderate absorption/desorption pressure, but they are expensive (USD >1428.6/ton) and are mainly used as negative electrodes for nickel–metal hydride batteries.
- (2)
- AB2-type metal hydrides such as ZrMn2 [53], TiCr2 [54] (Figure 2c,d), etc., with a mass hydrogen storage density of 1.8–2.2 wt.% and a volumetric hydrogen storage density of up to 130 kg/m3. The dehydrogenation plateau pressure is 0.1–1 MPa, and the dehydrogenation temperature is 25–150 °C. These materials have a high hydrogen storage capacity and good cyclic stability, but they are costly and difficult to activate. They are currently mainly used for large-scale stationary hydrogen storage.
- (3)
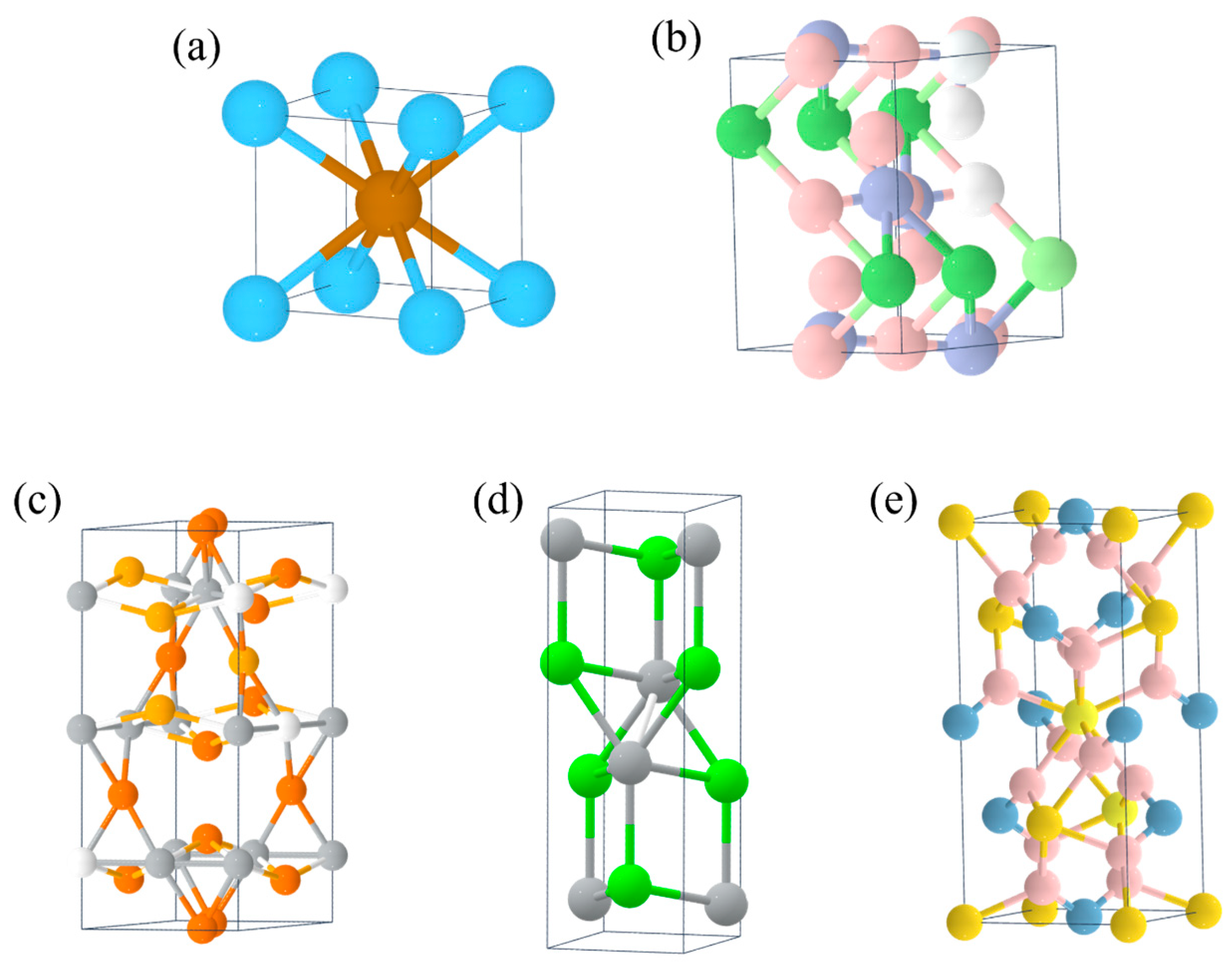
- AB-type metal hydrides such as TiFe [55], ZrNi [56] (Figure 3a,b), etc., with a mass hydrogen storage density of 1.5–2.0 wt.% and a volumetric hydrogen storage density of 105–115 kg/m3. The dehydrogenation pressure is 1–3 MPa, and the dehydrogenation temperature is 25–100 °C. These materials have a moderate hydrogen storage capacity and good cycling performance, but they require high hydrogen purity (>99.99%) and are mainly used for hydrogen purification and separation.
- (4)
- A2B-type metal hydrides such as Mg2Ni [57] (Figure 3c), etc., with a high mass hydrogen storage density of 3–5 wt.% and a volumetric hydrogen storage density of up to 110 kg/m3. The dehydrogenation pressure is 0.1–0.3 MPa, but the dehydrogenation temperature is high (250–400 °C). The notable feature of these materials is their high mass hydrogen storage density, but their dehydrogenation kinetics are poor and they are prone to pulverization, so their practical performance needs to be improved.
- (5)
- Complex hydrides such as NaAlH4 [58], LiNH2 [59] (Figure 3d,e), etc., with a mass hydrogen storage density of up to 5–10 wt.%, but a dehydrogenation temperature generally above 150 °C. The advantage of these materials is their high hydrogen storage capacity, but the dehydrogenation process is often accompanied by by-products (such as NH3), causing the reversible hydrogen storage capacity to decrease rapidly. They are currently still in the research stage.
- (1)
- High energy density. The volumetric hydrogen storage density of metal hydrides can reach 100–130 kg/m3, which is three times that of 70 MPa gaseous hydrogen and two times that of liquid hydrogen.
- (2)
- Good safety. Hydrogen storage materials can exist stably at room temperature and atmospheric pressure, and even if damaged, they will not cause a large amount of hydrogen leakage.
- (3)
- Simple system. Solid-state hydrogen storage does not require gas cylinders or compressors, let alone ultra-low-temperature insulation devices, greatly reducing the complexity of the system.
- (4)
- Good economy. Atmospheric storage can significantly reduce equipment costs, and there is no liquefaction power consumption, with operating costs that are only 1/3 of liquid hydrogen.
3. Advancements in Solid-State Hydrogen Storage Systems
3.1. Solid-State Hydrogen Storage System Architecture
- (1)
- Select materials with good thermal and hydrogen conductivity, such as metal materials such as stainless steel and aluminum alloy, or light-weight composite materials such as carbon fiber and glass fiber.
- (2)
- Optimize the size and shape parameters of the container to balance the hydrogen storage capacity, heat dissipation, and layout convenience. Commonly used shapes include cylinders, annuli, corrugated pipe, etc.
- (3)
- Set up porous baffles or fillers in the container, which can fix the hydrogen storage material particles, prevent stress concentration, and promote hydrogen diffusion and heat transfer.
- (4)
- Set up the fin and other enhanced heat transfer elements on the outside of the container to increase the heat transfer area. The fins can be arranged axially, radially or spirally.
- (5)
- When necessary, set up a skeleton, porous matrix, etc., in the container to guide hydrogen transport by capillary force and provide mechanical support.
- (1)
- AB5-type metal hydrides, such as LaNi5 [65,66]. Their hydrogen absorption platform pressure is moderate (0.1–0.3 MPa), and they can release hydrogen at room temperature, but they are expensive and the cost is too high for large-scale hydrogen storage. They are mostly used in small devices such as nickel–metal hydride batteries.
- (2)
- (3)
- Low-temperature AB2-type metal hydrides, such as Ti-Zr-V series [69,70,71]. Their hydrogen storage capacity is relatively high (≥2 wt.%), their dehydrogenation pressure is moderate (0.1–1 MPa), and their price is relatively cheap, but they are difficult to activate and require high-temperature treatment above 400 °C. They are currently mainly used in large-scale stationary hydrogen storage devices.
- (4)
- High-temperature Mg-based metal hydrides, such as Mg2Ni [72]. Their biggest feature are their high mass hydrogen storage density (≥3 wt.%), but their dehydrogenation temperature is also high (300–400 °C), which places high requirements on the heat and mass transfer process, and they are prone to pulverization, so their practical performance needs to be improved.
3.2. Solid-State Hydrogen Storage Device Integration
3.3. Key Technologies for Solid-State Hydrogen Storage
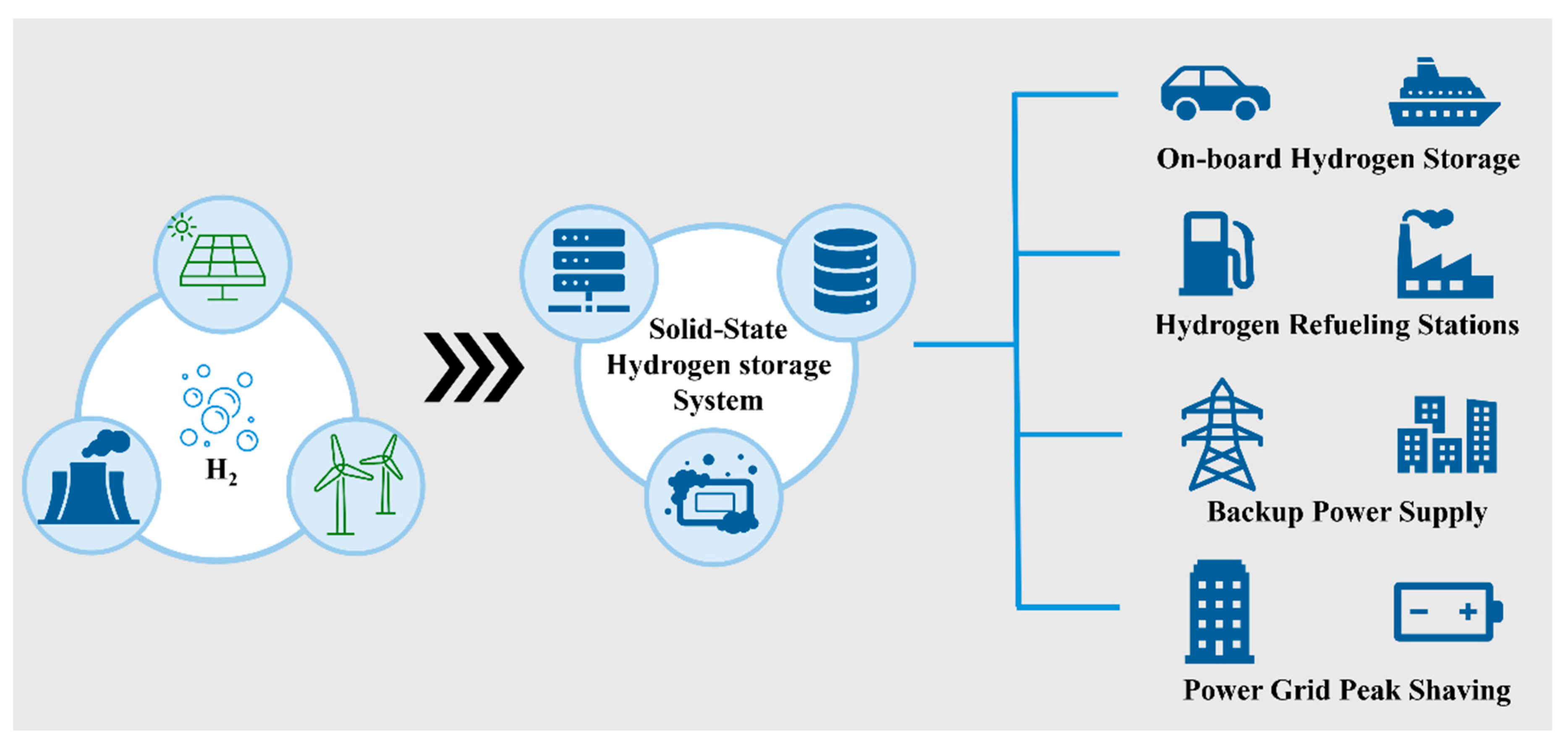
4. Application Scenarios and Market Prospects of Solid-State Hydrogen Storage Technology
4.1. On-Board Vehicular Applications
4.2. Hydrogen Refueling Stations
- (1)
- Atmospheric pressure storage, no need for compressors, saving electricity;
- (2)
- Adsorption at room temperature, no need for low-temperature insulation, simpler steel cylinders or tanks can be used;
- (3)
- Safe and environmentally friendly, even if the hydrogen storage container is damaged, it will not cause a large amount of hydrogen leakage;
- (4)
- Modular design, which can realize the integration of “storage–transportation–refueling”.
4.3. Backup Power Supply
4.4. Power Grid Peak Shaving
5. Challenges and Countermeasures for the Industrialization of Solid-State Hydrogen Storage
5.1. Key Materials and Equipment
- (1)
- High preparation cost. Take AB5-type hydrogen storage alloys as an example. The high-purity powder materials prepared by vacuum induction melting + hydrogen decrepitation treatment have a price as high as USD 11,428–14,285/ton, which is more than four times that of ordinary nickel–metal hydride battery negative electrode materials. Magnesium-based materials have similar problems. Due to the need for an oxygen-free and moisture-free environment in the preparation process, the production cost remains high and it is difficult to achieve large-scale application.
- (2)
- Difficult batch preparation. From laboratory small-scale to industrial scale-up, hydrogen storage materials often face many process bottlenecks. For example, the AB2 type has difficulty obtaining a uniform multi-element solid solution, and Mg2Ni has difficultly avoiding pulverization and sintering. As a result, the performance of materials produced in batches is difficult to guarantee, with rapid decay and a short service life.
- (3)
- Lack of equipment. A high preparation temperature (≥1000 °C), strict atmosphere requirements (high vacuum, high purity hydrogen), and long cycle (several days or even weeks) place very high requirements on the parameter control and air tightness of key equipment, such as melting and hydrogenation. At present, there is still a large gap between the technical level of domestic equipment and foreign equipment, especially large vacuum induction furnaces, plasma spheroidization machines, etc., and core components mostly rely on imports.
- (1)
- Explore new processes for preparing AB2 and AB5-type materials in non-vacuum and atmospheric environments, such as induction suspension melting, electromagnetic stirring heat treatment, etc., and strive to reduce production costs by more than 50%.
- (2)
- Develop new methods for the low-temperature preparation of magnesium-based hydrogen storage materials, such as reaction ball milling, plasma pyrolysis, etc., while reducing the sintering temperature (≤400 °C), suppressing material pulverization, and improving cyclic stability.
- (3)
- Implement the “Solid-State Hydrogen Storage Material Manufacturing Equipment Innovation Project” to focus on the development of ton-level vacuum induction furnaces, rapid hydrogen absorption/desorption systems, high-efficiency powder sorting systems, etc., to break through the industrialization bottlenecks of material preparation and system integration.
5.2. Testing Standards and Specifications
- (1)
- Non-uniform material testing methods. Common testing methods such as PCT and TDS are cumbersome to operate and have poor repeatability, and the test results from different laboratories often vary greatly. And there are a lack of evaluation standards for material aging, life, etc., making it difficult to comprehensively reflect the use performance of materials.
- (2)
- Lack of a system certification system. At present, there are no safety and reliability testing specifications for solid-state hydrogen storage systems in China, and no third-party certification bodies have been established. This not only restricts the promotion and application of solid-state hydrogen storage technology, but also sets obstacles for enterprises to participate in international market competition.
- (3)
- Establish rapid testing methods and testing specifications for the evaluation of hydrogen storage materials, formulate industry standards for key indicators such as PCT performance, thermal stability, anti-pulverization performance, and cycle life, and provide criteria for material selection.
- (4)
- Refer to testing specifications for fuel cells, lithium batteries, etc., and formulate safety testing standards for solid-state hydrogen storage systems as soon as possible, including high and low-temperature cycling, drop, vibration, electromagnetic compatibility, etc., to provide a basis for product safety performance evaluation.
- (5)
- Encourage scientific research institutes and testing institutions with strength to carry out the third-party certification of solid-state hydrogen storage systems, establish a scientific, standardized, and efficient testing and evaluation system, and provide a “pass” for products to enter the market.
5.3. Construction of Innovation Platforms
- (1)
- Relying on innovative carriers such as national key laboratories and manufacturing innovation centers, focusing on basic research and common technologies for solid-state hydrogen storage, and organizing and implementing the “Solid-State Hydrogen Storage Frontier Technology Research Special Project” to provide a continuous technology supply for industrial development.
- (2)
- Actively strive for the support of the national key R&D plan, organize leading enterprises, universities, and research institutes to carry out collaborative research on various links of the industrial chain, and focus on breakthroughs in key technologies, such as the large-scale preparation of hydrogen storage materials, system integration and optimization, and batch assembly.
- (3)
- Support the creation of “Solid-State Hydrogen Storage Industry Innovation Centers” in qualified regions, build public technology R&D platforms, pilot scale-up bases, and testing and certification centers, open up the innovation chain from materials and components to complete vehicles, and accelerate the transformation of scientific and technological achievements into real productivity.
- (4)
- Give full play to the guiding role of venture capital and industrial investment funds, promote the gathering of various innovative resources to key links of the industrial chain by setting up “Solid-State Hydrogen Storage Special Funds” and implementing “Solicitation for Solutions”, and accelerate the industrialization process of scientific and technological achievements.
- (5)
- Encourage local governments and industry associations to take the lead in regularly holding “Solid-State Hydrogen Storage Industry Development Forums”, inviting upstream and downstream enterprises, universities and research institutes, financial institutions, third-party service organizations, etc., to jointly “diagnose and treat” industrial development and form a joint force.
6. Conclusions and Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Momirlan, M.; Veziroglu, T.N. Current status of hydrogen energy. Renew. Sustain. Energy Rev. 2002, 6, 141–179. [Google Scholar] [CrossRef]
- Midilli, A.; Ay, M.; Dincer, I.; Rosen, M.A. On hydrogen and hydrogen energy strategies: I: Current status and needs. Renew. Sustain. Energy Rev. 2005, 9, 255–271. [Google Scholar] [CrossRef]
- Parra, D.; Valverde, L.; Pino, F.J.; Patel, M.K. A review on the role, cost and value of hydrogen energy systems for deep decarbonization. Renew. Sustain. Energy Rev. 2019, 101, 279–294. [Google Scholar] [CrossRef]
- Autrey, T.; Chen, P. Hydrogen energy carriers. J. Energy Chem. 2023, 77, 119–121. [Google Scholar] [CrossRef]
- Yan, J. Negative-emissions hydrogen energy. Nat. Clim. Chang. 2018, 8, 560–561. [Google Scholar] [CrossRef]
- Temiz, M.; Dincer, I. Development of solar and wind based hydrogen energy systems for sustainable communities. Energy Convers. Manag. 2022, 269, 116090. [Google Scholar] [CrossRef]
- Karayel, G.K.; Javani, N.; Dincer, I. A comprehensive assessment of energy storage options for green hydrogen. Energy Convers. Manag. 2023, 291, 117311. [Google Scholar] [CrossRef]
- Neumann, F.; Zeyen, E.; Victoria, M.; Brown, T. The potential role of a hydrogen network in Europe. Joule 2023, 7, 1793–1817. [Google Scholar] [CrossRef]
- Palmer, C. Hydrogen Power Focus Shifts from Cars to Heavy Vehicles. Engineering 2020, 6, 1333–1335. [Google Scholar] [CrossRef]
- Dincer, I.; Javani, N.; Karayel, G.K. Sustainable city concept based on green hydrogen energy. Sustain. Cities Soc. 2022, 87, 104154. [Google Scholar] [CrossRef]
- Ansarinasab, H.; Mehrpooya, M.; Sadeghzadeh, M. An exergy-based investigation on hydrogen liquefaction plant-exergy, exergoeconomic, and exergoenvironmental analyses. J. Clean. Prod. 2019, 210, 530–541. [Google Scholar] [CrossRef]
- Dincer, I.; Aydin, M.I. New paradigms in sustainable energy systems with hydrogen. Energy Convers. Manag. 2023, 283, 116950. [Google Scholar] [CrossRef]
- Qi, J.; Zhang, W.; Cao, R. Solar-to-Hydrogen Energy Conversion Based on Water Splitting. Adv. Energy Mater. 2018, 8, 1701620. [Google Scholar] [CrossRef]
- McPherson, M.; Johnson, N.; Strubegger, M. The role of electricity storage and hydrogen technologies in enabling global low-carbon energy transitions. Appl. Energy 2018, 216, 649–661. [Google Scholar] [CrossRef]
- He, Y.; Wang, D. Toward Practical Solar Hydrogen Production. Chem 2018, 4, 405–408. [Google Scholar] [CrossRef]
- Available online: https://www.nea.gov.cn/2022-05/07/c_1310587396.htm (accessed on 30 March 2024).
- Available online: http://zfxxgk.nea.gov.cn/1310525630_16479984022991n.pdf (accessed on 30 March 2024).
- Available online: https://file.vogel.com.cn/124/upload/resources/file/398627.pdf (accessed on 30 March 2024).
- Available online: https://web-assets.bcg.com/a4/e9/7bdf588340169730c3dad382b4b2/bcg-china-hydrogen-industry-outlook-cn-aug-2023.pdf (accessed on 30 March 2024).
- Available online: https://www.lightingchina.com.cn/upload/article/126449/file/1691910458643788.pdf (accessed on 30 March 2024).
- Li, X.; Huang, Q.; Liu, Y.; Zhao, B.; Li, J. Review of the Hydrogen Permeation Test of the Polymer Liner Material of Type IV On-Board Hydrogen Storage Cylinders. Materials 2023, 16, 5366. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.; Chen, G.; Gao, X.; Xu, Q.; Zhao, Y.; Su, S.; Xia, L.; Zhang, G.; Hu, K. Study on the methodology for evaluating the filling quality of type III hydrogen storage cylinders. Int. J. Hydrogen Energy 2023, 48, 36825–36835. [Google Scholar] [CrossRef]
- Li, J.; Chai, X.; Gu, Y.; Zhang, P.; Yang, X.; Wen, Y.; Xu, Z.; Jiang, B.; Wang, J.; Jin, G.; et al. Small-Scale High-Pressure Hydrogen Storage Vessels: A Review. Materials 2024, 17, 721. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zheng, S.; Zhang, Z.; Zheng, J.; Zhao, Y. Numerical study on the fast filling of on-bus gaseous hydrogen storage cylinder. Int. J. Hydrogen Energy 2020, 45, 9241–9251. [Google Scholar] [CrossRef]
- Yanxing, Z.; Maoqiong, G.; Yuan, Z.; Xueqiang, D.; Jun, S. Thermodynamics analysis of hydrogen storage based on compressed gaseous hydrogen, liquid hydrogen and cryo-compressed hydrogen. Int. J. Hydrogen Energy 2019, 44, 16833–16840. [Google Scholar] [CrossRef]
- Qiu, Y.; Yang, H.; Tong, L.; Wang, L. Research Progress of Cryogenic Materials for Storage and Transportation of Liquid Hydrogen. Metals 2021, 11, 1101. [Google Scholar] [CrossRef]
- Yatsenko, E.A.; Goltsman, B.M.; Novikov, Y.V.; Izvarin, A.I.; Rusakevich, I.V. Review on modern ways of insulation of reservoirs for liquid hydrogen storage. Int. J. Hydrogen Energy 2022, 47, 41046–41054. [Google Scholar] [CrossRef]
- Lee, J.; Park, B.; Kim, K.-H.; Ruy, W.-S. Multi-objective optimization of liquid hydrogen FPSO at the conceptual design stage. Int. J. Nav. Archit. Ocean. Eng. 2023, 15, 100511. [Google Scholar] [CrossRef]
- Gambini, M.; Guarnaccia, F.; Manno, M.; Vellini, M. Thermal design and heat transfer optimisation of a liquid organic hydrogen carrier batch reactor for hydrogen storage. Int. J. Hydrogen Energy 2023, 48, 37625–37636. [Google Scholar] [CrossRef]
- Chen, S.; Qiu, C.; Shen, Y.; Tao, X.; Gan, Z. Thermodynamic and economic analysis of new coupling processes with large-scale hydrogen liquefaction process and liquid air energy storage. Energy 2024, 286, 129563. [Google Scholar] [CrossRef]
- Zhu, X.Y.; Lee, J.H.; Kim, K.-H.; Lee, S.H. Computational design of vapor-cooled shield structure for liquid hydrogen storage tank. J. Mech. Sci. Technol. 2024, 38, 1575–1583. [Google Scholar] [CrossRef]
- Tsogt, N.; Gbadago, D.Q.; Hwang, S. Exploring the potential of liquid organic hydrogen carrier (LOHC) system for efficient hydrogen storage and Transport: A Techno-Economic and energy analysis perspective. Energy Convers. Manag. 2024, 299, 117856. [Google Scholar] [CrossRef]
- Milella, V.-O.; Koelpin, A.; Schluecker, E. Automation of the Storing-In Part of a Hydrogen-Storage System using Liquid Organic Hydrogen Carriers. Energy Technol. 2018, 6, 547–557. [Google Scholar] [CrossRef]
- Tang, J.; Li, J.; Pishva, P.; Xie, R.; Peng, Z. Aqueous, Rechargeable Liquid Organic Hydrogen Carrier Battery for High-Capacity, Safe Energy Storage. ACS Energy Lett. 2023, 8, 3727–3732. [Google Scholar] [CrossRef]
- Shiraz, H.G.; Vagin, M.; Ruoko, T.-P.; Gueskine, V.; Karoń, K.; Łapkowski, M.; Abrahamsson, T.; Ederth, T.; Berggren, M.; Crispin, X. Towards electrochemical hydrogen storage in liquid organic hydrogen carriers via proton-coupled electron transfers. J. Energy Chem. 2022, 73, 292–300. [Google Scholar] [CrossRef]
- Byun, M.; Lee, A.; Cheon, S.; Kim, H.; Lim, H. Preliminary feasibility study for hydrogen storage using several promising liquid organic hydrogen carriers: Technical, economic, and environmental perspectives. Energy Convers. Manag. 2022, 268, 116001. [Google Scholar] [CrossRef]
- Zhu, Q.-L.; Xu, Q. Liquid organic and inorganic chemical hydrides for high-capacity hydrogen storage. Energy Environ. Sci. 2015, 8, 478–512. [Google Scholar] [CrossRef]
- Modisha, P.M.; Ouma, C.N.M.; Garidzirai, R.; Wasserscheid, P.; Bessarabov, D. The Prospect of Hydrogen Storage Using Liquid Organic Hydrogen Carriers. Energy Fuels 2019, 33, 2778–2796. [Google Scholar] [CrossRef]
- Abdin, Z.; Tang, C.; Liu, Y.; Catchpole, K. Large-scale stationary hydrogen storage via liquid organic hydrogen carriers. iScience 2021, 24, 102966. [Google Scholar] [CrossRef] [PubMed]
- Mah, A.X.Y.; Ho, W.S.; Hassim, M.H.; Hashim, H.; Liew, P.Y.; Muis, Z.A. Targeting and scheduling of standalone renewable energy system with liquid organic hydrogen carrier as energy storage. Energy 2021, 218, 119475. [Google Scholar] [CrossRef]
- Eberle; Felderhoff, M.; Schüth, F. Chemical and Physical Solutions for Hydrogen Storage. Angew. Chem. Int. Ed. 2009, 48, 6608–6630. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Liao, Y.; Zhong, Y.; Cui, J.; Wang, D.; Wang, K. Improved reversible dehydrogenation performance of MgH2 by the synergistic effects of porous boron nitride and NbF5. J. Energy Storage 2020, 29, 101418. [Google Scholar] [CrossRef]
- Wang, K.; Wu, G.; Cao, H.; Li, H.; Zhao, X. Improved reversible dehydrogenation properties of MgH2 by the synergistic effects of graphene oxide-based porous carbon and TiCl3. Int. J. Hydrogen Energy 2018, 43, 7440–7446. [Google Scholar] [CrossRef]
- Han, Y.; Wu, C.; Wang, Q.; Sun, D.; Cheng, W.; Li, X.; Zhang, Y.; Cao, X.; Yan, Y.; Wang, Y.; et al. Phase evolution process and hydrogen storage performances of V72Ti18Cr10 alloy prepared by Co-precipitation-reduction method. Prog. Nat. Sci. Mater. Int. 2022, 32, 407–414. [Google Scholar] [CrossRef]
- Lv, L.; Lin, J.; Yang, G.; Ma, Z.; Xu, L.; He, X.; Han, X.; Liu, W. Hydrogen storage performance of LaNi3.95Al0.75Co0.3 alloy with different preparation methods. Prog. Nat. Sci. Mater. Int. 2022, 32, 206–214. [Google Scholar] [CrossRef]
- Ding, Z.; Wu, P.; Shaw, L. Solid-state hydrogen desorption of 2 MgH2 + LiBH4 nano-mixture: A kinetics mechanism study. J. Alloys Compd. 2019, 806, 350–360. [Google Scholar] [CrossRef]
- Ding, Z.; Li, S.; Zhou, Y.; Chen, Z.; Yang, W.; Ma, W.; Shaw, L. LiBH4 for hydrogen storage—New perspectives. Nano Mater. Sci. 2020, 2, 109–119. [Google Scholar] [CrossRef]
- Wang, K.; Kang, X.; Ren, J.; Wang, P. Nanostructured graphite-induced destabilization of LiBH4 for reversible hydrogen storage. J. Alloys Compd. 2016, 685, 242–247. [Google Scholar] [CrossRef]
- Salehabadi, A.; Ahmad, M.I.; Ismail, N.; Morad, N.; Enhessari, M. Energy, Society and the Environment: Solid-State Hydrogen Storage Materials; Springer: Singapore, 2020; p. 94. [Google Scholar]
- Hu, S.; Lv, L.; Yang, G.; Liu, W.; Miao, H.; Cheng, H.; Han, X.; Lin, Z. Improvement in hydrogen storage performance of Mg by mechanical grinding with molten salt etching Ti3C2Clx. Prog. Nat. Sci. Mater. Int. 2023, 33, 211–224. [Google Scholar] [CrossRef]
- Liang, G.; Huot, J.; Schulz, R. Hydrogen storage properties of the mechanically alloyed LaNi5-based materials. J. Alloys Compd. 2001, 320, 133–139. [Google Scholar] [CrossRef]
- Liang, G.; Huot, J.; Schulz, R. Mechanical alloying and hydrogen storage properties of CaNi5-based alloys. J. Alloys Compd. 2001, 321, 146–150. [Google Scholar] [CrossRef]
- Schlapbach, L. Surface properties of ZrMn2 and electronic structure of ZrMn2 hydride. Phys. Lett. 1982, 91, 303–306. [Google Scholar] [CrossRef]
- YHu, Q.; Zhang, H.F.; Yan, C.; Ye, L.; Ding, B.Z.; Hu, Z.Q. Preparation and hydrogenation of body-centered-cubic TiCr2 alloy. Mater. Lett. 2004, 58, 783–786. [Google Scholar]
- Gómez, E.I.L.; Edalati, K.; Antiqueira, F.J.; Coimbrão, D.D.; Zepon, G.; Leiva, D.R.; Ishikawa, T.T.; Cubero-Sesin, J.M.; Botta, W.J. Synthesis of Nanostructured TiFe Hydrogen Storage Material by Mechanical Alloying via High-Pressure Torsion. Adv. Eng. Mater. 2020, 22, 2000011. [Google Scholar] [CrossRef]
- Chen, B.-H.; Huang, C.-C.; Yeh, Y.-L.; Jang, M.-J. Semiempirical quantum model approach for hydrogen adsorption in ZrNi alloys. J. Alloys Compd. 2013, 580, S135–S139. [Google Scholar] [CrossRef]
- Kodera, Y.; Yamasaki, N.; Yamamoto, T.; Kawasaki, T.; Ohyanagi, M.; Munir, Z.A. Hydrogen storage Mg2Ni alloy produced by induction field activated combustion synthesis. J. Alloys Compd. 2007, 446–447, 138–141. [Google Scholar] [CrossRef]
- Sirsch, P.; Che, F.N.; Titah, J.T.; McGrady, G.S. Hydride–Hydride Bonding Interactions in the Hydrogen Storage Materials AlH3, MgH2, and NaAlH4. Chem. Eur. J. 2012, 18, 9476–9480. [Google Scholar] [CrossRef]
- Dong, X. Density functional theory on reaction mechanism between p-doped LiNH2 clusters and LiH and a new hydrogen storage and desorption mechanism. Acta Phys. Sin. 2023, 72, 153101. [Google Scholar] [CrossRef]
- Pentimalli, M.; Padella, F.; Pilloni, L.; Imperi, E.; Matricardi, P. AB5/ABS composite material for hydrogen storage. Int. J. Hydrogen Energy 2009, 34, 4592–4596. [Google Scholar] [CrossRef]
- Srivastava, S.; Upadhyaya, R.K. Investigations of AB5-type hydrogen storage materials with enhanced hydrogen storage capacity. Int. J. Hydrogen Energy 2011, 36, 7114–7121. [Google Scholar] [CrossRef]
- Zholdayakova, S.; Gemma, R.; Uchida, H.-H.; Sato, M.; Matsumura, Y. Mechanical Composition Control for Ti-Based Hydrogen Storage Alloys. e-J. Surf. Sci. Nanotechnol. 2018, 16, 298–301. [Google Scholar] [CrossRef]
- Manickam, K.; Grant, D.; Walker, G. Optimization of AB2 type alloy composition with superior hydrogen storage properties for stationary applications. Int. J. Hydrogen Energ. 2012, 40, 16288–16296. [Google Scholar] [CrossRef]
- Liu, J.; Sun, L.; Yang, J.; Guo, D.; Chen, D.; Yang, L.; Xiao, P. Ti–Mn hydrogen storage alloys: From properties to applications. RSC Advances 2022, 12, 35744–35755. [Google Scholar] [CrossRef]
- Ye, Y.; Yue, Y.; Lu, J.; Ding, J.; Wang, W.; Yan, J. Enhanced hydrogen storage of a LaNi5 based reactor by using phase change materials. Renew. Energy 2021, 180, 734–743. [Google Scholar] [CrossRef]
- Sato, T.; Saitoh, H.; Utsumi, R.; Ito, J.; Nakahira, Y.; Obana, K.; Takagi, S.; Orimo, S.-I. Hydrogen Absorption Reactions of Hydrogen Storage Alloy LaNi5 under High Pressure. Molecules 2023, 28, 1256. [Google Scholar] [CrossRef]
- Lanyin, S.; Fangjie, L.; Deyou, B. An advanced TiFe series hydrogen storage material with high hydrogen capacity and easily activated properties. Int. J. Hydrogen Energy 1990, 15, 259–262. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, J.; Sun, P.; Zhou, C.; Liu, Y.; Fang, Z.Z. Effect of oxygen on the hydrogen storage properties of TiFe alloys. J. Energy Storage 2022, 55, 105543. [Google Scholar] [CrossRef]
- Li, F.; Zhao, J.; Tian, D.; Zhang, H.; Ke, X.; Johansson, B. Hydrogen storage behavior in C15 Laves phase compound TiCr2 by first principles. J. Appl. Phys. 2009, 105, 043707. [Google Scholar] [CrossRef]
- Coduri, M.; Mauri, S.; Biffi, C.A.; Tuissi, A. A new method for simple quantification of Laves phases and precipitates in TiCr2 alloys. Intermetallics 2019, 109, 110–122. [Google Scholar] [CrossRef]
- Dai, L.; Wang, S.; Wang, L.; Yu, Y.; Shao, G.-J. Preparation of ZrMn2 hydrogen storage alloy by electro-deoxidation in molten calcium chloride. Trans. Nonferrous Met. Soc. China 2014, 24, 2883–2889. [Google Scholar] [CrossRef]
- Zheng, C.; Zhou, D.; Feng, D.; Ren, H.; Zhang, Y. Effect of Y content on the hydrogen storage properties of ball-milled Mg2.4-xYxNi (x = 0.05, 0.1, 0.15, 0.2) alloys. J. Phys. Chem. Solids 2023, 178, 111320. [Google Scholar] [CrossRef]
- Wan, H.; Ran, L.; Lu, H.; Qiu, J.; Zhang, H.; Yang, Y.; Chen, Y.A.; Wang, J.; Pan, F. Optimizing microstructure and enhancing hydrogen storage properties in Mg alloy via tailoring Ni and Si element. J. Magnes. Alloys 2024, in press. [Google Scholar] [CrossRef]
- Ding, Z.; Li, Y.; Yang, H.; Lu, Y.; Tan, J.; Li, J.; Li, Q.; Chen, Y.A.; Shaw, L.L.; Pan, F. Tailoring MgH2 for hydrogen storage through nanoengineering and catalysis. J. Magnes. Alloys 2022, 10, 2946–2967. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Y.; Wu, G.; Fan, C.; Zhang, L.; Han, S. Crucial role of Mg on phase transformation and stability of Sm–Mg–Ni-based AB2 type hydrogen storage alloy. Chem. Eng. J. 2023, 477, 147190. [Google Scholar] [CrossRef]
- Yong, H.; Wei, X.; Hu, J.; Yuan, Z.; Guo, S.; Zhao, D.; Zhang, Y. Hydrogen storage behavior of Mg-based alloy catalyzed by carbon-cobalt composites. J. Magnes. Alloys 2021, 9, 1977–1988. [Google Scholar] [CrossRef]
- Available online: https://automobiles.honda.com/clarity%20plug-in-hybrid (accessed on 30 March 2024).
- Available online: https://global.toyota/jp/detail/1605488 (accessed on 30 March 2024).
- Available online: https://www.allcarindex.com/concept/united-states/general-motors/sequel/ (accessed on 30 March 2024).
- Available online: https://www.gknhydrogen.com/wpcontent/uploads/2021/06/GKNHydrogen_HY2Mega_ProductSheet.pdf (accessed on 30 March 2024).
- Marques, F.; Balcerzak, M.; Winkelmann, F.; Zepon, G.; Felderhoff, M. Review and outlook on high-entropy alloys for hydrogen storage. Energy Environ. Sci. 2021, 14, 5191–5227. [Google Scholar] [CrossRef]
- Sealy, C. Complex Alloys Promise Lightweight Hydrogen Storage. Mater. Today 2023, 69, 4. [Google Scholar] [CrossRef]
- Yang, H.; Ding, Z.; Li, Y.-T.; Li, S.-Y.; Wu, P.-K.; Hou, Q.-H.; Zheng, Y.; Gao, B.; Huo, K.-F.; Du, W.-J.; et al. Recent advances in kinetic and thermodynamic regulation of magnesium hydride for hydrogen storage. Rare Met. 2023, 42, 2906–2927. [Google Scholar] [CrossRef]
- Zhou, H.; Ding, Z.; Chen, Y.A.; Lan, Z.Q.; Guo, S.F.; Spieckermann, F.; Zadorozhnyy, V.; Tan, J.; Pan, F.S.; Eckert, J. Enhancement of hydrogen storage properties from amorphous Mg85Ni5Y10 alloy. J. Non-Cryst. Solids 2023, 605, 122167. [Google Scholar] [CrossRef]
- Hubkowska, K.; Soszko, M.; Krajewski, M.; Czerwiński, A. Enhanced kinetics of hydrogen electrosorption in AB5 hydrogen storage alloy decorated with Pd nanoparticles. Electrochem. Commun. 2019, 100, 100–103. [Google Scholar] [CrossRef]
- Sule, R.; Mishra, A.K.; Nkambule, T.T. Recent advancement in consolidation of MOFs as absorbents for hydrogen storage. Int. J. Energy Res. 2021, 45, 12481–12499. [Google Scholar] [CrossRef]
- Téliz, E.; Abboud, M.; Faccio, R.; Esteves, M.; Zinola, F.; Díaz, V. Hydrogen storage in AB2 hydride alloys: Diffusion processes analysis. J. Electroanal. Chem. 2020, 879, 114781. [Google Scholar] [CrossRef]
- Li, Y.; Guo, Q.; Ding, Z.; Jiang, H.; Yang, H.; Du, W.; Zheng, Y.; Huo, K.; Shaw, L.L. MOFs-Based Materials for Solid-State Hydrogen Storage: Strategies and Perspectives. Chem. Eng. J. 2024, 485, 149665. [Google Scholar] [CrossRef]
- Liu, B.H.; Li, Z.P.; Suda, S. Improving MgH2 formation kinetics and its effect on NaBH4 synthesis. J. Alloys Compd. 2009, 474, 321–325. [Google Scholar] [CrossRef]
- Kato, S.; Borgschulte, A.; Bielmann, M.; Züttel, A. Interface reactions and stability of a hydride composite (NaBH4 + MgH2. Phys. Chem. Chem. Phys. 2021, 14, 8360–8368. [Google Scholar] [CrossRef]
- Ding, Z.; Shaw, L. Enhancement of Hydrogen Desorption from Nanocomposite Prepared by Ball Milling MgH2 with In Situ Aerosol Spraying LiBH4. ACS Sustain. Chem. Eng. 2019, 7, 15064–15072. [Google Scholar] [CrossRef]
- Ding, Z.; Lu, Y.; Li, L.; Shaw, L. High reversible capacity hydrogen storage through Nano-LiBH4 + Nano-MgH2 system. Energy Storage Mater. 2019, 20, 24–35. [Google Scholar] [CrossRef]
- Sreeraj, R.; Aadhithiyan, A.K.; Anbarasu, S. Comparison, advancement, and performance evaluation of heat exchanger assembly in solid-state hydrogen storage device. Renew. Energy 2022, 198, 667–678. [Google Scholar] [CrossRef]
- Keshari, V.; Maiya, P.M. Numerical Study of Solid State Hydrogen Storage System with Finned Tube Heat Exchanger. Heat Transf. Eng. 2020, 41, 484–496. [Google Scholar] [CrossRef]
- Wenger, D.; Polifke, W.; Schmidt-Ihn, E.; Abdel-Baset, T.; Maus, S. Comments on solid state hydrogen storage systems design for fuel cell vehicles. Int. J. Hydrogen Energy 2009, 34, 6265–6270. [Google Scholar] [CrossRef]
- Fontela, P.; Soria, A.; Mielgo, J.; Sierra, J.F.; De Blas, J.; Gauchia, L.; Martínez, J.M. Airport electric vehicle powered by fuel cell. J. Power Sources 2007, 169, 184–193. [Google Scholar] [CrossRef]
- Yuan, J.; Yao, M.; Zhao, B.; Lv, Y.; Huang, H.; Chen, J.; Liu, B.; Zhang, B.; Wu, Y. Influence of heat exchanger structure on hydrogen absorption-desorption performance of hydrogen storage vessel. Prog. Nat. Sci. Mater. Int. 2022, 32, 617–624. [Google Scholar] [CrossRef]
- Lajunen, A.; Lipman, T. Lifecycle cost assessment and carbon dioxide emissions of diesel, natural gas, hybrid electric, fuel cell hybrid and electric transit buses. Energy 2016, 106, 329–342. [Google Scholar] [CrossRef]
- Grüger, F.; Dylewski, L.; Robinius, M.; Stolten, D. Carsharing with fuel cell vehicles: Sizing hydrogen refueling stations based on refueling behavior. Appl. Energy 2018, 228, 1540–1549. [Google Scholar] [CrossRef]
- Lü, X.; Wang, P.; Meng, L.; Chen, C. Energy optimization of logistics transport vehicle driven by fuel cell hybrid power system. Energy Convers. Manag. 2019, 199, 111887. [Google Scholar] [CrossRef]
- Ajanovic, A.; Glatt, A.; Haas, R. Prospects and impediments for hydrogen fuel cell buses. Energy 2021, 235, 121340. [Google Scholar] [CrossRef]
- Robledo, C.B.; Oldenbroek, V.; Abbruzzese, F.; Van Wijk, A.J.M. Integrating a hydrogen fuel cell electric vehicle with vehicle-to-grid technology, photovoltaic power and a residential building. Appl. Energy 2018, 215, 615–629. [Google Scholar] [CrossRef]
- Han, R.; He, H.; Zhang, Z.; Quan, S.; Chen, J. A multi-objective hierarchical energy management strategy for a distributed fuel-cell hybrid electric tracked vehicle. J. Energy Storage 2024, 76, 109858. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, B.; Xu, S. Research on air mass flow-pressure combined control and dynamic performance of fuel cell system for vehicles application. Appl. Energy 2022, 309, 118446. [Google Scholar] [CrossRef]
- Jinquan, G.; Hongwen, H.; Jianwei, L.; Qingwu, L. Real-time energy management of fuel cell hybrid electric buses: Fuel cell engines friendly intersection speed planning. Energy 2021, 226, 120440. [Google Scholar] [CrossRef]
- Bui, V.G.; Bui, T.M.T.; Hoang, A.T.; Nižetić, S.; Sakthivel, R.; Tran, V.N.; Bui, V.H.; Engel, D.; Hadiyanto, H. Energy storage onboard zero-emission two-wheelers: Challenges and technical solutions. Sustain. Energy Technol. Assess. 2021, 47, 101435. [Google Scholar]
- Martinez-Boggio, S.; Di Blasio, D.; Fletcher, T.; Burke, R.; García, A.; Monsalve-Serrano, J. Optimization of the air loop system in a hydrogen fuel cell for vehicle application. Energy Convers. Manag. 2023, 283, 116911. [Google Scholar] [CrossRef]
- Breuer, J.L.; Samsun, R.C.; Stolten, D.; Peters, R. How to reduce the greenhouse gas emissions and air pollution caused by light and heavy duty vehicles with battery-electric, fuel cell-electric and catenary trucks. Environ. Int. 2021, 152, 106474. [Google Scholar] [CrossRef]
- Bauer, A.; Mayer, T.; Semmel, M.; Morales, M.A.G.; Wind, J. Energetic evaluation of hydrogen refueling stations with liquid or gaseous stored hydrogen. Int. J. Hydrogen Energy 2019, 44, 6795–6812. [Google Scholar] [CrossRef]
- Gye, H.-R.; Seo, S.-K.; Bach, Q.-V.; Ha, D.; Lee, C.-J. Quantitative risk assessment of an urban hydrogen refueling station. Int. J. Hydrogen Energy 2019, 44, 1288–1298. [Google Scholar] [CrossRef]
- Riedl, S.M. Development of a Hydrogen Refueling Station Design Tool. Int. J. Hydrogen Energy 2020, 45, 1–9. [Google Scholar] [CrossRef]
- Wang, X.; Gao, W. Hydrogen leakage risk assessment for hydrogen refueling stations. Int. J. Hydrogen Energy 2023, 48, 35795–35808. [Google Scholar] [CrossRef]
- Rong, Y.; Chen, S.; Li, C.; Chen, X.; Xie, L.; Chen, J.; Long, R. Techno-economic analysis of hydrogen storage and transportation from hydrogen plant to terminal refueling station. Int. J. Hydrogen Energy 2024, 52, 547–558. [Google Scholar] [CrossRef]
- Xiao, L.; Chen, J.; Wu, Y.; Zhang, W.; Ye, J.; Shao, S.; Xie, J. Effects of pressure levels in three-cascade storage system on the overall energy consumption in the hydrogen refueling station. Int. J. Hydrogen Energy 2021, 46, 31334–31345. [Google Scholar] [CrossRef]
- Serincan, M.F. Validation of hybridization methodologies of fuel cell backup power systems in real-world telecom applications. Int. J. Hydrogen Energy 2016, 41, 19129–19140. [Google Scholar] [CrossRef]
- Celestine, A.-D.N.; Sulic, M.; Wieliczko, M.; Stetson, N.T. Hydrogen-Based Energy Storage Systems for Large-Scale Data Center Applications. Sustainability 2021, 13, 12654. [Google Scholar] [CrossRef]
- Subedi, A.; Thapa, B.S. Parametric modeling of re-electrification by green hydrogen as an alternative to backup power. IOP Conf. Ser. Earth Environ. Sci. 2022, 1037, 012057. [Google Scholar] [CrossRef]
- Peng, P.; Anastasopoulou, A.; Brooks, K.; Furukawa, H.; Bowden, M.E.; Long, J.R.; Autrey, T.; Breunig, H. Cost and potential of metal–organic frameworks for hydrogen back-up power supply. Nat. Energy 2022, 7, 448–458. [Google Scholar] [CrossRef]
- Li, X.; Song, J.; Yang, P.; Dong, Z.; Yang, S.; He, J. Output analysis and capacity configuration of green backup power supply in data center. J. Phys. Conf. Ser. 2022, 2310, 012040. [Google Scholar] [CrossRef]
- Ziegler, M.S. Evaluating and improving technologies for energy storage and backup power. Joule 2021, 5, 1925–1927. [Google Scholar] [CrossRef]
- Mubaarak, S.; Zhang, D.; Wang, L.; Mohan, M.; Kumar, P.M.; Li, C.; Zhang, Y.; Li, M. Efficient photovoltaics-integrated hydrogen fuel cell-based hybrid system: Energy management and optimal configuration. J. Renew. Sustain. Energy 2021, 13, 013502. [Google Scholar] [CrossRef]
- Pan, G.; Gu, W.; Lu, Y.; Qiu, H.; Lu, S.; Yao, S. Optimal Planning for Electricity-Hydrogen Integrated Energy System Considering Power to Hydrogen and Heat and Seasonal Storage. IEEE Trans. Sustain. Energy 2020, 11, 2662–2676. [Google Scholar] [CrossRef]
- Shams, S.A.; Ahmadi, R. Dynamic optimization of solar-wind hybrid system connected to electrical battery or hydrogen as an energy storage system. Int. J. Energy Res. 2021, 45, 10630–10654. [Google Scholar] [CrossRef]
- Lu, Z.; Li, Z.; Guo, X.; Yang, B. Optimal Planning of Hybrid Electricity–Hydrogen Energy Storage System Considering Demand Response. Processes 2023, 11, 852. [Google Scholar] [CrossRef]
- Li, J.; Li, G.; Ma, S.; Liang, Z.; Li, Y.; Zeng, W. Modeling and Simulation of Hydrogen Energy Storage System for Power-to-gas and Gas-to-power Systems. J. Mod. Power Syst. Clean Energy 2023, 11, 885–895. [Google Scholar] [CrossRef]
- Miao, M.; Lou, S.; Zhang, Y.; Chen, X. Research on the Optimized Operation of Hybrid Wind and Battery Energy Storage System Based on Peak-Valley Electricity Price. Energies 2021, 14, 3707. [Google Scholar] [CrossRef]
- Dong, H.; Wu, Y.; Zhou, J.; Chen, W. Optimal selection for wind power coupled hydrogen energy storage from a risk perspective, considering the participation of multi-stakeholder. J. Clean. Prod. 2022, 356, 131853. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, N.; Dai, H.; Liu, L.; Zhou, Z.; Shi, Q.; Lu, J. Comparison of Different Coupling Modes between the Power System and the Hydrogen System Based on a Power–Hydrogen Coordinated Planning Optimization Model. Energies 2023, 16, 5374. [Google Scholar] [CrossRef]
- Zhao, P.; Xu, W.; Liu, A.; Wu, W.; Wang, J.; Wang, X. Assessment the hydrogen-electric coupled energy storage system based on hydrogen-fueled CAES and power-to-gas-to-power device considering multiple time-scale effect and actual operation constraints. Int. J. Hydrogen Energy 2023, 48, 9198–9218. [Google Scholar] [CrossRef]
- Han, Y.; Li, L.; Chen, W.; Hou, Y.; Zhang, J. A bi-cyclic co-optimization method for sizing of electricity-hydrogen hybrid energy storage microgrid. Sustain. Energy Fuels 2022, 6, 4048–4061. [Google Scholar] [CrossRef]
- Rejeb, O.; Alirahmi, S.M.; Assareh, E.; El Haj Assad, M.; Jemni, A.; Bettayeb, M.; Ghenai, C. Innovative integrated solar powered polygeneration system for green Hydrogen, Oxygen, electricity and heat production. Energy Convers. Manag. 2022, 269, 116073. [Google Scholar] [CrossRef]
- Deng, H.; Wang, J.; Shao, Y.; Zhou, Y.; Cao, Y.; Zhang, X.; Li, W. Optimization of configurations and scheduling of shared hybrid electric-hydrogen energy storages supporting to multi-microgrid system. J. Energy Storage 2023, 74, 109420. [Google Scholar] [CrossRef]
- Dong, H.; Shan, Z.; Zhou, J.; Xu, C.; Chen, W. Refined modeling and co-optimization of electric-hydrogen-thermal-gas integrated energy system with hybrid energy storage. Appl. Energy 2023, 351, 121834. [Google Scholar] [CrossRef]

| Type | Type I | Type II | Type III | Type IV |
|---|---|---|---|---|
| Pressure (MPa) | 17.5–20 | 20–30 | 30–45 | 45–70 |
| Volumetric density (kg/m3) | 10–20 | 20–25 | 30–40 | 30–40 |
| Gravimetric density (wt.%) | 1.6–1.8 | 3.6–4.2 | 6.2–7.0 | 9.0–10.0 |
| Cycle (years) | 15 | 15 | 15–20 | 15–20 |
| Technologies | Cryogenic Liquid Hydrogen Storage | Liquid Organic Hydrogen Carriers (LOHC) |
|---|---|---|
| Volumetric density (kg/m3) | 70 | 70 |
| Gravimetric density (wt.%) | 5.7 | 6 |
| Pressure (MPa) | 2–4 | 0.5–2 |
| Temperature (°C) | −235 | 25 |
| Safety | High | Middle |
| Materials | LaNi5 | CaNi5 | ZrMn2 | TiCr2 | TiFe | ZrNi | Mg2Ni | NaAlH4 | LiNH2 |
|---|---|---|---|---|---|---|---|---|---|
| Capacity (wt.%) | 1.8 | 1.4 | 1.5–2.0 | 2 | 1.8 | 1.6 | 3.6 | 5.6 | 10.5 |
| Temperature (°C) | 25 | 25 | 25–100 | 25–100 | 100 | 25–100 | 250–350 | 150 | 150 |
| Pressure (MPa) | 0.1–0.3 | 0.1 | 0.1–1 | 0.1–1 | 1–2 | 1–3 | 0.1–0.3 | 0.1–1 | 0.1–1 |
| Years | Sales Volume of Fuel Cell Vehicles (Units) | Stock of Fuel Cell Vehicles (Units) | ||
|---|---|---|---|---|
| China | World | China | World | |
| 2016 | 629 | 2755 | 639 | 2312 |
| 2017 | 1272 | 4575 | 1911 | 6475 |
| 2018 | 1527 | 5523 | 3438 | 12,900 |
| 2019 | 2737 | 10,409 | 6175 | 24,132 |
| 2020 | 1177 | 9006 | 7352 | 32,535 |
| 2021 | 1587 | 17,027 | 8939 | 49,562 |
| 2022 | 3367 | 18,631 | 12,306 | 67,488 |
| 2023 | 5805 | 14,451 | 12,682 | - |
| Years | China | World |
|---|---|---|
| 2016 | 10 | 274 |
| 2017 | 14 | 328 |
| 2018 | 31 | 369 |
| 2019 | 61 | 434 |
| 2020 | 128 | 560 |
| 2021 | 218 | 685 |
| 2022 | 274 | 815 |
| 2023 | 428 | 1089 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Y.; Zhou, Y.; Li, Y.; Ding, Z. Research Progress and Application Prospects of Solid-State Hydrogen Storage Technology. Molecules 2024, 29, 1767. https://doi.org/10.3390/molecules29081767
Xu Y, Zhou Y, Li Y, Ding Z. Research Progress and Application Prospects of Solid-State Hydrogen Storage Technology. Molecules. 2024; 29(8):1767. https://doi.org/10.3390/molecules29081767
Chicago/Turabian StyleXu, Yaohui, Yang Zhou, Yuting Li, and Zhao Ding. 2024. "Research Progress and Application Prospects of Solid-State Hydrogen Storage Technology" Molecules 29, no. 8: 1767. https://doi.org/10.3390/molecules29081767
APA StyleXu, Y., Zhou, Y., Li, Y., & Ding, Z. (2024). Research Progress and Application Prospects of Solid-State Hydrogen Storage Technology. Molecules, 29(8), 1767. https://doi.org/10.3390/molecules29081767