The Impact of Different Drying Methods on the Metabolomic and Lipidomic Profiles of Arthrospira platensis
Abstract
1. Introduction
2. Results and Discussion
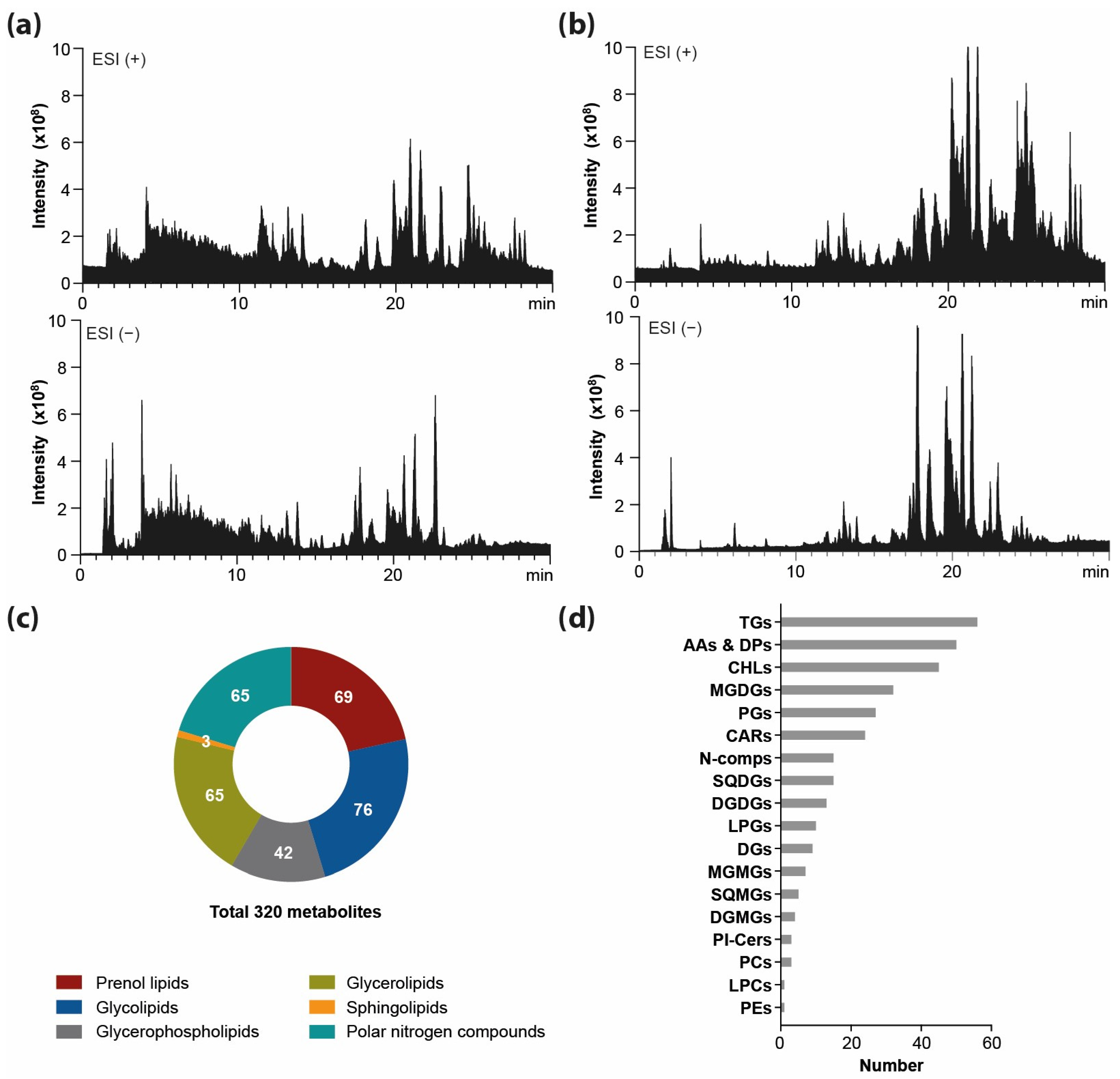
2.1. The Comprehensive Metabolome-Wide Profiling of Freeze-Dried Spirulina
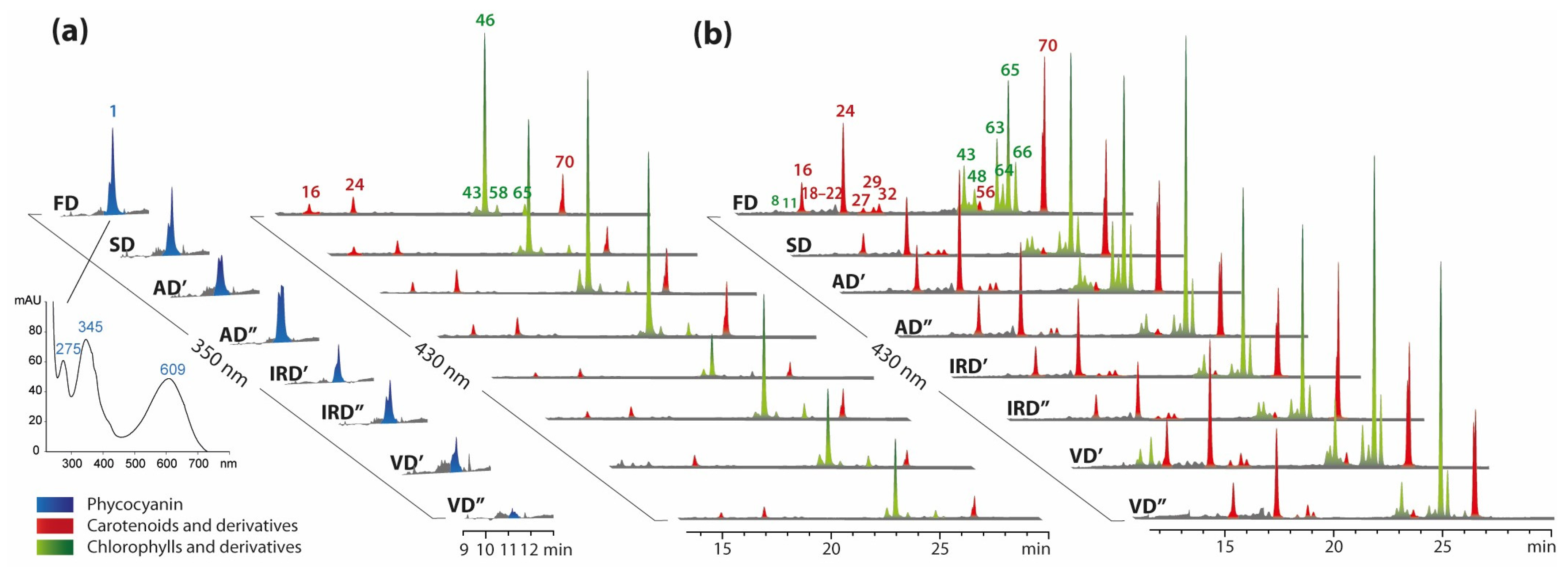
2.1.1. The Profiling of Pigments
2.1.2. The Profiling of Lipids
2.1.3. The Profiling of Polar Nitro-Organic Compounds
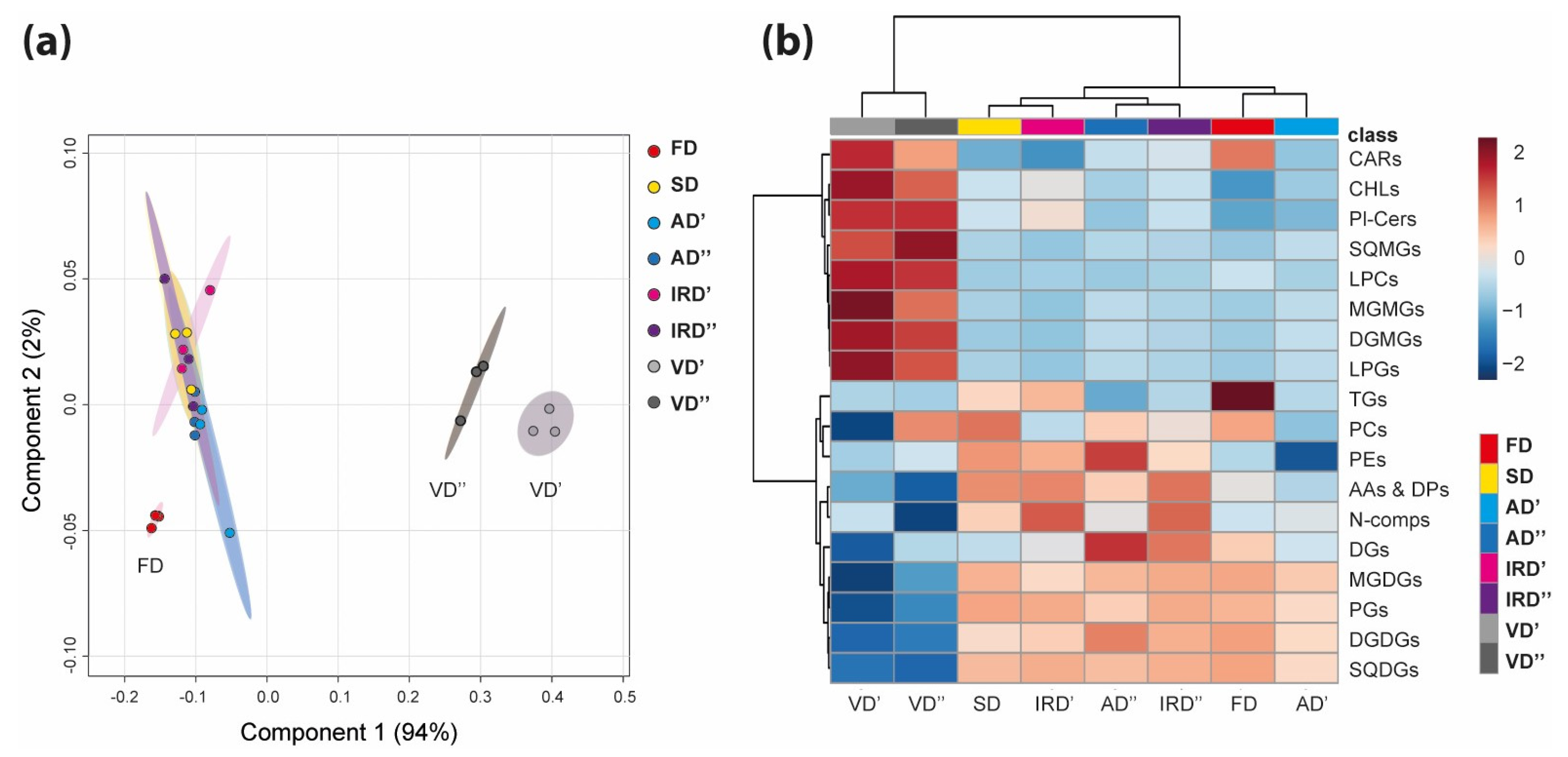
2.2. The Modulation of Metabolomic Profiles of Spirulina after Drying
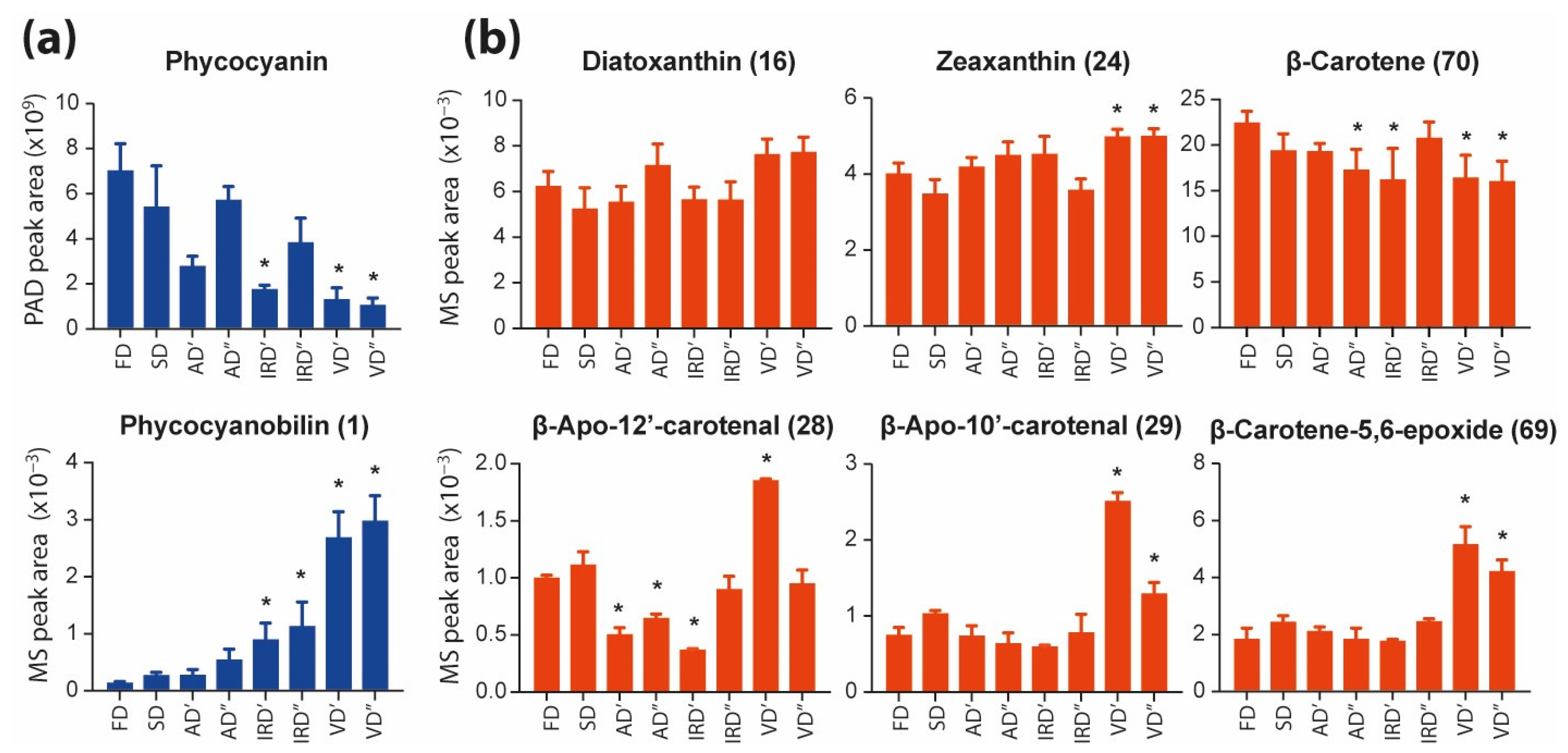
2.2.1. The Impacts of Drying Methods on Pigments
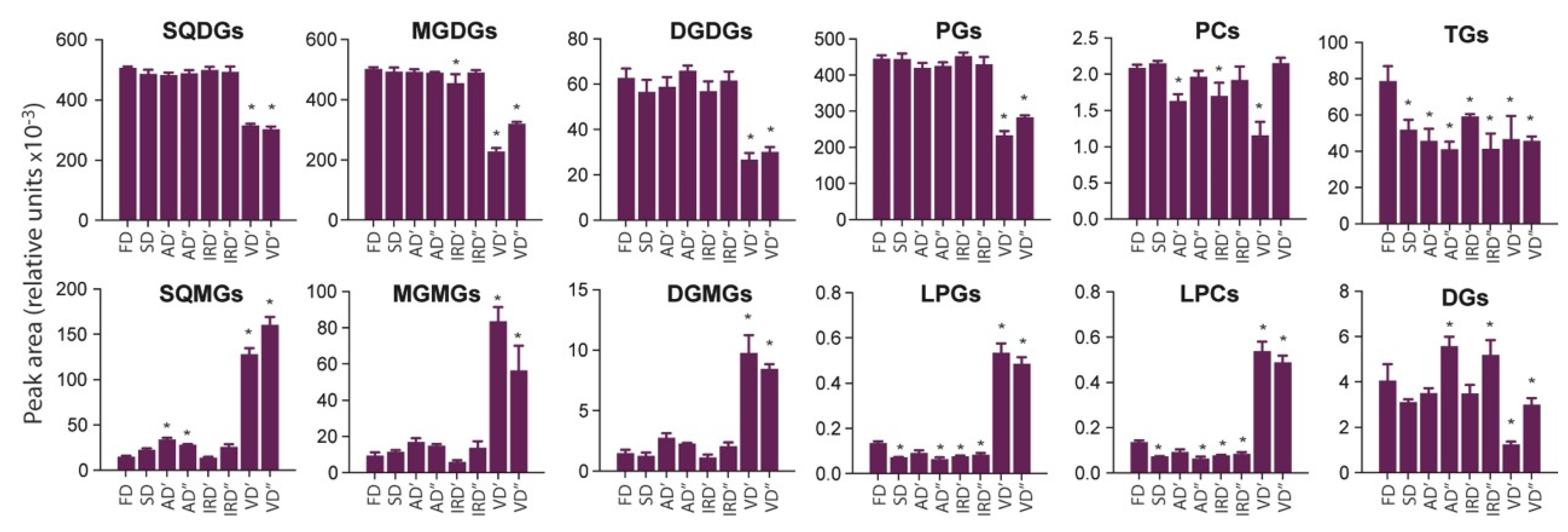
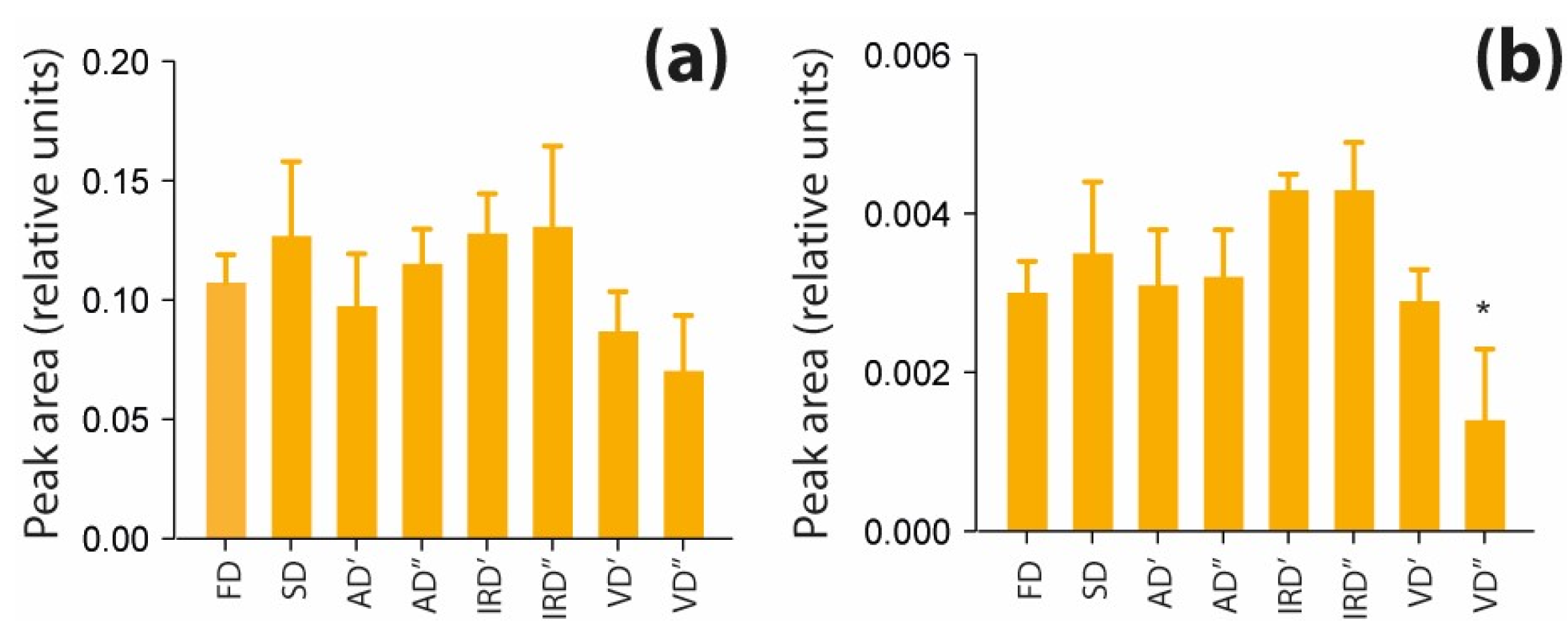
2.2.2. The Impact of Drying Methods on Lipids
2.2.3. The Impact of Drying Methods on Polar Nitro-organic Compounds
3. Materials and Methods
3.1. Strain and Growth Conditions
3.2. Drying Treatments
- (a)
- In freeze drying (FD), the batch of frozen spirulina paste was lyophilized in Christ Alpha 2–4 LSC freeze-dryer (Martin Christ Gefriertrocknungsanlagen GmbH, Osterode am Harz, Germany) at the temperature of −78 °C and a pressure of 0.94 mbar for 24 h.
- (b)
- In sun drying (SD), samples were dried in an open space under direct sunlight at a temperature of 25–28 °C for 24 h.
- (c)
- In air drying (AD), drying was performed in Florida Jerky air circulation oven (500 W, Klarestein, Berlin, Germany) at a temperature of 40 (AD′) or 75 °C (AD″) for 4 h.
- (d)
- In infrared drying (IRD), drying was conducted at a temperature of 40 (IRD′) or 75 °C (IRD″) for 4 h in a Florida Jerky dehydrator (500 W, Klarestein, Berlin, Germany).
- (e)
- In vacuum drying (VD), samples were dried in DZ-1BCII vacuum-drying oven (220 V, 800 W, ChemLand, Stargard, Poland) in two conditions: at a temperature of 40 °C (VD′) for 300 min or at 75 °C (VD″) for 240 min. The pressure inside the chamber in both tested conditions was reduced by 0.07–0.095 MPa compared to atmospheric pressure.
3.3. Extract Preparations
3.4. Q-Orbitrap HRMS Analysis
3.5. Data Processing
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chojnacka, K.; Noworyta, A. Evaluation of Spirulina sp. growth in photoautotrophic, heterotrophic and mixotrophic cultures. Enzym. Microb. Technol. 2004, 34, 461–465. [Google Scholar] [CrossRef]
- El-Baky, N.A.; Rezk, N.M.F.; Amara, A.A. Arthrospira platensis Variants: A Comparative Study Based on C-phycocyanin Gene and Protein, Habitat, and Growth Conditions. J. Mar. Sci. Eng. 2023, 11, 663. [Google Scholar] [CrossRef]
- Barnett, M. Arthrospira platensis: Brief history and description. Curr. Pharm. Biotechnol. 2005, 6, 373–379. [Google Scholar]
- Li, Y.; Aiello, G.; Bollati, C.; Bartolomei, M.; Arnoldi, A.; Lammi, C. Phycobiliproteins from Arthrospira platensis (Spirulina): A New Source of Peptides with Dipeptidyl Peptidase-IV Inhibitory Activity. Nutrients 2020, 12, 794. [Google Scholar] [CrossRef] [PubMed]
- Bui, H.T.H.; Thi Pham, T.; Thi Thu Nguyen, H.; Minh Do, T.; Thi Nga, V.; Bac, N.D.; Huyen, V.T.B.; Le, H.M.; Tran, Q.C. Transformation Chlorophyll a of Spirulina platensis to Chlorin E6 Derivatives and Several Applications. Open Access Maced. J. Med. Sci. 2019, 7, 4372–4377. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.K.; Saleh, A.M. Spirulina—An overview. Int. J. Pharm. Pharm. Sci. 2012, 4, 9–15. [Google Scholar]
- Marzorati, S.; Schievano, A.; Idà, A.; Verotta, L. Carotenoids, chlorophylls and phycocyanin from spirulina: Supercritical CO2 and water extraction methods for added value products cascade. Green Chem. 2020, 22, 187–196. [Google Scholar] [CrossRef]
- Hynstova, V.; Sterbova, D.; Klejdus, B.; Hedbavny, J.; Huska, D.; Adam, V. Separation, identification and quantification of carotenoids and chlorophylls in dietary supplements containing Chlorella vulgaris and Spirulina platensis using high performance thin layer chromatography. J. Pharm. Biomed. Anal. 2018, 148, 108–118. [Google Scholar] [CrossRef]
- Dos Santos, R.R.; Corrêa, P.S.; Dantas, F.M.L.; Teixeira, C.M.L.L. Evaluation of the Co-Production of Total Carotenoids, C-Phycocyanin and Polyhydroxyalkanoates by Arthrospira platensis. Bioresour. Technol. Rep. 2019, 7, 100226. [Google Scholar] [CrossRef]
- Ramadan, M.F.; Asker, M.M.S.; Ibrahim, Z.K. Functional bioactive compounds and biological activities of Spirulina platensis lipids. CAAS Agric. J. 2008, 26, 211–222. [Google Scholar] [CrossRef]
- Bashir, S.; Sharif, M.K.; Butt, M.S.; Shahid, M. Functional Properties and Amino Acid Profile of Spirulina Platensis Protein Isolates. Biol. Sci.—PJSIR 2016, 59, 12–19. [Google Scholar] [CrossRef]
- Çelekli, A.; Özbal, B.; Bozkurt, H. Challenges in Functional Food Products with the Incorporation of Some Microalgae. Foods 2024, 13, 725. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Wu, L.; Zhu, Z.; Hou, H.; Wang, Y. Investigation of Peptides Containing Branched-Chain Amino Acids from Arthrospira platensis through a Peptidomics Workflow. Food Bioprocess Technol. 2024, 17, 516–527. [Google Scholar] [CrossRef]
- El-Sayed, A.E.-K.; El-Sheekh, M. Outdoor Cultivation of Spirulina platensis for Mass Production. Not. Sci. Biol. 2018, 10, 38–44. [Google Scholar] [CrossRef]
- Ouedraogo, H.G.; Savadogo, B.; Traore, A. Storage influence on beta-carotene and alpha-tocopherol contents of solar-dried Spirulina platensis (Spirulina). Afr. J. Food Sci. 2015, 9, 546–554. [Google Scholar]
- Walker, T.H.; Drapcho, C.M.; Chen, F. Bioprocessing technology for production of nutraceutical compounds. In Functional Food Ingredients and Nutraceuticals: Processing Technologies; Shi, J., Ed.; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]
- Kharchuk, I.A.; Rylkova, O.A.; Beregovaya, N.M. State of cyanobacteria Arthrospira platensis and of associated microflora during long-term storage in the state of anhydrobiosis. Microbiology 2022, 91, 704–712. [Google Scholar] [CrossRef]
- Silva, N.C.; Graton, I.S.; Duarte, C.R.; Barrozo, M.A.S. Effects of Infrared and Microwave Radiation on the Bioactive Compounds of Microalga Spirulina platensis after Continuous and Intermittent Drying. Molecules 2023, 28, 5963. [Google Scholar] [CrossRef]
- Soni, R.; Sudhakar, K.; Rana, R.S. Spirulina—From growth to nutritional product: A review. Trends Food Sci. Technol. 2017, 69, 157–171. [Google Scholar] [CrossRef]
- Oliveira, E.G.; Duarte, J.H.; Moraes, K.; Crexi, V.T.; Pinto, L.A.A. Optimisation of Spirulina platensis convective drying: Evaluation of phycocyanin loss and lipid oxidation. Int. J. Food Sci. Technol. 2010, 45, 1572–1578. [Google Scholar] [CrossRef]
- Papalia, T.; Sidari, R.; Panuccio, M.R. Impact of Different Storage Methods on Bioactive Compounds in Arthrospira platensis Biomass. Molecules 2019, 24, 2810. [Google Scholar] [CrossRef]
- Demarco, M.; Moraes, J.O.d.; Ferrari, M.C.; Neves, F.d.F.; Laurindo, J.B.; Tribuzi, G. Production of Spirulina (Arthrospira platensis) powder by innovative and traditional drying techniques. J. Food Process Eng. 2022, 45, e13919. [Google Scholar] [CrossRef]
- Beaupeux, E.; Jayaprakash, P.; Dokmak, A.; Edorh, J.-M.; Gaiani, C.; Desobry, S.; Maudhuit, A. Electrostatic spray drying: Advantages for thermosensitive actives. Chem. Eng. Res. Des. 2024, 203, 688–696. [Google Scholar] [CrossRef]
- Guo, W.; Zeng, M.; Zhu, S.; Li, S.; Qian, Y.; Wu, H. Phycocyanin ameliorates mouse colitis via phycocyanobilin-dependent antioxidant and anti-inflammatory protection of the intestinal epithelial barrier. Food Funct. 2022, 13, 3294–3307. [Google Scholar] [CrossRef]
- Rivera, S.M.; Christou, P.; Canela-Garayoa, R. Identification of carotenoids using mass spectrometry. Mass Spectrom. Rev. 2014, 33, 353–372. [Google Scholar] [CrossRef]
- Bijttebier, S.K.; D’Hondt, E.; Hermans, N.; Apers, S.; Voorspoels, S. Unravelling ionization and fragmentation pathways of carotenoids using orbitrap technology: A first step towards identification of unknowns. J. Mass Spectrom. 2013, 48, 740–754. [Google Scholar] [CrossRef] [PubMed]
- Milenković, S.M.; Zvezdanović, J.; Anđelković, T.; Marković, D.Z. The identification of chlorophyll and its derivatives in the pigment mixtures: HPLC-chromatography, visible and mass spectroscopy studies. Adv. Technol. 2012, 1, 16–24. [Google Scholar]
- Chen, K.; Ríos, J.J.; Pérez-Gálvez, A.; Roca, M. Development of an accurate and high-throughput methodology for structural comprehension of chlorophylls derivatives. (I) Phytylated derivatives. J. Chromatogr. A 2015, 1406, 99–108. [Google Scholar] [CrossRef]
- Chen, K.; Ríos, J.J.; Roca, M.; Pérez-Gálvez, A. Development of an accurate and high-throughput methodology for structural comprehension of chlorophylls derivatives. (II) Dephytylated derivatives. J. Chromatogr. A 2015, 1412, 90–99. [Google Scholar] [CrossRef]
- Viera, I.; Roca, M.; Perez-Galvez, A. Mass spectrometry of non-allomerized chlorophylls a and b derivatives from plants. Curr. Org. Chem. 2018, 22, 842–876. [Google Scholar] [CrossRef]
- Yang, Y.; Du, L.; Hosokawa, M.; Miyashita, K. Effect of Spirulina lipids on high-fat and high-sucrose diet induced obesity and hepatic lipid accumulation in C57BL/6J mice. J. Funct. Foods 2020, 65, 103741. [Google Scholar] [CrossRef]
- Neag, E.; Stupar, Z.; Varaticeanu, C.; Senila, M.; Roman, C. Optimization of Lipid Extraction from Spirulina spp. by Ultrasound Application and Mechanical Stirring Using the Taguchi Method of Experimental Design. Molecules 2022, 27, 6794. [Google Scholar] [CrossRef] [PubMed]
- Conde, T.A.; Neves, B.F.; Couto, D.; Melo, T.; Neves, B.; Costa, M.; Silva, J.; Domingues, P.; Domingues, M.R. Microalgae as sustainable bio-factories of healthy lipids: Evaluating fatty acid content and antioxidant activity. Mar. Drugs 2021, 19, 357. [Google Scholar] [CrossRef] [PubMed]
- Couto, D.; Conde, T.A.; Melo, T.; Neves, B.; Costa, M.; Silva, J.; Domingues, R.; Domingues, P. The chemodiversity of polar lipidomes of microalgae from different taxa. Algal Res. 2023, 70, 103006. [Google Scholar] [CrossRef]
- Xue, C.; Hu, Y.; Saito, H.; Zhang, Z.; Li, Z.; Cai, Y.; Ou, C.; Lin, H.; Imbs, A.B. Molecular species composition of glycolipids from Spirulina platensis. Food Chem. 2002, 77, 9–13. [Google Scholar] [CrossRef]
- da Costa, E.; Silva, J.; Mendonça, S.H.; Abreu, M.H.; Domingues, M.R. Lipidomic approaches towards deciphering glycolipids from microalgae as a reservoir of bioactive lipids. Mar. Drugs 2016, 14, 101. [Google Scholar] [CrossRef] [PubMed]
- Lopes, D.; Moreira, A.S.; Rey, F.; da Costa, E.; Melo, T.; Maciel, E.; Rego, A.; Abreu, M.H.; Domingues, P.; Calado, R.; et al. Lipidomic signature of the green macroalgae Ulva rigida farmed in a sustainable integrated multi-trophic aquaculture. J. Appl. Phycol. 2019, 31, 1369–1381. [Google Scholar] [CrossRef]
- da Costa, E.; Melo, T.; Reis, M.; Domingues, P.; Calado, R.; Abreu, M.H.; Domingues, M.R. Polar lipids composition, antioxidant and anti-inflammatory activities of the Atlantic red seaweed Grateloupia turuturu. Mar. Drugs 2021, 19, 414. [Google Scholar] [CrossRef]
- Alves, E.; Melo, T.; Barros, M.P.; Domingues, M.R.M.; Domingues, P. Lipidomic profiling of the olive (Olea europaea L.) fruit towards its valorisation as a functional food: In-depth identification of triacylglycerols and polar lipids in Portuguese olives. Molecules 2019, 24, 2555. [Google Scholar] [CrossRef]
- da Costa, E.; Melo, T.; Moreira, A.S.; Alves, E.; Domingues, P.; Calado, R.; Abreeu, M.H.; Domingues, M.R. Decoding bioactive polar lipid profile of the macroalgae Codium tomentosum from a sustainable IMTA system using a lipidomic approach. Algal Res. 2015, 12, 388–397. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, M.; Li, Y.; Feng, X.; Chen, Y.; Lin, L. Analysis of lipids in green coffee by ultra-performance liquid chromatography-time-of-flight tandem mass spectrometry. Molecules 2022, 27, 5271. [Google Scholar] [CrossRef]
- Demir, B.S.; Tükel, S.S. Purification and characterization of lipase from Spirulina platensis. J. Mol. Catal. B Enzym. 2010, 64, 123–128. [Google Scholar] [CrossRef]
- da Fontoura Prates, D.; Duarte, H.; Vendruscolo, D.G.; Wagner, R.; Ballus, C.A.; da Silva Oliveira, W.; Godoy, H.T.; Barcia, M.T.; de Morais, M.G.; Radmann, E.M.; et al. Role of light emitting diode (LED) wavelengths on increase of protein productivity and free amino acid profile of Spirulina sp. cultures. Bioresour. Technol. 2020, 306, 123184. [Google Scholar] [CrossRef] [PubMed]
- Choi, A.; Kim, S.G.; Yoon, B.D.; Oh, H.M. Growth and amino acid contents of Spirulina platensis with different nitrogen sources. Biotechnol. Bioprocess Eng. 2003, 8, 368–372. [Google Scholar] [CrossRef]
- Zhang, P.; Chan, W.; Ang, I.L.; Wei, R.; Lam, M.M.; Lei, K.M.; Poon, T.C. Revisiting fragmentation reactions of protonated α-amino acids by High-Resolution Electrospray Ionization Tandem Mass Spectrometry with Collision-Induced Dissociation. Sci. Rep. 2019, 9, 6453. [Google Scholar] [CrossRef]
- Ma, X.; Dagan, S.; Somogyi, A.; Wysocki, V.H.; Scaraffia, P.Y. Low mass MS/MS fragments of protonated amino acids used for distinction of their 13C-isotopomers in metabolic studies. J. Am. Soc. Mass Spectrom. 2013, 24, 622–631. [Google Scholar] [CrossRef] [PubMed]
- Weimann, A.; Belling, D.; Poulsen, H.E. Quantification of 8-oxo-guanine and guanine as the nucleobase, nucleoside and deoxynucleoside forms in human urine by high-performance liquid chromatography-electrospray tandem mass spectrometry. Nucleic Acids Res. 2002, 30, e7. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, A.; Pitesky, M.E.; Steele, P.T.; Tobias, H.J.; Fergenson, D.P.; Horn, J.M.; Russell, S.C.; Czerwieniec, G.A.; Lebrilla, C.B.; Gard, E.E.; et al. Comprehensive assignment of mass spectral signatures from individual Bacillus atrophaeus spores in Matrix-Free Laser Desorption/Ionization Bioaerosol Mass Spectrometry. Anal. Chem. 2005, 77, 3315–3323. [Google Scholar] [CrossRef]
- Schram, B.L.; Kroes, H.H. Structure of phycocyanobilin. Eur. J. Biochem. 1971, 19, 581–594. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Dhar, D.W.; Pabbi, S.; Kumar, N.; Walia, S. Extraction and purification of C-phycocyanin from Spirulina platensis (CCC540). Indian J. Plant Physiol. 2014, 19, 184–188. [Google Scholar] [CrossRef]
- Choi, W.Y.; Lee, H.Y. Kinetic analysis of stabilizing C-phycocyanin in the Spirulina platensis extracts from ultrasonic process associated with effects of light and temperature. Appl. Sci. 2018, 8, 1662. [Google Scholar] [CrossRef]
- Abalde, J.; Betancourt, L.; Torres, E.; Cid, A.; Barwell, C. Purification and characterization of phycocyanin from the marine cyanobacterium Synechococcus sp. IO9201. Plant Sci. 1998, 136, 109–120. [Google Scholar] [CrossRef]
- Güroy, B.; Karadal, O.; Mantoğlu, S.; Cebeci, O.I. Effects of different drying methods on C-phycocyanin content of Spirulina platensis powder. Ege J. Fish. Aquatic. Sci. 2017, 34, 129–132. [Google Scholar]
- Wilkinson, I.V.L.; Castro-Falcón, G.; Roda-Serrat, M.C.; Purdy, T.N.; Straetener, J.; Brauny, M.M.; Maier, L.; Brötz-Oesterhelt, H.; Christensen, L.P.; Sieber, S.A.; et al. The cyanobacterial “nutraceutical” phycocyanobilin inhibits cysteine protease legumain. ChemBioChem 2023, 24, e202200455. [Google Scholar] [CrossRef]
- Roda-Serrat, M.C.; Christensen, K.V.; El-Houri, R.B.; Frette, X.; Christensen, L.P. Fast cleavage of phycocyanobilin from phycocyanin for use in food colouring. Food Chem. 2018, 240, 655–661. [Google Scholar] [CrossRef]
- Kamo, T.; Eki, T.; Hirose, Y. Pressurized liquid extraction of a phycocyanobilin chromophore and its reconstitution with a cyanobacteriochrome photosensor for efficient isotopic labeling. Plant Cell Physiol. 2021, 62, 334–347. [Google Scholar] [CrossRef]
- Souza, C.S.; Daood, H.G.; Duah, S.A.; Vinogradov, S.; Palotás, G.; Neményi, A.; Helyes, L.; Pék, Z. Stability of carotenoids, carotenoid esters, tocopherols and capsaicinoids in new chili pepper hybrids during natural and thermal drying. LWT 2022, 163, 113520. [Google Scholar] [CrossRef]
- Stramarkou, M.; Papadaki, S.; Kyriakopoulou, K.; Tzovenis, I.; Chronis, M.; Krokida, M. Comparative analysis of different drying techniques based on the qualitative characteristics of Spirulina platensis biomass. J. Aquat. Food Prod. 2021, 30, 498–516. [Google Scholar] [CrossRef]
- Pénicaud, C.; Achir, N.; Dhuique-Mayer, C.; Dornier, M.; Bohuon, P. Degradation of β-carotene during fruit and vegetable processing or storage: Reaction mechanisms and kinetic aspects: A review. Fruits 2011, 66, 417–440. [Google Scholar] [CrossRef]
- Marx, M.; Stuparic, M.; Schieber, A.; Carle, R. Effects of thermal processing on trans–cis-isomerization of β-carotene in carrot juices and carotene-containing preparations. Food Chem. 2003, 83, 609–617. [Google Scholar] [CrossRef]
- Nouri, E.; Abbasi, H.; Rahimi, E. Effects of processing on stability of water-and fat-soluble vitamins, pigments (C-phycocyanin, carotenoids, chlorophylls) and colour characteristics of Spirulina platensis. Qual. Assur. Saf. Crops Foods 2018, 10, 335–349. [Google Scholar] [CrossRef]
- Ritchie, R.J. Universal chlorophyll equations for estimating chlorophylls a, b, c, and d and total chlorophylls in natural assemblages of photosynthetic organisms using acetone, methanol, or ethanol solvents. Photosynthetica 2008, 46, 115–126. [Google Scholar] [CrossRef]
- Hu, X.; Tanaka, A.; Tanaka, R. Simple extraction methods that prevent the artifactual conversion of chlorophyll to chlorophyllide during pigment isolation from leaf samples. Plant Methods 2013, 9, 19. [Google Scholar] [CrossRef]
- Bórquez, R.; Melo, D.; Saavedra, C. Microwave–vacuum drying of strawberries with automatic temperature control. Food Bioprocess Technol. 2015, 8, 266–276. [Google Scholar] [CrossRef]
- Roca, M.; Chen, K.; Perez-Galvez, A. Chlorophylls. In Handbook on Natural Pigments in Food and Beverages; Carle, R., Schweiggert, R., Eds.; Elsevier: New York, NY, USA, 2016; pp. 125–158. [Google Scholar]
- Balduyck, L.; Dejonghe, C.; Goos, P.; Jooken, E.; Muylaert, K.; Foubert, I. Inhibition of lipolytic reactions during wet storage of T-Isochrysis lutea biomass by heat treatment. Algal Res. 2019, 38, 101388. [Google Scholar] [CrossRef]
- Balduyck, L.; Stock, T.; Bijttebier, S.; Bruneel, C.; Jacobs, G.; Voorspoels, S.; Muylaert, K.; Foubert, I. Integrity of the microalgal cell plays a major role in the lipolytic stability during wet storage. Algal Res. 2017, 25, 516–524. [Google Scholar] [CrossRef]
- Machado, L.; Carvalho, G.; Pereira, R.N. Effects of innovative processing methods on microalgae cell wall: Prospects towards digestibility of protein-rich biomass. Biomass 2022, 2, 80–102. [Google Scholar] [CrossRef]
- Hashiro, S.; Fujiuchi, K.; Sugimori, D.; Yasueda, H. A novel galactolipase from a green microalga Chlorella kessleri: Purification, characterization, molecular cloning, and heterologous expression. Appl. Microbiol. Biotechnol. 2018, 102, 1711–1723. [Google Scholar] [CrossRef]
- Piotrowski, D.; Lenart, A.; Borkowska, O. Temperature changes during vacuum drying of defrosted and osmotically dehydrated strawberries. Pol. J. Food Nutr. Sci. 2007, 57, 141–146. [Google Scholar]
- Saddhe, A.A.; Potocký, M. Comparative phylogenomic and structural analysis of canonical secretory PLA2 and novel PLA2-like family in plants. Front. Plant Sci. 2023, 14, 1118670. [Google Scholar] [CrossRef]
- Vega-Galvez, A.; Uribe, E.; Pasten, A.; Camus, J.; Rojas, M.; Garcia, V.; Araya, M.; Valenzuela-Barra, G.; Zambrano, A.; Goñi, M.G. Low-Temperature Vacuum Drying on Broccoli: Enhanced Anti-Inflammatory and Anti-Proliferative Properties Regarding Other Drying Methods. Foods 2023, 12, 3311. [Google Scholar] [CrossRef]
- Yang, R.L.; Li, Q.; Hu, Q.P. Physicochemical properties, microstructures, nutritional components, and free amino acids of Pleurotus eryngii as affected by different drying methods. Sci. Rep. 2020, 10, 121. [Google Scholar] [CrossRef]
- Zarrouk, C. Contribution a L’etude D’une Cyanobacterie: Influence de Divers Facteurs Physiques et Chimiques sur la Croissance et la Photosynthese de Spirulina Maxima (Setchell et Gardner) Geitler. Ph.D. Thesis, University of Paris, Paris, France, 1966. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mróz, M.; Parchem, K.; Jóźwik, J.; Domingues, M.R.; Kusznierewicz, B. The Impact of Different Drying Methods on the Metabolomic and Lipidomic Profiles of Arthrospira platensis. Molecules 2024, 29, 1747. https://doi.org/10.3390/molecules29081747
Mróz M, Parchem K, Jóźwik J, Domingues MR, Kusznierewicz B. The Impact of Different Drying Methods on the Metabolomic and Lipidomic Profiles of Arthrospira platensis. Molecules. 2024; 29(8):1747. https://doi.org/10.3390/molecules29081747
Chicago/Turabian StyleMróz, Marika, Karol Parchem, Joanna Jóźwik, M. Rosário Domingues, and Barbara Kusznierewicz. 2024. "The Impact of Different Drying Methods on the Metabolomic and Lipidomic Profiles of Arthrospira platensis" Molecules 29, no. 8: 1747. https://doi.org/10.3390/molecules29081747
APA StyleMróz, M., Parchem, K., Jóźwik, J., Domingues, M. R., & Kusznierewicz, B. (2024). The Impact of Different Drying Methods on the Metabolomic and Lipidomic Profiles of Arthrospira platensis. Molecules, 29(8), 1747. https://doi.org/10.3390/molecules29081747





