Recycling and Degradation of Polyamides
Abstract
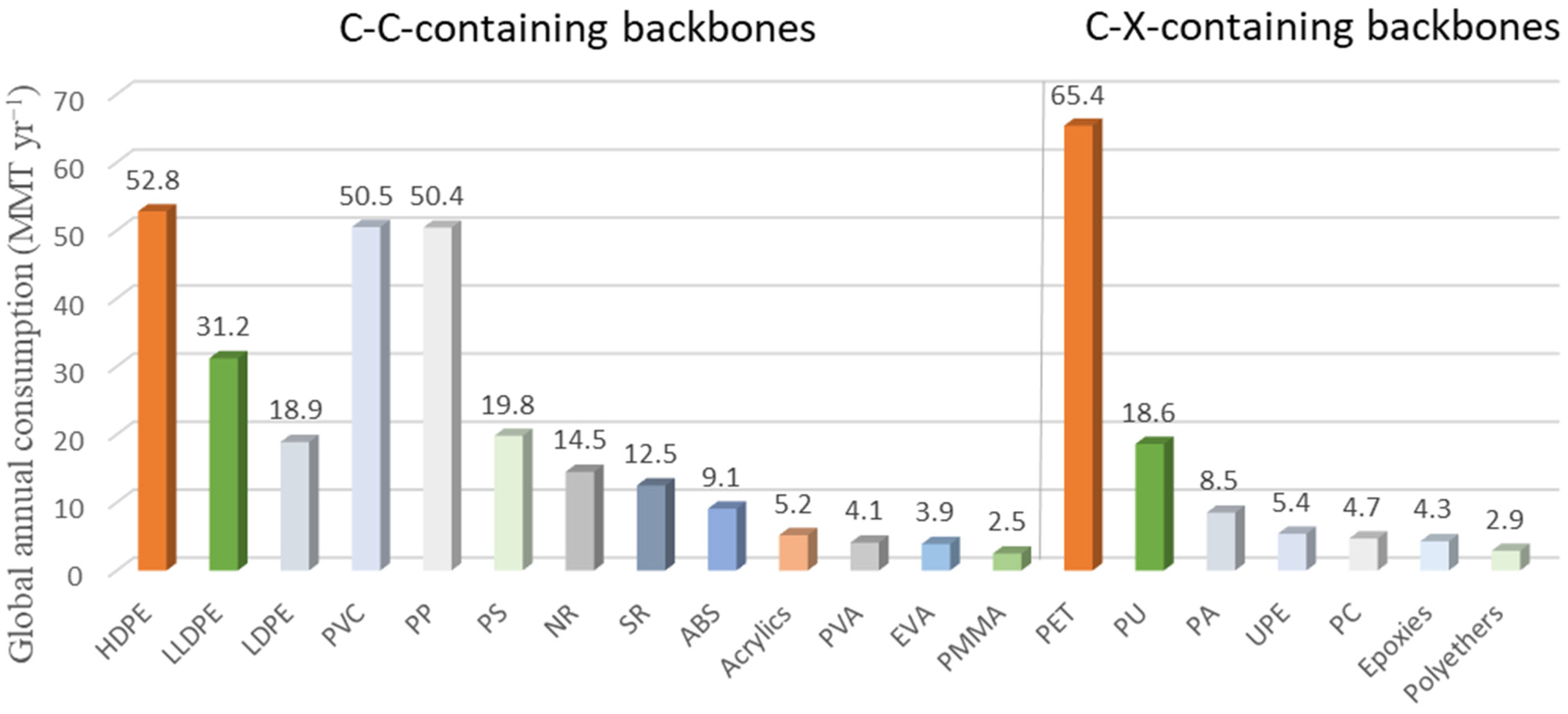
1. Introduction
2. Energy Recovery and Recycling Process
2.1. Filling and Landfilling
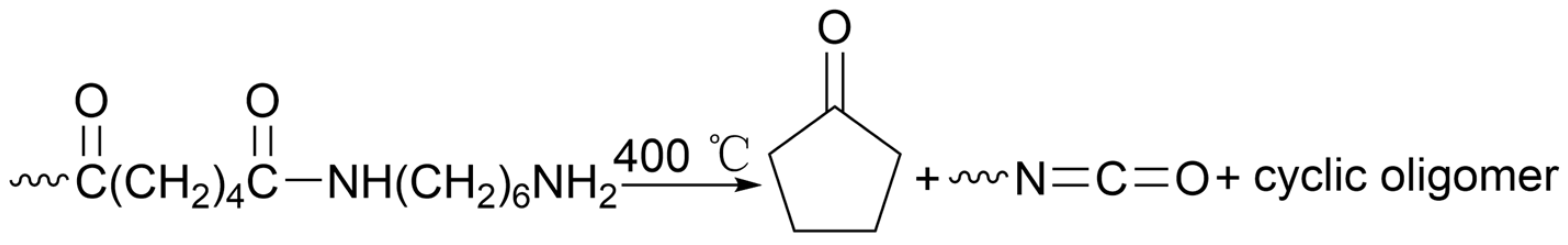
2.2. Energy Recovery and Pyrolysis
2.3. Physical Recycling
2.3.1. Mechanical Recycling
2.3.2. Solvent Precipitation Recovery
2.4. Chemical Recycling
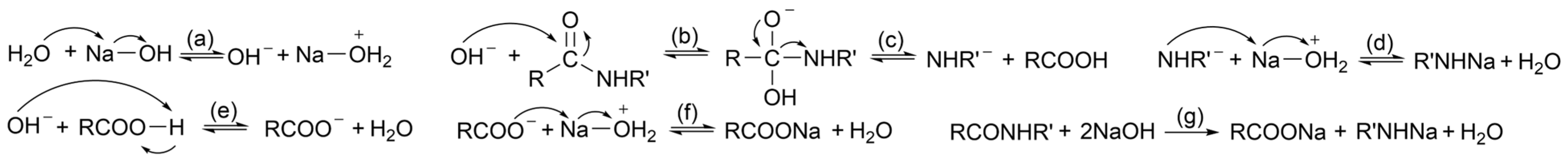
2.4.1. Alkaline Hydrolysis
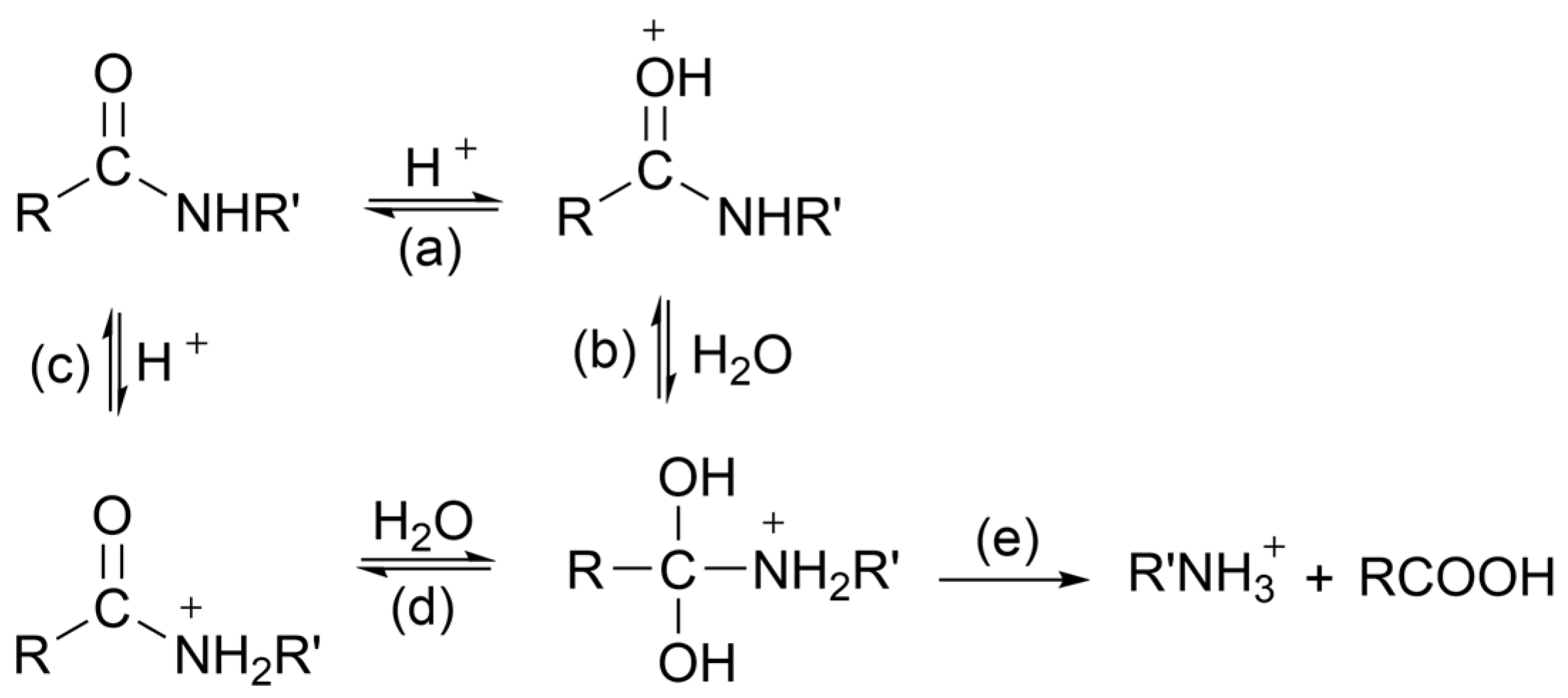
2.4.2. Acidic Hydrolysis
2.4.3. Hydrothermal Reaction
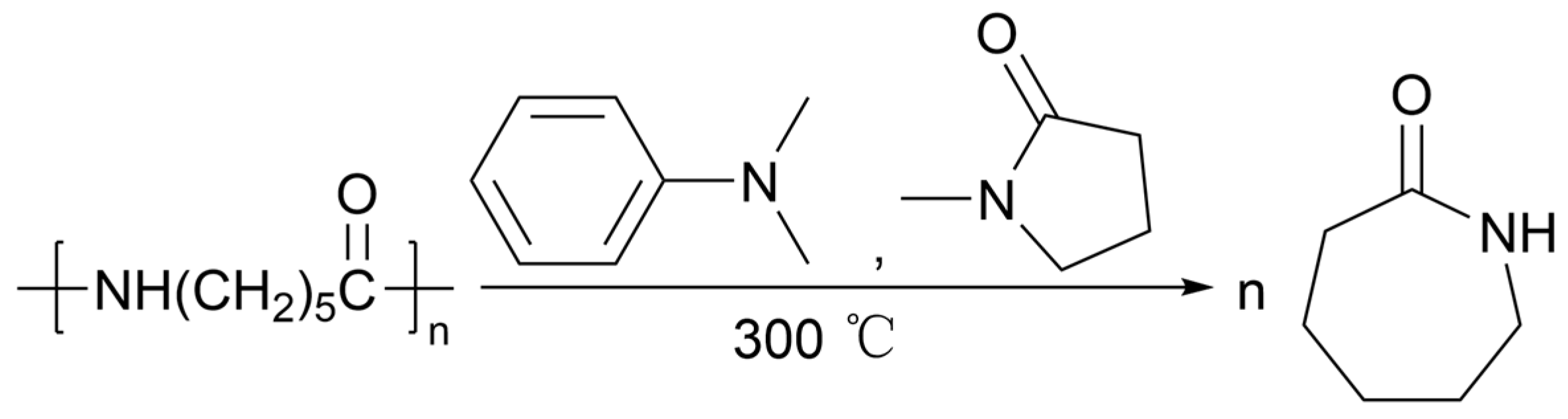
2.4.4. Pyrolysis under Alkali Catalysis
2.4.5. Microwave-Assisted Hydrolysis
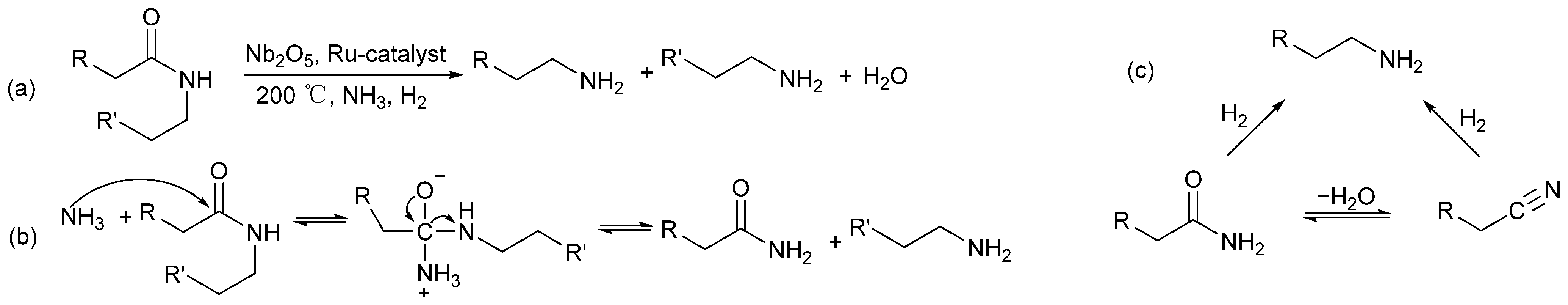
2.4.6. Ammonolysis
2.4.7. Alcoholysis
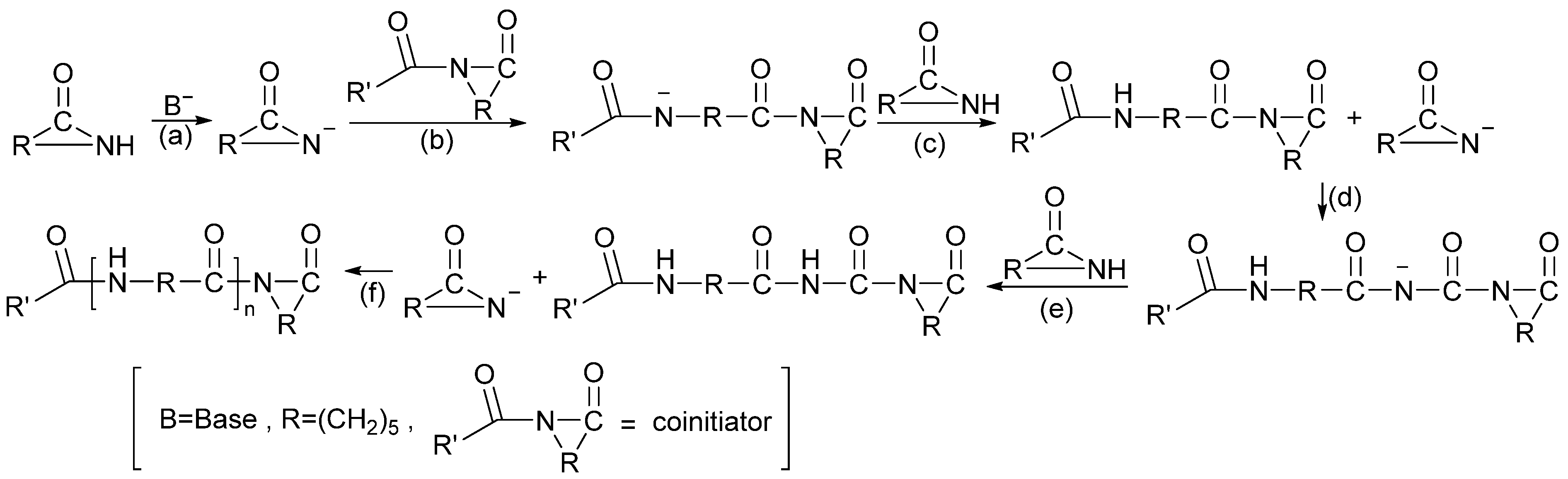
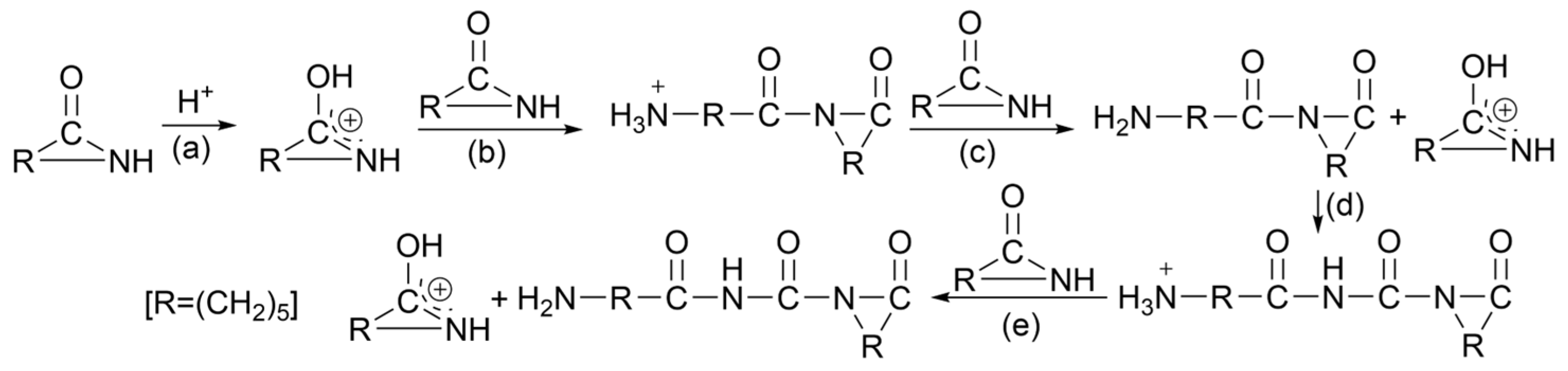
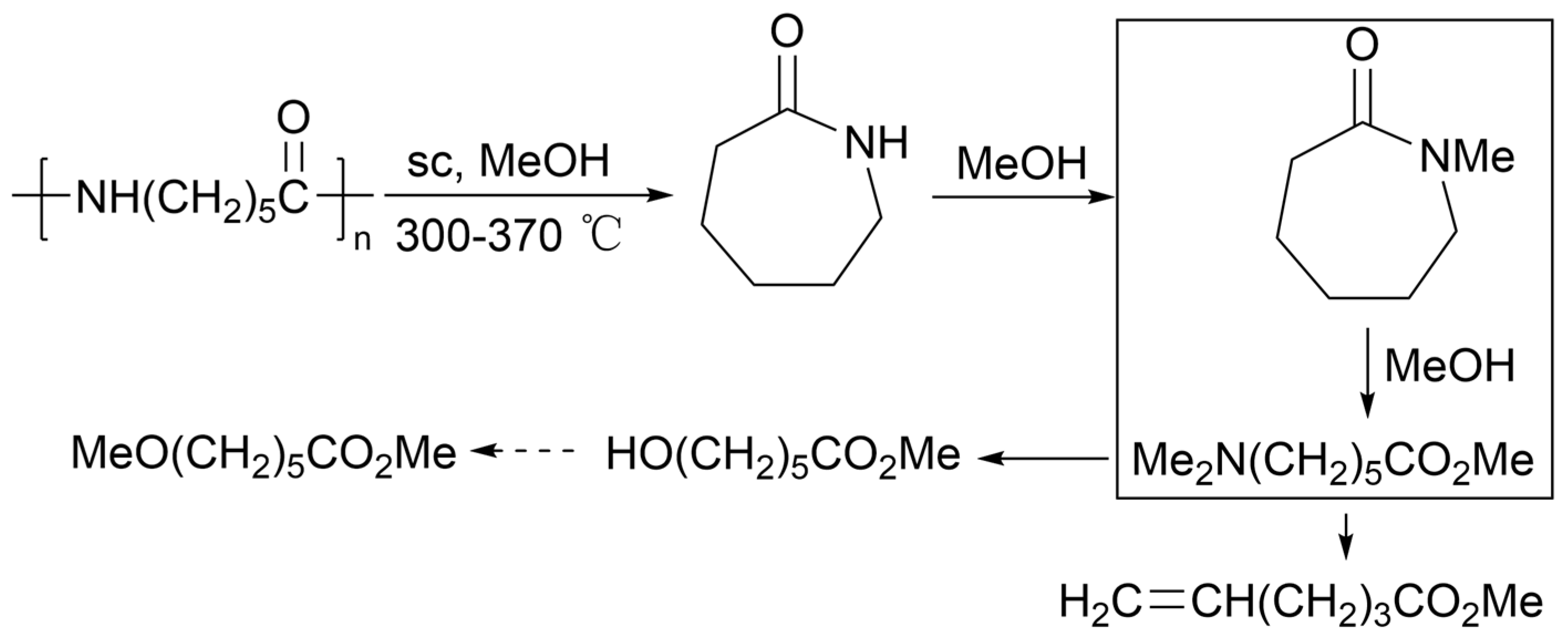
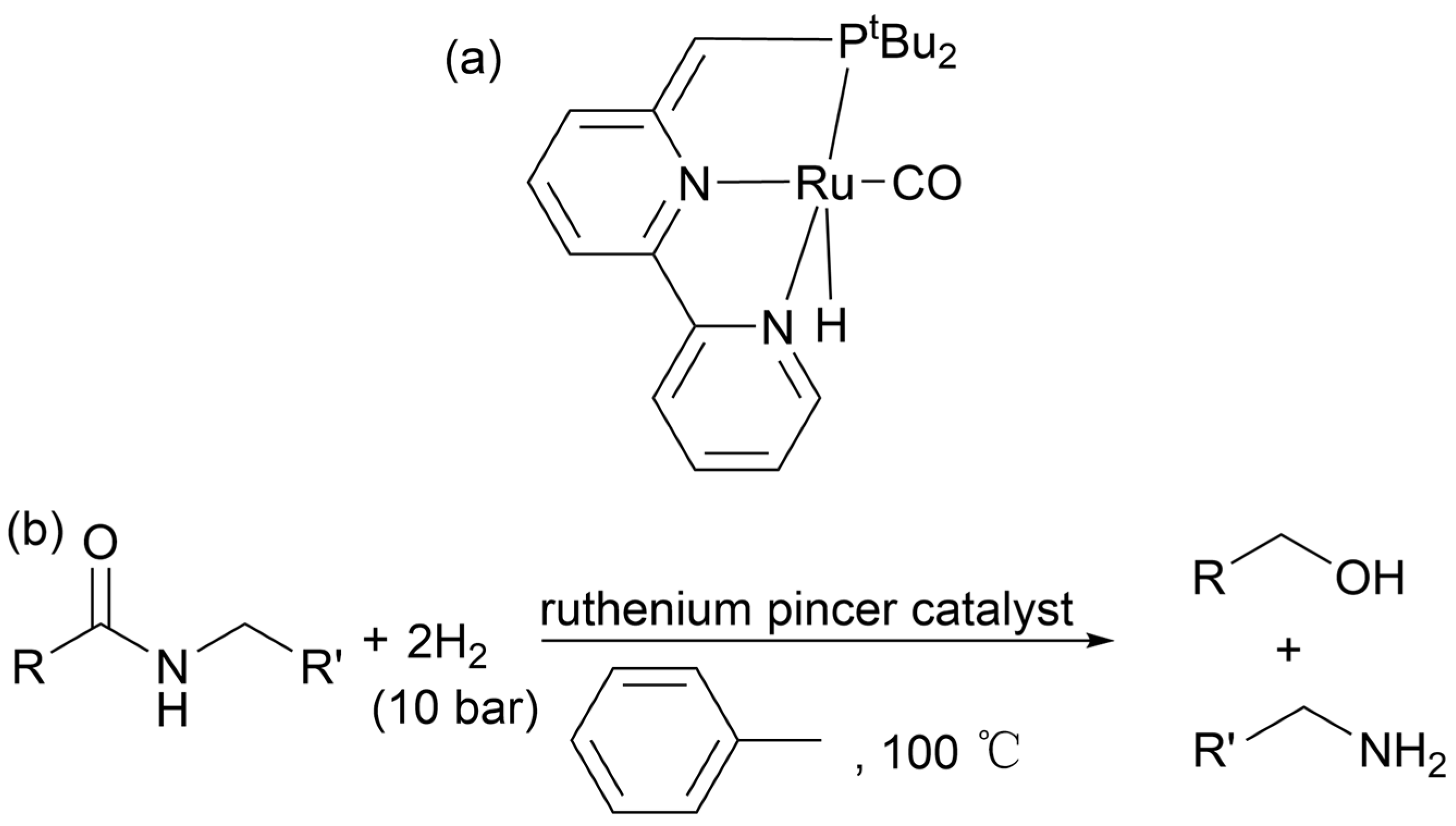
2.4.8. Hydrogen Dissolving Polymerization
2.4.9. Depolymerization in Ionic Liquids
3. Environmental Degradation
3.1. Weather Degradation
3.1.1. Thermal Oxidative Degradation
3.1.2. Other Natural Environmental Degradations
3.2. Enzymatic Hydrolysis
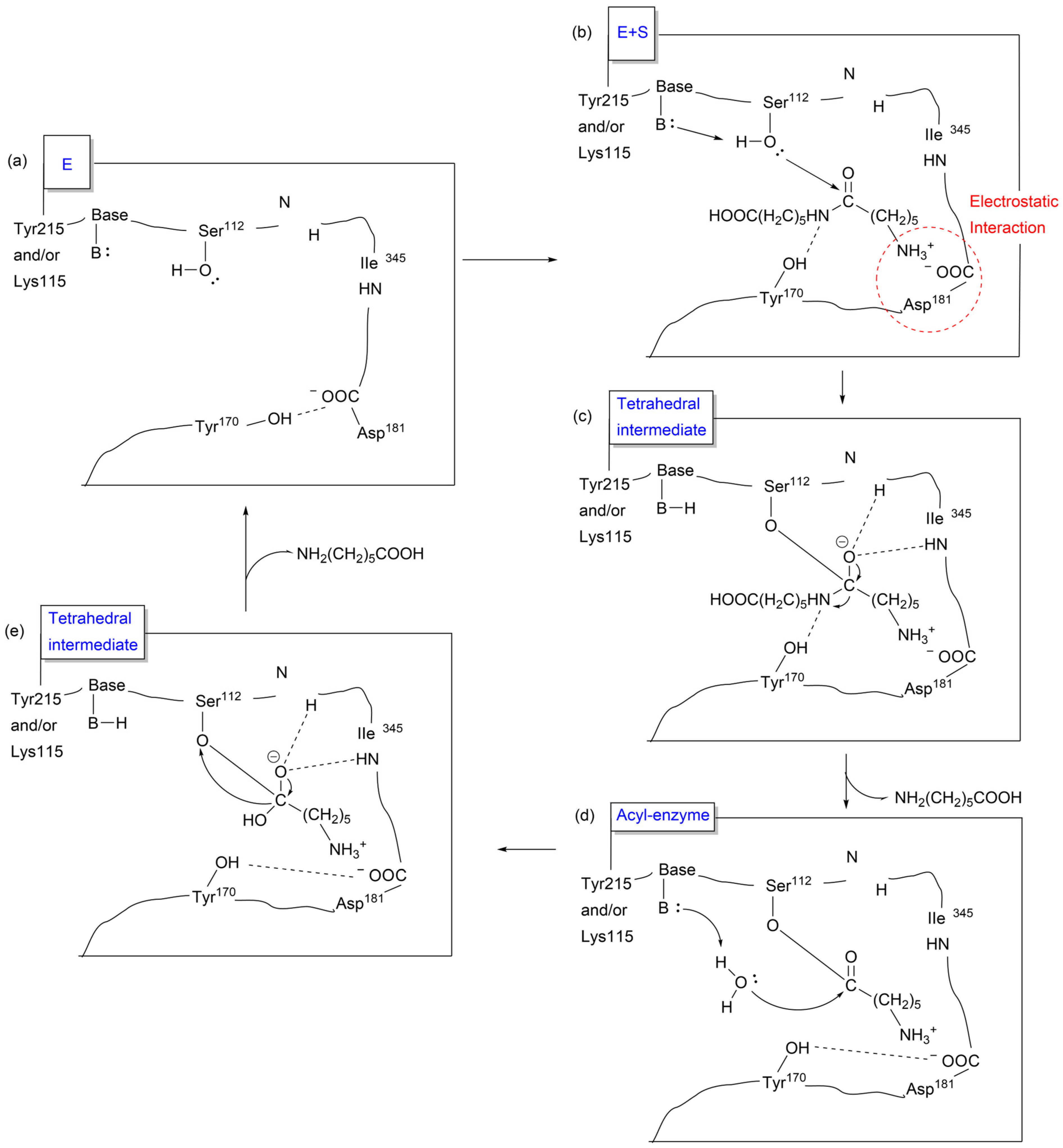
3.2.1. Proteases
3.2.2. Cutinases
3.2.3. Amidase
4. Prospects
4.1. Find Enzymes That Can Degrade High-Molecular-Weight Polyamides
4.2. Explore Methods for Recycling Polyamides under Mild Conditions
4.3. Synthesize Degradable Polyamides in Natural Environments through Copolymerization and Molecular Design
4.4. Degradable Bio-Based Polyamides
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pervaiz, M.; Faruq, M.; Jawaid, M.; Sain, M. Polyamides: Developments and applications towards next-generation engineered plastics. Curr. Org. Synth. 2017, 14, 146–155. [Google Scholar] [CrossRef]
- Troughton, M.J. Handbook of Plastics Joining: A Practical Guide; William Andrew: New York, NY, USA, 2008. [Google Scholar]
- McKeen, L.W. The Effect of UV Light and Weather on Plastics and Elastomers; William Andrew: New York, NY, USA, 2019. [Google Scholar]
- Babu, R.P.; O’connor, K.; Seeram, R. Current progress on bio-based polymers and their future trends. Prog. Biomater. 2013, 2, 8. [Google Scholar] [CrossRef]
- Kawaguchi, H.; Ogino, C.; Kondo, A. Microbial conversion of biomass into bio-based polymers. Bioresour. Technol. 2017, 245, 1664–1673. [Google Scholar] [CrossRef]
- Wang, L.; Li, G.; Deng, Y. Diamine biosynthesis: Research progress and application prospects. Appl. Environ. Microbiol. 2020, 86, e01972-20. [Google Scholar] [CrossRef]
- Yang, H.; Wentao, L. Bio-based Polyamide 56: Recent advances in basic and applied research. Polym. Eng. Sci. 2023, 63, 2484–2497. [Google Scholar] [CrossRef]
- Vert, M.; Doi, Y.; Hellwich, K.-H.; Hess, M.; Hodge, P.; Kubisa, P.; Rinaudo, M.; Schué, F. Terminology for biorelated polymers and applications (IUPAC Recommendations 2012). Pure Appl. Chem. 2012, 84, 377–410. [Google Scholar] [CrossRef]
- Lee, J.A.; Kim, J.Y.; Ahn, J.H.; Ahn, Y.-J.; Lee, S.Y. Current advancements in the bio-based production of polyamides. Trends Chem. 2023, 5, 873–891. [Google Scholar] [CrossRef]
- Yang, K.; Liu, Y.; Zheng, Z.; Lu, G.; Tang, Z.; Chen, X. Synthesis and thermal degradation mechanism of a semi-aromatic copolyamide from renewable sources. Polym. Degrad. Stab. 2022, 203, 110089. [Google Scholar] [CrossRef]
- Haider, S.; Kausar, A.; Muhammad, B. Research advancement in high-performance polyamides and polyamide blends loaded with layered silicate. Polym. Plast. Technol. Eng. 2016, 55, 1536–1556. [Google Scholar] [CrossRef]
- Reglero Ruiz, J.A.; Trigo-López, M.; García, F.C.; García, J.M. Functional aromatic polyamides. Polymers 2017, 9, 414. [Google Scholar] [CrossRef]
- Turk, S.C.; Kloosterman, W.P.; Ninaber, D.K.; Kolen, K.P.; Knutova, J.; Suir, E.; Schurmann, M.; Raemakers-Franken, P.C.; Muller, M.; de Wildeman, S.M. Metabolic engineering toward sustainable production of nylon-6. ACS Synth. Biol. 2016, 5, 65–73. [Google Scholar] [CrossRef]
- Chanda, M.; Roy, S.K. Plastics Technology Handbook; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar]
- Sterner, E.S. Three Ways to Polyamides: The Impact of Polymerization Mechanism on Polymer Properties. J. Chem. Educ. 2019, 96, 2003–2008. [Google Scholar] [CrossRef]
- Katzer, J. Hydrolytic caprolactam polymerization–progress in dynamic simulation. Macromol. React. Eng. 2014, 8, 658–665. [Google Scholar] [CrossRef]
- Varghese, M.; Grinstaff, M.W. Beyond nylon 6: Polyamides via ring opening polymerization of designer lactam monomers for biomedical applications. Chem. Soc. Rev. 2022, 51, 8258–8275. [Google Scholar] [CrossRef]
- Fang, H.; Su, S.; Luo, Y.; Jiang, Y.; Luo, Z. Unveiling the Mechanisms of Hydrolytic Ring-Opening Polymerization of Caprolactam and Amino-Assisted Ring Opening of Cyclic Dimers: A DFT Study. Ind. Eng. Chem. Res. 2022, 62, 136–144. [Google Scholar] [CrossRef]
- Zou, W.; Xia, M.; Jiang, K.; Cao, Z.; Zhang, X.; Hu, X. Photo-oxidative degradation mitigated the developmental toxicity of polyamide microplastics to zebrafish larvae by modulating macrophage-triggered proinflammatory responses and apoptosis. Environ. Sci. Technol. 2020, 54, 13888–13898. [Google Scholar] [CrossRef]
- Nicholson, S.R.; Rorrer, N.A.; Carpenter, A.C.; Beckham, G.T. Manufacturing energy and greenhouse gas emissions associated with plastics consumption. Joule 2021, 5, 673–686. [Google Scholar] [CrossRef]
- Ellis, L.D.; Rorrer, N.A.; Sullivan, K.P.; Otto, M.; McGeehan, J.E.; Román-Leshkov, Y.; Wierckx, N.; Beckham, G.T. Chemical and biological catalysis for plastics recycling and upcycling. Nat. Catal. 2021, 4, 539–556. [Google Scholar] [CrossRef]
- Kind, S.; Neubauer, S.; Becker, J.; Yamamoto, M.; Völkert, M.; von Abendroth, G.; Zelder, O.; Wittmann, C. From zero to hero–production of bio-based nylon from renewable resources using engineered Corynebacterium glutamicum. Metab. Eng. 2014, 25, 113–123. [Google Scholar] [CrossRef]
- Scopetani, C.; Chelazzi, D.; Cincinelli, A.; Esterhuizen-Londt, M. Assessment of microplastic pollution: Occurrence and characterisation in Vesijärvi lake and Pikku Vesijärvi pond, Finland. Environ. Monit. Assess. 2019, 191, 652. [Google Scholar] [CrossRef]
- Magni, S.; Binelli, A.; Pittura, L.; Avio, C.G.; Della Torre, C.; Parenti, C.C.; Gorbi, S.; Regoli, F. The fate of microplastics in an Italian Wastewater Treatment Plant. Sci. Total Environ. 2019, 652, 602–610. [Google Scholar] [CrossRef]
- Cincinelli, A.; Scopetani, C.; Chelazzi, D.; Martellini, T.; Pogojeva, M.; Slobodnik, J. Microplastics in the Black Sea sediments. Sci. Total Environ. 2021, 760, 143898. [Google Scholar] [CrossRef]
- Scopetani, C.; Chelazzi, D.; Martellini, T.; Pellinen, J.; Ugolini, A.; Sarti, C.; Cincinelli, A. Occurrence and characterization of microplastic and mesoplastic pollution in the Migliarino San Rossore, Massaciuccoli Nature Park (Italy). Mar. Pollut. Bull. 2021, 171, 112712. [Google Scholar] [CrossRef]
- Li, X.; Chen, L.; Mei, Q.; Dong, B.; Dai, X.; Ding, G.; Zeng, E.Y. Microplastics in sewage sludge from the wastewater treatment plants in China. Water Res. 2018, 142, 75–85. [Google Scholar] [CrossRef]
- Maheswaran, B.; Karmegam, N.; Al-Ansari, M.; Subbaiya, R.; Al-Humaid, L.; Raj, J.S.; Govarthanan, M. Assessment, characterization, and quantification of microplastics from river sediments. Chemosphere 2022, 298, 134268. [Google Scholar] [CrossRef]
- Pietrelli, L.; Di Gennaro, A.; Menegoni, P.; Lecce, F.; Poeta, G.; Acosta, A.T.; Battisti, C.; Iannilli, V. Pervasive plastisphere: First record of plastics in egagropiles (Posidonia spheroids). Environ. Pollut. 2017, 229, 1032–1036. [Google Scholar] [CrossRef]
- Li, H.; Yang, Z.; Jiang, F.; Li, L.; Li, Y.; Zhang, M.; Qi, Z.; Ma, R.; Zhang, Y.; Fang, J. Detection of microplastics in domestic and fetal pigs’ lung tissue in natural environment: A preliminary study. Environ. Res. 2023, 216, 114623. [Google Scholar] [CrossRef]
- Lusher, A.L.; Mchugh, M.; Thompson, R.C. Occurrence of microplastics in the gastrointestinal tract of pelagic and demersal fish from the English Channel. Mar. Pollut. Bull. 2013, 67, 94–99. [Google Scholar] [CrossRef]
- Trindade, P.A.; Brabo, L.D.; Andrades, R.; Azevedo-Santos, V.M.; Andrade, M.C.; Candore, L.; Cabigliera, S.B.; Chelazzi, D.; Cincinelli, A.; Jeffres, C.A. First record of plastic ingestion by a freshwater stingray. Sci. Total Environ. 2023, 880, 163199. [Google Scholar] [CrossRef]
- Martinez-Tavera, E.; Duarte-Moro, A.; Sujitha, S.; Rodriguez-Espinosa, P.; Rosano-Ortega, G.; Exposito, N. Microplastics and metal burdens in freshwater Tilapia (Oreochromis niloticus) of a metropolitan reservoir in Central Mexico: Potential threats for human health. Chemosphere 2021, 266, 128968. [Google Scholar] [CrossRef]
- Tournier, V.; Duquesne, S.; Guillamot, F.; Cramail, H.; Taton, D.; Marty, A.; André, I. Enzymes’ power for plastics degradation. Chem. Rev. 2023, 123, 5612–5701. [Google Scholar] [CrossRef]
- Soares dos Santos, L.; Souto Martinez, A. A Simpler Lotka-Volterra Model Under Microplastic Particles Influence. Braz. J. Phys. 2023, 53, 47. [Google Scholar] [CrossRef]
- Yang, S.; Cheng, Y.; Liu, T.; Huang, S.; Yin, L.; Pu, Y.; Liang, G. Impact of waste of COVID-19 protective equipment on the environment, animals and human health: A review. Environ. Chem. Lett. 2022, 20, 2951–2970. [Google Scholar] [CrossRef]
- Van Sebille, E.; Wilcox, C.; Lebreton, L.; Maximenko, N.; Hardesty, B.D.; Van Franeker, J.A.; Eriksen, M.; Siegel, D.; Galgani, F.; Law, K.L. A global inventory of small floating plastic debris. Environ. Res. Lett. 2015, 10, 124006. [Google Scholar] [CrossRef]
- Bhatt, P.; Pathak, V.M.; Bagheri, A.R.; Bilal, M. Microplastic contaminants in the aqueous environment, fate, toxicity consequences, and remediation strategies. Environ. Res. 2021, 200, 111762. [Google Scholar] [CrossRef]
- Hirschberg, V.; Rodrigue, D. Recycling of polyamides: Processes and conditions. J. Polym. Sci. 2023, 61, 1937–1958. [Google Scholar] [CrossRef]
- Li, H.; Aguirre-Villegas, H.A.; Allen, R.D.; Bai, X.; Benson, C.H.; Beckham, G.T.; Bradshaw, S.L.; Brown, J.L.; Brown, R.C.; Cecon, V.S. Expanding plastics recycling technologies: Chemical aspects, technology status and challenges. Green Chem. 2022, 24, 8899–9002. [Google Scholar] [CrossRef]
- Zheng, Y.; Yanful, E.K.; Bassi, A.S. A review of plastic waste biodegradation. Crit. Rev. Biotechnol. 2005, 25, 243–250. [Google Scholar] [CrossRef]
- Müller, R.-J.; Kleeberg, I.; Deckwer, W.-D. Biodegradation of polyesters containing aromatic constituents. J. Biotechnol. 2001, 86, 87–95. [Google Scholar] [CrossRef]
- Bonhomme, S.; Cuer, A.; Delort, A.; Lemaire, J.; Sancelme, M.; Scott, G. Environmental biodegradation of polyethylene. Polym. Degrad. Stab. 2003, 81, 441–452. [Google Scholar] [CrossRef]
- Arnaud, R.; Dabin, P.; Lemaire, J.; Al-Malaika, S.; Chohan, S.; Coker, M.; Scott, G.; Fauve, A.; Maaroufi, A. Photooxidation and biodegradation of commercial photodegradable polyethylenes. Polym. Degrad. Stab. 1994, 46, 211–224. [Google Scholar] [CrossRef]
- Nishida, H.; Tokiwa, Y. Distribution of poly (β-hydroxybutyrate) and poly (ε-caprolactone) aerobic degrading microorganisms in different environments. J. Environ. Polym. Degrad. 1993, 1, 227–233. [Google Scholar] [CrossRef]
- Oda, K.; Wlodawer, A. Development of Enzyme-Based Approaches for Recycling PET on an Industrial Scale. Biochemistry 2024, 63, 369–401. [Google Scholar] [CrossRef]
- Wei, R.; Zimmermann, W. Microbial enzymes for the recycling of recalcitrant petroleum-based plastics: How far are we? Microb. Biotechnol. 2017, 10, 1308–1322. [Google Scholar] [CrossRef] [PubMed]
- Koshti, R.; Mehta, L.; Samarth, N. Biological recycling of polyethylene terephthalate: A mini-review. J. Polym. Environ. 2018, 26, 3520–3529. [Google Scholar] [CrossRef]
- Carniel, A.; de Abreu Waldow, V.; de Castro, A.M. A comprehensive and critical review on key elements to implement enzymatic PET depolymerization for recycling purposes. Biotechnol. Adv. 2021, 52, 107811. [Google Scholar] [CrossRef]
- Zimmermann, W. Biocatalytic recycling of polyethylene terephthalate plastic. Philos. Trans. R. Soc. A 2020, 378, 20190273. [Google Scholar] [CrossRef]
- Carr, C.M.; Clarke, D.J.; Dobson, A.D. Microbial polyethylene terephthalate hydrolases: Current and future perspectives. Front. Microbiol. 2020, 11, 571265. [Google Scholar] [CrossRef] [PubMed]
- Kakudo, S.; Negoro, S.; Urabe, I.; Okada, H. Nylon oligomer degradation gene, nylC, on plasmid pOAD2 from a Flavobacterium strain encodes endo-type 6-aminohexanoate oligomer hydrolase: Purification and characterization of the nylC gene product. Appl. Environ. Microbiol. 1993, 59, 3978–3980. [Google Scholar] [CrossRef]
- Kawasaki, N.; Nakayama, A.; Yamano, N.; Takeda, S.; Kawata, Y.; Yamamoto, N.; Aiba, S.-I. Synthesis, thermal and mechanical properties and biodegradation of branched polyamide 4. Polymer 2005, 46, 9987–9993. [Google Scholar] [CrossRef]
- Yamano, N.; Nakayama, A.; Kawasaki, N.; Yamamoto, N.; Aiba, S. Mechanism and characterization of polyamide 4 degradation by Pseudomonas sp. J. Polym. Environ. 2008, 16, 141–146. [Google Scholar] [CrossRef]
- Hashimoto, K.; Hamano, T.; Okada, M. Degradation of several polyamides in soils. J. Appl. Polym. Sci. 1994, 54, 1579–1583. [Google Scholar] [CrossRef]
- Wang, M.; Li, Y.; Zheng, L.; Hu, T.; Yan, M.; Wu, C. Recycling and depolymerisation of poly (ethylene terephthalate): A review. Polym. Chem. 2023, 15, 585–608. [Google Scholar] [CrossRef]
- Fuchs, H.; Ritz, J.; Neubauer, G. Recovery of Caprolactam from Oligomers and/or Polymers of Caprolactam. U.S. Patent No. 5,700,358, 23 December 1997. [Google Scholar]
- Kotek, R. Semi-Continuous Depolymerization of Nylon 6 Polymer. U.S. Patent No. 5,294,707, 15 March 1994. [Google Scholar]
- Corbin, T.F.; Handermann, A.C.; Kotek, R.; Porter, W.D.; Dellinger, J.A.; Davis, E.A. Reclaiming Epsilon-Caprolactam from Nylon 6 Carpet. U.S. Patent No. 5,977,193, 2 November 1999. [Google Scholar]
- Fuchs, H.; Neubauer, G.; Ritz, J.; Priester, C.-U. Recovery of Caprolactam from Polycaprolactam. U.S. Patent No. 5,359,062, 25 October 1994. [Google Scholar]
- Moran, E.F., Jr.; McKinney, R.J. Conversion of Nylon 6 and/or Nylon 6, 6 to Adipic Acid. U.S. Patent No. 5,468,900, 21 November 1995. [Google Scholar]
- Moran, E.F., Jr. Nylon Component Reclamation. U.S. Patent No. 5,266,694, 30 November 1993. [Google Scholar]
- Halderit, A.H.; Booij, M.; Hendrix, J.A.; Frentzen, Y.H. Reclaiming ε-Caprolactam from Carpet Waste. U.S. Patent No. 5,556,890, 17 September 1996. [Google Scholar]
- Thomissen, P.J. Depolymerization of Polyamides. U.S. Patent No. 6087494A, July 2000. [Google Scholar]
- Frentzen, Y.H.; Thijert, M.P.; Zwart, R.L. Process for the Recovery of Caprolactam from Waste Containing Nylon. U.S. Patent No. 6,111,099, 29 August 2000. [Google Scholar]
- Dijkstra, H.; van Beukering, P.; Brouwer, R. In the business of dirty oceans: Overview of startups and entrepreneurs managing marine plastic. Mar. Pollut. Bull. 2021, 162, 111880. [Google Scholar] [CrossRef] [PubMed]
- Charter, M.; Carruthers, R. Products from waste fishing nets: Accessories, clothing, footwear, home ware and recreation. Circular Ocean. 2022, 36–37. [Google Scholar]
- Uddin, M.; Williams, D.; Blencowe, A. Recycling of selective laser sintering waste nylon powders into fused filament fabrication parts reinforced with Mg particles. Polymers 2021, 13, 2046. [Google Scholar] [CrossRef] [PubMed]
- Al-Mazrouei, N.; Al-Marzouqi, A.H.; Ahmed, W. Characterization and sustainability potential of recycling 3D-printed nylon composite wastes. Sustainability 2022, 14, 10458. [Google Scholar] [CrossRef]
- Braun, M.; Levy, A.; Sifniades, S. Recycling nylon 6 carpet to caprolactam. Polym. Plast. Technol. Eng. 1999, 38, 471–484. [Google Scholar] [CrossRef]
- Bockhorn, H.; Donner, S.; Gernsbeck, M.; Hornung, A.; Hornung, U. Pyrolysis of polyamide 6 under catalytic conditions and its application to reutilization of carpets. J. Anal. Appl. Pyrolysis 2001, 58, 79–94. [Google Scholar] [CrossRef]
- Sotayo, A.; Green, S.; Turvey, G. Carpet recycling: A review of recycled carpets for structural composites. Environ. Technol. Innov. 2015, 3, 97–107. [Google Scholar] [CrossRef]
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef] [PubMed]
- Soto-Salcido, L.A.; Pihlajamäki, A.; Mänttäri, M. Reuse of end—of—Life membranes through accelerated polyamide degradation. Waste Manag. 2023, 171, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Aldousiri, B.; Dhakal, H.; Onuh, S.; Zhang, Z.; Bennett, N. Nanoindentation behaviour of layered silicate filled spent polyamide-12 nanocomposites. Polym. Test. 2011, 30, 688–692. [Google Scholar] [CrossRef]
- Patankar, K.A.; Ginzburg, V.V.; Billovits, G.F. Rheological characterization of filled polyamide 11 and polyamide 12 solutions in polyols. J. Appl. Polym. Sci. 2019, 136, 48244. [Google Scholar] [CrossRef]
- Soni, V.K.; Singh, G.; Vijayan, B.K.; Chopra, A.; Kapur, G.S.; Ramakumar, S. Thermochemical recycling of waste plastics by pyrolysis: A review. Energy Fuels 2021, 35, 12763–12808. [Google Scholar] [CrossRef]
- Perna, A.; Angotzi, G.N.; Berdondini, L.; Ribeiro, J.F. Advancing the interfacing performances of chronically implantable neural probes in the era of CMOS neuroelectronics. Front. Neurosci. 2023, 17, 1275908. [Google Scholar] [CrossRef]
- Pannase, A.M.; Singh, R.K.; Ruj, B.; Gupta, P. Decomposition of polyamide via slow pyrolysis: Effect of heating rate and operating temperature on product yield and composition. J. Anal. Appl. Pyrolysis 2020, 151, 104886. [Google Scholar] [CrossRef]
- Holland, B.J.; Hay, J.N. Thermal degradation of nylon polymers. Polym. Int. 2000, 49, 943–948. [Google Scholar] [CrossRef]
- Liu, X.; Bertilsson, H. Recycling of ABS and ABS/PC blends. J. Appl. Polym. Sci. 1999, 74, 510–515. [Google Scholar] [CrossRef]
- Bernasconi, A.; Davoli, P.; Rossin, D.; Armanni, C. Effect of reprocessing on the fatigue strength of a fibreglass reinforced polyamide. Compos. Part A Appl. Sci. Manuf. 2007, 38, 710–718. [Google Scholar] [CrossRef]
- Reddy, M.M.; Vivekanandhan, S.; Misra, M.; Bhatia, S.K.; Mohanty, A.K. Biobased plastics and bionanocomposites: Current status and future opportunities. Prog. Polym. Sci. 2013, 38, 1653–1689. [Google Scholar] [CrossRef]
- Bockhorn, H.; Hornung, A.; Hornung, U.; Weichmann, J. Kinetic study on the non-catalysed and catalysed degradation of polyamide 6 with isothermal and dynamic methods. Thermochim. Acta 1999, 337, 97–110. [Google Scholar] [CrossRef]
- Kowalska, E.; Choroś, M.; Kuczyńska, L.; Wielgosz, Z. Recykling odpadów dywanów i wykładzin dywanowych. Polimery 2006, 51, 671–679. [Google Scholar] [CrossRef]
- Arif, Z.U.; Khalid, M.Y.; Sheikh, M.F.; Zolfagharian, A.; Bodaghi, M. Biopolymeric sustainable materials and their emerging applications. J. Environ. Chem. Eng. 2022, 10, 108159. [Google Scholar] [CrossRef]
- Bernasconi, A.; Rossin, D.; Armanni, C. Analysis of the effect of mechanical recycling upon tensile strength of a short glass fibre reinforced polyamide 6, 6. Eng. Fract. Mech. 2007, 74, 627–641. [Google Scholar] [CrossRef]
- Casado, J.A.; Carrascal, I.; Diego, S.; Polanco, J.A.; Gutiérrez-Solana, F.; García, A. Mechanical behavior of recycled reinforced polyamide railway fasteners. Polym. Compos. 2010, 31, 1142–1149. [Google Scholar] [CrossRef]
- Ferreira, C.T.; Fonseca, J.B.d.; Saron, C. Reciclagem de rejeitos de poli (tereftalato de etileno) (PET) e de poliamida (PA) por meio de extrusão reativa para a preparação de blendas. Polímeros 2011, 21, 118–122. [Google Scholar] [CrossRef]
- Moritzer, E.; Heiderich, G. Mechanical recycling of continuous fiber-reinforced thermoplastic sheets. In Proceedings of the AIP Conference Proceedings, Jeju Island, Republic of Korea, 7–11 June 2015. [Google Scholar]
- Zhao, P.; Xie, J.; Gu, F.; Sharmin, N.; Hall, P.; Fu, J. Separation of mixed waste plastics via magnetic levitation. Waste Manag. 2018, 76, 46–54. [Google Scholar] [CrossRef]
- Xie, J.; Zhang, C.; Jia, Y.; Fu, J.; Zhao, P. Automatic and continuous separation of mixed waste plastics via magneto-Archimedes levitation. Sep. Purif. Technol. 2022, 287, 120537. [Google Scholar] [CrossRef]
- Moreno, D.D.P.; de Camargo, R.V.; dos Santos Luiz, D.; Branco, L.T.P.; Grillo, C.C.; Saron, C. Composites of recycled polypropylene from cotton swab waste with pyrolyzed rice husk. J. Polym. Environ. 2021, 29, 350–362. [Google Scholar] [CrossRef]
- Moreno, D.D.P.; Saron, C. Low-density polyethylene/polyamide 6 blends from multilayer films waste. J. Appl. Polym. Sci. 2019, 136, 47456. [Google Scholar] [CrossRef]
- Lesiak, P.; Kisielowska, A.; Walkowiak, K.; Wiktorczyk, A.; Kramek, G.; Wypych, M.; Sadkowski, Ł.; Zieliński, J.; Paszkiewicz, S.; Irska, I. Preparation and characterization of polymer blends based on the wastes from automotive coverings. Polimery 2020, 65, 232–239. [Google Scholar] [CrossRef]
- Pietroluongo, M.; Padovano, E.; Frache, A.; Badini, C. Mechanical recycling of an end-of-life automotive composite component. Sustain. Mater. Technol. 2020, 23, e00143. [Google Scholar] [CrossRef]
- Demets, R.; Grodent, M.; Van Kets, K.; De Meester, S.; Ragaert, K. Macromolecular Insights into the Altered Mechanical Deformation Mechanisms of Non-Polyolefin Contaminated Polyolefins. Polymers 2022, 14, 239. [Google Scholar] [CrossRef]
- Krause, A.; Lange, A.; Ezrin, M.; Ruby, K. Plastics Analysis Guide: Chemical and Instrumental Methods; Hanser Publishers: Munich, Germany, 1983. [Google Scholar]
- Wang, Y. Fiber and textile waste utilization. Waste Biomass Valorization 2010, 1, 135–143. [Google Scholar] [CrossRef]
- Busquets-Fité, M.; Fernandez, E.; Janer, G.; Vilar, G.; Vázquez-Campos, S.; Zanasca, R.; Citterio, C.; Mercante, L.; Puntes, V. Exploring release and recovery of nanomaterials from commercial polymeric nanocomposites. J. Phys. Conf. Ser. 2013, 429, 012048. [Google Scholar] [CrossRef]
- Kartalis, C.; Poulakis, J.; Tsenoglou, C.; Papaspyrides, C. Pure component recovery from polyamide 6/6 6 mixtures by selective dissolution and reprecipitation. J. Appl. Polym. Sci. 2002, 86, 1924–1930. [Google Scholar] [CrossRef]
- Puhan, M.R.; Sutariya, B.; Karan, S. Revisiting the alkali hydrolysis of polyamide nanofiltration membranes. J. Membr. Sci. 2022, 661, 120887. [Google Scholar] [CrossRef]
- Derombise, G.; Van Schoors, L.V.; Davies, P. Degradation of aramid fibers under alkaline and neutral conditions: Relations between the chemical characteristics and mechanical properties. J. Appl. Polym. Sci. 2010, 116, 2504–2514. [Google Scholar] [CrossRef]
- Jun, B.-M.; Yoon, Y.; Park, C.M. Post-treatment of nanofiltration polyamide membrane through alkali-catalyzed hydrolysis to treat dyes in model wastewater. Water 2019, 11, 1645. [Google Scholar] [CrossRef]
- Khan, M.N. Experimental versus theoretical evidence for the rate-limiting steps in uncatalyzed and H+-and HO−-catalyzed hydrolysis of the amide bond. Int. J. Chem. Kinet. 2009, 41, 599–611. [Google Scholar] [CrossRef]
- East, A.L. On the hydrolysis mechanisms of amides and peptides. Int. J. Chem. Kinet. 2018, 50, 705–709. [Google Scholar] [CrossRef]
- Serpe, G.; Chaupart, N.; Verdu, J. Ageing of polyamide 11 in acid solutions. Polymer 1997, 38, 1911–1917. [Google Scholar] [CrossRef]
- Meyer, A.; Jones, N.; Lin, Y.; Kranbuehl, D. Characterizing and modeling the hydrolysis of polyamide-11 in a pH 7 water environment. Macromolecules 2002, 35, 2784–2798. [Google Scholar] [CrossRef]
- Hood, D.K. Monitoring and Modeling of Infiltration, Polymerization, and Degradation Phenomena in Polymeric Systems. Ph.D. Thesis, The College of William and Mary, Williamsburg, VA, USA, 1996. [Google Scholar]
- Ehrenstein, M.L.P. Polyamides x. 34: A New Class of Polymers between Polyethylene and Polyamides. Ph.D. Thesis, Technische Universität Clausthal, Clausthal, Germany, 2002. [Google Scholar]
- Moiseev, Y.V.; Zaikov, G.E. Chemical Resistance of Polymers in Aggressive Media; Springer Science & Business Media: New York, NY, USA, 1987. [Google Scholar]
- Hocker, S.; Rhudy, A.K.; Ginsburg, G.; Kranbuehl, D.E. Polyamide hydrolysis accelerated by small weak organic acids. Polymer 2014, 55, 5057–5064. [Google Scholar] [CrossRef]
- Reiter, G.; Strobl, G.R. Progress in Understanding of Polymer Crystallization; Springer: Berlin/Heidelberg, Germany, 2007; Volume 714. [Google Scholar]
- Wang, Z.-l.; Xu, J.-l.; Yuan, Q.; Shibraen, M.H.; Xu, J.; Yang, S.-G. Hydrothermal treatment of polyamide 6 with presence of lanthanum chloride. Chin. J. Polym. Sci. 2016, 34, 399–406. [Google Scholar] [CrossRef]
- Touchaleaume, F.; Soulestin, J.; Sclavons, M.; Devaux, J.; Cordenier, F.; Van Velthem, P.; Flat, J.; Lacrampe, M.-F.; Krawczak, P. Efficient one-step melt-compounding of copolyetheramide/pristine clay nanocomposites using water-injection as intercalating/exfoliating aid. Express Polym. Lett 2011, 5, 1085–1101. [Google Scholar] [CrossRef]
- Wang, Z.; Wu, Z.; Mai, S.; Yang, C.; Wang, X.; An, Y.; Zhou, Z. Research and applications of membrane bioreactors in China: Progress and prospect. Sep. Purif. Technol. 2008, 62, 249–263. [Google Scholar] [CrossRef]
- Goto, M. Chemical recycling of plastics using sub-and supercritical fluids. J. Supercrit. Fluids 2009, 47, 500–507. [Google Scholar] [CrossRef]
- Smith, R., Jr.; Fang, Z.; Inomata, H.; Arai, K. Phase behavior and reaction of nylon 6/6 in water at high temperatures and pressures. J. Appl. Polym. Sci. 2000, 76, 1062–1073. [Google Scholar] [CrossRef]
- Meng, L.; Zhang, Y.; Huang, Y.; Shibata, M.; Yosomiya, R. Studies on the decomposition behavior of nylon-66 in supercritical water. Polym. Degrad. Stab. 2004, 83, 389–393. [Google Scholar] [CrossRef]
- Chen, J.; Liu, G.; Jin, L.; Ni, P.; Li, Z.; He, H.; Xu, Y.; Zhang, J.; Dong, J. Catalytic hydrolysis of waste nylon 6 to produce ɛ-caprolactam in sub-critical water. J. Anal. Appl. Pyrolysis 2010, 87, 50–55. [Google Scholar] [CrossRef]
- Barontini, F.; Cozzani, V. Formation of hydrogen bromide and organobrominated compounds in the thermal degradation of electronic boards. J. Anal. Appl. Pyrolysis 2006, 77, 41–55. [Google Scholar] [CrossRef]
- Iwaya, T.; Sasaki, M.; Goto, M. Kinetic analysis for hydrothermal depolymerization of nylon 6. Polym. Degrad. Stab. 2006, 91, 1989–1995. [Google Scholar] [CrossRef]
- Ikushima, Y.; Hatakeda, K.; Sato, M.; Sato, O.; Arai, M. Innovation in a chemical reaction process using a supercritical water microreaction system: Environmentally friendly production of ε-caprolactam. Chem. Commun. 2002, 19, 2208–2209. [Google Scholar] [CrossRef]
- Ikushima, Y.; Sato, M. A one-step production of fine chemicals using supercritical water: An environmental benign application to the synthesis of monoterpene alcohol. Chem. Eng. Sci. 2004, 59, 4895–4901. [Google Scholar] [CrossRef]
- Levchik, S.V.; Weil, E.D.; Lewin, M. Thermal decomposition of aliphatic nylons. Polym. Int. 1999, 48, 532–557. [Google Scholar] [CrossRef]
- Zakharyan, E.; Maksimov, A. Pyrolysis of polyamide-containing materials. Process features and composition of reaction products. Russ. J. Appl. Chem. 2022, 95, 895–928. [Google Scholar] [CrossRef]
- Wang, N.; Wang, P. Study and application status of microwave in organic wastewater treatment—A review. Chem. Eng. J. 2016, 283, 193–214. [Google Scholar] [CrossRef]
- Češarek, U.; Pahovnik, D.; Žagar, E. Chemical recycling of aliphatic polyamides by microwave-assisted hydrolysis for efficient monomer recovery. ACS Sustain. Chem. Eng. 2020, 8, 16274. [Google Scholar] [CrossRef]
- Bäckström, E.; Odelius, K.; Hakkarainen, M. Microwave assisted selective hydrolysis of polyamides from multicomponent carpet waste. Glob. Chall. 2021, 5, 2000119. [Google Scholar] [CrossRef]
- Choi, H.-Y.; Choe, E.-K.; Yang, E.-K.; Jang, S.-W.; Park, C.-R. Characterization of synthetic polyamides by MALDI-TOF mass spectrometry. Bull. Korean Chem. Soc. 2007, 28, 2354–2358. [Google Scholar]
- McKinney, R.J. Ammonolysis of Nylon. U.S. Patent No. 5,302,756, 12 April 1994. [Google Scholar]
- McKinney, R.J. Lewis Acid Catalyzed Ammonolysis of Nylon. U.S. Patent No. 5,395,974, 7 March 1995. [Google Scholar]
- Coeck, R.; De Bruyne, A.; Borremans, T.; Stuyck, W.; De Vos, D.E. Ammonolytic hydrogenation of secondary amides: An efficient method for the recycling of long-chain polyamides. ACS Sustain. Chem. Eng. 2022, 10, 3048–3056. [Google Scholar] [CrossRef]
- Kamimura, A.; Kaiso, K.; Suzuki, S.; Oishi, Y.; Ohara, Y.; Sugimoto, T.; Kashiwagi, K.; Yoshimoto, M. Direct conversion of polyamides to ω-hydroxyalkanoic acid derivatives by using supercritical MeOH. Green Chem. 2011, 13, 2055–2061. [Google Scholar] [CrossRef]
- Kamimura, A.; Ikeda, K.; Suzuki, S.; Kato, K.; Akinari, Y.; Sugimoto, T.; Kashiwagi, K.; Kaiso, K.; Matsumoto, H.; Yoshimoto, M. Efficient Conversion of Polyamides to ω-Hydroxyalkanoic Acids: A New Method for Chemical Recycling of Waste Plastics. ChemSusChem 2014, 7, 2473–2477. [Google Scholar] [CrossRef]
- Matsumoto, H.; Akinari, Y.; Kaiso, K.; Kamimura, A. Efficient depolymerization and chemical conversion of polyamide 66 to 1, 6-hexanediol. J. Mater. Cycles Waste Manag. 2017, 19, 326–331. [Google Scholar] [CrossRef]
- Rylander, P.N. Hydrogenation Methods; Academic Press: Cambridge, MA, USA, 1985. [Google Scholar]
- Balaraman, E.; Gnanaprakasam, B.; Shimon, L.J.; Milstein, D. Direct hydrogenation of amides to alcohols and amines under mild conditions. J. Am. Chem. Soc. 2010, 132, 16756–16758. [Google Scholar] [CrossRef]
- Ito, M.; Sakaguchi, A.; Kobayashi, C.; Ikariya, T. Chemoselective hydrogenation of imides catalyzed by Cp* Ru (PN) complexes and its application to the asymmetric synthesis of paroxetine. J. Am. Chem. Soc. 2007, 129, 290–291. [Google Scholar] [CrossRef]
- Ito, M.; Kobayashi, C.; Himizu, A.; Ikariya, T. Highly enantioselective hydrogenative desymmetrization of bicyclic imides leading to multiply functionalized chiral cyclic compounds. J. Am. Chem. Soc. 2010, 132, 11414–11415. [Google Scholar] [CrossRef]
- Ito, M.; Ootsuka, T.; Watari, R.; Shiibashi, A.; Himizu, A.; Ikariya, T. Catalytic hydrogenation of carboxamides and esters by well-defined Cp* Ru complexes bearing a protic amine ligand. J. Am. Chem. Soc. 2011, 133, 4240–4242. [Google Scholar] [CrossRef]
- Takebayashi, S.; John, J.M.; Bergens, S.H. Desymmetrization of meso-cyclic imides via enantioselective monohydrogenation. J. Am. Chem. Soc. 2010, 132, 12832–12834. [Google Scholar] [CrossRef] [PubMed]
- John, J.M.; Bergens, S.H. A highly active catalyst for the hydrogenation of amides to alcohols and amines. Angew. Chem. Int. Ed. 2011, 50, 10377–10380. [Google Scholar] [CrossRef] [PubMed]
- John, J.M.; Loorthuraja, R.; Antoniuk, E.; Bergens, S.H. Catalytic hydrogenation of functionalized amides under basic and neutral conditions. Catal. Sci. Technol. 2015, 5, 1181–1186. [Google Scholar] [CrossRef]
- Cabrero-Antonino, J.R.; Alberico, E.; Drexler, H.-J.; Baumann, W.; Junge, K.; Junge, H.; Beller, M. Efficient base-free hydrogenation of amides to alcohols and amines catalyzed by well-defined pincer imidazolyl–ruthenium complexes. ACS Catal. 2016, 6, 47–54. [Google Scholar] [CrossRef]
- Miura, T.; Naruto, M.; Toda, K.; Shimomura, T.; Saito, S. Multifaceted catalytic hydrogenation of amides via diverse activation of a sterically confined bipyridine–ruthenium framework. Sci. Rep. 2017, 7, 1586. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Tan, X.; Long, J.; Xiong, X.; Yang, S.; Xue, P.; Lv, H.; Zhang, X. Direct catalytic hydrogenation of simple amides: A highly efficient approach from amides to amines and alcohols. Chem. A Eur. J. 2017, 23, 546–548. [Google Scholar] [CrossRef] [PubMed]
- Kita, Y.; Higuchi, T.; Mashima, K. Hydrogenation of amides catalyzed by a combined catalytic system of a Ru complex with a zinc salt. Chem. Commun. 2014, 50, 11211–11213. [Google Scholar] [CrossRef] [PubMed]
- Jayarathne, U.; Zhang, Y.; Hazari, N.; Bernskoetter, W.H. Selective iron-catalyzed deaminative hydrogenation of amides. Organometallics 2017, 36, 409–416. [Google Scholar] [CrossRef]
- Schneck, F.; Assmann, M.; Balmer, M.; Harms, K.; Langer, R. Selective hydrogenation of amides to amines and alcohols catalyzed by improved iron pincer complexes. Organometallics 2016, 35, 1931–1943. [Google Scholar] [CrossRef]
- Rezayee, N.M.; Samblanet, D.C.; Sanford, M.S. Iron-catalyzed hydrogenation of amides to alcohols and amines. ACS Catal. 2016, 6, 6377–6383. [Google Scholar] [CrossRef]
- Daw, P.; Chakraborty, S.; Garg, J.A.; Ben-David, Y.; Milstein, D. Direct synthesis of pyrroles by dehydrogenative coupling of diols and amines catalyzed by cobalt pincer complexes. Angew. Chem. 2016, 128, 14585–14589. [Google Scholar] [CrossRef]
- Papa, V.; Cabrero-Antonino, J.R.; Alberico, E.; Spanneberg, A.; Junge, K.; Junge, H.; Beller, M. Efficient and selective hydrogenation of amides to alcohols and amines using a well-defined manganese–PNN pincer complex. Chem. Sci. 2017, 8, 3576–3585. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.M.; Whyman, R. Review of methods for the catalytic hydrogenation of carboxamides. Chem. Rev. 2014, 114, 5477–5510. [Google Scholar] [CrossRef] [PubMed]
- Chardon, A.; Morisset, E.; Rouden, J.; Blanchet, J. Recent advances in amide reductions. Synthesis 2018, 50, 984–997. [Google Scholar]
- Kumar, A.; von Wolff, N.; Rauch, M.; Zou, Y.-Q.; Shmul, G.; Ben-David, Y.; Leitus, G.; Avram, L.; Milstein, D. Hydrogenative depolymerization of nylons. J. Am. Chem. Soc. 2020, 142, 14267–14275. [Google Scholar] [CrossRef] [PubMed]
- Kamimura, A.; Yamamoto, S. An efficient method to depolymerize polyamide plastics: A new use of ionic liquids. Org. Lett. 2007, 9, 2533–2535. [Google Scholar] [CrossRef] [PubMed]
- Fernández-González, V.; Andrade, J.M.; Ferreiro, B.; López-Mahía, P.; Muniategui-Lorenzo, S. Monitorization of polyamide microplastics weathering using attenuated total reflectance and microreflectance infrared spectrometry. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 263, 120162. [Google Scholar] [CrossRef] [PubMed]
- Shi, K.; Gao, L.; Tao, Y.; Ye, L.; Li, G.; Jie, G. Outdoor weathering behavior of polyamide 6 under various climates in C hina. J. Appl. Polym. Sci. 2017, 134, 44231. [Google Scholar] [CrossRef]
- Wang, M.; Song, H.; He, W.; Wang, J.; Zhou, D.; Guo, J. Thermal oxidation and thermal degradation kinetics of brominated epoxy resin/Sb2O3 flame retardant PA10T/GF composites. Polym. Eng. Sci. 2018, 58, 1583–1595. [Google Scholar] [CrossRef]
- Wang, J.; He, Y.; Jin, L.; Zhou, D.; Guo, J. Influence of thermo-oxidative aging on the dynamical mechanical properties and thermal degradation kinetics of glass fiber-reinforced PA10T composites. Polym. Eng. Sci. 2019, 59, 643–656. [Google Scholar] [CrossRef]
- Li, R.; Shi, K.; Ye, L.; Li, G. Intercalation structure and enhanced thermal oxidative stability of polyamide 6/graphene nanocomposites prepared through in situ polymerization. Ind. Eng. Chem. Res. 2017, 56, 13715–13724. [Google Scholar] [CrossRef]
- Yebra-Rodriguez, A.; Fernandez-Barranco, C.; La Rubia, M.; Yebra, A.; Rodríguez-Navarro, A.; Jiménez-Millán, J. Thermooxidative degradation of injection-moulded sepiolite/polyamide 66 nanocomposites. Mineral. Mag. 2014, 78, 1227–1239. [Google Scholar] [CrossRef]
- Zuo, X.; Shao, H.; Zhang, D.; Hao, Z.; Guo, J. Effects of thermal-oxidative aging on the flammability and thermal-oxidative degradation kinetics of tris (tribromophenyl) cyanurate flame retardant PA6/LGF composites. Polym. Degrad. Stab. 2013, 98, 2774–2783. [Google Scholar] [CrossRef]
- Sang, L.; Wang, C.; Wang, Y.; Wei, Z. Thermo-oxidative ageing effect on mechanical properties and morphology of short fibre reinforced polyamide composites–comparison of carbon and glass fibres. RSC Adv. 2017, 7, 43334–43344. [Google Scholar] [CrossRef]
- Boonkongkaew, M.; Hornsby, P.; Sirisinha, K. Structural effect of secondary antioxidants on mechanical properties and stabilization efficiency of polyamide 6/halloysite nanotube composites during heat ageing. J. Appl. Polym. Sci. 2017, 134, 45360. [Google Scholar] [CrossRef]
- Song, H.; Zhou, D.; Guo, J. Thermal-oxidative aging effects on the properties of long glass fiber reinforced polyamide 10T composites. Polym. Compos. 2018, 39, 2117–2125. [Google Scholar] [CrossRef]
- Li, R.; Shi, K.; Ye, L.; Li, G. Polyamide 6/graphene oxide-g-hindered phenol antioxidant nano-composites: Intercalation structure and synergistic thermal oxidative stabilization effect. Compos. Part B Eng. 2019, 162, 11–20. [Google Scholar] [CrossRef]
- White, G.V.; Clough, R.L.; Hochrein, J.M.; Bernstein, R. Application of isotopic labeling, and gas chromatography mass spectrometry, to understanding degradation products and pathways in the thermal-oxidative aging of Nylon 6.6. Polym. Degrad. Stab. 2013, 98, 2452–2465. [Google Scholar] [CrossRef]
- Smith, J.N.; White, G.V.; White, M.I.; Bernstein, R.; Hochrein, J.M. Characterization of volatile nylon 6.6 thermal-oxidative degradation products by selective isotopic labeling and cryo-GC/MS. J. Am. Soc. Mass Spectrom. 2012, 23, 1579–1592. [Google Scholar] [CrossRef]
- Shackleford, A.S.; Williams, R.J.; Brown, R.; Wingham, J.R.; Majewski, C. Degradation of Laser Sintered polyamide 12 parts due to accelerated exposure to ultraviolet radiation. Addit. Manuf. 2021, 46, 102132. [Google Scholar] [CrossRef]
- Deguchi, T.; Kakezawa, M.; Nishida, T. Nylon biodegradation by lignin-degrading fungi. Appl. Environ. Microbiol. 1997, 63, 329–331. [Google Scholar] [CrossRef]
- Deguchi, T.; Kitaoka, Y.; Kakezawa, M.; Nishida, T. Purification and characterization of a nylon-degrading enzyme. Appl. Environ. Microbiol. 1998, 64, 1366–1371. [Google Scholar] [CrossRef]
- Negoro, S.; Shinagawa, H.; Nakata, A.; Kinoshita, S.; Hatozaki, T.; Okada, H. Plasmid control of 6-aminohexanoic acid cyclic dimer degradation enzymes of Flavobacterium sp. K172. J. Bacteriol. 1980, 143, 238–245. [Google Scholar] [CrossRef]
- Negoro, S.; Taniguchi, T.; Kanaoka, M.; Kimura, H.; Okada, H. Plasmid-determined enzymatic degradation of nylon oligomers. J. Bacteriol. 1983, 155, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Tachibana, K.; Hashimoto, K.; Yoshikawa, M.; Okawa, H. Isolation and characterization of microorganisms degrading nylon 4 in the composted soil. Polym. Degrad. Stab. 2010, 95, 912–917. [Google Scholar] [CrossRef]
- Yamano, N.; Kawasaki, N.; Ida, S.; Nakayama, Y.; Nakayama, A. Biodegradation of polyamide 4 in vivo. Polym. Degrad. Stab. 2017, 137, 281–288. [Google Scholar] [CrossRef]
- Yamano, N.; Kawasaki, N.; Ida, S.; Nakayama, A. Biodegradation of polyamide 4 in seawater. Polym. Degrad. Stab. 2019, 166, 230–236. [Google Scholar] [CrossRef]
- Araujo, R.; Casal, M.; Cavaco-Paulo, A. Application of enzymes for textile fibres processing. Biocatal. Biotransform. 2008, 26, 332–349. [Google Scholar] [CrossRef]
- Almansa, E.; Heumann, S.; Eberl, A.; Kaufmann, F.; Cavaco-Paulo, A.; Gübitz, G.M. Surface hydrolysis of polyamide with a new polyamidase from Beauveria brongniartii. Biocatal. Biotransform. 2008, 26, 371–377. [Google Scholar] [CrossRef]
- Cavaco-Paulo, A.; Gubitz, G. Textile Processing with Enzymes; Woodhead Publishing: Cambridge, UK, 2003; Volume 29. [Google Scholar]
- Klun, U.; Friedrich, J.; Kržan, A. Polyamide-6 fibre degradation by a lignolytic fungus. Polym. Degrad. Stab. 2003, 79, 99–104. [Google Scholar] [CrossRef]
- Heumann, S.; Eberl, A.; Pobeheim, H.; Liebminger, S.; Fischer-Colbrie, G.; Almansa, E.; Cavaco-Paulo, A.; Gübitz, G.M. New model substrates for enzymes hydrolysing polyethyleneterephthalate and polyamide fibres. J. Biochem. Biophys. Methods 2006, 69, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.; Oliver, C.; Williams, D. The enzymatic degradation of polymers in vitro. J. Biomed. Mater. Res. 1987, 21, 991–1003. [Google Scholar] [CrossRef] [PubMed]
- Song, J.E.; Kim, H.R.; Lee, S.H. Effect of enzymatic hydrolysis on developing support of polyamide woven fabric for enzyme immobilization. Text. Res. J. 2019, 89, 1345–1360. [Google Scholar] [CrossRef]
- Miettinen-Oinonen, A.; Puolakka, A.; Buchert, J. Method for Modifying Polyamide. WO2005121438A2, 22 December 2005. [Google Scholar]
- Kanelli, M.; Vasilakos, S.; Ladas, S.; Symianakis, E.; Christakopoulos, P.; Topakas, E. Surface modification of polyamide 6.6 fibers by enzymatic hydrolysis. Process Biochem. 2017, 59, 97–103. [Google Scholar] [CrossRef]
- Silva, C.; Araújo, R.; Casal, M.; Gübitz, G.M.; Cavaco-Paulo, A. Influence of mechanical agitation on cutinases and protease activity towards polyamide substrates. Enzym. Microb. Technol. 2007, 40, 1678–1685. [Google Scholar] [CrossRef]
- Silva, C.; Cavaco-Paulo, A. Biotransformations in synthetic fibres. Biocatal. Biotransform. 2008, 26, 350–356. [Google Scholar] [CrossRef]
- Silva, C.M.; Carneiro, F.; O’Neill, A.; Fonseca, L.P.; Cabral, J.S.; Guebitz, G.; Cavaco-Paulo, A. Cutinase—A new tool for biomodification of synthetic fibers. J. Polym. Sci. Part A Polym. Chem. 2005, 43, 2448–2450. [Google Scholar] [CrossRef]
- Kato, D.-i.; Shibata, N.; Negoro, S. Structural analysis of nylon hydrolase and enzymatic approach to hydrolyze polyamide nylon. In Photo-Switched Biodegradation of Bioplastics in Marine Environments; Springer: Berlin/Heidelberg, Germany, 2023; pp. 121–133. [Google Scholar]
- Negoro, S.; Ohki, T.; Shibata, N.; Mizuno, N.; Wakitani, Y.; Tsurukame, J.; Matsumoto, K.; Kawamoto, I.; Takeo, M.; Higuchi, Y. X-ray crystallographic analysis of 6-aminohexanoate-dimer hydrolase: Molecular basis for the birth of a nylon oligomer-degrading enzyme. J. Biol. Chem. 2005, 280, 39644–39652. [Google Scholar] [CrossRef] [PubMed]
- Negoro, S.; Ohki, T.; Shibata, N.; Sasa, K.; Hayashi, H.; Nakano, H.; Yasuhira, K.; Kato, D.-I.; Takeo, M.; Higuchi, Y. Nylon-oligomer degrading enzyme/substrate complex: Catalytic mechanism of 6-aminohexanoate-dimer hydrolase. J. Mol. Biol. 2007, 370, 142–156. [Google Scholar] [CrossRef]
- Takehara, I.; Fujii, T.; Tanimoto, Y.; Kato, D.-I.; Takeo, M.; Negoro, S. Metabolic pathway of 6-aminohexanoate in the nylon oligomer-degrading bacterium Arthrobacter sp. KI72: Identification of the enzymes responsible for the conversion of 6-aminohexanoate to adipate. Appl. Microbiol. Biotechnol. 2018, 102, 801–814. [Google Scholar] [CrossRef]
- Yasuhira, K.; Shibata, N.; Mongami, G.; Uedo, Y.; Atsumi, Y.; Kawashima, Y.; Hibino, A.; Tanaka, Y.; Lee, Y.-H.; Kato, D.-I. X-ray crystallographic analysis of the 6-aminohexanoate cyclic dimer hydrolase: Catalytic mechanism and evolution of an enzyme responsible for nylon-6 byproduct degradation. J. Biol. Chem. 2010, 285, 1239–1248. [Google Scholar] [CrossRef]
- Negoro, S. Biodegradation of nylon and other synthetic polyamides. Biopolym. Online Biol. Chem. Biotechnol. Appl. 2005, 9, 395–415. [Google Scholar]
- Heumann, S.; Eberl, A.; Fischer-Colbrie, G.; Pobeheim, H.; Kaufmann, F.; Ribitsch, D.; Cavaco-Paulo, A.; Guebitz, G.M. A novel aryl acylamidase from Nocardia farcinica hydrolyses polyamide. Biotechnol. Bioeng. 2009, 102, 1003–1011. [Google Scholar] [CrossRef]
- Acero, E.H.; Ribitsch, D.; Rodriguez, R.D.; Dellacher, A.; Zitzenbacher, S.; Marold, A.; Greimel, K.J.; Schroeder, M.; Kandelbauer, A.; Heumann, S. Two-step enzymatic functionalisation of polyamide with phenolics. J. Mol. Catal. B Enzym. 2012, 79, 54–60. [Google Scholar] [CrossRef]
- Guo, Y.; Chen, S.; Su, L.; Wu, J.; Chen, J. Cloning, expression, and characterization of polyamidase from Nocardia farcinica and its application to polyamide modification. Biotechnol. Bioprocess Eng. 2013, 18, 1067–1075. [Google Scholar] [CrossRef]
- Biundo, A.; Subagia, R.; Maurer, M.; Ribitsch, D.; Syrén, P.-O.; Guebitz, G.M. Switched reaction specificity in polyesterases towards amide bond hydrolysis by enzyme engineering. RSC Adv. 2019, 9, 36217–36226. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.R.; Seo, H.Y. Enzymatic hydrolysis of polyamide fabric by using acylase. Text. Res. J. 2013, 83, 1181–1189. [Google Scholar] [CrossRef]
- Ragupathy, L.; Ziener, U.; Dyllick-Brenzinger, R.; von Vacano, B.; Landfester, K. Enzyme-catalyzed polymerizations at higher temperatures: Synthetic methods to produce polyamides and new poly (amide-co-ester) s. J. Mol. Catal. B Enzym. 2012, 76, 94–105. [Google Scholar] [CrossRef]
- Mohanty, A.; Misra, M.A.; Hinrichsen, G. Biofibres, biodegradable polymers and biocomposites: An overview. Macromol. Mater. Eng. 2000, 276, 1–24. [Google Scholar] [CrossRef]
- Jin, J.; Arciszewski, J.; Auclair, K.; Jia, Z. Polyethylene Biodegradation by Enzymes: Progress and Hurdles. Available at SSRN 4435398. Available online: https://papers.ssrn.com/sol3/papers.cfm?abstract_id=4435398 (accessed on 30 March 2024).
- Rahimi, A.; García, J.M. Chemical recycling of waste plastics for new materials production. Nat. Rev. Chem. 2017, 1, 46. [Google Scholar] [CrossRef]
- Chen, X. Synthesis, Characterization, Hydrolysis and Biodegradation Studies on Glycine-Containing Nylons and Polyesteramides; Stevens Institute of Technology: Hoboken, NJ, USA, 1993. [Google Scholar]
- Parente, J.F.; Sousa, V.I.; Marques, J.F.; Forte, M.A.; Tavares, C.J. Biodegradable polymers for microencapsulation systems. Adv. Polym. Technol. 2022, 2022, 4640379. [Google Scholar] [CrossRef]
- Miller, S.A. Sustainable polymers: Replacing polymers derived from fossil fuels. Polym. Chem. 2014, 5, 3117–3118. [Google Scholar] [CrossRef]
- Colucci, G.; Ostrovskaya, O.; Frache, A.; Martorana, B.; Badini, C. The effect of mechanical recycling on the microstructure and properties of PA66 composites reinforced with carbon fibers. J. Appl. Polym. Sci. 2015, 132, 42275. [Google Scholar] [CrossRef]

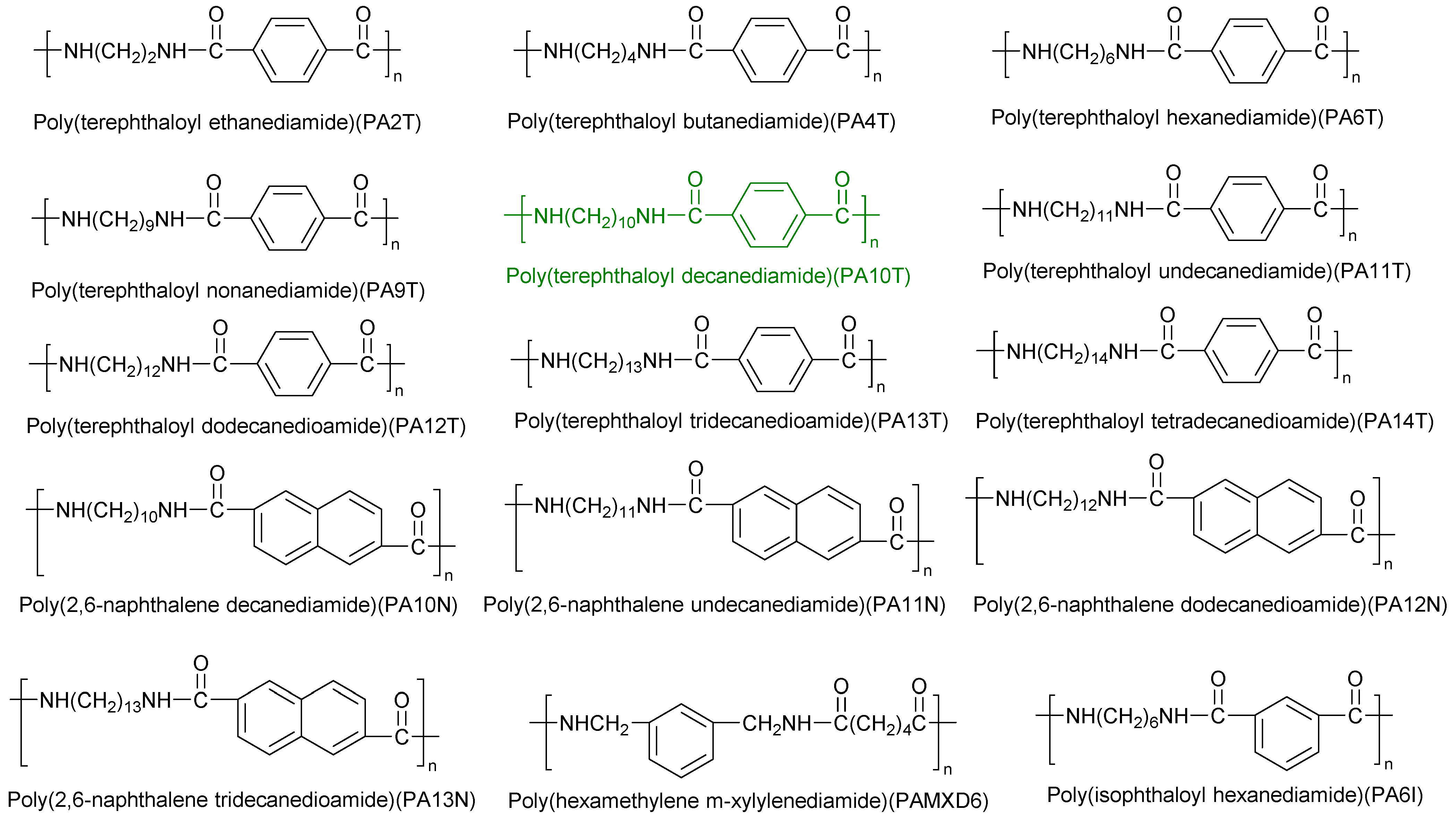
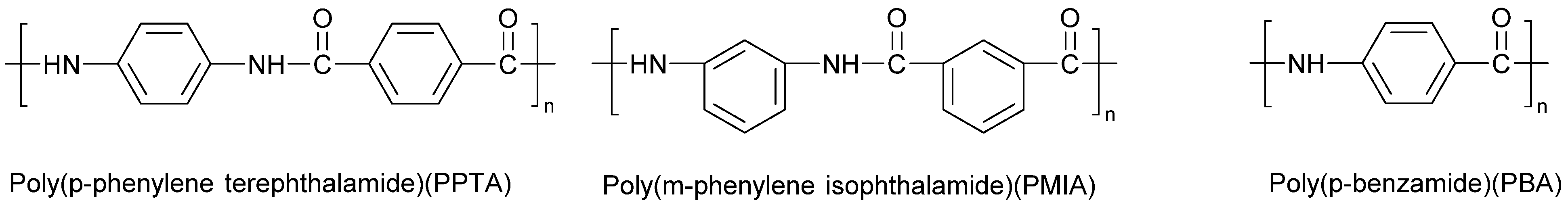
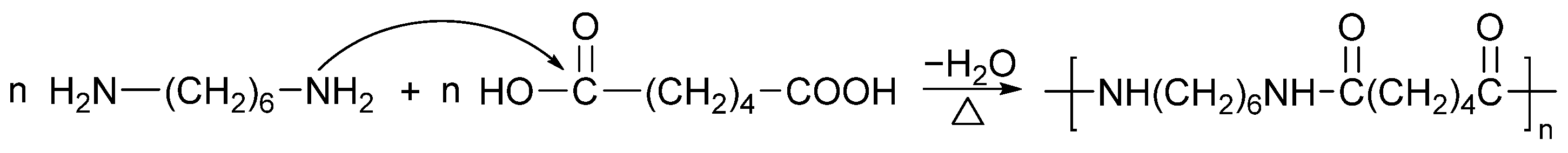
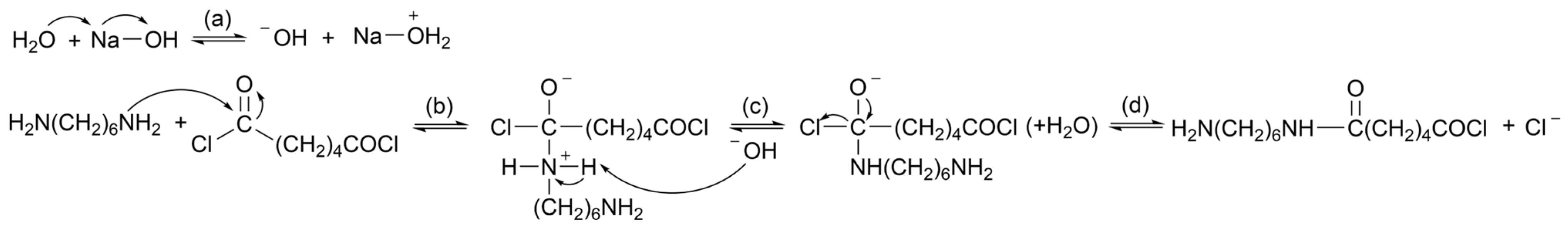
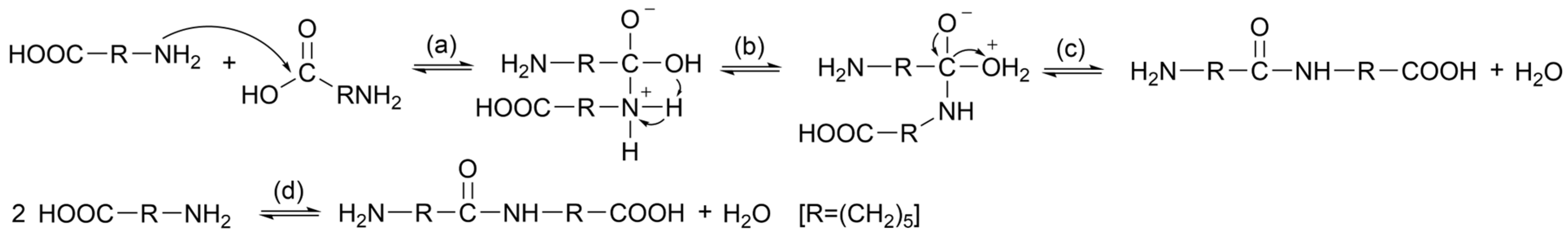





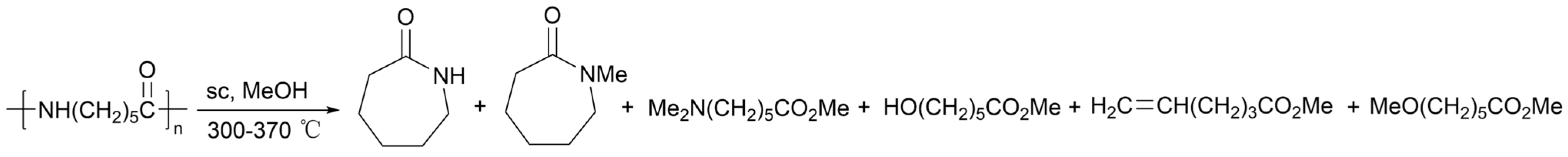
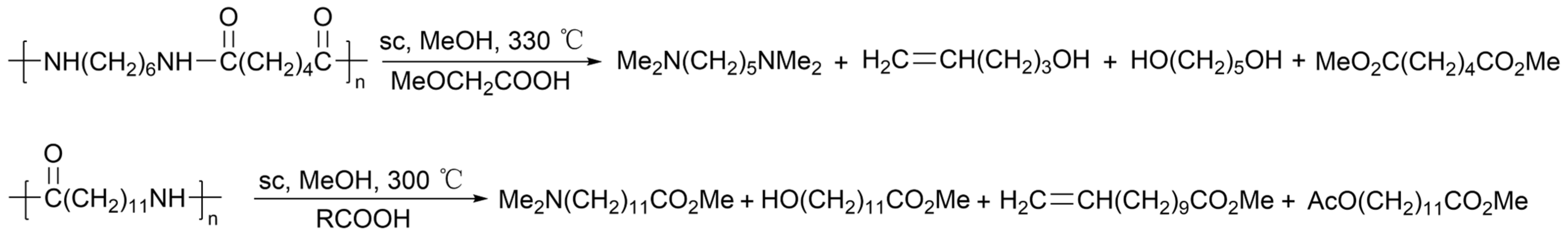
| PA Grade | Other Materials | Processing Method | Reference |
|---|---|---|---|
| PA-6, PA-66 | ABS, PC, PMMA | EXT, INJ | [84] |
| PA-66 | PAN | INJ | [85] |
| PA-66 | GF | INJ | [86] |
| PA-66 | GF | INJ | [87] |
| PA-66 | GF | INJ | [88] |
| PA | RUBBER | EXT | [89] |
| PA-6, PA-66 | RUBBER | - | [84] |
| PA-66 | GF | INJ | [21] |
| PA-6 | GF | EXT, INJ | [90] |
| PA-6 | ABS, PC, PET, PP, PTFE | - | [91] |
| PA-6 | PTFE, PU | - | [92] |
| PA-6 | LDPE, MAPE | EXT, INJ | [93] |
| PA-6 | LDPE, MAPE | EXT, INJ | [94] |
| PA-6 | PP, CHALK | EXT, INJ | [95] |
| PA-66 | GF | INJ | [96] |
| PA-6 | HDPE, LDPE, LLDPE, PP | INJ | [97] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, L.; Wang, M.; Li, Y.; Xiong, Y.; Wu, C. Recycling and Degradation of Polyamides. Molecules 2024, 29, 1742. https://doi.org/10.3390/molecules29081742
Zheng L, Wang M, Li Y, Xiong Y, Wu C. Recycling and Degradation of Polyamides. Molecules. 2024; 29(8):1742. https://doi.org/10.3390/molecules29081742
Chicago/Turabian StyleZheng, Lin, Mengjin Wang, Yaoqin Li, Yan Xiong, and Chonggang Wu. 2024. "Recycling and Degradation of Polyamides" Molecules 29, no. 8: 1742. https://doi.org/10.3390/molecules29081742
APA StyleZheng, L., Wang, M., Li, Y., Xiong, Y., & Wu, C. (2024). Recycling and Degradation of Polyamides. Molecules, 29(8), 1742. https://doi.org/10.3390/molecules29081742






