Antiprotozoal Activity of Plants Used in the Management of Sleeping Sickness in Angola and Bioactivity-Guided Fractionation of Brasenia schreberi J.F.Gmel and Nymphaea lotus L. Active against T. b. rhodesiense
Abstract
1. Introduction
2. Results and Discussion
2.1. Selection of the Candidate Plants
2.2. Screening of Extracts against Trypanosoma brucei rhodesiense
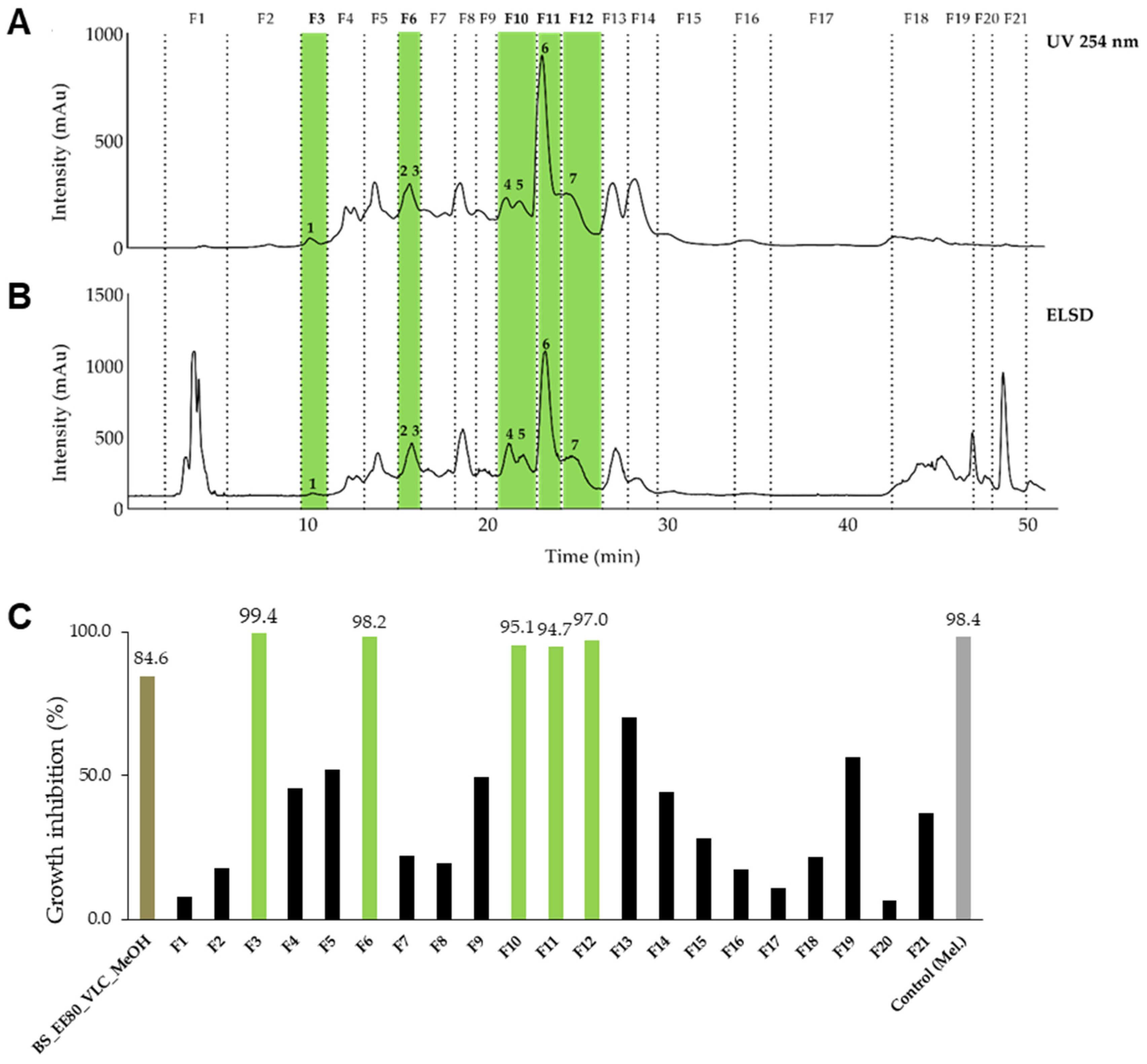
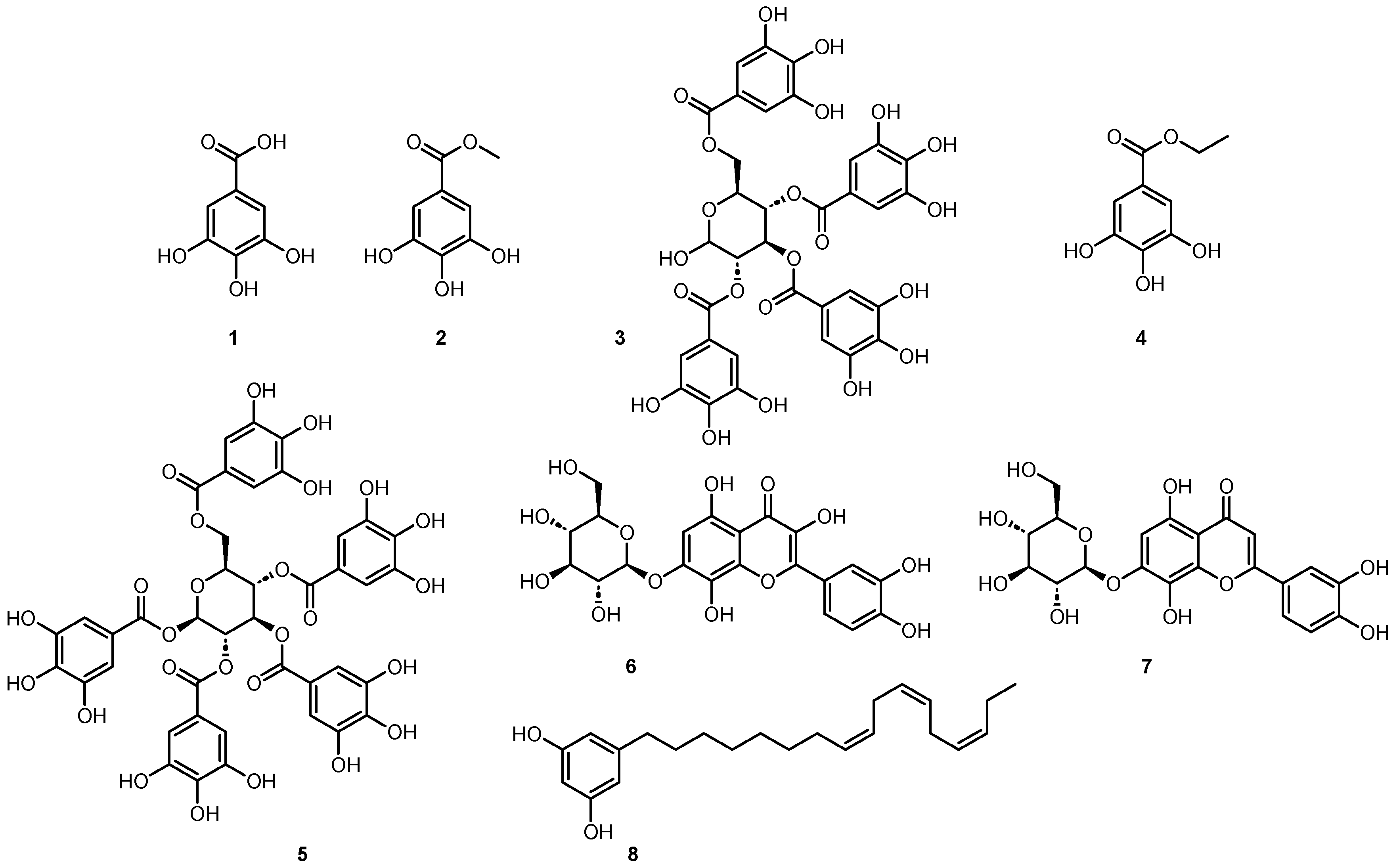
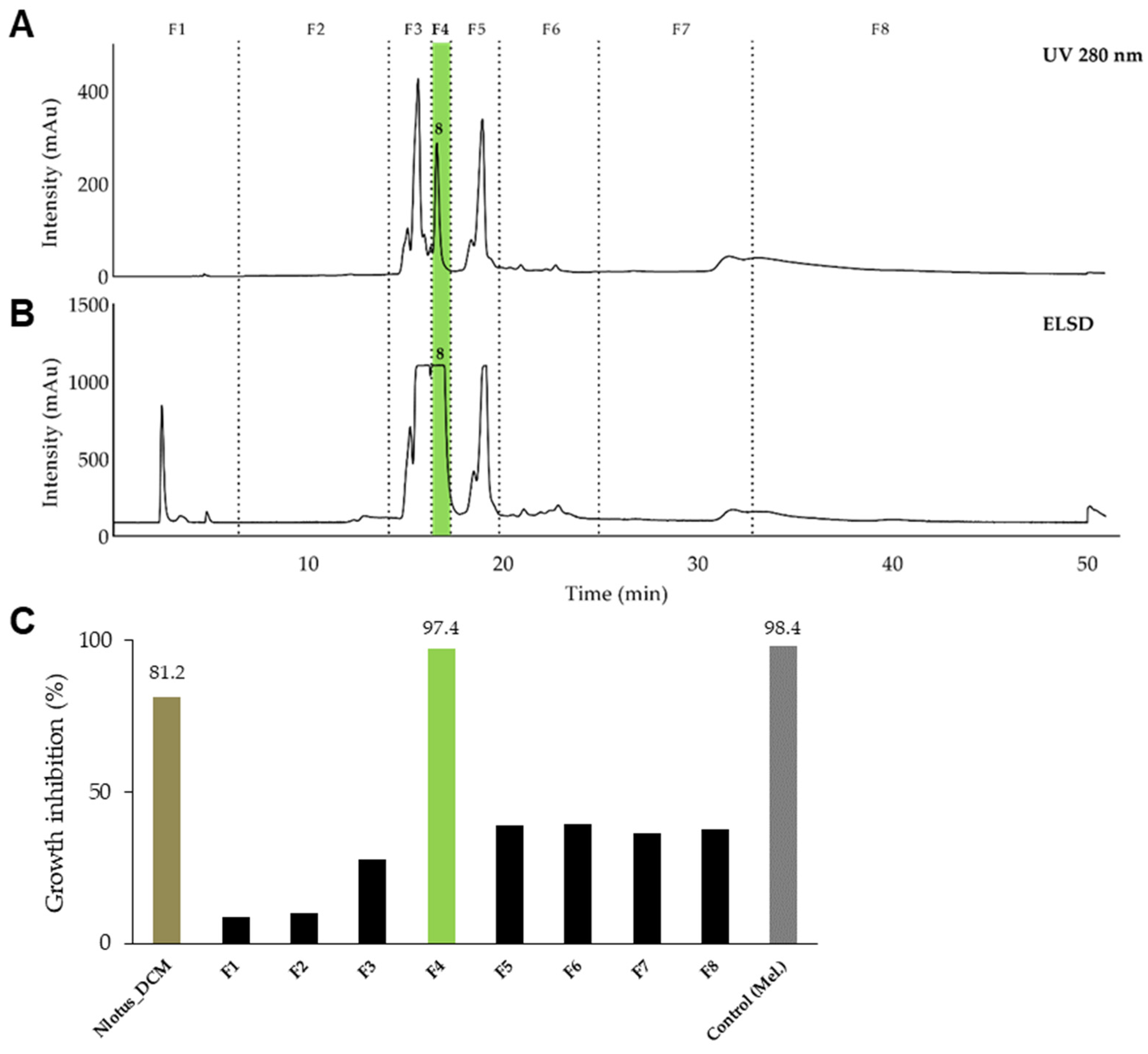
2.3. Isolation of Active Constituents from Brasenia schreberi J.F.Gmel and Nymphaea lotus L.
2.4. Antiprotozoal Activity of the Identified Components
2.5. Active Constituents in Local Herbal Preparation
3. Materials and Methods
3.1. Chemicals
3.2. Selection of the Candidate Plants
3.3. Plant Collection, Identification and Exportation
3.4. Extract Preparation
3.5. General Chromatographic Procedures
3.6. Fractionation and Isolation of Active Constituents
3.7. UHPLC_-HRMS/MS Analysis
3.8. HPLC-DAD-ELSD Analysis
3.9. NMR Spectroscopic Data
3.10. Quantification of Active Pure Compounds
3.11. Antiprotozoal Activity and Cytotoxicity Testing
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Stratégie de l’OMS Pour la Médecine Traditionnelle Pour 2002–2005; World Health Organization: Geneva, Switzerland, 2002. [Google Scholar]
- Büscher, P.; Cecchi, G.; Jamonneau, V.; Priotto, G. Human African trypanosomiasis. Lancet 2017, 390, 2397–2409. [Google Scholar] [CrossRef] [PubMed]
- Simarro, P.P.; Cecchi, G.; Franco, J.R.; Paone, M.; Diarra, A.; Ruiz-Postigo, J.A.; Fevre, E.M.; Mattioli, R.C.; Jannin, J.G. Estimating and mapping the population at risk of sleeping sickness. PLoS Neglected Trop. Dis. 2012, 6, e1859. [Google Scholar] [CrossRef] [PubMed]
- Truc, P.; Grebaut, P.; Lando, A.; Makiadi Donzoau, F.; Penchenier, L.; Herder, S.; Geiger, A.; Vatunga, G.; Josenando, T. Epidemiological aspects of the transmission of the parasites causing human African trypanosomiasis in Angola. Ann. Trop. Med. Parasitol. 2011, 105, 261–265. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, P.G. Clinical features, diagnosis, and treatment of human African trypanosomiasis (sleeping sickness). Lancet Neurol. 2013, 12, 186–194. [Google Scholar] [CrossRef]
- Valverde Mordt, O.; Tarral, A.; Strub-Wourgaft, N. Development and Introduction of Fexinidazole into the Global Human African Trypanosomiasis Program. Am. J. Trop. Med. Hyg. 2022, 106, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Bernhard, S.; Kaiser, M.; Burri, C.; Maser, P. Fexinidazole for Human African Trypanosomiasis, the Fruit of a Successful Public-Private Partnership. Diseases 2022, 10, 90. [Google Scholar] [CrossRef] [PubMed]
- Vahekeni, N.; Neto, P.M.; Kayimbo, M.K.; Maser, P.; Josenando, T.; da Costa, E.; Falquet, J.; van Eeuwijk, P. Use of herbal remedies in the management of sleeping sickness in four northern provinces of Angola. J. Ethnopharmacol. 2020, 256, 112382. [Google Scholar] [CrossRef] [PubMed]
- Gurib-Fakim, A.; Mahomoodally, M. African Flora as potential sources of medicinal plants: Towards the chemotherapy of major parasitic and other infectious diseases-a review. Jordan J. Biol. Sci. 2013, 6, 77–84. [Google Scholar] [CrossRef]
- Ibrahim, M.A.; Mohammed, A.; Isah, M.B.; Aliyu, A.B. Anti-trypanosomal activity of African medicinal plants: A review update. J. Ethnopharmacol. 2014, 154, 26–54. [Google Scholar] [CrossRef]
- Schmidt, T.; Khalid, S.; Romanha, A.; Alves, T.; Biavatti, M.; Brun, R.; Da Costa, F.; De Castro, S.; Ferreira, V.; de Lacerda, M. The potential of secondary metabolites from plants as drugs or leads against protozoan neglected diseases-part I. Curr. Med. Chem. 2012, 19, 2128–2175. [Google Scholar] [CrossRef]
- Ogungbe, I.; Setzer, W. The potential of secondary metabolites from plants as drugs or leads against protozoan neglected diseases—Part III: In-silico molecular docking investigations. Molecules 2016, 21, 1389. [Google Scholar] [CrossRef] [PubMed]
- Mahomoodally, M.F. Traditional Medicines in Africa: An Appraisal of Ten Potent African Medicinal Plants. Evid. Based Complement. Altern. Med. 2013, 2013, 617459. [Google Scholar] [CrossRef] [PubMed]
- Simoben, C.V.; Ntie-Kang, F.; Akone, S.H.; Sippl, W. Compounds from African Medicinal Plants with Activities Against Selected Parasitic Diseases: Schistosomiasis, Trypanosomiasis and Leishmaniasis. Nat. Prod. Bioprospecting 2018, 8, 151–169. [Google Scholar] [CrossRef] [PubMed]
- Nwodo, N.J.; Ibezim, A.; Ntie-Kang, F.; Adikwu, M.U.; Mbah, C.J. Anti-Trypanosomal Activity of Nigerian Plants and Their Constituents. Molecules 2015, 20, 7750–7771. [Google Scholar] [CrossRef] [PubMed]
- Gehrig, S.; Efferth, T. Development of drug resistance in Trypanosoma brucei rhodesiense and Trypanosoma brucei gambiense. Treatment of human African trypanosomiasis with natural products (Review). Int. J. Mol. Med. 2008, 22, 411–419. [Google Scholar]
- Mwangi, V.I.; Mumo, R.M.; Nyachieo, A.; Onkoba, N. Herbal medicine in the treatment of poverty associated parasitic diseases: A case of sub-Saharan Africa. J. Herb. Med. 2017, 10, 1–7. [Google Scholar] [CrossRef]
- Tullius Scotti, M.; Scotti, L.; Ishiki, H.; Fávaro Ribeiro, F.; Marques Duarte da Cruz, R.; Pedrosa de Oliveira, M.; Jaime Bezerra Mendonça, F. Natural products as a source for antileishmanial and antitrypanosomal agents. Comb. Chem. High Throughput Screen. 2016, 19, 537–553. [Google Scholar] [CrossRef] [PubMed]
- Plaatjie, M.; Onyiche, T.; Legoabe, L.; Ramatla, T.; Nyembe, N.; Suganuma, K.; Thekisoe, O. Medicinal plants as potential therapeutic agents for trypanosomosis: A systematic review. Adv. Trad. Med. 2022, 23, 1–23. [Google Scholar] [CrossRef]
- Mesia, G.K.; Tona, G.L.; Nanga, T.H.; Cimanga, R.K.; Apers, S.; Cos, P.; Maes, L.; Pieters, L.; Vlietinck, A.J. Antiprotozoal and cytotoxic screening of 45 plant extracts from Democratic Republic of Congo. J. Ethnopharmacol. 2008, 115, 409–415. [Google Scholar] [CrossRef] [PubMed]
- Bizimana, N.; Tietjen, U.; Zessin, K.H.; Diallo, D.; Djibril, C.; Melzig, M.F.; Clausen, P.H. Evaluation of medicinal plants from Mali for their in vitro and in vivo trypanocidal activity. J. Ethnopharmacol. 2006, 103, 350–356. [Google Scholar] [CrossRef]
- Freiburghaus, F.; Ogwal, E.N.; Nkunya, M.H.; Kaminsky, R.; Brun, R. In vitro antitrypanosomal activity of African plants used in traditional medicine in Uganda to treat sleeping sickness. Trop. Med. Int. Health 1996, 1, 765–771. [Google Scholar] [CrossRef] [PubMed]
- Freiburghaus, F.; Steck, A.; Pfander, H.; Brun, R. Bioassay-guided isolation of a diastereoisomer of kolavenol from Entada abyssinica active on Trypanosoma brucei rhodesiense. J. Ethnopharmacol. 1998, 61, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Sempombe, J.; Mugoyela, V.; Mihale, M.J.; Zacharia, A.; Ipagala, P.; Kilulya, K.F. Preliminary in vivo antitrypanosomal activity and cytotoxicity of Entada abyssinica, Securinega virosa and Ehretia amoena. East. Cent. Afr. J. Pharm. Sci. 2014, 17, 37–43. [Google Scholar]
- Garba, M.H.; Kabiru, A.Y.; Yusuf, A.M.; Muhammad, A.H.; Lekene, B.J.; Kabir, M.; Joseph, A. In vivo trypanocidal activity of Nymphaea lotus Linn. methanol extract against Trypanosoma brucei brucei. Asian Pac. J. Trop. Dis. 2015, 5, 808–812. [Google Scholar] [CrossRef]
- Nwodo, N.; Okoye, F.; Lai, D.; Debbab, A.; Kaiser, M.; Brun, R.; Proksch, P. Evaluation of the in vitro trypanocidal activity of methylated flavonoid constituents of Vitex simplicifolia leaves. BMC Complement. Altern. Med. 2015, 15, 82. [Google Scholar] [CrossRef]
- Atindehou, K.K.; Schmid, C.; Brun, R.; Koné, M.; Traore, D. Antitrypanosomal and antiplasmodial activity of medicinal plants from Côte d’Ivoire. J. Ethnopharmacol. 2004, 90, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Nibret, E.; Ashour, M.L.; Rubanza, C.D.; Wink, M. Screening of some Tanzanian medicinal plants for their trypanocidal and cytotoxic activities. Phytother. Res. 2010, 24, 945–947. [Google Scholar] [CrossRef]
- Nyasse, B.; Ngantchou, I.; Tchana, E.; Sonké, B.; Denier, C.; Fontaine, C. Inhibition of both Trypanosoma brucei bloodstream form and related glycolytic enzymes by a new kolavic acid derivative isolated from Entada abyssinica. Die Pharm. An. Int. J. Pharm. Sci. 2004, 59, 873–875. [Google Scholar] [CrossRef]
- Oguntoye, S.O.; Bello, O.M.; Fasinu, P.S.; Khan, I.A.; Ali, Z.; Khan, S.I.; Usman, L.A. Evaluation of Selected Nigerian Medicinal Plants for in vitro Antiprotozoal Activity. Nat. Prod. J. 2018, 8, 175–184. [Google Scholar] [CrossRef]
- Challal, S.; Queiroz, E.F.; Debrus, B.; Kloeti, W.; Guillarme, D.; Gupta, M.P.; Wolfender, J.-L. Rational and efficient preparative isolation of natural products by MPLC-UV-ELSD based on HPLC to MPLC gradient transfer. Planta Medica 2015, 81, 1636–1643. [Google Scholar] [CrossRef]
- Kamatham, S.; Kumar, N.; Gudipalli, P. Isolation and characterization of gallic acid and methyl gallate from the seed coats of Givotia rottleriformis Griff. and their anti-proliferative effect on human epidermoid carcinoma A431 cells. Toxicol. Rep. 2015, 2, 520–529. [Google Scholar] [CrossRef] [PubMed]
- Cammann, J.; Denzel, K.; Schilling, G.; Gross, G.G. Biosynthesis of gallotannins: β-glucogallin-dependent formation of 1, 2, 3, 4, 6-pentagalloylglucose by enzymatic galloylation of 1, 2, 3, 6-tetragalloylglucose. Arch. Biochem. Biophys. 1989, 273, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Leela, V.; Saraswathy, A. Isolation and characterization of phytoconstituents from Acacia leucophloea flowers (Roxb) wild. Int. Res. J. Pharm. 2013, 4, 107–109. [Google Scholar]
- Zhao, W.-H.; Gao, C.-C.; Ma, X.-F.; Bai, X.-Y.; Zhang, Y.-X. The isolation of 1, 2, 3, 4, 6-penta-O-galloyl-beta-D-glucose from Acer truncatum Bunge by high-speed counter-current chromatography. J. Chromatogr. B 2007, 850, 523–527. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Shi, J.-G.; Mo, S.-Y.; Yang, Y.-C. Chemical constituents of Pyrrosia petiolosa. J. Asian Nat. Prod. Res. 2003, 5, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Zapesochnaya, G.; Pangarova, T. Hypolaetin 7-glucoside from Caryopteris monolica. Chem. Nat. Compd. 1973, 9, 521. [Google Scholar] [CrossRef]
- Choubey, S.; Varughese, L.R.; Kumar, V.; Beniwal, V. Medicinal importance of gallic acid and its ester derivatives: A patent review. Pharm. Pat. Anal. 2015, 4, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Daglia, M.; Di Lorenzo, A.; Nabavi, S.F.; Talas, Z.S.; Nabavi, S.M. Polyphenols: Well beyond the antioxidant capacity: Gallic acid and related compounds as neuroprotective agents: You are what you eat! Curr. Pharm. Biotechnol. 2014, 15, 362–372. [Google Scholar] [CrossRef]
- Kroes, B.v.; Van den Berg, A.; Van Ufford, H.Q.; Van Dijk, H.; Labadie, R. Anti-inflammatory activity of gallic acid. Planta Medica 1992, 58, 499–504. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Lee, H.-J.; Jiang, C.; Zhang, J.; Wang, L.; Zhao, Y.; Xiang, Q.; Lee, E.-O.; Kim, S.-H.; Lü, J. Penta-1, 2, 3, 4, 6-O-galloyl-β-d-glucose induces p53 and inhibits STAT3 in prostate cancer cells in vitro and suppresses prostate xenograft tumor growth in vivo. Mol. Cancer Ther. 2008, 7, 2681–2691. [Google Scholar] [CrossRef]
- Kant, R.; Yen, C.-H.; Hung, J.-H.; Lu, C.-K.; Tung, C.-Y.; Chang, P.-C.; Chen, Y.-H.; Tyan, Y.-C.; Chen, Y.-M.A. Induction of GNMT by 1, 2, 3, 4, 6-penta-O-galloyl-beta-D-glucopyranoside through proteasome-independent MYC downregulation in hepatocellular carcinoma. Sci. Rep. 2019, 9, 1968. [Google Scholar] [CrossRef] [PubMed]
- Locatelli, C.; Filippin-Monteiro, F.B.; Creczynski-Pasa, T.B. Alkyl esters of gallic acid as anticancer agents: A review. Eur. J. Med. Chem. 2013, 60, 233–239. [Google Scholar] [CrossRef]
- Lee, H.; Lee, H.; Kwon, Y.; Lee, J.-H.; Kim, J.; Shin, M.-K.; Kim, S.-H.; Bae, H. Methyl gallate exhibits potent antitumor activities by inhibiting tumor infiltration of CD4+ CD25+ regulatory T cells. J. Immunol. 2010, 185, 6698–6705. [Google Scholar] [CrossRef] [PubMed]
- Borges, A.; Ferreira, C.; Saavedra, M.J.; Simões, M. Antibacterial activity and mode of action of ferulic and gallic acids against pathogenic bacteria. Microb. Drug Resist. 2013, 19, 256–265. [Google Scholar] [CrossRef]
- Buzzini, P.; Arapitsas, P.; Goretti, M.; Branda, E.; Turchetti, B.; Pinelli, P.; Ieri, F.; Romani, A. Antimicrobial and antiviral activity of hydrolysable tannins. Mini Rev. Med. Chem. 2008, 8, 1179–1187. [Google Scholar] [CrossRef]
- Choi, J.G.; Mun, S.H.; Chahar, H.S.; Bharaj, P.; Kang, O.H.; Kim, S.G.; Shin, D.W.; Kwon, D.Y. Methyl gallate from Galla rhois successfully controls clinical isolates of Salmonella infection in both in vitro and in vivo systems. PLoS ONE 2014, 9, e102697. [Google Scholar] [CrossRef]
- Legault, J.; Perron, T.; Mshvildadze, V.; Girard-Lalancette, K.; Perron, S.; Laprise, C.; Sirois, P.; Pichette, A. Antioxidant and anti-inflammatory activities of quercetin 7-O-β-D-glucopyranoside from the leaves of Brasenia schreberi. J. Med. Food 2011, 14, 1127–1134. [Google Scholar] [CrossRef] [PubMed]
- Shimoda, H.; Nakamura, S.; Hitoe, S.; Terazawa, S.; Tanaka, J.; Matsumoto, T.; Matsuda, H. Anti-adipogenic polyphenols of water shield suppress TNF-alpha-induced cell damage and enhance expression of HAS2 and HABP2 in adiponectin. Nat. Prod. Chem. Res. 2014, 2, 146. [Google Scholar]
- Braunberger, C.; Zehl, M.; Conrad, J.; Fischer, S.; Adhami, H.-R.; Beifuss, U.; Krenn, L. LC–NMR, NMR, and LC–MS identification and LC–DAD quantification of flavonoids and ellagic acid derivatives in Drosera peltata. J. Chromatogr. B 2013, 932, 111–116. [Google Scholar] [CrossRef]
- Veit, M.; Beckert, C.; Höhne, C.; Bauer, K.; Geiger, H.J.P. Interspecific and intraspecific variation of phenolics in the genus Equisetum subgenus Equisetum. Phytochemistry 1995, 38, 881–891. [Google Scholar] [CrossRef]
- Barrow, R.; Capon, R. Alkyl and alkenyl resorcinols from an Australian marine sponge, Haliclona Sp (Haplosclerida: Haliclonidae). Aust. J. Chem. 1991, 44, 1393–1405. [Google Scholar] [CrossRef]
- Kozubek, A.; Tyman, J.H. Resorcinolic lipids, the natural non-isoprenoid phenolic amphiphiles and their biological activity. Chem. Rev. 1999, 99, 1–26. [Google Scholar] [CrossRef]
- Kozubek, A.; Zarnowski, R.; Stasiuk, M.; Gubernator, J. Natural amphiphilic phenols as bioactive compounds. Cell. Mol. Biol. Lett. 2001, 6, 351–355. [Google Scholar]
- Tasdemir, D.; Kaiser, M.; Brun, R.; Yardley, V.; Schmidt, T.J.; Tosun, F.; Rüedi, P. Antitrypanosomal and antileishmanial activities of flavonoids and their analogues: In vitro, in vivo, structure-activity relationship, and quantitative structure-activity relationship studies. Antimicrob. Agents Chemother. 2006, 50, 1352–1364. [Google Scholar] [CrossRef] [PubMed]
- Koide, T.; Nose, M.; Inoue, M.; Ogihara, Y.; Yabu, Y.; Ohta, N. Trypanocidal effects of gallic acid and related compounds. Planta Medica 1998, 64, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Nose, M.; Koide, T.; Morikawa, K.; Inoue, M.; Ogihara, Y.; Yabu, Y.; Ohta, N. Formation of reactive oxygen intermediates might be involved in the trypanocidal activity of gallic acid. Biol. Pharm. Bull. 1998, 21, 583–587. [Google Scholar] [CrossRef] [PubMed]
- Grady, R.W.; Bienen, E.J.; Clarkson Jr, A.B. Esters of 3, 4-dihydroxybenzoic acid, highly effective inhibitors of the sn-glycerol-3-phosphate oxidase of Trypanosoma brucei brucei. Mol. Biochem. Parasitol. 1986, 21, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Amisigo, C.M.; Antwi, C.A.; Adjimani, J.P.; Gwira, T.M. In vitro anti-trypanosomal effects of selected phenolic acids on Trypanosoma brucei. PLoS ONE 2019, 14, e0216078. [Google Scholar] [CrossRef] [PubMed]
- Andjelković, M.; Van Camp, J.; De Meulenaer, B.; Depaemelaere, G.; Socaciu, C.; Verloo, M.; Verhe, R. Iron-chelation properties of phenolic acids bearing catechol and galloyl groups. Food Chem. 2006, 98, 23–31. [Google Scholar]
- Wink, M. Medicinal plants: A source of anti-parasitic secondary metabolites. Molecules 2012, 17, 12771–12791. [Google Scholar] [CrossRef]
- Jeacock, L.; Baker, N.; Wiedemar, N.; Maser, P.; Horn, D. Aquaglyceroporin-null trypanosomes display glycerol transport defects and respiratory-inhibitor sensitivity. PLoS Pathog 2017, 13, e1006307. [Google Scholar] [CrossRef]
- Andréo, R.; Regasini, L.O.; Petrônio, M.S.; Chiari-Andréo, B.G.; Tansini, A.; Silva, D.H.S.; Cicarelli, R.M.B. Toxicity and loss of mitochondrial membrane potential induced by alkyl gallates in trypanosoma cruzi. Int. Sch. Res. Not. 2015, 2015, 1–7. [Google Scholar] [CrossRef]
- Calderon, A.I.; Romero, L.I.; Ortega-Barria, E.; Brun, R.; Correa, A.M.D.; Gupta, M.P. Evaluation of Larvicidal and in Vitro. Antiparasitic Activities of Plants in a Biodiversity Plot in the Altos de Campana National Park, Panama. Pharm. Biol. 2006, 44, 487–498. [Google Scholar] [CrossRef]
- Khasanah, U.; WidyaWaruyanti, A.; Hafid, A.F.; Tanjung, M. Antiplasmodial activity of isolated polyphenols from Alectryon serratus leaves against 3D7 Plasmodium falciparum. Pharmacogn. Res. 2017, 9, S57. [Google Scholar]
- Arsianti, A.; Astuti, H.; Simadibrata, D.M.; Adyasa, Z.M.; Amartya, D.; Bahtiar, A.; Tanimoto, H.; Kakiuchi, K. Synthesis and in Vitro Antimalarial Activity of Alkyl Esters Gallate as a Growth Inhibitors of Plasmodium Falciparum. Orient. J. Chem. 2018, 34, 655. [Google Scholar] [CrossRef]
- Torres-Leon, C.; Ventura-Sobrevilla, J.; Serna-Cock, L.; Ascacio-Valdes, J.A.; Contreras-Esquivel, J.; Aguilar, C.N. Pentagalloylglucose (PGG): A valuable phenolic compound with functional properties. J. Funct. Foods 2017, 37, 176–189. [Google Scholar] [CrossRef]
- Kolodziej, H.; Kayser, O.; Kiderlen, A.; Ito, H.; Hatano, T.; Yoshida, T.; Foo, L. Antileishmanial activity of hydrolyzable tannins and their modulatory effects on nitric oxide and tumour necrosis factor-α release in macrophages in vitro. Planta Medica 2001, 67, 825–832. [Google Scholar] [CrossRef] [PubMed]
- Jin, W.; Zjawiony, J.K. 5-Alkylresorcinols from Merulius i ncarnatus. J. Nat. Prod. 2006, 69, 704–706. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Romero, C.; Torres-Mendoza, D.; González, L.D.U.; Ortega-Barría, E.; McPhail, K.L.; Gerwick, W.H.; Cubilla-Rios, L. Hydroxyalkenylresorcinols from Stylogyne turbacensis. J. Nat. Prod. 2007, 70, 1249–1252. [Google Scholar] [CrossRef]
- Belmonte-Reche, E.; Martínez-García, M.; Peñalver, P.; Gomez-Perez, V.; Lucas, R.; Gamarro, F.; Pérez-Victoria, J.M.; Morales, J.C. Tyrosol and hydroxytyrosol derivatives as antitrypanosomal and antileishmanial agents. Eur. J. Med. Chem. 2016, 119, 132–140. [Google Scholar] [CrossRef]
- Matutino Bastos, T.; Mannochio Russo, H.; Silvio Moretti, N.; Schenkman, S.; Marcourt, L.; Gupta, M.P.; Wolfender, J.L.; Ferreira Queiroz, E.; Botelho Pereira Soares, M. Chemical Constituents of Anacardium occidentale as Inhibitors of Trypanosoma cruzi Sirtuins. Molecules 2019, 24, 1299. [Google Scholar] [CrossRef]
- Choo, L.M.; Forest, F.; Wieringa, J.J.; Bruneau, A.; de la Estrella, M. Phylogeny and biogeography of the Daniellia clade (Leguminosae: Detarioideae), a tropical tree lineage largely threatened in Africa and Madagascar. Mol. Phylogenet Evol. 2020, 146, 106752. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, A.B.; Maser, P.; Kaiser, M.; Hamburger, M.; Khalid, S. Mining Sudanese Medicinal Plants for Antiprotozoal Agents. Front. Pharmacol. 2020, 11, 865. [Google Scholar] [CrossRef] [PubMed]
- Oyeyemi, I.T.; Akanni, O.O.; Adaramoye, O.A.; Bakare, A.A. Methanol extract of Nymphaea lotus ameliorates carbon tetrachloride-induced chronic liver injury in rats via inhibition of oxidative stress. J. Basic Clin. Physiol. Pharmacol. 2017, 28, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Cai, X.; Fan, Y.; Luo, A. Antioxidant activity of water-soluble polysaccharides from Brasenia schreberi. Pharmacogn. Mag. 2016, 12, 193. [Google Scholar]
- Adelakun, K.M.; Mustapha, M.K.; Muazu, M.M.; Omotayo, O.L.; Olaoye, O. Phytochemical screening and antibacterial activities of crude extract of Nymphaea lotus (water lily) against fish pathogens. J. Biomed. Sci. 2015, 2, 38–42. [Google Scholar] [CrossRef]
- Akinjogunla, O.; Adegoke, A.; Udokang, I.; Adebayo-Tayo, B. Antimicrobial potential of Nymphaea lotus (Nymphaeaceae) against wound pathogens. J. Med. Plants Res. 2009, 3, 138–141. [Google Scholar]
- Akinjogunla, O.; Yah, C.; Eghafona, N.; Ogbemudia, F. Antibacterial activity of leave extracts of Nymphaea lotus (Nymphaeaceae) on Methicillin resistant Staphylococcus aureus (MRSA) and Vancomycin resistant Staphylococcus aureus (VRSA) isolated from clinical samples. Ann. Biol. Res. 2010, 1, 174–184. [Google Scholar]
- Elakovich, S.D.; Wooten, J.W. An examination of the phytotoxicity of the water shield, Brasenia schreberi. J. Chem. Ecol. 1987, 13, 1935–1940. [Google Scholar] [CrossRef]
- Kim, H.; Wang, Q.; Shoemaker, C.F.; Zhong, F.; Bartley, G.E.; Yokoyama, W.H. Polysaccharide gel coating of the leaves of Brasenia schreberi lowers plasma cholesterol in hamsters. J. Tradit. Complement. Med. 2015, 5, 56–61. [Google Scholar] [CrossRef]
- Hisayoshi, T.; Shinomura, M.; Konishi, A.; Tanaka, J.; Shimoda, H.; Hata, K.; Takahashi, S.; Yasukawa, K. Inhibition of HIV-1 reverse transcriptase activity by Brasenia schreberi (Junsai) components. J. Biol. Macromol. 2014, 14, 59–65. [Google Scholar] [CrossRef][Green Version]
- Hisayoshi, T.; Shinomura, M.; Yokokawa, K.; Kuze, I.; Konishi, A.; Kawaji, K.; Kodama, E.N.; Hata, K.; Takahashi, S.; Nirasawa, S.; et al. Inhibition of the DNA polymerase and RNase H activities of HIV-1 reverse transcriptase and HIV-1 replication by Brasenia schreberi (Junsai) and Petasites japonicus (Fuki) components. J. Nat. Med. 2015, 69, 432–440. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.B.; Zhao, Q.C.; Cai, B.; Yao, X.S.; Osadsa, K. Two new and four known polyphenolics obtained as new cell-cycle inhibitors from Rubus aleaefolius Poir. J. Asian. Nat. Prod. Res. 2002, 4, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Nonaka, G.I.; Nishioka, I. Tannins and Related-Compounds.14. 7-O-Galloyl-(+)-Catechin and 3-O-Galloylprocyanidin-B-3 from Sanguisorba officinalis. Phytochemistry 1983, 22, 2575–2578. [Google Scholar] [CrossRef]


| Plant | Family | Collection Number |
|---|---|---|
| Brillantaisia owariensis P.beauv. | Acanthaceae | 7925 |
| Brasenia schreberi J.F. Gmel | Cabombaceae | n.d. |
| Palisota schweinfurthii C.B.Clarke | Commelinaceae | 894 |
| Momordica charantia L. | Cucurbitaceae | 8591 |
| Entada abyssinica A.Rich. | Fabaceae | 3468 |
| Vitex madiensis Oliv. | Lamiaceae | 7186 |
| Nymphaea lotus L. | Nymphaeaceae | 2513 |
| Crossopteryx febrifuga (Afzel.ex G.Don) Benth | Rubiaceae | 8212 |
| Sarcocephalus latifolius (Sm.)E.A.Bruce | Rubiaceae | 8231 |
| Extract ID | Plant Name | Plant Part | Solvent | GI (%) 1 |
|---|---|---|---|---|
| 46 | E. abyssinica | Ri | Aqueous | 103 |
| 47 | E. abyssinica | Ri | EtOH 80% | 101 |
| 91 | N. lotus | AeP | Hexane | 98 |
| 54 | E. abyssinica | Rb | EtOH 80% | 98 |
| 109 | B. schreberi | L | Aqueous | 99 |
| 110 | B. schreberi | L | EtOH 80% | 96 |
| 111 | B. schreberi | L | MeOH 70% | 96 |
| 92 | N. lotus | AeP | DCM | 74 |
| 115 | N. lotus | AeP | Hexane | 96 |
| 116 | N. lotus | AeP | DCM | 81 |
| 69 | V. madiensis | R | Hexane | 79 |
| 20 | C. febrifuga | L | Hexane | 85 |
| 28 | V. madiensis | L | Hexane | 96 |
| 64 | M. charantia | AeP | DCM | 72 |
| 35 | B. owariensis | L | Hexane | 96 |
| Extract ID | Plant plant part | T. b. rhodesiense | T. cruzi | L. donovani | P. falciparum | L6 | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| IC50 | SI 1 | IC50 | SI | IC50 | SI | IC50 | SI | IC50 | ||
| 46 | E. abyssinica Ri | 1.8 | 4.5 | 14.0 | 0.6 | 29.9 | 0.3 | 6.5 | 1.2 | 6.3 |
| 47 | E. abyssinica Ri | 4.1 | 4.0 | 16.1 | 1.0 | 43.4 | 0.4 | 12.7 | 1.3 | 16.3 |
| 91 | N. lotus AeP | 4.8 | 5.8 | 36.8 | 0.8 | 44.2 | 0.6 | 10.3 | 2.7 | 32.9 |
| 54 | E. abyssinica Rb | 5.1 | 3.6 | 26.4 | 0.7 | 45.8 | 0.4 | 10.4 | 1.8 | 16.0 |
| 109 | B. schreberi L | 5.9 | 2.9 | 26.7 | 0.6 | 53.0 | 0.3 | 3.5 | 4.9 | 33.8 |
| 110 | B. schreberi L | 7.1 | 4.3 | 61.5 | 0.5 | 48.1 | 0.6 | 8.1 | 3.8 | 33.8 |
| 111 | B. schreberi L | 7.9 | 4.0 | 65.9 | 0.5 | 42.4 | 0.7 | 7.5 | 4.2 | 36.0 |
| 92 | N. lotus L | 9.8 | 3.8 | 56.7 | 0.7 | 14.5 | 2.5 | 6.3 | 5.9 | 42.4 |
| 115 | N. lotus L | 11.9 | 2.5 | 45.5 | 0.6 | 20.1 | 1.5 | 14.7 | 2.0 | 34.5 |
| 116 | N. lotus L | 12.2 | 3.6 | 56.3 | 0.8 | 17.7 | 2.5 | 7.9 | 5.5 | 49.7 |
| 69 | V. madiensis R | 12.8 | 2.2 | 53.0 | 0.5 | 11.7 | 2.4 | 20.7 | 1.4 | 41.9 |
| 20 | C. febrifuga L | 13.1 | 3.5 | 64.1 | 0.7 | 46.9 | 1.0 | 21.2 | 2.2 | 47.0 |
| 28 | V. madiensis L | 13.6 | 1.7 | 42.2 | 0.6 | 23.2 | 1.0 | 23.9 | 1.0 | 22.8 |
| 64 | M. charantia Wp | 30.5 | 1.1 | 48.1 | 0.7 | 25.5 | 1.3 | 8.7 | 3.9 | 26.0 |
| 35 | B. owariensis L | 40.2 | 1.2 | 55.9 | 0.9 | 62.1 | 0.8 | >50 | n.d | 48.2 |
| T. b. rhodesiense | T. cruzi | L. donovani | P. falciparum | L6 | |||||
|---|---|---|---|---|---|---|---|---|---|
| IC50 | SI | IC50 | SI | IC50 | SI | IC50 | SI | IC50 | |
| Gallic acid (1) | 0.5 | 34 | 66 | 0.2 | 56 | 0.3 | >10 | n.d. | 16 |
| Methyl gallate (2) | 1.1 | 15 | 16 | 1.0 | 8.5 | 1.9 | 2.1 | 7.8 | 16 |
| Ethyl gallate (4) | 0.6 | 25 | 16 | 0.9 | 6.8 | 2.2 | 3.0 | 4.9 | 15 |
| Pentagalloyl-β-glucopyranoside (5) | 20.0 | 1.0 | 44 | 0.5 | 15 | 1.4 | 6.7 | 3.1 | 21 |
| Gossypetin-7-O-β-glucopyranoside (6) | 5.5 | 1.6 | 12 | 0.8 | 53 | 0.2 | n.d. | n.d. | 8.9 |
| Hypolaetin-7-O-glucoside (7) | 5.7 | 3.2 | 49 | 0.4 | 52 | 0.4 | n.d. | n.d. | 19 |
| Resorcinol-alkyl (8) | 5.3 | 2.5 | 9.1 | 1.4 | 2.5 | 5.2 | n.d. | n.d. | 13 |
| Active Component | B. schreberi Decoction | N. lotus Decoction | ||
|---|---|---|---|---|
| Raw Material | Extract | Raw Material | Extract | |
| Gallic acid (1) | 8.8 | 50 | 5.6 | 22 |
| Methyl gallate (2) | 0.007 | 0.04 | 0.005 | 0.022 |
| Ethyl gallate (4) | n.d. | <19 ppm | n.d. | <19 ppm |
| Pentagalloyl-β-glucopyranoside (5) | 0.39 | 2.3 | 0.09 | 0.36 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vahekeni, N.; Brillatz, T.; Rahmaty, M.; Cal, M.; Keller-Maerki, S.; Rocchetti, R.; Kaiser, M.; Sax, S.; Mattli, K.; Wolfram, E.; et al. Antiprotozoal Activity of Plants Used in the Management of Sleeping Sickness in Angola and Bioactivity-Guided Fractionation of Brasenia schreberi J.F.Gmel and Nymphaea lotus L. Active against T. b. rhodesiense. Molecules 2024, 29, 1611. https://doi.org/10.3390/molecules29071611
Vahekeni N, Brillatz T, Rahmaty M, Cal M, Keller-Maerki S, Rocchetti R, Kaiser M, Sax S, Mattli K, Wolfram E, et al. Antiprotozoal Activity of Plants Used in the Management of Sleeping Sickness in Angola and Bioactivity-Guided Fractionation of Brasenia schreberi J.F.Gmel and Nymphaea lotus L. Active against T. b. rhodesiense. Molecules. 2024; 29(7):1611. https://doi.org/10.3390/molecules29071611
Chicago/Turabian StyleVahekeni, Nina, Théo Brillatz, Marjan Rahmaty, Monica Cal, Sonja Keller-Maerki, Romina Rocchetti, Marcel Kaiser, Sibylle Sax, Kevin Mattli, Evelyn Wolfram, and et al. 2024. "Antiprotozoal Activity of Plants Used in the Management of Sleeping Sickness in Angola and Bioactivity-Guided Fractionation of Brasenia schreberi J.F.Gmel and Nymphaea lotus L. Active against T. b. rhodesiense" Molecules 29, no. 7: 1611. https://doi.org/10.3390/molecules29071611
APA StyleVahekeni, N., Brillatz, T., Rahmaty, M., Cal, M., Keller-Maerki, S., Rocchetti, R., Kaiser, M., Sax, S., Mattli, K., Wolfram, E., Marcourt, L., Queiroz, E. F., Wolfender, J.-L., & Mäser, P. (2024). Antiprotozoal Activity of Plants Used in the Management of Sleeping Sickness in Angola and Bioactivity-Guided Fractionation of Brasenia schreberi J.F.Gmel and Nymphaea lotus L. Active against T. b. rhodesiense. Molecules, 29(7), 1611. https://doi.org/10.3390/molecules29071611









