Analgesic Peptides: From Natural Diversity to Rational Design
Abstract
1. Introduction: Opioid Peptides and Their Receptors
2. Antinociceptive Peptides of Animal Origin
2.1. Peptides Derived from Sea Snails
2.2. Peptides Derived from Spiders
2.3. Peptides from Scorpion Venom
2.4. Peptides Isolated from Other Arthropods
2.5. Peptides of Amphibian Origin
2.6. Peptides from Snake Venom
3. Structural Modifications of Opioid Peptides
3.1. The First Step: Amino Acid Replacement
3.2. Biphalin—A Prominent Example of SAR Study
3.3. Cyclic Analogs: Frozen Structure
3.4. Bifunctional Analogs: Hybrid Peptides
3.5. Adding Novel Functionality: Peptide Conjugates
4. Drug Delivery Systems for Antinociceptive Peptides
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pathan, H.; Williams, J. Basic opioid pharmacology: An update. Br. J. Pain 2012, 6, 11–16. [Google Scholar] [CrossRef]
- Janecka, A.; Fichna, J.; Janecki, T. Opioid receptors and their ligands. Curr. Top. Med. Chem. 2004, 4, 1–17. [Google Scholar] [CrossRef]
- Gentilucci, L. New trends in the development of opioid peptide analogues as advanced remedies for pain relief. Curr. Top. Med. Chem. 2004, 4, 19–38. [Google Scholar] [CrossRef]
- Przewłocki, R.; Przewłocka, B. Opioids in chronic pain. Eur. J. Pharmacol. 2001, 429, 79–91. [Google Scholar] [CrossRef]
- Schuckit, M.A. Treatment of Opioid-Use Disorders. N. Engl. J. Med. 2016, 375, 357–368. [Google Scholar] [CrossRef]
- Mollereau, C.; Parmentier, M.; Mailleux, P.; Butour, J.L.; Moisand, C.; Chalon, P.; Caput, D.; Vassart, G.; Meunier, J.C. ORL1, a novel member of the opioid receptor family. Cloning, functional expression and localization. FEBS Lett. 1994, 341, 33–38. [Google Scholar] [CrossRef]
- Daga, P.R.; Zaveri, N.T. Homology modeling and molecular dynamics simulations of the active state of the nociceptin receptor reveal new insights into agonist binding and activation. Proteins 2012, 80, 1948–1961. [Google Scholar] [CrossRef]
- Senese, N.B.; Kandasamy, R.; Kochan, K.E.; Traynor, J.R. Regulator of G-Protein Signaling (RGS) Protein Modulation of Opioid Receptor Signaling as a Potential Target for Pain Management. Front. Mol. Neurosci. 2020, 13, 5. [Google Scholar] [CrossRef]
- Stein, C. Opioid Receptors. Annu. Rev. Med. 2016, 67, 433–451. [Google Scholar] [CrossRef]
- Hosseinzadeh Sahafi, O.; Sardari, M.; Alijanpour, S.; Rezayof, A. Shared Mechanisms of GABAergic and Opioidergic Transmission Regulate Corticolimbic Reward Systems and Cognitive Aspects of Motivational Behaviors. Brain Sci. 2023, 13, 815. [Google Scholar] [CrossRef]
- Janecka, A.; Perlikowska, R.; Gach, K.; Wyrebska, A.; Fichna, J. Development of opioid peptide analogs for pain relief. Curr. Pharm. Des. 2010, 16, 1126–1135. [Google Scholar] [CrossRef]
- Hughes, J.; Smith, T.W.; Kosterlitz, H.W.; Fothergill, L.A.; Morgan, B.A.; Morris, H.R. Identification of two related pentapeptides from the brain with potent opiate agonist activity. Nature 1975, 258, 577–580. [Google Scholar] [CrossRef]
- Goldstein, A.; Tachibana, S.; Lowney, L.I.; Hunkapiller, M.; Hood, L. Dynorphin-(1-13), an extraordinarily potent opioid peptide. Proc. Natl. Acad. Sci. USA 1979, 76, 6666–6670. [Google Scholar] [CrossRef]
- Chavkin, C.; James, I.F.; Goldstein, A. Dynorphin is a specific endogenous ligand of the kappa opioid receptor. Science 1982, 215, 413–415. [Google Scholar] [CrossRef]
- Li, C.H.; Chung, D. Isolation and structure of an untriakontapeptide with opiate activity from camel pituitary glands. Proc. Natl. Acad. Sci. USA 1976, 73, 1145–1148. [Google Scholar] [CrossRef]
- Zadina, J.E.; Hackler, L.; Ge, L.J.; Kastin, A.J. A potent and selective endogenous agonist for the mu-opiate receptor. Nature 1997, 386, 499–502. [Google Scholar] [CrossRef]
- Hackler, L.; Zadina, J.E.; Ge, L.J.; Kastin, A.J. Isolation of relatively large amounts of endomorphin-1 and endomorphin-2 from human brain cortex. Peptides 1997, 18, 1635–1639. [Google Scholar] [CrossRef]
- Meunier, J.C.; Mollereau, C.; Toll, L.; Suaudeau, C.; Moisand, C.; Alvinerie, P.; Butour, J.L.; Guillemot, J.C.; Ferrara, P.; Monsarrat, B. Isolation and structure of the endogenous agonist of opioid receptor-like ORL1 receptor. Nature 1995, 377, 532–535. [Google Scholar] [CrossRef]
- Reinscheid, R.K.; Nothacker, H.P.; Bourson, A.; Ardati, A.; Henningsen, R.A.; Bunzow, J.R.; Grandy, D.K.; Langen, H.; Monsma, F.J., Jr.; Civelli, O. Orphanin FQ: A neuropeptide that activates an opioidlike G protein-coupled receptor. Science 1995, 270, 792–794. [Google Scholar] [CrossRef]
- Toll, L.; Bruchas, M.R.; Calo’, G.; Cox, B.M.; Zaveri, N.T. Nociceptin/Orphanin FQ Receptor Structure, Signaling, Ligands, Functions, and Interactions with Opioid Systems. Pharmacol. Rev. 2016, 68, 419–457. [Google Scholar] [CrossRef]
- Brantl, V.; Teschemacher, H.; Henschen, A.; Lottspeich, F. Novel opioid peptides derived from casein (beta-casomorphins). I. Isolation from bovine casein peptone. Hoppe Seylers Z. Physiol. Chem. 1979, 360, 1211–1216. [Google Scholar] [CrossRef]
- Chang, K.J.; Lillian, A.; Hazum, E.; Cuatrecasas, P.; Chang, J.K. Morphiceptin (NH4-Tyr-Pro-Phe-Pro-COHN2): A potent and specific agonist for morphine (mu) receptors. Science 1981, 212, 75–77. [Google Scholar] [CrossRef]
- Chang, K.J.; Su, Y.F.; Brent, D.A.; Chang, J.K. Isolation of a specific mu-opiate receptor peptide, morphiceptin, from an enzymatic digest of milk proteins. J. Biol. Chem. 1985, 260, 9706–9712. [Google Scholar] [CrossRef]
- Nyberg, F.; Sanderson, K.; Glämsta, E.L. The hemorphins: A new class of opioid peptides derived from the blood protein hemoglobin. Biopolymers 1997, 43, 147–156. [Google Scholar] [CrossRef]
- Montecucchi, P.C.; de Castiglione, R.; Piani, S.; Gozzini, L.; Erspamer, V. Amino acid composition and sequence of dermorphin, a novel opiate-like peptide from the skin of Phyllomedusa sauvagei. Int. J. Pept. Protein Res. 1981, 17, 275–283. [Google Scholar] [CrossRef]
- Amiche, M.; Sagan, S.; Mor, A.; Delfour, A.; Nicolas, P. Dermenkephalin (Tyr-D-Met-Phe-His-Leu-Met-Asp-NH2): A potent and fully specific agonist for the delta opioid receptor. Mol. Pharmacol. 1989, 35, 774–779. [Google Scholar]
- Kreil, G.; Barra, D.; Simmaco, M.; Erspamer, V.; Erspamer, G.F.; Negri, L.; Severini, C.; Corsi, R.; Melchiorri, P. Deltorphin, a novel amphibian skin peptide with high selectivity and affinity for delta opioid receptors. Eur. J. Pharmacol. 1989, 162, 123–128. [Google Scholar] [CrossRef]
- Bodnar, R.J. Endogenous opiates and behavior: 2022. Peptides 2023, 169, 171095. [Google Scholar] [CrossRef]
- Wtorek, K.; Janecka, A. Potential of Nociceptin/Orphanin FQ Peptide Analogs for Drug Development. Chem. Biodivers. 2021, 18, e2000871. [Google Scholar] [CrossRef]
- Wtorek, K.; Ghidini, A.; Gentilucci, L.; Adamska-Bartłomiejczyk, A.; Piekielna-Ciesielska, J.; Ruzza, C.; Sturaro, C.; Calò, G.; Pieretti, S.; Kluczyk, A.; et al. Synthesis, Biological Activity and Molecular Docking of Chimeric Peptides Targeting Opioid and NOP Receptors. Int. J. Mol. Sci. 2022, 23, 12700. [Google Scholar] [CrossRef]
- Lee, Y.S. Peptidomimetics and Their Applications for Opioid Peptide Drug Discovery. Biomolecules 2022, 12, 1241. [Google Scholar] [CrossRef] [PubMed]
- Aldrich, J.V.; McLaughlin, J.P. Opioid Peptides: Potential for Drug Development. Drug Discov. Today Technol. 2012, 9, e23–e31. [Google Scholar] [CrossRef] [PubMed]
- Gu, Z.H.; Wang, B.; Kou, Z.Z.; Bai, Y.; Chen, T.; Dong, Y.L.; Li, H.; Li, Y.Q. Endomorphins: Promising Endogenous Opioid Peptides for the Development of Novel Analgesics. Neurosignals 2017, 25, 98–116. [Google Scholar] [CrossRef] [PubMed]
- Yam, M.F.; Loh, Y.C.; Tan, C.S.; Khadijah Adam, S.; Abdul Manan, N.; Basir, R. General Pathways of Pain Sensation and the Major Neurotransmitters Involved in Pain Regulation. Int. J. Mol. Sci. 2018, 19, 2164. [Google Scholar] [CrossRef] [PubMed]
- Muratspahić, E.; Freissmuth, M.; Gruber, C.W. Nature-Derived Peptides: A Growing Niche for GPCR Ligand Discovery. Trends Pharmacol. Sci. 2019, 40, 309–326. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.J.; Qiu, Y.; Hua, Z. The Emerging Perspective of Morphine Tolerance: MicroRNAs. Pain Res. Manag. 2019, 2019, 9432965. [Google Scholar] [CrossRef]
- Quah, Y.; Tong, S.-R.; Bojarska, J.; Giller, K.; Tan, S.-A.; Ziora, Z.M.; Esatbeyoglu, T.; Chai, T.-T. Bioactive Peptide Discovery from Edible Insects for Potential Applications in Human Health and Agriculture. Molecules 2023, 28, 1233. [Google Scholar] [CrossRef]
- Pennington, M.W.; Czerwinski, A.; Norton, R.S. Peptide therapeutics from venom: Current status and potential. Bioorg. Med. Chem. 2018, 26, 2738–2758. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, A.N.; Soares, A.M.; Da Silva, S.L. Why to Study Peptides from Venomous and Poisonous Animals? Int. J. Pept. Res. Ther. 2023, 29, 76. [Google Scholar] [CrossRef]
- Diochot, S. Pain-related toxins in scorpion and spider venoms: A face to face with ion channels. J. Venom. Anim. Toxins Incl. Trop. Dis. 2021, 27, e20210026. [Google Scholar] [CrossRef]
- Wu, T.; Wang, M.; Wu, W.; Luo, Q.; Jiang, L.; Tao, H.; Deng, M. Spider venom peptides as potential drug candidates due to their anticancer and antinociceptive activities. J. Venom. Anim. Toxins Incl. Trop. Dis. 2019, 25, e146318. [Google Scholar] [CrossRef]
- Dos Santos, A.T.; Cruz, G.S.; Baptista, G.R. Anti-inflammatory activities of arthropod peptides: A systematic review. J. Venom. Anim. Toxins Incl. Trop. Dis. 2021, 27, e20200152. [Google Scholar] [CrossRef]
- Schroeder, C.I.; Lewis, R.J. ω-Conotoxins GVIA, MVIIA and CVID: SAR and Clinical Potential. Mar. Drugs 2006, 4, 193–214. [Google Scholar] [CrossRef]
- Wen, L.; Yang, S.; Qiao, H.; Liu, Z.; Zhou, W.; Zhang, Y.; Huang, P. SO-3, a new O-superfamily conopeptide derived from Conus striatus, selectively inhibits N-type calcium currents in cultutured hipppcampal neurons. Br. J. Pharmacol. 2005, 145, 728–739. [Google Scholar] [CrossRef]
- Sousa, S.R.; McArthur, J.R.; Brust, A.; Bhola, R.F.; Rosengren, K.J.; Ragnarsson, L.; Dutertre, S.; Alewood, P.F.; Christie, M.J.; Adams, D.J.; et al. Novel analgesic ω-conotoxins from the vermivorous cone snail Conus moncuri provide new insights into the evolution of conopeptides. Sci. Rep. 2018, 8, 13397. [Google Scholar] [CrossRef]
- Margiotta, F.; Micheli, L.; Ciampi, C.; Ghelardini, C.; McIntosh, J.M.; Di Cesare Mannelli, L. Conus regius-Derived Conotoxins: Novel Therapeutic Opportunities from a Marine Organism. Mar. Drugs 2022, 20, 773. [Google Scholar] [CrossRef]
- Liu, Z.; Bartels, P.; Sadeghi, M.; Du, T.; Dai, Q.; Zhu, C.; Yu, S.; Wang, S.; Dong, M.; Sun, T.; et al. A novel α-conopeptide Eu1.6 inhibits N-type (Cav2.2) calcium channels and exhibits potent analgesic activity. Sci. Rep. 2018, 8, 1004. [Google Scholar] [CrossRef]
- Trevisan, G.; Oliveira, S.M. Animal Venom Peptides Cause Antinociceptive Effects by Voltage-gated Calcium Channels Activity Blockage. Curr. Neuropharmacol. 2022, 20, 1579–1599. [Google Scholar] [CrossRef]
- Lee, S.; Kim, Y.; Back, S.K.; Choi, H.W.; Lee, J.Y.; Jung, H.H.; Ryu, J.H.; Suh, H.W.; Na, H.S.; Kim, H.J.; et al. Analgesic effect of highly reversible ω-conotoxin FVIA on N type Ca2+ channels. Mol. Pain 2010, 6, 97. [Google Scholar] [CrossRef]
- Berecki, G.; Motin, L.; Haythornthwaite, A.; Vink, S.; Bansal, P.; Drinkwater, R.; Wang, C.I.; Moretta, M.; Lewis, R.J.; Alewood, P.F.; et al. Analgesic (ω)-conotoxins CVIE and CVIF selectively and voltage-dependently block recombinant and native N-type calcium channels. Mol. Pharmacol. 2011, 80, 356. [Google Scholar] [CrossRef]
- Bernáldez, J.; Román-González, S.A.; Martínez, O.; Jiménez, S.; Vivas, O.; Arenas, I.; Corzo, G.; Arreguín, R.; García, D.E.; Possani, L.D.; et al. A Conus regularis Conotoxin with a Novel Eight-Cysteine Framework Inhibits Cav2.2 Channels and Displays an Anti-Nociceptive Activity. Mar. Drugs 2013, 11, 1188–1202. [Google Scholar] [CrossRef]
- Guo, R.; Guo, G.; Wang, A.; Xu, G.; Lai, R.; Jin, H. Spider-Venom Peptides: Structure, Bioactivity, Strategy, and Research Applications. Molecules 2024, 29, 35. [Google Scholar] [CrossRef]
- Grishin, E.V.; Savchenko, G.A.; Vassilevski, A.A.; Korolkova, Y.V.; Boychuk, Y.A.; Viatchenko-Karpinski, V.Y.; Nadezhdin, K.D.; Arseniev, A.S.; Pluzhnikov, K.A.; Kulyk, V.B.; et al. Novel peptide from spider venom inhibits P2X3 receptors and inflammatory pain. Ann. Neurol. 2010, 67, 680–683. [Google Scholar] [CrossRef]
- Kabanova, N.V.; Vassilevski, A.A.; Rogachevskaja, O.A.; Bystrova, M.F.; Korolkova, Y.V.; Pluzhnikov, K.A.; Romanov, R.A.; Grishin, E.V.; Kolesnikov, S.S. Modulation of P2X3 receptors by spider toxins. Biochim. Biophys. Acta-Biomembr. 2012, 1818, 2868–2875. [Google Scholar] [CrossRef]
- Matavel, A.; Fleury, C.; Oliveira, L.C.; Molina, F.; de Lima, M.E.; Cruz, J.S.; Cordeiro, M.N.; Richardson, M.; Ramos, C.H.I.; Beirão, P.S.L. Structure and Activity Analysis of Two Spider Toxins That Alter Sodium Channel Inactivation Kinetics. Biochemistry 2009, 48, 3078–3088. [Google Scholar] [CrossRef]
- Peigneur, S.; de Lima, M.E.; Tytgat, J. Phoneutria nigriventer venom: A pharmacological treasure. Toxicon 2018, 151, 96–110. [Google Scholar] [CrossRef]
- Leão, R.M.; Cruz, J.S.; Diniz, C.R.; Cordeiro, M.N.; Beirão, P.S.L. Inhibition of neuronal high-voltage activated calcium channels by the ω-Phoneutria nigriventer Tx3-3 peptide toxin. Neuropharmacology 2000, 39, 1756–1767. [Google Scholar] [CrossRef]
- Lyukmanova, E.N.; Mironov, P.A.; Kulbatskii, D.S.; Shulepko, M.A.; Paramonov, A.S.; Chernaya, E.M.; Logashina, Y.A.; Andreev, Y.A.; Kirpichnikov, M.P.; Shenkarev, Z.O. Recombinant Production, NMR Solution Structure, and Membrane Interaction of the Phα1β Toxin, a TRPA1 Modulator from the Brazilian Armed Spider Phoneutria nigriventer. Toxins 2023, 15, 378. [Google Scholar] [CrossRef]
- Oliveira, S.M.; Silva, C.R.; Trevisan, G.; Villarinho, J.G.; Cordeiro, M.N.; Richardson, M.; Borges, M.H.; Castro, C.J., Jr.; Gomez, M.V.; Ferreira, J. Antinociceptive effect of a novel armed spider peptide Tx3-5 in pathological pain models in mice. Pflugers Arch.-Eur. J. Physiol. 2016, 468, 881–894. [Google Scholar] [CrossRef]
- Figueiredo, S.G.; Garcia, M.E.; Valentim, A.C.; Cordeiro, M.N.; Diniz, C.R.; Richardson, M. Purification and amino acid sequence of the insecticidal neurotoxin Tx4(6-1) from the venom of the ‘armed’ spider Phoneutria nigriventer (Keys). Toxicon 1995, 33, 83–93. [Google Scholar] [CrossRef]
- Sousa, S.R.; Wingerd, J.S.; Brust, A.; Bladen, C.; Ragnarsson, L.; Herzig, V.; Deuis, J.R.; Dutertre, S.; Vetter, I.; Zamponi, G.W.; et al. Discovery and mode of action of a novel analgesic β-toxin from the African spider Ceratogyrus darlingi. PLoS ONE 2017, 12, e0182848. [Google Scholar] [CrossRef]
- Lopez, L.; De Waard, S.; Meudal, H.; Caumes, C.; Khakh, K.; Peigneur, S.; Oliveira-Mendes, B.; Lin, S.; De Waele, J.; Montnach, J.; et al. Structure-function relationship of new peptides activating human Nav1.1. Biomed. Pharmacother. 2023, 165, 115173. [Google Scholar] [CrossRef]
- Liu, Y.; Tang, J.; Zhang, Y.; Xun, X.; Tang, D.; Peng, D.; Yi, J.; Liu, Z.; Shi, X. Synthesis and Analgesic Effects of μ-TRTX-Hhn1b on Models of Inflammatory and Neuropathic Pain. Toxins 2014, 6, 2363–2378. [Google Scholar] [CrossRef]
- Liang, S. An overview of peptide toxins from the venom of the Chinese bird spider Selenocosmia huwena Wang [=Ornithoctonus huwena (Wang)]. Toxicon 2004, 43, 575–585. [Google Scholar] [CrossRef]
- Wang, M.; Guan, X.; Liang, S. The cross channel activities of spider neurotoxin huwentoxin-I on rat dorsal root ganglion neurons. Biochem. Biophys. Res. Commun. 2007, 357, 579–583. [Google Scholar] [CrossRef]
- Deng, M.; Luo, X.; Xiao, Y.; Sun, Z.; Jiang, L.; Liu, Z.; Zeng, X.; Chen, H.; Tang, J.; Zeng, W.; et al. Huwentoxin-XVI, an analgesic, highly reversible mammalian N-type calcium channel antagonist from Chinese tarantula Ornithoctonus huwena. Neuropharmacology 2014, 79, 657–667. [Google Scholar] [CrossRef]
- Newcomb, R.; Szoke, B.; Palma, A.; Wang, G.; Chen, X.; Hopkins, W.; Cong, R.; Miller, J.; Urge, L.; Tarczy-Hornoch, K.; et al. Selective Peptide Antagonist of the Class E Calcium Channel from the Venom of the Tarantula Hysterocrates gigas. Biochemistry 1998, 37, 15353–15362. [Google Scholar] [CrossRef]
- Nicolas, S.; Zoukimian, C.; Bosmans, F.; Montnach, J.; Diochot, S.; Cuypers, E.; De Waard, S.; Béroud, R.; Mebs, D.; Craik, D.; et al. Chemical Synthesis, Proper Folding, Nav Channel Selectivity Profile and Analgesic Properties of the Spider Peptide Phlotoxin 1. Toxins 2019, 11, 367. [Google Scholar] [CrossRef]
- Pringos, E.; Vignes, M.; Martinez, J.; Rolland, V. Peptide Neurotoxins That Affect Voltage-Gated Calcium Channels: A Close-Up on ω-Agatoxins. Toxins 2011, 3, 17–42. [Google Scholar] [CrossRef]
- Liang, J.G.; Zhang, J.; Lai, R.; Rees, H.H. An opioid peptide from synganglia of the tick, Amblyomma testindinarium. Peptides 2005, 26, 603–606. [Google Scholar] [CrossRef]
- Cordeiro Mdo, N.; Diniz, C.R.; Valentim Ado, C.; von Eickstedt, V.R.; Gilroy, J.; Richardson, M. The purification and amino acid sequences of four Tx2 neurotoxins from the venom of the Brazilian ‘armed’ spider Phoneutria nigriventer (Keys). FEBS Lett. 1992, 310, 153–156. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, C.N.; Nunes, K.P.; Dourado, L.F.N.; Vieira, T.O.; Mariano, X.M.; Cunha Junior, A.D.S.; de Lima, M.E. From the PnTx2-6 Toxin to the PnPP-19 Engineered Peptide: Therapeutic Potential in Erectile Dysfunction, Nociception, and Glaucoma. Front. Mol. Biosci. 2022, 9, 831823. [Google Scholar] [CrossRef] [PubMed]
- da Silva, J.F.; Binda, N.S.; Pereira, E.M.R.; de Lavor, M.S.L.; Vieira, L.B.; de Souza, A.H.; Rigo, F.K.; Ferrer, H.T.; de Castro, C.J.; Ferreira, J.; et al. Analgesic effects of Phα1β toxin: A review of mechanisms of action involving pain pathways. J. Venom. Anim. Toxins Incl. Trop. Dis. 2021, 27, e20210001. [Google Scholar] [CrossRef] [PubMed]
- Emerich, B.L.; Ferreira, R.C.M.; Cordeiro, M.N.; Borges, M.H.; Pimenta, A.M.C.; Figueiredo, S.G.; Duarte, I.D.G.; De Lima, M.E. δ-Ctenitoxin-Pn1a, a Peptide from Phoneutria nigriventer Spider Venom, Shows Antinociceptive Effect Involving Opioid and Cannabinoid Systems, in Rats. Toxins 2016, 8, 106. [Google Scholar] [CrossRef] [PubMed]
- Emerich, B.L.; Ferreira, R.C.M.; Machado-de-Avila, R.A.; Resende, J.M.; Duarte, I.D.G.; de Lima, M.E. PnAn13, an antinociceptive synthetic peptide inspired in the Phoneutria nigriventer toxin PnTx4(6–1) (δ-Ctenitoxin-Pn1a). Toxicon X 2020, 7, 100045. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Li, P.; Chen, B.; Huang, J.; Lai, R.; Liu, J.; Rong, M. Selective Closed-State Nav1.7 Blocker JZTX-34 Exhibits Analgesic Effects against Pain. Toxins 2018, 10, 64. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wu, Z.; Tang, D.; Xun, X.; Liu, L.; Li, X.; Nie, D.; Xiang, Y.; Yi, J.; Yi, J. Analgesic Effects of Huwentoxin-IV on Animal Models of Inflammatory and Neuropathic Pain. Protein Pept. Lett. 2014, 21, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Peter Muiruri, K.; Zhong, J.; Yao, B.; Lai, R.; Luo, L. Bioactive peptides from scorpion venoms: Therapeutic scaffolds and pharmacological tools. Chin. J. Nat. Med. 2023, 21, 19–35. [Google Scholar] [CrossRef] [PubMed]
- Kampo, S.; Anabah, W.T.; Bayor, F.; Wilfred, S.-A. Scorpion Venom Component; BmK AGAP Potentiates the Analgesic Effects of Lidocaine During Sciatic Nerve Block. Venoms Toxins 2023, 3, 63–68. [Google Scholar] [CrossRef]
- Shao, J.-H.; Cui, Y.; Zhao, M.-Y.; Wu, C.-F.; Liu, Y.-F.; Zhang, J.-H. Purification, characterization, and bioactivity of a new analgesic-antitumor peptide from Chinese scorpion Buthus martensii Karsch. Peptides 2014, 53, 89–96. [Google Scholar] [CrossRef]
- Ji, Y.-H.; Li, Y.-J.; Zhang, J.-W.; Song, B.-L.; Yamaki, T.; Mochizuki, T.; Hoshino, M.; Yanaihara, N. Covalent structures of BmK AS and BmK AS-1, two novel bioactive polypeptides purified from Chinese scorpion Buthus martensi Karsch. Toxicon 1999, 37, 519–536. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-J.; Tan, Z.-Y.; Ji, Y.-H. The binding of BmK IT2, a depressant insect-selective scorpion toxin on mammal and insect sodium channels. Neurosci. Res. 2000, 38, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xu, J.; Wang, Z.; Zhang, X.; Liang, X.; Civelli, O. BmK-YA, an enkephalin-like peptide in scorpion venom. PLoS ONE 2012, 7, e40417. [Google Scholar] [CrossRef] [PubMed]
- Guan, R.J.; Wang, M.; Wang, D.; Wang, D.C. A new insect neurotoxin AngP1 with analgesic effect from the scorpion Buthus martensii Karsch: Purification and characterization. J. Pept. Res. 2001, 58, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Cologna, C.T.; Marcussi, S.; Giglio, J.R.; Soares, A.M.; Arantes, E.C. Tityus serrulatus scorpion venom and toxins: An overview. Protein Pept. Lett. 2009, 16, 920–932. [Google Scholar] [CrossRef]
- Hoang, A.N.; Vo, H.D.; Vo, N.P.; Kudryashova, K.S.; Nekrasova, O.V.; Feofanov, A.V.; Kirpichnikov, M.P.; Andreeva, T.V.; Serebryakova, M.V.; Tsetlin, V.I.; et al. Vietnamese Heterometrus laoticus scorpion venom: Evidence for analgesic and anti-inflammatory activity and isolation of new polypeptide toxin acting on Kv1.3 potassium channel. Toxicon 2014, 77, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Konopińska, D.; Rosiński, G. Proctolin, an insect neuropeptide. J. Pept. Sci. 1999, 5, 533–546. [Google Scholar] [CrossRef]
- Ryszka, F.; Dolińska, B.; Suszka-Świtek, A.; Rykaczewska-Czerwińska, M.; Konopińska, D.; Kuczer, M.; Plech, A. Distribution in Rats Internal Organs of Intraperitoneally Given 125I-Labeled Heptapeptide [2-8]-Leucopyrokinin ([2-8]-LPK), a Truncated Analog of Insect Neuropeptide Leucopyrokinin. Adv. Clin. Exp. Med. 2015, 24, 579–584. [Google Scholar] [CrossRef] [PubMed]
- Plech, A.; Kuczer, M.; Szewczyk, A.; Konopińska, D. Antinociceptive effect of insect hexapeptide, insect trypsin modulating oostatic factor (Neb-TMOF) in rats. Pestycydy/Pestic. 2005, 1–2, 5–13. [Google Scholar]
- Rykaczewska-Czerwińska, M.; Oleś, P.; Oleś, M.; Kuczer, M.; Konopińska, D.; Plech, A. Effect of alloferon 1 on central nervous system in rats. Acta Pol. Pharm. 2015, 72, 205–211. [Google Scholar] [CrossRef]
- Rykaczewska-Czerwinska, M.; Radosz, A.; Szymanowska-Dziubasik, K.; Konopinska, D.; Plech, A. Antinociceptive effect of MAS MT in rats. Pestycydy/Pestic. 2008, 3–4, 139–146. [Google Scholar]
- Rykaczewska-Czerwinska, M.; Radosz, A.; Konopińska, D.; Wrobel, M.; Plech, A. Antinociceptive effect of poneratoxin [PoTX] in rats. Pestycydy/Pestic. 2008, 1–2, 135–141. [Google Scholar]
- Mortari, M.R.; Cunha, A.O.; Carolino, R.O.; Coutinho-Netto, J.; Tomaz, J.C.; Lopes, N.P.; Coimbra, N.C.; Dos Santos, W.F. Inhibition of acute nociceptive responses in rats after i.c.v. injection of Thr6-bradykinin, isolated from the venom of the social wasp, Polybia occidentalis. Br. J. Pharmacol. 2007, 151, 860–869. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, J.; Rangel, M.; Biolchi, A.; Alves, E.; Moreira, K.; Silva, L.; Mortari, M. Antinociceptive properties of the mastoparan peptide Agelaia-MPI isolated from social wasps. Toxicon 2016, 120, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Galante, P.; Campos, G.A.A.; Moser, J.C.G.; Martins, D.B.; dos Santos Cabrera, M.P.; Rangel, M.; Coelho, L.C.; Simon, K.S.; Amado, V.M.; de AIMuller, J.; et al. Exploring the therapeutic potential of an antinociceptive and anti-inflammatory peptide from wasp venom. Sci. Rep. 2023, 13, 12491. [Google Scholar] [CrossRef] [PubMed]
- Merlo, L.A.; Bastos, L.F.; Godin, A.M.; Rocha, L.T.; Nascimento, E.B., Jr.; Paiva, A.L.; Moraes-Santos, T.; Zumpano, A.A.; Bastos, E.M.; Heneine, L.G.; et al. Effects induced by Apis mellifera venom and its components in experimental models of nociceptive and inflammatory pain. Toxicon 2011, 57, 764–771. [Google Scholar] [CrossRef] [PubMed]
- Ullah, A.; Aldakheel, F.M.; Anjum, S.I.; Raza, G.; Khan, S.A.; Tlak Gajger, I. Pharmacological properties and therapeutic potential of honey bee venom. Saudi. Pharm. J. 2023, 31, 96–109. [Google Scholar] [CrossRef] [PubMed]
- Wehbe, R.; Frangieh, J.; Rima, M.; El Obeid, D.; Sabatier, J.-M.; Fajloun, Z. Bee Venom: Overview of Main Compounds and Bioactivities for Therapeutic Interests. Molecules 2019, 24, 2997. [Google Scholar] [CrossRef] [PubMed]
- Shi, P.; Xie, S.; Yang, J.; Zhang, Y.; Han, S.; Su, S.; Yao, H. Pharmacological effects and mechanisms of bee venom and its main components: Recent progress and perspective. Front. Pharmacol. 2022, 13, 1001553. [Google Scholar] [CrossRef]
- Malpezzi-Marinho, E.L.A.; Zanoni, C.I.S.; Molska, G.R.; Paraventi, C.; Wuo-Silva, R.; Berro, L.F.; Parada, C.A.; Tamura, E.K.; Marinho, E.A.V. Antinociceptive Activity of the Skin Secretion of Phyllomedusa rohdei (Amphibia, Anura). Toxins 2020, 12, 589. [Google Scholar] [CrossRef]
- Xu, X.; Lai, R. The chemistry and biological activities of peptides from amphibian skin secretions. Chem. Rev. 2015, 115, 1760–1846. [Google Scholar] [CrossRef]
- Smith, M.T.; Kong, D.; Kuo, A.; Imam, M.Z.; Williams, C.M. Analgesic Opioid Ligand Discovery Based on Nonmorphinan Scaffolds Derived from Natural Sources. J. Med. Chem. 2022, 65, 1612–1661. [Google Scholar] [CrossRef]
- Usenko, A.B.; Emel’yanova, T.G.; Myasoedov, N.F. Dermorphins are Natural Opioids with an Unique Primary Structure That Determines Their Biological Specificity. Biol. Bull. Russ. Acad. Sci. 2002, 29, 154–164. [Google Scholar] [CrossRef]
- Chen, W.; Yang, X.; Chen, L.; Yang, X.; Feng, F.; He, W.; Liu, J.; Yu, H. Gene cloning and characterization of novel antinociceptive peptide from the brain of the frog, Odorrana grahami. Biochimie 2011, 93, 1110–1114. [Google Scholar] [CrossRef]
- Negri, L.; Melchiorri, P.; Lattanzi, R. Pharmacology of Amphibian Opiate Peptides. Peptides 2000, 21, 1639–1647. [Google Scholar] [CrossRef]
- Ellis-Steinborner, S.T.; Scanlon, D.; Musgrave, I.F.; Tran, T.T.N.; Hack, S.; Wang, T.; Abell, A.D.; Tyler, M.J.; Bowie, J.H. An unusual kynurenine-containing opioid tetrapeptide from the skin gland secretion of the Australian red tree frog Litoria rubella. Sequence determination by electrospray mass spectrometry. Rapid Commun. Mass Spectrom. 2011, 25, 1735–1740. [Google Scholar] [CrossRef]
- Garcia-Garcia, F.; Corrales-Garcia, L.L.; Olamendi-Portugal, T.; Restano-Cassulini, R.; Vega, R.; Vega, M.; Ortiz, E.; Sandoval-Lopez, G.; Soto, E.; Corzo, G. Identification, chemical synthesis and heterologous expression of an antinociceptive peptide from the veined tree frog Trachycephalus typhonius. Process Biochem. 2017, 62, 205–214. [Google Scholar] [CrossRef]
- Mourier, G.; Salinas, M.; Kessler, P.; Stura, E.A.; Leblanc, M.; Tepshi, L.; Besson, T.; Diochot, S.; Baron, A.; Douguet, D.; et al. Mambalgin-1 Pain-relieving Peptide, Stepwise Solid-phase Synthesis, Crystal Structure, and Functional Domain for Acid-sensing Ion Channel 1a Inhibition. J. Biol. Chem. 2016, 291, 2616–2629. [Google Scholar] [CrossRef]
- Le Goas, R.; LaPlante, S.R.; Mikou, A.; Delsuc, M.A.; Guittet, E.; Robin, M.; Charpentier, I.; Lallemand, J.Y. Alpha-cobratoxin: Proton NMR assignment and solution structure. Biochemistry 1992, 31, 4867–4875. [Google Scholar] [CrossRef] [PubMed]
- Konno, K.; Picolo, G.; Gutierrez, V.P.; Brigatte, P.; Zambelli, V.O.; Camargo, A.C.M.; Cury, Y. Crotalphine, a novel potent analgesic peptide from the venom of the South American rattlesnake Crotalus durissus terrificus. Peptides 2008, 29, 1293–1304. [Google Scholar] [CrossRef] [PubMed]
- Vidya, V.; Raghu RamAchar Himathi, M.U.; Akshita, N.; Yogish Somayaji, T.; Vivek Hamse Kameshwar, K.; Byrappa, K.; Ramadas, D. Venom peptides—A comprehensive translational perspective in pain management. Curr. Res. Toxicol. 2021, 2, 329–340. [Google Scholar] [CrossRef]
- Brigatte, P.; Konno, K.; Gutierrez, V.P.; Sampaio, S.C.; Zambelli, V.O.; Picolo, G.; Curi, R.; Cury, Y. Peripheral kappa and delta opioid receptors are involved in the antinociceptive effect of crotalphine in a rat model of cancer pain. Pharmacol. Biochem. Behav. 2013, 109, 1–7. [Google Scholar] [CrossRef]
- Hayashi, M.A.F.; Campeiro, J.D.; Yonamine, C.M. Revisiting the potential of South American rattlesnake Crotalus durissus terrificus toxins as therapeutic, theranostic and/or biotechnological agents. Toxicon 2022, 206, 1–13. [Google Scholar] [CrossRef]
- Boutin, J.A.; Leprince, J. Why Search for Alternative GPCR Agonists? Receptors 2023, 2, 16–33. [Google Scholar] [CrossRef]
- Hellinger, R.; Sigurdsson, A.; Wu, W.; Romanova, E.V.; Li, L.; Sweedler, J.V.; Süssmuth, R.D.; Gruber, C.W. Peptidomics. Nat. Rev. Methods Primers 2023, 3, 25. [Google Scholar] [CrossRef]
- Wang, L.; Wang, N.; Zhang, W.; Cheng, X.; Yan, Z.; Shao, G.; Wang, X.; Wang, R.; Fu, C. Therapeutic peptides: Current applications and future directions. Signal Transduct. Target. Ther. 2022, 7, 48. [Google Scholar] [CrossRef]
- Perlikowska, R.; Janecka, A. Bioavailability of endomorphins and the blood–brain barrier—A review. Med. Chem. 2014, 10, 2–17. [Google Scholar] [CrossRef]
- De Marco, R.; Janecka, A. Strategies to Improve Bioavailability and In Vivo Efficacy of the Endogenous Opioid Peptides Endomorphin-1 and Endomorphin-2. Curr. Top. Med. Chem. 2015, 16, 141–155. [Google Scholar] [CrossRef]
- Piekielna, J.; Perlikowska, R.; Gach, K.; Janecka, A. Cyclization in opioid peptides. Curr. Drug Targets 2013, 14, 798–816. [Google Scholar] [CrossRef]
- Remesic, M.; Lee, Y.S.; Hruby, V.J. Cyclic Opioid Peptides. Curr. Med. Chem. 2016, 23, 1288–1303. [Google Scholar] [CrossRef] [PubMed]
- Piekielna-Ciesielska, J.; Mollica, A.; Pieretti, S.; Fichna, J.; Szymaszkiewicz, A.; Zielińska, M.; Kordek, R.; Janecka, A. Antinociceptive potency of a fluorinated cyclopeptide Dmt-c[D-Lys-Phe-p-CF3-Phe-Asp]NH2. J. Enzym. Inhib. Med. Chem. 2018, 33, 560–566. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Xu, B.; Li, N.; Zhang, R.; Zhang, Q.; Shi, X.; Xu, K.; Xiao, J.; Chen, D.; Niu, J.; et al. Development of Multifunctional and Orally Active Cyclic Peptide Agonists of Opioid/Neuropeptide FF Receptors that Produce Potent, Long-Lasting, and Peripherally Restricted Antinociception with Diminished Side Effects. J. Med. Chem. 2021, 64, 13394–13409. [Google Scholar] [CrossRef] [PubMed]
- Schwyzer, R. ACTH: A short introductory review. Ann. N. Y. Acad. Sci. 1977, 297, 3–26. [Google Scholar] [CrossRef] [PubMed]
- Beddell, C.R.; Clark, R.B.; Hardy, G.W.; Lowe, L.A.; Ubatuba, F.B.; Vane, J.R.; Wilkinson, S. Structural requirements for opioid activity of analogues of the enkephalins. Proc. R. Soc. Lond. B Biol. Sci. 1977, 198, 249–265. [Google Scholar] [CrossRef] [PubMed]
- Gentilucci, L.; De Marco, R.; Cerisoli, L. Chemical Modifications Designed to Improve Peptide Stability: Incorporation of Non-Natural Amino Acids, Pseudo-Peptide Bonds, and Cyclization. Curr. Pharm. Des. 2010, 16, 3185–3203. [Google Scholar] [CrossRef] [PubMed]
- Beaudeau, J.L.; Blais, V.; Holleran, B.J.; Bergeron, A.; Pineyro, G.; Guérin, B.; Gendron, L.; Dory, Y.L. N-Guanidyl and C-Tetrazole Leu-Enkephalin Derivatives: Efficient Mu and Delta Opioid Receptor Agonists with Improved Pharmacological Properties. ACS Chem. Neurosci. 2019, 10, 1615–1626. [Google Scholar] [CrossRef] [PubMed]
- Handa, B.K.; Land, A.C.; Lord, J.A.; Morgan, B.A.; Rance, M.J.; Smith, C.F. Analogues of beta-LPH61-64 possessing selective agonist activity at mu-opiate receptors. Eur. J. Pharmacol. 1981, 70, 531–540. [Google Scholar] [CrossRef] [PubMed]
- Gacel, G.; Fournie-Zaluski, M.C.; Roques, B.P. D-Tyr-Ser-Gly-Phe-Leu-Thr, a highly preferential ligand for delta-opiate receptors. FEBS Lett. 1980, 118, 245–247. [Google Scholar] [CrossRef] [PubMed]
- Belknap, J.K.; Laursen, S.E. DSLET (D-Ser2-Leu5-Enkephalin-Thr6) produces analgesia on the hot plate by mechanisms largely different from DAGO and morphine-like opioids. Life Sci. 1987, 41, 391–395. [Google Scholar] [CrossRef] [PubMed]
- Lung, F.D.; Meyer, J.P.; Lou, B.S.; Xiang, L.; Li, G.; Davis, P.; DeLeon, I.A.; Yamamura, H.I.; Porreca, F.; Hruby, V.J. Effects of modifications of residues in position 3 of dynorphin A(1-11)-NH2 on kappa receptor selectivity and potency. J. Med. Chem. 1996, 39, 2456–2460. [Google Scholar] [CrossRef] [PubMed]
- Frączak, O.; Lasota, A.; Tymecka, D.; Kosson, P.; Muchowska, A.; Misicka, A.; Olma, A. Synthesis, binding affinities and metabolic stability of dimeric dermorphin analogs modified with β3-homo-amino acids. J. Pept. Sci. 2016, 22, 222–227. [Google Scholar] [CrossRef]
- Liu, X.; Wang, Y.; Xing, Y.; Yu, J.; Ji, H.; Kai, M.; Wang, Z.; Wang, D.; Zhang, Y.; Zhao, D.; et al. Design, synthesis, and pharmacological characterization of novel endomorphin-1 analogues as extremely potent μ-opioid agonists. J. Med. Chem. 2013, 56, 3102–3114. [Google Scholar] [CrossRef]
- Schiller, P.W.; Nguyen, T.M.; Berezowska, I.; Dupuis, S.; Weltrowska, G.; Chung, N.N.; Lemieux, C. Synthesis and in vitro opioid activity profiles of DALDA analogues. Eur. J. Med. Chem. 2000, 35, 895–901. [Google Scholar] [CrossRef]
- Cai, Y.; Lu, D.; Chen, Z.; Ding, Y.; Chung, N.N.; Li, T.; Schiller, P.W. [Dmt(1)]DALDA analogues modified with tyrosine analogues at position 1. Bioorg. Med. Chem. Lett. 2016, 26, 3629–3631. [Google Scholar] [CrossRef]
- Hiramatsu, M.; Inoue, K.; Ambo, A.; Sasaki, Y.; Kameyama, T. Long-lasting antinociceptive effects of a novel dynorphin analogue, Tyr-D-Ala-Phe-Leu-Arg psi (CH2NH)Arg-NH2, in mice. Br. J. Pharmacol. 2001, 132, 1948–1956. [Google Scholar] [CrossRef]
- Lu, Y.; Nguyen, M.D.; Weltrowska, G.; Berezowska, I.; Lemieux, C.; Chung, N.N.; Schiller, P. [2′,6′-Dimethyltyrosine]Dynorphin A(1–11)-NH2 Analogues Lacking an N-Terminal Amino Group: Potent and Selective κ Opioid Antagonists. J. Med. Chem. 2001, 44, 3048–3053. [Google Scholar] [CrossRef] [PubMed]
- Neilan, C.L.; Nguyen, T.M.; Schiller, P.W.; Pasternak, G.W. Pharmacological characterization of the dermorphin analog [Dmt(1)]DALDA, a highly potent and selective mu-opioid peptide. Eur. J. Pharmacol. 2001, 419, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Schiller, P.W.; Nguyen, T.M.; Saray, A.; Poon, A.W.; Laferrière, A.; Coderre, T.J. The bifunctional μ opioid agonist/antioxidant [Dmt(1)]DALDA is a superior analgesic in an animal model of complex regional pain syndrome-type i. ACS Chem. Neurosci. 2015, 6, 1789–1793. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, C.P.; Tome, M.E.; Davis, T.P. The opioid epidemic: A central role for the blood brain barrier in opioid analgesia and abuse. Fluids Barriers CNS 2017, 14, 32. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.M.; Liu, X.F.; Yao, J.L.; Wang, C.L.; Yu, Y.; Wang, R. Utilization of combined chemical modifications to enhance the blood–brain barrier permeability and pharmacological activity of endomorphin-1. J. Pharmacol. Exp. Ther. 2006, 319, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Schiller, P.W. Bi- or multifunctional opioid peptide drugs. Life Sci. 2010, 86, 598–603. [Google Scholar] [CrossRef] [PubMed]
- Dietis, N.; Guerrini, R.; Calo, G.; Salvadori, S.; Rowbotham, D.J.; Lambert, D.G. Simultaneous targeting of multiple opioid receptors: A strategy to improve side-effect profile. Br. J. Anaesth. 2009, 103, 38–49. [Google Scholar] [CrossRef]
- Günther, T.; Dasgupta, P.; Mann, A.; Miess, E.; Kliewer, A.; Fritzwanker, S.; Steinborn, R.; Schulz, S. Targeting multiple opioid receptors–improved analgesics with reduced side effects? Br. J. Pharmacol. 2018, 175, 2857–2868. [Google Scholar] [CrossRef] [PubMed]
- Schiller, P.W.; Fundytus, M.E.; Merovitz, L.; Weltrowska, G.; Nguyen, T.M.; Lemieux, C.; Chung, N.N.; Coderre, T.J. The opioid mu agonist/delta antagonist DIPP-NH(2)[Psi] produces a potent analgesic effect, no physical dependence, and less tolerance than morphine in rats. J. Med. Chem. 1999, 42, 3520–3526. [Google Scholar] [CrossRef] [PubMed]
- Morphy, R.; Rankovic, Z. Designing multiple ligands- medicinal chemistry strategies and challenges. Curr. Pharm. Des. 2009, 15, 587–600. [Google Scholar] [CrossRef] [PubMed]
- Pfeiffer, A.; Brantl, V.; Herz, A.; Emrich, H.M. Psychotomimesis mediated by kappa opiate receptors. Science 1986, 233, 774–776. [Google Scholar] [CrossRef] [PubMed]
- Mello, N.K.; Negus, S.S. Interactions between kappa opioid agonists and cocaine. Preclinical studies. Ann. N. Y. Acad. Sci. 2000, 909, 104–132. [Google Scholar] [CrossRef] [PubMed]
- Ananthan, S. Opioid ligands with mixed mu/delta opioid receptor interactions: An emerging approach to novel analgesics. AAPS J. 2006, 8, E118–E125. [Google Scholar] [CrossRef] [PubMed]
- Lipkowski, A.W.; Konecka, A.M.; Sroczyńska, I. Double-enkephalins—Synthesis, activity on guinea-pig ileum, and analgesic effect. Peptides 1982, 3, 697–700. [Google Scholar] [CrossRef]
- Redkiewicz, P.; Dyniewicz, J.; Misicka, A. Biphalin—A Potent Opioid Agonist—As a Panacea for Opioid System-Dependent Pathophysiological Diseases? Int. J. Mol. Sci. 2021, 22, 11347. [Google Scholar] [CrossRef]
- Yamazaki, M.; Suzuki, T.; Narita, M.; Lipkowski, A.W. The opioid peptide analogue biphalin induces less physical dependence than morphine. Life Sci. 2001, 69, 1023–1028. [Google Scholar] [CrossRef]
- Mollica, A.; Davis, P.; Ma, S.W.; Porreca, F.; Lai, J.; Hruby, V.J. Synthesis and biological activity of the first cyclic biphalin analogues. Bioorg. Med. Chem. Lett. 2006, 16, 367–372. [Google Scholar] [CrossRef] [PubMed]
- Feliciani, F.; Pinnen, F.; Stefanucci, A.; Costante, R.; Cacciatore, I.; Lucente, G.; Mollica, A. Structure–activity relationships of biphalin analogs and their biological evaluation on opioid receptors. Mini Rev. Med. Chem. 2013, 13, 11–33. [Google Scholar] [CrossRef] [PubMed]
- Lipkowski, A.W.; Konecka, A.M.; Sadowski, B. Double enkephalins. J. Pharmacol. Pharm. 1982, 34, 69–71. [Google Scholar]
- Mollica, A.; Davis, P.; Ma, S.W.; Lai, J.; Porreca, F.; Hruby, V.J. Synthesis and biological evaluation of new biphalin analogues with non-hydrazine linkers. Bioorg. Med. Chem. Lett. 2005, 15, 2471–2475. [Google Scholar] [CrossRef] [PubMed]
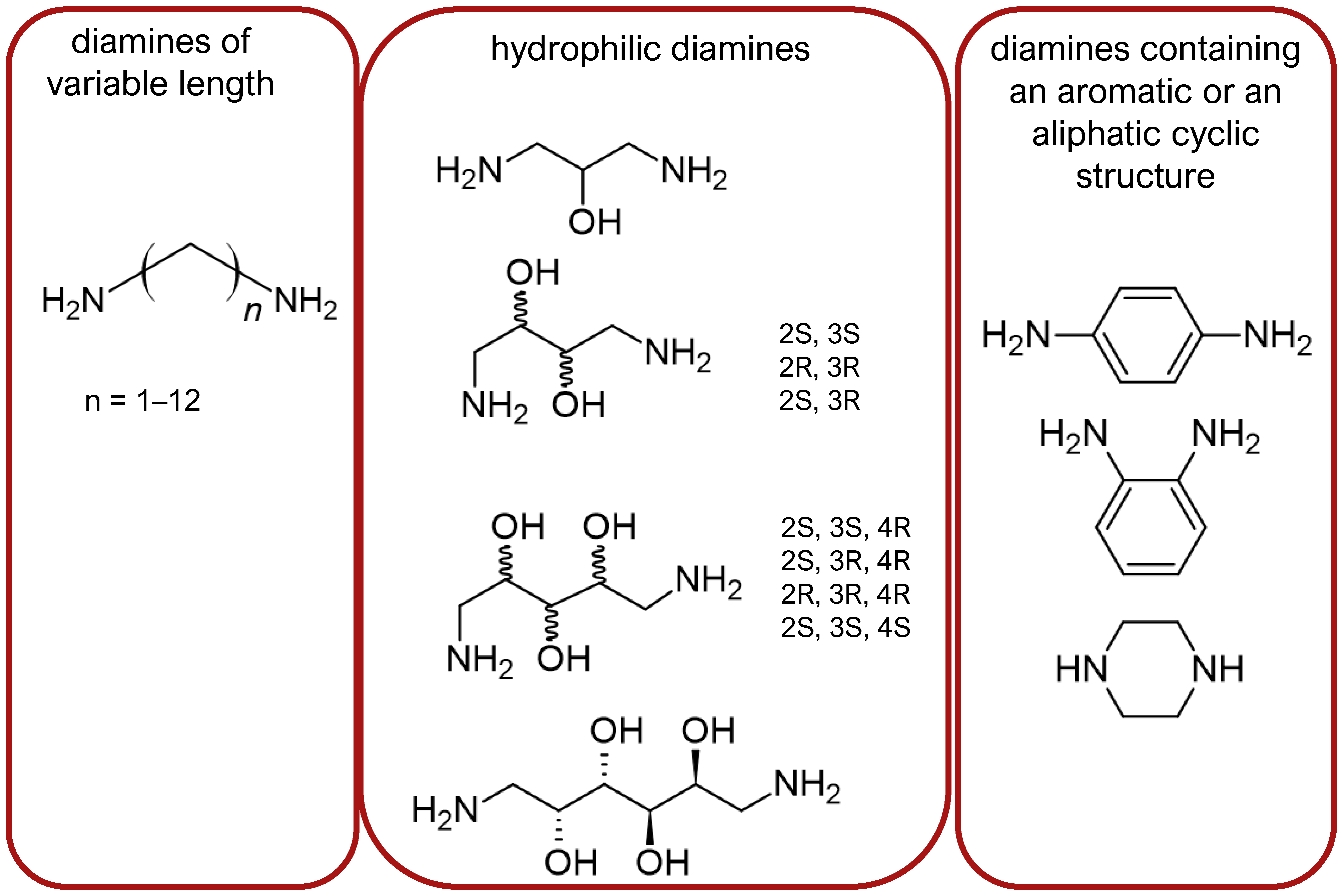
- Stepinski, J.; Zajaczkowski, I.; Kazem-Bek, D.; Temeriusz, A.; Lipkowski, A.W.; Tam, S.W. Use of hydrophilic diamines for bridging of two opioid peptide pharmacophores. Int. J. Pept. Protein Res. 1991, 38, 588–592. [Google Scholar] [CrossRef] [PubMed]
- Misicka, A.; Lipkowski, A.W.; Horvath, R.; Davis, P.; Porreca, F.; Yamamura, H.I.; Hruby, V.J. Structure–activity relationship of biphalin. The synthesis and biological activities of new analogs with modifications in positions 3 and 4. Life Sci. 1997, 60, 1263–1269. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Haq, W.; Xiang, L.; Lou, B.-S.; Hughes, R.; De Leon, I.A.; Davis, P.; Gillespie, T.J.; Romanowski, M.; Zhu, X.; et al. Modifications of the 4,4′-residues and SAR studies of biphalin, a highly potent opioid receptor active peptide. Bioorg. Med. Chem. Lett. 1998, 8, 555–560. [Google Scholar] [CrossRef] [PubMed]
- Mollica, A.; Pinnen, F.; Feliciani, F.; Stefanucci, A.; Lucente, G.; Davis, P.; Porreca, F.; Ma, S.-W.; Lai, J. New potent biphalin analogues containing p-F-L-phenylalanine at the 4,4′ positions and non-hydrazine linkers. Amino Acids 2011, 40, 1503–1511. [Google Scholar] [CrossRef] [PubMed]
- Mollica, A.; Pinnen, F.; Costante, R.; Locatelli, M.; Stefanucci, A.; Pieretti, S.; Davis, P.; Lai, J.; Rankin, D.; Porreca, F.; et al. Biological active analogues of the opioid peptide biphalin: Mixed α/β(3)-peptides. J. Med. Chem. 2013, 56, 3419–3423. [Google Scholar] [CrossRef]
- Frączak, O.; Lasota, A.; Kosson, P.; Leśniak, A.; Muchowska, A.; Lipkowski, A.W.; Olma, A. Biphalin analogs containing β(3)-homo-amino acids at the 4,4′ positions: Synthesis and opioid activity profiles. Peptides 2015, 66, 13–18. [Google Scholar] [CrossRef]
- DiMaio, J.; Schiller, P.W. A cyclic enkephalin analog with high in vitro opiate activity. Proc. Natl. Acad. Sci. USA 1980, 77, 7162–7166. [Google Scholar] [CrossRef] [PubMed]
- DiMaio, J.; Nguyen, T.M.; Lemieux, C.; Schiller, P.W. Synthesis and pharmacological characterization in vitro of cyclic enkephalin analogues: Effect of conformational constraints on opiate receptor selectivity. J. Med. Chem. 1982, 25, 1432–1438. [Google Scholar] [CrossRef]
- Schiller, P.W.; DiMaio, J. Opiate receptor subclasses differ in their conformational requirements. Nature 1982, 297, 74–76. [Google Scholar] [CrossRef] [PubMed]
- Janecka, A.; Fichna, J.; Kruszynski, R.; Sasaki, Y.; Ambo, A.; Costentin, J.; do-Rego, J.C. Synthesis and antinociceptive activity of cyclic endomorphin-2 and morphiceptin analogs. Biochem. Pharmacol. 2005, 71, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Perlikowska, R.; do-Rego, J.C.; Cravezic, A.; Fichna, J.; Wyrebska, A.; Toth, G.; Janecka, A. Synthesis and biological evaluation of cyclic endomorphin-2 analogs. Peptides 2010, 31, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Perlikowska, R.; Piekielna, J.; Fichna, J.; do-Rego, J.C.; Toth, G.; Janecki, T.; Janecka, A. Pharmacological Properties of Novel Cyclic Pentapeptides with u-opioid Receptor Agonist Activity. Med. Chem. 2014, 10, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Gentilucci, L.; Tolomelli, A.; De Marco, R.; Artali, R. Molecular docking of opiates and opioid peptides, a tool for the design of selective agonists and antagonists, and for the investigation of atypical ligand-receptor interactions. Curr. Med. Chem. 2012, 19, 1587–1601. [Google Scholar] [CrossRef] [PubMed]
- Fichna, J.; Perlikowska, R.; Wyrębska, A.; Gach, K.; Piekielna, J.; do-Rego, J.C.; Toth, G.; Kluczyk, A.; Janecki, T.; Janecka, A. Effect of 2′,6′-dimethyl-L-tyrosine (Dmt) on pharmacological activity of cyclic endomorphin-2 and morphiceptin analogs. Bioorg. Med. Chem. 2011, 19, 6977–6981. [Google Scholar] [CrossRef] [PubMed]
- Perlikowska, R.; Malfacini, D.; Cerlesi, M.C.; Calo’, G.; Piekielna, J.; Floriot, L.; Henry, T.; do-Rego, J.C.; Tömböly, C.; Kluczyk, A.; et al. Pharmacological characterization of endomorphin-2-based cyclic pentapeptides with methylated phenylalanine residues. Peptides 2014, 55, 145–150. [Google Scholar] [CrossRef]
- Zadina, J.E.; Nilges, M.R.; Morgenweck, J.; Zhang, X.; Hackler, L.; Fasold, M.B. Endomorphin analog analgesics with reduced abuse liability, respiratory depression, motor impairment, tolerance, and glial activation relative to morphine. Neuropharmacology 2016, 105, 215–227. [Google Scholar] [CrossRef]
- Perlikowska, R.; Piekielna, J.; Gentilucci, L.; De Marco, R.; Cerlesi, M.C.; Calo, G.; Artali, R.; Tömböly, C.; Kluczyk, A.; Janecka, A. Synthesis of mixed MOR/KOR efficacy cyclic opioid peptide analogs with antinociceptive activity after systemic administration. Eur. J. Med. Chem. 2016, 109, 276–286. [Google Scholar] [CrossRef]
- Piekielna, J.; Perlikowska, R.; do-Rego, J.C.; do-Rego, J.L.; Cerlesi, M.C.; Calo, G.; Kluczyk, A.; Łapiński, K.; Tömböly, C.; Janecka, A. Synthesis of mixed opioid affinity cyclic endomorphin-2 analogues with fluorinated phenylalanines. ACS Med. Chem. Lett. 2015, 6, 579–583. [Google Scholar] [CrossRef]
- Feehan, A.K.; Morgenweck, J.; Zhang, X.; Amgott-Kwan, A.T.; Zadina, J.E. Novel Endomorphin Analogs Are More Potent and Longer-Lasting Analgesics in Neuropathic, Inflammatory, Postoperative, and Visceral Pain Relative to Morphine. J. Pain 2017, 18, 1526–1541. [Google Scholar] [CrossRef]
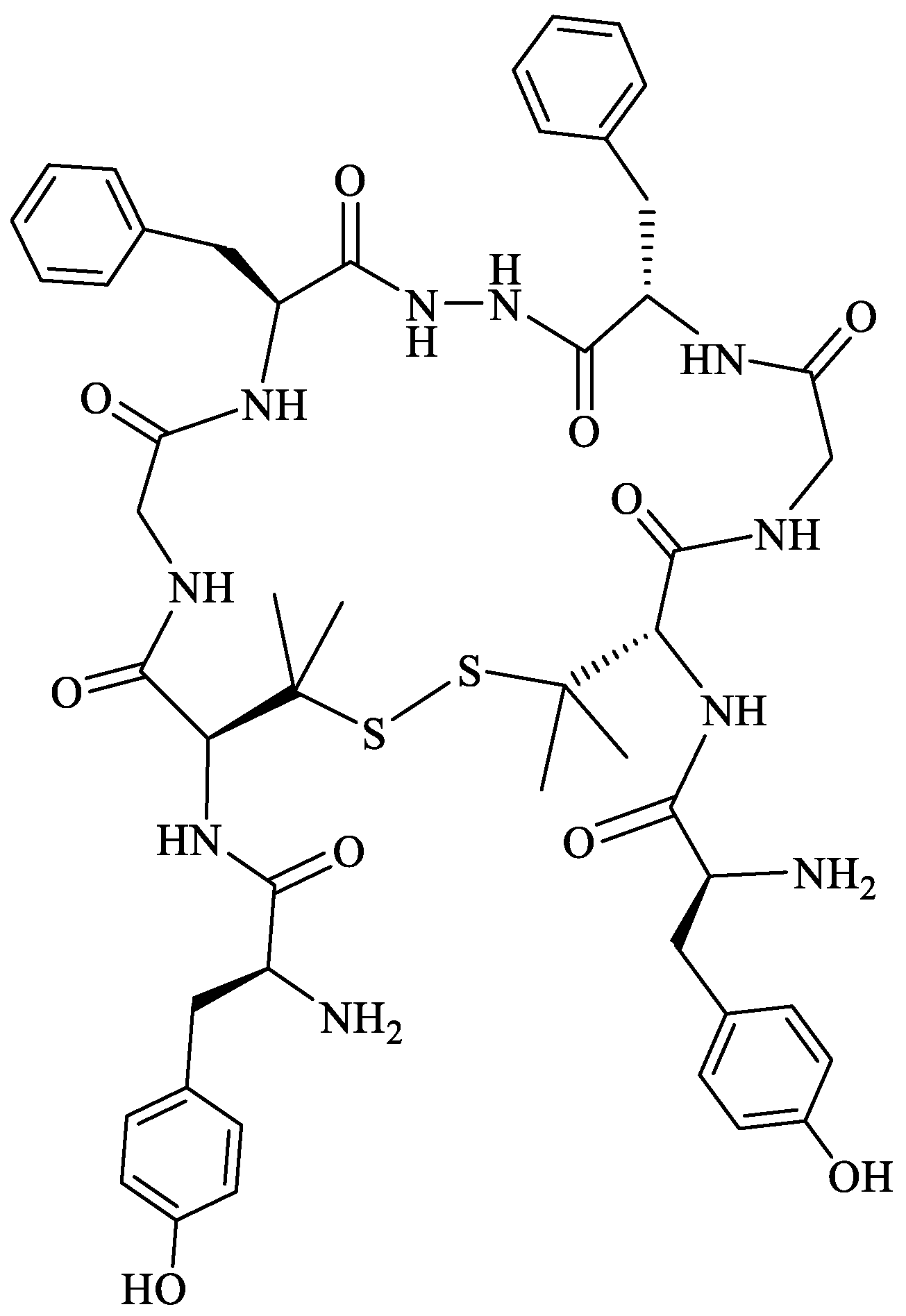
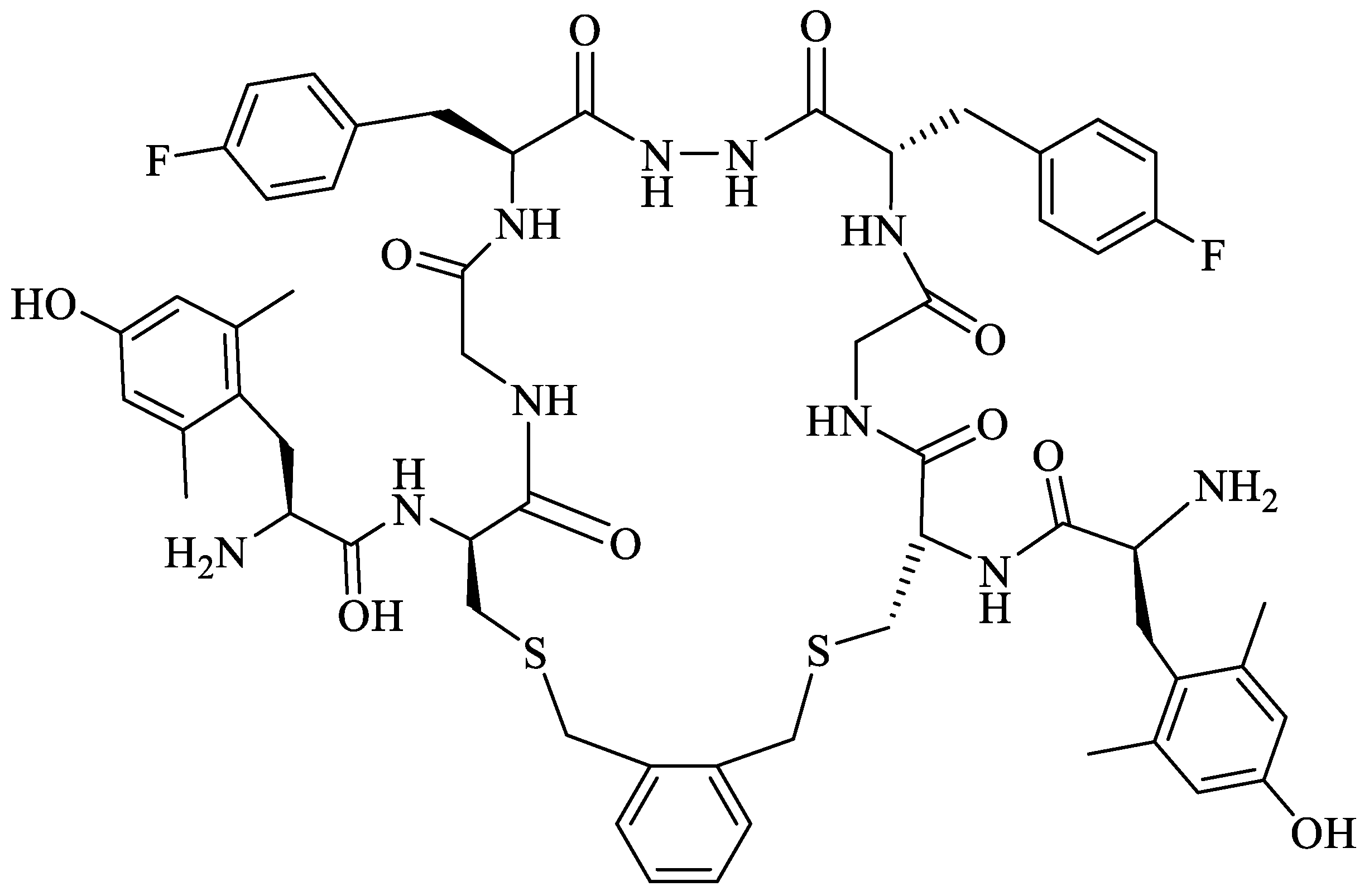
- Mollica, A.; Carotenuto, A.; Novellino, E.; Limatola, A.; Costante, R.; Pinnen, F.; Stefanucci, A.; Pieretti, S.; Borsodi, A.; Samavati, R.; et al. Novel cyclic biphalin analogue with improved antinociceptive properties. ACS Med. Chem. Lett. 2014, 5, 1032–1036. [Google Scholar] [CrossRef]
- Stefanucci, A.; Carotenuto, A.; Macedonio, G.; Novellino, E.; Pieretti, S.; Marzoli, F.; Szűcs, E.; Erdei, A.I.; Zádor, F.; Benyhe, S.; et al. Cyclic Biphalin Analogues Incorporating a Xylene Bridge: Synthesis, Characterization, and Biological Profile. ACS Med. Chem. Lett. 2017, 8, 858–863. [Google Scholar] [CrossRef]
- Stefanucci, A.; Novellino, E.; Mirzaie, S.; Macedonio, G.; Pieretti, S.; Minosi, P.; Szücs, E.; Erdei, A.I.; Zádor, F.; Benyhe, S.; et al. Opioid receptor activity and analgesic potency of DPDPE peptide analogues containing a xylene bridge. ACS Med. Chem. Lett. 2017, 8, 449–454. [Google Scholar] [CrossRef]
- Stefanucci, A.; Dimmito, M.P.; Macedonio, G.; Ciarlo, L.; Pieretti, S.; Novellino, E.; Lei, W.; Barlow, D.; Houseknecht, K.L.; Streicher, J.M.; et al. Potent, Efficacious, and Stable Cyclic Opioid Peptides with Long Lasting Antinociceptive Effect after Peripheral Administration. J. Med. Chem. 2020, 63, 2673–2687. [Google Scholar] [CrossRef]
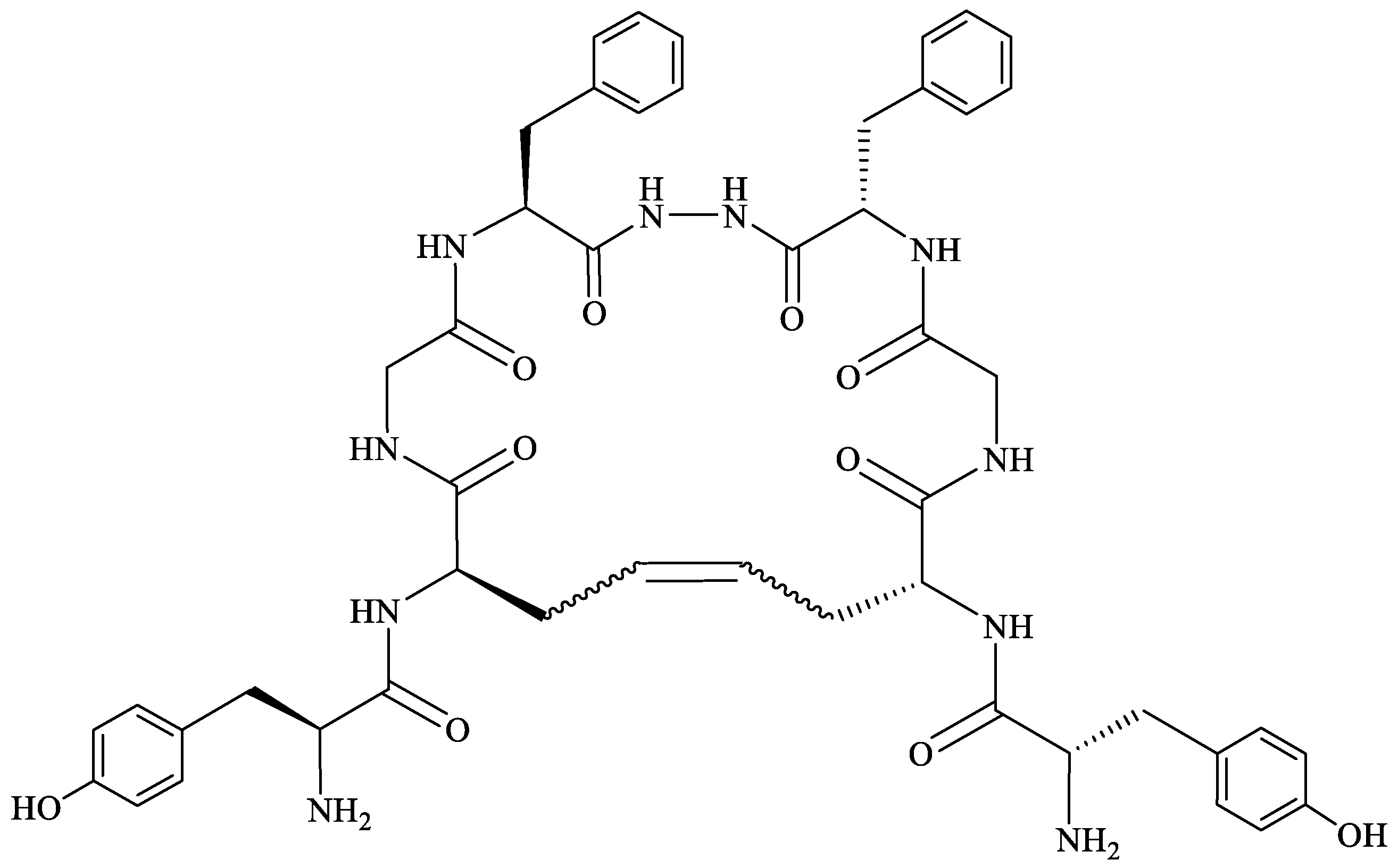
- Gary, S.; Bloom, S. Peptide Carbo cycles: From -SS- to -CC- via a Late-Stage “Snip-and-Stitch”. ACS Cent. Sci. 2022, 8, 1537–1547. [Google Scholar] [CrossRef]
- Stefanucci, A.; Lei, W.; Pieretti, S.; Dimmito, M.P.; Luisi, G.; Novellino, E.; Nowakowski, M.; Koźmiński, W.; Mirzaie, S.; Zengin, G.; et al. Novel Cyclic Biphalin Analogues by Ruthenium-Catalyzed Ring Closing Metathesis: In Vivo and in Vitro Biological Profile. ACS Med. Chem. Lett. 2019, 10, 450–456. [Google Scholar] [CrossRef]
- Fujii, H. Twin and triplet drugs in opioid research. Top. Curr. Chem. 2011, 299, 239–275. [Google Scholar] [CrossRef]
- Kleczkowska, P.; Lipkowski, A.W.; Tourwé, D.; Ballet, S. Hybrid opioid/non-opioid ligands in pain research. Curr. Pharm. Des. 2013, 19, 7435–7450. [Google Scholar] [CrossRef]
- Kleczkowska, P.; Nowicka, K.; Bujalska-Zadrozny, M.; Hermans, E. Neurokinin-1 receptor-based bivalent drugs in pain management: The journey to nowhere? Pharmacol. Ther. 2019, 196, 44–58. [Google Scholar] [CrossRef]
- Dvoracsko, S.; Stefanucci, A.; Novellino, E.; Mollica, A. The design of multitarget ligands for chronic and neuropathic pain. Future Med. Chem. 2015, 7, 2469–2483. [Google Scholar] [CrossRef]
- Wtorek, K.; Adamska-Bartłomiejczyk, A.; Piekielna-Ciesielska, J.; Ferrari, F.; Ruzza, C.; Kluczyk, A.; Piasecka-Zelga, J.; Calo’, G.; Janecka, A. Synthesis and Pharmacological Evaluation of Hybrids Targeting Opioid and Neurokinin Receptors. Molecules 2019, 24, 4460. [Google Scholar] [CrossRef]
- Cunningham, C.W.; Elballa, W.M.; Vold, S.U. Bifunctional opioid receptor ligands as novel analgesics. Neuropharmacology 2019, 151, 195–207. [Google Scholar] [CrossRef]
- Kiguchi, N.; Ding, H.; Ko, M.C. Therapeutic potentials of NOP and MOP receptor coactivation for the treatment of pain and opioid abuse. J. Neurosci. Res. 2020, 100, 191–202. [Google Scholar] [CrossRef]
- Guillemyn, K.; Starnowska, J.; Lagard, C.; Dyniewicz, J.; Rojewska, E.; Mika, J.; Chung, N.N.; Utard, V.; Kosson, P.; Lipkowski, A.W.; et al. Bifunctional Peptide-Based Opioid Agonist-Nociceptin Antagonist Ligands for Dual Treatment of Acute and Neuropathic Pain. J. Med. Chem. 2016, 59, 3777–3792. [Google Scholar] [CrossRef]
- Nguyen, T.; Marusich, J.; Li, J.X.; Zhang, Y. Neuropeptide FF and Its Receptors: Therapeutic Applications and Ligand Development. J. Med. Chem. 2020, 63, 12387–12402. [Google Scholar] [CrossRef]
- Zhang, M.; Xu, B.; Li, N.; Zhang, R.; Zhang, Q.; Chen, D.; Rizvi, S.F.A.; Xu, K.; Shi, Y.; Yu, B.; et al. OFP011 Cyclic Peptide as a Multifunctional Agonist for Opioid/Neuropeptide FF Receptors with Improved Blood–brain Barrier Penetration. ACS Chem. Neurosci. 2022, 13, 3078–3092. [Google Scholar] [CrossRef]
- Zhang, M.; Xu, B.; Li, N.; Liu, H.; Shi, X.; Zhang, Q.; Shi, Y.; Xu, K.; Xiao, J.; Chen, D.; et al. Synthesis and biological characterization of cyclic disulfide-containing peptide analogs of the multifunctional opioid/neuropeptide FF receptor agonists that produce long-lasting and non-tolerant antinociception. J. Med. Chem. 2020, 63, 15709–15725. [Google Scholar] [CrossRef]
- Drieu la Rochelle, A.; Guillemyn, K.; Dumitrascuta, M.; Martin, C.; Utard, V.; Quillet, R.; Schneider, S.; Daubeuf, F.; Willemse, T.; Mampuys, P.; et al. A bifunctional-biased mu-opioid agonist-neuropeptide FF receptor antagonist as analgesic with improved acute and chronic side effects. Pain 2018, 159, 1705–1718. [Google Scholar] [CrossRef] [PubMed]
- Dumitrascuta, M.; Martin, C.; Ballet, S.; Spetea, M. Bifunctional Peptidomimetic G Protein-Biased Mu-Opioid Receptor Agonist and Neuropeptide FF Receptor Antagonist KGFF09 Shows Efficacy in Visceral Pain without Rewarding Effects after Subcutaneous Administration in Mice. Molecules 2022, 27, 8785. [Google Scholar] [CrossRef]
- Futaki, S. Oligoarginine vectors for intracellular delivery: Design and cellular-uptake mechanisms. Biopolymers 2006, 84, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Z.; Wang, M.M.; Wang, S.Y.; Wang, X.F.; Yang, W.J.; Zhao, Y.N.; Han, F.T.; Zhang, Y.; Gu, N.; Wang, C.L. Novel Cyclic Endomorphin Analogues with Multiple Modifications and Oligoarginine Vector Exhibit Potent Antinociception with Reduced Opioid-like Side Effects. J. Med. Chem. 2021, 64, 16801–16819. [Google Scholar] [CrossRef] [PubMed]
- McFadyen, I.J.; Ho, J.C.; Mosberg, H.I.; Traynor, J.R. Modifications of the cyclic mu receptor selective tetrapeptide Tyr-c[D-Cys-Phe-D-Pen]NH2 (Et): Effects on opioid receptor binding and activation. J. Pept. Res. 2000, 55, 255–261. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mosberg, H.I.; Omnaas, J.R.; Lomize, A.; Heyl, D.L.; Nordan, I.; Mousigian, C.; Davis, P.; Porreca, F. Development of a model for the delta opioid receptor pharmacophore. 2. Conformationally restricted Phe3 replacements in the cyclic delta receptor selective tetrapeptide Tyr-c[D-Cys-Phe-D-Pen]OH (JOM-13). J. Med. Chem. 1994, 37, 4384–4391. [Google Scholar] [CrossRef] [PubMed]
- Purington, L.C.; Sobczyk-Kojiro, K.; Pogozheva, I.D.; Traynor, J.R.; Mosberg, H.I. Development and in vitro characterization of a novel bifunctional μ-agonist/δ-antagonist opioid tetrapeptide. ACS Chem. Biol. 2011, 6, 1375–1381. [Google Scholar] [CrossRef] [PubMed]
- Mosberg, H.I.; Yeomans, L.; Anand, J.P.; Porter, V.; Sobczyk-Kojiro, K.; Traynor, J.R.; Jutkiewicz, E.M. Development of a bioavailable μ opioid receptor (MOPr) agonist, δ opioid receptor (DOPr) antagonist peptide that evokes antinociception without development of acute tolerance. J. Med. Chem. 2014, 57, 3148–3153. [Google Scholar] [CrossRef] [PubMed]
- Varga, B.R.; Streicher, J.M.; Majumdar, S. Strategies towards safer opioid analgesics-A review of old and upcoming targets. Br. J. Pharmacol. 2023, 180, 975–993. [Google Scholar] [CrossRef] [PubMed]
- Rossino, G.; Marchese, E.; Galli, G.; Verde, F.; Finizio, M.; Serra, M.; Linciano, P.; Collina, S. Peptides as Therapeutic Agents: Challenges and Opportunities in the Green Transition Era. Molecules. 2023, 28, 7165. [Google Scholar] [CrossRef]
- Mu, R.; Zhu, D.; Abdulmalik, S.; Wijekoon, S.; Wei, G.; Kumbar, S.G. Stimuli-responsive peptide assemblies: Design, self-assembly, modulation, and biomedical applications. Bioact. Mater. 2024, 35, 181–207. [Google Scholar] [CrossRef]
- Coluzzi, F.; Rullo, L.; Scerpa, M.S.; Losapio, L.M.; Rocco, M.; Billeci, D.; Candeletti, S.; Romualdi, P. Current and Future Therapeutic Options in Pain Management: Multi-mechanistic Opioids Involving Both MOR and NOP Receptor Activation. CNS Drugs 2022, 36, 617–632. [Google Scholar] [CrossRef]
- Martin, C.; De Baerdemaeker, A.; Poelaert, J.; Madder, A.; Hoogenboom, R.; Ballet, S. Controlled-release of opioids for improved pain management. Mater. Today 2016, 19, 491–502. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, J.; Li, P.; Shi, L.; Zhang, S.; Guo, Q.; Yang, Y. Advances in the use of local anesthetic extended-release systems in pain management. Drug Deliv. 2024, 31, 2296349. [Google Scholar] [CrossRef]
- Hohenwarter, L.; Böttger, R.; Li, S.D. Modification and Delivery of Enkephalins for Pain Modulation. Int. J. Pharm. 2023, 646, 123425. [Google Scholar] [CrossRef]
- Ali, M.; Manolios, N. Peptide delivery systems. Lett. Pept. Sci. 2001, 8, 289–294. [Google Scholar] [CrossRef]
- Knauer, N.; Pashkina, E.; Apartsin, E. Topological Aspects of the Design of Nanocarriers for Therapeutic Peptides and Proteins. Pharmaceutics 2019, 11, 91. [Google Scholar] [CrossRef]
- Dillon, C.; Hughes, H.; O’Reilly, N.J.; McLoughlin, P. Formulation and characterisation of dissolving microneedles for the transdermal delivery of therapeutic peptides. Int. J. Pharm. 2017, 526, 125–136. [Google Scholar] [CrossRef]
- Mdanda, S.; Ubanako, P.; Kondiah, P.P.D.; Kumar, P.; Choonara, Y.E. Recent Advances in Microneedle Platforms for Transdermal Drug Delivery Technologies. Polymers 2021, 13, 2405. [Google Scholar] [CrossRef] [PubMed]
- Umeyor, C.E.; Shelke, V.; Pol, A.; Kolekar, P.; Jadhav, S.; Tiwari, N.; Anure, A.; Nayak, A.; Bairagi, G.; Agale, A.; et al. Biomimetic microneedles: Exploring the recent advances on a microfabricated system for precision delivery of drugs, peptides, and proteins. Future J. Pharm. Sci. 2023, 9, 103. [Google Scholar] [CrossRef]
- Lochhead, J.J.; Thorne, R.G. Intranasal delivery of biologics to the central nervous system. Adv. Drug Deliv. Rev. 2012, 64, 614–628. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.; Dumitrascuta, M.; Mannes, M.; Lantero, A.; Bucher, D.; Walker, K.; Van Wanseele, Y.; Oyen, E.; Hernot, S.; Van Eeckhaut, A.; et al. Biodegradable Amphipathic Peptide Hydrogels as Extended-Release System for Opioid Peptides. J. Med. Chem. 2018, 61, 9784–9789. [Google Scholar] [CrossRef] [PubMed]
- Heremans, J.; Chevillard, L.; Mannes, M.; Mangialetto, J.; Leroy, K.; White, J.F.; Lamouroux, A.; Vinken, M.; Gardiner, J.; Van Mele, B.; et al. Impact of doubling peptide length on in vivo hydrogel stability and sustained drug release. J. Control. Release 2022, 350, 514–524. [Google Scholar] [CrossRef] [PubMed]
- Ruan, Y.; Yao, L.; Zhang, B.; Zhang, S.; Guo, J. Antinociceptive properties of nasal delivery of neurotoxin-loaded nanoparticles coated with polysorbate-80. Peptides 2011, 32, 1526–1529. [Google Scholar] [CrossRef] [PubMed]
- Mirra, D.; Spaziano, G.; Esposito, R.; Santonocito, D.; Filosa, R.; Roviezzo, F.; Malgieri, G.; D’Abrosca, G.; Iovino, P.; Gallelli, L.; et al. Formulation of Solid Lipid Nanoparticles Loaded with Nociceptin/Orphanin FQ (N/OFQ) and Characterization in a Murine Model of Airway Hyperresponsiveness. Pharmaceuticals 2022, 15, 1210. [Google Scholar] [CrossRef] [PubMed]
- Mura, P.; Mennini, N.; Nativi, C.; Richichi, B. In situ mucoadhesive-thermosensitive liposomal gel as a novel vehicle for nasal extended delivery of opiorphin. Eur. J. Pharm. Biopharm. 2018, 122, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Cooper, B.M.; Iegre, J.; O’ Donovan, D.H.; Ölwegård Halvarsson, M.; Spring, D.R. Peptides as a platform for targeted therapeutics for cancer: Peptide-drug conjugates (PDCs). Chem. Soc. Rev. 2021, 50, 1480–1494. [Google Scholar] [CrossRef]
- Wan, J.; Brust, A.; Bhola, R.F.; Jha, P.; Mobli, M.; Lewis, R.J.; Christie, M.J.; Alewood, P.F. Inhibition of the norepinephrine transporter by χ-conotoxin dendrimers. J. Pept. Sci. 2016, 22, 280–289. [Google Scholar] [CrossRef] [PubMed]
- Pacifico, S.; Albanese, V.; Illuminati, D.; Fantinati, A.; Marzola, E.; Ferrari, F.; Neto, J.A.; Sturaro, C.; Ruzza, C.; Calò, G.; et al. Tetrabranched Hetero-Conjugated Peptides as Bifunctional Agonists of the NOP and Mu Opioid Receptors. Bioconjug. Chem. 2019, 30, 2444–2451. [Google Scholar] [CrossRef]
- Kiguchi, N.; Ding, H.; Kishioka, S.; Ko, M.C. Nociceptin/Orphanin FQ Peptide Receptor-Related Ligands as Novel Analgesics. Curr. Top. Med. Chem. 2020, 20, 2878–2888. [Google Scholar] [CrossRef]
- Pacifico, S.; Albanese, V.; Illuminati, D.; Marzola, E.; Fabbri, M.; Ferrari, F.; Holanda, V.A.D.; Sturaro, C.; Malfacini, D.; Ruzza, C.; et al. Novel Mixed NOP/Opioid Receptor Peptide Agonists. J. Med. Chem. 2021, 64, 6656–6669. [Google Scholar] [CrossRef] [PubMed]
- El Daibani, A.; Che, T. Spotlight on Nociceptin/Orphanin FQ Receptor in the Treatment of Pain. Molecules 2022, 27, 595. [Google Scholar] [CrossRef]
- Stefanucci, A.; Iobbi, V.; Della Valle, A.; Scioli, G.; Pieretti, S.; Minosi, P.; Mirzaie, S.; Novellino, E.; Mollica, A. In Silico Identification of Tripeptides as Lead Compounds for the Design of KOR Ligands. Molecules 2021, 26, 4767. [Google Scholar] [CrossRef]
- Ebrahimi, S.B.; Samanta, D. Engineering protein-based therapeutics through structural and chemical design. Nat. Commun. 2023, 14, 2411. [Google Scholar] [CrossRef]
- Alfei, S.; Schito, A.M. From Nanobiotechnology, Positively Charged Biomimetic Dendrimers as Novel Antibacterial Agents: A Review. Nanomaterials 2020, 10, 2022. [Google Scholar] [CrossRef]
- Alshawwa, S.Z.; Kassem, A.A.; Farid, R.M.; Mostafa, S.K.; Labib, G.S. Nanocarrier Drug Delivery Systems: Characterization, Limitations, Future Perspectives and Implementation of Artificial Intelligence. Pharmaceutics 2022, 14, 883. [Google Scholar] [CrossRef]


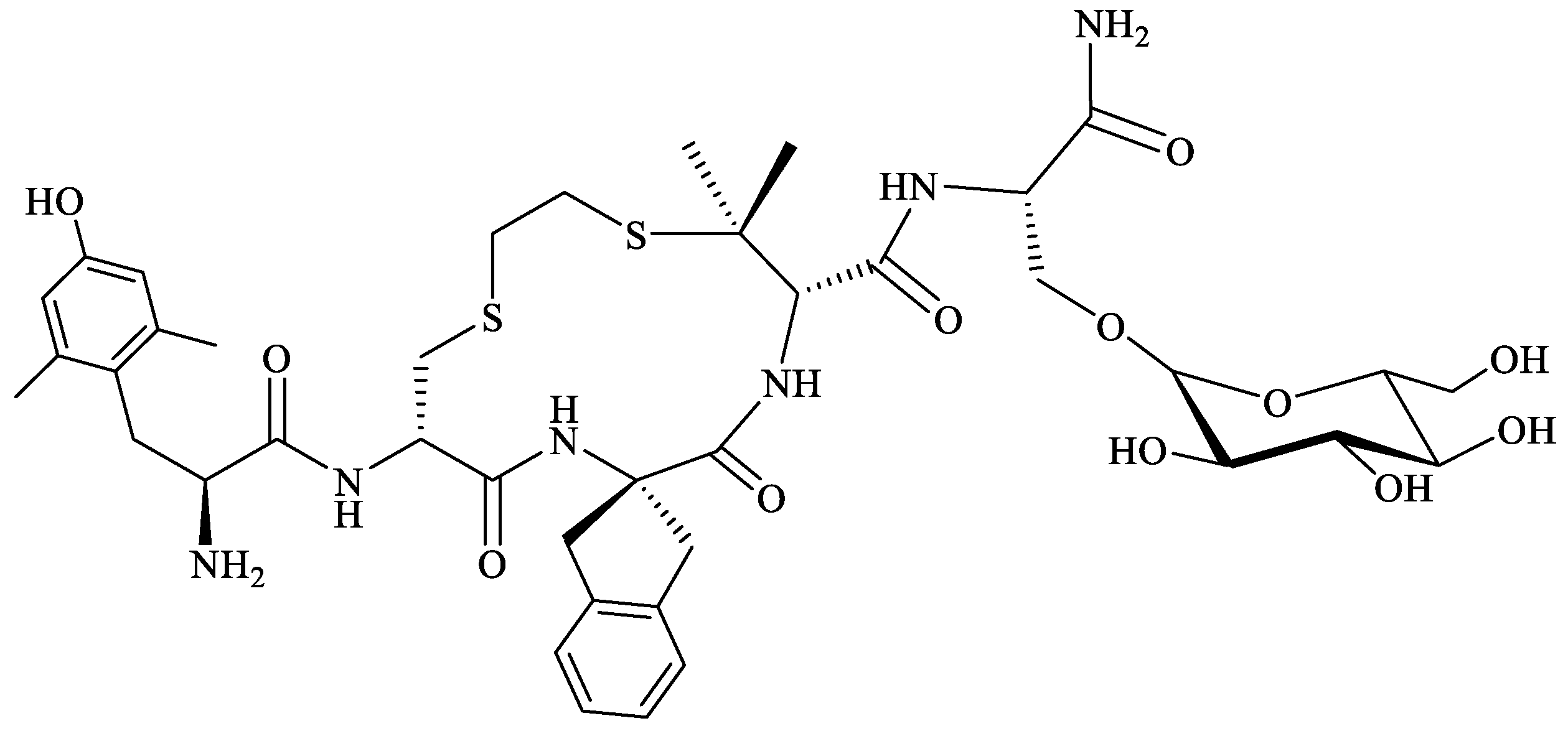
| Endogenous Peptide | Amino Acid Sequence | Opioid Receptor Affinity |
|---|---|---|
| Mammalian | ||
| [Met]enkephalin | H-Tyr-Gly-Gly-Phe-Met-OH | DOR, MOR |
| [Leu]enkephalin | H-Tyr-Gly-Gly-Phe-Leu-OH | (DOR >> MOR) |
| dynorphin A | H-Tyr-Gly-Gly-Phe-Leu-Arg-Arg-Ile-Arg-Pro-Lys-Leu-Lys-Trp-Asp-Asn-Gln-OH | KOR, MOR, DOR |
| dynorphin B | H-Tyr-Gly-Gly-Phe-Leu-Arg-Arg-Gln-Phe-Lys-Val-Val-Thr-OH | (KOR >> MOR and DOR) |
| β-endorphin | H-Tyr-Gly-Gly-Phe-Met-Thr-Ser-Glu-Lys-Ser-Gln-Thr-Pro-Leu-Val-Thr-Leu-Phe-Lys-Asn-Ala-Ile-Ile-Lys-Asn-Ala-Tyr-Lys-Lys-Gly-Glu-OH | MOR, DOR |
| (MOR = DOR) | ||
| endomorphin-1 | H-Tyr-Pro-Trp-Phe-NH2 | MOR |
| endomorphin-2 | H-Tyr-Pro-Phe-Phe-NH2 | |
| nociceptin/orphanin FQ (N/OFQ) | H-Phe-Gly-Gly-Phe-Thr-Gly-Ala-Arg-Lys-Ser-Ala-Arg-Lys-Leu-Ala-Asn-Gln-OH | NOP |
| Origin | Peptide | Amino Acid Sequence | Opioid Receptor Affinity |
|---|---|---|---|
| β-casein | bovine β-casomorphin(1–7) | H-Tyr-Pro-Phe-Pro-Gly-Pro-Ile-OH | MOR |
| human β-casomorphin(1–7) | H-Tyr-Pro-Phe-Val-Glu-Pro-Ile-OH | MOR | |
| morphiceptin | H-Tyr-Pro-Phe-Pro-NH2 | MOR | |
| hemoglobin | hemorphin-4 | H-Tyr-Pro-Trp-Thr-OH | MOR |
| amphibian skin * | dermorphin | H-Tyr-d-Ala-Phe-Gly-Tyr-Pro-Ser-NH2 | MOR |
| dermenkephalin | H-Tyr-d-Met-Phe-His-Leu-Met-Asp-NH2 | DOR | |
| deltorphin I | H-Tyr-d-Ala-Phe-Asp-Val-Val-Gly-NH2 | DOR | |
| deltorphin II | H-Tyr-d-Ala-Phe-Glu-Val-Val-Gly-NH2 | DOR |
| Peptide | Sequence | Organism | Ref. |
|---|---|---|---|
| α-conotoxin RgIA | GCCSDPRCRYRCR disulfide bonds: C2–C8, C3–C12 | Conus regius | [46] |
| α-conopeptide Eu1.6 | GCCSNPACMLKNPNLCa disulfide bonds: C2–C8, C3–C16 | Conus eburneus | [47] |
| ω-conotoxin MVIIA (SNX-111, ziconotide, or Prialt®) | CKGKGAKCSRLMYDCCTGSCRSGKCa disulfide bonds: C1–C16, C8–C20, C15–C25 | Conus magus | [43] |
| ω-conotoxin GVIA (SNX-124) | CKSOGSSCSOTSYNCCRSCNOYTKRCYa disulfide bonds: C1–C16, C8–C19, C15–C26 | Conus geographus | [43] |
| CVIID ω-conotoxin | CKSKGAKCSKLMYDCCSGSCSGTVGRCa disulfide bonds: C1–C16, C8–C20, C15–C27 | Conus catus | [43,48] |
| ω-conotoxin SO-3 | CKAAGKPCSRIAYNCCTGSCRSGKCa disulfide bonds: C1–C16, C8–C20, C15–C25 | Conus striatus | [43,44] |
| FVIA ω-conotoxin | CKGTGKSCSRIAYNCCTGSCRSGKCa disulfide bonds: C1–C16, C8–C20, C15–C25 | Conus fulmen | [49] |
| CVIE ω-conotoxins | CKGKGASCRRTSYDCCTGSCRSGRCa disulfide bonds: C1–C16, C8–C20, C15–C25 | Conus catus | [50] |
| CVIF ω-conotoxins | CKGKGASCRRTSYDCCTGSCRLGRCa disulfide bonds: C1–C16, C8–C20, C15–C25 | Conus catus | [50] |
| MoVIA ω-conotoxins | CKPOGSKCSOSMRDCCTTCISYTKRCRKYYN disulfide bonds: C1–C16, C8–C19, C15–C26 | Conus moncuri | [45] |
| MoVIB ω-conotoxins | CKPOGSKCSOSMRDCCTTCISYTKRCRKYY disulfide bonds: C1–C16, C8–C19, C15–C26 | Conus moncuri | [45] |
| RsXXIVA | CKGQSCSSCSTKEFCLSKGSRLMYDCCTGSCCGVKTAGVT disulfide bonds: location not reported | Conus regularis | [51] |
| Peptide | Sequence | Organism | Ref. |
|---|---|---|---|
| Purotoxin-1 (PT1) | GYCAEKGIRCDDIHCCTGLKCKCNASGYNCVCRKKa disulfide bonds: C3–C16, C10–C21, C15–C32, C23–C30 | Lycosa kazakhstanicus | [54] |
| Purotoxin-2 (PT2) | AKACTPLLHDCSHDRHSCCRGDMFKYVCDCFYPEGEDKTEVCSCQQPKSHKIAEKIIDKAKTTLa disulfide bonds: C4–C19, C11–C28, C18–C44, C30–C42 | Lycosa kazakhstanicus | [54] |
| PnTx2-6 (δ-CNTX-Pn2a) | ATCAGQDQPCKETCDCCGERGECVCGGPCICRQGYFWIAWYKLANCKK disulfide bonds: C3–C17, C10–C23, C14–C46, C16–C31, C25–C29 | Phoneutria nigriventer | [55,56] |
| PnTx2-5 | ATCAGQDQTCKVTCDCCGERGECVCGGPCICRQGNFLIAWYKLASCKK disulfide bonds: C3–C17, C10–C23, C14–C46, C16–C31, C25–C29 | Phoneutria nigriventer | [55,56] |
| PnTx3-1 | AECAAVYERCGKGYKRCCEERPCKCNIVMDNCTCKKFISEL disulfide bonds: C3–C18, C10–C23, C17–C34, C25–C32 | Phoneutria nigriventer | [56] |
| PnTx3-2 (Tx3-2, PNTx3-2) | ACAGLYKKCGKGASPCCEDRPCKCDLAMGNCICKKKFIEFFGGGK disulfide bonds: C2–C17, C9–C22, C16–C33, C24–C31 | Phoneutria nigriventer | [56] |
| PnTx3-3 (Phα1β) | GCANAYKSCNGPHTCCWGYNGYKKACICSGXNWK disulfide bonds: C2–C16, C9–C26, C15–C28 | Phoneutria nigriventer | [56,57] |
| PnTx3-6 (Phα1β) | ACIPRGEICTDDCECCGCDNQCYCPPGSSLGIFKCSCAHANKYFCNRKKEKCKKA disulfide bonds: C2–C16, C9–C22, C13–C52, C15–C37, C18–C45, C24–C35 | Phoneutria nigriventer | [58] |
| PnTx3-4 | SCSINVGDFCDGKKDDCQCCRDNAFCSCVIFGYKTNCRCEVGTTATSYGICMAKHKCGRQTTCTKPCLSKRCKKNHG disulfide bonds: C2–C20, C10–C26, C17–C51, C19–C39, C28–C37, C57–C63, C67–C72 | Phoneutria nigriventer | [56] |
| PnTx3-5 | GCIGRNESCKFDRHGCCWPWSCSCWNKEGQPESDVWCECSLKIGK disulfide bonds: C2–C17, C9–C22, C16–C39, C24–C37 | Phoneutria nigriventer | [56,59] |
| PnTx4(6-1) (δ-Ctenitoxin-Pn1a, δ-CNTX-Pn1a) | CGDINAACKEDCDCCGYTTACDCYWSKSCKCREAAIVIYTAPKKKLTC disulfide bonds: C1–C15, C8–C21, C12–C48, C14–C31, C23–C29 | Phoneutria nigriventer | [56,60] |
| β-TRTX-Cd1a | DCLGWFKSCDPKNDKCCKNYSCSRRDRWCKYDLa disulfide bonds: C2–C17, C9–C22, C16–C29 | Ceratogyrus darling | [61] |
| JzTx-34 | ACREWLGGCSKDADCCAHLECRKKWPYHCVWDWTV disulfide bonds: C2–C16, C9–C21, C15–C29 | Chilobrachys guangxiensis | [62] |
| Hainantoxin-IV (μ-TRTX-Hhn1b, HNTX-IV) | ECLGFGKGCNPSNDQCCKSSNLVCSRKHRWCKYEIa disulfide bonds: C2–C17, C9–C24, C16–C31 | Ornithoctonus hainana | [63] |
| Huwentoxin-I (HWTX-I or HWAP-I) | ACKGVFDACTPGKNECCPNRVCSDKHKWCKWKL disulfide bonds: C2–C17, C9–C22, C16–C29 | Ornithoctonus huwena | [64,65] |
| Huwentoxin-XVI (HWTX-XVI) | CIGEGVPCDENDPRCCSGLVCLKPTLHGIWYKSYYCYKK disulfide bonds: C1–C16, C8–C21, C15–C36 | Ornithoctonus huwena | [66] |
| SNX-482 | GVDKAGCRYMFGGCSVNDDCCPRLGCHSLFSYCAWDLTFSD disulfide bonds: C7–C21, C14–C26, C20–C33 | Hysterocrates gigas | [67] |
| Phlotoxin 1 (Ph1Tx1) | ACLGQWDSCDPKASKCCPNYACEWKYPWCRYKLF disulfide bonds: C2–C17, C9–C22, C16–C29 | Phlogiellus spider | [68] |
| ω-Agatoxin IVA | KKKCIAKDYGRCKWGGTPCCRGRGCICSIMGTNCECKPRLIMEGLGLA disulfide bonds: C4–C20, C12–C25, C19–C36, C27–C34 | Agelenopsis aperta | [69] |
| Tick peptide | LVVYPWTKM | Amblyomma testindiarium (tick) | [70] |
| Peptide | Sequence | Organism | Ref. |
|---|---|---|---|
| BmK AGAP | VRDGYIADDKNCAYFCGRNAYCDDECKKNGAESGYCQWAGVYGNACWCYKLPDKVPIRVPGKCNGG disulfide bonds; C12–C63, C16–C36, C22–C46, C26–C48 | Buthus martensi | [80] |
| BmK AGAP-SYPU2 | VKDGYIVDDKNCAYFCGRNAYCDDECEKNGAESGYCQWAGVYGNACWCYKLPDKVPIRVPGRCNG disulfide bonds: C12–C63, C16–C36, C22–C46, C26–C48 | Buthus martensi | [80] |
| BmK AS | DNGYLLDKYTGCKVWCVINNESCNSECKIRGGYYGYCYFWKLACFCQGARKSELWNYNTNKCDGKL disulfide bonds: C12–C62, C16–C37, C23–C44, C27–C46 | Buthus martensi | [78,81] |
| BmK AS1 | DNGYLLNKYTGCKIWCVINNESCNSECKLRRGNYGYCYFWKLACYCEGAPKSELWAYETNKCDGKL disulfide bonds: C12–C62, C16–C37, C23–C44, C27–C46 | Buthus martensi | [81] |
| BmK IT2 | DGYIKGKSGCRVACLIGNQGCLKDCRAYGASYGYCWTWGLACWCEGLPDNKTWKSESNTCG disulfide bonds: C10–C60, C14–C35, C21–C42, C25–C44 | Buthus marten | [78,82] |
| BmK-YA | YGGYMNPAa | Buthus marten | [83] |
| BmK Ang P1 | KKNGYAVDSSGKVAE | Buthus marten | [84] |
| TsNTxP | MKRMILFISCLLLIDIVVGGREGYPADSKGCKITCFLTAAGYCNTECTLKKGSSGYCAWPACYCYGLPDSVKIWTSETNKCGKK disulfide bonds: C31–C81, C35–C57, C43–C62, C47–C64 | Tityus serrulatus | [85] |
| Hetlaxin | ISCTGSKQCYDPCKKKTGCPNAKCMNKSCXCYGCa disulfide bonds: C3–C24, C9–C29, C13–C31, C19–C34 | Heterometrus laoticus | [86] |
| Peptide | Sequence | Organism | Ref. |
|---|---|---|---|
| [Thr6]-bradykinin (Thr6-BK) | RPPGFTPFR | Polybia occidentalis (wasp) | [93] |
| Pallipin-III | SIKKHKCIALLKRRGGSKLPFCa | Agelaia pallipes pallipes (wasp) | [94] |
| Protonectin | ILGTILGLLKGLa | Parachartergus fraternus (wasp) | [95] |
| Agelaia-MP I | INWLKLGKAIIDALa | Parachartergus fraternus (wasp) | [94] |
| Melittin | GIGAVLKVLTTGLPALISWIKRKRQQ | Apis mellifera (bee) | [96] |
| Proctolin | RYLPT | Perplaneta americana (cockroach) | [87] |
| Neb-TMOF | NPTNLH | Neobellieria bullata (fly) | [89] |
| Alloferon | HGVSGHGQHGVHG | Calliphora vicina (fly) | [90] |
| LPK | pETSFTPRLa | Leucophaea madera (cockroach) | [88] |
| MAS MT I | pEDVVHSFLRFa | Manduca sexta (cockroach) | [91] |
| Poneratoxin | FLPLLILGSLLMTPPVIQAIHDAQRa | Paraponera clavata (ant) | [92] |
| Peptide | Sequence | Organism | Ref. |
|---|---|---|---|
| Dermorphin | H-Tyr-d-Ala-Phe-Gly-Tyr-Pro-Ser-NH2 | Phyllomedusa sauvagei | [100] |
| [Hyp6]-dermorphin | H-Tyr-d-Ala-Phe-Gly-Tyr-Hyp-Ser-NH2 | Phyllomedusa sauvagei | [105] |
| [Lys7]-dermorphin-OH | H-Tyr-d-Ala-Phe-Gly-Tyr-Pro-Lys-OH | Phyllomedusa bicolor | [101] |
| [Lys7]-dermorphin-NH2 | H-Tyr-d-Ala-Phe-Gly-Tyr-Pro-Lys-NH2 | Phyllomedusa bicolor | [101] |
| [Trp4-NH2]-dermorphin | H-Tyr-d-Ala-Phe-Trp-Tyr-Pro-Ser-NH2 | Phyllomedusa bicolor | [101] |
| [Trp4, Asn7]-dermorphin | H-Tyr-d-Ala-Phe-Trp-Tyr-Pro-Asn-OH | Phyllomedusa bicolor | [105] |
| [Trp4, Asn7-NH2]-dermorphin | H-Tyr-d-Ala-Phe-Trp-Tyr-Pro-Asn-NH2 | Phyllomedusa bicolor | [101] |
| [Trp4, Asn-OH5]-dermorphin | H-Tyr-d-Ala-Phe-Trp-Asn-OH | Phyllomedusa bicolor | [101] |
| Y10A | H-Tyr-d-Ala-Phe-Gly-Tyr-Pro-Ser-Gly-Glu-Ala-OH | Phyllomedusa sauvagei | [101] |
| Y131 | H-Tyr-d-Ala-Phe-Gly-Tyr-Pro-Ser-Gly-Glu-Ala-Lys-Lys-Ile-OH | Phyllomedusa sauvagei | [101] |
| DAla-deltorphin I | H-Tyr-d-Ala-Phe-Asp-Val-Val-Gly-NH2 | Phyllomedusa bicolor | [105] |
| DAla-deltorphin II | H-Tyr-d-Ala-Phe-Glu-Val-Val-Gly-NH2 | Phyllomedusa bicolor | [105] |
| DMet-deltorphin, dermenkephalin, Deltrophin | H-Tyr-d-Met-Phe-His-Leu-Met-Asp-NH2 | Phyllomedusa bicolor | [105] |
| Deltorphin | H-Tyr-Ala-Phe-Gly-Tyr-Pro-Ser-NH2 | Phasmahyla jandaia | [101] |
| Dlle-deltorphin | H-Tyr-d-Ile-Phe-His-Leu-Met-Asp-NH2 | Pachymedusa dacnicolor, Agalychnis annae | [101] |
| [Met(Ox)]6-deltorphin | H-Tyr-Met-Phe-His-Leu-Met(O)-Asp-NH2 | Phasmahyla jandaia | [101] |
| DLeu-deltorphin-17 | H-Tyr-d-Leu-Phe-Ala-Asp-Val-Ala-Ser-Thr-Ile-Gly-Asp-Phe-Phe-His-Ser-Ile-NH2 | Phasmahyla jandaia | [101] |
| Odorranaopin | H-Asp-Tyr-Thr-Ile-Arg-Thr-Arg-Leu-His-Gln-Glu-Ser-Ser-Arg-Lys-Val-Leu-OH | Odorrana graham | [104] |
| Tryptophyllin L1.2 | H-Phe-Pro-Trp-Leu-NH2 | Litoria rubella | [101] |
| Tryptophyllin L3.1 | H-Phe-Pro-Trp-Pro-NH2 | Litoria rubella | [101] |
| - | H-Phe-Pro-Kyn-Leu-NH2 | Litoria rubella | [101,106] |
| Adenoregulin | GLWSKIKEVGKEAAKAAAKAAGKAALGAVSEAV | Phyllomedusa bicolor | [101] |
| Tt7 | EQDPKCLLPRNLGKGKGSTIRYYYDKSAGT disulfide bonds: C6–C4, C2–C3 | Trachycephalus typhonius | [107] |
| Peptide | Sequence | Organism | Ref. |
|---|---|---|---|
| Mambalgin-1 | LKCYQHGKVVTCHRDMKFCYHNTGMPFRNLKLILQGCSSSCSETENNKCCSTDRCNK disulfide bonds: C3–C19, C12–C37, C41–C49, C50–C55 | Dendroaspis polylepis | [108] |
| Mambalgin-2 | LKCFQHGKVVTCHRDMKFCYHNTGMPFRNLKLILQGCSSSCSETENNKCCSTDRCNK disulfide bonds: C3–C19, C12–C37, C41–C49, C50–C55 | Dendroaspis polylepis | [108] |
| Mambalgin-3 | LKCYQHGKVVTCHRDMKFCYHNIGMPFRNLKLILQGCSSSCSETENNKCCSTDRCNK disulfide bonds: C3–C19, C12–C37, C41–C49, C50–C55 | Dendroaspis angusticeps | [108] |
| α-cobratoxin (α-CbTX, α-elapitoxin–Nk2a) | IRCFITPDITSKDCPNGHVCYTKTWCDAFCSIRGKRVDLGCAATCPTVKTGVDIQCCSTDNCNPFPTRKRP disulfide bonds: C3–C20, C14–C41, C26–C30, C45–C56, C57–C62 | Naja naja kaouthia | [48,109] |
| Crotalphine | pEFSPENCQGESQPC disulfide bond: C7–C14 | Crotalus durissus terrificus | [110] |
| μ-EPTX-Na1a | LKCHNTQLPFIYKTCPEGKNLCFKATLKKFPLKFPKRGCADNCPKNSALLKYVCCSTDKCN disulfide bonds: C3–C22, C15–C40, C44–C55, C56–C61 | Naja atra | [111] |
| Type of Modification | Peptidomimetic | Ref. |
|---|---|---|
| d-amino acids | [d-Ala2, d-Leu5]-Enkephalin (DADLE) 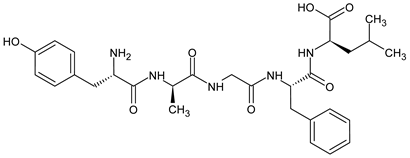 | [124] |
| [d-Ser2, d-Leu5, Thr6]-Enkephalin (DSLET) 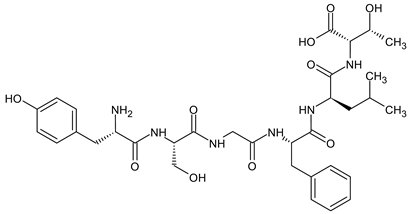 | [128,129] | |
| [d-Ala3]-Dynorphin A-(1-11)-NH2 H-Tyr-Gly-d-Ala-Phe-Leu-Arg-Arg-Ile-Arg-Pro-Lys-NH2 | [130] | |
| β amino acids Homo-amino acids | (H-Tyr-d-Ala-Phe-Gly-β3-homo-Tyr)2hydrazine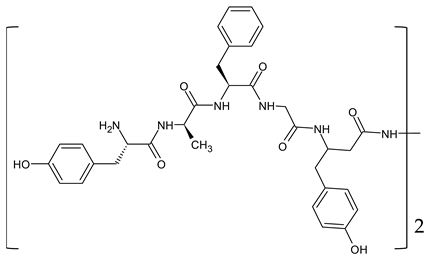 | [131] |
| 2′,6′-dimethyltyrosine (Dmt) derivatives | H-Dmt1-βPro2-Trp3-(2-furyl)Map4 -Endomorphin 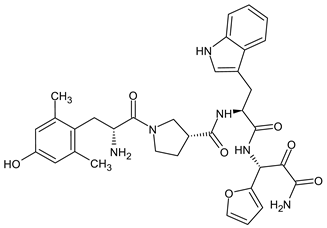 | [132] |
H-Dmt-d-Arg-Phe-Lys-NH2 ([Dmt1]DALDA)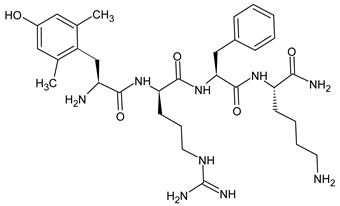 | [133,134] | |
| N- and C-terminal modifications | [d-Ala2,N-MePhe4,Gly-ol]-Enkephalin (DAMGO) 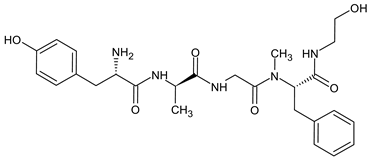 | [127] |
guanidyl-Tyr-d-Ala-Gly-Phe-Leu-tetrazole | [126] | |
| Replacement of the peptide bond | H-Tyr-d-Ala-Phe-Leu-Arg ψ (CH2NH) Arg-NH2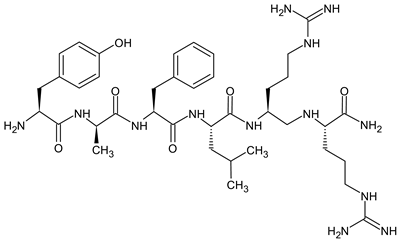 | [135] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gach-Janczak, K.; Biernat, M.; Kuczer, M.; Adamska-Bartłomiejczyk, A.; Kluczyk, A. Analgesic Peptides: From Natural Diversity to Rational Design. Molecules 2024, 29, 1544. https://doi.org/10.3390/molecules29071544
Gach-Janczak K, Biernat M, Kuczer M, Adamska-Bartłomiejczyk A, Kluczyk A. Analgesic Peptides: From Natural Diversity to Rational Design. Molecules. 2024; 29(7):1544. https://doi.org/10.3390/molecules29071544
Chicago/Turabian StyleGach-Janczak, Katarzyna, Monika Biernat, Mariola Kuczer, Anna Adamska-Bartłomiejczyk, and Alicja Kluczyk. 2024. "Analgesic Peptides: From Natural Diversity to Rational Design" Molecules 29, no. 7: 1544. https://doi.org/10.3390/molecules29071544
APA StyleGach-Janczak, K., Biernat, M., Kuczer, M., Adamska-Bartłomiejczyk, A., & Kluczyk, A. (2024). Analgesic Peptides: From Natural Diversity to Rational Design. Molecules, 29(7), 1544. https://doi.org/10.3390/molecules29071544








