Lipase-Catalyzed Preparation and Optimization of Structured Phosphatidylcholine Containing Nervonic Acid
Abstract
1. Introduction
2. Results and Discussion
2.1. Immobilization of PLA1
2.1.1. Screening of Carrier
2.1.2. Specific Activity of Immobilized Enzyme
2.1.3. Immobilization Time
2.2. Lipase Screening
2.3. Fatty acid Composition of SPC
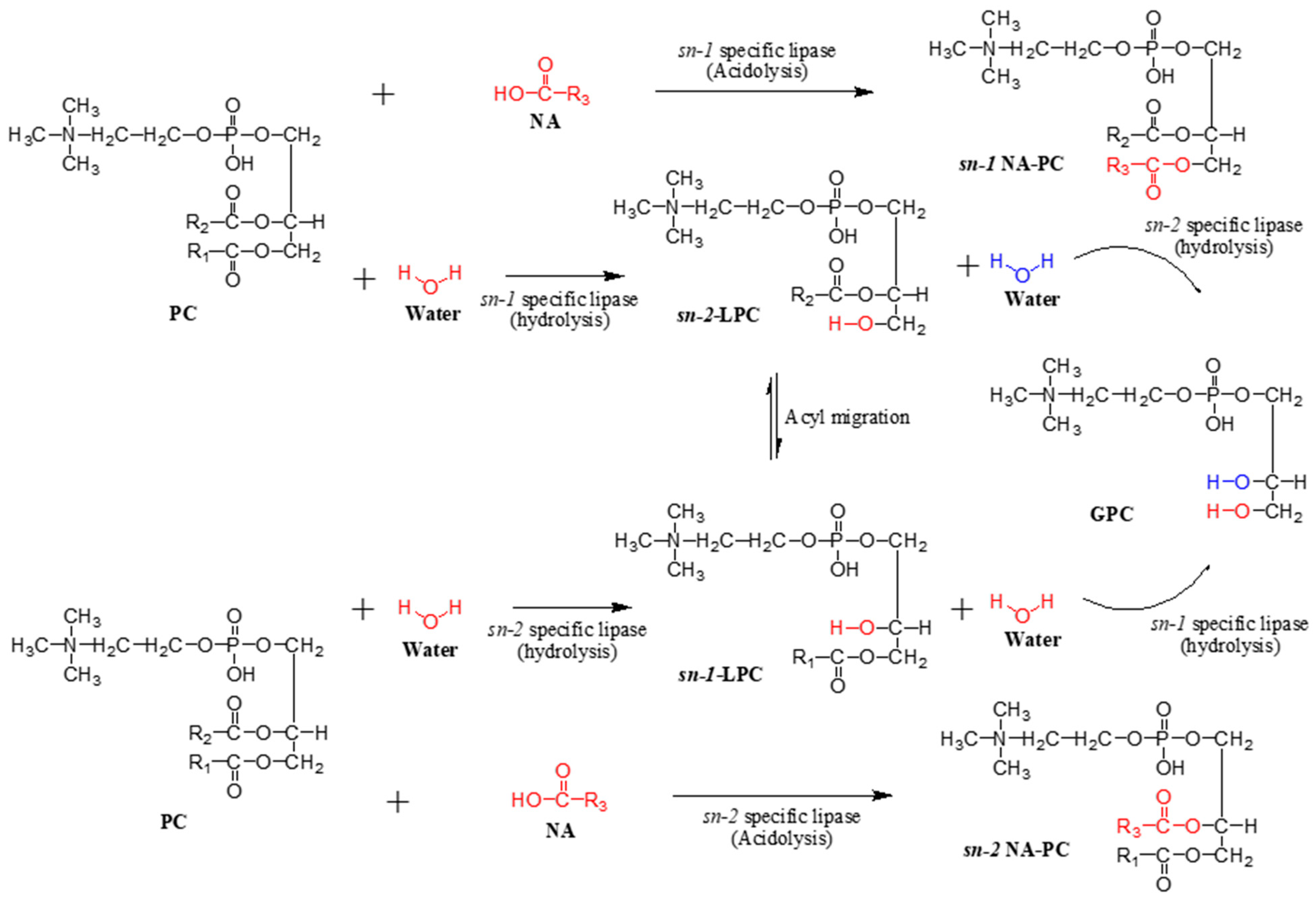
2.4. Stereospecific Position of SPC
2.5. Parameters Affecting Acidolysis of PC and NA
2.5.1. Substrate Ratio
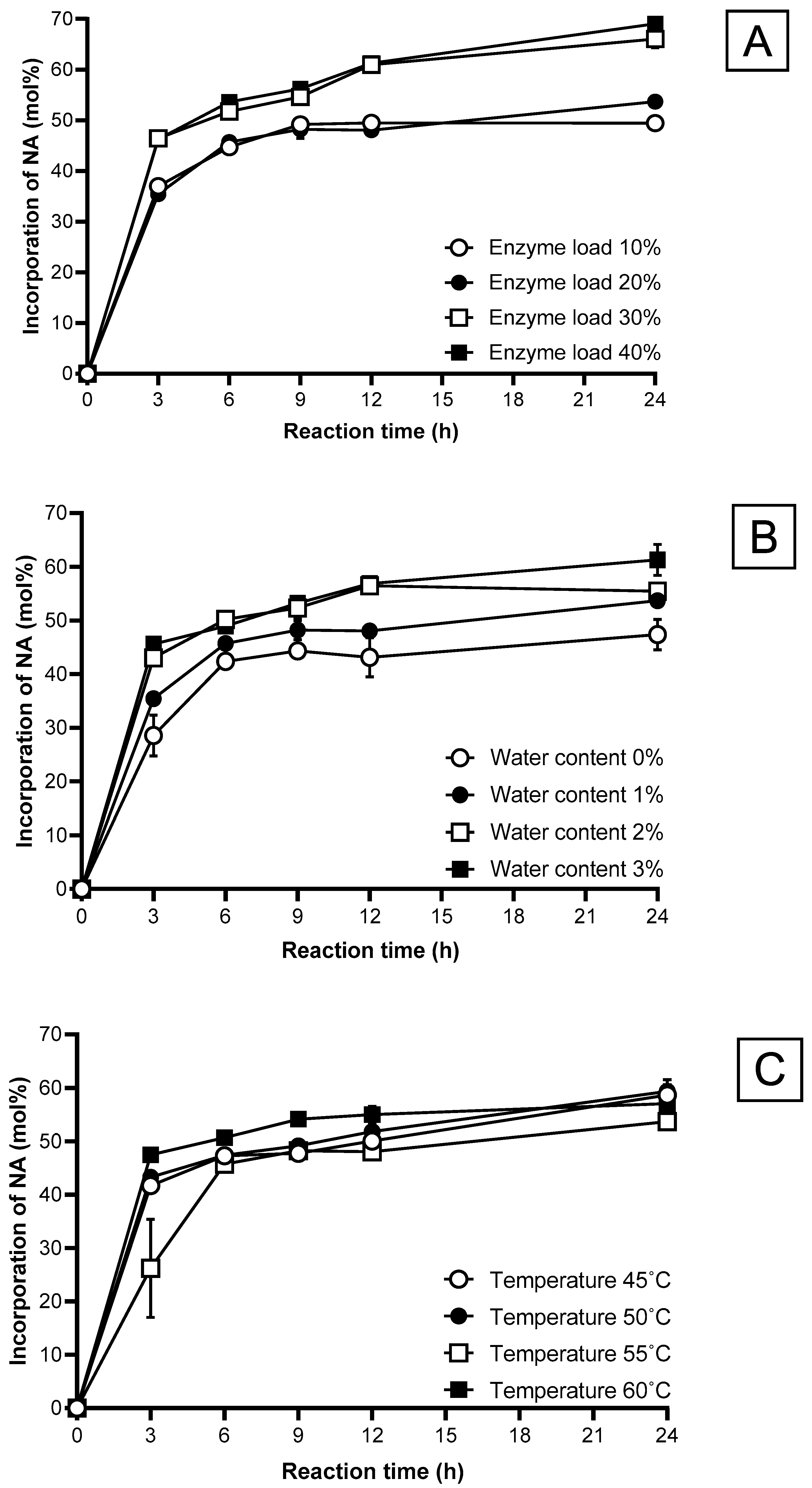
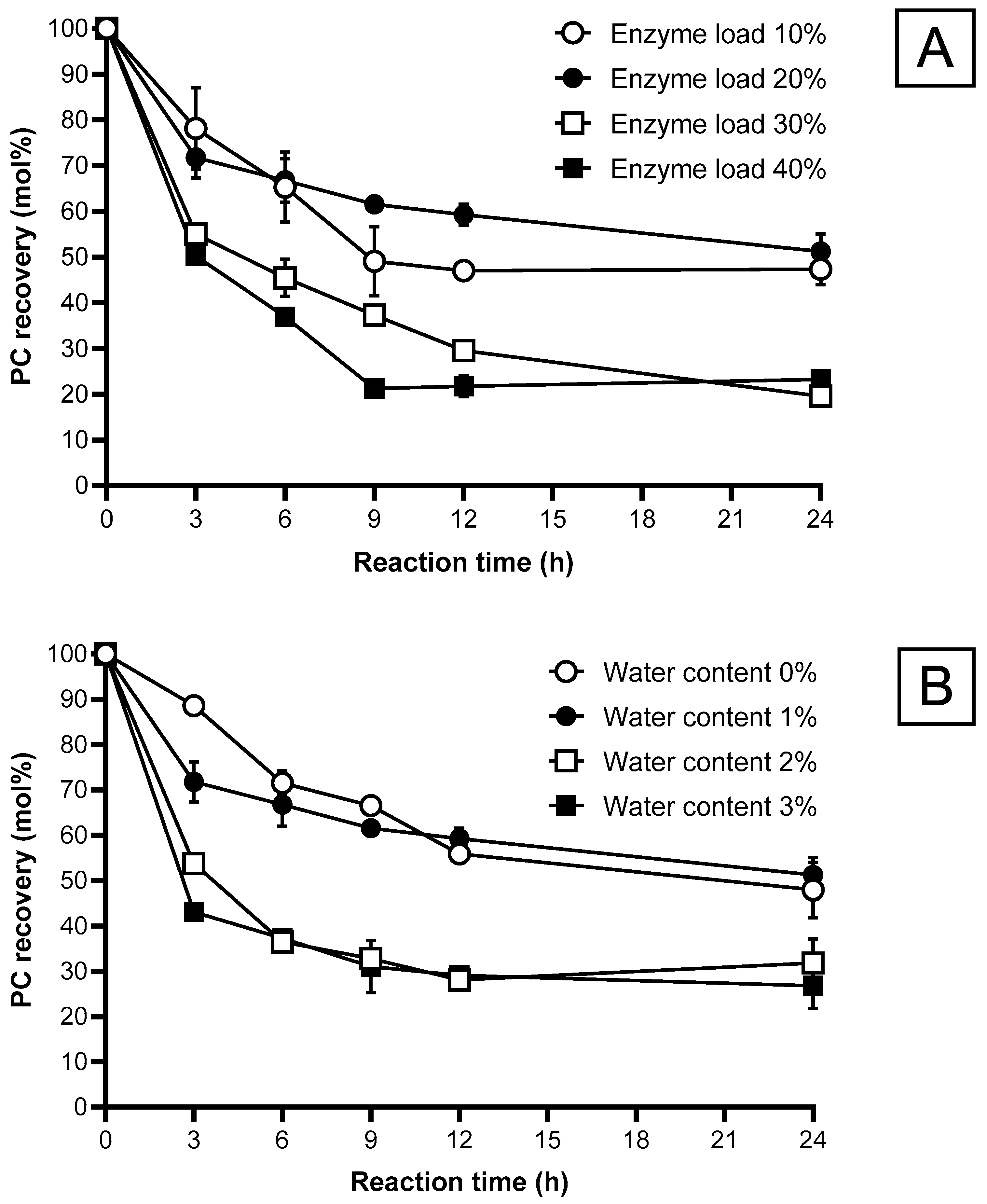
2.5.2. Enzyme Load
2.5.3. Water Content
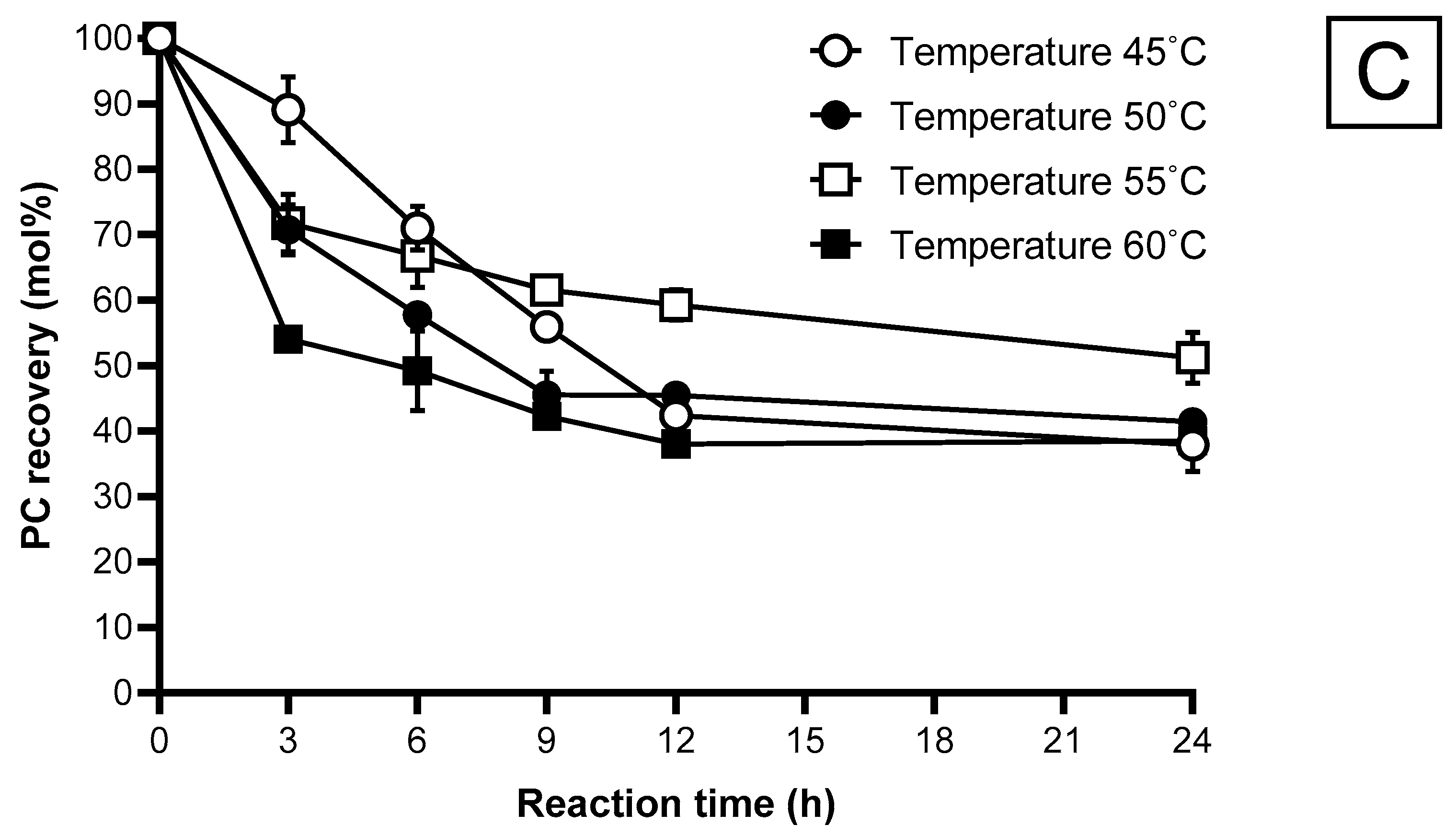
2.5.4. Reaction Temperature
2.5.5. Reaction Time
2.6. Purification of Structured Phosphatidylcholine
3. Materials and Methods
3.1. Materials
3.2. Immobilization of Phospholipase A1 (PLA1)
3.3. Determination of Enzyme Activity for Immobilized PLA1
3.4. Lipase-Catalyzed Acidolysis
3.5. Analysis of Fatty Acid Composition by Gas Chromatography (GC)
3.6. Analysis of Fatty Acids in sn-1 and sn-2 Position of Structured Phosphatidylcholine
3.7. Purification of Structured Phosphatidylcholine
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sargent, J.R.; Coupland, K.; Wilson, R. Nervonic acid and demyelinating disease. Med. Hypotheses 1994, 42, 237–242. [Google Scholar] [CrossRef]
- Tang, T.-F.; Liu, X.-M.; Ling, M.; Lai, F.; Zhang, L.; Zhou, Y.-H.; Sun, R.-R. Constituents of the essential oil and fatty acid from Malania oleifera. Ind. Crops Prod. 2013, 43, 1–5. [Google Scholar] [CrossRef]
- Lewkowicz, N.; Piątek, P.; Namiecińska, M.; Domowicz, M.; Bonikowski, R.; Szemraj, J.; Przygodzka, P.; Stasiołek, M.; Lewkowicz, P. Naturally Occurring Nervonic Acid Ester Improves Myelin Synthesis by Human Oligodendrocytes. Cells 2019, 8, 786. [Google Scholar] [CrossRef]
- Hu, P.; Xu, X.; Yu, L.L. Interesterified trans-free fats rich in sn-2 nervonic acid prepared using Acer truncatum oil, palm stearin and palm kernel oil, and their physicochemical properties. LWT Food Sci. Technol. 2017, 76, 156–163. [Google Scholar] [CrossRef]
- Yamazaki, Y.; Kondo, K.; Maeba, R.; Nishimukai, M.; Nezu, T.; Hara, H. The Proportion of Nervonic Acid in Serum Lipids is Associated with Serum Plasmalogen Levels and Metabolic Syndrome. J. Oleo Sci. 2014, 63, 527–537. [Google Scholar] [CrossRef]
- Guo, Z.; Vikbjerg, A.F.; Xu, X. Enzymatic modification of phospholipids for functional applications and human nutrition. Biotechnol. Adv. 2005, 23, 203–259. [Google Scholar] [CrossRef]
- Ulven, S.M.; Holven, K.B. Comparison of bioavailability of krill oil versus fish oil and health effect. Vasc. Health Risk Manag. 2015, 11, 511–524. [Google Scholar] [CrossRef]
- Galli, C.; Sirtori, C.R.; Mosconi, C.; Medini, L.; Gianfranceschi, G.; Vaccarino, V.; Scolastico, C. Prolonged retention of doubly labeled phosphatidylcholine in human plasma and erythrocytes after oral administration. Lipids 1992, 27, 1005–1012. [Google Scholar] [CrossRef]
- Ramprasath, V.R.; Eyal, I.; Zchut, S.; Jones, P.J.H. Enhanced increase of omega-3 index in healthy individuals with response to 4-week n-3 fatty acid supplementation from krill oil versus fish oil. Lipids Health Dis. 2013, 12, 178. [Google Scholar] [CrossRef]
- Küllenberg, D.; Taylor, L.A.; Schneider, M.; Massing, U. Health effects of dietary phospholipids. Lipids Health Dis. 2012, 11, 3. [Google Scholar] [CrossRef]
- Domoto, N.; Koenen, M.E.; Havenaar, R.; Mikajiri, A.; Chu, B.S. The bioaccessibility of eicosapentaenoic acid was higher from phospholipid food products than from mono- and triacylglycerol food products in a dynamic gastrointestinal model. Food Sci. Nutr. 2013, 1, 409–415. [Google Scholar] [CrossRef]
- Lemaitre-Delaunay, D.; Pachiaudi, C.; Laville, M.; Pousin, J.; Armstrong, M.; Lagarde, M. Blood compartmental metabolism of docosahexaenoic acid (DHA) in humans after ingestion of a single dose of [(13)C]DHA in phosphatidylcholine. J. Lipid Res. 1999, 40, 1867–1874. [Google Scholar] [CrossRef]
- Ang, X.; Chen, H.; Xiang, J.-Q.; Wei, F.; Quek, S.Y. Preparation and functionality of lipase-catalysed structured phospholipid—A review. Trends Food Sci. Technol. 2019, 88, 373–383. [Google Scholar] [CrossRef]
- Mateo, C.; Palomo, J.M.; Fernandez-Lorente, G.; Guisan, J.M.; Fernandez-Lafuente, R. Improvement of enzyme activity, stability and selectivity via immobilization techniques. Enzym. Microb. Technol. 2007, 40, 1451–1463. [Google Scholar] [CrossRef]
- Li, X.; Chen, J.-F.; Yang, B.; Li, D.-M.; Wang, Y.-H.; Wang, W.-F. Production of Structured Phosphatidylcholine with High Content of DHA/EPA by Immobilized Phospholipase A(1)-Catalyzed Transesterification. Int. J. Mol. Sci. 2014, 15, 15244–15258. [Google Scholar] [CrossRef]
- Zhao, T.; Kim, B.H.; Garcia, H.S.; Kim, Y.; Kim, I.-H. Immobilized phospholipase A1-catalyzed modification of phosphatidylcholine with n − 3 polyunsaturated fatty acid. Food Chem. 2014, 157, 132–140. [Google Scholar] [CrossRef]
- Kim, J.H.; Yoon, S.H. Effects of organic solvents on transesterification of phospholipids using phospholipase A2 and lipase. Food Sci. Biotechnol. 2014, 23, 1207–1211. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Madoery, R.; Gattone, C.G.; Fidelio, G. Bioconversion of phospholipids by immobilized phospholipase A2. J. Biotechnol. 1995, 40, 145–153. [Google Scholar] [CrossRef]
- Xi, X.; Feng, X.; Shi, N.; Ma, X.; Lin, H.; Han, Y. Immobilized phospholipase A1-catalyzed acidolysis of phosphatidylcholine from Antarctic krill (Euphausia superba) for docosahexaenoic acid enrichment under supercritical conditions. J. Mol. Catal. B Enzym. 2016, 126, 46–55. [Google Scholar] [CrossRef]
- Hossen, M.; Hernandez, E. Enzyme-catalyzed synthesis of structured phospholipids with conjugated linoleic acid. Eur. J. Lipid Sci. Technol. 2005, 107, 730–736. [Google Scholar] [CrossRef]
- Kim, B.H.; Akoh, C.C. Recent Research Trends on the Enzymatic Synthesis of Structured Lipids. J. Food Sci. 2015, 80, C1713–C1724. [Google Scholar] [CrossRef]
- Vikbjerg, A.F.; Mu, H.; Xu, X. Synthesis of structured phospholipids by immobilized phospholipase A2 catalyzed acidolysis. J. Biotechnol. 2007, 128, 545–554. [Google Scholar] [CrossRef]
- Verdasco-Martín, C.M.; Corchado-Lopo, C.; Fernández-Lafuente, R.; Otero, C. Rapid and high yield production of phospholipids enriched in CLA via acidolysis: The critical role of the enzyme immobilization protocol. Food Chem. 2019, 296, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Chojnacka, A.; Gładkowski, W.; Gliszczyńska, A.; Niezgoda, N.; Kiełbowicz, G.; Wawrzeńczyk, C. Synthesis of structured phosphatidylcholine containing punicic acid by the lipase-catalyzed transesterification with pomegranate seed oil. Catal. Commun. 2016, 75 (Suppl. C), 60–64. [Google Scholar] [CrossRef]
- Vikbjerg, A.F.; Mu, H.; Xu, X. Elucidation of acyl migration during lipase-catalyzed production of structured phospholipids. J. Am. Oil Chem. Soc. 2006, 83, 609–614. [Google Scholar] [CrossRef]
- Lopes, P.A.; Bandarra, N.M.; Martins, S.V.; Madeira, M.S.; Ferreira, J.; Guil-Guerrero, J.L.; Prates, J.A.M. Docosahexaenoic acid (DHA) at the sn-2 position of triacylglycerols increases DHA incorporation in brown, but not in white adipose tissue, of hamsters. Int. J. Food Sci. Nutr. 2018, 69, 458–471. [Google Scholar] [CrossRef]
- Ochoa, A.A.; Hernández-Becerra, J.A.; Cavazos-Garduño, A.; García, H.S.; Vernon-Carter, E.J. Phosphatidylcholine enrichment with medium chain fatty acids by immobilized phospholipase A1-catalyzed acidolysis. Biotechnol. Prog. 2013, 29, 230–236. [Google Scholar] [CrossRef]
- Vikbjerg, A.F.; Peng, L.; Mu, H.; Xu, X. Continuous production of structured phospholipids in a packed bed reactor with lipase from Thermomyces lanuginosa. J. Am. Oil Chem. Soc. 2005, 82, 237–242. [Google Scholar] [CrossRef]
- Kumari, A.; Mahapatra, P.; Garlapati, V.K.; Banerjee, R. Enzymatic transesterification of Jatropha oil. Biotechnol. Biofuels 2009, 2, 1. [Google Scholar] [CrossRef]
- Vikbjerg, A.F.; Mu, H.; Xu, X. Parameters affecting incorporation and by-product formation during the production of structured phospholipids by lipase-catalyzed acidolysis in solvent-free system. J. Mol. Catal. B Enzym. 2005, 36, 14–21. [Google Scholar] [CrossRef]
- Yang, G.; Yang, L. Increase of Oleic Acid Content in Phosphatidylcholine through Lipase-catalyzed Interesterification: Optimization by Response Surface Methodology. J. Oleo Sci. 2015, 64, 673–682. [Google Scholar] [CrossRef]
- Niezgoda, N.; Gliszczyńska, A. Lipase Catalyzed Acidolysis for Efficient Synthesis of Phospholipids Enriched with Isomerically Pure cis-9,trans-11 and trans-10,cis-12 Conjugated Linoleic Acid. Catalysts 2019, 9, 1012. [Google Scholar] [CrossRef]
- Egger, D.; Wehtje, E.; Adlercreutz, P. Characterization and optimization of phospholipase A2 catalyzed synthesis of phosphatidylcholine. Biochim. Biophys. Acta (BBA) Protein Struct. Mol. Enzymol. 1997, 1343, 76–84. [Google Scholar] [CrossRef]
- Li, D.; Qin, X.; Wang, W.; Li, Z.; Yang, B.; Wang, Y. Synthesis of DHA/EPA-rich phosphatidylcholine by immobilized phospholipase A1: Effect of water addition and vacuum condition. Bioprocess Biosyst. Eng. 2016, 39, 1305–1314. [Google Scholar] [CrossRef] [PubMed]
- Doig, S.D.; Diks, R.M.M. Toolbox for exchanging constituent fatty acids in lecithins. Eur. J. Lipid Sci. Technol. 2003, 105, 359–367. [Google Scholar] [CrossRef]
- Haraldsson, G.G.; Thorarensen, A. Preparation of phospholipids highly enriched with n-3 polyunsaturated fatty acids by lipase. J. Am. Oil Chem. Soc. 1999, 76, 1143–1149. [Google Scholar] [CrossRef]
- Vikbjerg, A.F.; Rusig, J.-Y.; Jonsson, G.; Mu, H.; Xu, X. Strategies for lipase-catalyzed production and the purification of structured phospholipids. Eur. J. Lipid Sci. Technol. 2006, 108, 802–811. [Google Scholar] [CrossRef]
- Wei, F.; Zhao, Q.; Lv, X.; Dong, X.-Y.; Feng, Y.-Q.; Chen, H. Rapid Magnetic Solid-Phase Extraction Based on Monodisperse Magnetic Single-Crystal Ferrite Nanoparticles for the Determination of Free Fatty Acid Content in Edible Oils. J. Agric. Food Chem. 2013, 61, 76–83. [Google Scholar] [CrossRef]
- Kiełbowicz, G.; Gładkowski, W.; Chojnacka, A.; Wawrzeńczyk, C. A simple method for positional analysis of phosphatidylcholine. Food Chem. 2012, 135, 2542–2548. [Google Scholar] [CrossRef]


| Carrier | Fixation Level (%) | Specific Activity (µmol/g Protein/min) |
|---|---|---|
| Amberlite XAD-2 | 24.58 ± 4.2 | 9.4 × 10−4 |
| Amberlite XAD-7HP | 94.16 ± 0.31 | 2.9 × 10−3 |
| Diaion HP-20 | 69.94 ± 2.22 | 2.2 × 10−3 |
| Supelite DAX-8 | 70.65 ± 0.28 | 2.9 × 10−3 |
| Fatty Acid | NA | PC95 | SPC | sn-1 | sn-2 |
|---|---|---|---|---|---|
| C16:0 | - | 24.19 ± 0.3 | 4.41 ± 0.71 | 3.28 ± 0.51 | 2.94 ± 0.61 |
| C18:0 | - | 5.22 ± 0.04 | 0.61 ± 0.46 | 1.15 ± 0.19 | 2.59 ± 2.39 |
| C18:1 | - | 9.27 ± 0.03 | 6.12 ± 0.28 | 3.93 ± 0.23 | 10.91 ± 2.92 |
| C18:2 | - | 50.09 ± 0.25 | 31.8 ± 1.32 | 23.22 ± 1.15 | 44.18 ± 1.30 |
| C18:3 | - | 11.4 ± 0.12 | 6.73 ± 0.30 | 2.27 ± 0.20 | 4.22 ± 0.92 |
| C22:1 | 3.00 ± 0.18 | - | 1.09 ± 0.50 | 1.18 ± 0.22 | 0.89 ± 0.08 |
| C24:0 | 4.64 ± 0.54 | - | 2.52 ± 0.19 | 1.48 ± 0.35 | 1.49 ± 0.35 |
| C24:1 | 92.37 ± 0.71 | - | 46.72 ± 2.72 | 63.50 ± 1.59 | 33.44 ± 2.73 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ang, X.; Chen, H.; Xiang, J.; Wei, F.; Quek, S.Y. Lipase-Catalyzed Preparation and Optimization of Structured Phosphatidylcholine Containing Nervonic Acid. Molecules 2024, 29, 1539. https://doi.org/10.3390/molecules29071539
Ang X, Chen H, Xiang J, Wei F, Quek SY. Lipase-Catalyzed Preparation and Optimization of Structured Phosphatidylcholine Containing Nervonic Acid. Molecules. 2024; 29(7):1539. https://doi.org/10.3390/molecules29071539
Chicago/Turabian StyleAng, Xun, Hong Chen, Jiqian Xiang, Fang Wei, and Siew Young Quek. 2024. "Lipase-Catalyzed Preparation and Optimization of Structured Phosphatidylcholine Containing Nervonic Acid" Molecules 29, no. 7: 1539. https://doi.org/10.3390/molecules29071539
APA StyleAng, X., Chen, H., Xiang, J., Wei, F., & Quek, S. Y. (2024). Lipase-Catalyzed Preparation and Optimization of Structured Phosphatidylcholine Containing Nervonic Acid. Molecules, 29(7), 1539. https://doi.org/10.3390/molecules29071539







