Achieving Long-Cycle-Life Zinc-Ion Batteries through a Zincophilic Prussian Blue Analogue Interphase
Abstract
1. Introduction
2. Results and Discussion
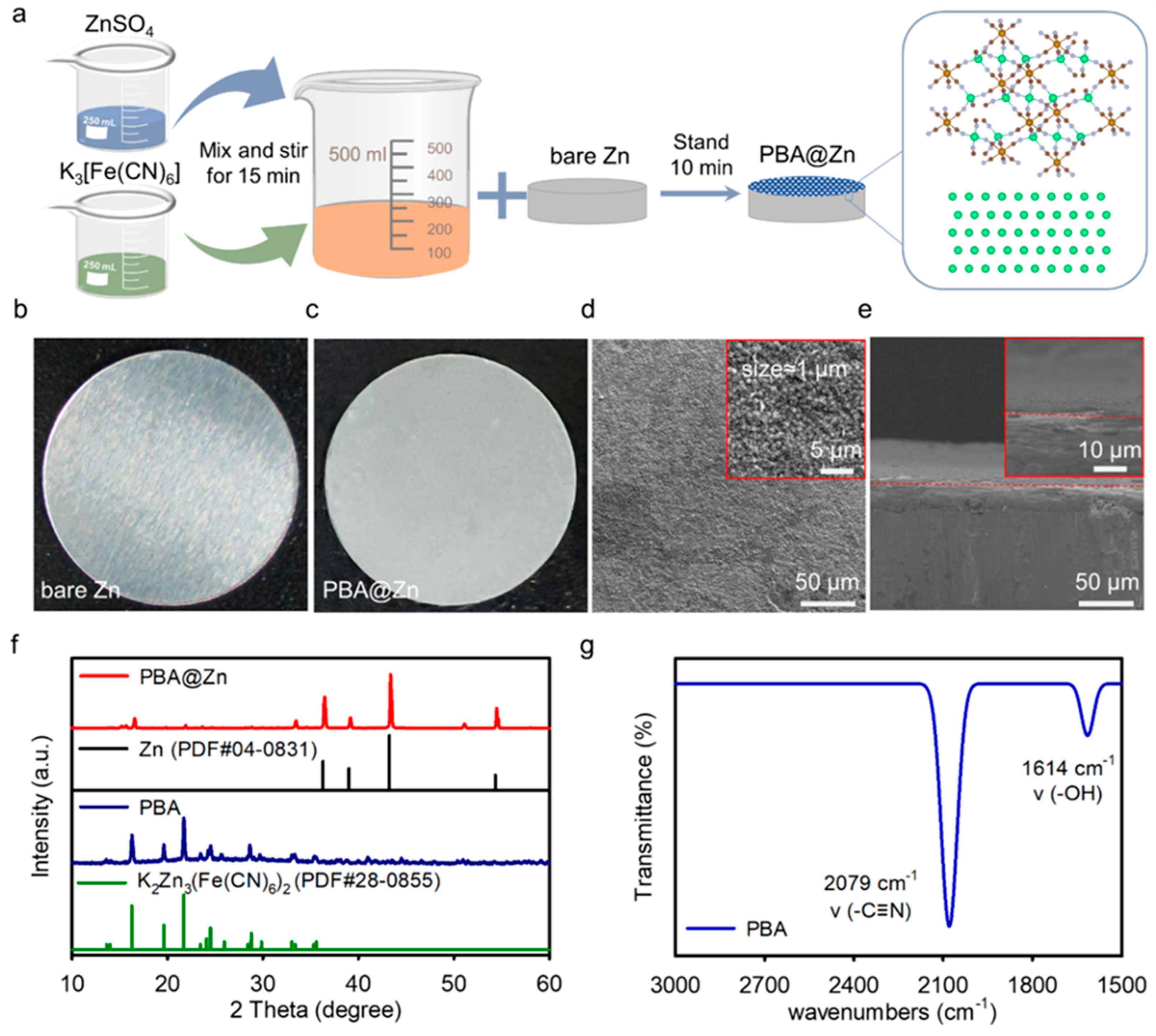
2.1. Characterizations
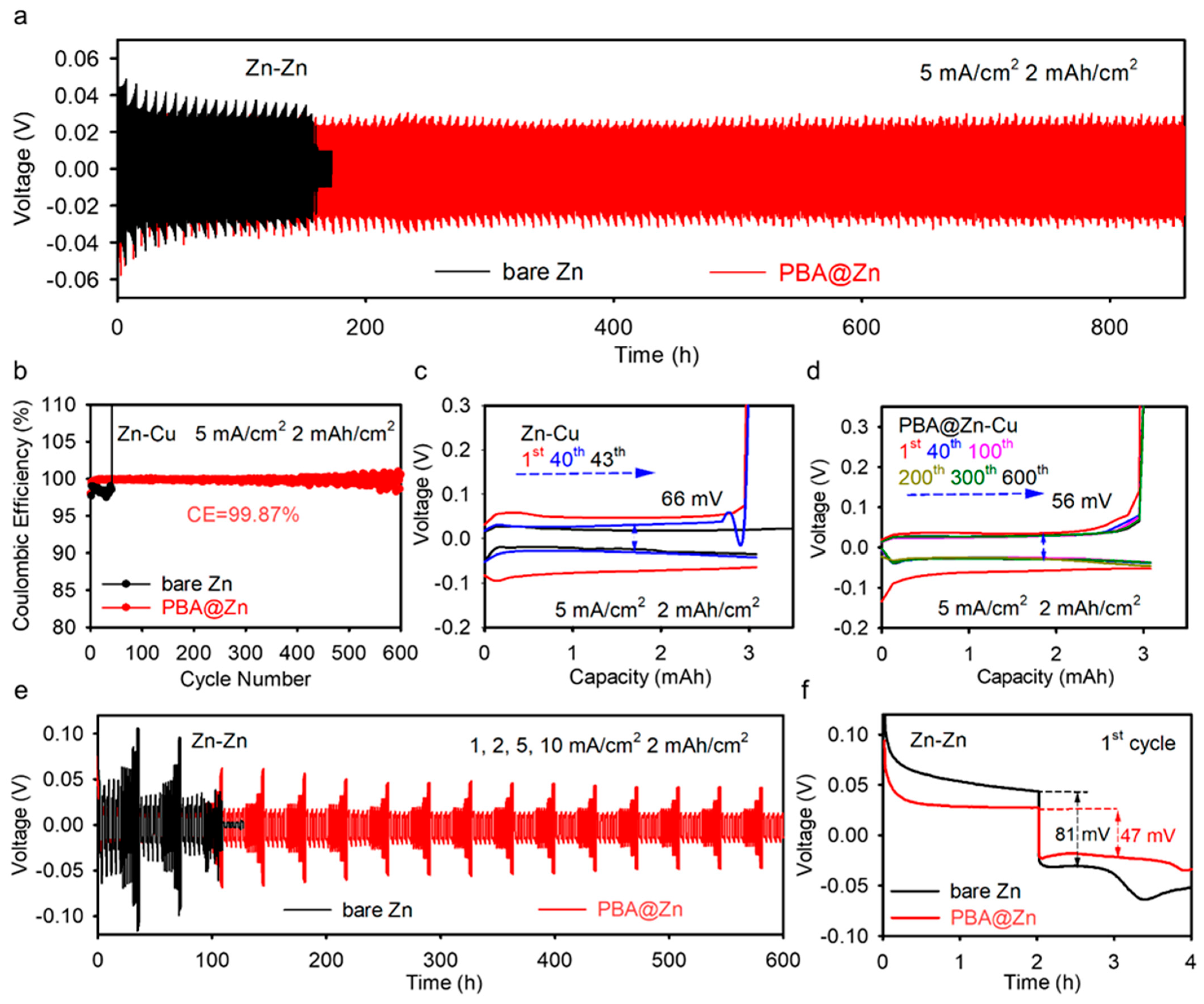
2.2. Electrochemical Characterizations
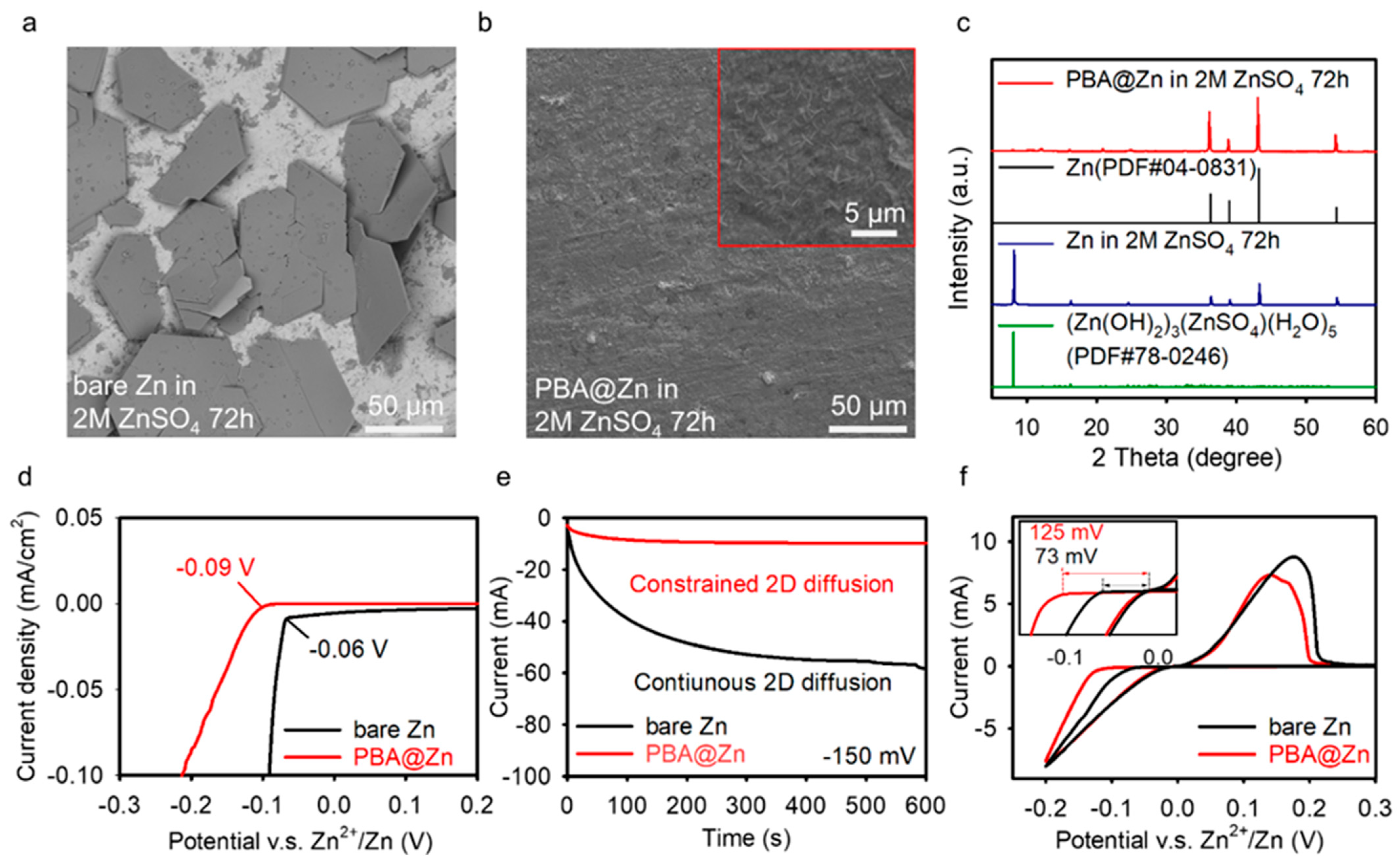
2.3. Mechanism
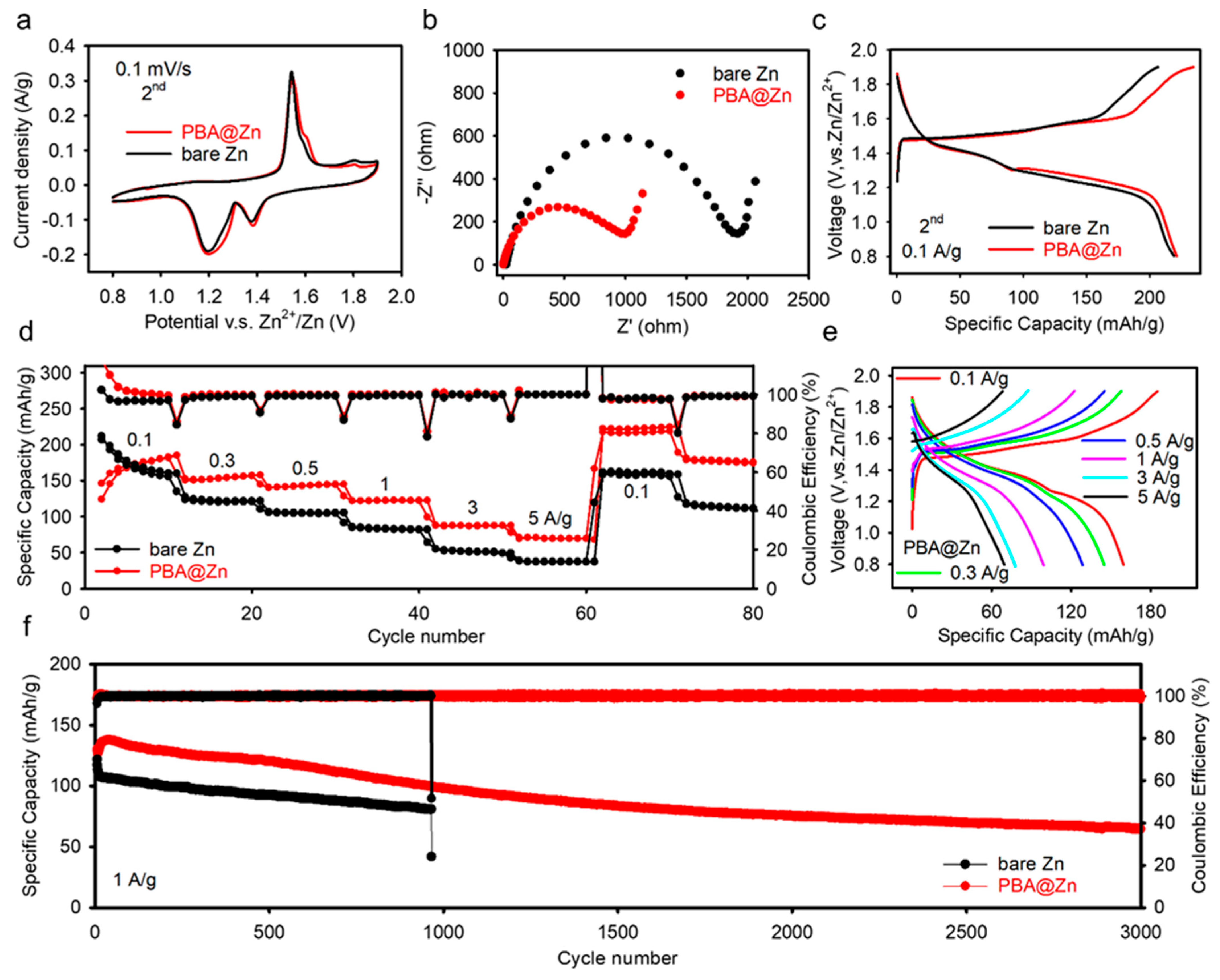
2.4. Full Cell Examination
3. Materials and Methods
3.1. Materials
3.2. Synthesis of the PBA Coating
3.3. Synthesis of α-MnO2 Cathode Material by Hydrothermal Reactions
3.4. Synthesis of the α-MnO2 Composite Electrode
3.5. Electrochemical Evaluation
3.6. Material Characterization
3.7. Details of Density Functional Theory (DFT) Calculation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, J.; Tian, L.; Lai, X. Outlook of Electrical Energy Storage Technologies Under Energy Internet Background. Autom. Electr. Power Syst. 2015, 39, 15–25. [Google Scholar]
- Hao, J.; Li, X.; Zeng, X.; Li, D.; Mao, J.; Guo, Z. Deeply understanding the Zn anode behaviour and corresponding improvement strategies in different aqueous Zn-based batteries. Energy Environ. Sci. 2020, 13, 3917–3949. [Google Scholar] [CrossRef]
- Wang, T.; Li, C.; Xie, X.; Lu, B.; He, Z.; Liang, S.; Zhou, J. Anode Materials for Aqueous Zinc Ion Batteries: Mechanisms, Properties, and Perspectives. ACS Nano 2020, 14, 16321–16347. [Google Scholar] [CrossRef]
- Mallick, S.; Raj, C.R. Aqueous Rechargeable Zn-ion Batteries: Strategies for Improving the Energy Storage Performance. ChemSusChem 2021, 14, 1987–2022. [Google Scholar] [CrossRef]
- Yang, Q.; Li, L.; Hussain, T.; Wang, D.; Hui, L.; Guo, Y.; Liang, G.; Li, X.; Chen, Z.; Huang, Z.; et al. Stabilizing Interface pH by N-Modified Graphdiyne for Dendrite-Free and High-Rate Aqueous Zn-Ion Batteries. Angew. Chem. Int. Ed. 2021, 61, e202112304. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Tan, H.; Chao, D.; Fan, H.J. Recent Advances in Zn-Ion Batteries. Adv. Funct. Mater. 2018, 28, 1802564. [Google Scholar] [CrossRef]
- Tang, M.; Zhu, Q.; Hu, P.; Jiang, L.; Liu, R.; Wang, J.; Cheng, L.; Zhang, X.; Chen, W.; Wang, H. Ultrafast Rechargeable Aqueous Zinc-Ion Batteries Based on Stable Radical Chemistry. Adv. Funct. Mater. 2021, 31, 2102011. [Google Scholar] [CrossRef]
- Blanc, L.E.; Kundu, D.; Nazar, L.F. Scientific Challenges for the Implementation of Zn-Ion Batteries. Joule 2020, 4, 771–799. [Google Scholar] [CrossRef]
- Guo, W.B.; Cong, Z.F.; Guo, Z.H.; Chang, C.Y.; Liang, X.Q.; Liu, Y.D.; Hu, W.G.; Pu, X. Dendrite-free Zn anode with dual channel 3D porous frameworks for rechargeable Zn batteries. Energy Storage Mater. 2020, 30, 104–112. [Google Scholar] [CrossRef]
- Wang, S.B.; Ran, Q.; Yao, R.Q.; Shi, H.; Wen, Z.; Zhao, M.; Lang, X.Y.; Jiang, Q. Lamella-nanostructured eutectic zinc-aluminum alloys as reversible and dendrite-free anodes for aqueous rechargeable batteries. Nat. Commun. 2020, 11, 9. [Google Scholar] [CrossRef]
- Zheng, J.; Zhu, G.; Liu, X.; Xie, H.; Lin, Y.; Zeng, Y.; Zhang, Y.; Gandi, A.N.; Qi, Z.; Wang, Z.; et al. Simultaneous Dangling Bond and Zincophilic Site Engineering of SiNx Protective Coatings toward Stable Zinc Anodes. ACS Energy Lett. 2022, 7, 4443–4450. [Google Scholar] [CrossRef]
- Di, S.; Nie, X.; Ma, G.; Yuan, W.; Wang, Y.; Liu, Y.; Shen, S.; Zhang, N. Zinc anode stabilized by an organic-inorganic hybrid solid electrolyte interphase. Energy Storage Mater. 2021, 43, 375–382. [Google Scholar] [CrossRef]
- Zhang, C.; Holoubek, J.; Wu, X.Y.; Daniyar, A.; Zhu, L.D.; Chen, C.; Leonard, D.P.; Rodriguez-Perez, I.A.; Jiang, J.X.; Fang, C.; et al. A ZnCl2 water-in-salt electrolyte for a reversible Zn metal anode. Chem. Commun. 2018, 54, 14097–14099. [Google Scholar] [CrossRef]
- Zhang, S.J.; Hao, J.N.; Luo, D.; Zhang, P.F.; Zhang, B.K.; Davey, K.; Lin, Z.; Qiao, S.Z. Dual-Function Electrolyte Additive for Highly Reversible Zn Anode. Adv. Energy Mater. 2021, 11, 9. [Google Scholar] [CrossRef]
- Qin, Y.; Liu, P.; Zhang, Q.; Wang, Q.; Sun, D.; Tang, Y.; Ren, Y.; Wang, H. Advanced Filter Membrane Separator for Aqueous Zinc-Ion Batteries. Small 2020, 16, e2003106. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Dong, L.; Huang, W.; Jia, H.; Zhao, Q.; Wang, Y.; Fei, B.; Pan, F. Simultaneously Regulating Uniform Zn2+ Flux and Electron Conduction by MOF/rGO Interlayers for High-Performance Zn Anodes. Nanomicro Lett. 2021, 13, 73. [Google Scholar] [CrossRef] [PubMed]
- Geng, Y.; Pan, L.; Peng, Z.; Sun, Z.; Lin, H.; Mao, C.; Wang, L.; Dai, L.; Liu, H.; Pan, K.; et al. Electrolyte additive engineering for aqueous Zn ion batteries. Energy Storage Mater. 2022, 51, 733–755. [Google Scholar] [CrossRef]
- Pu, X.; Jiang, B.; Wang, X.; Liu, W.; Dong, L.; Kang, F.; Xu, C. High-Performance Aqueous Zinc-Ion Batteries Realized by MOF Materials. Nano-Micro Lett. 2020, 12, 152. [Google Scholar] [CrossRef]
- Wang, Z.; Hu, J.; Han, L.; Wang, Z.; Wang, H.; Zhao, Q.; Liu, J.; Pan, F. A MOF-based single-ion Zn2+ solid electrolyte leading to dendrite-free rechargeable Zn batteries. Nano Energy 2019, 56, 92–99. [Google Scholar] [CrossRef]
- Xue, P.; Guo, C.; Li, L.; Li, H.; Luo, D.; Tan, L.; Chen, Z. A MOF-Derivative Decorated Hierarchical Porous Host Enabling Ultrahigh Rates and Superior Long-Term Cycling of Dendrite-Free Zn Metal Anodes. Adv. Mater. 2022, 34, 2110047. [Google Scholar] [CrossRef]
- Yang, H.; Qiao, Y.; Chang, Z.; Deng, H.; He, P.; Zhou, H. A Metal–Organic Framework as a Multifunctional Ionic Sieve Membrane for Long-Life Aqueous Zinc–Iodide Batteries. Adv. Mater. 2020, 32, e2004240. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Zhao, J.; Wan, Z.; Jiang, W.; Ling, M.; Yan, L.; Liang, C. Controllably Electrodepositing ZIF-8 Protective Layer for Highly Reversible Zinc Anode with Ultralong Lifespan. J. Phys. Chem. Lett. 2021, 12, 9055–9059. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yang, F.; Xu, W.; Zeng, Y.; He, J.; Lu, X. Zeolitic Imidazolate Frameworks as Zn2+ Modulation Layers to Enable Dendrite-Free Zn Anodes. Adv. Sci. 2020, 7, 2002173. [Google Scholar] [CrossRef] [PubMed]
- Yuksel, R.; Buyukcakir, O.; Seong, W.K.; Ruoff, R.S. Metal-Organic Framework Integrated Anodes for Aqueous Zinc-Ion Batteries. Adv. Energy Mater. 2020, 10, 1904215. [Google Scholar] [CrossRef]
- Yi, H.; Qin, R.; Ding, S.; Wang, Y.; Li, S.; Zhao, Q.; Pan, F. Structure and Properties of Prussian Blue Analogues in Energy Storage and Conversion Applications. Adv. Funct. Mater. 2020, 31, 2006970. [Google Scholar] [CrossRef]
- Jayalakshmi, M.; Scholz, F. Performance characteristics of zinc hexacyanoferrate/Prussian blue and copper hexacyanoferrate/Prussian blue solid state secondary cells. J. Power Sources 2000, 91, 217–223. [Google Scholar] [CrossRef]
- Li, M.; Maisuradze, M.; Sciacca, R.; Hasa, I.; Giorgetti, M. A Structural Perspective on Prussian Blue Analogues for Aqueous Zinc-Ion Batteries. Batter. Supercaps 2023, 6, e202300340. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, J.; Hu, Q.; Hao, T.; Cao, H.; Huang, X.; Liu, Y.; Zhang, Y.; Lin, D.; Tang, Y.; et al. Prussian blue analogs cathodes for aqueous zinc ion batteries. Mater. Today Energy 2022, 29, 101095. [Google Scholar] [CrossRef]
- Li, Z.; Liu, T.; Meng, R.; Gao, L.; Zou, Y.; Peng, P.; Shao, Y.; Liang, X. Insights into the Structure Stability of Prussian Blue for Aqueous Zinc Ion Batteries. Energy Environ. Mater. 2020, 4, 111–116. [Google Scholar] [CrossRef]
- Wang, Z.; Huang, W.; Hua, J.; Wang, Y.; Yi, H.; Zhao, W.; Zhao, Q.; Jia, H.; Fei, B.; Pan, F. An Anionic-MOF-Based Bifunctional Separator for Regulating Lithium Deposition and Suppressing Polysulfides Shuttle in Li–S Batteries. Small Methods 2020, 4, 2000082. [Google Scholar] [CrossRef]
- Liu, J.; Shen, Z.; Lu, C.-Z. Research progress of Prussian blue and its analogues for cathodes of aqueous zinc ion batteries. J. Mater. Chem. A 2024, 12, 2647–2672. [Google Scholar] [CrossRef]
- Paolella, A.; Faure, C.; Timoshevskii, V.; Marras, S.; Bertoni, G.; Guerfi, A.; Vijh, A.; Armand, M.; Zaghib, K. A review on hexacyanoferrate-based materials for energy storage and smart windows: Challenges and perspectives. J. Mater. Chem. A 2017, 5, 18919–18932. [Google Scholar] [CrossRef]
- Zampardi, G.; La Mantia, F. Prussian blue analogues as aqueous Zn-ion batteries electrodes: Current challenges and future perspectives. Curr. Opin. Electrochem. 2020, 21, 84–92. [Google Scholar] [CrossRef]
- Zhang, H.; Peng, J.; Li, L.; Zhao, Y.; Gao, Y.; Wang, J.; Cao, Y.; Dou, S.; Chou, S. Low-Cost Zinc Substitution of Iron-Based Prussian Blue Analogs as Long Lifespan Cathode Materials for Fast Charging Sodium-Ion Batteries. Adv. Funct. Mater. 2022, 33, 2210725. [Google Scholar] [CrossRef]
- Sun, H.; Huyan, Y.; Li, N.; Lei, D.; Liu, H.; Hua, W.; Wei, C.; Kang, F.; Wang, J.-G. A Seamless Metal–Organic Framework Interphase with Boosted Zn2+ Flux and Deposition Kinetics for Long-Living Rechargeable Zn Batteries. Nano Lett. 2023, 23, 1726–1734. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Yuan, W.; Ma, G.; Qiu, K.; Nie, X.; Liu, Y.; Shen, S.; Zhang, N. In-Situ Integration of a Hydrophobic and Fast-Zn2+-Conductive Inorganic Interphase to Stabilize Zn Metal Anodes. Angew. Chem. Int. Ed. 2023, 62, e202304444. [Google Scholar] [CrossRef] [PubMed]
- Jie, W. Principle and Technology of Crystal Growth; Science Press: Beijing, China, 2010; pp. 110–111. [Google Scholar]
- Cahn, J.W. Theory of crystal growth and interface motion in crystalline materials. Acta Metall. 1960, 8, 554–562. [Google Scholar] [CrossRef]
- Zhao, Q.; Zachman, M.J.; Al Sadat, W.I.; Zheng, J.; Kourkoutis, L.F.; Archer, L. Solid electrolyte interphases for high-energy aqueous aluminum electrochemical cells. Sci. Adv. 2018, 4, eaau8131. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B Condens. Matter 1996, 54, 11169–11186. [Google Scholar] [CrossRef]
- Blöchl, P.E. Projector augmented-wave method. Phys. Rev. B Condens. Matter 1994, 50, 17953–17979. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Maxisch, T.; Ceder, G. Oxidation energies of transition metal oxides within the GGA + Uframework. Phys. Rev. B 2006, 73, 195107. [Google Scholar] [CrossRef]
- Ong, S.P.; Richards, W.D.; Jain, A.; Hautier, G.; Kocher, M.; Cholia, S.; Gunter, D.; Chevrier, V.L.; Persson, K.A.; Ceder, G. Python Materials Genomics (pymatgen): A robust, open-source python library for materials analysis. Comput. Mater. Sci. 2013, 68, 314–319. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, K.; Zhao, S.; Deng, W. Achieving Long-Cycle-Life Zinc-Ion Batteries through a Zincophilic Prussian Blue Analogue Interphase. Molecules 2024, 29, 1501. https://doi.org/10.3390/molecules29071501
Chang K, Zhao S, Deng W. Achieving Long-Cycle-Life Zinc-Ion Batteries through a Zincophilic Prussian Blue Analogue Interphase. Molecules. 2024; 29(7):1501. https://doi.org/10.3390/molecules29071501
Chicago/Turabian StyleChang, Kun, Shuangying Zhao, and Wenzhuo Deng. 2024. "Achieving Long-Cycle-Life Zinc-Ion Batteries through a Zincophilic Prussian Blue Analogue Interphase" Molecules 29, no. 7: 1501. https://doi.org/10.3390/molecules29071501
APA StyleChang, K., Zhao, S., & Deng, W. (2024). Achieving Long-Cycle-Life Zinc-Ion Batteries through a Zincophilic Prussian Blue Analogue Interphase. Molecules, 29(7), 1501. https://doi.org/10.3390/molecules29071501





