Development and Validation of a High-Performance Liquid Chromatography Method to Quantify Marker Compounds in Lysimachia vulgaris var. davurica and Its Effects in Diarrhea-Predominant Irritable Bowel Syndrome
Abstract
1. Introduction
2. Results and Discussion
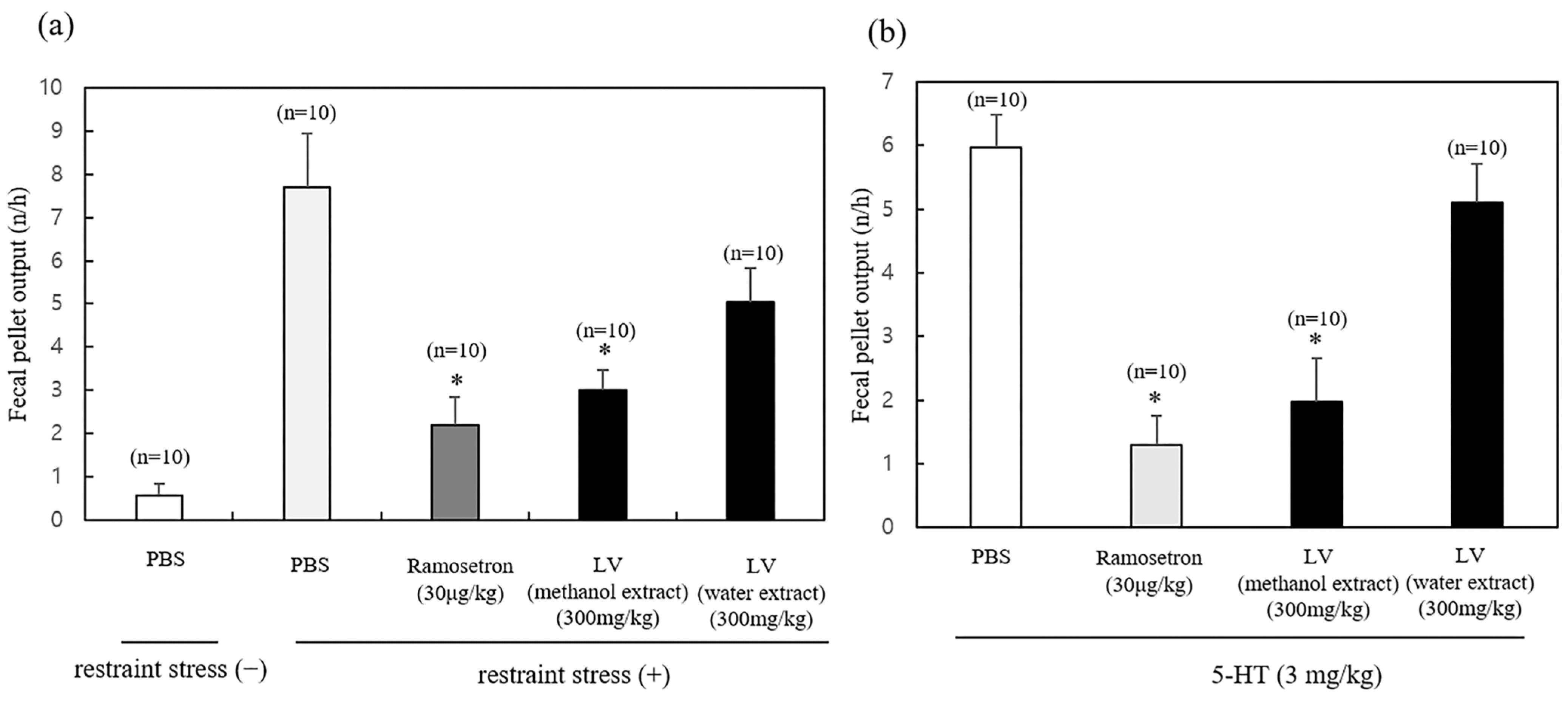
2.1. Effect of the LV Extract on Fecal Pellet Output in Mice
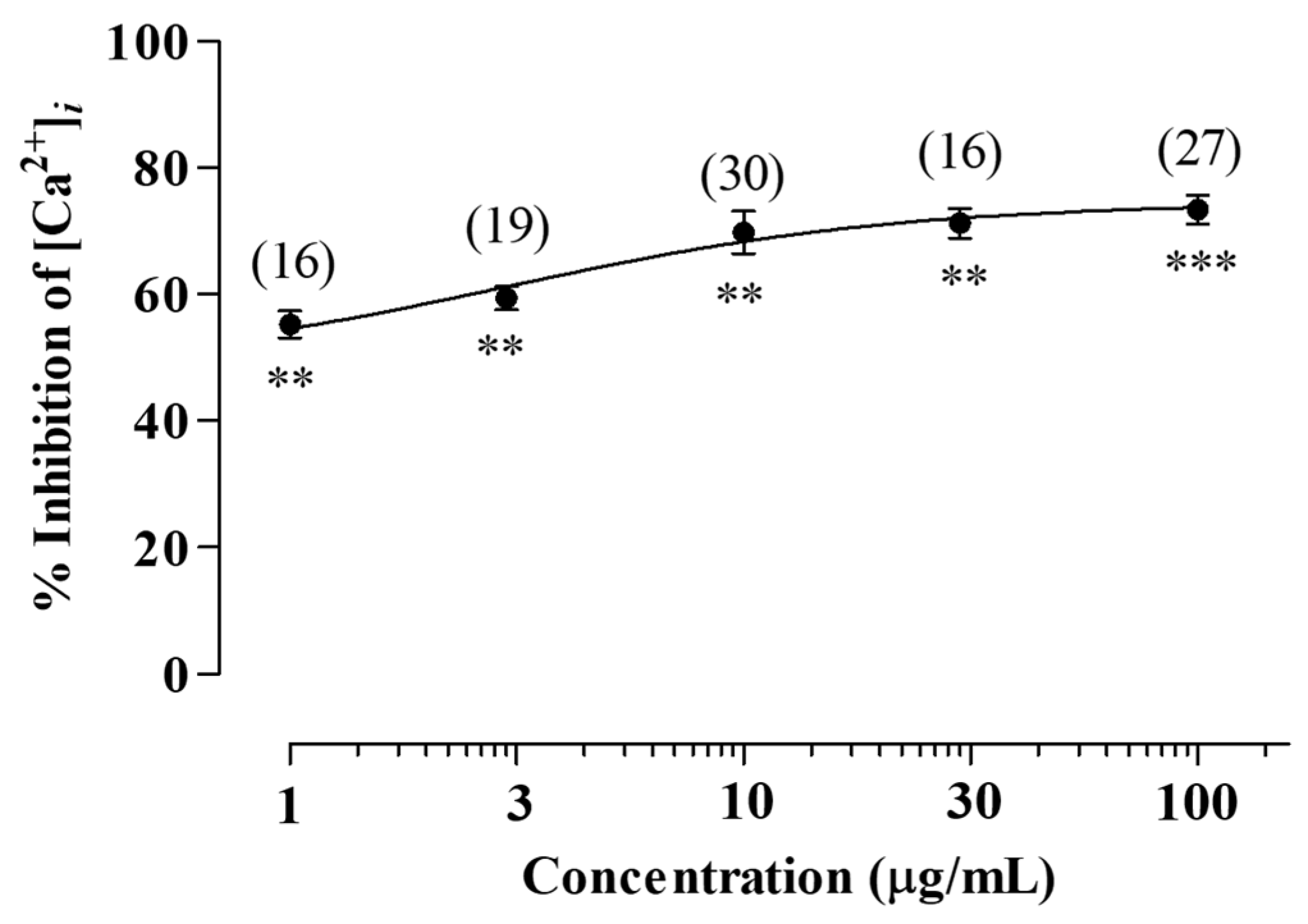
2.2. Inhibitory Effects of the LV Methanol Extract on 5-HT3 Receptor-Induced [Ca2+]i Increase
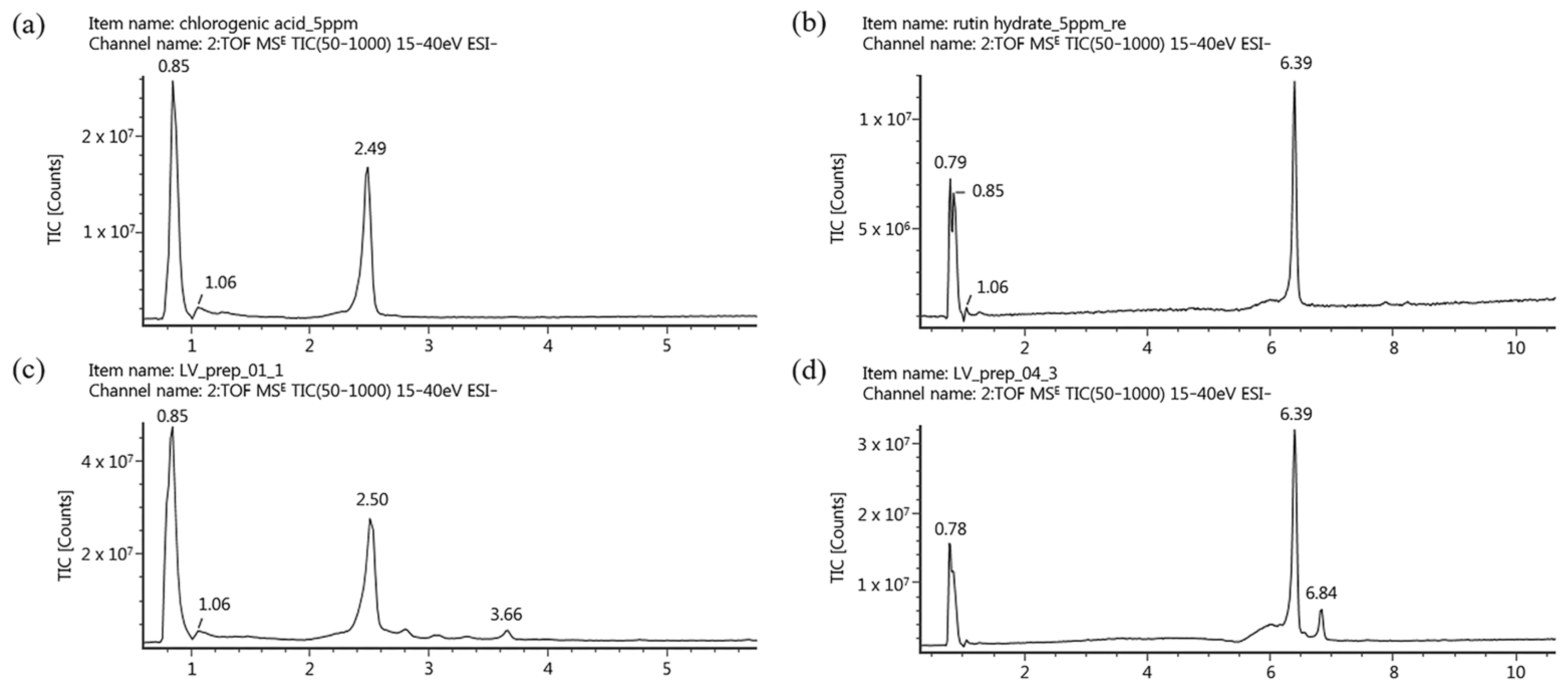
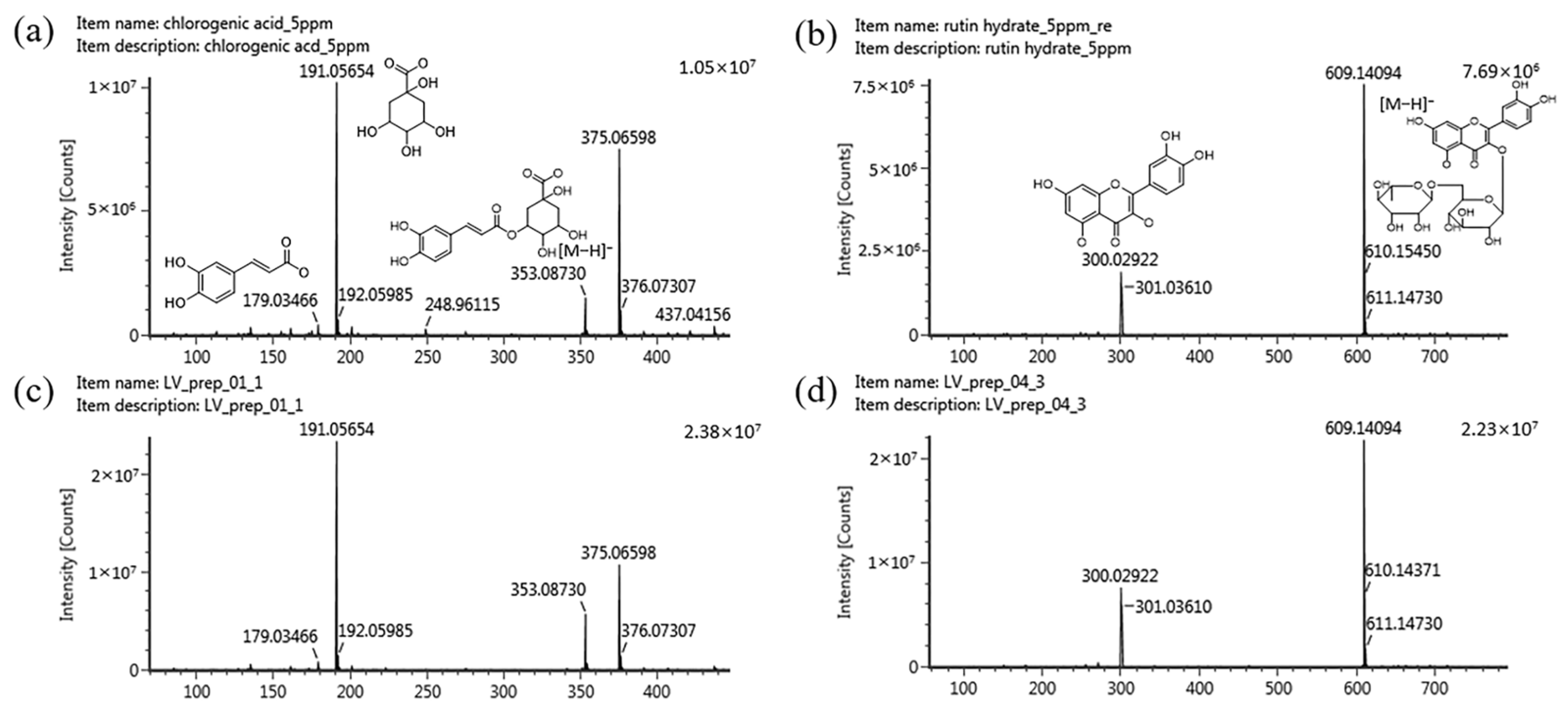
2.3. Identification of Marker Compounds in the LV Methanol Extract
2.4. Validation of the HPLC Method
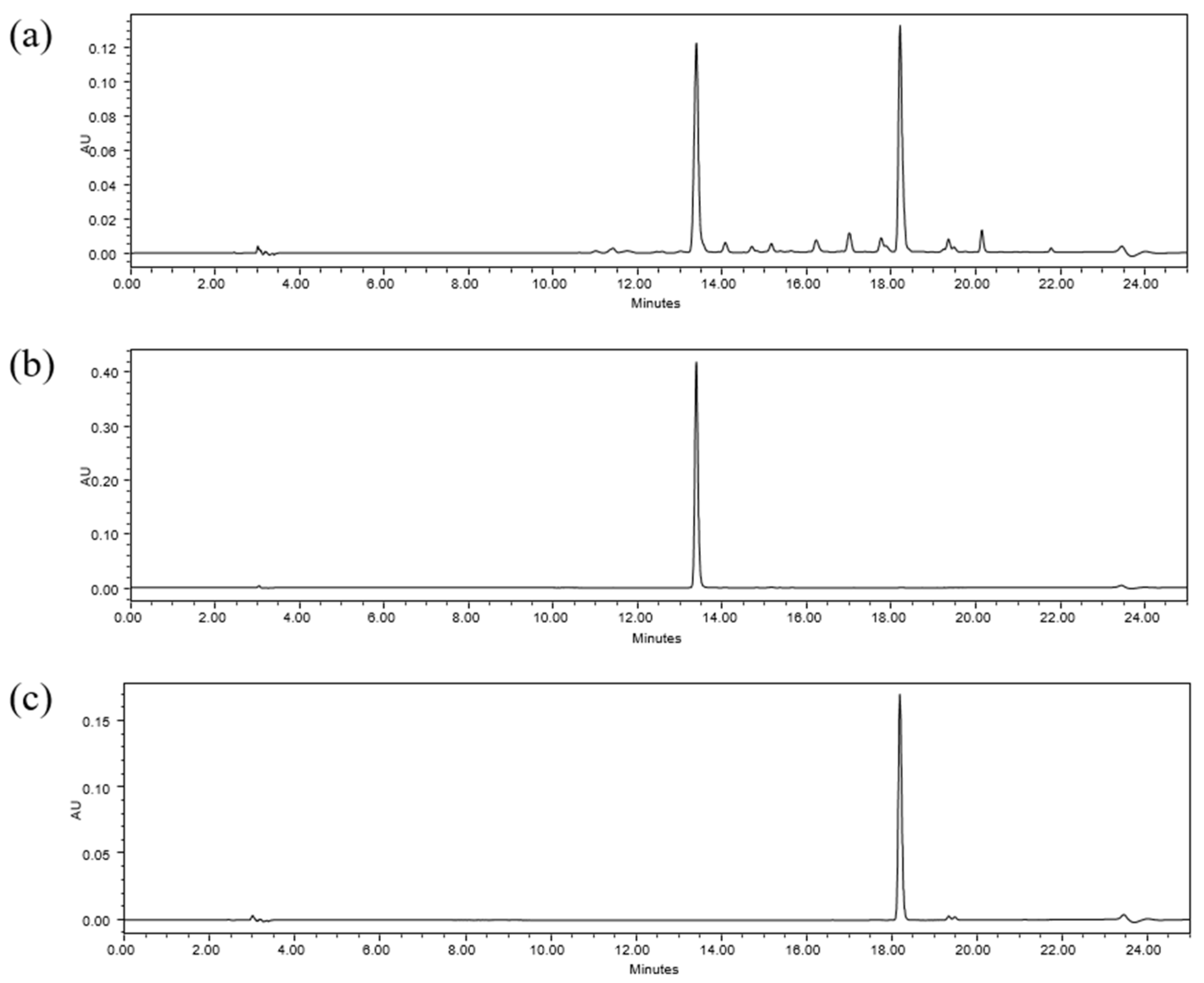
2.4.1. Specificity
2.4.2. Linearity, LOD, and LOQ
2.4.3. Intra- and Inter-Day Precision
2.4.4. Recovery
2.4.5. Solution Stability
2.4.6. Quantification of the Marker Compounds in the LV Methanol Extract
3. Materials and Methods
3.1. Plant Material and Chemicals
3.2. Animals
3.3. Analysis of Fecal Pellet Output Restraint Stress- or 5-HT-Induced Diarrhea in Mice
3.4. Expression of the Human 5-HT3 Receptor Gene
3.5. Measurement of Intracellular Ca2+ ([Ca2+]i)
3.6. Preparation of the Extract and Standard Solutions
3.7. HPLC Instrumentation and Analytical Method
3.8. Identification of the Marker Compounds
3.9. Validation of the HPLC Method
3.9.1. Specificity
3.9.2. Linearity, LOD, and LOQ
3.9.3. Precision
3.9.4. Recovery
3.9.5. Solution Stability
3.10. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Camilleri, M. Management of the Irritable Bowel Syndrome. Gastroenterology 2001, 120, 652–668. [Google Scholar] [CrossRef] [PubMed]
- Weaver, K.R.; Melkus, G.D.; Henderson, W.A. Irritable Bowel Syndrome: A review. Am. J. Nurs. 2017, 117, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Canavan, C.; West, J.; Card, T. The Epidemiology of Irritable Bowel Syndrome. Clin. Epidemiol. 2014, 6, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Lovell, R.M.; Ford, A.C. Global Prevalence of and Risk Factors for Irritable Bowel Syndrome: A Meta-Analysis. Clin. Gastroenterol. Hepatol. 2012, 10, 712–721. [Google Scholar] [CrossRef] [PubMed]
- Sperber, A.D.; Dumitrascu, D.; Fukudo, S.; Gerson, C.; Ghoshal, U.C.; Gwee, K.A.; Hungin, A.P.S.; Kang, J.; Minhu, C.; Schmulson, M. The Global Prevalence of IBS in Adults Remains Elusive due to the Heterogeneity of Studies: A Rome Foundation Working Team Literature Review. Gut 2017, 66, 1075–1082. [Google Scholar] [CrossRef] [PubMed]
- Mathew, P.; Bhatia, S.J. Pathogenesis and Management of Irritable Bowel Syndrome. Trop. Gastroenterol. 2009, 30, 19–25. [Google Scholar] [PubMed]
- Naeem, H.U.B.; Nisa, F.U.; Qudsia, F.; Abdullah, A.; Qumber, F.; Tariq, B.; Sarfaraz, E.; Khan, F.; Saboor, A.; Aziz, F. Impact of Irritable Bowel Syndrome (IBS) on Health-Related Quality of Life in South East Asia. J. Soc. Prev. Advocacy Res. KEMU 2022, 1, 1–12. [Google Scholar]
- Longstreth, G.F.; Thompson, W.G.; Chey, W.D.; Houghton, L.A.; Mearin, F.; Spiller, R.C. Functional Bowel Disorders. Gastroenterology 2006, 130, 1480–1491. [Google Scholar] [CrossRef]
- Hadjivasilis, A.; Tsioutis, C.; Michalinos, A.; Ntourakis, D.; Christodoulou, D.K.; Agouridis, A.P. New Insights into Irritable Bowel Syndrome: From Pathophysiology to Treatment. Ann. Gastroenterol. 2019, 32, 554–564. [Google Scholar] [CrossRef]
- Joć, E.B.; Mądro, A.; Celiński, K.; Słomka, M.; Kasztelan-Szczerbińska, B.; Pacian, A.; Kulik, T. Quality of Life of Patients with Irritable Bowel Syndrome before and After Education. Psychiatr. Pol. 2015, 49, 821–833. [Google Scholar] [CrossRef]
- Grundmann, O.; Yoon, S.L. Complementary and Alternative Medicines in Irritable Bowel Syndrome: An Integrative View. World J. Gastroenterol. 2014, 20, 346–362. [Google Scholar] [CrossRef] [PubMed]
- Colomier, E.; Algera, J.; Melchior, C. Pharmacological Therapies and their Clinical Targets in Irritable Bowel Syndrome with Diarrhea. Front. Pharmacol. 2021, 11, 629026. [Google Scholar] [CrossRef] [PubMed]
- Aronson, J.K. Meyler’s Side Effects of Drugs: The International Encyclopedia of Adverse Drug Reactions and Interactions; Elsevier: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Rahimi, R.; Abdollahi, M. Herbal Medicines for the Management of Irritable Bowel Syndrome: A Comprehensive Review. World J. Gastroenterol. 2012, 18, 589–600. [Google Scholar] [CrossRef] [PubMed]
- Roudsari, N.M.; Lashgari, N.; Momtaz, S.; Farzaei, M.H.; Marques, A.M.; Abdolghaffari, A.H. Natural Polyphenols for the Prevention of Irritable Bowel Syndrome: Molecular Mechanisms and Targets; a Comprehensive Review. DARU J. Pharm. Sci. 2019, 27, 755–780. [Google Scholar] [CrossRef] [PubMed]
- Davis, P.H. Flora of Turkey and the East Aegean Islands; Edinburgh University Press: Edinburgh, UK, 1978; Volume 6. [Google Scholar]
- Podolak, I.; Elas, M.; Cieszka, K. In Vitro Antifungal and Cytotoxic Activity of Triterpene Saponosides and Quinoid Pigments from Lysimachia vulgaris L. Phytother. Res. 1998, 12, S70–S73. [Google Scholar] [CrossRef]
- Turker, A.U.; Guner, B. Efficient Plant Regeneration of Yellow Loosestrife (Lysimachia vulgaris L.), a Medicinal Plant. Acta. Biol. Hung. 2013, 64, 218–230. [Google Scholar] [CrossRef]
- Yildirim, A.B.; Guner, B.; Karakas, F.P.; Turker, A.U. Evaluation of Antibacterial, Antitumor, Antioxidant Activities and Phenolic Constituents of Field-Grown and in Vitro-Grown Lysimachia vulgaris L. Afr. J. Tradit. Complement. Altern. Med. 2017, 14, 177–187. [Google Scholar] [CrossRef]
- Kanbolat, Ş.; Badem, M.; Şener, S.Ö.; Korkmaz, N.; Kulaber, A.; Aliyazıcıoğlu, R.; Yenilmez, E.; Özgen, U.; Karaoğlu, Ş.A. Investigation of Wound Healing Potentials of Lysimachia Verticillaris and Lysimachia vulgaris: In Vivo and In Vitro Studies. S. Afr. J. Bot. 2023, 163, 770–776. [Google Scholar] [CrossRef]
- Tanaka, T. Tanaka’s Cyclopedia of Edible Plants of the World; Keigaku Pub. Co.: Tokyo, Japan, 1976. [Google Scholar]
- Hanganu, D.; Olah, N.K.; Mocan, A.; Vlase, L.; Benedec, D.; Raita, O.; Toma, C.C. Comparative Polyphenolic Content and Antioxidant Activities of Two Romanian Lysimachia. Rev. Chim. 2016, 67, 227–231. [Google Scholar] [CrossRef]
- Cojocariu, R.; Ciobica, A.; Balmus, I.; Guenne, S.; Trifan, A.; Stanciu, C.; Hrițcu, L.; Lefter, R. Antioxidant Capacity and Behavioral Relevance of a Polyphenolic Extract of Chrysanthellum Americanum in a Rat Model of Irritable Bowel Syndrome. Oxid. Med. Cell. Longev. 2019, 2019, 3492767. [Google Scholar] [CrossRef]
- Curci, F.; Corbo, F.; Clodoveo, M.L.; Salvagno, L.; Rosato, A.; Corazza, I.; Budriesi, R.; Micucci, M.; Mattioli, L.B. Polyphenols from Olive-Mill Wastewater and Biological Activity: Focus on Irritable Bowel Syndrome. Nutrients 2022, 14, 1264. [Google Scholar] [CrossRef] [PubMed]
- Rasheed, N.; Nagaiah, K.; Goud, P.R.; Sharma, V. Chemical Marker Compounds and their Essential Role in Quality Control of Herbal Medicines. Ann. Phytomed. 2012, 1, 1–8. [Google Scholar]
- Kushwaha, S.K.; Kushwaha, N.; Maurya, N.; Rai, A.K. Role of Markers in the Standardization of Herbal Drugs: A Review. Arch. Appl. Sci. Res. 2010, 2, 225–229. [Google Scholar]
- Chevalier, A. The Encyclopedia of Medicinal Plants; Dorling Kindersley: London, UK, 1996. [Google Scholar]
- Asano, T.; Tanaka, K.; Suemasu, S.; Ishihara, T.; Tahara, K.; Suzuki, T.; Suzuki, H.; Fukudo, S.; Mizushima, T. Effects of Β-(1, 3–1, 6)-D-Glucan on Irritable Bowel Syndrome-Related Colonic Hypersensitivity. Biochem. Biophys. Res. Commun. 2012, 420, 444–449. [Google Scholar] [CrossRef]
- Son, Y.; Jung, D.S.; Shin, J.M.; Kim, M.; Yoo, G.; Nho, C.W. Yellow Loosestrife (Lysimachia vulgaris var. Davurica) Ameliorates Liver Fibrosis in Db/Db Mice with Methionine-and Choline-Deficient Diet-Induced Nonalcoholic Steatohepatitis. BMC Complement. Med. Ther. 2021, 21, 44. [Google Scholar] [CrossRef]
- Saito, T.; Mizutani, F.; Iwanaga, Y.; Morikawa, K.; Kato, H. Laxative and Anti-Diarrheal Activity of Polycarbophil in Mice and Rats. Jpn. J. Pharmacol. 2002, 89, 133–141. [Google Scholar] [CrossRef][Green Version]
- Browning, K.N. Role of Central Vagal 5-HT3 Receptors in Gastrointestinal Physiology and Pathophysiology. Front. Neurosci. 2015, 9, 413. [Google Scholar] [CrossRef] [PubMed]
- Zhong, W.; Hutchinson, T.E.; Chebolu, S.; Darmani, N.A. Serotonin 5-HT3 Receptor-Mediated Vomiting Occurs Via the Activation of Ca2+/CaMKII-Dependent ERK1/2 Signaling in the Least Shrew (Cryptotis parva). PLoS ONE 2014, 9, e104718. [Google Scholar] [CrossRef]
- Fakhfouri, G.; Rahimian, R.; Dyhrfjeld-Johnsen, J.; Zirak, M.R.; Beaulieu, J. 5-HT3 Receptor Antagonists in Neurologic and Neuropsychiatric Disorders: The Iceberg Still Lies Beneath the Surface. Pharmacol. Rev. 2019, 71, 383–412. [Google Scholar] [CrossRef]
- Merecz, K.; Hirsa, M.; Biniszewska, O.; Fichna, J.; Tarasiuk, A. An Overview of 5-HT3 Receptor Antagonists as a Treatment Option for Irritable Bowel Syndrome with Diarrhea. Expert Opin. Pharmacother. 2023, 24, 1189–1198. [Google Scholar] [CrossRef]
- Cremonini, F.; Nicandro, J.P.; Atkinson, V.; Shringarpure, R.; Chuang, E.; Lembo, A. Randomised Clinical Trial: Alosetron Improves Quality of Life and Reduces Restriction of Daily Activities in Women with Severe Diarrhoea-predominant IBS. Aliment. Pharmacol. Ther. 2012, 36, 437–448. [Google Scholar] [CrossRef]
- Kunle, O.F.; Egharevba, H.O.; Henry, O.; Ahmadu, P.O. Standardization of Herbal Medicines-A Review. Int. J. Biodivers. Conserv. 2012, 4, 101–112. [Google Scholar] [CrossRef]
- Tóth, A.; Riethmüller, E.; Alberti, Á.; Végh, K.; Kéry, Á. Comparative Phytochemical Screening of Phenoloids in Lysimachia Species. Eur. Chem. Bull. 2012, 2, 27–30. [Google Scholar]
- Toth, A.; Toth, G.; Kery, A. Polyphenol Composition and Antioxidant Capacity of Three Lysimachia Species. Nat. Prod. Commun. 2014, 9, 1473–1478. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Mao, L.; Wu, C.; Ye, W.; Wang, X. Chlorogenic Acid Improves Intestinal Barrier Function by Downregulating CD14 to Inhibit the NF-κB Signaling Pathway. J. Funct. Foods 2021, 85, 104640. [Google Scholar] [CrossRef]
- Zheng, D.; Liwinski, T.; Elinav, E. Interaction between Microbiota and Immunity in Health and Disease. Cell Res. 2020, 30, 492–506. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Zhong, Y.; Zhang, W.; Wang, Z.; Xiao, H.; Zhang, W.; Xie, J.; Peng, X.; Luo, J.; Xu, W. Chlorogenic Acid Ameliorates Post-Infectious Irritable Bowel Syndrome by Regulating Extracellular Vesicles of Gut Microbes. Adv. Sci. 2023, 10, 2302798. [Google Scholar] [CrossRef]
- Gullon, B.; Lú-Chau, T.A.; Moreira, M.T.; Lema, J.M.; Eibes, G. Rutin: A Review on Extraction, Identification and Purification Methods, Biological Activities and Approaches to Enhance its Bioavailability. Trends Food Sci. Technol. 2017, 67, 220–235. [Google Scholar] [CrossRef]
- Kwon, K.H.; Murakami, A.; Tanaka, T.; Ohigashi, H. Dietary Rutin, but Not its Aglycone Quercetin, Ameliorates Dextran Sulfate Sodium-Induced Experimental Colitis in Mice: Attenuation of Pro-Inflammatory Gene Expression. Biochem. Pharmacol. 2005, 69, 395–406. [Google Scholar] [CrossRef]
- Kim, H.; Kong, H.; Choi, B.; Yang, Y.; Kim, Y.; Lim, M.J.; Neckers, L.; Jung, Y. Metabolic and Pharmacological Properties of Rutin, a Dietary Quercetin Glycoside, for Treatment of Inflammatory Bowel Disease. Pharm. Res. 2005, 22, 1499–1509. [Google Scholar] [CrossRef]
- International Council for Harmonisation (ICH). Validation of Analytical Procedures Q2(R2). ICH, Geneva, Switzerland. 24 March 2022. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/ich-guideline-q2r2-validation-analytical-procedures-step-2b_en.pdf (accessed on 13 December 2023).
- Marson, B.M.; Concentino, V.; Junkert, A.M.; Fachi, M.M.; Vilhena, R.O.; Pontarolo, R. Validation of Analytical Methods in a Pharmaceutical Quality System: An Overview Focused on HPLC Methods. Quím. Nova 2020, 43, 1190–1203. [Google Scholar] [CrossRef]
- Ferenczi-Fodor, K.; Végh, Z.; Nagy-Turák, A.; Renger, B.; Zeller, M. Validation and Quality Assurance of Planar Chromatographic Procedures in Pharmaceutical Analysis. J. AOAC Int. 2001, 84, 1265–1276. [Google Scholar] [CrossRef] [PubMed]
- Betz, J.M.; Brown, P.N.; Roman, M.C. Accuracy, Precision, and Reliability of Chemical Measurements in Natural Products Research. Fitoterapia 2011, 82, 44–52. [Google Scholar] [CrossRef]
- Prenesti, E.; Gosmaro, F. Trueness, Precision and Accuracy: A Critical Overview of the Concepts as Well as Proposals for Revision. Accreditation Qual. Assur. 2015, 20, 33–40. [Google Scholar] [CrossRef]
- Shabir, G.A. Step-by-Step Analytical Methods Validation and Protocol in the Quality System Compliance Industry. J. Valid. Technol. 2005, 10, 314–325. [Google Scholar]
- AOAC International. Appendix F: Guidelines for Standard Method Performance Requirements. 2016. Available online: https://www.aoac.org/wp-content/uploads/2019/08/app_f.pdf (accessed on 13 December 2023).
- Zhang, M.; Shi, T.; Lei, Y.; Chen, W.; Mu, S.; Pan, Y.; Zhao, H. Developing and Validating an HPLC Method for Related Substance of Camostat Mesilate in Bulk. Am. J. Biomed. Sci. Res. 2022, 15, 145–151. [Google Scholar] [CrossRef]
- Šamec, D.; Karalija, E.; Šola, I.; Vujčić Bok, V.; Salopek-Sondi, B. The Role of Polyphenols in Abiotic Stress Response: The Influence of Molecular Structure. Plants 2021, 10, 118. [Google Scholar] [CrossRef]
| Compound | Linear Equation | Correlation Coefficient (r2) | LOD (μg/mL) | LOQ (μg/mL) |
|---|---|---|---|---|
| Chlorogenic acid | y = 47,062x − 39,362 | 0.99969 | 0.39 | 1.17 |
| Rutin | y = 20,210x − 1351 | 0.99999 | 0.27 | 0.82 |
| Compound | Intra-Day Precision (n = 5) | Inter-Day Precision (n = 3) | |||
|---|---|---|---|---|---|
| Actual Conc. of Analyte (μg/mL) | Observed Conc. of Analyte (μg/mL) | RSD (%) | Observed Conc. of Analyte (μg/mL) | RSD (%) | |
| Chlorogenic acid | 5.00 | 5.21 | 0.07 | 5.39 | 0.62 |
| 25.00 | 23.75 | 0.39 | 24.36 | 0.45 | |
| 50.00 | 48.07 | 0.22 | 49.42 | 0.75 | |
| Rutin | 5.00 | 5.05 | 0.32 | 5.06 | 0.32 |
| 25.00 | 24.69 | 0.15 | 24.77 | 0.24 | |
| 50.00 | 49.68 | 0.15 | 49.76 | 0.30 | |
| Compound | Spiked Conc. (μg/mL) | Observed Conc. (μg/mL) | Recovery (%) | RSD (%) |
|---|---|---|---|---|
| Chlorogenic acid | 5.00 | 5.22 | 104.47 ± 1.24 | 1.18 |
| 25.00 | 25.41 | 101.65 ± 0.36 | 0.36 | |
| 50.00 | 50.81 | 101.61 ± 0.55 | 0.54 | |
| Rutin | 5.00 | 5.18 | 103.53 ± 1.75 | 1.69 |
| 25.00 | 25.04 | 100.15 ± 0.50 | 0.50 | |
| 50.00 | 51.39 | 102.78 ± 0.54 | 0.53 |
| Time | % Differences in Peak Areas under Storage Condition of Room Temperature | % Differences in Peak Areas under Storage Condition of 0–4 °C | ||||||
|---|---|---|---|---|---|---|---|---|
| Chlorogenic Acid | Rutin | Chlorogenic Acid | Rutin | |||||
| Standard | Extract | Standard | Extract | Standard | Extract | Standard | Extract | |
| 12 | 1.34 | 0.13 | 0.39 | 0.08 | 1.18 | 0.21 | 0.33 | 0.04 |
| 24 | 0.83 | 0.19 | 0.27 | 0.22 | 0.38 | 0.06 | 0.02 | 0.06 |
| 48 | 0.57 | 0.61 | 0.59 | 0.15 | 0.74 | 0.25 | 0.52 | 0.1 |
| Compound | Content (mg/g) | RSD (%) |
|---|---|---|
| Chlorogenic acid | 24.31 ± 0.23 | 0.95 |
| Rutin | 54.89 ± 0.38 | 0.69 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.-Y.; Kim, C.-E.; Oh, D.-R.; Kim, Y.; Choi, C.-Y.; Kim, J. Development and Validation of a High-Performance Liquid Chromatography Method to Quantify Marker Compounds in Lysimachia vulgaris var. davurica and Its Effects in Diarrhea-Predominant Irritable Bowel Syndrome. Molecules 2024, 29, 1489. https://doi.org/10.3390/molecules29071489
Kim H-Y, Kim C-E, Oh D-R, Kim Y, Choi C-Y, Kim J. Development and Validation of a High-Performance Liquid Chromatography Method to Quantify Marker Compounds in Lysimachia vulgaris var. davurica and Its Effects in Diarrhea-Predominant Irritable Bowel Syndrome. Molecules. 2024; 29(7):1489. https://doi.org/10.3390/molecules29071489
Chicago/Turabian StyleKim, Hye-Youn, Cho-Een Kim, Dool-Ri Oh, Yonguk Kim, Chul-Yung Choi, and Jaeyong Kim. 2024. "Development and Validation of a High-Performance Liquid Chromatography Method to Quantify Marker Compounds in Lysimachia vulgaris var. davurica and Its Effects in Diarrhea-Predominant Irritable Bowel Syndrome" Molecules 29, no. 7: 1489. https://doi.org/10.3390/molecules29071489
APA StyleKim, H.-Y., Kim, C.-E., Oh, D.-R., Kim, Y., Choi, C.-Y., & Kim, J. (2024). Development and Validation of a High-Performance Liquid Chromatography Method to Quantify Marker Compounds in Lysimachia vulgaris var. davurica and Its Effects in Diarrhea-Predominant Irritable Bowel Syndrome. Molecules, 29(7), 1489. https://doi.org/10.3390/molecules29071489







