Long-Term Impact of Diffuse Traumatic Brain Injury on Neuroinflammation and Catecholaminergic Signaling: Potential Relevance for Parkinson’s Disease Risk
Abstract
1. Introduction
2. Results
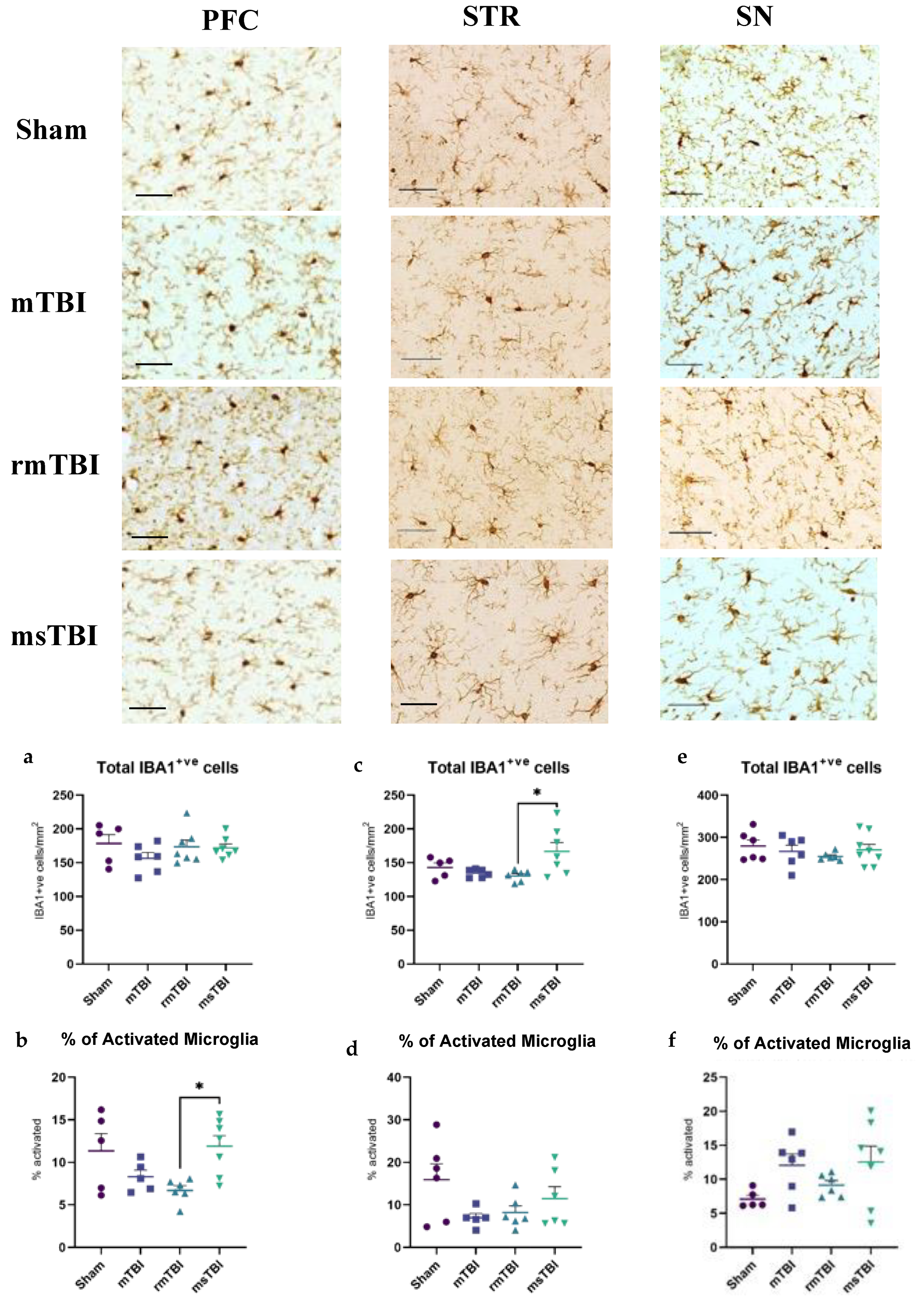
2.1. Subtle Alterations in Neuroinflammation Observed following Moderate–Severe TBI at 12-Months Post-Injury
2.2. DRD1 Elevation Observed in PFC, but Not STR or SN, following Single Mild TBI at 12 Months Post-Injury
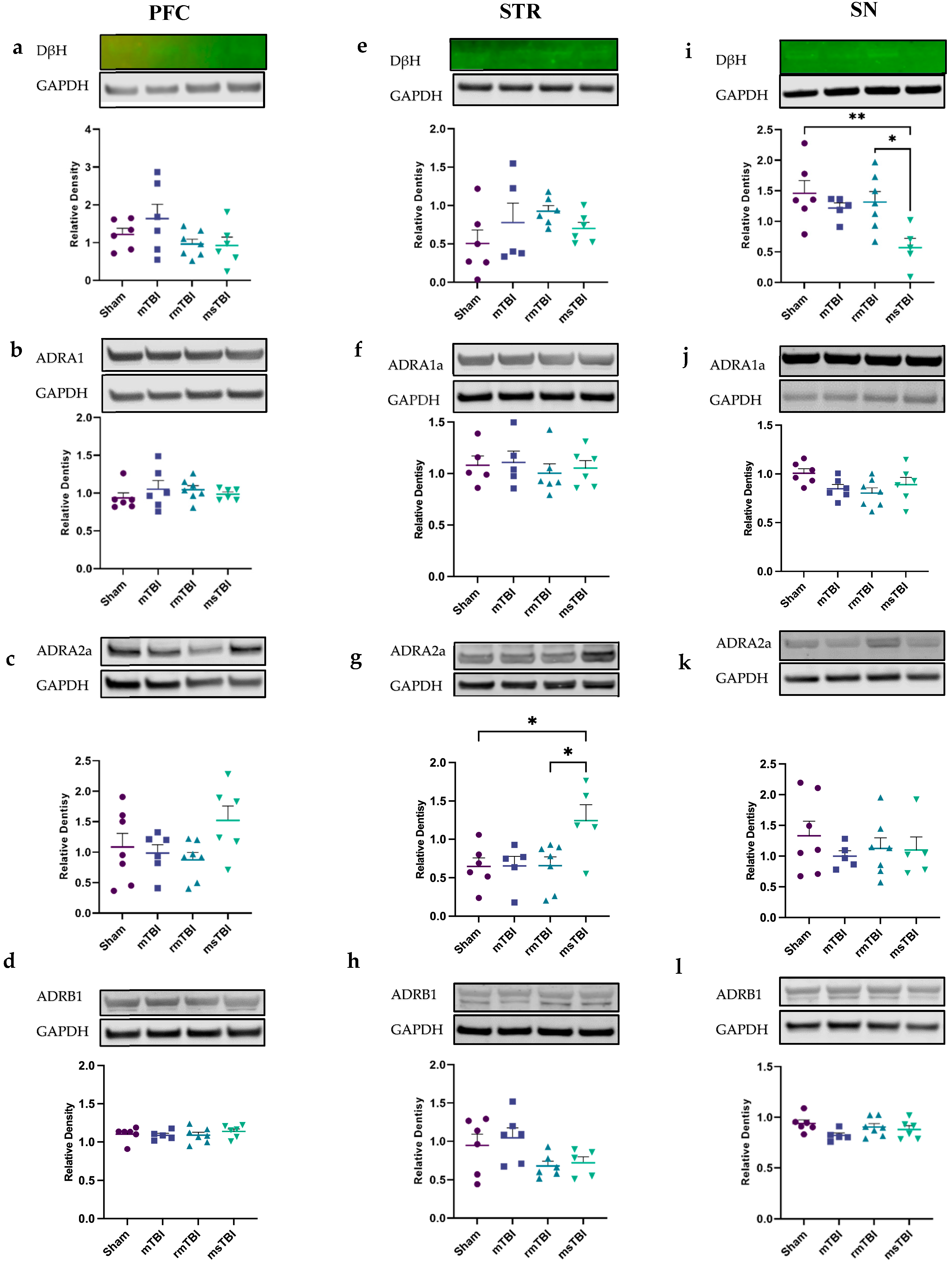
2.3. Moderate–Severe TBI Leads to Chronic Changes in STR ADRA2A and SN DβH Levels at 12 Months Post-Injury
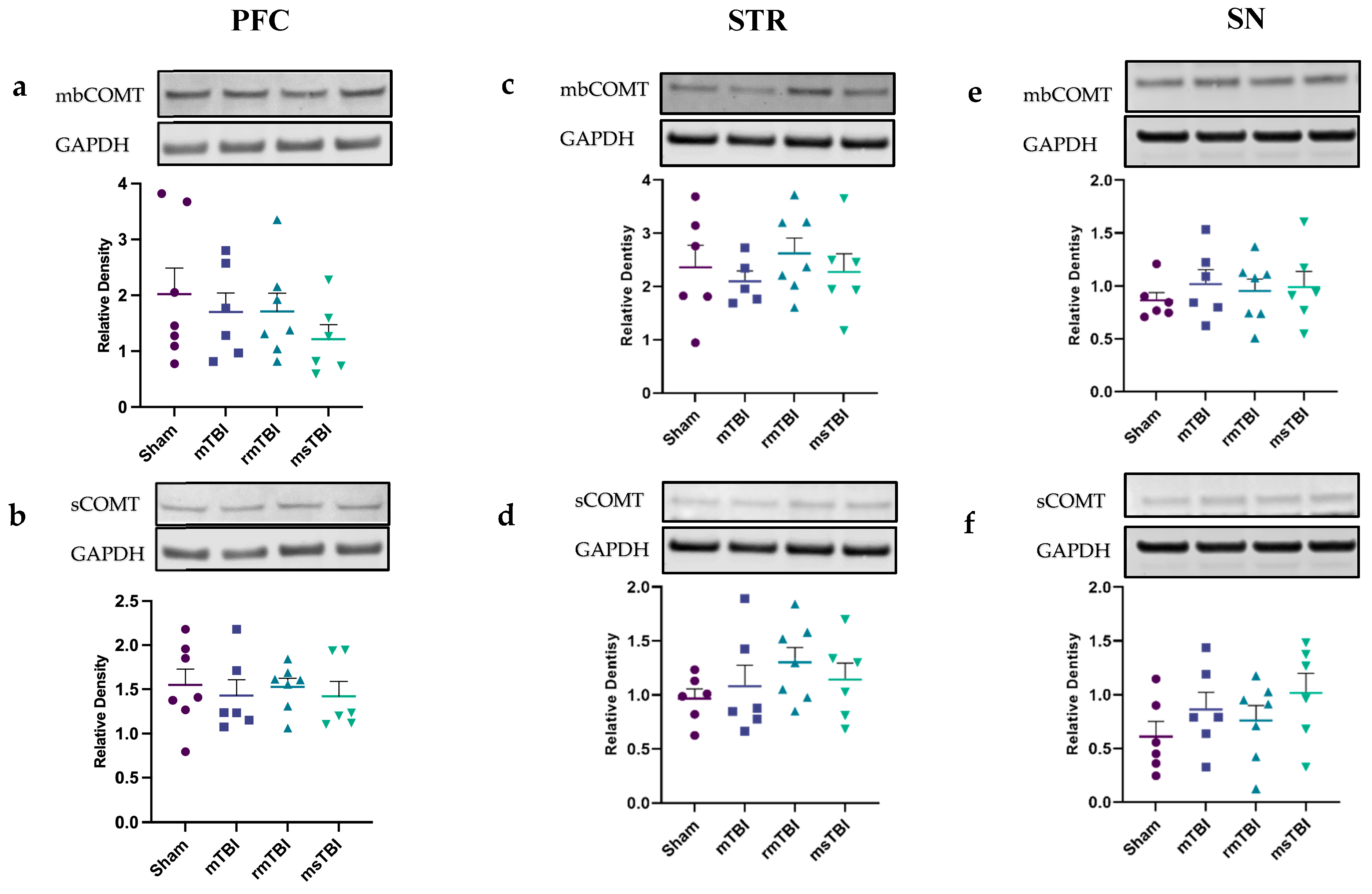
2.4. Expression of COMT Was Not Altered by Chronic TBI
3. Materials and Methods
3.1. Animals
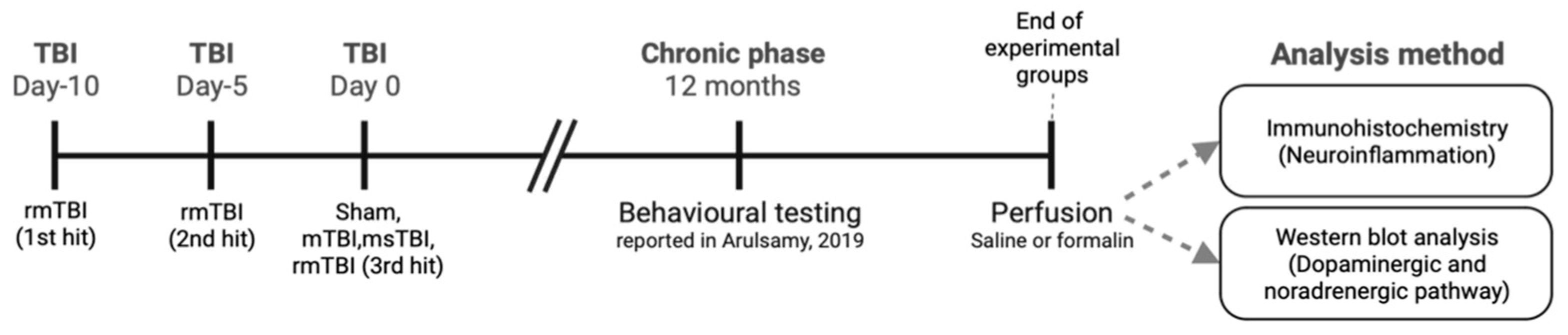
3.2. Experimental Groups and Study Design
3.3. Injury Protocol and Postoperative Care
3.4. Immunohistochemistry
3.5. Image Analysis
3.6. Western Blot
3.7. Statistics
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dewan, M.C.; Rattani, A.; Gupta, S.; Baticulon, R.E.; Hung, Y.-C.; Punchak, M.; Agrawal, A.; Adeleye, A.O.; Shrime, M.G.; Rubiano, A.M.; et al. Estimating the Global Incidence of Traumatic Brain Injury. J. Neurosurg. 2019, 130, 1080–1097. [Google Scholar] [CrossRef] [PubMed]
- Taylor, C.A.; Bell, J.M.; Breiding, M.J.; Xu, L. Traumatic Brain Injury–Related Emergency Department Visits, Hospitalizations, and Deaths—United States, 2007 and 2013. MMWR Surveill. Summ. 2017, 66, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Faul, M.; Wald, M.M.; Xu, L.; Coronado, V.G. Traumatic Brain Injury in the United States: Emergency Department Visits, Hospitalization and Deaths 2002–2006. Cent. Dis. Control Prev. Natl. Cent. Inj. Prev. Control 2010. Available online: https://stacks.cdc.gov/view/cdc/5571 (accessed on 20 March 2024).
- James, S.L.; Theadom, A.; Ellenbogen, R.G.; Bannick, M.S.; Montjoy-Venning, W.; Lucchesi, L.R.; Abbasi, N.; Abdulkader, R.; Abraha, H.N.; Adsuar, J.C.; et al. Global, Regional, and National Burden of Traumatic Brain Injury and Spinal Cord Injury, 1990–2016: A Systematic Analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 56–87. [Google Scholar] [CrossRef]
- Feigin, V.L.; Theadom, A.; Barker-Collo, S.; Starkey, N.J.; McPherson, K.; Kahan, M.; Dowell, A.; Brown, P.; Parag, V.; Kydd, R.; et al. Incidence of Traumatic Brain Injury in New Zealand: A Population-Based Study. Lancet Neurol. 2013, 12, 53–64. [Google Scholar] [CrossRef]
- Brett, B.L.; Gardner, R.C.; Godbout, J.; Dams-O’Connor, K.; Keene, C.D. Traumatic Brain Injury and Risk of Neurodegenerative Disorder. Biol. Psychiatry 2022, 91, 498–507. [Google Scholar] [CrossRef] [PubMed]
- Gardner, R.C.; Burke, J.F.; Nettiksimmons, J.; Goldman, S.; Tanner, C.M.; Yaffe, K. Traumatic Brain Injury in Later Life Increases Risk for Parkinson Disease: TBI Increases Risk for PD. Ann. Neurol. 2015, 77, 987–995. [Google Scholar] [CrossRef] [PubMed]
- Braak, H.; Tredici, K.D.; Rüb, U.; de Vos, R.A.I.; Jansen Steur, E.N.H.; Braak, E. Staging of Brain Pathology Related to Sporadic Parkinson’s Disease. Neurobiol. Aging 2003, 24, 197–211. [Google Scholar] [CrossRef] [PubMed]
- Dauer, W.; Przedborski, S. Parkinson’s Disease: Mechanisms and Models. Neuron 2003, 39, 889–909. [Google Scholar] [CrossRef]
- Ehringer, H.; Hornykiewicz, O. Distribution of Noradrenaline and Dopamine (3-Hydroxytyramine) in the Human Brain and Their Behavior in Diseases of the Extrapyramidal System. Park. Relat. Disord. 1998, 4, 53–57. [Google Scholar] [CrossRef]
- Klein, M.O.; Battagello, D.S.; Cardoso, A.R.; Hauser, D.N.; Bittencourt, J.C.; Correa, R.G. Dopamine: Functions, Signaling, and Association with Neurological Diseases. Cell. Mol. Neurobiol. 2019, 39, 31–59. [Google Scholar] [CrossRef]
- Nandhagopal, R.; Kuramoto, L.; Schulzer, M.; Mak, E.; Cragg, J.; Lee, C.S.; McKenzie, J.; McCormick, S.; Samii, A.; Troiano, A.; et al. Longitudinal Progression of Sporadic Parkinson’s Disease: A Multi-Tracer Positron Emission Tomography Study. Brain 2009, 132, 2970–2979. [Google Scholar] [CrossRef]
- Booij, J.; Tissingh, G.; Boer, G.J.; Speelman, J.D.; Stoof, J.C.; Janssen, A.G.; Wolters, E.C.; Van Royen, E.A. [123I]FP-CIT SPECT Shows a Pronounced Decline of Striatal Dopamine Transporter Labelling in Early and Advanced Parkinson’s Disease. J. Neurol. Neurosurg. Psychiatry 1997, 62, 133–140. [Google Scholar] [CrossRef]
- Ziebell, M.; Andersen, B.B.; Thomsen, G.; Pinborg, L.H.; Karlsborg, M.; Hasselbalch, S.G.; Knudsen, G.M. Predictive Value of Dopamine Transporter SPECT Imaging with [123I]PE2I in Patients with Subtle Parkinsonian Symptoms. Eur. J. Nucl. Med. Mol. Imaging 2012, 39, 242–250. [Google Scholar] [CrossRef]
- Kish, S.J.; Shannak, K.; Hornykiewicz, O. Uneven Pattern of Dopamine Loss in the Striatum of Patients with Idiopathic Parkinson’s Disease. N. Engl. J. Med. 1988, 318, 876–880. [Google Scholar] [CrossRef] [PubMed]
- Fearnley, J.M.; Lees, A.J. Ageing and parkinson’s disease: Substantia nigra regional selectivity. Brain 1991, 114, 2283–2301. [Google Scholar] [CrossRef]
- Kamath, T.; Abdulraouf, A.; Burris, S.J.; Langlieb, J.; Gazestani, V.; Nadaf, N.M.; Balderrama, K.; Vanderburg, C.; Macosko, E.Z. Single-Cell Genomic Profiling of Human Dopamine Neurons Identifies a Population That Selectively Degenerates in Parkinson’s Disease. Nat. Neurosci. 2022, 25, 588–595. [Google Scholar] [CrossRef]
- Otsuka, M.; Ichiya, Y.; Kuwabara, Y.; Hosokawa, S.; Sasaki, M.; Yoshida, T.; Fukumura, T.; Masuda, K.; Kato, M. Differences in the Reduced 18F-Dopa Uptakes of the Caudate and the Putamen in Parkinson’s Disease: Correlations with the Three Main Symptoms. J. Neurol. Sci. 1996, 136, 169–173. [Google Scholar] [CrossRef] [PubMed]
- Rinne, J.O.; Ruottinen, H.; Bergman, J.; Haaparanta, M.; Sonninen, P.; Solin, O. Usefulness of a Dopamine Transporter PET Ligand [18F]Beta-CFT in Assessing Disability in Parkinson’s Disease. J. Neurol. Neurosurg. Psychiatry 1999, 67, 737–741. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zarow, C.; Lyness, S.A.; Mortimer, J.A.; Chui, H.C. Neuronal Loss Is Greater in the Locus Coeruleus Than Nucleus Basalis and Substantia Nigra in Alzheimer and Parkinson Diseases. Arch. Neurol. 2003, 60, 337. [Google Scholar] [CrossRef]
- Ohtsuka, C.; Sasaki, M.; Konno, K.; Koide, M.; Kato, K.; Takahashi, J.; Takahashi, S.; Kudo, K.; Yamashita, F.; Terayama, Y. Changes in Substantia Nigra and Locus Coeruleus in Patients with Early-Stage Parkinson’s Disease Using Neuromelanin-Sensitive MR Imaging. Neurosci. Lett. 2013, 541, 93–98. [Google Scholar] [CrossRef]
- Oertel, W.H.; Henrich, M.T.; Janzen, A.; Geibl, F.F. The Locus Coeruleus: Another Vulnerability Target in Parkinson’s Disease. Mov. Disord. 2019, 34, 1423–1429. [Google Scholar] [CrossRef] [PubMed]
- Sitte, H.H.; Pifl, C.; Rajput, A.H.; Hörtnagl, H.; Tong, J.; Lloyd, G.K.; Kish, S.J.; Hornykiewicz, O. Dopamine and Noradrenaline, but Not Serotonin, in the Human Claustrum Are Greatly Reduced in Patients with Parkinson’s Disease: Possible Functional Implications. Eur. J. Neurosci. 2017, 45, 192–197. [Google Scholar] [CrossRef] [PubMed]
- Braak, E.; Sandmann-Keil, D.; Rüb, U.; Gai, W.P.; De Vos, R.A.I.; Jansen Steur, E.N.H.; Arai, K.; Braak, H. α-Synuclein Immunopositive Parkinson’s Disease-Related Inclusion Bodies in Lower Brain Stem Nuclei. Acta Neuropathol. 2001, 101, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Abbott, R.D.; Ross, G.W.; White, L.R.; Tanner, C.M.; Masaki, K.H.; Nelson, J.S.; Curb, J.D.; Petrovitch, H. Excessive Daytime Sleepiness and Subsequent Development of Parkinson Disease. Neurology 2005, 65, 1442–1446. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, L.M.; Tuppy, M.; Moreira, T.S.; Takakura, A.C. Role of the Locus Coeruleus Catecholaminergic Neurons in the Chemosensory Control of Breathing in a Parkinson’s Disease Model. Exp. Neurol. 2017, 293, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Guo, T.; Bai, X.; Wu, J.; Gao, T.; Guan, X.; Liu, X.; Gu, L.; Huang, P.; Xuan, M.; et al. Locus Coeruleus Degeneration Is Associated with Disorganized Functional Topology in Parkinson’s Disease. NeuroImage Clin. 2021, 32, 102873. [Google Scholar] [CrossRef] [PubMed]
- Mastroeni, D.; Grover, A.; Leonard, B.; Joyce, J.N.; Coleman, P.D.; Kozik, B.; Bellinger, D.L.; Rogers, J. Microglial Responses to Dopamine in a Cell Culture Model of Parkinson’s Disease. Neurobiol. Aging 2009, 30, 1805–1817. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, Y.; Wu, J.; Manaenko, A.; Yang, P.; Tang, J.; Fu, W.; Zhang, J.H. Activation of Dopamine D2 Receptor Suppresses Neuroinflammation through αB-Crystalline by Inhibition of NF-κB Nuclear Translocation in Experimental ICH Mice Model. Stroke 2015, 46, 2637–2646. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Nowrangi, D.; Yu, L.; Lu, T.; Tang, J.; Han, B.; Ding, Y.; Fu, F.; Zhang, J.H. Activation of Dopamine D1 Receptor Decreased NLRP3-Mediated Inflammation in Intracerebral Hemorrhage Mice. J. Neuroinflamm. 2018, 15, 2. [Google Scholar] [CrossRef]
- Marinova-Mutafchieva, L.; Sadeghian, M.; Broom, L.; Davis, J.B.; Medhurst, A.D.; Dexter, D.T. Relationship between Microglial Activation and Dopaminergic Neuronal Loss in the Substantia Nigra: A Time Course Study in a 6-Hydroxydopamine Model of Parkinson’s Disease. J. Neurochem. 2009, 110, 966–975. [Google Scholar] [CrossRef]
- Shao, W.; Zhang, S.; Tang, M.; Zhang, X.; Zhou, Z.; Yin, Y.; Zhou, Q.; Huang, Y.; Liu, Y.; Wawrousek, E.; et al. Suppression of Neuroinflammation by Astrocytic Dopamine D2 Receptors via αB-Crystallin. Nature 2013, 494, 90–94. [Google Scholar] [CrossRef]
- Färber, K.; Pannasch, U.; Kettenmann, H. Dopamine and Noradrenaline Control Distinct Functions in Rodent Microglial Cells. Mol. Cell. Neurosci. 2005, 29, 128–138. [Google Scholar] [CrossRef] [PubMed]
- Evans, A.K.; Defensor, E.; Shamloo, M. Selective Vulnerability of the Locus Coeruleus Noradrenergic System and Its Role in Modulation of Neuroinflammation, Cognition, and Neurodegeneration. Front. Pharmacol. 2022, 13, 1030609. [Google Scholar] [CrossRef] [PubMed]
- Cebrián, C.; Loike, J.D.; Sulzer, D. Neuroinflammation in Parkinson’s Disease Animal Models: A Cell Stress Response or a Step in Neurodegeneration? In Behavioral Neurobiology of Huntington’s Disease and Parkinson’s Disease; Nguyen, H.H.P., Cenci, M.A., Eds.; Current Topics in Behavioral Neurosciences; Springer: Berlin/Heidelberg, Germany, 2014; Volume 22, pp. 237–270. ISBN 978-3-662-46343-7. [Google Scholar]
- Troncoso-Escudero, P.; Parra, A.; Nassif, M.; Vidal, R.L. Outside in: Unraveling the Role of Neuroinflammation in the Progression of Parkinson’s Disease. Front. Neurol. 2018, 9, 860. [Google Scholar] [CrossRef]
- Wang, Q.; Oyarzabal, E.A.; Song, S.; Wilson, B.; Santos, J.H.; Hong, J.-S. Locus Coeruleus Neurons Are Most Sensitive to Chronic Neuroinflammation-Induced Neurodegeneration. Brain. Behav. Immun. 2020, 87, 359–368. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.-F.; Wu, S.-Y.; Pan, B.-S.; Tsai, S.-F.; Kuo, Y.-M. Inhibition of Nigral Microglial Activation Reduces Age-Related Loss of Dopaminergic Neurons and Motor Deficits. Cells 2022, 11, 481. [Google Scholar] [CrossRef]
- McGeer, P.L.; Itagaki, S.; Boyes, B.E.; McGeer, E.G. Reactive Microglia Are Positive for HLA-DR in the Substantia Nigra of Parkinson’s and Alzheimer’s Disease Brains. Neurology 1988, 38, 1285. [Google Scholar] [CrossRef]
- Mogi, M.; Harada, M.; Riederer, P.; Narabayashi, H.; Fujita, K.; Nagatsu, T. Tumor Necrosis Factor-α (TNF-α) Increases Both in the Brain and in the Cerebrospinal Fluid from Parkinsonian Patients. Neurosci. Lett. 1994, 165, 208–210. [Google Scholar] [CrossRef] [PubMed]
- Mogi, M.; Harada, M.; Kondo, T.; Riederer, P.; Inagaki, H.; Minami, M.; Nagatsu, T. Interleukin-1β, Interleukin-6, Epidermal Growth Factor and Transforming Growth Factor-α Are Elevated in the Brain from Parkinsonian Patients. Neurosci. Lett. 1994, 180, 147–150. [Google Scholar] [CrossRef]
- Mogi, M.; Harada, M.; Narabayashi, H.; Inagaki, H.; Minami, M.; Nagatsu, T. Interleukin (IL)-1β, IL-2, IL-4, IL-6 and Transforming Growth Factor-α Levels Are Elevated in Ventricular Cerebrospinal Fluid in Juvenile Parkinsonism and Parkinson’s Disease. Neurosci. Lett. 1996, 211, 13–16. [Google Scholar] [CrossRef]
- Butkovich, L.M.; Houser, M.C.; Tansey, M.G. α-Synuclein and Noradrenergic Modulation of Immune Cells in Parkinson’s Disease Pathogenesis. Front. Neurosci. 2018, 12, 626. [Google Scholar] [CrossRef]
- McIntosh, T.K.; Vink, R.; Noble, L.; Yamakami, I.; Fernyak, S.; Soares, H.; Faden, A.L. Traumatic Brain Injury in the Rat: Characterization of a Lateral Fluid-Percussion Model. Neuroscience 1989, 28, 233–244. [Google Scholar] [CrossRef] [PubMed]
- Huger, F.; Patrick, G. Effect of concussive head injury on central catecholamine levels and synthesis rates in rat brain regions. J. Neurochem. 1979, 33, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Bales, J.W.; Kline, A.E.; Wagner, A.K.; Dixon, C.E. Targeting Dopamine in Acute Traumatic Brain Injury. Open Drug Discov. J. 2010, 2, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Mautes, A.E.M.; Müller, M.; Cortbus, F.; Schwerdtfeger, K.; Maier, B.; Holanda, M.; Nacimiento, A.; Marzi, I.; Steudel, W.-I. Alterations of Norepinephrine Levels in Plasma and CSF of Patients after Traumatic Brain Injury in Relation to Disruption of the Blood-Brain Barrier. Acta Neurochir. 2001, 143, 51–58. [Google Scholar] [CrossRef] [PubMed]
- van Bregt, D.R.; Thomas, T.C.; Hinzman, J.M.; Cao, T.; Liu, M.; Bing, G.; Gerhardt, G.A.; Pauly, J.R.; Lifshitz, J. Substantia Nigra Vulnerability after a Single Moderate Diffuse Brain Injury in the Rat. Exp. Neurol. 2012, 234, 8–19. [Google Scholar] [CrossRef]
- Wagner, A.K.; Sokoloski, J.E.; Ren, D.; Chen, X.; Khan, A.S.; Zafonte, R.D.; Michael, A.C.; Dixon, C.E. Controlled Cortical Impact Injury Affects Dopaminergic Transmission in the Rat Striatum. J. Neurochem. 2005, 95, 457–465. [Google Scholar] [CrossRef]
- Impellizzeri, D.; Campolo, M.; Bruschetta, G.; Crupi, R.; Cordaro, M.; Paterniti, I.; Cuzzocrea, S.; Esposito, E. Traumatic Brain Injury Leads to Development of Parkinson’s Disease Related Pathology in Mice. Front. Neurosci. 2016, 10, 196837. [Google Scholar] [CrossRef] [PubMed]
- Levin, B.E.; Brown, K.L.; Pawar, G.; Dunn-Meynell, A. Widespread and Lateralized Effects of Acute Traumatic Brain Injury on Norepinephrine Turnover in the Rat Brain. Brain Res. 1995, 674, 307–313. [Google Scholar] [CrossRef]
- Fujinaka, T.; Kohmura, E.; Yuguchi, T.; Yoshimine, T. The Morphological and Neurochemical Effects of Diffuse Brain Injury on Rat Central Noradrenergic System. Neurol. Res. 2003, 25, 35–41. [Google Scholar] [CrossRef]
- Donnemiller, E.; Brenneis, C.; Wissel, J.; Scherfler, C.; Poewe, W.; Riccabona, G.; Wenning, G.K. Impaired Dopaminergic Neurotransmission in Patients with Traumatic Brain Injury: A SPET Study Using 123I-β-CIT and 123I-IBZM. Eur. J. Nucl. Med. 2000, 27, 1410–1414. [Google Scholar] [CrossRef] [PubMed]
- Tschuor, C.; Asmis, L.M.; Lenzlinger, P.M.; Tanner, M.; Härter, L.; Keel, M.; Stocker, R.; Stover, J.F. In Vitro Norepinephrine Significantly Activates Isolated Platelets from Healthy Volunteers and Critically Ill Patients Following Severe Traumatic Brain Injury. Crit. Care 2008, 12, R80. [Google Scholar] [CrossRef]
- Lloyd-Donald, P.; Spencer, W.; Cheng, J.; Romero, L.; Jithoo, R.; Udy, A.; Fitzgerald, M.C. In Adult Patients with Severe Traumatic Brain Injury, Does the Use of Norepinephrine for Augmenting Cerebral Perfusion Pressure Improve Neurological Outcome? A Systematic Review. Injury 2020, 51, 2129–2134. [Google Scholar] [CrossRef]
- Dunn-Meynell, A.A.; Hassanain, M.; Levin, B.E. Norepinephrine and Traumatic Brain Injury: A Possible Role in Post-Traumatic Edema. Brain Res. 1998, 800, 245–252. [Google Scholar] [CrossRef]
- Chien, Y.J.; Chien, Y.C.; Liu, C.T.; Wu, H.C.; Chang, C.Y.; Wu, M.Y. Effects of Methylphenidate on Cognitive Function in Adults with Traumatic Brain Injury: A Meta-Analysis. Brain Sci. 2019, 9, 291. [Google Scholar] [CrossRef]
- Johnson, V.E.; Stewart, J.E.; Begbie, F.D.; Trojanowski, J.Q.; Smith, D.H.; Stewart, W. Inflammation and White Matter Degeneration Persist for Years after a Single Traumatic Brain Injury. Brain 2013, 136, 28–42. [Google Scholar] [CrossRef] [PubMed]
- Loane, D.J.; Kumar, A.; Stoica, B.A.; Cabatbat, R.; Faden, A.I. Progressive Neurodegeneration after Experimental Brain Trauma: Association with Chronic Microglial Activation. J. Neuropathol. Exp. Neurol. 2014, 73, 14–29. [Google Scholar] [CrossRef] [PubMed]
- Rusiecki, J.; Levin, L.I.; Wang, L.; Byrne, C.; Krishnamurthy, J.; Chen, L.; Galdzicki, Z.; French, L.M. Blast Traumatic Brain Injury and Serum Inflammatory Cytokines: A Repeated Measures Case-Control Study among U.S. Military Service Members. J. Neuroinflamm. 2020, 17, 20. [Google Scholar] [CrossRef]
- Chen, W.; Man, X.; Zhang, Y.; Yao, G.; Chen, J. Medial Prefrontal Cortex Oxytocin Mitigates Epilepsy and Cognitive Impairments Induced by Traumatic Brain Injury through Reducing Neuroinflammation in Mice. Sci. Rep. 2023, 13, 5214. [Google Scholar] [CrossRef]
- Liu, M.; Bachstetter, A.D.; Cass, W.A.; Lifshitz, J.; Bing, G. Pioglitazone Attenuates Neuroinflammation and Promotes Dopaminergic Neuronal Survival in the Nigrostriatal System of Rats after Diffuse Brain Injury. J. Neurotrauma 2017, 34, 414–422. [Google Scholar] [CrossRef]
- Acosta, S.A.; Tajiri, N.; Shinozuka, K.; Ishikawa, H.; Grimmig, B.; Diamond, D.; Sanberg, P.R.; Bickford, P.C.; Kaneko, Y.; Borlongan, C.V. Long-Term Upregulation of Inflammation and Suppression of Cell Proliferation in the Brain of Adult Rats Exposed to Traumatic Brain Injury Using the Controlled Cortical Impact Model. PLoS ONE 2013, 8, e53376. [Google Scholar] [CrossRef]
- Ramlackhansingh, A.F.; Brooks, D.J.; Greenwood, R.J.; Bose, S.K.; Turkheimer, F.E.; Kinnunen, K.M.; Gentleman, S.; Heckemann, R.A.; Gunanayagam, K.; Gelosa, G.; et al. Inflammation after Trauma: Microglial Activation and Traumatic Brain Injury. Ann. Neurol. 2011, 70, 374–383. [Google Scholar] [CrossRef] [PubMed]
- Hutson, C.B.; Lazo, C.R.; Mortazavi, F.; Giza, C.C.; Hovda, D.; Chesselet, M.-F. Traumatic Brain Injury in Adult Rats Causes Progressive Nigrostriatal Dopaminergic Cell Loss and Enhanced Vulnerability to the Pesticide Paraquat. J. Neurotrauma 2011, 28, 1783–1801. [Google Scholar] [CrossRef] [PubMed]
- Nagamoto-Combs, K.; McNeal, D.W.; Morecraft, R.J.; Combs, C.K. Prolonged Microgliosis in the Rhesus Monkey Central Nervous System after Traumatic Brain Injury. J. Neurotrauma 2007, 24, 1719–1742. [Google Scholar] [CrossRef]
- Mouzon, B.C.; Bachmeier, C.; Ferro, A.; Ojo, J.-O.; Crynen, G.; Acker, C.M.; Davies, P.; Mullan, M.; Stewart, W.; Crawford, F. Chronic Neuropathological and Neurobehavioral Changes in a Repetitive Mild Traumatic Brain Injury Model: Chronic Effects of r-mTBI. Ann. Neurol. 2014, 75, 241–254. [Google Scholar] [CrossRef] [PubMed]
- Mouzon, B.C.; Bachmeier, C.; Ojo, J.O.; Acker, C.M.; Ferguson, S.; Paris, D.; Ait-Ghezala, G.; Crynen, G.; Davies, P.; Mullan, M.; et al. Lifelong Behavioral and Neuropathological Consequences of Repetitive Mild Traumatic Brain Injury. Ann. Clin. Transl. Neurol. 2018, 5, 64–80. [Google Scholar] [CrossRef]
- Molinoff, P.B.; Axelrod, J. Biochemistry of Catecholamines. Annu. Rev. Biochem. 1971, 40, 465–500. [Google Scholar] [CrossRef] [PubMed]
- Daubner, S.C.; Le, T.; Wang, S. Tyrosine Hydroxylase and Regulation of Dopamine Synthesis. Arch. Biochem. Biophys. 2011, 508, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Weinshilboum, R.; Axelrod, J. Serum Dopamine-Beta-Hydroxylase Activity. Circ. Res. 1971, 28, 307–315. [Google Scholar] [CrossRef]
- Grossman, M.H.; Emanuel, B.S.; Budarf, M.L. Chromosomal Mapping of the Human Catechol-O-Methyltransferase Gene to 22q11.1→q11.2. Genomics 1992, 12, 822–825. [Google Scholar] [CrossRef]
- Arulsamy, A.; Corrigan, F.; Collins-Praino, L.E. Cognitive and Neuropsychiatric Impairments Vary as a Function of Injury Severity at 12 Months Post-Experimental Diffuse Traumatic Brain Injury: Implications for Dementia Development. Behav. Brain Res. 2019, 365, 66–76. [Google Scholar] [CrossRef]
- Arulsamy, A.; Corrigan, F.; Collins-Praino, L.E. Age, but Not Severity of Injury, Mediates Decline in Executive Function: Validation of the Rodent Touchscreen Paradigm for Preclinical Models of Traumatic Brain Injury. Behav. Brain Res. 2019, 368, 111912. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, P. The Laboratory Rat: Relating Its Age with Human’s. Int. J. Prev. Med. 2013, 4, 624–630. [Google Scholar]
- Postuma, R.B.; Aarsland, D.; Barone, P.; Burn, D.J.; Hawkes, C.H.; Oertel, W.; Ziemssen, T. Identifying Prodromal Parkinson’s Disease: Pre-Motor Disorders in Parkinson’s Disease. Mov. Disord. 2012, 27, 617–626. [Google Scholar] [CrossRef]
- Marmarou, A.; Foda, M.A.; van den Brink, W.; Campbell, J.; Kita, H.; Demetriadou, K. A New Model of Diffuse Brain Injury in Rats. Part I: Pathophysiology and Biomechanics. J. Neurosurg. 1994, 80, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Mahmood, A.; Chopp, M. Animal Models of Traumatic Brain Injury. Nat. Rev. Neurosci. 2013, 14, 128–142. [Google Scholar] [CrossRef] [PubMed]
- Arulsamy, A.; Teng, J.; Colton, H.; Corrigan, F.; Collins-Praino, L. Evaluation of Early Chronic Functional Outcomes and Their Relationship to Pre-Frontal Cortex and Hippocampal Pathology Following Moderate-Severe Traumatic Brain Injury. Behav. Brain Res. 2018, 348, 127–138. [Google Scholar] [CrossRef]
- Corrigan, F.; Arulsamy, A.; Shultz, S.R.; Wright, D.K.; Collins-Praino, L.E. Initial Severity of Injury Has Little Effect on the Temporal Profile of Long-Term Deficits in Locomotion, Anxiety, and Cognitive Function after Diffuse Traumatic Brain Injury. Neurotrauma Rep. 2023, 4, 41–50. [Google Scholar] [CrossRef]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates: Hard Cover Edition; Elsevier: Amsterdam, The Netherlands, 2006; ISBN 0-08-047515-9. [Google Scholar]
- Delic, V.; Beck, K.D.; Pang, K.C.H.; Citron, B.A. Biological Links between Traumatic Brain Injury and Parkinson’s Disease. Acta Neuropathol. Commun. 2020, 8, 45. [Google Scholar] [CrossRef]
- Bower, J.H.; Maraganore, D.M.; Peterson, B.J.; McDonnell, S.K.; Ahlskog, J.E.; Rocca, W.A. Head Trauma Preceding PD: A Case-Control Study. Neurology 2003, 60, 1610–1615. [Google Scholar] [CrossRef]
- Gardner, R.C.; Byers, A.L.; Barnes, D.E.; Li, Y.; Boscardin, J.; Yaffe, K. Mild TBI and Risk of Parkinson Disease: A Chronic Effects of Neurotrauma Consortium Study. Neurology 2018, 90, e1771–e1779. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Lopez, E.; Vrana, K.E. Dopamine Beta-hydroxylase and Its Genetic Variants in Human Health and Disease. J. Neurochem. 2020, 152, 157–181. [Google Scholar] [CrossRef] [PubMed]
- Robertson, D.; Goldberg, M.R.; Onrot, J.; Hollister, A.S.; Wiley, R.; Thompson, J.G.; Robertson, R.M. Isolated Failure of Autonomic Noradrenergic Neurotransmission. N. Engl. J. Med. 1986, 314, 1494–1497. [Google Scholar] [CrossRef] [PubMed]
- Jones, B.E.; Moore, R.Y. Ascending Projections of the Locus Coeruleus in the Rat. II. Autoradiographic Study. Brain Res. 1977, 127, 23–53. [Google Scholar] [CrossRef]
- Collingridge, G.L.; James, T.A.; MacLeod, N.K. Neurochemical and Electrophysiological Evidence for a Projection from the Locus Coeruleus to the Substantia Nigra [Proceedings]. J. Physiol. 1979, 290, 44P. [Google Scholar] [PubMed]
- Sugama, S.; Takenouchi, T.; Hashimoto, M.; Ohata, H.; Takenaka, Y.; Kakinuma, Y. Stress-Induced Microglial Activation Occurs through β-Adrenergic Receptor: Noradrenaline as a Key Neurotransmitter in Microglial Activation. J. Neuroinflamm. 2019, 16, 266. [Google Scholar] [CrossRef] [PubMed]
- Smits, R.P.; Steinbusch, H.W.; Mulder, A.H. Distribution of Dopamine-Immunoreactive Cell Bodies in the Guinea-Pig Brain. J. Chem. Neuroanat. 1990, 3, 101–123. [Google Scholar] [PubMed]
- Fang, Y.; Malik, M.; England, S.K.; Imoukhuede, P.I. Absolute Quantification of Plasma Membrane Receptors Via Quantitative Flow Cytometry. Methods Mol. Biol. Clifton NJ 2022, 2475, 61–77. [Google Scholar] [CrossRef]
- Wassall, R.D.; Teramoto, N.; Cunnane, T.C. Noradrenaline. In Encyclopedia of Neuroscience; Elsevier: Amsterdam, The Netherlands, 2009; pp. 1221–1230. ISBN 978-0-08-045046-9. [Google Scholar]
- Taylor, F.B.; Russo, J. Comparing Guanfacine and Dextroamphetamine for the Treatment of Adult Attention-Deficit/Hyperactivity Disorder. J. Clin. Psychopharmacol. 2001, 21, 223–228. [Google Scholar] [CrossRef]
- Avery, R. The Alpha-2A-Adrenoceptor Agonist, Guanfacine, Increases Regional Cerebral Blood Flow in Dorsolateral Prefrontal Cortex of Monkeys Performing a Spatial Working Memory Task. Neuropsychopharmacology 2000, 23, 240–249. [Google Scholar] [CrossRef]
- Arnsten, A.; Cai, J.; Goldman-Rakic, P. The Alpha-2 Adrenergic Agonist Guanfacine Improves Memory in Aged Monkeys without Sedative or Hypotensive Side Effects: Evidence for Alpha-2 Receptor Subtypes. J. Neurosci. 1988, 8, 4287–4298. [Google Scholar] [CrossRef] [PubMed]
- Carlson, S.; Tanila, H.; Rama, P.; Mecke, E.; Pertovaara, A. Effects of Medetomidine, an α-2 Adrenoceptor Agonist, and Atipamezole, an α-2 Antagonist, on Spatial Memory Performance in Adult and Aged Rats. Behav. Neural Biol. 1992, 58, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Rämä, P.; Linnankoski, I.; Tanila, H.; Pertovaara, A.; Carlson, S. Medetomidine, Atipamezole, and Guanfacine in Delayed Response Performance of Aged Monkeys. Pharmacol. Biochem. Behav. 1996, 55, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Novak, J.C.; Hillier, A.; Votolato, N.A.; Beversdorf, D.Q. The Effect of α-2 Adrenergic Agonists on Memory and Cognitive Flexibility. Cogn. Behav. Neurol. 2006, 19, 204–207. [Google Scholar] [CrossRef] [PubMed]
- Jäkälä, P. Guanfacine, but Not Clonidine, Improves Planning and Working Memory Performance in Humans. Neuropsychopharmacology 1999, 20, 460–470. [Google Scholar] [CrossRef] [PubMed]
- Sysoev, Y.I.; Prikhodko, V.A.; Chernyakov, R.T.; Idiyatullin, R.D.; Musienko, P.E.; Okovityi, S.V. Effects of Alpha-2 Adrenergic Agonist Mafedine on Brain Electrical Activity in Rats after Traumatic Brain Injury. Brain Sci. 2021, 11, 981. [Google Scholar] [CrossRef]
- Wu, J.; Vogel, T.; Gao, X.; Lin, B.; Kulwin, C.; Chen, J. Neuroprotective Effect of Dexmedetomidine in a Murine Model of Traumatic Brain Injury. Sci. Rep. 2018, 8, 4935. [Google Scholar] [CrossRef] [PubMed]
- McAllister, T.W.; McDonald, B.C.; Flashman, L.A.; Ferrell, R.B.; Tosteson, T.D.; Yanofsky, N.N.; Grove, M.R.; Saykin, A.J. Alpha-2 Adrenergic Challenge with Guanfacine One Month after Mild Traumatic Brain Injury: Altered Working Memory and BOLD Response. Int. J. Psychophysiol. Off. J. Int. Organ. Psychophysiol. 2011, 82, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Li, B.-M.; Mei, Z.-T. Delayed-Response Deficit Induced by Local Injection of the A2-Adrenergic Antagonist Yohimbine into the Dorsolateral Prefrontal Cortex in Young Adult Monkeys. Behav. Neural Biol. 1994, 62, 134–139. [Google Scholar] [CrossRef]
- Tanila, H.; Rämä, P.; Carlson, S. The Effects of Prefrontal Intracortical Microinjections of an Alpha-2 Agonist, Alpha-2 Antagonist and Lidocaine on the Delayed Alternation Performance of Aged Rats. Brain Res. Bull. 1996, 40, 117–119. [Google Scholar] [CrossRef]
- Ferrucci, M.; SGiorgi, F.; Bartalucci, A.; LBusceti, C.; Fornai, F. The Effects of Locus Coeruleus and Norepinephrine in Methamphetamine Toxicity. Curr. Neuropharmacol. 2013, 11, 80–94. [Google Scholar] [CrossRef][Green Version]
- Smith, Y.; Kieval, J.Z. Anatomy of the Dopamine System in the Basal Ganglia. Trends Neurosci. 2000, 23, S28–S33. [Google Scholar] [CrossRef] [PubMed]
- Grueschow, M.; Kleim, B.; Ruff, C.C. Functional Coupling of the Locus Coeruleus Is Linked to Successful Cognitive Control. Brain Sci. 2022, 12, 305. [Google Scholar] [CrossRef] [PubMed]
- Cragg, S.J.; Rice, M.E.; Greenfield, S.A. Heterogeneity of Electrically Evoked Dopamine Release and Reuptake in Substantia Nigra, Ventral Tegmental Area, and Striatum. J. Neurophysiol. 1997, 77, 863–873. [Google Scholar] [CrossRef] [PubMed]
- Carboni, E.; Tanda, G.L.; Frau, R.; Chiara, G.D. Blockade of the Noradrenaline Carrier Increases Extracellular Dopamine Concentrations in the Prefrontal Cortex: Evidence That Dopamine Is Taken up In Vivo by Noradrenergic Terminals. J. Neurochem. 1990, 55, 1067–1070. [Google Scholar] [CrossRef] [PubMed]
- af Bjerkén, S.; Stenmark Persson, R.; Barkander, A.; Karalija, N.; Pelegrina-Hidalgo, N.; Gerhardt, G.A.; Virel, A.; Strömberg, I. Noradrenaline Is Crucial for the Substantia Nigra Dopaminergic Cell Maintenance. Neurochem. Int. 2019, 131, 104551. [Google Scholar] [CrossRef] [PubMed]
- Hassani, O.K.; Rymar, V.V.; Nguyen, K.Q.; Huo, L.; Cloutier, J.-F.; Miller, F.D.; Sadikot, A.F. The Noradrenergic System Is Necessary for Survival of Vulnerable Midbrain Dopaminergic Neurons: Implications for Development and Parkinson’s Disease. Neurobiol. Aging 2020, 85, 22–37. [Google Scholar] [CrossRef] [PubMed]
- Tong, J.; Hornykiewicz, O.; Kish, S.J. Inverse Relationship Between Brain Noradrenaline Level and Dopamine Loss in Parkinson Disease: A Possible Neuroprotective Role for Noradrenaline. Arch. Neurol. 2006, 63, 1724. [Google Scholar] [CrossRef] [PubMed]
- Isaias, I.U.; Marotta, G.; Pezzoli, G.; Sabri, O.; Schwarz, J.; Crenna, P.; Classen, J.; Cavallari, P. Enhanced Catecholamine Transporter Binding in the Locus Coeruleus of Patients with Early Parkinson Disease. BMC Neurol. 2011, 11, 88. [Google Scholar] [CrossRef]
- Delaville, C.; Deurwaerdère, P.D.; Benazzouz, A. Noradrenaline and Parkinson’s Disease. Front. Syst. Neurosci. 2011, 5, 31. [Google Scholar] [CrossRef]
- Braak, H.; Del Tredici, K. Invited Article: Nervous System Pathology in Sporadic Parkinson Disease. Neurology 2008, 70, 1916–1925. [Google Scholar] [CrossRef] [PubMed]
- Walf, A.A.; Frye, C.A. The Use of the Elevated plus Maze as an Assay of Anxiety-Related Behavior in Rodents. Nat. Protoc. 2007, 2, 322–328. [Google Scholar] [CrossRef] [PubMed]
- Tucker, L.B.; Fu, A.H.; McCabe, J.T. Performance of Male and Female C57BL/6J Mice on Motor and Cognitive Tasks Commonly Used in Pre-Clinical Traumatic Brain Injury Research. J. Neurotrauma 2016, 33, 880–894. [Google Scholar] [CrossRef] [PubMed]
- Budinich, C.S.; Tucker, L.B.; Lowe, D.; Rosenberger, J.G.; McCabe, J.T. Short and Long-Term Motor and Behavioral Effects of Diazoxide and Dimethyl Sulfoxide Administration in the Mouse after Traumatic Brain Injury. Pharmacol. Biochem. Behav. 2013, 108, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Nolan, A.; Hennessy, E.; Krukowski, K.; Guglielmetti, C.; Chaumeil, M.M.; Sohal, V.S.; Rosi, S. Repeated Mild Head Injury Leads to Wide-Ranging Deficits in Higher-Order Cognitive Functions Associated with the Prefrontal Cortex. J. Neurotrauma 2018, 35, 2425–2434. [Google Scholar] [CrossRef] [PubMed]
- Shultz, S.R.; MacFabe, D.F.; Foley, K.A.; Taylor, R.; Cain, D.P. A Single Mild Fluid Percussion Injury Induces Short-Term Behavioral and Neuropathological Changes in the Long–Evans Rat: Support for an Animal Model of Concussion. Behav. Brain Res. 2011, 224, 326–335. [Google Scholar] [CrossRef] [PubMed]
- Hare, B.D.; Shinohara, R.; Liu, R.J.; Pothula, S.; DiLeone, R.J.; Duman, R.S. Optogenetic Stimulation of Medial Prefrontal Cortex Drd1 Neurons Produces Rapid and Long-Lasting Antidepressant Effects. Nat. Commun. 2019, 10, 223. [Google Scholar] [CrossRef] [PubMed]
- Beyer, D.K.E.; Mattukat, A.; Freund, N. Prefrontal Dopamine D1 Receptor Manipulation Influences Anxiety Behavior and Induces Neuroinflammation within the Hippocampus. Int. J. Bipolar Disord. 2021, 9, 9. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Peng, C.; Li, Q.; Yan, X.; Yang, L.; Li, M.; Cao, X.; Xie, X.; Chen, D.; Rao, C.; et al. Dopamine Homeostasis Imbalance and Dopamine Receptors-Mediated AC/cAMP/PKA Pathway Activation Are Involved in Aconitine-Induced Neurological Impairment in Zebrafish and SH-SY5Y Cells. Front. Pharmacol. 2022, 13, 837810. [Google Scholar] [CrossRef]
- Jones-Tabah, J.; Martin, R.D.; Chen, J.J.; Tanny, J.C.; Clarke, P.B.S.; Hébert, T.E. Dopamine D1 Receptor Activation and cAMP/PKA Signalling Mediate Brd4 Recruitment to Chromatin to Regulate Gene Expression in Rat Striatal Neurons. Neuroscience 2021. [Google Scholar] [CrossRef]
- Keil, M.F.; Briassoulis, G.; Stratakis, C.A.; Wu, T.J. Protein Kinase A and Anxiety-Related Behaviors: A Mini-Review. Front. Endocrinol. 2016, 7, 83. [Google Scholar] [CrossRef] [PubMed]
- Miguel, T.T.; Gomes, K.S.; Nunes-de-Souza, R.L. Tonic Modulation of Anxiety-like Behavior by Corticotropin-Releasing Factor (CRF) Type 1 Receptor (CRF1) within the Medial Prefrontal Cortex (mPFC) in Male Mice: Role of Protein Kinase A (PKA). Horm. Behav. 2014, 66, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Kurowski, B.G.; Backeljauw, B.; Zang, H.; Zhang, N.; Martin, L.J.; Pilipenko, V.; Yeates, K.; Taylor, H.G.; Wade, S. Influence of Catechol-O-Methyltransferase on Executive Functioning Longitudinally after Early Childhood Traumatic Brain Injury: Preliminary Findings. J. Head Trauma Rehabil. 2016, 31, E1–E9. [Google Scholar] [CrossRef] [PubMed]
- Lachman, H.M.; Papolos, D.F.; Saito, T.; Yu, Y.-M.; Szumlanski, C.L.; Weinshilboum, R.M. Human Catechol-O-Methyltransferase Pharmacogenetics: Description of a Functional Polymorphism and Its Potential Application to Neuropsychiatric Disorders. Pharmacogenet. Genom. 1996, 6, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Lotta, T.; Vidgren, J.; Tilgmann, C.; Ulmanen, I.; Melen, K.; Julkunen, I.; Taskinen, J. Kinetics of Human Soluble and Membrane-Bound Catechol O-Methyltransferase: A Revised Mechanism and Description of the Thermolabile Variant of the Enzyme. Biochemistry 1995, 34, 4202–4210. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-H.; Fan, J.-Y.; Lin, H.-I.; Chang, C.-W.; Wu, Y.-R. Catechol-O-Methyltransferase (COMT) Genetic Variants Are Associated with Cognitive Decline in Patients with Parkinson’s Disease. Park. Relat. Disord. 2018, 50, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Wang, W.; Shi, M.; Zhang, N.; Zhou, X.; Li, X.; Ma, C.; Chen, G.; Xiang, J.; Gao, D. Meta-Analysis of the Effects of the Catechol-O-Methyltransferase Val158/108Met Polymorphism on Parkinson’s Disease Susceptibility and Cognitive Dysfunction. Front. Genet. 2019, 10, 644. [Google Scholar] [CrossRef] [PubMed]
- Lipsky, R.H.; Sparling, M.B.; Ryan, L.M.; Xu, K.; Salazar, A.M.; Goldman, D.; Warden, D.L. Association of COMT Val158Met Genotype with Executive Functioning Following Traumatic Brain Injury. J. Neuropsychiatry Clin. Neurosci. 2005, 17, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Redell, J.B.; Dash, P.K. Traumatic Brain Injury Stimulates Hippocampal Catechol-O-Methyl Transferase Expression in Microglia. Neurosci. Lett. 2007, 413, 36–41. [Google Scholar] [CrossRef]
- Wang, D.; Xu, X.; Wu, Y.-G.; Lyu, L.; Zhou, Z.-W.; Zhang, J.-N. Dexmedetomidine Attenuates Traumatic Brain Injury: Action Pathway and Mechanisms. Neural Regen. Res. 2018, 13, 819. [Google Scholar] [CrossRef]
- Gyoneva, S.; Traynelis, S.F. Norepinephrine Modulates the Motility of Resting and Activated Microglia via Different Adrenergic Receptors. J. Biol. Chem. 2013, 288, 15291–15302. [Google Scholar] [CrossRef] [PubMed]
- Vidal-Itriago, A.; Radford, R.A.W.; Aramideh, J.A.; Maurel, C.; Scherer, N.M.; Don, E.K.; Lee, A.; Chung, R.S.; Graeber, M.B.; Morsch, M. Microglia Morphophysiological Diversity and Its Implications for the CNS. Front. Immunol. 2022, 13, 997786. [Google Scholar] [CrossRef] [PubMed]
- Wake, H.; Moorhouse, A.J.; Jinno, S.; Kohsaka, S.; Nabekura, J. Resting Microglia Directly Monitor the Functional State of Synapses In Vivo and Determine the Fate of Ischemic Terminals. J. Neurosci. 2009, 29, 3974–3980. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Cheng, Z.; Zhou, L.; Darmanis, S.; Neff, N.F.; Okamoto, J.; Gulati, G.; Bennett, M.L.; Sun, L.O.; Clarke, L.E.; et al. Developmental Heterogeneity of Microglia and Brain Myeloid Cells Revealed by Deep Single-Cell RNA Sequencing. Neuron 2019, 101, 207–223.e10. [Google Scholar] [CrossRef] [PubMed]
- Kenkhuis, B.; Somarakis, A.; Kleindouwel, L.R.T.; Van Roon-Mom, W.M.C.; Höllt, T.; Van Der Weerd, L. Co-Expression Patterns of Microglia Markers Iba1, TMEM119 and P2RY12 in Alzheimer’s Disease. Neurobiol. Dis. 2022, 167, 105684. [Google Scholar] [CrossRef]
- Bélanger, M.; Magistretti, P.J. The Role of Astroglia in Neuroprotection. Dialogues Clin. Neurosci. 2009, 11, 281–295. [Google Scholar] [CrossRef] [PubMed]
- Myer, D.J. Essential Protective Roles of Reactive Astrocytes in Traumatic Brain Injury. Brain 2006, 129, 2761–2772. [Google Scholar] [CrossRef]
- Karve, I.P.; Taylor, J.M.; Crack, P.J. The Contribution of Astrocytes and Microglia to Traumatic Brain Injury. Br. J. Pharmacol. 2016, 173, 692–702. [Google Scholar] [CrossRef]
- Valles, S.L.; Singh, S.K.; Campos-Campos, J.; Colmena, C.; Campo-Palacio, I.; Alvarez-Gamez, K.; Caballero, O.; Jorda, A. Functions of Astrocytes under Normal Conditions and after a Brain Disease. Int. J. Mol. Sci. 2023, 24, 8434. [Google Scholar] [CrossRef]
- Reeve, A.; Simcox, E.; Turnbull, D. Ageing and Parkinson’s Disease: Why Is Advancing Age the Biggest Risk Factor? Ageing Res. Rev. 2014, 14, 19–30. [Google Scholar] [CrossRef]
- Sparkman, N.L.; Johnson, R.W. Neuroinflammation Associated with Aging Sensitizes the Brain to the Effects of Infection or Stress. Neuroimmunomodulation 2008, 15, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Rieckmann, A.; Johnson, K.A.; Sperling, R.A.; Buckner, R.L.; Hedden, T. Dedifferentiation of Caudate Functional Connectivity and Striatal Dopamine Transporter Density Predict Memory Change in Normal Aging. Proc. Natl. Acad. Sci. USA 2018, 115, 10160–10165. [Google Scholar] [CrossRef] [PubMed]
- Dahl, M.J.; Bachman, S.L.; Dutt, S.; Düzel, S.; Bodammer, N.C.; Lindenberger, U.; Kühn, S.; Werkle-Bergner, M.; Mather, M. The Integrity of Dopaminergic and Noradrenergic Brain Regions Is Associated with Different Aspects of Late-Life Memory Performance. Nat. Aging 2023, 3, 1128–1143. [Google Scholar] [CrossRef] [PubMed]
- Mather, M. Noradrenaline in the Aging Brain: Promoting Cognitive Reserve or Accelerating Alzheimer’s Disease? Semin. Cell Dev. Biol. 2021, 116, 108–124. [Google Scholar] [CrossRef] [PubMed]
- Guneykaya, D.; Ivanov, A.; Hernandez, D.P.; Haage, V.; Wojtas, B.; Meyer, N.; Maricos, M.; Jordan, P.; Buonfiglioli, A.; Gielniewski, B.; et al. Transcriptional and Translational Differences of Microglia from Male and Female Brains. Cell Rep. 2018, 24, 2773–2783.e6. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Fan, Y.; Zhou, K.; Blomgren, K.; Harris, R.A. Uncovering Sex Differences of Rodent Microglia. J. Neuroinflamm. 2021, 18, 74. [Google Scholar] [CrossRef] [PubMed]
- Santos-Galindo, M.; Acaz-Fonseca, E.; Bellini, M.J.; Garcia-Segura, L.M. Sex Differences in the Inflammatory Response of Primary Astrocytes to Lipopolysaccharide. Biol. Sex Differ. 2011, 2, 7. [Google Scholar] [CrossRef] [PubMed]
- Gillies, G.E.; McArthur, S. Independent Influences of Sex Steroids of Systemic and Central Origin in a Rat Model of Parkinson’s Disease: A Contribution to Sex-Specific Neuroprotection by Estrogens. Horm. Behav. 2010, 57, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Gillies, G.E.; Virdee, K.; McArthur, S.; Dalley, J.W. Sex-Dependent Diversity in Ventral Tegmental Dopaminergic Neurons and Developmental Programing: A Molecular, Cellular and Behavioral Analysis. Neuroscience 2014, 282, 69–85. [Google Scholar] [CrossRef]
- Gillies, G.E.; Pienaar, I.S.; Vohra, S.; Qamhawi, Z. Sex Differences in Parkinson’s Disease. Front. Neuroendocrinol. 2014, 35, 370–384. [Google Scholar] [CrossRef]
- Baldereschi, M.; Di Carlo, A.; Rocca, W.A.; Vanni, P.; Maggi, S.; Perissinotto, E.; Grigoletto, F.; Amaducci, L.; Inzitari, D. Parkinson’s Disease and Parkinsonism in a Longitudinal Study: Two-Fold Higher Incidence in Men. Neurology 2000, 55, 1358–1363. [Google Scholar] [CrossRef] [PubMed]
- Cerri, S.; Mus, L.; Blandini, F. Parkinson’s Disease in Women and Men: What’s the Difference? J. Park. Dis. 2019, 9, 501–515. [Google Scholar] [CrossRef] [PubMed]


| Marker Analysis | PFC | STR | SN | |||
|---|---|---|---|---|---|---|
| TBI Effect | Post hoc | TBI Effect | Post hoc | TBI Effect | Post hoc | |
| Total GFAP+ cells | ns; p = 0.64 | ΔmTBI = −19.22 ΔrmTBI = 12.85 ΔmsTBI = −6.70 | ns; p = 0.3 | ΔmTBI = −44.45 ΔrmTBI = −27.20 ΔmsTBI = −26.55 | * p = 0.02 | ΔmTBI = −73.03 ΔrmTBI = 52.48 ΔmsTBI = 121.9 * |
| % of Reactive Astrocyte | ns; p = 0.53 | ΔmTBI = −1.7 ΔrmTBI = 0.71 ΔmsTBI = 0.13 | ns; p = 0.08 | ΔmTBI = 0.25 ΔrmTBI = 3.58 ΔmsTBI = 1.11 | ns; p = 0.81 | ΔmTBI = −2.15 ΔrmTBI = −0.75 ΔmsTBI = −1.18 |
| Total IBA1+ cells | ns; p = 0.42 | ΔmTBI = 21.90 ΔrmTBI = 4.67 ΔmsTBI = 6.38 | * p = 0.02 | ΔmTBI = 8.32 ΔrmTBI = 12.38 ΔmsTBI = −23.91 ΔrmTBI-msTBI = −36.29 * | ns; p = 0.62 | ΔmTBI = 12.77 ΔrmTBI = 24.57 ΔmsTBI = 9.06 |
| % of Activated Microglial | * p = 0.02 | ΔmTBI = 3.04 ΔrmTBI = 4.64 ΔmsTBI = −0.54 ΔrmTBI-msTBI = −5.2 * | ns; p = 0.11 | ΔmTBI = 8.94 ΔrmTBI = 7.70 ΔmsTBI = 4.43 | ns; p = 0.11 | ΔmTBI = −4.98 ΔrmTBI = −2.05 ΔmsTBI = −5.46 |
| Marker Analysis | PFC | STR | SN | |||
|---|---|---|---|---|---|---|
| TBI Effect | Post hoc | TBI Effect | Post hoc | TBI Effect | Post hoc | |
| TH | ns; p = 0.15 | ΔS-mTBI = 0.19 ΔS-rmTBI = −0.19 ΔS-msTBI = −0.32 | ns; p = 0.38 | ΔS-mTBI = 0.23 ΔS-rmTBI = 0.13 ΔS-msTBI = 0.25 | ns; p = 0.8 | ΔS-mTBI = −0.37 ΔS-rmTBI = −0.14 ΔS-msTBI = −0.13 |
| DRD1 | ** p = 0.0013 | ΔS-mTBI = −0.86 ** ΔS-rmTBI = −0.002 ΔS-msTBI = −0.43 ΔmTBI-rmTBI = 0.85 ** | ns; p = 0.97 | ΔS-mTBI = 0.008 ΔS-rmTBI = 0.03 ΔS-msTBI = 0.06 | ns; p = 0.94 | ΔS-mTBI = 0.015 ΔS-rmTBI = −0.06 ΔS-msTBI = 0.02 |
| DβH | ns; p = 0.16 | ΔS-mTBI = −0.4 ΔS-rmTBI = 0.26 ΔS-msTBI = 0.29 | ns; p = 0.29 | ΔS-mTBI = −0.27 ΔS-rmTBI = −0.42 ΔS-msTBI = −0.20 | * p = 0.01 | ΔS-mTBI = 0.24 ΔS-rmTBI = 0.14 ΔS-msTBI = 0.89 ** ΔrmTBI-msTBI = 0.74 * |
| ADRA1a | ns; p = 0.63 | ΔS-mTBI = −0.12 ΔS-rmTBI = −0.11 ΔS-msTBI = −0.05 | ns; p = 0.86 | ΔS-mTBI = −0.03 ΔS-rmTBI = 0.08 ΔS-msTBI = 0.03 | ns; p = 0.09 | ΔS-mTBI = 0.16 ΔS-rmTBI = 0.20 ΔS-msTBI = 0.12 |
| ADRA2a | ns; p = 0.12 | ΔS-mTBI = −0.10 ΔS-rmTBI = 0.21 ΔS-msTBI = −0.44 | * p = 0.02 | ΔS-mTBI = −0.006 ΔS-rmTBI = −0.01 ΔS-msTBI = −0.60 * ΔrmTBI-msTBI = −0.59 * | ns; p = 0.68 | ΔS-mTBI = 0.33 ΔS-rmTBI = 0.21 ΔS-msTBI = 0.23 |
| ADRB1 | ns; p = 0.74 | ΔS-mTBI = 0.01 ΔS-rmTBI = 0.01 ΔS-msTBI = −0.04 | ns; p = 0.09 | ΔS-mTBI = −0.1 ΔS-rmTBI = 0.27 ΔS-msTBI = 0.23 | ns; p = 0.17 | ΔS-mTBI = 0.12 ΔS-rmTBI = 0.03 ΔS-msTBI = 0.06 |
| mbCOMT | ns; p = 0.5 | ΔS-mTBI = 0.32 ΔS-rmTBI = 0.30 ΔS-msTBI = 0.80 | ns; p = 0.72 | ΔS-mTBI = 0.27 ΔS-rmTBI = −0.26 ΔS-msTBI = 0.09 | ns; p = 0.83 | ΔS-mTBI = −0.15 ΔS-rmTBI = −0.09 ΔS-msTBI = −0.13 |
| sCOMT | ns; p = 0.92 | ΔS-mTBI = 0.12 ΔS-rmTBI = 0.02 ΔS-msTBI = 0.13 | ns; p = 0.44 | ΔS-mTBI = −0.11 ΔS-rmTBI = −0.34 ΔS-msTBI = −0.18 | ns; p = 0.35 | ΔS-mTBI = −0.25 ΔS-rmTBI = −0.15 ΔS-msTBI = −0.41 |
| Region | Coronal Coordinates (Bregma) [81] | Region of Interest (Both Left and Right) | |
|---|---|---|---|
| Prefrontal cortex | 3.7 mm to 3.2 mm | Prelimbic Area Anterior cingulate area Infralimbic Area | |
| Striatum | Early | 1.0 mm to 0.48 mm | Caudoputamen |
| Middle I | 0.20 mm to −0.40 mm | ||
| Middle II | −0.80 mm to −1.30 mm | ||
| Late | −1.5 mm to −2.10 mm | ||
| Substantia Nigra | Early | −4.5 mm to −5.2 mm | -Substantia nigra, compact part -Substantia nigra, reticular part |
| Middle | −5.2 mm to −5.8 mm | ||
| Late | −5.8 mm to −6.3 mm | ||
| Primary Antibody | Species | Conc. | Catalogue# | Analysis Target | Analysis Platform and Parameters |
|---|---|---|---|---|---|
| Ionized calcium-binding adaptor molecule 1 (IBA1) | Rabbit | 1:20,000 | Wako- 019-19741 | Microglial reactivity | Halo microglial activation module: Min cell body diameter—3.4 μm ∙Contrast threshold—0.3 pixel ∙Min process OD—0.25 pixel ∙Max process Radius—12 μm ∙Max fragmentation length—2.5 μm ∙Activation process thickness—2.12 μm |
| Glial fibrillary acidic protein (GFAP) | Rabbit | 1:40,000 | Dako- Z0334 | Astrocyte reactivity | Image J [version 1.53b]: ∙Manual identification of astrocyte morphology |
| Primary Antibody | Species | Conc. | Catalogue# | Analysis Target |
|---|---|---|---|---|
| Tyrosine Hydroxylase (TH) | Rabbit | 1:1000 | Abcam-ab112 | Catalytic enzyme for conversion of tyrosine to DA |
| Dopamine Beta Hydroxylase (DβH) | Rabbit | 1:500 | Abcam-ab209487 | Enzyme converts dopamine to norepinephrine |
| Dopamine receptor D1 (DrD1) | Rabbit | 1:1000 | Abcam-ab20066 | Receptor from D1R family |
| Rabbit anti-Dopamine receptor D4 (DrD4) | Rabbit | 1:1000 | Abcam-ab20424 | Receptor from D2R family |
| Rabbit anti-Catechol-O-methyltransferase (COMT) | Rabbit | 1:1000 | Abcam-ab226938 | Enzyme that degrades catecholamines |
| Rabbit anti-alpha 1a Adrenergic receptor (ADRA1A) | Rabbit | 1:1000 | Abcam-ab137123 | Alpha-1 adrenergic receptor subtypes |
| Rabbit anti-alpha 2a Adrenergic receptor (ADRA2A) | Rabbit | 1:1000 | Abcam-ab85570 | Alpha-1 adrenergic receptor subtypes |
| Rabbit anti-beta 1 Adrenergic receptor (ADRB1) | Rabbit | 1:1000 | Abcam-ab3442 | A beta-adrenergic receptor |
| Chicken anti-GAPDH | Chicken | 1:10,000 | Abcam-108162 | Housekeeping protein |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wee, I.C.; Arulsamy, A.; Corrigan, F.; Collins-Praino, L. Long-Term Impact of Diffuse Traumatic Brain Injury on Neuroinflammation and Catecholaminergic Signaling: Potential Relevance for Parkinson’s Disease Risk. Molecules 2024, 29, 1470. https://doi.org/10.3390/molecules29071470
Wee IC, Arulsamy A, Corrigan F, Collins-Praino L. Long-Term Impact of Diffuse Traumatic Brain Injury on Neuroinflammation and Catecholaminergic Signaling: Potential Relevance for Parkinson’s Disease Risk. Molecules. 2024; 29(7):1470. https://doi.org/10.3390/molecules29071470
Chicago/Turabian StyleWee, Ing Chee, Alina Arulsamy, Frances Corrigan, and Lyndsey Collins-Praino. 2024. "Long-Term Impact of Diffuse Traumatic Brain Injury on Neuroinflammation and Catecholaminergic Signaling: Potential Relevance for Parkinson’s Disease Risk" Molecules 29, no. 7: 1470. https://doi.org/10.3390/molecules29071470
APA StyleWee, I. C., Arulsamy, A., Corrigan, F., & Collins-Praino, L. (2024). Long-Term Impact of Diffuse Traumatic Brain Injury on Neuroinflammation and Catecholaminergic Signaling: Potential Relevance for Parkinson’s Disease Risk. Molecules, 29(7), 1470. https://doi.org/10.3390/molecules29071470






