Sonocatalytic Activity of Porous Carbonaceous Materials for the Selective Oxidation of 4-Hydroxy-3,5-dimethoxybenzyl Alcohol
Abstract
1. Introduction
2. Results and Discussion
2.1. Characterization
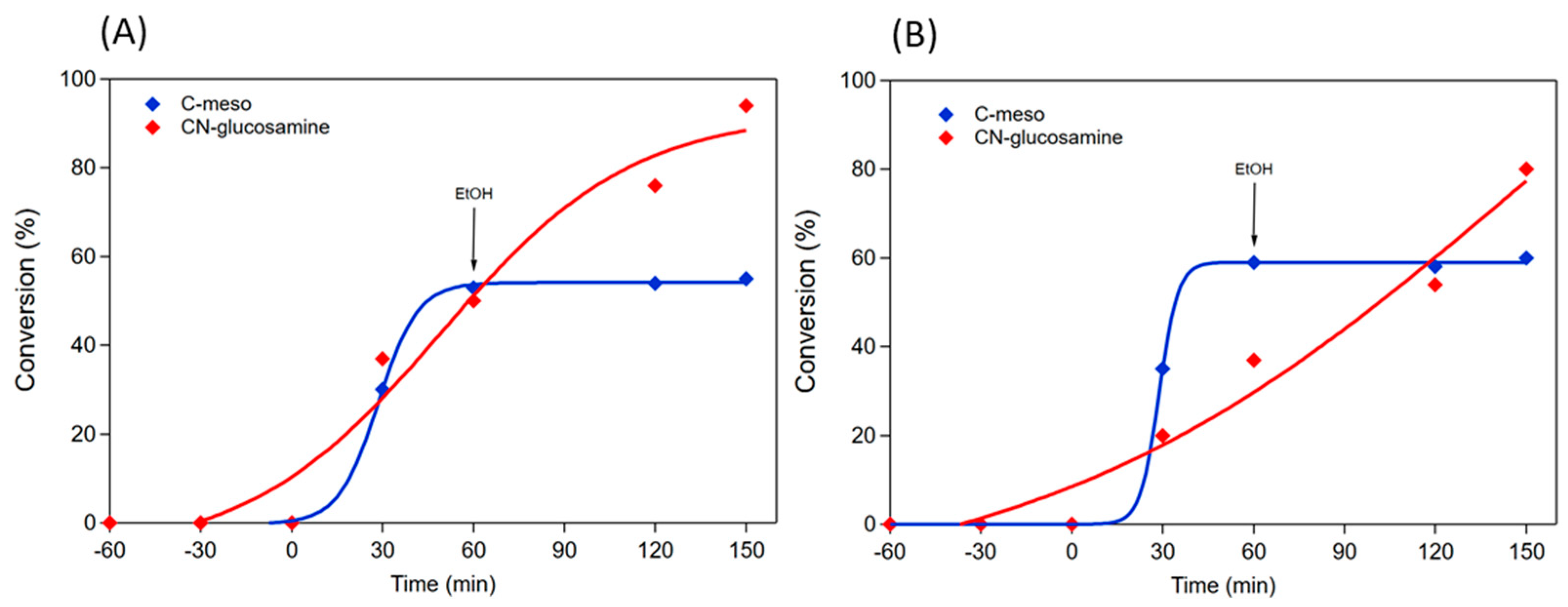
2.2. Catalytic Performance
3. Materials and Methods
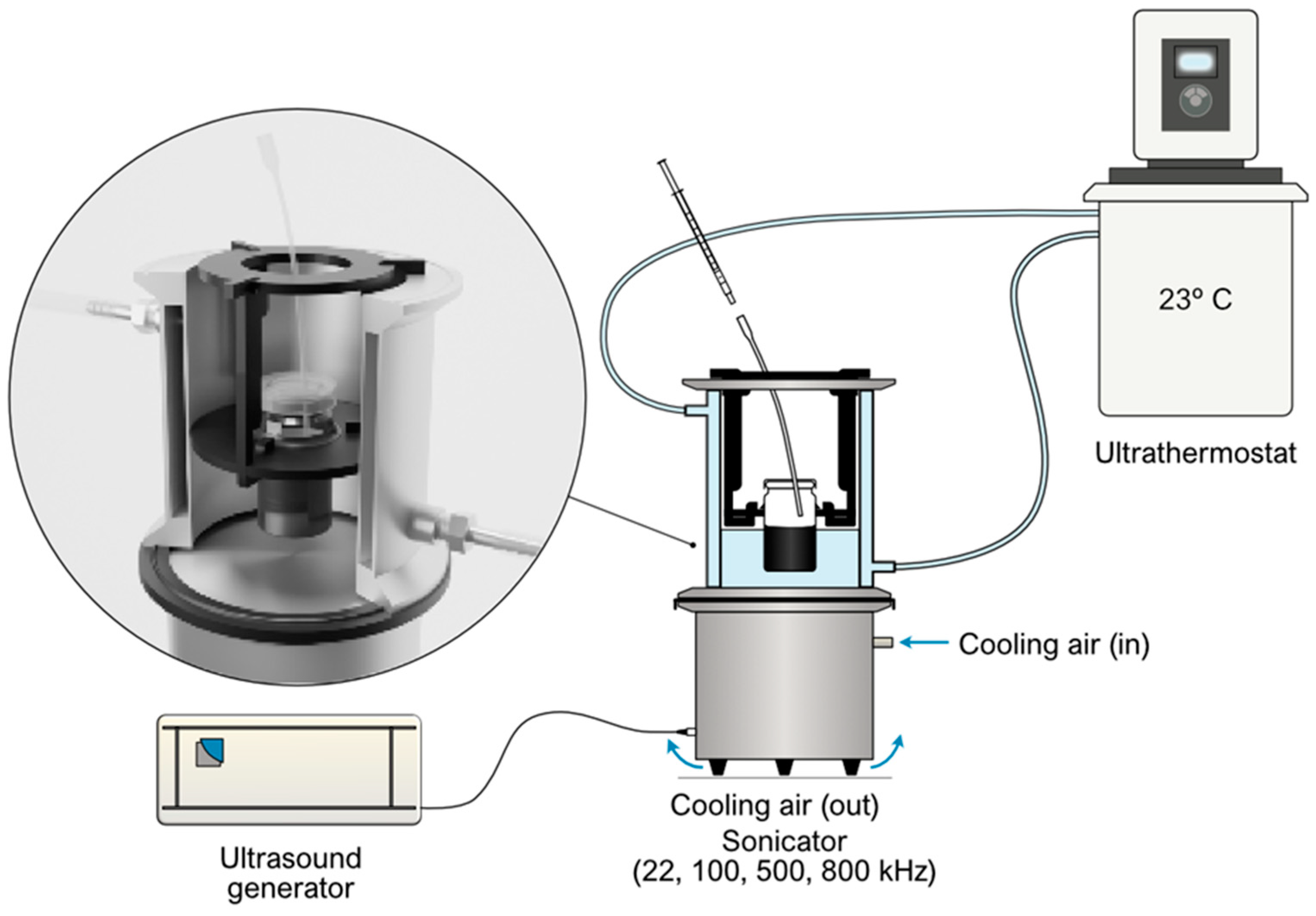
3.1. Experimental
Synthetic Methods for Porous Heteroatom-Doped Carbons
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Andrew, J.J.; Dhakal, H.N. Sustainable biobased composites for advanced applications: Recent trends and future opportunities—A critical review. Compos. Part C Open Access 2022, 7, 100220. [Google Scholar] [CrossRef]
- Cheng, C.; Shen, D.; Gu, S.; Luo, K.H. State-of-the-art catalytic hydrogenolysis of lignin for the production of aromatic chemicals. Catal. Sci. Technol. 2018, 8, 6275–6296. [Google Scholar] [CrossRef]
- Prasetyo, D.W.; Putra, Z.A.; Bilad, M.R.; Mahlia, T.M.I.; Wibisono, Y.; Nordin, N.A.H.; Wirzal, M.D.H. Insight into the sustainable integration of bio- and petroleum refineries for the production of fuels and chemicals. Polymers 2020, 12, 1091. [Google Scholar] [CrossRef]
- Behling, R.; Oliveira, S.V.W.B. Sonochemical oxidation of vanillyl alcohol to vanillin in the presence of a cobalt oxide catalyst under mild conditions. Ultrason. Sonochem. 2017, 36, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Abdelaziz, O.Y.; Clemmensen, I.; Meier, S.; Costa, C.A.E.; Rodrigues, A.E.; Hulteberg, C.P.; Riisager, A. On the oxidative valorization of lignin to high-value chemicals: A critical review of opportunities and challenges. ChemSusChem 2022, 15, e202201232. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Prawirasatya, M.; Zhao, W.; Wang, Z.; Zhu, Y. Selective lignin oxidation towards vanillin in phenol media. ChemistrySelect 2016, 1, 4596–4601. [Google Scholar] [CrossRef]
- Abughalia, H.A. Synthesis and utilization of natural fiber-reinforced poly (lactic acid) bionanocomposites. In Lignocellulosic Fibre and Biomass-Based Composite Materials; Jawaid, M., Md Tahir, P., Saba, N., Eds.; Woodhead Publishing: Sawstonm UK, 2017; pp. 313–345. [Google Scholar] [CrossRef]
- Davis, S.E.; Ide, M.S.; Davis, R.J. Selective oxidation of alcohols and aldehydes over supported metal nanoparticles. Green Chem. 2013, 15, 17–45. [Google Scholar] [CrossRef]
- Lagerblom, K.; Keskiväli, J.; Parviainen, A.; Mannisto, J.; Repo, T. Selective Aerobic Oxidation of Alcohols with NO3− Activated Nitroxyl Radical/Manganese Catalyst System. ChemCatChem 2018, 10, 2908–2914. [Google Scholar] [CrossRef]
- Andrade, M.A.; Martins, L.M.D.R.S. Selective Styrene Oxidation to Benzaldehyde over Recently Developed Heterogeneous Catalysts. Molecules 2021, 26, 1680. [Google Scholar] [CrossRef]
- Yadav, G.D.; Mewada, R.K.; Wagh, D.P.; Manyar, H.G. Advances and future trends in selective oxidation catalysis: A critical review. Catal. Sci. Technol. 2022, 12, 7245–7269. [Google Scholar] [CrossRef]
- Descorme, C.; Gallezot, P.; Geantet, C.; George, C. Heterogeneous Catalysis: A Key Tool toward Sustainability. ChemCatChem 2012, 4, 1897–1906. [Google Scholar] [CrossRef]
- Liu, L.; Corma, A. Metal Catalysts for Heterogeneous Catalysis: From Single Atoms to Nanoclusters and Nanoparticles. Chem. Rev. 2018, 118, 4981–5079. [Google Scholar] [CrossRef]
- Osman, A.I.; Elgarahy, A.M.; Eltaweil, A.S.; El-Monaem, E.M.A.; El-Aqapa, H.G.; Park, Y.; Hwang, Y.; Ayati, A.; Farghali, M.; Ihara, I.; et al. Biofuel production, hydrogen production and water remediation by photocatalysis, biocatalysis and electrocatalysis. Environ. Chem. Lett. 2023, 21, 1315–1379. [Google Scholar] [CrossRef]
- Sarmah, B.; Satpati, B.; Srivastava, R. Selective Oxidation of Biomass-Derived Alcohols and Aromatic and Aliphatic Alcohols to Aldehydes with O2/Air Using a RuO2-Supported Mn3O4 Catalyst. ACS Omega 2018, 3, 7944–7954. [Google Scholar] [CrossRef]
- Oliveira, R.L.; Zanchet, D.; Kiyohara, P.K.; Rossi, L.M. On the stabilization of gold nanoparticles over silica-based magnetic supports modified with organosilanes. Chem.—A Eur. J. 2011, 17, 4626–4631. [Google Scholar] [CrossRef]
- Khan, A.; Goepel, M.; Kubas, A.; Łomot, D.; Lisowski, W.; Lisovytskiy, D.; Nowicka, A.; Colmenares, J.C.; Gläser, R. Selective oxidation of 5-hydroxymethylfurfural to 2,5-diformylfuran by visible light-driven photocatalysis over in situ substrate-sensitized titania. ChemSusChem 2021, 14, 1351–1362. [Google Scholar] [CrossRef]
- Oliveira, R.L.; Pisarek, M.; Ledwa, K.A.; Pasternak, G.; Kepinski, L. Enhanced activation of persulfate improves the selective oxidation of alcohols catalyzed by earth-abundant metal oxides embedded on porous N-doped carbon derived from chitosan. React. Chem. Eng. 2023, 8, 1061–1071. [Google Scholar] [CrossRef]
- Al-Hamadani, Y.A.; Jung, C.; Im, J.-K.; Boateng, L.K.; Flora, J.R.; Jang, M.; Heo, J.; Park, C.M.; Yoon, Y. Sonocatalytic degradation coupled with single-walled carbon nanotubes for removal of ibuprofen and sulfamethoxazole. Chem. Eng. Sci. 2017, 162, 300–308. [Google Scholar] [CrossRef]
- Qayyum, A.; Giannakoudakis, D.A.; LaGrow, A.P.; Bondarchuk, O.; Łomot, D.; Colmenares, J.C. High-frequency sonication for the synthesis of nanocluster-decorated titania nanorods: Making a better photocatalyst for the selective oxidation of monoaromatic alcohol. Catal. Commun. 2022, 163, 106406. [Google Scholar] [CrossRef]
- Giannakoudakis, D.A.; Łomot, D.; Colmenares, J.C. When sonochemistry meets heterogeneous photocatalysis: Designing a sonophotoreactor towards sustainable selective oxidation. Green Chem. 2020, 22, 4896–4905. [Google Scholar] [CrossRef]
- Salavati, H.; Tangestaninejad, S.; Moghadam, M.; Mirkhani, V.; Mohammadpoor-Baltork, I. Sonocatalytic epoxidation of alkenes by vanadium-containing polyphosphomolybdate immobilized on multi-wall carbon nanotubes. Ultrason. Sonochem. 2010, 17, 453–459. [Google Scholar] [CrossRef]
- Bhalerao, M.S.; Kulkarni, V.M.; Patwardhan, A.V. Ultrasound-assisted chemoenzymatic epoxidation of soybean oil by using lipase as biocatalyst. Ultrason. Sonochem. 2018, 40, 912–920. [Google Scholar] [CrossRef]
- Salavati, H.; Tangestaninejad, S.; Moghadam, M.; Mirkhani, V.; Mohammadpoor-Baltork, I. Zirconia-supported Keggin phosphomolybdovanadate nanocomposite: A heterogeneous and reusable catalyst for alkene epoxidation under thermal and ultrasonic irradiation conditions. Comptes Rendus Chim. 2011, 14, 588–596. [Google Scholar] [CrossRef]
- Kuna, E.; Behling, R.; Valange, S.; Chatel, G.; Colmenares, J.C. Sonocatalysis: A Potential Sustainable Pathway for the Valorization of Lignocellulosic Biomass and Derivatives. Top. Curr. Chem. (Z) 2017, 375, 41. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, A.; Mohammadi, Y.; Ramazani, A.; Zheng, H. Ultrasound-assisted pseudohomogeneous tungstate catalyst for selective oxidation of alcohols to aldehydes. Sci. Rep. 2022, 12, 3367. [Google Scholar] [CrossRef] [PubMed]
- Cintas, P.; Luche, J.L. Green chemistry: The sonochemical approach. Green Chem. 1999, 1, 115–125. [Google Scholar] [CrossRef]
- Oliveira, R.L.; Nicinski, K.; Pisarek, M.; Kaminska, A.; Thomas, A.; Pasternak, G.; Colmenares, J.C. Porous heteroatom-doped carbons: Efficient catalysts for selective oxidation of alcohols by activated persulfate. ChemCatChem 2022, 14, e202200787. [Google Scholar] [CrossRef]
- Qu, G.; Jia, P.; Tang, S.; Pervez, N.; Pang, Y.; Li, B.; Cao, C.; Zhao, Y. Enhanced peroxymonosulfate activation via heteroatomic doping defects of pyridinic and pyrrolic N in 2D N doped carbon nanosheets for BPA degradation. J. Hazard. Mater. 2024, 461, 132626. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, M.; Sun, H.; Ao, Z.; Wang, S.; Liu, S. Understanding of the oxidation behavior of benzyl alcohol by peroxymonosulfate via carbon nanotubes activation. ACS Catal. 2020, 10, 3516–3525. [Google Scholar] [CrossRef]
- Li, M.; Xu, F.; Li, H.; Wang, Y. Nitrogen-doped porous carbon materials: Promising catalysts or catalyst supports for heterogeneous hydrogenation and oxidation. Catal. Sci. Technol. 2016, 6, 3670–3693. [Google Scholar] [CrossRef]
- Zhu, S.; Chen, Y.; Gao, X.; Lv, Z.; He, Y.; Wang, J.; Fan, W. Kraft lignin derived S and O co-doped porous graphene for metal-free benzylic alcohol oxidation. Catal. Sci. Technol. 2020, 10, 2786–2796. [Google Scholar] [CrossRef]
- Gholami, P.; Khataee, A.; Cheshmeh Soltani, R.D.; Bhatnagar, A. A review on carbon-based materials for heterogeneous sonocatalysis: Fundamentals, properties, and applications. Ultrason. Sonochem. 2019, 58, 104681. [Google Scholar] [CrossRef]
- Camparotto, N.G.; Paixão, G.R.; Brião, G.d.V.; Oliveira, R.L.; Prediger, P.; Vieira, M.G.A. Comparative effect of mesoporous carbon doping on the adsorption of pharmaceutical drugs in water: Theoretical calculations and mechanism study. Environ. Toxicol. Pharmacol. 2023, 99, 104105. [Google Scholar] [CrossRef]
- Paixão, G.R.; Camparotto, N.G.; Brião, G.d.V.; Oliveira, R.d.L.; Colmenares, J.C.; Prediger, P.; Vieira, M.G.A. Synthesis of mesoporous P-doped carbon and its application in propranolol drug removal: Characterization, kinetics, and isothermal studies. Chem. Eng. Res. Des. 2022, 187, 225–239. [Google Scholar] [CrossRef]
- Selim, A.; Kaur, S.; Dar, A.H.; Sartaliya, S.; Jayamurugan, G. Synergistic Effects of Carbon Dots and Palladium Nanoparticles Enhance the Sonocatalytic Performance for Rhodamine B Degradation in the Absence of Light. ACS Omega 2020, 5, 22603–22613. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Dai, J.; Zhao, Y.; Wu, J.; Ji, C.; Zhang, Y. Advances in preparation, application in contaminant removal, and environmental risks of biochar-based catalysts: A review. Biochar 2022, 4, 51. [Google Scholar] [CrossRef]
- do Nascimento, B.F.; de Araújo, C.M.B.; del Carmen Pinto Osorio, D.; Silva LF, O.; Dotto, G.L.; Cavalcanti JV, F.L.; da Motta Sobrinho, M.A. Adsorption of chloroquine, propranolol, and metformin in aqueous solutions using magnetic graphene oxide nanocomposite. Environ. Sci. Pollut. Res. 2023, 30, 85344–85358. [Google Scholar] [CrossRef]
- Oliveira, R.L.; Ben Ghorbel, M.C.; Praetz, S.; Meiling, D.; Schlesiger, C.; Schomäcker, R.; Thomas, A. Confinement of Cobalt Species in Mesoporous N-Doped Carbons and the Impact on Nitroarene Hydrogenation. ACS Sustain. Chem. Eng. 2020, 8, 11171–11182. [Google Scholar] [CrossRef]
- Fiorio, J.L.; Garcia, M.A.; Gothe, M.L.; Galvan, D.; Troise, P.C.; Conte-Junior, C.A.; Vidinha, P.; Camargo, P.H.; Rossi, L.M. Recent advances in the use of nitrogen-doped carbon materials for the design of noble metal catalysts. Coord. Chem. Rev. 2023, 481, 215053. [Google Scholar] [CrossRef]
- Jorio, A.; Ferreira, E.H.M.; Moutinho, M.V.O.; Stavale, F.; Achete, C.A.; Capaz, R.B. Measuring disorder in graphene with the G and D bands. Phys. Stat. Sol. 2010, 247, 2980–2982. [Google Scholar] [CrossRef]
- Smidt, E.; Gomes, J.A.G.; Santos, J.B.O. The applicability of Fourier transform infrared (FT-IR) spectroscopy in waste management. Waste Manag. 2007, 27, 268–276. [Google Scholar] [CrossRef] [PubMed]
- Campos-Roldán, C.A.; Ramos-Sánchez, G.; Gonzalez-Huerta, R.G.; Vargas García, J.R.; Balbuena, P.B.; Alonso-Vante, N. Influence of sp3–sp2 Carbon Nanodomains on Metal/Support Interaction, Catalyst Durability, and Catalytic Activity for the Oxygen Reduction Reaction. ACS Appl. Mater. Interfaces 2016, 8, 23260–23269. [Google Scholar] [CrossRef]
- Liu, Y.; Quan, X.; Fan, X.; Wang, H.; Chen, S. High-Yield Electrosynthesis of Hydrogen Peroxide from Oxygen Reduction by Hierarchically Porous Carbon. Angew. Chem. Int. Ed. 2015, 54, 6837–6841. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, F.; Yang, Q.; Wang, S.; Sun, H.; Yang, Q.; Tang, J.; Liu, S. Tailoring collaborative N–O functionalities of graphene oxide for enhanced selective oxidation of benzyl alcohol. Carbon 2021, 182, 715–724. [Google Scholar] [CrossRef]
- Babu, D.D.; Huang, Y.; Anandhababu, G.; Wang, X.; Si, R.; Wu, M.; Li, Q.; Wang, Y.; Yao, J. Atomic iridium@cobalt nanosheets for dinuclear tandem water oxidation. J. Mater. Chem. A 2019, 7, 8376–8383. [Google Scholar] [CrossRef]
- Bai, L.; Gong, K.; Ye, S.; Mao, Y.; Lin, Z.; Liu, Z.; Luo, Y. Catalytic activities enhanced by abundant structural defects and balanced N distribution of N-doped graphene in oxygen reduction reaction. J. Power Sources 2016, 306, 85–91. [Google Scholar] [CrossRef]
- Zheng, Y.; Cheng, P.; Xu, J.; Han, J.; Wang, D.; Hao, C.; Alanagh, H.R.; Long, C.; Shi, X.; Tang, Z. MOF-derived nitrogen-doped nanoporous carbon for electroreduction of CO2 to CO: The calcining temperature effect and the mechanism. Nanoscale 2019, 11, 4911–4917. [Google Scholar] [CrossRef]
- Lazar, P.; Mach, R.; Otyepka, M. Spectroscopic fingerprints of graphitic, pyrrolic, pyridinic, and chemisorbed nitrogen in N-doped graphene. J. Phys. Chem. C 2019, 123, 10695–10702. [Google Scholar] [CrossRef]
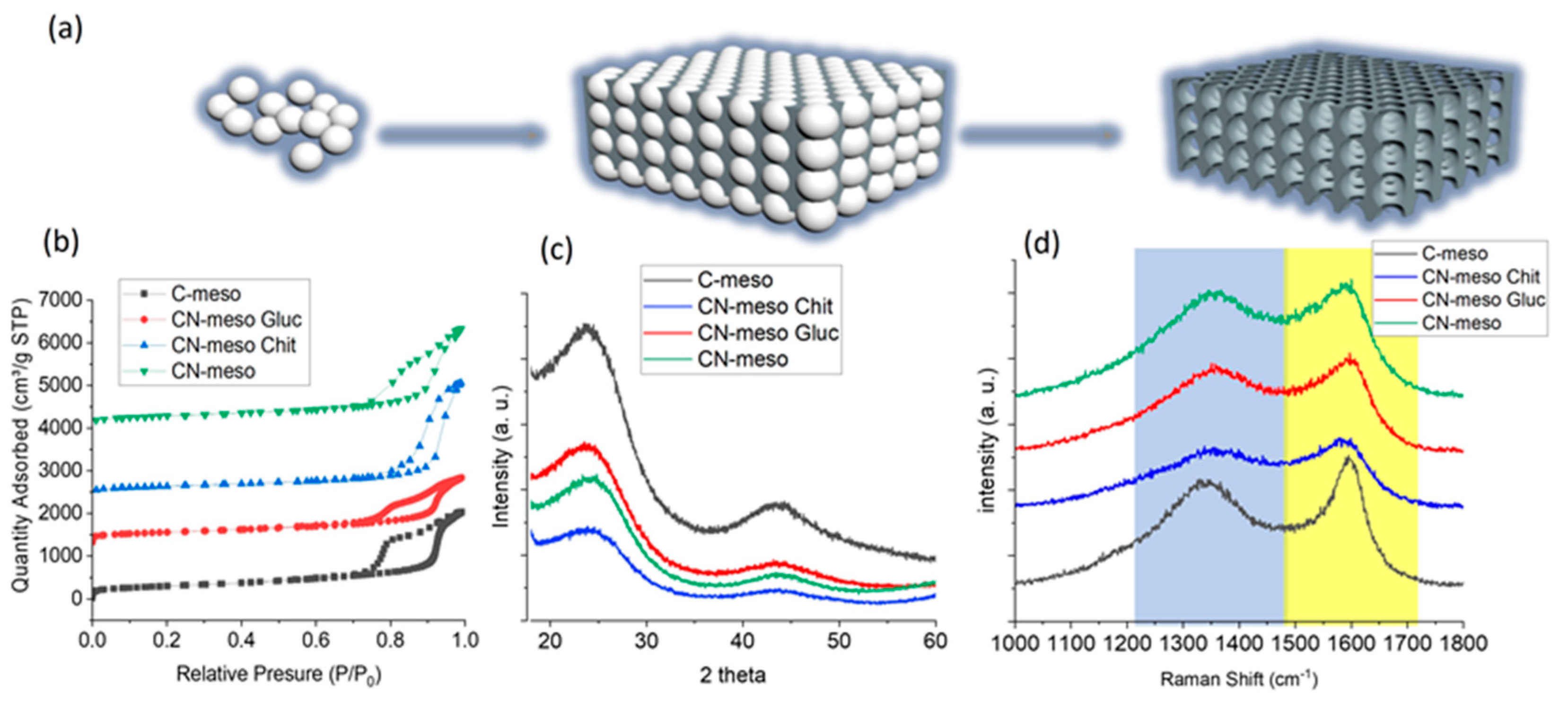


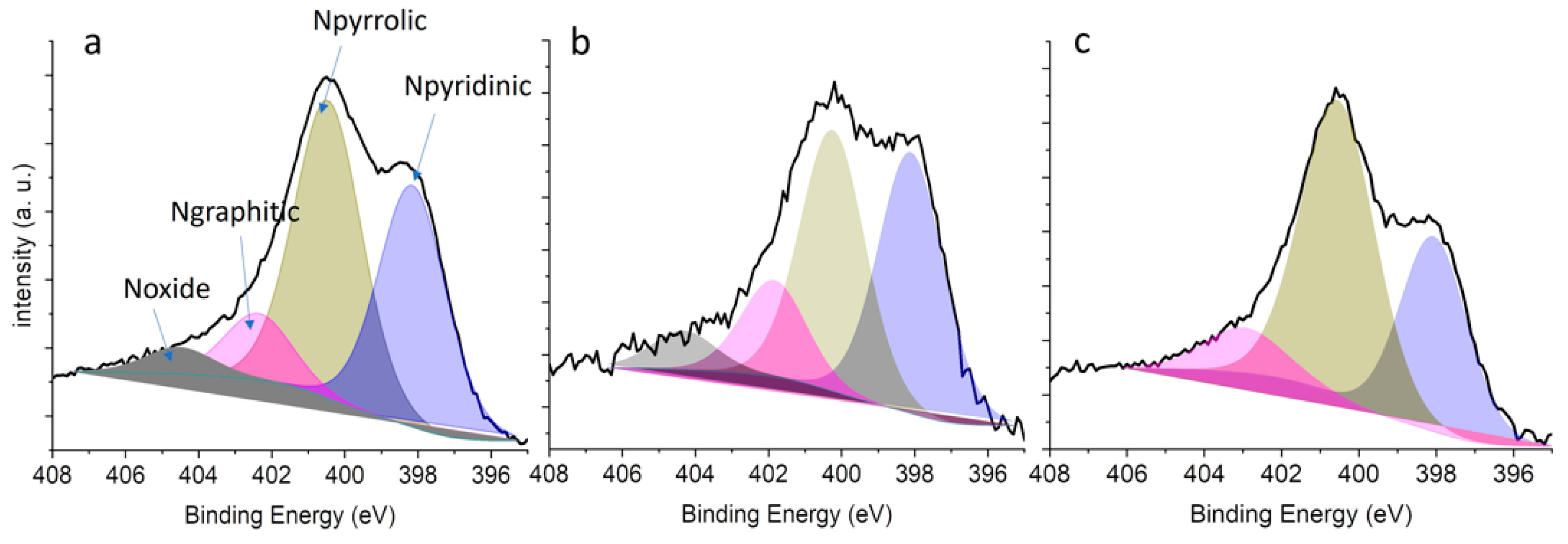
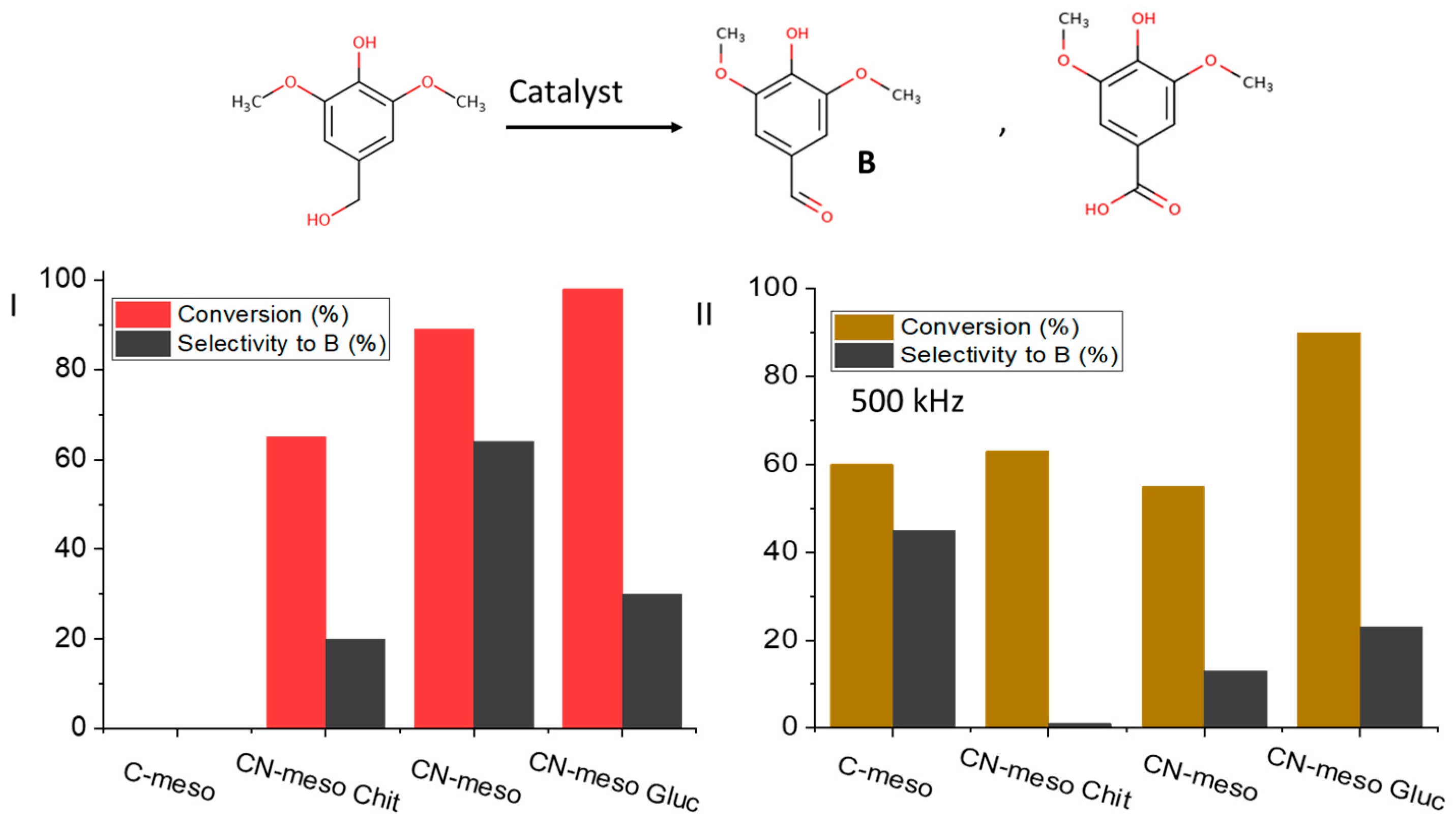
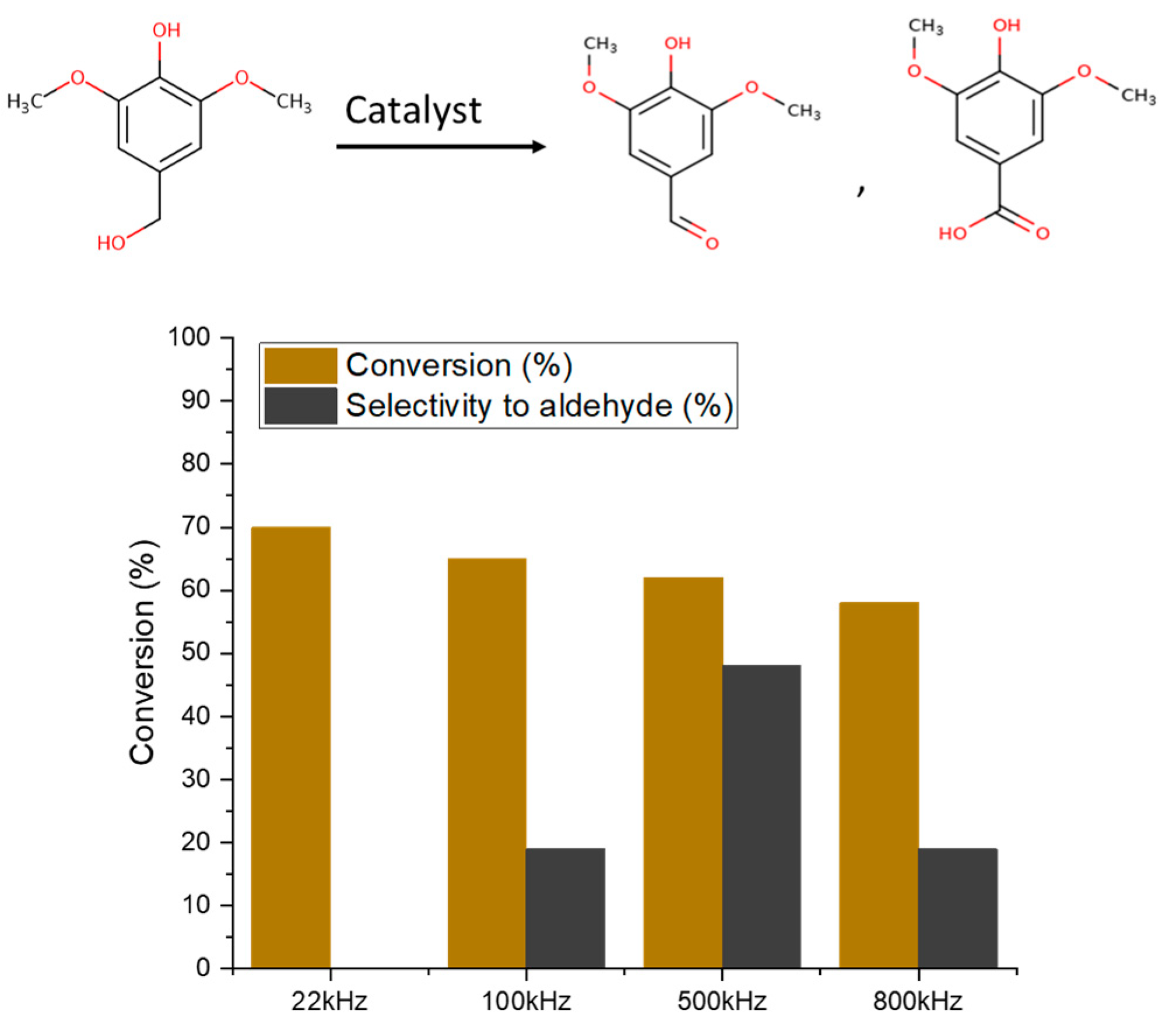
| Catalyst Name | Surface Area [m2/g] | Pore Volume [cm3/g] | ID/IG |
|---|---|---|---|
| C-meso | 1044 | 3.15 | 1.6 |
| CN-meso | 873 | 2.3 | 1.8 |
| CN-meso chit | 834 | 3.3 | 1.4 |
| CN-glucosamine | 904 | 2.38 | 1.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hashemi Hosseini, B.; Oliveira, R.L.; Łomot, D.; Chernyayeva, O.; Colmenares Quintero, J.C. Sonocatalytic Activity of Porous Carbonaceous Materials for the Selective Oxidation of 4-Hydroxy-3,5-dimethoxybenzyl Alcohol. Molecules 2024, 29, 1436. https://doi.org/10.3390/molecules29071436
Hashemi Hosseini B, Oliveira RL, Łomot D, Chernyayeva O, Colmenares Quintero JC. Sonocatalytic Activity of Porous Carbonaceous Materials for the Selective Oxidation of 4-Hydroxy-3,5-dimethoxybenzyl Alcohol. Molecules. 2024; 29(7):1436. https://doi.org/10.3390/molecules29071436
Chicago/Turabian StyleHashemi Hosseini, Behdokht, Rafael L. Oliveira, Dariusz Łomot, Olga Chernyayeva, and Juan C. Colmenares Quintero. 2024. "Sonocatalytic Activity of Porous Carbonaceous Materials for the Selective Oxidation of 4-Hydroxy-3,5-dimethoxybenzyl Alcohol" Molecules 29, no. 7: 1436. https://doi.org/10.3390/molecules29071436
APA StyleHashemi Hosseini, B., Oliveira, R. L., Łomot, D., Chernyayeva, O., & Colmenares Quintero, J. C. (2024). Sonocatalytic Activity of Porous Carbonaceous Materials for the Selective Oxidation of 4-Hydroxy-3,5-dimethoxybenzyl Alcohol. Molecules, 29(7), 1436. https://doi.org/10.3390/molecules29071436







