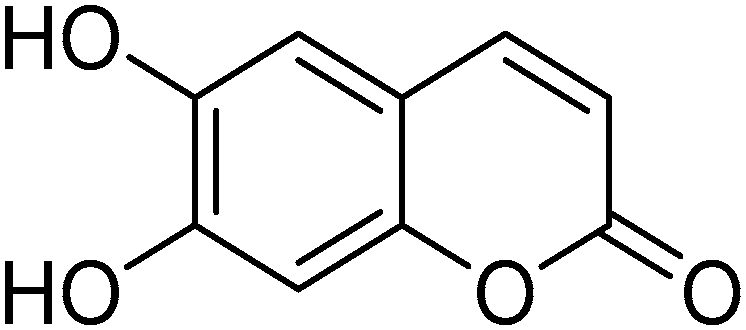
Protective Effects and Mechanisms of Esculetin against H2O2-Induced Oxidative Stress, Apoptosis, and Pyroptosis in Human Hepatoma HepG2 Cells
Abstract
1. Introduction
2. Results
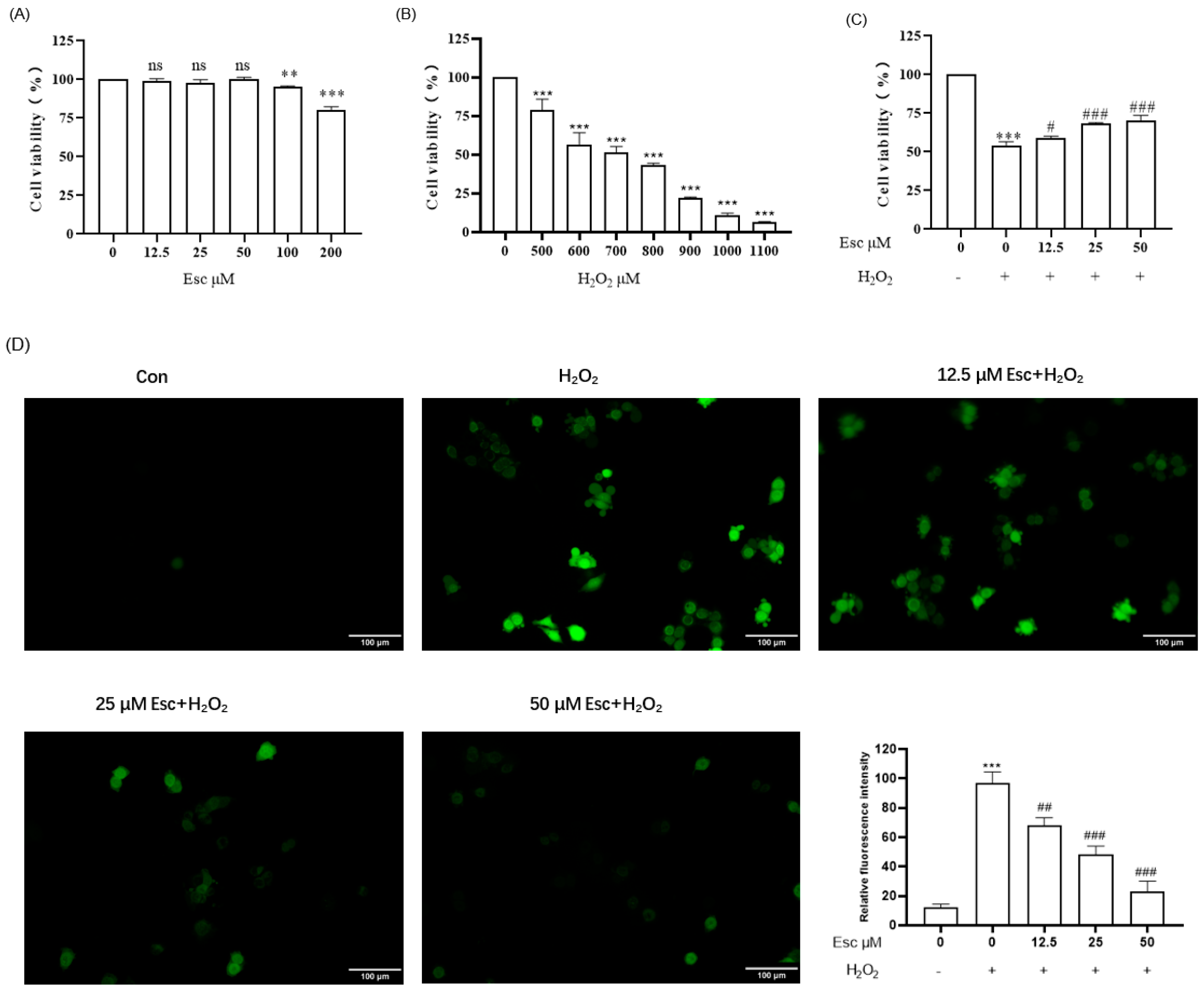
2.1. Esc Protected HepG2 Cells against H2O2-Induced Oxidative Stress
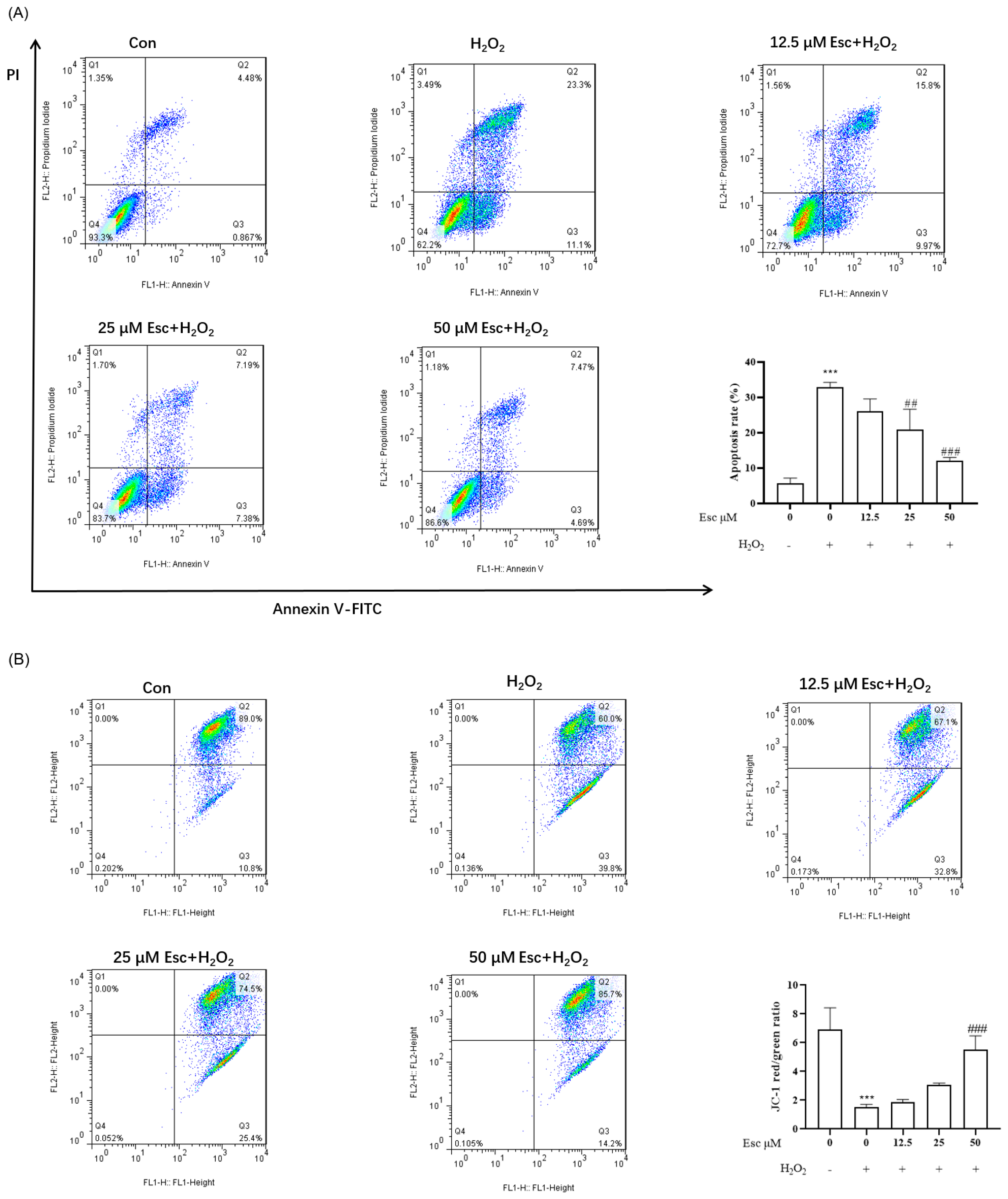
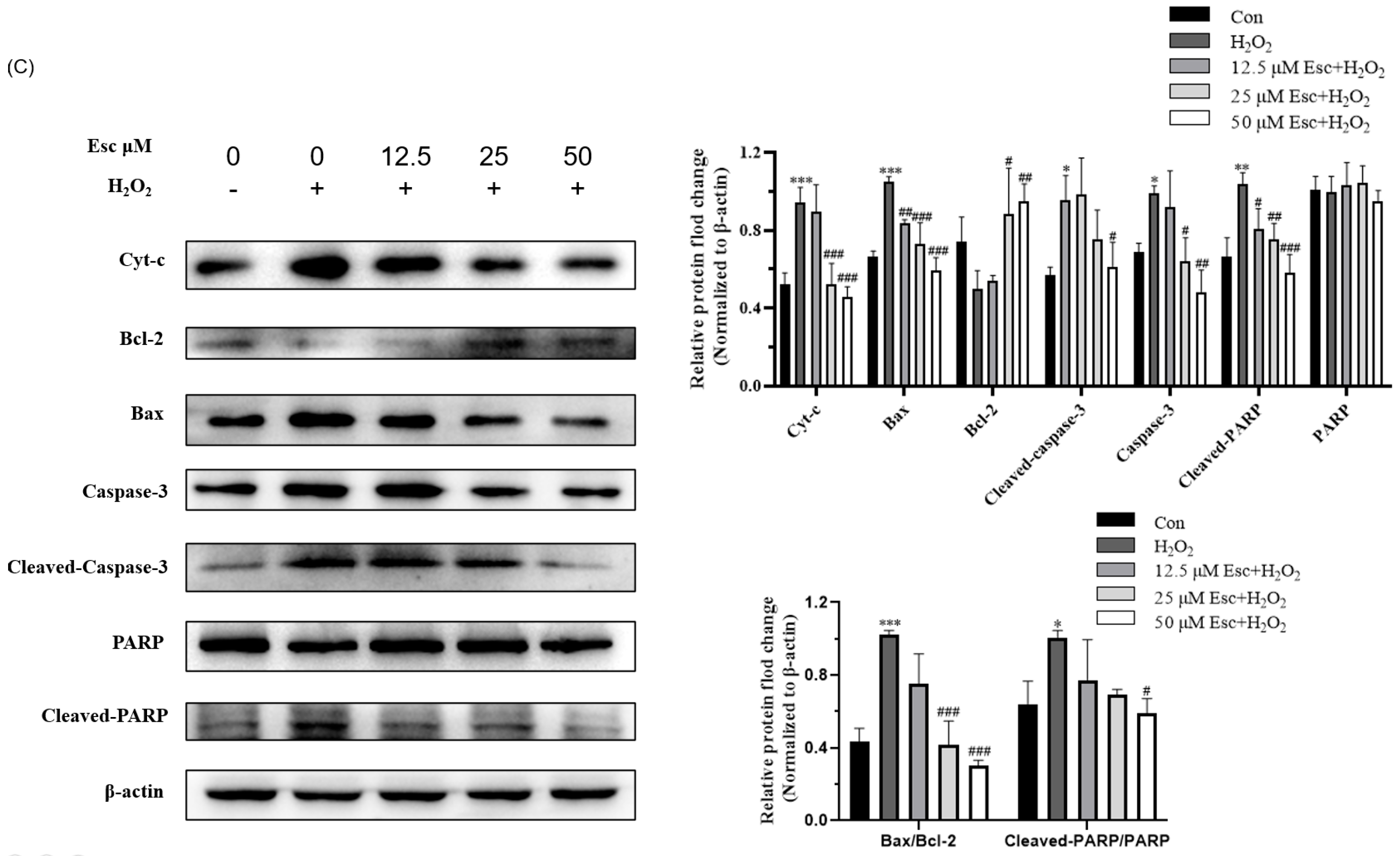
2.2. Esc Protected HepG2 Cells from H2O2-Induced Apoptosis
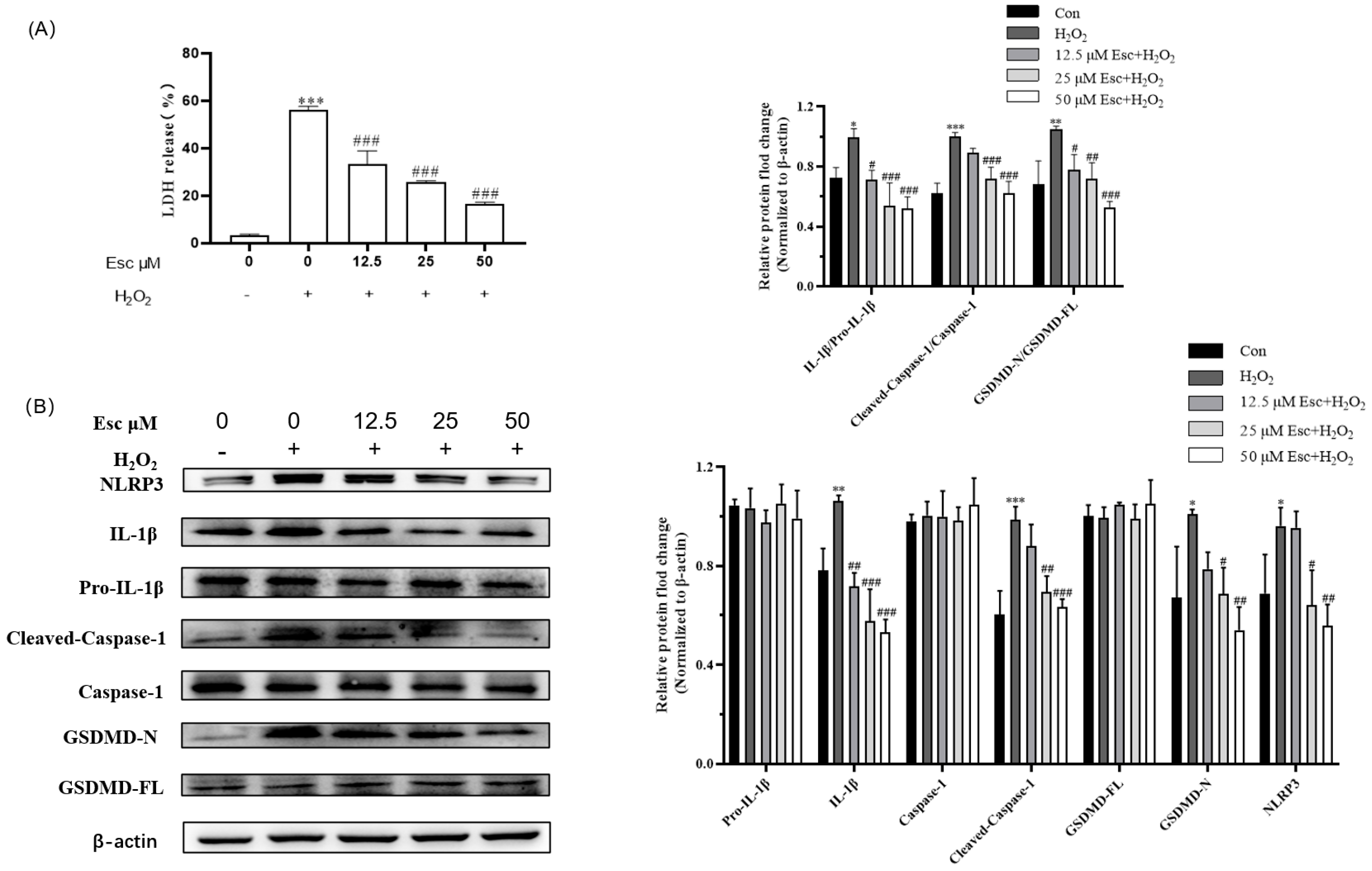
2.3. Esc Protected HepG2 Cells from H2O2-Induced Pyroptosis
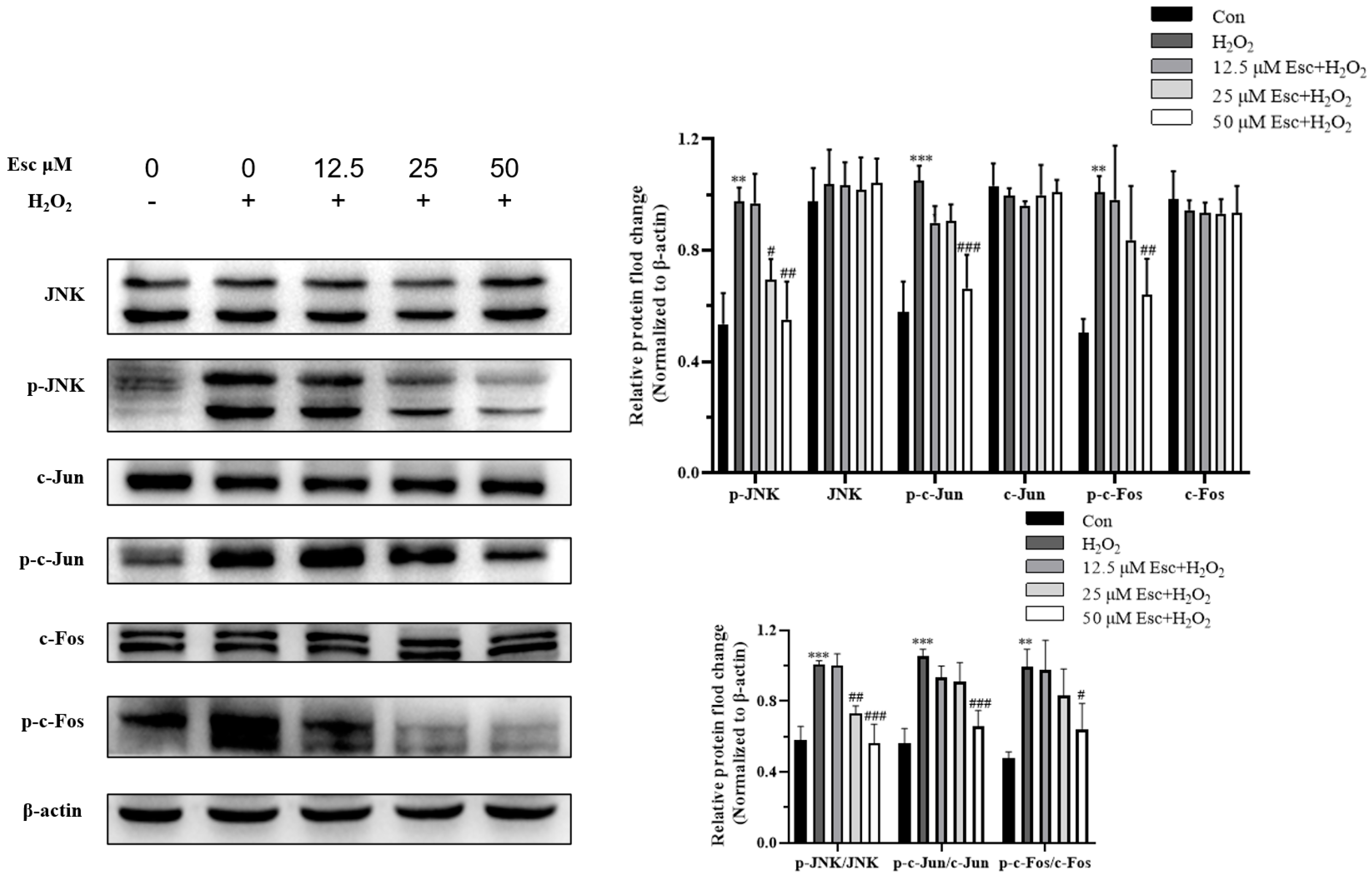
2.4. Esc Protected HepG2 Cells against H2O2-Induced Oxidative Stress via the JNK Signaling Pathway
2.5. Ani Reversed the Protection of Esc against H2O2-Induced HepG2 Cells’ Oxidative Stress, Apoptosis, and Pyroptosis
3. Discussion
4. Materials and Methods
4.1. Reagents and Antibodies
4.2. Cell Culture
4.3. Cell Viability Assay
4.4. ROS Production Assay
4.5. Apoptosis Rate Assay
4.6. Mitochondrial Membrane Potential Assay
4.7. LDH Release Assay
4.8. Western Blot Analysis
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lister, I.N.E.; Ginting, C.N.; Girsang, E.; Nataya, E.D.; Azizah, A.M.; Widowati, W. Hepatoprotective properties of red betel (Piper crocatum Ruiz and Pav) leaves extract towards H2O2-induced HepG2 cells via anti-inflammatory, antinecrotic, antioxidant potency. Saudi Pharm. J. 2020, 28, 1182–1189. [Google Scholar] [CrossRef]
- Jian, L.Y.; Xue, Y.; Gao, Y.F.; Wang, B.; Qu, Y.H.; Li, S.H.; Li, H.Q.; Li, Z.; Wang, B.; Luo, H.L. Vitamin E can ameliorate oxidative damage of ovine hepatocytes in vitro by regulating genes expression associated with apoptosis and pyroptosis, but not ferroptosis. Molecules 2021, 26, 4520. [Google Scholar] [CrossRef]
- Doustimotlagh, A.H.; Taheri, S.; Mansourian, M.; Eftekhari, M. Extraction and identification of two flavonoids in Phlomoides hyoscyamoides as an endemic plant of Iran: The role of quercetin in the activation of the glutathione peroxidase, the improvement of the hydroxyproline and protein oxidation in bile duct-ligated rats. Curr. Comput.-Aided Drug Des. 2020, 16, 629–640. [Google Scholar]
- Wang, Y.J.; Liu, F.J.; Liu, M.R.; Zhou, X.; Wang, M.; Cao, K.X.; Jin, S.J.; Shan, A.S.; Feng, X.J. Curcumin mitigates aflatoxin B1-induced liver injury via regulating the NLRP3 inflammasome and Nrf2 signaling pathway. Food Chem. Toxicol. 2022, 161, 112823. [Google Scholar] [CrossRef]
- Wu, C.R.; Huang, M.Y.; Lin, Y.T.; Ju, H.Y.; Ching, H. Antioxidant properties of Cortex Fraxini and its simple coumarins. Food Chem. 2007, 104, 1464–1471. [Google Scholar] [CrossRef]
- Kim, S.H.; Kang, K.A.; Zhang, R.; Piao, M.J.; Ko, D.O.; Wang, Z.H.; Chae, S.W.; Kang, S.S.; Lee, K.H.; Kang, H.K.; et al. Protective effect of esculetin against oxidative stress-induced cell damage via scavenging reactive oxygen species. Acta Pharmacol. Sin. 2008, 29, 1319–1326. [Google Scholar] [CrossRef] [PubMed]
- Jung, W.K.; Park, S.B.; Yu, H.Y.; Kim, Y.H.; Kim, J. Antioxidant efficacy of esculetin against tert-butyl hydroperoxide-induced oxidative stress in HEK293 cells. Curr. Issues Mol. Biol. 2022, 44, 5986–5994. [Google Scholar] [CrossRef]
- Jung, W.K.; Park, S.B.; Yu, H.Y.; Kim, Y.H.; Kim, J. Effect of esculetin on tert-butyl hydroperoxide-induced oxidative injury in retinal pigment epithelial cells in vitro. Molecules 2022, 27, 8970. [Google Scholar] [CrossRef] [PubMed]
- Pruccoli, L.; Morroni, F.; Sita, G.; Hrelia, P.; Tarozzi, A. Esculetin as a bifunctional antioxidant prevents and counteracts the oxidative stress and neuronal death induced by amyloid protein in SH-SY5Y cells. Antioxidants 2020, 9, 551. [Google Scholar] [CrossRef] [PubMed]
- Subramaniam, S.R.; Ellis, E.M. Esculetin-induced protection of human hepatoma HepG2 cells against hydrogen peroxide is associated with the Nrf2-dependent induction of the NAD(P)H: Quinone oxidoreductase 1 gene. Toxicol. Appl. Pharmacol. 2011, 250, 130–136. [Google Scholar] [CrossRef]
- Lin, W.L.; Wang, C.J.; Tsai, Y.Y.; Liu, C.L.; Hwang, J.M.; Tseng, T.H. Inhibitory effect of esculetin on oxidative damage induced by t-butyl hydroperoxide in rat liver. Arch. Toxicol. 2000, 74, 467–472. [Google Scholar] [CrossRef]
- Lee, J.; Yang, J.; Yang, J.; Jeon, J.; Jeong, Y.; Jeong, H.S.; Lee, J.; Sun, J. Hepatoprotective effect of esculetin on ethanol-induced liver injury in human HepG2 cells and C57BL/6J mice. J. Funct. Foods 2018, 40, 536–543. [Google Scholar] [CrossRef]
- Murat Bilgin, H.; Atmaca, M.; Deniz Obay, B.; Ozekinci, S.; Taşdemir, E.; Ketani, A. Protective effects of coumarin and coumarin derivatives against carbon tetrachloride-induced acute hepatotoxicity in rats. Exp. Toxicol. Pathol. 2011, 63, 325–330. [Google Scholar] [CrossRef]
- Garg, S.S.; Gupta, J.; Sahu, D.; Liu, C.J. Pharmacological and therapeutic applications of esculetin. Int. J. Mol. Sci. 2022, 23, 12643. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.J.; Zhang, Y.W.; Ren, G.F.; Yang, R.G.; Chen, J.F.; Xiang, X.J.; Qin, H.; Chen, J.H. Inhibitory effect of delphinidin on oxidative stress induced by H2O2 in HepG2 Cells. Oxid. Med. Cell. Longev. 2020, 2020, 4694760. [Google Scholar] [CrossRef] [PubMed]
- Basu, C.; Sur, R. S-Allyl cysteine alleviates hydrogen peroxide induced oxidative injury and apoptosis through upregulation of Akt/Nrf-2/HO-1 signaling pathway in HepG2 cells. BioMed Res. Int. 2018, 2018, 3169431. [Google Scholar] [CrossRef]
- Jiang, J.Y.; Yu, S.N.; Jiang, Z.C.; Liang, C.H.; Yu, W.B.; Li, J.; Du, X.D.; Wang, H.L.; Gao, X.H.; Wang, X. N-acetyl-serotonin protects HepG2 cells from oxidative stress injury induced by hydrogen peroxide. Oxid. Med. Cell. Longev. 2014, 2014, 310504. [Google Scholar] [CrossRef] [PubMed]
- Su, M.; Yu, T.F.; Zhang, H.; Wu, Y.; Wang, X.Q.; Li, G. The antiapoptosis effect of glycyrrhizate on HepG2 cells induced by hydrogen peroxide. Oxid. Med. Cell. Longev. 2016, 2016, 6849758. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Li, X.; Liu, L.F.; Xiao, X.; Zhang, L.; Zhang, S.L.; Lin, P.P.; Wang, X.J.; Wang, Y.W.; Li, Q.S. Attenuation of doxorubicin-induced cardiotoxicity by esculetin through modulation of Bmi-1 expression. Exp. Ther. Med. 2017, 14, 2216–2220. [Google Scholar] [CrossRef]
- Mai, W.J.; Xu, Y.Z.; Xu, J.H.; Zhao, D.; Ye, L.Y.; Yu, G.X.; Wang, Z.L.; Lu, Q.T.; Lin, J.E.; Yang, T.; et al. Berberine inhibits Nod-like receptor family pyrin domain containing 3 inflammasome activation and pyroptosis in nonalcoholic steatohepatitis via the ROS/TXNIP axis. Front. Pharmacol. 2020, 11, 185. [Google Scholar] [CrossRef]
- Xie, W.H.; Ding, J.; Xie, X.X.; Yang, X.H.; Wu, X.F.; Chen, Z.X.; Guo, Q.L.; Gao, W.Y.; Wang, X.Z.; Li, D. Hepatitis B virus X protein promotes liver cell pyroptosis under oxidative stress through NLRP3 inflammasome activation. Inflamm. Res. 2020, 69, 683–696. [Google Scholar] [CrossRef]
- Yao, T.; Chen, J.M.; Shen, L.E.; Yu, Y.S.; Tang, Z.H.; Zang, G.Q.; Zhang, Y.; Chen, X.H. Astragalus polysaccharide alleviated hepatocyte senescence via autophagy pathway. Kaohsiung J. Med. Sci. 2022, 38, 457–468. [Google Scholar] [CrossRef]
- Wang, S.K.; Chen, T.X.; Wang, W.; Xu, L.L.; Zhang, Y.Q.; Jin, Z.; Liu, Y.B.; Tang, Y.Z. Aesculetin exhibited anti-inflammatory activities through inhibiting NF-κB and MAPKs pathway in vitro and in vivo. J. Ethnopharmacol. 2022, 296, 115489. [Google Scholar] [CrossRef]
- Ren, W.; Zhou, Q.Y.; Yu, R.Y.; Liu, Z.J.; Hu, Y.S. Esculetin inhibits the pyroptosis of microvascular endothelial cells through NF-κB /NLRP3 signaling pathway. Arch. Biochem. Biophys. 2022, 720, 109173. [Google Scholar] [CrossRef]
- Ishikawa, Y.; Kitamura, M. Anti-apoptotic effect of quercetin: Intervention in the JNK- and ERK-mediated apoptotic pathways. Kidney Int. 2000, 58, 1078–1087. [Google Scholar] [CrossRef]
- Kitamura, M.; Ishikawa, Y.; Moreno-Manzano, V.; Xu, Q.; Konta, T.; Lucio-cazana, J.; Furusu, A.; Nakayama, K. Intervention by retinoic acid in oxidative stress-induced apoptosis. Nephrol. Dial. Transplant. 2002, 9, 84–87. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yan, Y.Y.; Zheng, D.D.; Zhang, J.F.; Wang, J.N. Inhibiting microRNA-200a-3p attenuates pyroptosis via targeting the SIRT1/NF-κB/NLRP3 pathway in H2O2-induced HAEC. Aging 2023, 15, 11184–11200. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.Y.; Han, J.; Li, Y.; Dong, B. Esculetin inhibits the apoptosis in H9c2 cardiomyocytes via the MAPK signaling pathway following hypoxia/reoxygenation injury. Biomed. Pharmacother. 2017, 88, 1206–1210. [Google Scholar] [CrossRef]
- Zhen, A.X.; Piao, M.J.; Kang, K.A.; Fernando, P.D.S.M.; Kang, H.K.; Koh, Y.S.; Hyun, J.W. Esculetin prevents the induction of matrix metalloproteinase-1 by hydrogen peroxide in skin keratinocytes. J. Cancer Prev. 2019, 24, 123–128. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, Y.; Chang, T.; Huang, S.; Xiang, J.; Tang, S.; Shen, H. Protective Effects and Mechanisms of Esculetin against H2O2-Induced Oxidative Stress, Apoptosis, and Pyroptosis in Human Hepatoma HepG2 Cells. Molecules 2024, 29, 1415. https://doi.org/10.3390/molecules29071415
Luo Y, Chang T, Huang S, Xiang J, Tang S, Shen H. Protective Effects and Mechanisms of Esculetin against H2O2-Induced Oxidative Stress, Apoptosis, and Pyroptosis in Human Hepatoma HepG2 Cells. Molecules. 2024; 29(7):1415. https://doi.org/10.3390/molecules29071415
Chicago/Turabian StyleLuo, Ying, Tenglong Chang, Shiting Huang, Jing Xiang, Shuangyang Tang, and Haiyan Shen. 2024. "Protective Effects and Mechanisms of Esculetin against H2O2-Induced Oxidative Stress, Apoptosis, and Pyroptosis in Human Hepatoma HepG2 Cells" Molecules 29, no. 7: 1415. https://doi.org/10.3390/molecules29071415
APA StyleLuo, Y., Chang, T., Huang, S., Xiang, J., Tang, S., & Shen, H. (2024). Protective Effects and Mechanisms of Esculetin against H2O2-Induced Oxidative Stress, Apoptosis, and Pyroptosis in Human Hepatoma HepG2 Cells. Molecules, 29(7), 1415. https://doi.org/10.3390/molecules29071415





