Study on the Properties of FEVE Modified with Ag2O/OH-MWCNTS Nanocomposites for Use as Adhesives for Wooden Heritage Objects
Abstract
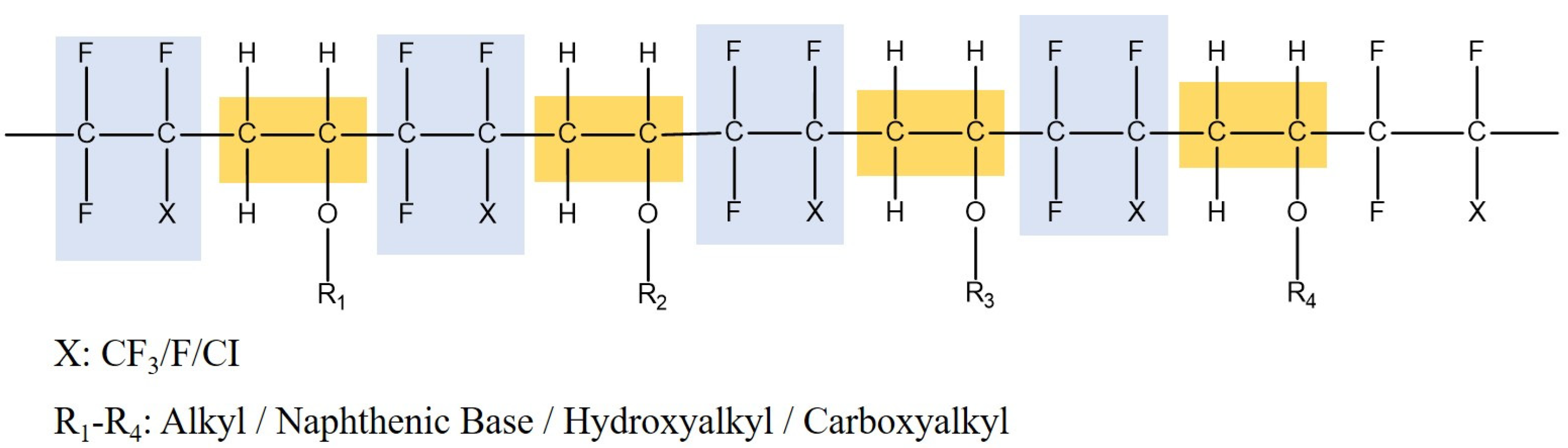
1. Introduction
2. Results and Discussion
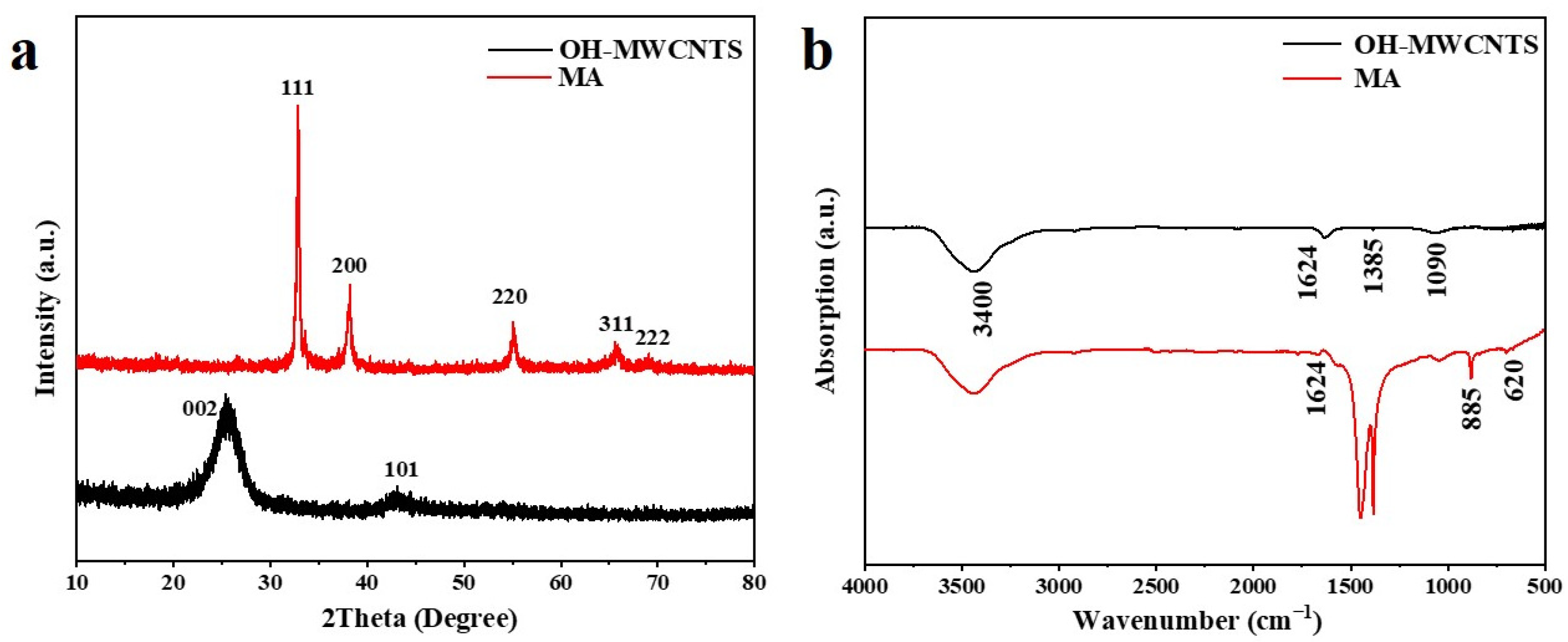
2.1. X-ray Diffraction Analysis
2.2. Infrared Absorption Spectroscopy Analysis
2.3. SEM Analysis
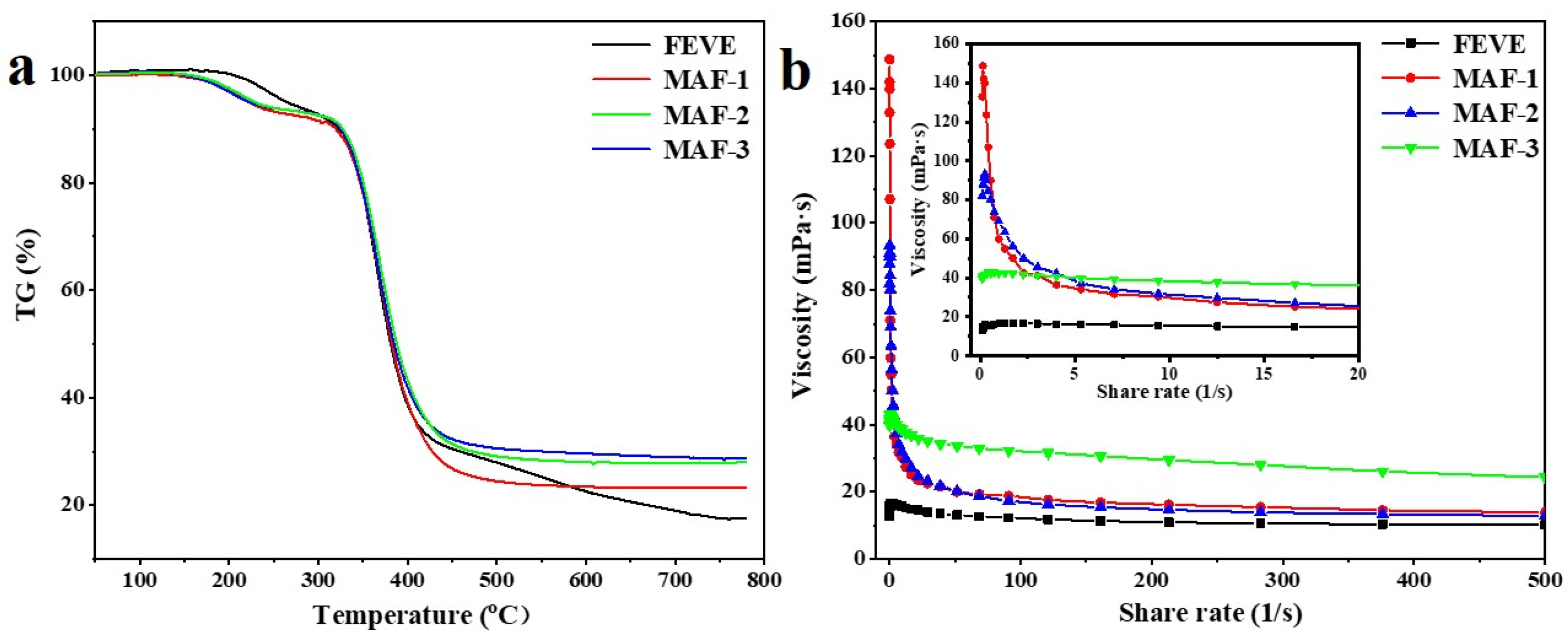
2.4. Thermal Stability Analysis
2.5. Viscoelastic Measurement
2.6. Analysis of Adhesive Bonding Shear Strength
2.7. Antimicrobial Effects of Ag2O/OH-MWCNTS-FEVE Adhesives
3. Materials and Methods
3.1. Materials
3.2. Preparation of Ag2O/OH-MWCNTS-FEVE (MAF) Adhesives and Plywooden Samples
3.3. Characterization
3.3.1. X-ray Diffraction Analysis
3.3.2. Infrared Absorption Spectroscopy
3.3.3. Scanning Electron Microscopy
3.3.4. Thermogravimetric Analysis
3.3.5. Viscoelastic Measurement
3.3.6. Shear Strength
3.3.7. Antifungal and Antibacterial Effects of Ag2O/OH-MWCNTS-FEVE Adhesives
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Unger, A.; Schniewind, A.P.; Unger, W. Conservation of Wooden Artifacts: A Handbook; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2001. [Google Scholar]
- Peng, Y.; Wang, Y.; Zhang, R.; Wang, W.; Cao, J. Improvement of wooden against uv weathering and decay by using plant origin substances: Tannin acid and tung oil. Ind. Crops Prod. 2021, 168, 113606. [Google Scholar] [CrossRef]
- Walsh-Korb, Z. Sustainability in heritage wooden conservation: Challenges and directions for future research. Forests 2022, 13, 18. [Google Scholar] [CrossRef]
- Thieme, H. Lower palaeolithic hunting spears from germany. Nature 1997, 385, 807–810. [Google Scholar] [CrossRef]
- Ambrose, S.H. Paleolithic technology and human evolution. Science 2001, 291, 1748–1753. [Google Scholar] [CrossRef]
- Timar, M.C.; Sandu, I.C.A.; Beldean, E.C.; Sandu, I.G. Ftir investigation of paraloid b72 as consolidant for old wooden artefacts principle and methods. Mater. Plast. 2014, 51, 382–387. [Google Scholar]
- Emmanuel, V.; Odile, B.; Céline, R. Ftir spectroscopy of woodens: A new approach to study the weathering of the carving face of a sculpture. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 136, 1255–1259. [Google Scholar] [CrossRef]
- Lionetto, F.; Frigione, M. Mechanical and natural durability properties of wooden treated with a novel organic preservative/consolidant product. Mater. Des. 2009, 30, 3303–3307. [Google Scholar] [CrossRef]
- Almkvist, G.; Persson, I. Fenton-induced degradation of polyethylene glycol and oak holocellulose. A model experiment in comparison to changes observed in conserved waterlogged wooden. Holzforschung 2008, 62, 704–708. [Google Scholar] [CrossRef]
- Fors, Y.; Sandström, M. Sulfur and iron in shipwrecks cause conservation concerns. Chem. Soc. Rev 2006, 35, 399–415. [Google Scholar] [CrossRef] [PubMed]
- Fors, Y.; Grudd, H.; Rindby, A.; Jalilehvand, F.; Sandström, M.; Cato, I.; Bornmalm, L. Sulfur and iron accumulation in three marine-archaeological shipwrecks in the baltic sea: The ghost, the crown and the sword. Sci. Rep. 2014, 4, 4222. [Google Scholar] [CrossRef]
- Fors, Y.; Nilsson, T.; Risberg, E.D.; Sandström, M.; Torssander, P. Sulfur accumulation in pinewooden (pinus sylvestris) induced by bacteria in a simulated seabed environment: Implications for marine archaeological wooden and fossil fuels. Int. Biodeterior. Biodegrad. 2008, 62, 336–347. [Google Scholar] [CrossRef]
- Fors, Y.; Jalilehvand, F.; Damian Risberg, E.; Björdal, C.; Phillips, E.; Sandström, M. Sulfur and iron analyses of marine archaeological wooden in shipwrecks from the baltic sea and scandinavian waters. J. Archaeol. Sci. Rep. 2012, 39, 2521–2532. [Google Scholar] [CrossRef]
- Sandström, M.; Jalilehvand, F.; Damian, E.; Fors, Y.; Gelius, U.; Jones, M.; Salomé, M. Sulfur accumulation in the timbers of king henry viii’s warship mary rose: A pathway in the sulfur cycle of conservation concern. Proc. Natl. Acad. Sci. USA 2005, 102, 14165–14170. [Google Scholar] [CrossRef]
- Nagarajappa, G.B.; Pandey, K.K. Uv resistance and dimensional stability of wooden modified with isopropenyl acetate. J. Photochem. Photobiol. B Biol. 2016, 155, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Hon, D.N.S.; Chang, S.T. Surface degradation of wooden by ultraviolet light. J. Polym. Sci. Polym. Chem. Ed. 2003, 22, 2227–2241. [Google Scholar] [CrossRef]
- Nair, S.; Nagarajappa, G.B.; Pandey, K.K. Uv stabilization of wooden by nano metal oxides dispersed in propylene glycol. J. Photochem. Photobiol. B Biol. 2018, 183, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Magdy, M. Analytical techniques for the preservation of cultural heritage: Frontiers in knowledge and application. Crit. Rev. Anal. Chem. 2022, 52, 1171–1196. [Google Scholar] [CrossRef] [PubMed]
- Pike, R.A. Adhesive. Encyclopedia Britannica Online. 2022. Available online: https://www.britannica.com/technology/adhesive (accessed on 7 March 2024).
- Chiavari, G.; Mazzeo, R. Characterisation of paint layers in chinese archaelogical relics by pyrolysis-gc-ms. Chromatographia 1999, 49, 268–272. [Google Scholar] [CrossRef]
- Calvano, C.D.; van der Werf, I.D.; Palmisano, F.; Sabbatini, L. Fingerprinting of egg and oil binders in painted artworks by matrix-assisted laser desorption ionization time-of-flight mass spectrometry analysis of lipid oxidation by-products. Anal. Bioanal. Chem. 2011, 400, 2229–2240. [Google Scholar] [CrossRef]
- Luo, W.; Li, T.; Wang, C.; Huang, F. Discovery of beeswax as binding agent on a 6th-century bc chinese turquoise-inlaid bronze sword. J. Archaeol. Sci. 2012, 39, 1227–1237. [Google Scholar] [CrossRef]
- Mitkidou, S.; Dimitrakoudi, E.; Urem-Kotsou, D.; Papadopoulou, D.; Kotsakis, K.; Stratis, J.A.; Stephanidou-Stephanatou, I. Organic residue analysis of neolithic pottery from north greece. Microchim. Acta 2008, 160, 493–498. [Google Scholar] [CrossRef]
- Regert, M. Investigating the history of prehistoric glues by gas chromatography-mass spectrometry. J. Sep. Sci. 2004, 27, 244–254. [Google Scholar] [CrossRef]
- Wei, S.; Pintus, V.; Pitthard, V.; Schreiner, M.; Song, G. Analytical characterization of lacquer objects excavated from a chu tomb in china. J. Archaeol. Sci. 2011, 38, 2667–2674. [Google Scholar] [CrossRef]
- Unoki, M.; Kimura, I.; Yamauchi, M. Solvent-soluble fluoropolymers for coatings—Chemical structure and weatherability. Surf. Coat. Int. Part B Coat. Trans. 2002, 85, 209–213. [Google Scholar] [CrossRef]
- Zhong, B.; Shen, L.; Zhang, X.; Li, C.; Bao, N. Reduced graphene oxide/silica nanocomposite-reinforced anticorrosive fluorocarbon coating. J. Appl. Polym. Sci. 2021, 138, 49689. [Google Scholar] [CrossRef]
- Zhao, P.Y. Study on Preparation and Properties of Nano- Titanium Fluorocarbon Anticorrosion Coating. Master’s Thesis, Harbin Institute of Technology, Harbin, China, 2010. (In Chinese with English Abstract). [Google Scholar]
- Deacon, J. (Ed.) Fungal ecology: Saprotrophs. In Fungal Biology; Blackwell Publishing: Oxford, UK, 2005; pp. 213–236. [Google Scholar] [CrossRef]
- Björdal, C.G.; Nilsson, T. Waterlogged archaeological wooden-a substrate for white rot fungi during drainage of wetlands. Int. Biodeterior. Biodegrad. 2002, 50, 17–23. [Google Scholar] [CrossRef]
- Blanchette, R.A. A review of microbial deterioration found in archaeological wooden from different environments. Int. Biodeterior. Biodegrad. 2000, 46, 189–204. [Google Scholar] [CrossRef]
- He, W.; Zhang, Y.; Li, J.; Gao, Y.; Luo, F.; Tan, H.; Wang, K.; Fu, Q. A novel surface structure consisting of contact-active antibacterial upper-layer and antifouling sub-layer derived from gemini quaternary ammonium salt polyurethanes. Sci. Rep. 2016, 6, 32140. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, G.G.; Soliman, M.H. Synthesis, spectroscopic and thermal characterization of sulpiride complexes of iron, manganese, copper, cobalt, nickel, and zinc salts. Antibacterial and antifungal activity. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2010, 76, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Hameed, A.S.H.; Karthikeyan, C.; Ahamed, A.P.; Thajuddin, N.; Alharbi, N.S.; Alharbi, S.A.; Ravi, G. In vitro antibacterial activity of ZnO and nd doped ZnO nanoparticles against esbl producing escherichia coli and klebsiella pneumoniae. Sci. Rep. 2016, 6, 24312. [Google Scholar] [CrossRef]
- Kasinathan, K.; Kennedy, J.; Elayaperumal, M.; Henini, M.; Malik, M. Photodegradation of organic pollutants rhb dye using uv simulated sunlight on ceria based TiO2 nanomaterials for antibacterial applications. Sci. Rep. 2016, 6, 38064. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Zhan, S.; Jia, Y.; Zhou, Q. Superior antibacterial activity of Fe3O4-TiO2 nanosheets under solar light. ACS Appl. Mater. Interfaces 2015, 7, 21875–21883. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Hu, X.; Stanislaus, M.S.; Zhang, N.; Xiao, R.; Liu, N.; Yang, Y. A novel P/Ag/Ag2O/Ag3PO4/TiO2 composite film for water purification and antibacterial application under solar light irradiation. Sci. Total Environ. 2017, 577, 236–244. [Google Scholar] [CrossRef] [PubMed]
- Grandcolas, M.; Ye, J.; Hanagata, N. Combination of photocatalytic and antibacterial effects of silver oxide loaded on titania nanotubes. Mater. Lett. 2011, 65, 236–239. [Google Scholar] [CrossRef]
- Sun, D.; Zhang, W.; Mou, Z.; Chen, Y.; Guo, F.; Yang, E.; Wang, W. Transcriptome analysis reveals silver nanoparticle-decorated quercetin antibacterial molecular mechanism. ACS Appl. Mater. Interfaces 2017, 9, 10047–10060. [Google Scholar] [CrossRef]
- Rajabi, A.; Ghazali, M.J.; Mahmoudi, E.; Azizkhani, S.; Sulaiman, N.H.; Mohammad, A.W.; Mustafah, N.M.; Ohnmar, H.; Naicker, A.S. Development and antibacterial application of nanocomposites: Effects of molar ratio on Ag2O–CuO nanocomposite synthesised via the microwave-assisted route. Ceram. Int. 2018, 44, 21591–21598. [Google Scholar] [CrossRef]
- Tripathi, S.; Mehrotra, G.K.; Dutta, P.K. Chitosan–silver oxide nanocomposite film: Preparation and antimicrobial activity. Bull. Mater. Sci. 2011, 34, 29–35. [Google Scholar] [CrossRef]
- Hu, Z.; Chan, W.L.; Szeto, Y.S. Nanocomposite of chitosan and silver oxide and its antibacterial property. J. Appl. Polym. Sci 2008, 108, 52–56. [Google Scholar] [CrossRef]
- Trang, V.T.; Tam, L.T.; Van Quy, N.; Huy, T.Q.; Thuy, N.T.; Tri, D.Q.; Cuong, N.D.; Tuan, P.A.; Van Tuan, H.; Le, A.-T.; et al. Functional iron oxide–silver hetero-nanocomposites: Controlled synthesis and antibacterial activity. J. Electron. Mater. 2017, 46, 3381–3389. [Google Scholar] [CrossRef]
- Sen, R.; Govindaraj, A.; Rao, C.N.R. Carbon nanotubes by the metallocene route. Chem. Phys. Lett. 1997, 267, 276–280. [Google Scholar] [CrossRef]
- Moreno, V.; Llorent-Martínez, E.J.; Zougagh, M.; Ríos, A. Decoration of multi-walled carbon nanotubes with metal nanoparticles in supercritical carbon dioxide medium as a novel approach for the modification of screen-printed electrodes. Talanta 2016, 161, 775–779. [Google Scholar] [CrossRef]
- Shi, L.; Zhang, G.; Wang, Y. Tailoring catalytic performance of carbon nanotubes confined CuO-CeO2 catalysts for CO preferential oxidation. Int. J. Hydrogen Energy 2018, 43, 18211–18219. [Google Scholar] [CrossRef]
- Obaidullah, I. Carbon nanotube membranes for water purification: Developments, challenges, and prospects for the future. Sep. Purif. Technol. 2019, 209, 307–337. [Google Scholar] [CrossRef]
- Jha, R.; Singh, A.; Sharma, P.K.; Fuloria, N.K. Smart carbon nanotubes for drug delivery system: A comprehensive study. J. Drug Deliv. Sci. Technol. 2020, 58, 101811. [Google Scholar] [CrossRef]
- Wang, P.; Gao, S.; Chen, X.; Yang, L.; Wu, X.; Feng, S.; Hu, X.; Liu, J.; Xu, P.; Ding, Y. Effect of hydroxyl and carboxyl-functionalized carbon nanotubes on phase morphology, mechanical and dielectric properties of poly(lactide)/poly(butylene adipate-co-terephthalate) composites. Int. J. Biol. Macromol. 2022, 206, 661–669. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, T.; Ding, W.; Kameta, N. Soft-matter nanotubes: A platform for diverse functions and applications. Chem. Rev. 2020, 120, 2347–2407. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Mauter, M.S.; Elimelech, M. Physicochemical determinants of multiwalled carbon nanotube bacterial cytotoxicity. Environ. Sci. Technol. 2008, 42, 7528–7534. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Qi, Y.; Zhai, H. Surface Characteristics and Electric Conductivity of Mwcnts/Feve Copolymer Composite Coatings; Springer International Publishing: Cham, Switzerland, 2016; pp. 2117–2122. [Google Scholar]
- Epp, J. 4—X-ray diffraction (xrd) techniques for materials characterization. In Materials Characterization Using Nondestructive Evaluation (Nde) Methods; Hübschen, G., Altpeter, I., Tschuncky, R., Herrmann, H.-G., Eds.; Woodenhead Publishing: Cambridge, UK, 2016; pp. 81–124. [Google Scholar]
- Ananth, A.; Mok, Y.S. Dielectric barrier discharge (DBD) plasma assisted synthesis of Ag2O nanomaterials and Ag2O/RuO2 nanocomposites. Nanomaterials 2016, 6, 42. [Google Scholar] [CrossRef]
- Sarode, V.B.; Patil, R.D.; Chaudhari, G.E. Characterization of functionalized multi-walled carbon nanotubes. Mater. Today Proc. 2023. [CrossRef]
- Ravichandran, S.; Paluri, V.; Kumar, G.; Loganathan, K.; Kokati Venkata, B.R. A novel approach for the biosynthesis of silver oxide nanoparticles using aqueous leaf extract of callistemon lanceolatus (myrtaceae) and their therapeutic potential. J. Exp. Nanosci. 2016, 11, 445–458. [Google Scholar] [CrossRef]
- Lucacel, R.C.; Marcus, C.; Timar, V.; Ardelean, I.I. Ft-ir and raman spectroscopic studies on B2O3-PbO-Ag2O glasses dopped with manganese ions. Solid State Sci. 2007, 9, 850–854. [Google Scholar] [CrossRef]
- Hootifard, G.; Sheikhhosseini, E.; Ahmadi, S.A.; Yahyazadehfar, M. Synthesis and characterization of CO-MOF@Ag2O nanocomposite and its application as a nano-organic catalyst for one-pot synthesis of pyrazolopyranopyrimidines. Sci. Rep. 2023, 13, 17500. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Chen, S.; Zhang, K.; Gao, N.; Zhang, M.; Albasher, G.; Shi, J.; Wang, C. The interaction of Ag2O nanoparticles with Escherichia coli: Inhibition–sterilization process. Sci. Rep. 2021, 11, 1703. [Google Scholar] [CrossRef]
- Çakır, Ü.; Kestel, F.; Kızılduman, B.K.; Bicil, Z.; Doğan, M. Multi walled carbon nanotubes functionalized by hydroxyl and schiff base and their hydrogen storage properties. Diam. Relat. Mater. 2021, 120, 108604. [Google Scholar] [CrossRef]
- Liu, H.; Li, C.; Sun, X.S. Soy-oil-based waterborne polyurethane improved wet strength of soy protein adhesives on wooden. Int. J. Adhes. Adhes. 2017, 73, 66–74. [Google Scholar] [CrossRef]
- Fang, Y.; Qin, L.; Zhao, W.; Qin, B.; Zhang, X.; Wu, X. Research progress and development trend on corrosion resistant fluorocarbon paint. J. Chin. Soc. Corros. Prot. 2016, 36, 97–106. [Google Scholar]
- Shi, Y.; Wang, G. Influence of molecular weight of peg on thermal and fire protection properties of pepa-containing polyether flame retardants with high water solubility. Prog. Org. Coat. 2016, 90, 390–398. [Google Scholar] [CrossRef]
- Mo, M.; Zhao, W.; Chen, Z.; Yu, Q.; Zeng, Z.; Wu, X.; Xue, Q. Excellent tribological and anti-corrosion performance of polyurethane composite coatings reinforced with functionalized graphene and graphene oxide nanosheets. RSC Adv. 2015, 5, 56486–56497. [Google Scholar] [CrossRef]
- Li, M.; Liu, Q.; Jia, Z.; Xu, X.; Cheng, Y.; Zheng, Y.; Xi, T.; Wei, S. Graphene oxide/hydroxyapatite composite coatings fabricated by electrophoretic nanotechnology for biological applications. Carbon 2014, 67, 185–197. [Google Scholar] [CrossRef]
- Mišković-Stanković, V.; Jevremović, I.; Jung, I.; Rhee, K. Electrochemical study of corrosion behavior of graphene coatings on copper and aluminum in a chloride solution. Carbon 2014, 75, 335–344. [Google Scholar] [CrossRef]
- Alahmadi, N.S.; Betts, J.W.; Cheng, F.; Francesconi, M.G.; Kelly, S.M.; Kornherr, A.; Prior, T.J.; Wadhawan, J.D. Synthesis and antibacterial effects of cobalt–cellulose magnetic nanocomposites. RSC Adv. 2017, 7, 20020–20026. [Google Scholar] [CrossRef]

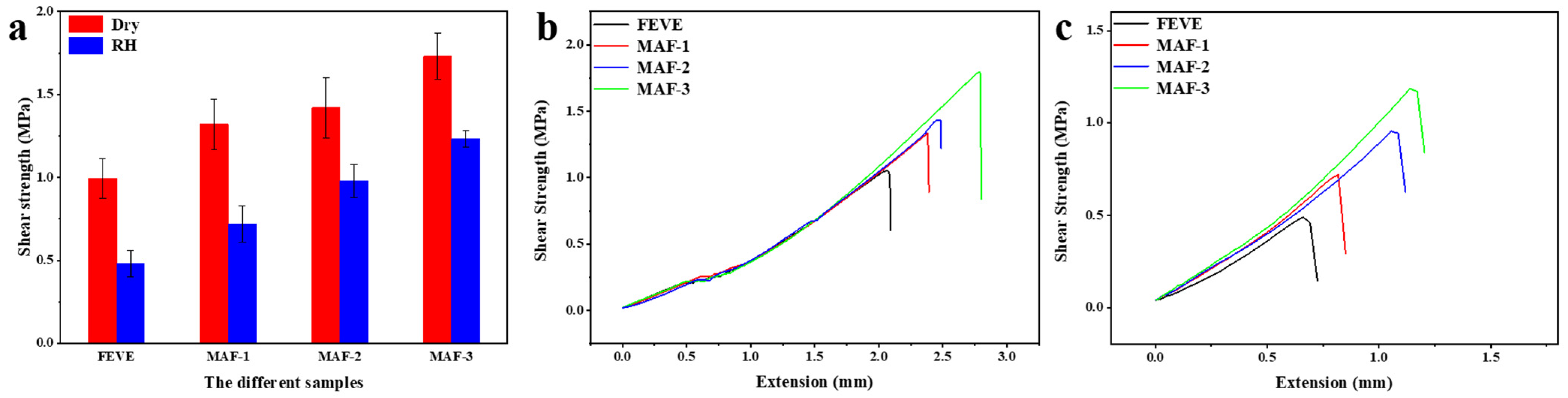
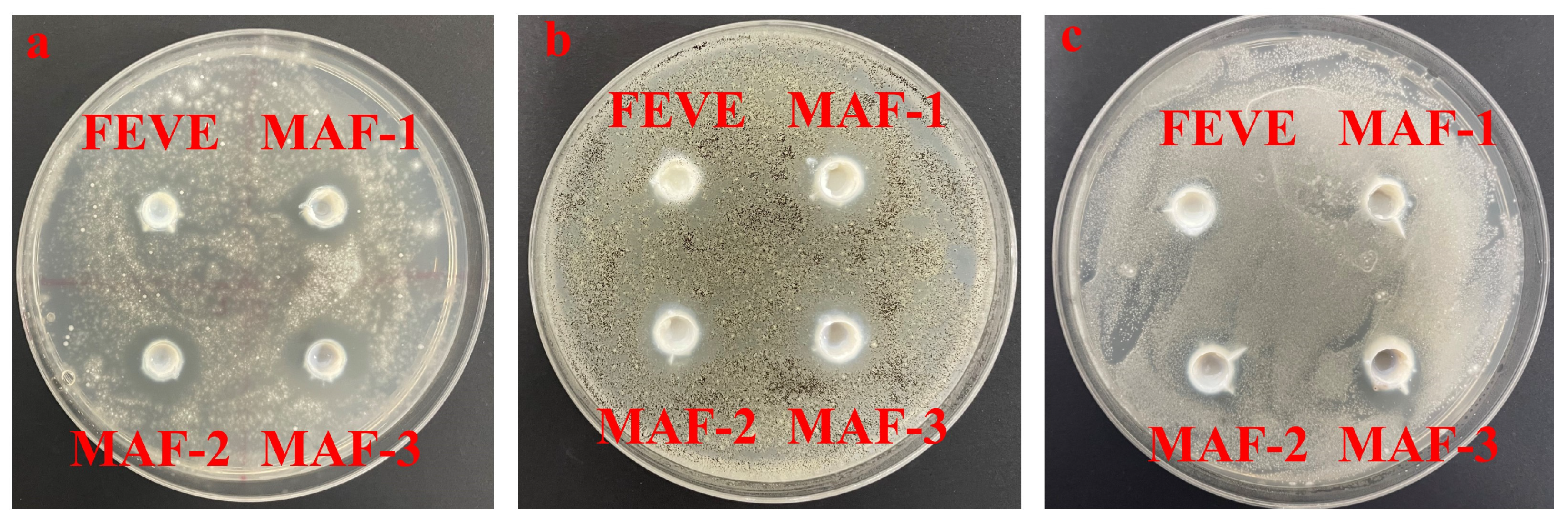
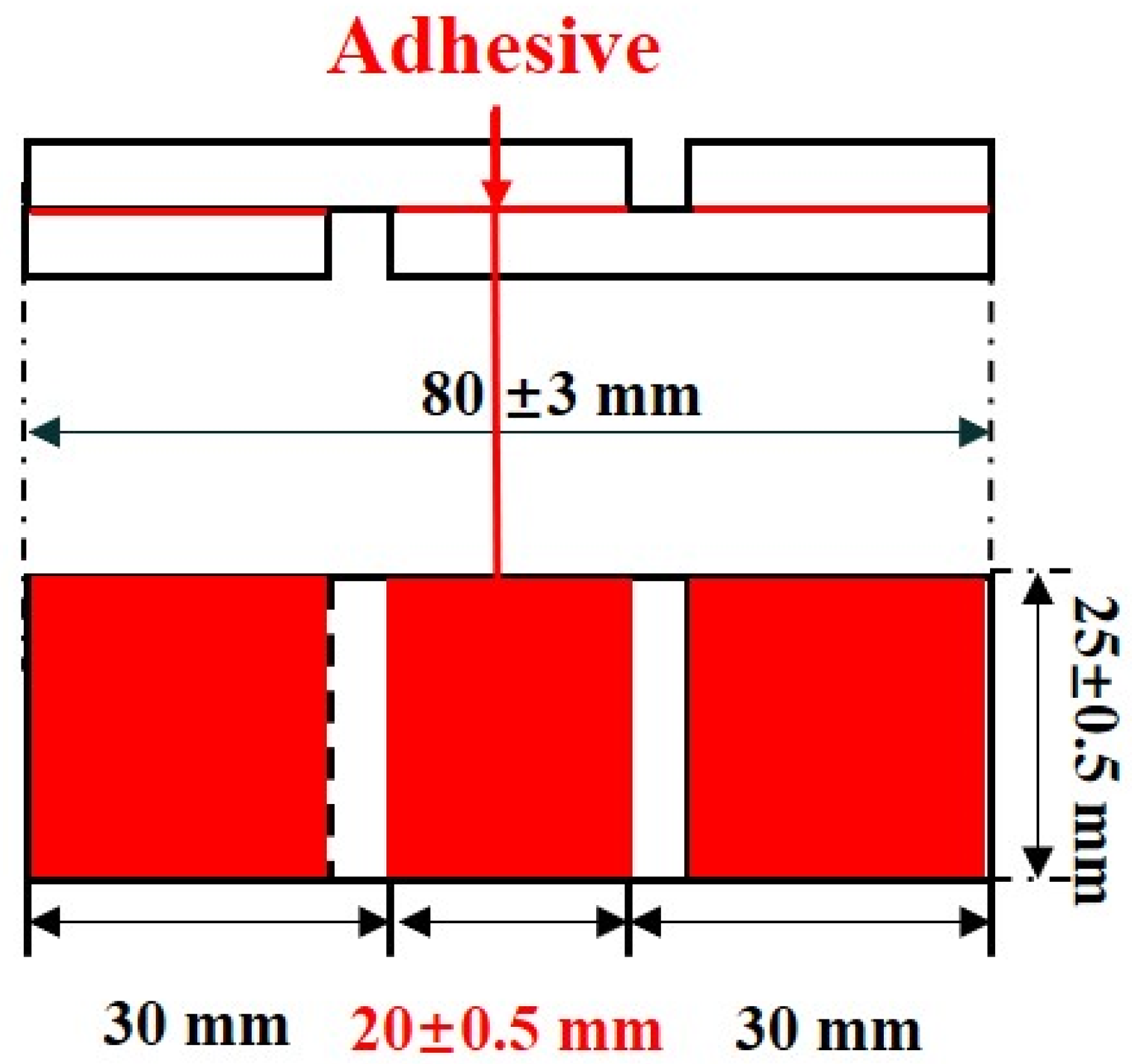

| Adhesives | FEVE | MAF-1 | MAF-2 | MAF-3 |
|---|---|---|---|---|
| Dry strength (MPa) | 0.99 | 1.32 | 1.42 | 1.73 |
| RH strength (MPa) | 0.48 | 0.72 | 0.98 | 1.23 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teri, G.; Cheng, C.; Han, K.; Huang, D.; Li, J.; Luo, Y.; Fu, P.; Li, Y. Study on the Properties of FEVE Modified with Ag2O/OH-MWCNTS Nanocomposites for Use as Adhesives for Wooden Heritage Objects. Molecules 2024, 29, 1365. https://doi.org/10.3390/molecules29061365
Teri G, Cheng C, Han K, Huang D, Li J, Luo Y, Fu P, Li Y. Study on the Properties of FEVE Modified with Ag2O/OH-MWCNTS Nanocomposites for Use as Adhesives for Wooden Heritage Objects. Molecules. 2024; 29(6):1365. https://doi.org/10.3390/molecules29061365
Chicago/Turabian StyleTeri, Gele, Cong Cheng, Kezhu Han, Dan Huang, Jing Li, Yujia Luo, Peng Fu, and Yuhu Li. 2024. "Study on the Properties of FEVE Modified with Ag2O/OH-MWCNTS Nanocomposites for Use as Adhesives for Wooden Heritage Objects" Molecules 29, no. 6: 1365. https://doi.org/10.3390/molecules29061365
APA StyleTeri, G., Cheng, C., Han, K., Huang, D., Li, J., Luo, Y., Fu, P., & Li, Y. (2024). Study on the Properties of FEVE Modified with Ag2O/OH-MWCNTS Nanocomposites for Use as Adhesives for Wooden Heritage Objects. Molecules, 29(6), 1365. https://doi.org/10.3390/molecules29061365








