Steroidal Saponins from Water Eggplant (Fruits of Solanum torvum) Exhibit Anti-Epileptic Activity against Pentylenetetrazole-Induced Seizure Model in Zebrafish
Abstract
1. Introduction
2. Results
2.1. Structural Elucidation
2.1.1. Torvoside U (1)
2.1.2. Torvoside V (2)
2.1.3. Torvoside W (3)
2.1.4. Torvoside X (4)
2.1.5. Torvoside Y (5)
2.1.6. Torvoside Z (6)
2.2. Biological Activity Results
2.2.1. Screening of Anti-Epileptic Activity of the Compounds
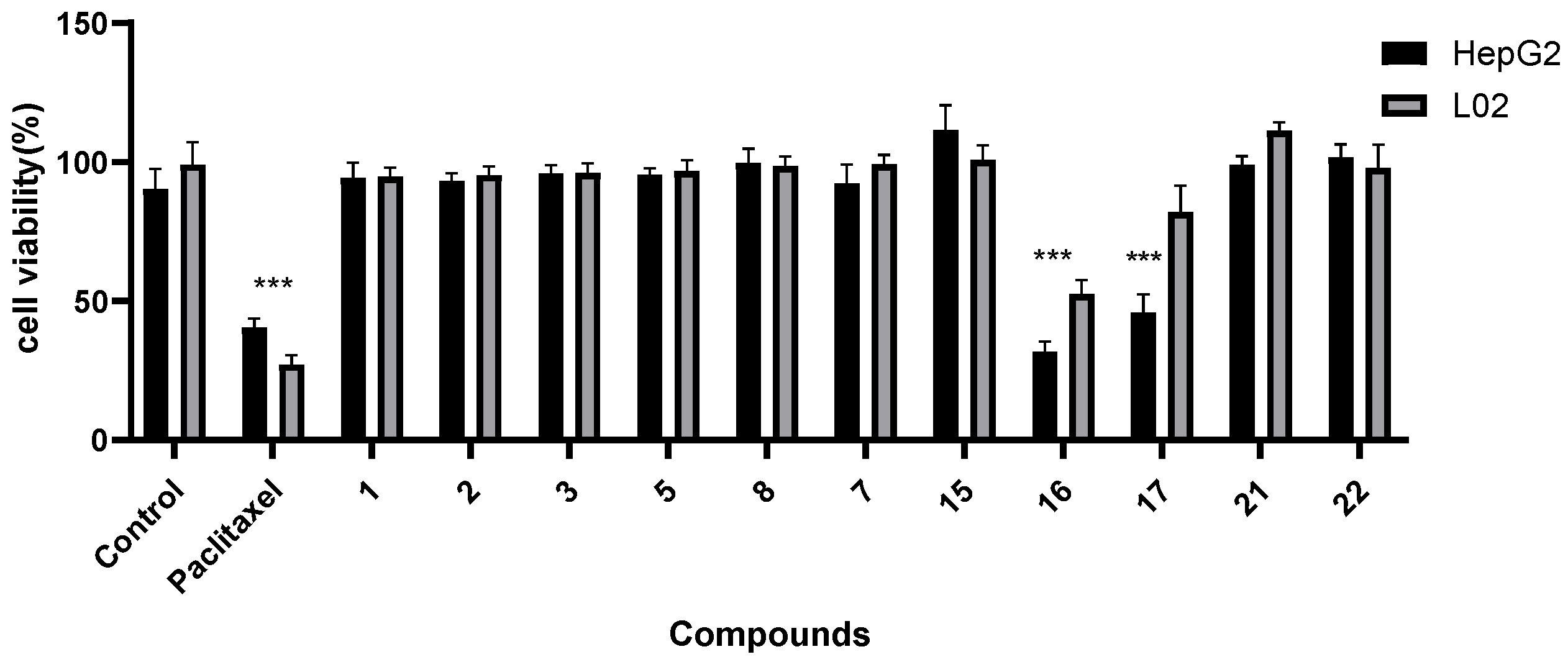
2.2.2. Screening for Anti-Liver-Cancer Activity
3. Discussion
4. Materials and Methods
4.1. Equipment
4.2. Chemicals and Reagents
4.3. Plant Material
4.4. Extraction and Isolation
4.5. Bioactivity Assays
4.5.1. Anti-Epileptic Activity Studies
4.5.2. Screening for Hepatotoxicity
4.6. Statistical Analysis
4.7. Animal Ethics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chah, K.F.; Muko, K.N.; Oboegbulem, S.I. Antimicrobial activity of methanolic extract of Solanum torvum fruit. Fitoterapia 2000, 71, 187–189. [Google Scholar] [CrossRef]
- Arthan, D.; Svasti, J.; Kittakoop, P.; Pittayakhachonwut, D.; Tanticharoen, M.; Thebtaranonth, Y. Antiviral isoflavonoid sulfate and steroidal glycosides from the fruits of Solanum torvum. Phytochemistry 2002, 59, 459–463. [Google Scholar] [CrossRef]
- Lu, Y.; Luo, J.; Huang, X.; Kong, L. Four new steroidal glycosides from Solanum torvum and their cytotoxic activities. Steroids 2009, 74, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Arthan, D.; Kittakoop, P.; Esen, A.; Svasti, J. Furostanol glycoside 26-o-β-glucosidase from the leaves of Solanum torvum. Phytochemistry 2006, 67, 27–33. [Google Scholar] [CrossRef]
- Yahara, S.; Yamashita, T.; Fujimura, N.N.N.; Nohara, T. Steroidal glycosides from Solanum torvum. Phytochemistry 1996, 43, 1069–1074. [Google Scholar] [CrossRef]
- Colmenares, A.P.; Rojas, L.B.; Mitaine-Offer, A.; Pouységu, L.; Quideau, S.; Miyamoto, T.; Tanaka, C.; Paululat, T.; Usubillaga, A.; Lacaille-Dubois, M. Steroidal saponins from the fruits of Solanum torvum. Phytochemistry 2013, 86, 137–143. [Google Scholar] [CrossRef]
- Challal, S.; Buenafe, O.E.; Queiroz, E.F.; Maljevic, S.; Marcourt, L.; Bock, M.; Kloeti, W.; Dayrit, F.M.; Harvey, A.L.; Lerche, H.; et al. Zebrafish bioassay-guided microfractionation identifies anticonvulsant steroid glycosides from the philippine medicinal plant Solanum torvum. ACS Chem. Neurosci. 2014, 5, 993–1004. [Google Scholar] [CrossRef]
- Sani, S.; Lawal, B.; Ejeje, J.N.; Aliu, T.B.; Onikanni, A.S.; Uchewa, O.O.; Ovoh, J.C.; Ekpa, F.U.; Ozoagu, C.D.; Akuma, T.S.; et al. Biochemical and tissue physiopathological evaluation of the preclinical efficacy of Solanum torvum swartz leaves for treating oxidative impairment in rats administered a β-cell-toxicant (stz). Biomed. Pharmacother. 2022, 154, 113605. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, G.R.; Ignacimuthu, S.; Paulraj, M.G.; Sasikumar, P. Antihyperglycemic activity and antidiabetic effect of methyl caffeate isolated from Solanum torvum swartz. Fruit in streptozotocin induced diabetic rats. Eur. J. Pharmacol. 2011, 670, 623–631. [Google Scholar] [CrossRef] [PubMed]
- Mohan, M.; Jaiswal, B.S.; Kasture, S. Effect of Solanum torvum on blood pressure and metabolic alterations in fructose hypertensive rats. J. Ethnopharmacol. 2009, 126, 86–89. [Google Scholar] [CrossRef]
- Sultana, N.; Afolayan, A.J. A novel daucosterol derivative and antibacterial activity of compounds from arctotis arctotoides. Nat. Prod. Res. 2007, 21, 889–896. [Google Scholar] [CrossRef]
- Lu, Y.; Luo, J.; Kong, L. Structure elucidation and complete nmr spectral assignments of new furostanol glycosides from Solanum torvum. Magn. Reson. Chem. 2009, 47, 808–812. [Google Scholar] [CrossRef]
- Patel, D.C.; Tewari, B.P.; Chaunsali, L.; Sontheimer, H. Neuron-glia interactions in the pathophysiology of epilepsy. Nat. Rev. Neurosci. 2019, 20, 282–297. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Cao, M.; Peng, Y.; Dong, B.; Jiang, Y.; Hu, C.; Zhu, P.; Xing, W.; Yu, L.; Xu, R.; et al. Research progress on the treatment of epilepsy with traditional chinese medicine. Phytomedicine 2023, 120, 155022. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Xu, M.; Zhang, Y.; Li, H.; Yang, C. Steroidal saponins from Disporopsis pernyi. Helv. Chim. Acta 2004, 87, 1248–1253. [Google Scholar] [CrossRef]
- Vil’, V.A.; Dos Passos Gomes, G.; Bityukov, O.V.; Lyssenko, K.A.; Nikishin, G.I.; Alabugin, I.V.; Terent’Ev, A.O. Interrupted baeyer–villiger rearrangement: Building a stereoelectronic trap for the criegee intermediate. Angew. Chem. Int. Ed. 2018, 57, 3372–3376. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Hwang, T.; Yang, J.; Cheng, H.; He, W.; Yen, C.; Kuo, C.; Chen, C.; Chang, W.; Wu, Y. Anti-inflammatory spirostanol and furostanol saponins from Solanum macaonense. J. Nat. Prod. 2014, 77, 1770–1783. [Google Scholar] [CrossRef]
- Qin, X.J.; Lunga, P.K.; Zhao, Y.L.; Liu, Y.P.; Luo, X.D. Chemical constituents of Solanum coagulans and their antimicrobial activities. Chin. J. Nat. Med. 2016, 14, 308–312. [Google Scholar] [CrossRef]
- Chou, C.; Hsu, Y.; Huang, T.; Liu, F.; Weng, J. Sterodial sapogenins from Solanum torvum. Biochem. Syst. Ecol. 2012, 45, 108–110. [Google Scholar] [CrossRef]
- Lee, C.; Hwang, T.; He, W.; Tsai, Y.; Yen, C.; Yen, H.; Chen, C.; Chang, W.; Wu, Y. Anti-neutrophilic inflammatory steroidal glycosides from Solanum torvum. Phytochemistry 2013, 95, 315–321. [Google Scholar] [CrossRef]
- Iid, Y.; Yanai, Y.; Ono, M.; Ikeda, T.; Nohara, T. Three unusual 22-b-o-23-hydroxy-(5a)-spirostanol glycosides from the fruits of Solanum torvum. Chem. Pharm. Bull. 2005, 53, 1122–1125. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chang, L.; Tsai, T.; Wang, J.; Lin, C.; Kuo, K. The rhamnose moiety of solamargine plays a crucial role in triggering cell death by apoptosis. Biochem. Biophys. Res. Commun. 1998, 242, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Kozukue, N.; Han, J.; Park, J.; Chang, E.; Baek, E.; Chang, J.; Friedman, M. Glycoalkaloids and metabolites inhibit the growth of human colon (ht29) and liver (hepg2) cancer cells. J. Agric. Food. Chem. 2004, 52, 2832–2839. [Google Scholar] [CrossRef] [PubMed]

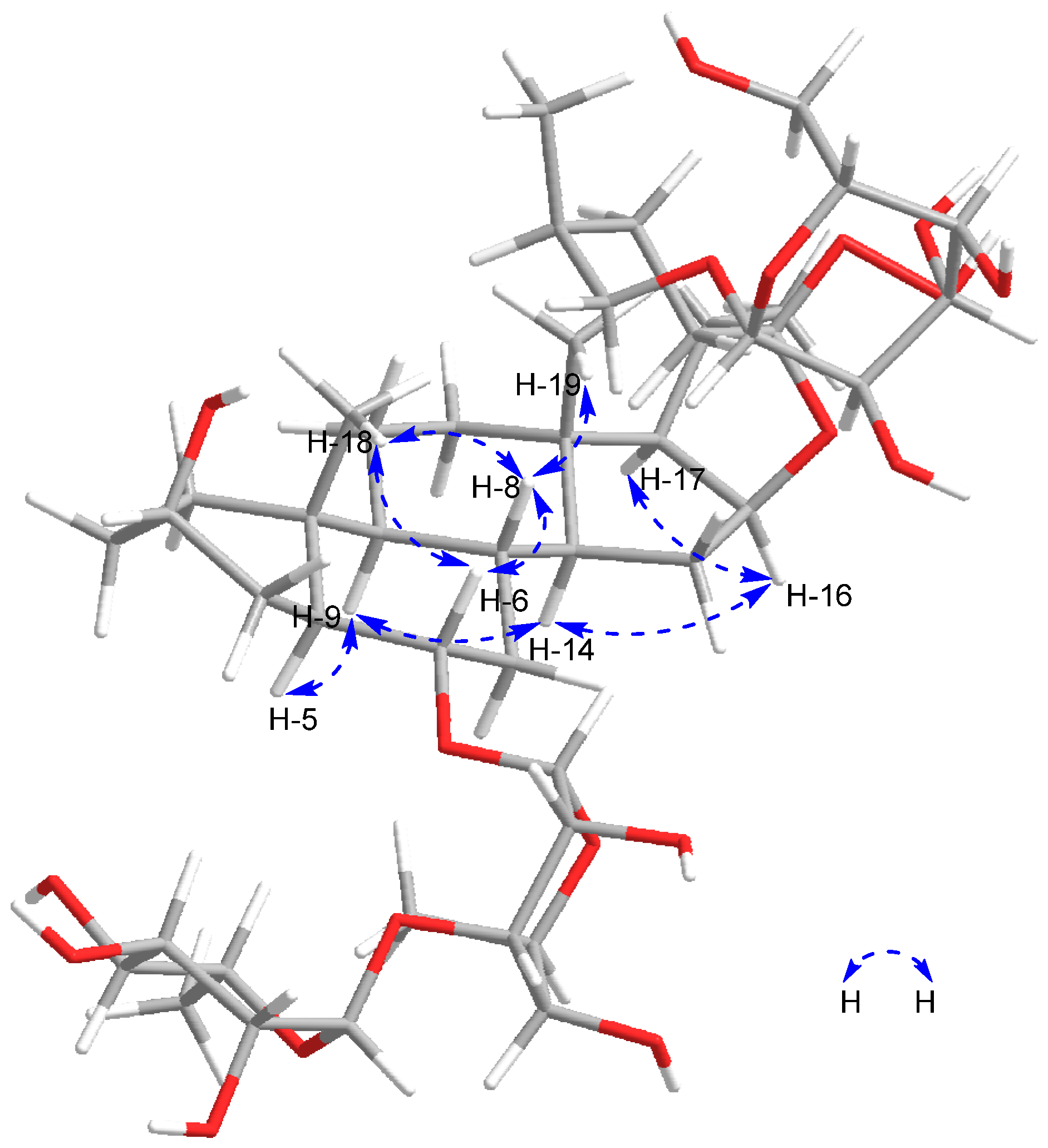
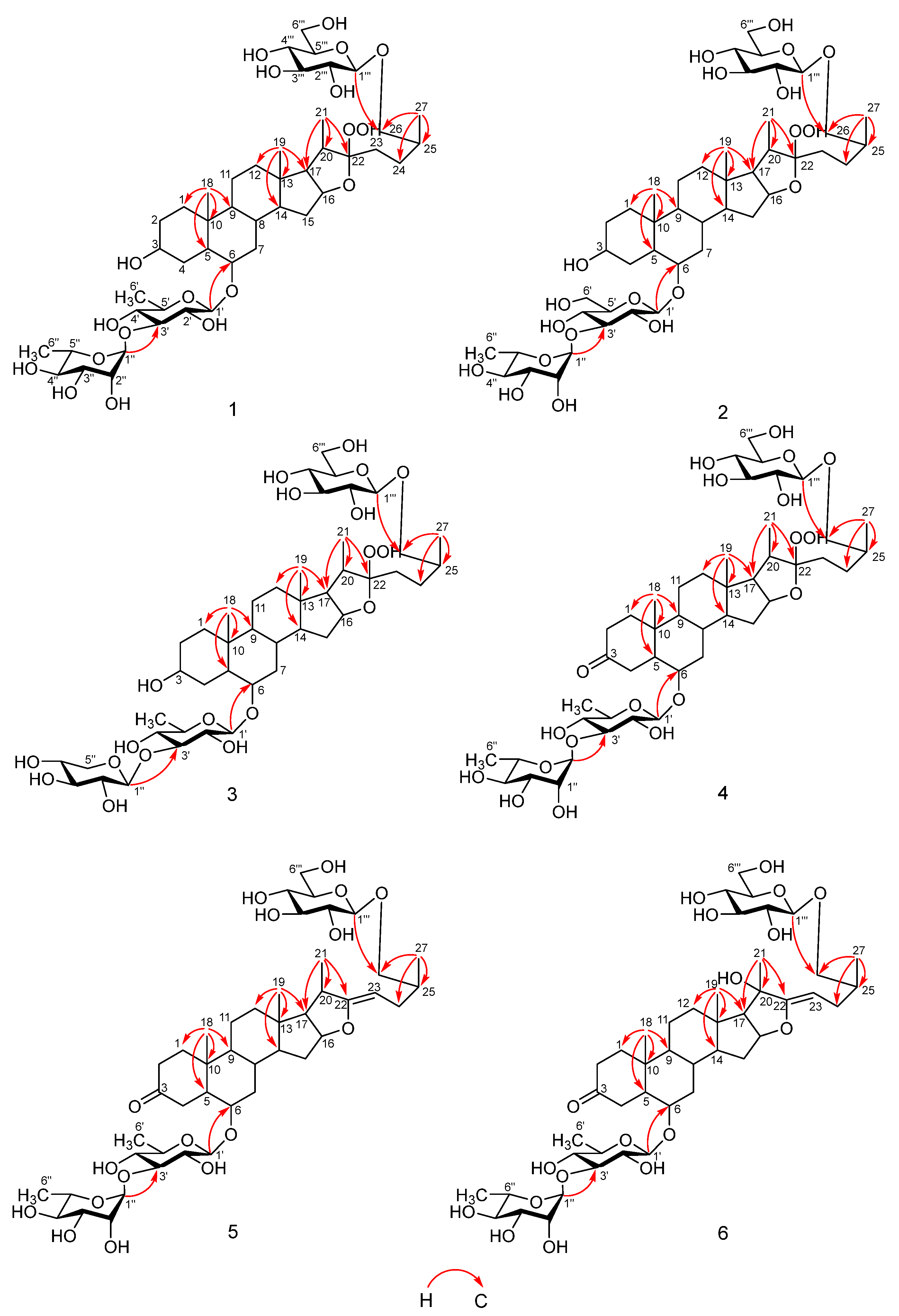
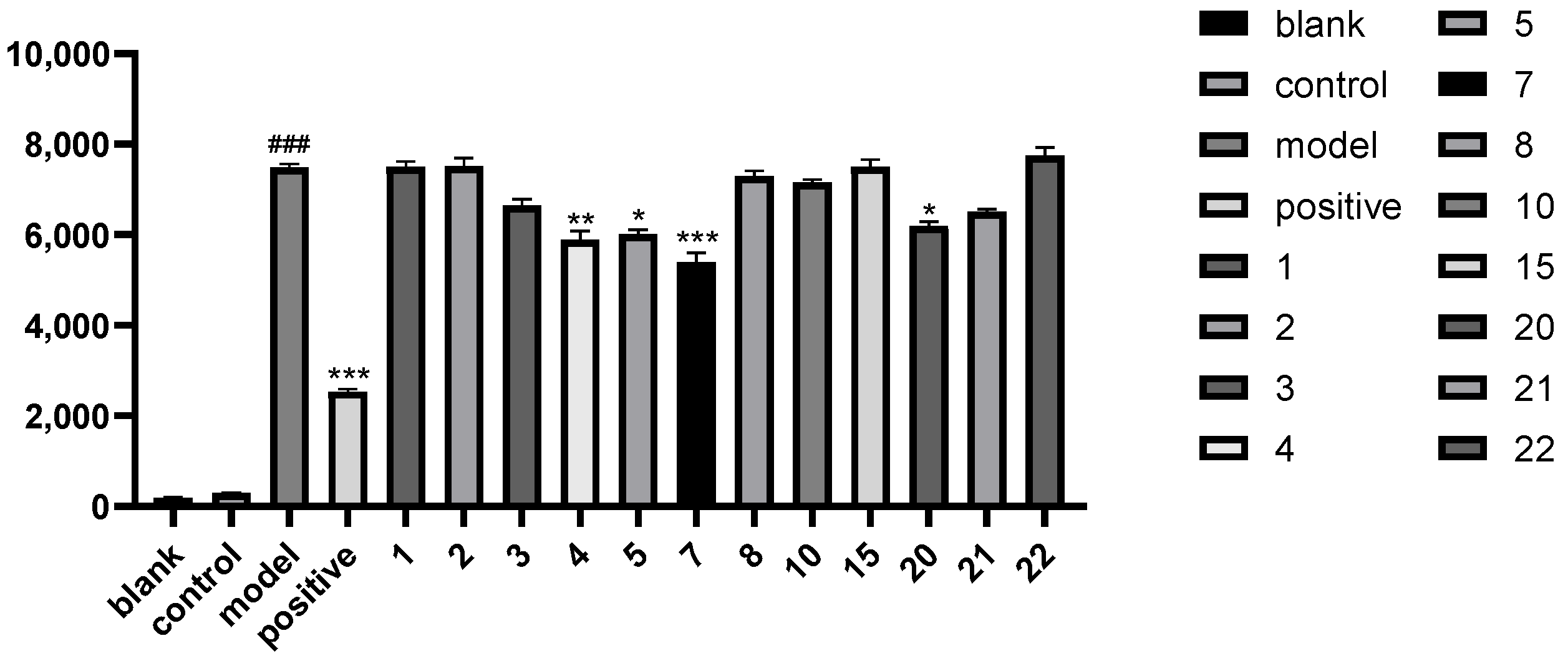
| No. | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|
| 1 | 0.88 m | 0.88 m | 1.16 m | 0.91 m | 1.15 m | 1.14 m |
| 1.59 m a | 1.58 m a | 1.77 m | 1.60 m a | 1.77 m | 1.77 m | |
| 2 | 1.34 m | 1.24 m | 2.27 m | 1.37 m | 2.29 m | 2.28 m |
| 1.90 m | 1.80 m | 2.41 m | 1.91 m | 2.39 m | 2.39 m | |
| 3 | 3.70 m a | 3.72 m | / | 3.81 m | / | / |
| 4 | 1.64 m | 1.66 m | 2.44 m | 1.72 m | 2.44 m | 2.45 m |
| 3.18 dd (14.5, 15.0) | 3.21 m | 3.55 m | 3.25 dd (14.1, 14.5) | 3.56 m a | 3.58 m | |
| 5 | 1.26 m | 1.25 m | 1.57 m a | 1.39 m | 1.59 m a | 1.58 m a |
| 6 | 3.66 m | 3.70 m | 3.69 m | 3.74 m a | 3.74 m | 3.70 m |
| 7 | 1.11 m | 1.06 m | 1.14 m | 1.17 m | 1.16 m | 1.08 m |
| 2.45 m | 2.45 m | 2.44 m | 2.43 m a | 2.45 m | 2.42 m | |
| 8 | 1.60 m a | 1.51 m a | 1.62 m | 1.58 m | 1.60 m a | 1.59 m a |
| 9 | 0.54 m | 0.53 m | 0.56 m | 0.57 m | 0.56 m | 0.51 m |
| 10 | / | / | / | / | / | / |
| 11 | 1.17 dd (3.9, 12.2) | 1.17 dd (3.7, 4.2) | 1.27 dd (7.2, 8.0) | 1.22 dd (5.8, 6.1) | 1.25 m | 1.24 m |
| 1.41 m | 1.41 m | 1.39 m | 1.43 m | 1.39 m | 1.39 m | |
| 12 | 0.98 br d (10.4) | 0.99 d (4.6) | 1.02 d (8.1) | 1.01 m | 1.11 d (6.2) | 1.13 d (6.6) |
| 1.65 m | 1.64 m | 1.66 m | 1.65 m | 1.78 m | 1.74 m | |
| 13 | / | / | / | / | / | / |
| 14 | 0.95 m | 0.86 m | 0.94 m | 0.94 m | 0.93 m | 0.87 m |
| 15 | 1.71 m | 1.75 m | 1.39 m | 1.77 m a | 1.45 m | 1.37 m |
| 2.02 m | 2.03 m a | 1.90 m | 2.05 m | 2.05 m | 1.98 m | |
| 16 | 4.77 m | 4.73 m | 4.81 m | 4.80 m | 5.18 m | 4.90 m a |
| 17 | 1.80 m | 1.76 m | 1.81 m | 1.80 m | 2.22 m | 2.03 m |
| 18 | 0.81 s | 0.82 s | 0.98 s | 0.81 s | 1.03 s | 0.99 s |
| 19 | 0.80 s | 0.77 s | 0.83 s | 0.80 s | 0.89 s | 0.82 s |
| 20 | 2.39 m | 2.37 m a | 2.40 m | 2.41 m a | 2.43 m | / |
| 21 | 1.25 d (6.0) | 1.23 d (7.0) | 1.25 d (7.1) | 1.25 d (6.8) | 1.72 d (5.6) | 1.36 s |
| 22 | / | / | / | / | / | / |
| 23 | 2.24 m | 2.22 m | 1.31 m | 1.26 m | 4.55 t (6.9) | 4.34 m |
| 2.33 m | 2.31 m a | 1.28 m | 1.32 m | |||
| 24 | 1.57 m | 1.55 m | 1.58 m | 1.57 m a | 2.14 m | 2.20 m |
| 2.04 m | 2.04 m a | 2.05 m | 2.04 m | 2.56 m | 2.52 m | |
| 25 | 1.96 m | 1.96 m | 1.96 m | 1.96 m | 2.15 m | 2.14 m |
| 26 | 3.53 m | 3.52 m | 3.53 m | 3.51 m | 3.54 m a | 3.58 m |
| 4.16 m | 4.15 m | 4.17 m | 4.16 m | 4.22 m | 4.21 m | |
| 27 | 1.10 d (5.6) | 1.10 d (6.5) | 1.10 d (6.7) | 1.10 d (6.5) | 1.12 d (5.8) | 1.13 d (6.2) |
| 6-O-Qui | 6-O-Glc | 6-O-Qui | 6-O-Qui | 6-O-Qui | 6-O-Qui | |
| 1′ | 4.80 d (6.0) | 4.85 d (6.0) | 4.71 d (7.7) | 4.86 d (7.1) | 4.74 d (7.7) | 4.71 d (7.8) |
| 2′ | 4.04 dd (3.9, 4.2) | 4.07 m | 4.02 m | 4.08 m | 4.03 dd (8.7, 8.9) | 4.01 m |
| 3′ | 4.29 t (9.0) a | 4.41 m | 4.28 m a | 4.12 m | 4.28 m a | 4.26 m a |
| 4′ | 3.62 t (4.6) | 5.16 m | 3.64 t (9.4) | 3.64 t (9.1) | 3.62 t (9.5) | 3.64 m |
| 5′ | 4.64 m | 3.90 dd (9.2, 9.5) | 4.65 m | 4.64 m | 4.64 m | 4.62 m |
| 6′ | 1.73 d (6.0) | 4.58 m | 1.65 d (6.0) | 1.60 d (5.8) a | 1.64 d (6.1) | 1.62 d (5.9) |
| 4.48 m | ||||||
| Rha | Rha | Rha | Xyl | Rha | Rha | |
| 1″ | 6.36 br s | 6.39 br s | 6.32 br s | 5.30 d (7.4) | 6.32 br s | 6.30 br s |
| 2″ | 3.76 m a | 4.65 m | 3.76 m | 4.29 m | 3.76 m | 3.74 m |
| 3″ | 4.85 dd (4.5, 4.8) | 4.87 dd (7.8, 8.0) | 4.86 dd (3.9, 9.0) | 4.22 m a | 4.87 dd (7.8, 8.0) | 4.85 m |
| 4″ | 4.39 t (4.8) | 4.39 t (5.0) | 4.40 t (9.5) | 4.22 m a | 4.38 t (9.3) | 4.38 t (9.6) |
| 5″ | 5.07 m | 5.06 m | 5.05 m | 3.77 m | 5.06 m | 5.03 m |
| 4.34 m | ||||||
| 6″ | 1.64 d (6.2) | 1.72 d (6.5) | 1.72 d (6.2) | 1.73 d (5.6) a | 1.71 d (6.0) | |
| 26-O-Glc | 26-O-Glc | 26-O-Glc | 26-O-Glc | 26-O-Glc | 26-O-Glc | |
| 1‴ | 4.86 d (7.6) | 4.88 d (7.7) | 4.87 d (7.7) | 4.87 d (7.6) | 4.89 d (7.7) | 4.92 d (7.7) |
| 2‴ | 4.06 m | 4.08 m | 4.08 m | 4.10 m | 4.09 m | 4.09 m |
| 3‴ | 4.29 m a | 4.28 m a | 4.28 m a | 4.29 m a | 4.29 m a | 4.28 m a |
| 4‴ | 4.29 m a | 4.30 m a | 4.29 m a | 4.30 m a | 4.31 m a | 4.29 m a |
| 5‴ | 3.98 m | 3.98 m | 3.99 m | 3.99 m | 3.98 m | 3.98 m |
| 6‴ | 4.58 dd (4.6, 11.6) | 4.60 dd (4.0, 11.7) | 4.59 dd (4.0, 9.4) | 4.44 dd (4.9, 11.8) | 4.40 dd (9.7, 11.3) | 4.43 dd (6.0, 11.6) |
| 4.44 dd (5.9, 11.6) | 4.43 dd (4.8, 11.7) | 4.41 dd (5.4, 9.4) | 4.59 dd (4.5, 11.8) | 4.59 dd (9.4, 11.3) | 4.58 dd (5.1, 11.6) |
| No. | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|
| 1 | 38.0 (CH2) | 38.0 (CH2) | 38.9 (CH2) | 38.0 (CH2) | 39.0 (CH2) | 38.9 (CH2) |
| 2 | 32.7 (CH2) | 32.6 (CH2) | 38.4 (CH2) | 32.6 (CH2) | 38.4 (CH2) | 38.4 (CH2) |
| 3 | 70.9 (CH) | 70.9 (CH) | 211.1 (C) | 70.9 (CH) | 211.1 (C) | 211.1 (C) |
| 4 | 33.5 (CH2) | 33.5 (CH2) | 39.9 (CH2) | 33.6 (CH2) | 40.2 (CH2) | 40.1 (CH2) |
| 5 | 51.7 (C) | 51.7 (C) | 52.8 (C) | 51.7 (C) | 52.8 (C) | 52.8 (C) |
| 6 | 79.4 (CH) | 79.8 (CH) | 80.0 (CH) | 79.4 (CH) | 80.2 (CH) | 80.1 (CH) |
| 7 | 41.7 (CH2) | 41.6 (CH2) | 41.3 (CH2) | 41.7 (CH2) | 41.1 (CH2) | 41.1 (CH2) |
| 8 | 34.4 (CH) | 34.3 (CH) | 34.2 (CH) | 34.4 (CH) | 33.7 (CH) | 33.6 (CH) |
| 9 | 54.1 (CH) | 54.1 (CH) | 53.4 (CH) | 54.1 (CH) | 53.2 (CH) | 53.2 (CH) |
| 10 | 37.0 (C) | 37.0 (C) | 37.0 (C) | 37.0 (C) | 37.1 (C) | 37.1 (C) |
| 11 | 21.5 (CH2) | 21.4 (CH2) | 21.5 (CH2) | 21.5 (CH2) | 21.1 (CH2) | 21.1 (CH2) |
| 12 | 40.1 (CH2) | 40.1 (CH2) | 40.1 (CH2) | 40.1 (CH2) | 39.5 (CH2) | 39.4 (CH2) |
| 13 | 41.6 (C) | 41.6 (C) | 41.6 (C) | 41.6 (C) | 40.9 (C) | 40.8 (C) |
| 14 | 56.4 (CH) | 56.4 (CH) | 56.1 (CH) | 56.3 (CH) | 56.7 (CH) | 56.5 (CH) |
| 15 | 32.5 (CH2) | 32.5 (CH2) | 32.6 (CH2) | 32.5 (CH2) | 33.6 (CH2) | 33.5 (CH2) |
| 16 | 82.6 (CH) | 82.6 (CH) | 82.6 (CH) | 82.6 (CH) | 84.4 (CH) | 84.1 (CH) |
| 17 | 64.1 (CH) | 64.3 (CH) | 64.3 (CH) | 64.4 (CH) | 68.1 (CH) | 67.0 (CH) |
| 18 | 13.9 (CH3) | 13.8 (CH3) | 12.8 (CH3) | 13.9 (CH3) | 12.9 (CH3) | 12.8 (CH3) |
| 19 | 16.7 (CH3) | 16.7 (CH3) | 16.7 (CH3) | 16.7 (CH3) | 14.0 (CH3) | 14.1 (CH3) |
| 20 | 40.4 (CH) | 40.4 (CH) | 40.4 (CH) | 40.4 (CH) | 40.8 (CH) | 82.6 (C) |
| 21 | 16.2 (CH3) | 16.2 (CH3) | 16.2 (CH3) | 16.2 (CH3) | 22.2 (CH3) | 15.5 (CH3) |
| 22 | 117.6 (C) | 117.6 (C) | 117.6 (C) | 117.6 (C) | 164.0 (C) | 157.5 (C) |
| 23 | 32.3 (CH2) | 32.2 (CH2) | 30.3 (CH2) | 30.3 (CH2) | 91.6 (CH) | 96.5 (CH) |
| 24 | 28.7 (CH2) | 28.7 (CH2) | 28.7 (CH2) | 28.7 (CH2) | 30.0 (CH2) | 30.0 (CH2) |
| 25 | 34.9 (CH) | 34.9 (CH) | 34.9 (CH) | 34.9 (CH) | 35.2 (CH) | 35.2 (CH) |
| 26 | 75.6 (CH2) | 75.6 (CH2) | 75.6 (CH2) | 75.6 (CH2) | 75.8 (CH2) | 75.8 (CH2) |
| 27 | 17.9 (CH3) | 17.9 (CH3) | 17.9 (CH3) | 17.9 (CH3) | 17.9 (CH3) | 17.9 (CH3) |
| 1′ | 105.9(CH) | 106.2 (CH) | 105.9 (CH) | 105.6 (CH) | 106.0 (CH) | 106.1 (CH) |
| 2′ | 76.7 (CH) | 76.6 (CH) | 76.4 (CH) | 75.1 (CH) | 77.0 (CH) | 76.4 (CH) |
| 3′ | 83.3 (CH) | 83.5 (CH) | 83.8 (CH) | 87.9 (CH) | 83.8(CH) | 83.8 (CH) |
| 4′ | 75.6 (CH) | 70.2 (CH) | 75.5 (CH) | 75.3 (CH) | 76.3 (CH) | 75.5 (CH) |
| 5′ | 73.0 (CH) | 78.4 (CH) | 73.1 (CH) | 72.7 (CH) | 74.5 (CH) | 73.1 (CH) |
| 6′ | 19.1 (CH3) | 62.8 (CH2) | 19.0 (CH3) | 19.0 (CH3) | 19.0 (CH3) | 19.2 (CH3) |
| 1″ | 103.4 (CH) | 103.3 (CH) | 103.6 (CH) | 106.9 (CH) | 103.6 (CH) | 103.6 (CH) |
| 2″ | 73.1 (CH) | 73.1 (CH) | 73.1 (CH) | 75.6 (CH) | 73.1 (CH) | 73.1 (CH) |
| 3″ | 73.0 (CH) | 73.0 (CH) | 72.9 (CH) | 78.7 (CH) | 72.9 (CH) | 72.9 (CH) |
| 4″ | 74.5 (CH) | 74.5 (CH) | 74.5 (CH) | 71.3 (CH) | 75.5 (CH) | 74.5 (CH) |
| 5″ | 70.2 (CH) | 69.9 (CH) | 70.3 (CH) | 67.8 (CH2) | 70.3 (CH) | 70.3 (CH) |
| 6″ | 19.1 (CH3) | 19.1 (CH3) | 19.1 (CH3) | / | 19.2 (CH3) | 19.0 (CH3) |
| 1‴ | 105.6 (CH) | 105.6 (CH) | 105.6 (CH) | 105.5 (CH) | 105.5 (CH) | 105.6(CH) |
| 2‴ | 75.5 (CH) | 75.6 (CH) | 75.6 (CH) | 75.8 (CH) | 75.6 (CH) | 75.7 (CH) |
| 3‴ | 78.9 (CH) | 78.9 (CH) | 78.9 (CH) | 78.9 (CH) | 78.9 (CH) | 78.9 (CH) |
| 4‴ | 71.9 (CH) | 71.9 (CH) | 71.9 (CH) | 71.9 (CH) | 71.9 (CH) | 71.9 (CH) |
| 5‴ | 78.9 (CH) | 78.9 (CH) | 78.9 (CH) | 78.9 (CH) | 79.0 (CH) | 79.0 (CH) |
| 6‴ | 63.1 (CH2) | 63.1 (CH2) | 63.1 (CH2) | 63.1 (CH2) | 63.0 (CH2) | 63.1 (CH2) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, R.; Zhang, M.-y.; Shu, T.; Kong, Y.-t.; Su, L.-h.; Li, H.-z. Steroidal Saponins from Water Eggplant (Fruits of Solanum torvum) Exhibit Anti-Epileptic Activity against Pentylenetetrazole-Induced Seizure Model in Zebrafish. Molecules 2024, 29, 1316. https://doi.org/10.3390/molecules29061316
Ren R, Zhang M-y, Shu T, Kong Y-t, Su L-h, Li H-z. Steroidal Saponins from Water Eggplant (Fruits of Solanum torvum) Exhibit Anti-Epileptic Activity against Pentylenetetrazole-Induced Seizure Model in Zebrafish. Molecules. 2024; 29(6):1316. https://doi.org/10.3390/molecules29061316
Chicago/Turabian StyleRen, Rui, Ming-yan Zhang, Tengyun Shu, Ya-ting Kong, Li-hua Su, and Hai-zhou Li. 2024. "Steroidal Saponins from Water Eggplant (Fruits of Solanum torvum) Exhibit Anti-Epileptic Activity against Pentylenetetrazole-Induced Seizure Model in Zebrafish" Molecules 29, no. 6: 1316. https://doi.org/10.3390/molecules29061316
APA StyleRen, R., Zhang, M.-y., Shu, T., Kong, Y.-t., Su, L.-h., & Li, H.-z. (2024). Steroidal Saponins from Water Eggplant (Fruits of Solanum torvum) Exhibit Anti-Epileptic Activity against Pentylenetetrazole-Induced Seizure Model in Zebrafish. Molecules, 29(6), 1316. https://doi.org/10.3390/molecules29061316







