Abstract
Gas hydrates, a type of inclusion compound capable of trapping gas molecules within a lattice structure composed of water molecules, are gaining attention as an environmentally benign gas storage or separation platform. In general, the formation of gas hydrates from water requires high-pressure and low-temperature conditions, resulting in significant energy consumption. In this study, tetrabutylammonium fluoride (TBAF) was utilized as a thermodynamic promoter forming a semi-clathrate-type hydrate, enabling gas capture or separation at room temperature. Those TBAF hydrate systems were explored to check their capability of CO2 separation from flue gas, the mixture of CO2 and N2 gases. The formation rates and gas storage capacities of TBAF hydrates were systematically investigated under various concentrations of CO2, and they presented selective CO2 capture behavior during the hydrate formation process. The maximum gas storage capacities were achieved at 2.36 and 2.38 mmol/mol for TBAF·29.7 H2O and TBAF·32.8 H2O hydrate, respectively, after the complete enclathration of the feed gas of CO2 (80%) + N2 (20%). This study provides sufficient data to support the feasibility of TBAF hydrate systems to be applied to CO2 separation from CO2/N2 gas mixtures based on their CO2 selectivity.
1. Introduction
The steady increase in greenhouse gas emissions worldwide has highlighted various environmental issues, including climate change [1]. The atmospheric concentration of CO2 exceeded 415 ppm in 2022 and is expected to continue rising in the coming years [2]. Consequently, to address the severity of global warming and reduce emissions of carbon dioxide, a 2050 carbon neutrality scenario has been proposed. In order to achieve this goal, research on carbon capture, utilization, and storage (CCUS) is actively underway [3]. Among this, the carbon dioxide capture process incurs the highest cost, necessitating the development of economically viable and efficient capture technology. One of the recently notable technologies is the process of separating mixed gases and extracting desired gases through hydrate formation, known as hydrate-based gas separation (HBGS) [4,5,6].
Clathrate hydrates, crystalline structures formed through water molecule hydrogen bonding, possess the ability to encapsulate low-molecular-weight gas molecules within their lattice framework [7]. They can effectively capture various gases, and their high gas storage capacity per unit volume makes them efficient gas storage media. Particularly notable is their non-explosive nature due to being composed of water. Research has extensively explored the hydrate-based stable storage of energy gases like natural gas [8,9], hydrogen [10,11,12], and their mixtures [13,14,15,16,17], as well as greenhouse gases such as carbon dioxide [18,19,20,21] and nitrous oxide [22,23,24]. In particular, based on the gas capture capability of clathrate hydrates, hydrate-based CO2 capture (HBCC), which separates and stores carbon dioxide in hydrate media, has garnered most attention. HBCC offers the advantages of environmental friendliness and high selectivity for carbon dioxide due to its thermodynamically stable characteristics in hydrate [25]. Additionally, HBCC can be designed as a continuous process suitable for large-scale capture and enables cost-effective carbon dioxide treatment. However, traditional hydrate-based technologies typically demand low-temperature, high-pressure conditions, resulting in substantial energy consumption. To address this limitation, this study explores the utilization of ionic species as thermodynamic promoters, enabling hydrate stability even at ambient temperatures.
As previously discussed, pure gas hydrates containing gas species exclusively within the hydrate medium typically necessitate high-pressure and low-temperature conditions to sustain their structural integrity. Consequently, to enhance the feasibility of hydrate-based gas storage techniques, researchers have identified and implemented various thermodynamic promoters. The three most prominent structures of clathrate hydrates are Structure I (sI, comprising two small cages (512) and six large cages (51262)), Structure II (sII, characterized by sixteen small cages (512) and eight large cages (51264)), and Structure H (sH, featuring three small cages (512), two medium cages (435663), and one large cage (51268)). These thermodynamic promoter molecules typically occupy the large cages of sII (51264) or sH (51268) hydrates, stabilizing the overall structure and enabling secure storage of small gaseous guest molecules within the small cages of sII (512), or the small and medium cages of sH (512, 435663) hydrates under relatively lower-pressure and higher-temperature conditions. Similarly, certain ionic species can stabilize hydrate structures, leading to the formation of various types of semi-clathrate hydrates. Semi-clathrate hydrates represent unique structures capable of capturing large cations or anions within confined cages, with counterions being incorporated into the hydrate lattice. Typically, the size of the captured ionic species exceeds that of a single large cage of sI (51262) or sII (51264) hydrates, necessitating the partial breaking of cages to accommodate the larger ions. Due to ionic interactions between the captured species in confined cages and the incorporated counterions within the hydrate lattice, some ionic semi-clathrate hydrates exhibit exceptional thermodynamic stability, facilitating the capture of gaseous guest molecules under higher-temperature and lower-pressure conditions [26,27]. This property underscores their potential utility in gas storage and separation applications.
In this study, TBAF hydrates were employed to facilitate the formation of hydrates from mixed gases containing various ratios of carbon dioxide. This study conducted experiments under conditions not previously explored and also confirmed results from a structural change perspective [28,29]. Initially, we investigated the phase equilibrium conditions of TBAF hydrates with different compositions of gas mixtures. Once the temperature and pressure conditions conducive to the formation of TBAF + gas hydrates were confirmed, we conducted an analysis of their structure. Semi-clathrate hydrates exhibit three distinct crystal structures: tetragonal structure-I (TS-I) [10(512)·16(51262)·4(51263)·172 H2O], cubic superstructure-I (CSS-I) [2(512)·6(51262)·46 H2O], and hexagonal structure-I (HS-I) [3(512)·2(51262)·2(51263)·40 H2O] [30]. Since the hydration number determines the crystal structure and chemical properties of ionic hydrates, it was imperative to identify the structures of TBAF·29.7 H2O and TBAF·32.8 H2O hydrates. Subsequently, we investigated the gas uptake behaviors of TBAF hydrates during the hydrate formation process. The findings of this study yield valuable insights for implementing an energy-efficient carbon dioxide capture and separation process based on semi-clathrate hydrates.
2. Results and Discussion
2.1. Phase Equilibria of a Semi-Clathrate Hydrate with a Secondary Gaseous Guest
The phase equilibria of TBAF-enhanced systems consisting of TBAF·29.7/32.8 H2O + CO2 (20, 50, 80%) + balanced N2 + water were measured using the customized experimental setup (Figure 1), and the detailed procedures are described in Section 4. As depicted in Figure 2, the addition of TBAF had a significant impact on the equilibrium conditions of the gas hydrate system (the detailed temperature and pressure values for each point are summarized in Table 1). Notably, the presence of TBAF caused a notable shift in the equilibrium curve of hydrate formation toward higher temperatures and lower pressures compared to hydrates formed without promoters. This shift implies that under equivalent pressure conditions, hydrate formation becomes achievable at elevated temperatures, and under equivalent temperature conditions, it becomes feasible at reduced pressures. The utilization of TBAF as a thermodynamic promoter resulted in a shift of the equilibrium curve to temperatures, exceeding 30 K higher than that of mixed gas hydrates, thereby enabling the stability of the structure even at ambient conditions. Additionally, akin to hydrates formed without promoters, an increase in the proportion of CO2 in the gas mixture led to a rightward shift in the equilibrium curve. This consistent effect across all mixed gases suggests that TBAF exhibited reliable performance. Furthermore, it was observed that there was no significant discrepancy in the equilibrium curve shifts between TBAF·29.7 H2O and TBAF·32.8 H2O hydrates. Despite the differences in hydration numbers, the thermodynamic promotion effects of TBAF hydrates remained nearly identical, indicating similar crystalline structures and secondary gaseous guest enclathration within the hydrate lattice for both semi-clathrate hydrates.
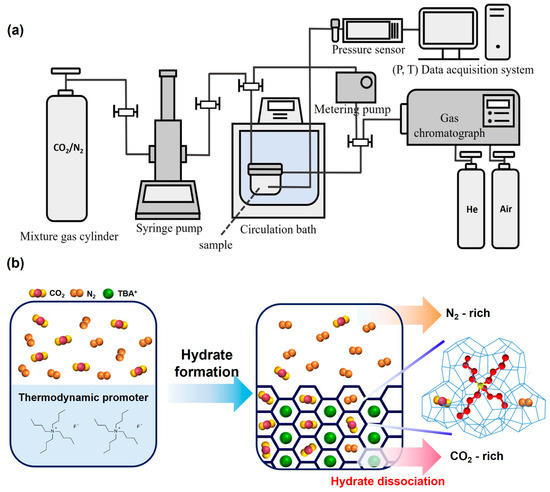
Figure 1.
(a) Schematic diagram of the overall semi-clathrate hydrate formation system. (b) Schematic procedure of TBAF hydrate-based gas separation.
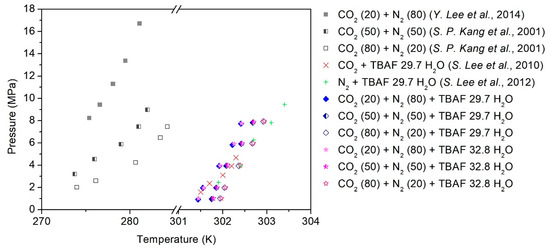
Figure 2.
Phase equilibria of the TBAF + CO2 (20, 50, 80%) + balanced N2 hydrates [31,32,33,34].

Table 1.
Phase equilibrium data for the TBAF + CO2 + N2 systems.
2.2. Spectroscopic Analysis: Structural Identification and Guest Enclathration Behavior
2.2.1. PXRD Analysis of Semi-Clathrate Hydrates
Understanding the structure of gas hydrates is crucial for accurately predicting and assessing their gas storage capabilities, particularly in applications such as gas separation and storage. In our study, we employed PXRD analysis to delve into the structural characteristics of semi-clathrate hydrates formed under varying compositions of CO2 in the feed gas. As depicted in Figure 3a, the TBAF·29.7 H2O hydrate devoid of secondary gaseous guests exhibited the anticipated cubic structure of I-43d, a well-known configuration. However, upon the introduction of CO2 and N2 molecules as secondary guests, the semi-clathrate hydrate with an I-43d space group transitioned into a tetragonal structure of P42/m, as illustrated in Figure 3b–e. In contrast, the tetragonal structure of TBAF·32.8 H2O hydrate remained unchanged even under gas pressurization conditions, as depicted in Figure 3f–j. These findings were consistent with the phase equilibria data of TBAF·29.7/32.8 H2O + CO2 (20, 50, 80%) + balanced N2 + water (Figure 2), indicating that despite the differences in hydration numbers, both systems originated from the same crystalline structure. The cubic I-43d structure features two small cages (512 cages) per unit cell, suitable for accommodating secondary gaseous guests. In contrast, the tetragonal P42/m structure contains ten small cages, offering greater capacity for gaseous guest molecules within the hydrate lattice. Thus, in the presence of CO2 and N2, the semi-clathrate hydrate of TBAF·29.7 H2O favored the formation of the tetragonal P42/m structure to accommodate more gaseous guest molecules.
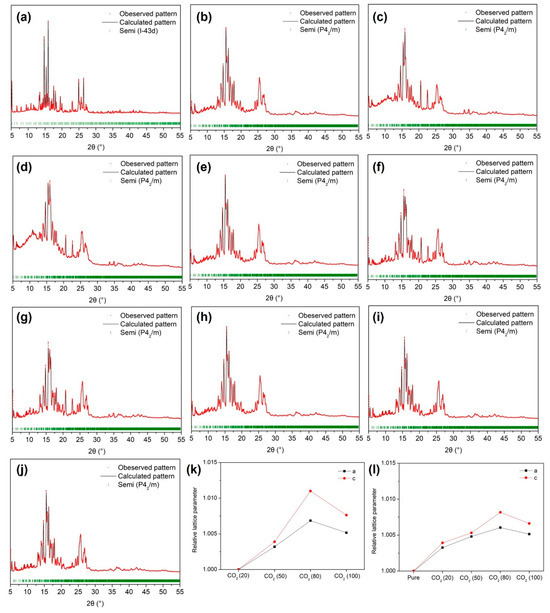
Figure 3.
PXRD patterns of the TBAF·29.7 H2O hydrates: (a) pure; (b) CO2 (20%) + N2 (80%), (c) CO2 (50%) + N2 (50%), (d) CO2 (80%) + N2 (20%), (e) CO2. PXRD patterns of the TBAF·32.8 H2O hydrates: (f) pure, (g), CO2 (20%) + N2 (80%), (h) CO2 (50%) + N2 (50%), (i) CO2 (80%) + N2 (20%) (j) CO2. Relative lattice parameter ratio as a function of CO2 composition in the mixed gas: (k) TBAF·29.7 H2O hydrate, (l) TBAF·32.8 H2O hydrate.
Following the identification of the crystalline structure of TBAF·29.7 H2O and TBAF·32.8 H2O hydrates, we investigated the lattice parameters obtained from PXRD patterns. Initially, considering the molecular sizes of CO2 and N2 [35], we anticipated that the lattice parameters would increase proportionally with the CO2 composition in the feed gas. However, contrary to expectations, the lattice parameters of both TBAF hydrates increased proportionally with CO2 composition up to 80%, but decreased when only CO2 was present (i.e., 100% CO2 in the feed gas), as shown in Figure 3k,l. To elucidate these results, Raman analysis was conducted.
2.2.2. Raman Analysis of Semi-Clathrate Hydrates
Raman spectroscopy was employed to validate the stable enclathration of secondary gaseous guest molecules within the lattice structures of semi-clathrate hydrates [33]. As depicted in Figure 4, confirmation of guest molecule capture in TBAF·29.7/32.8 H2O + CO2 (20, 50, 80%) + balanced N2 hydrate was obtained through comprehensive Raman spectroscopy analysis. In all semi-clathrate hydrates, discernible peaks corresponding to the C−C stretching mode of the tetrabutylammonium cation (TBA+) were observed within the range of 950 to 1400 cm−1, along with peaks attributed to the C−H bending mode from 1400 to 1500 cm−1, indicating the stable formation of TBAF hydrates. Additionally, the presence of CO2 and N2 within the small cages (512 cages) of semi-clathrate hydrates was evidenced by peaks observed at 1280 and 2324 cm−1, respectively.
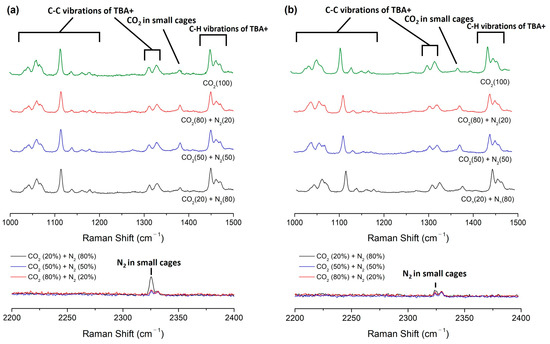
Figure 4.
Raman spectra of the TBAF + CO2 (20, 50, 80%) + balanced N2 hydrates. (a) CO2 and N2 captured in TBAF·29.7 H2O hydrates; (b) CO2 and N2 captured in TBAF·32.8 H2O hydrates.
To further elucidate the PXRD findings described earlier, normalization of Raman peak intensities of CO2 and N2 was performed using the peak at 1113 cm−1, corresponding to the C–C stretching mode of TBA+ (Table 2). This normalization process was essential due to the inherent variability in Raman intensity values across measurements. While exact quantitative values from Raman intensity may pose challenges, qualitative comparisons can be made. Notably, as the molar composition of CO2 to N2 in the feed gas increased, the ratio of Raman peak intensity of enclathrated CO2 to N2 molecule also showed a corresponding increase in all semi-clathrates. Interestingly, when CO2 gas was the sole constituent in the feed gas, the Raman peak intensity of enclathrated CO2 appeared almost identical compared to the hydrate formed from a feed gas composition of CO2 (80%) + N2 (20%). However, in the latter case, additional enclathrated N2 was observed within the hydrate lattice, resulting in a larger lattice parameter of the unit cell structure of semi-clathrate hydrates. This finding implies that the presence of N2 within the semi-clathrate hydrates contributes to an augmented gas storage capacity compared to scenarios where only CO2 is present.

Table 2.
Raman peak intensity of the guest molecules in semi-clathrate hydrates.
2.3. Semi-Clathrate Hydrate-Based Gas Capture and Separation
2.3.1. Gas Uptake during Hydrate Formation
To evaluate the viability of utilizing TBAF hydrates for gas capture and separation processes, we conducted experiments to measure gas storage capacities during hydrate formation from mixed gases. The formation of TBAF hydrates from CO2 and N2 mixed gas necessitates specific temperature and pressure conditions, which were established based on data obtained from equilibrium experiments (Figure 2). Experimental parameters were set at 40 bar and 299 K with a driving force of ∆T = 3 K. Gas composition inside the reactor was continuously monitored using GC during the hydrate formation experiments, and the number of moles of gases was calculated using the measured temperature and pressure inside the reactor, following the method outlined in Section 3.5. The change in moles of gas in the vapor phase was considered indicative of the moles of gas captured within the hydrate, and thus, the moles of gas captured within the hydrate were determined based on the moles of gas in the vapor phase.
Figure 5a,b illustrates the overall gas uptake curves of the TBAF + CO2 + N2 hydrates during hydrate formation over a period of 200 min. The attainment of equilibrium in gas uptake confirms that sufficient hydrate formation occurred within the specified time frame. Gas uptake was expressed as the ratio of consumed gas moles to initially charged water moles. We compared the differences in gas storage capacity based on the composition of mixed gases for the same hydrate, as well as variations in hydrate numbers due to adjustments in mixed gas composition. Following hydrate formation, the total gas storage capacities were measured for each gas mixture: CO2 (20%) + N2 (80%), CO2 (50%) + N2 (50%), and CO2 (80%) + N2 (20%). For the TBAF·29.7 H2O hydrate, the gas storage capacities were 2.25, 2.33, and 2.38 mmol/mol, respectively. Similarly, for TBAF·32.8 H2O hydrate, the gas storage capacities were 2.25, 2.34, and 2.38 mmol/mol, respectively. Comparing the two TBAF hydrates under the same gas composition revealed similar results. Moreover, this total amount of enclathrated gases corresponded to the tendencies of lattice parameter changes (Figure 3) and the Raman peak intensities of two TBAF hydrates under various mixed gas compositions (Figure 4 and Table 2).
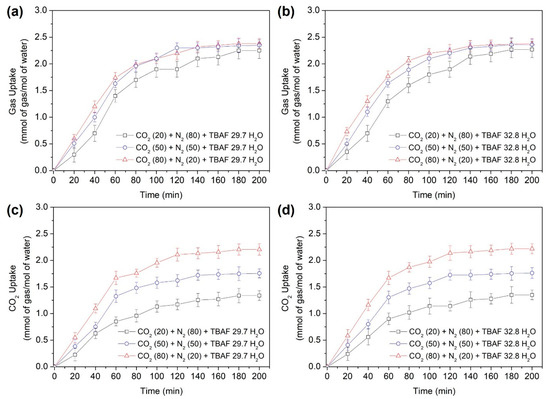
Figure 5.
Gas uptake of the semi-clathrate hydrates: (a) TBAF·29.7 H2O, (b) TBAF·32.8 H2O; CO2 uptake of the semi-clathrate hydrates: (c) TBAF·29.7 H2O, (d) TBAF·32.8 H2O.
Overall, as the CO2 composition in the injected mixed gas increased, the total gas uptake also increased, indicating that CO2 is more efficiently captured within the hydrate cages compared to N2. This trend is particularly evident from the CO2 uptake results illustrated in Figure 5c,d, where the amount of CO2 captured within the hydrate increases with a higher CO2 composition in the injected gas. Additionally, the storage capacities of the two TBAF hydrates were comparable. When CO2 (20%) + N2 (80%), CO2 (50%) + N2 (50%), and CO2 (80%) + N2 (20%) were injected, the CO2 storage after hydrate formation was 1.23, 1.53, and 2.19 mmol/mol, respectively, for the TBAF·29.7 H2O hydrate, and 1.23, 1.54, and 2.21 mmol/mol, respectively, for the TBAF·32.8 H2O hydrate. This highlights the higher proportion of CO2 stored within the hydrate compared to N2, resulting in the enhanced total gas storage capacity.
2.3.2. Separation Efficiency of Semi-Clathrate Hydrates-Based Gas Separation
In our quest to comprehend gas separation and storage utilizing semi-clathrate hydrates, we meticulously monitored the changes in gas composition and pressure within the reactor throughout the hydrate formation process. Figure 6 depicts the dynamic evolution of gas composition during hydrate formation from the initial mixed gas for each hydrate, while the gradual decrease in pressure over time attests to the stable capture of gas within the hydrate lattice. This phenomenon underscores the efficacy of gas capture within the hydrate matrix.
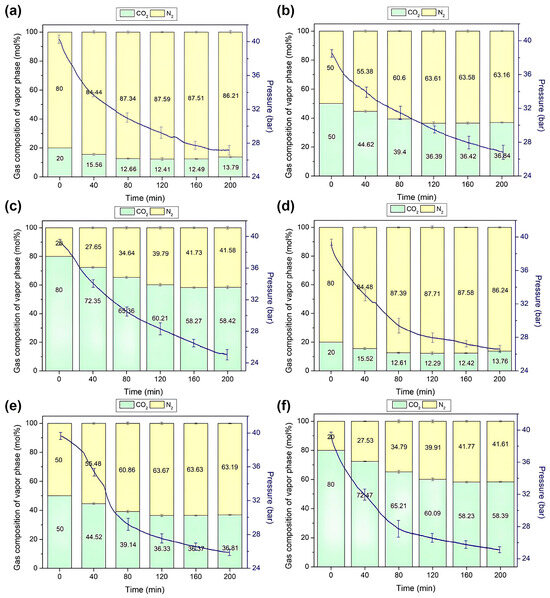
Figure 6.
Changes the pressure and gas composition in the vapor phase during the formation of TBAF·29.7 H2O hydrates: (a) CO2 (20%) + N2 (80%), (b) CO2 (50%) + N2 (50%), (c) CO2 (80%) + N2 (20%); TBAF·32.8 H2O hydrates: (d) CO2 (20%) + N2 (80%), (e) CO2 (50%) + N2 (50%), (f) CO2 (80%) + N2 (20%) over time.
Upon pressurizing the TBAF·29.7 H2O hydrate with a mixed gas containing 20% CO2, an intriguing sequence of events unfolded: initially, CO2 was stored within the hydrate lattice, causing a noticeable reduction in CO2 composition in the gas phase, as illustrated in Figure 6a. However, around the 160 min mark post-initiation of formation, a discernible uptick in N2 uptake appeared, leading to a subsequent increase in CO2 composition within the gas phase. Analogous trends were observed for other mixed gases (Figure 6b,c). As the proportion of CO2 in the mixed gas increased, the diminishment in CO2 concentration within the gas phase became more pronounced, yet over time, N2 depletion also became more conspicuous. This intricate interplay elucidates the differential capture kinetics of CO2 and N2 within the hydrate structure [36,37,38], ultimately impacting separation efficiency.
Similarly, TBAF·32.8 H2O hydrates exhibited a comparable phenomenon, with CO2 preferentially captured initially, followed by N2 (Figure 6d–f). Moreover, Figure 7 demonstrates the final CO2 concentrations in both the vapor and hydrate phases post-semi-clathrate hydrate formation. Across all compositions, it was revealed that the CO2 composition decreased in the vapor phase while concurrently augmenting within the hydrate phase. This disparity underscores the selective separation of CO2 from the injected gas via hydrate formation, with a conspicuous augmentation in separation efficiency as the CO2 proportion in the mixed gas increased.
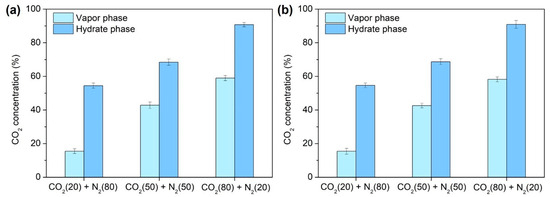
Figure 7.
CO2 concentration in the vapor phase and hydrate phase after the formation of semi-clathrate hydrates: (a) TBAF·29.7 H2O, (b) TBAF·32.8 H2O.
Further quantitative analysis yielded two pivotal parameters to gauge the separation efficiency of TBAF hydrates, as tabulated in Table 3. The separation factor presents the extent to which CO2 was sequestered relative to other gases in the initial injected gas, while CO2 recovery (%) quantifies the proportion of CO2 stored from the initial injected gas into the hydrate phase. Encouragingly, the calculation results unveiled highly favorable values for both parameters across various CO2 compositions in the mixed gas, with the two TBAF hydrates displaying strikingly similar values. Typically, a separation factor exceeding 10 is deemed substantial [39,40,41,42], underscoring the profound potential application of TBAF hydrates in gas separation and capture technologies, as substantiated by our findings.

Table 3.
Separation performance of the semi-clathrate hydrates.
3. Materials and Methods
3.1. Materials
Ultrahigh-purity deionized water was acquired from a Direct-Q3 water purification unit (Millipore, Burlington, MA, USA). The gas mixture of CO2 (20, 50, 80%) + balanced N2 used for this study was supplied by Seoul Specialty Gas Co. (Seoul, Republic of Korea). TBAF solution with a concentration of 75% in water was purchased from Sigma-Aldrich (St. Louis, MA, USA).
3.2. Phase Equilibrium Measurements of a Semi-Clathrate Hydrate
The conventional isochoric method was employed to determine the phase equilibrium conditions of TBAF·29.7 H2O and TBAF·32.8 H2O with CO2 (20, 50, 80%) + balanced N2 [43]. The prepared TBAF solutions having a hydration number of TBAF·29.7 H2O and TBAF·32.8 H2O were loaded into the stirring reactor used for phase equilibrium measurements. The reactor was placed in a circulating water bath (RW-2025G, Jeio Tech., Daejeon, Republic of Korea) capable of temperature control, and mixed gas was pressurized into the reactor to the desired pressure using a syringe pump. The internal temperature of the reactor was maintained at a temperature where hydrate formation does not occur, and air existing inside the cell was flushed out by continuous injection of feed gas for each sample. The stirrer started stirring at 100 rpm. Then, sufficient time was allowed for stabilization without changes in temperature and pressure before starting the temperature changes.
Once the equilibrium state was confirmed without temperature and pressure change, the temperature was gradually lowered to initiate and facilitate the hydrate formation process. After the sudden pressure drop due to the gas enclathration and the completion of the formation process, the temperature was increased very slowly at a rate of 0.1 K/h to provide a sufficient time for an equilibrated dissociation state at each temperature. Pressure and temperature changes during the hydrate formation and dissociation processes were measured using a pressure transducer (PSHB0250BCPG, Sensys Co., Ansan-si, Republic of Korea, full-scale accuracy of ±0.15%, 0–250 bar) and a four-wire type Pt-100 Ω probe (full-scale accuracy of ±0.05%, 13–923 K), respectively, and the data were monitored in real time using a DAQ system (LabVIEW). This data were then utilized to plot the formation-dissociation curve and identify the hydrate equilibrium point at each initial temperature and pressure condition. This procedure was repeated multiple times for each sample to determine the phase equilibrium line of TBAF·29.7 H2O and TBAF·32.8 H2O with various feed gas compositions.
3.3. Structure and Guest Enclathration Analysis of Semi-Clathrate Hydrates
The semi-clathrate hydrate (TBAF + CO2 + N2) samples formed at 40 bar were prepared for the PXRD and Raman spectroscopic analysis. Prior to retrieving the semi-clathrate hydrate powder samples, the reactor was rapidly cooled using liquid nitrogen to minimize sample damage during the recovery of samples. After a brief period, the reactor was depressurized, and the semi-clathrate hydrates were collected and finely ground to a 40 μm size using a sieve immersed in liquid nitrogen.
Powder X-ray diffraction (PXRD) was utilized to analyze the crystalline structure of semi-clathrate hydrates. The PXRD data were collected at the supramolecular crystallography beamline (2D) facility located at the Pohang Accelerator Laboratory (PAL) in the Republic of Korea. A synchrotron radiation source with a wavelength of 0.900 Å and an ADSC Quantum 210 CCD diffractometer (Pohang, Republic of Korea) were employed for data acquisition. The sample powder, stored in liquid nitrogen, was promptly transferred into pre-cooled polyimide tubing (Cole-Parmer, Vernon Hills, IL, USA) to minimize potential sample damage, and the experiments were conducted at approximately 93 K. The crystal structures of the samples were determined using the Le Bail fitting method, which involves profile matching implemented in the FullProf Program (version 2.00).
Raman spectroscopy was employed to determine the guest enclathration within semi-clathrate hydrates. The Raman spectra of the semi-clathrate hydrates were obtained using a dispersive Raman spectrometer (ARAMIS, Horiba Jobin Yvon Inc., Palaiseau, France), with a 514.53 nm Ar ion laser serving as the excitation source. The scattered light was dispersed by the 1800 grating of the spectrometer and detected by a charge-coupled device (CCD) with electrical cooling (203 K). The sample temperature was maintained at 93 K using a THMSG 600 unit (Linkam Scientific, Redhill, UK).
3.4. Gas Composition Analysis
The prepared 100 mL of TBAF solution was injected into the stirring reactor with a volume of 200 mL. Subsequently, the reactor was placed in a circulating water bath. The solution was stirred at a speed of 100 rpm, and mixed gas was pressurized into the reactor to conduct the hydrate formation experiment. The conditions for hydrate formation were set at a pressure of 40 bar, with the formation temperature determined to be 299 K, corresponding to a driving force of ∆T = 3 K at the specified pressure.
To analyze the gas composition inside the hydrate, real-time gas composition in the reactor was measured using a gas chromatograph (GC) (YL6500 GC, Young Lin Instrument Co, Anyang, Republic of Korea) during the hydrate formation process. Gas composition in the reactor was analyzed at 20 min intervals for 200 min, from the pressurization of mixed gas into the reactor to the completion of hydrate formation. To minimize pressure loss due to GC measurements, a metering pump (Eldex, Napa, CA, USA) was used. The constructed hydrate formation system is illustrated in Figure 1.
3.5. Quantitative Analysis of Gas Separation Performance
The gas storage capacity within the hydrate was calculated using the gas composition obtained from experiments of semi-clathrate hydrate formation conducted with an in situ GC system. Initially, the critical temperature, critical pressure, and acentric factor of the entire mixed gas were determined by multiplying the factors of each component obtained from the gas composition of the vapor phase analyzed by GC.
TC and PC are the critical temperature and pressure, respectively, and is the acentric factor. These are used to determine the reduced temperature and pressure of the entire mixed gas, as shown below:
and present the reduced temperature and pressure, respectively. T and P present the temperature and pressure obtained through experiment, respectively. We calculated by putting the previously obtained parameters into Pitzer’s correlation.
and are the virial coefficients and presents the compressibility factor. and are variables related to the correlation for compressibility factors. We used the calculated values to determine the total gas storage capacity.
presents the moles of gas trapped inside the hydrate up to time t. R is the universal gas constant, and is the volume of the gas (mL). , , , and present the moles of gas (mol), temperature (K), pressure (bar), and compressibility factor at time t, respectively. , , , and present the initial moles of gas (mol), temperature (K), pressure (bar), and compressibility factor of the feed gas, respectively. We used the obtained moles of gas from the above calculation to determine the concentration of CO2 in the gas phase and in the hydrate phase after hydrate formation.
and represent the concentration of CO2 in the vapor phase and in the hydrate phase, respectively. is the initial moles of the mixed gas, is the moles of the mixed gas after hydrate formation, is the initial moles of CO2, and is the moles of CO2 after hydrate formation. Additionally, two parameters were calculated to evaluate the efficiency of gas separation in the mixed gas.
where is separation factor, and is CO2 recovery %. and present the moles of CO2 and N2 in the hydrate phase, respectively, and present the moles of CO2 and N2 in the vapor phase, respectively, and presents the moles of CO2 in the feed gas.
4. Conclusions
This study investigated the potential of semi-clathrate hydrates containing tetrabutylammonium fluoride (TBAF) for CO2/N2 gas capture and separation. Structural analysis conducted through PXRD unveiled structural transitions in TBAF·29.7 H2O hydrates upon gas capture, suggesting a shift toward structures with a higher number of small cages to accommodate secondary gaseous guest molecules. Raman spectroscopy corroborated the stable enclathration of guest molecules within TBAF hydrates. Moreover, the gas uptake capacities of TBAF hydrates during formation from CO2 and N2 mixed gases were evaluated. Experimental findings revealed varying gas storage capacities contingent upon the ratio of CO2 and N2 mixtures, with notably superior storage capacity observed for CO2. These results underscore the potential efficacy of TBAF hydrates in the gas separation and capture of CO2 and N2 mixed gases. Additionally, the separation efficiency of TBAF hydrates was examined, with the observed similarity between the two types of TBAF hydrates underscoring their consistent performance. Hence, this research underscores the significance of TBAF as a promising additive in advancing the practical utilization of semi-clathrate hydrate for gas separation and storage technologies.
Author Contributions
Conceptualization, Y.-H.A. and H.K.; methodology, Y.-H.A. and H.K.; validation, H.K.; formal analysis, Y.-H.A. and H.K.; investigation, Y.-H.A. and H.K.; data curation, H.K.; writing—original draft preparation, Y.-H.A. and H.K.; writing—review and editing, Y.-H.A.; visualization, H.K.; supervision, Y.-H.A.; project administration, Y.-H.A.; funding acquisition, Y.-H.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (no. 2021R1F1A1047108).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are openly available.
Acknowledgments
PXRD experiments were carried out at Beamline 2D of the Pohang Accelerator Laboratory (PAL).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Sévellec, F.; Drijfhout, S.S. A novel probabilistic forecast system predicting anomalously warm 2018–2022 reinforcing the long-term global warming trend. Nat. Commun. 2018, 9, 3024. [Google Scholar] [CrossRef]
- Dlugokencky, P.T.E. Trends in Atmospheric Carbon Dioxide (Global). 2022. Available online: https://gml.noaa.gov/ccgg/trends/ (accessed on 10 March 2024).
- International Energy Agency. Energy Technology Perspectives 2020; International Energy Agency: Paris, France, 2020. [CrossRef]
- Babu, P.; Linga, P.; Kumar, R.; Englezos, P. A review of the hydrate based gas separation (HBGS) process forcarbon dioxide pre-combustion capture. Energy 2015, 85, 261–279. [Google Scholar] [CrossRef]
- Lee, Y.; Lee, S.; Seo, D.; Moon, S.; Ahn, Y.H.; Park, Y. Highly efficient separation and equilibrium recovery of H2/CO2 in hydrate-based pre-combustion CO2 capture. Chem. Eng. J. 2024, 481, 148709. [Google Scholar] [CrossRef]
- Han, G.; Lee, W.; Kim, M.-K.; Lee, J.W.; Ahn, Y.-H. Hydrogen separation from hydrogen-compressed natural gas blends through successive hydrate formations. Chem. Eng. J. 2024, 483, 149409. [Google Scholar] [CrossRef]
- Sloan, E.D.; Koh, C.A. Clathrate Hydrates of Natural Gases, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2007; ISBN 9781420008494. [Google Scholar]
- Kang, D.W.; Lee, W.; Ahn, Y.H.; Lee, J.W. Confined tetrahydrofuran in a superabsorbent polymer for sustainable methane storage in clathrate hydrates. Chem. Eng. J. 2021, 411, 128512. [Google Scholar] [CrossRef]
- Kim, M.K.; Han, G.; Kim, H.; Yu, J.; Lee, Y.; Song, T.; Park, J.; Kim, Y.H.; Ahn, Y.H. Optimization of water-saturated superabsorbent polymers for hydrate-based gas storage. Korean J. Chem. Eng. 2023, 40, 1063–1070. [Google Scholar] [CrossRef]
- Kim, M.K.; Ahn, Y.H. Gas Hydrates for Hydrogen Storage: A Comprehensive Review and Future Prospects. Korean J. Chem. Eng. 2024, 41, 73–94. [Google Scholar] [CrossRef]
- Kang, D.W.; Lee, W.; Ahn, Y.H.; Lee, J.W. Exploring tuning phenomena of THF-H2 hydrates via molecular dynamics simulations. J. Mol. Liq. 2022, 349, 118490. [Google Scholar] [CrossRef]
- Moon, S.; Hong, S.; Lee, Y.; Lee, J.S.; Ahn, Y.H.; Park, Y. Enhancing Hydrogen Cluster Storage in Clathrate Hydrates via Defect-Mediated Lattice Engineering. J. Phys. Chem. C 2021, 125, 1767–1773. [Google Scholar] [CrossRef]
- Mahajan, D.; Tan, K.; Venkatesh, T.; Kileti, P.; Clayton, C.R. Hydrogen Blending in Gas Pipeline Networks—A Review. Energies 2022, 15, 3582. [Google Scholar] [CrossRef]
- Lee, W.; Kang, D.W.; Ahn, Y.H.; Lee, J.W. Blended hydrate seed and liquid promoter for the acceleration of hydrogen hydrate formation. Renew. Sustain. Energy Rev. 2023, 177, 113217. [Google Scholar] [CrossRef]
- Lee, W.; Kang, D.W.; Ahn, Y.H.; Lee, J.W. Rapid Formation of Hydrogen-Enriched Hydrocarbon Gas Hydrates under Static Conditions. ACS Sustain. Chem. Eng. 2021, 9, 8414–8424. [Google Scholar] [CrossRef]
- Moon, S.; Lee, Y.; Seo, D.; Lee, S.; Hong, S.; Ahn, Y.H.; Park, Y. Critical hydrogen concentration of hydrogen-natural gas blends in clathrate hydrates for blue hydrogen storage. Renew. Sustain. Energy Rev. 2021, 141, 110789. [Google Scholar] [CrossRef]
- Ahn, Y.H.; Moon, S.; Koh, D.Y.; Hong, S.; Lee, H.; Lee, J.W.; Park, Y. One-step formation of hydrogen clusters in clathrate hydrates stabilized via natural gas blending. Energy Storage Mater. 2020, 24, 655–661. [Google Scholar] [CrossRef]
- Moon, S.; Lee, S.; Ahn, Y.H.; Park, Y. Abnormal thermodynamic promotion and tuning behavior of epoxycyclopentane for its implications in CO2 storage. Chem. Eng. J. 2021, 425, 130647. [Google Scholar] [CrossRef]
- Moon, S.; Ahn, Y.H.; Kim, H.; Hong, S.; Koh, D.Y.; Park, Y. Secondary gaseous guest-dependent structures of binary neopentyl alcohol hydrates and their tuning behavior for potential application to CO2 capture. Chem. Eng. J. 2017, 330, 890–898. [Google Scholar] [CrossRef]
- Kang, D.W.; Lee, W.; Ahn, Y.H. Superabsorbent polymer for improved CO2 hydrate formation under a quiescent system. J. CO2 Util. 2022, 61, 102005. [Google Scholar] [CrossRef]
- Lee, Y.; Kim, H.; Lee, W.; Kang, D.W.; Lee, J.W.; Ahn, Y.H. Thermodynamic and kinetic properties of CO2 hydrates and their applications in CO2 capture and separation. J. Environ. Chem. Eng. 2023, 11, 110933. [Google Scholar] [CrossRef]
- Zhao, J.; Guo, X.; Sun, M.; Zhao, Y.; Yang, L.; Song, Y. N2O hydrate formation in porous media: A potential method to mitigate N2O emissions. Chem. Eng. J. 2019, 361, 12–20. [Google Scholar] [CrossRef]
- Ahn, Y.H.; Lim, D.; Min, J.; Kim, J.; Lee, B.; Lee, J.W.; Shin, K. Clathrate nanocage reactor for the decomposition of greenhouse gas. Chem. Eng. J. 2019, 359, 1629–1634. [Google Scholar] [CrossRef]
- Yang, Y.; Shin, D.; Choi, S.; Woo, Y.; Lee, J.W.; Kim, D.; Shin, H.Y.; Cha, M.; Yoon, J.H. Selective Encaging of N2O in N2O-N2 Binary Gas Hydrates via Hydrate-Based Gas Separation. Environ. Sci. Technol. 2017, 51, 3550–3557. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.; Choi, W.; Mok, J.; Seo, Y. Kinetic CO2 selectivity in clathrate-based CO2 capture for upgrading CO2-rich natural gas and biogas. Chem. Eng. J. 2019, 369, 686–693. [Google Scholar] [CrossRef]
- Trueba, A.T.; Radović, I.R.; Zevenbergen, J.F.; Peters, C.J.; Kroon, M.C. Kinetic measurements and in situ Raman spectroscopy study of the formation of TBAF semi-hydrates with hydrogen and carbon dioxide. Int. J. Hydrogen Energy 2013, 38, 7326–7334. [Google Scholar] [CrossRef]
- Mohammadi, A. The Effect of Various Concentrations of Tetra-n-butylammonium Fluoride on the Dissociation Enthalpy of Gas Hydrates. Iran. J. Energy Environ. 2022, 13, 151–157. [Google Scholar] [CrossRef]
- Pandey, G.; Poothia, T.; Kumar, A. Hydrate based carbon capture and sequestration (HBCCS): An innovative approach towards decarbonization. Appl. Energy 2022, 326, 119900. [Google Scholar] [CrossRef]
- Hashimoto, H.; Ozeki, H.; Yamamoto, Y.; Muromachi, S. CO2 Capture from Flue Gas Based on Tetra-n-butylammonium Fluoride Hydrates at near Ambient Temperature. ACS Omega 2020, 5, 7115–7123. [Google Scholar] [CrossRef]
- Shi, L.L.; Liang, D. qing Thermodynamic model of phase equilibria of tetrabutyl ammonium halide (fluoride, chloride, or bromide) plus methane or carbon dioxide semiclathrate hydrates. Fluid Phase Equilib. 2015, 386, 149–154. [Google Scholar] [CrossRef]
- Lee, Y.; Lee, S.; Lee, J.; Seo, Y. Structure identification and dissociation enthalpy measurements of the CO2+N2 hydrates for their application to CO2 capture and storage. Chem. Eng. J. 2014, 246, 20–26. [Google Scholar] [CrossRef]
- Kang, S.P.; Lee, H.; Lee, C.S.; Sung, W.M. Hydrate phase equilibria of the guest mixtures containing CO2, N2 and tetrahydrofuran. Fluid Phase Equilib. 2001, 185, 101–109. [Google Scholar] [CrossRef]
- Lee, S.; Lee, Y.; Park, S.; Kim, Y.; Lee, J.D.; Seo, Y. Thermodynamic and spectroscopic identification of guest gas enclathration in the double tetra-n-butylammonium fluoride semiclathrates. J. Phys. Chem. B 2012, 116, 9075–9081. [Google Scholar] [CrossRef]
- Lee, S.; Lee, Y.; Park, S.; Seo, Y. Phase equilibria of semiclathrate hydrate for nitrogen in the presence of tetra-n-butylammonium bromide and fluoride. J. Chem. Eng. Data 2010, 55, 5883–5886. [Google Scholar] [CrossRef]
- Sizova, A.A.; Grintsevich, S.A.; Kochurin, M.A.; Sizov, V.V.; Brodskaya, E.N. Molecular Simulations of CO2/CH4, CO2/N2 and N2/CH4 Binary Mixed Hydrates. Colloid J. 2021, 83, 372–378. [Google Scholar] [CrossRef]
- Koh, D.Y.; Kang, H.; Kim, D.O.; Park, J.; Cha, M.; Lee, H. Recovery of methane from gas hydrates intercalated within natural sediments using CO2 and a CO2/N2 gas mixture. ChemSusChem 2012, 5, 1443–1448. [Google Scholar] [CrossRef]
- Koh, D.; Ahn, Y.; Kang, H.; Park, S.; Lee, J.Y.; Kim, S.; Lee, J.; Lee, H. One-dimensional productivity assessment for on-field methane hydrate production using CO2/N2 mixture gas. AIChE J. 2015, 61, 1004–1014. [Google Scholar] [CrossRef]
- Belosludov, V.R.; Bozhko, Y.Y.; Subbotin, O.S.; Belosludov, R.V.; Zhdanov, R.K.; Gets, K.V.; Kawazoe, Y. Influence of N2 on formation conditions and guest distribution of mixed CO2 + CH4 gas hydrates. Molecules 2018, 23, 3336. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, H.; Maruyama, K.; Yamagiwa, K.; Tajima, H. Separation processes for carbon dioxide capture with semi-clathrate hydrate slurry based on phase equilibria of CO2 + N2 + tetra-n-butylammonium bromide + water systems. Chem. Eng. Res. Des. 2019, 150, 289–298. [Google Scholar] [CrossRef]
- Ma, Z.W.; Zhang, P.; Bao, H.S.; Deng, S. Review of fundamental properties of CO2 hydrates and CO2 capture and separation using hydration method. Renew. Sustain. Energy Rev. 2016, 53, 1273–1302. [Google Scholar] [CrossRef]
- Ye, N.; Zhang, P. Phase equilibrium conditions and carbon dioxide separation efficiency of tetra-n-butylphosphonium bromide hydrate. J. Chem. Eng. Data 2014, 59, 2920–2926. [Google Scholar] [CrossRef]
- Li, S.; Fan, S.; Wang, J.; Lang, X.; Liang, D. CO2 capture from binary mixture via forming hydrate with the help of tetra-n-butyl ammonium bromide. J. Nat. Gas Chem. 2009, 18, 15–20. [Google Scholar] [CrossRef]
- Ahn, Y.H.; Kang, H.; Koh, D.Y.; Park, Y.; Lee, H. Gas hydrate inhibition by 3-hydroxytetrahydrofuran: Spectroscopic identifications and hydrate phase equilibria. Fluid Phase Equilib. 2016, 413, 65–70. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).