The Causality between Human Immunoglobulin G (IgG) N-Glycosylation and Aging: A Mendelian Randomization Study
Abstract
1. Introduction
2. Results
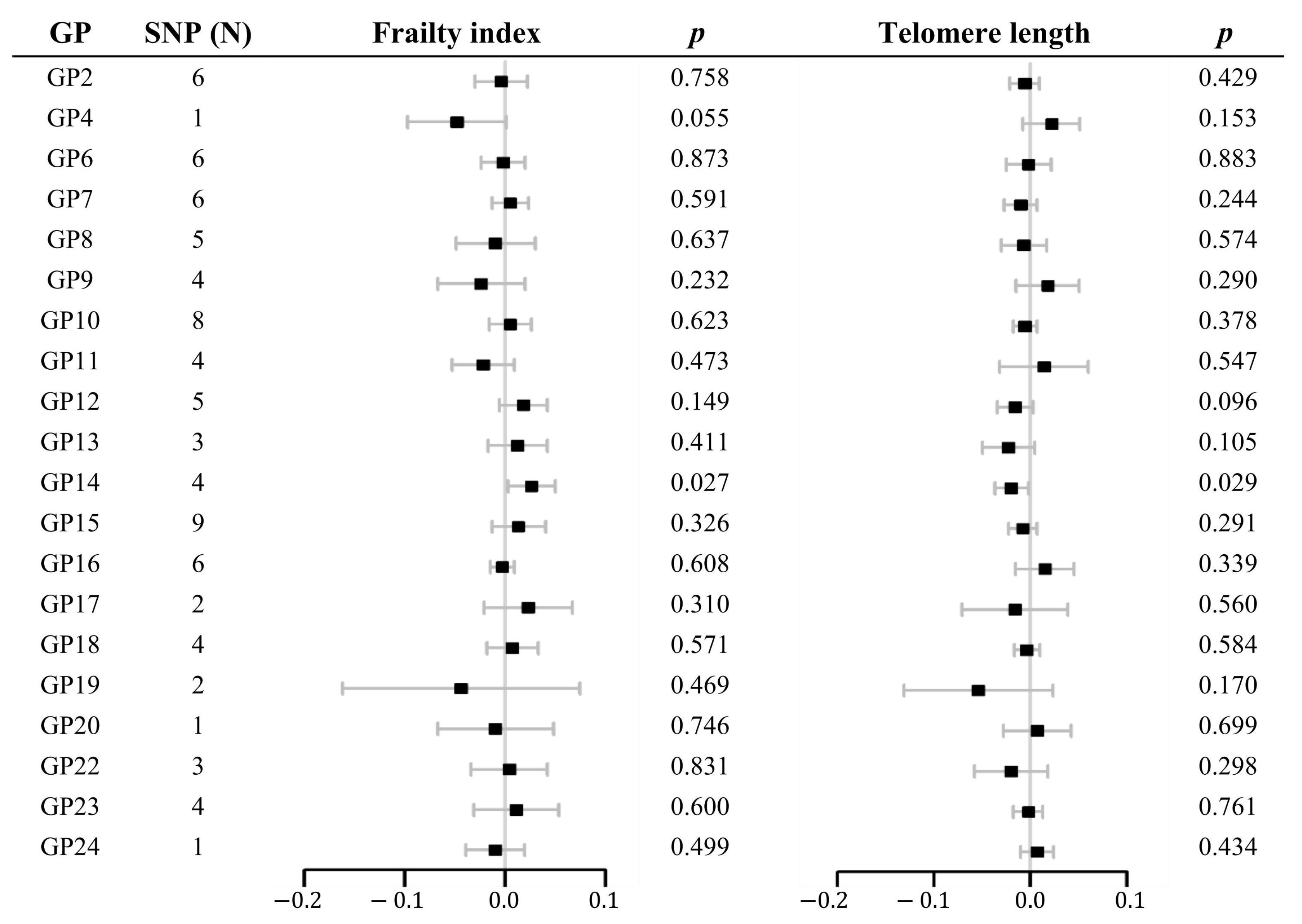
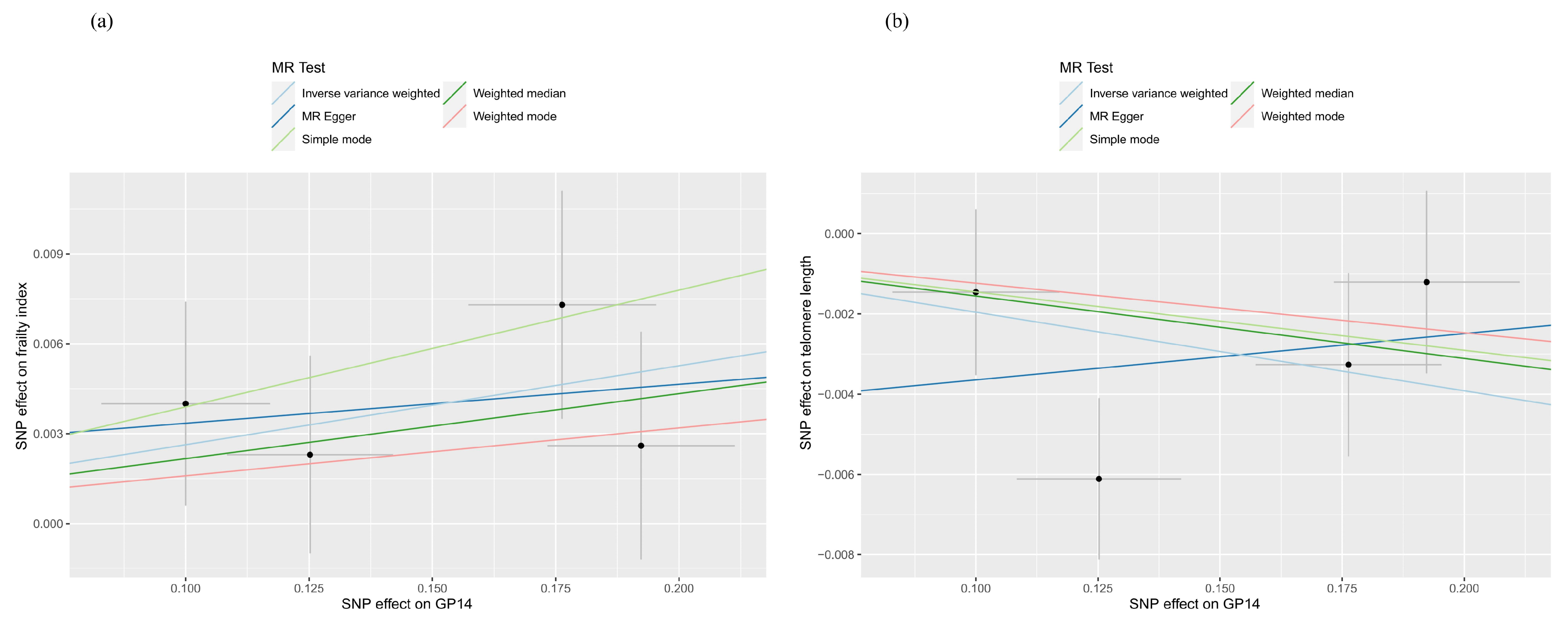
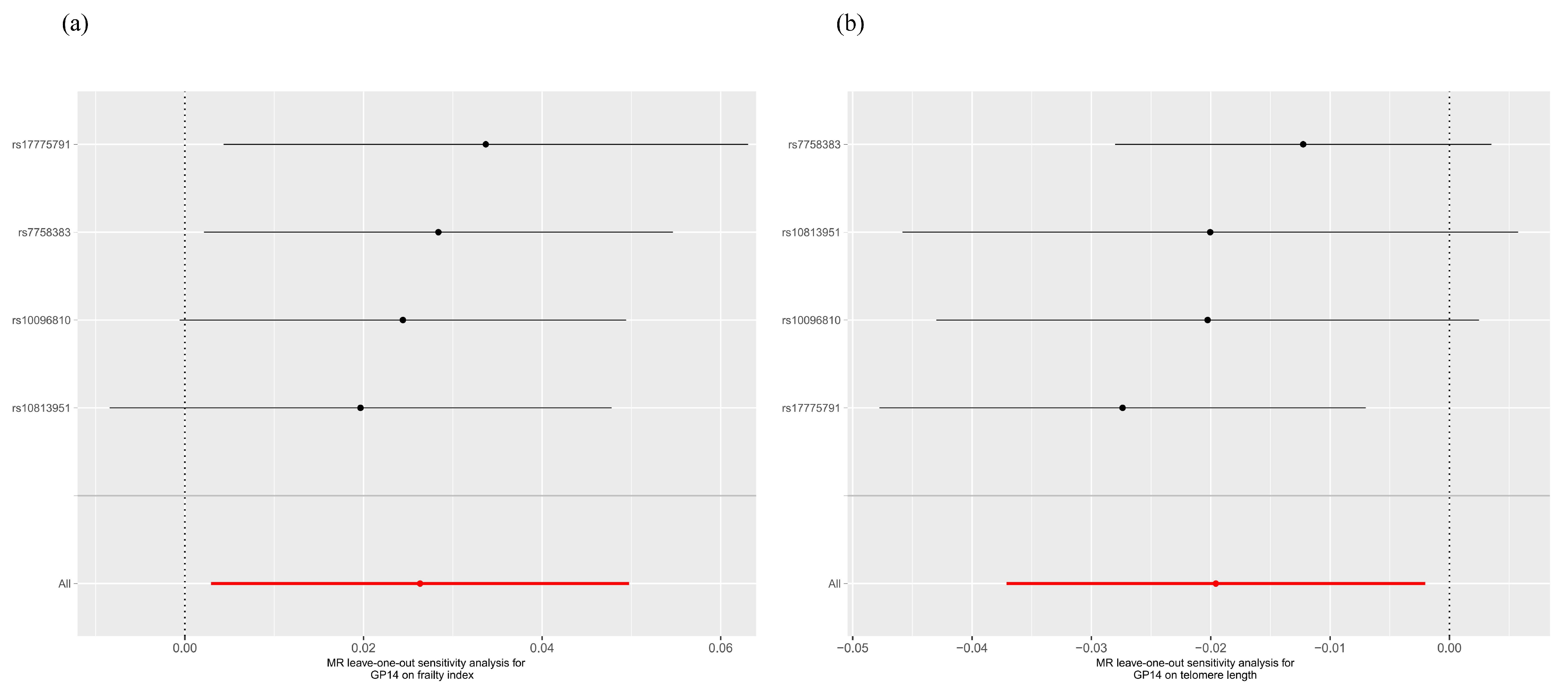
2.1. Two-Sample MR Analysis
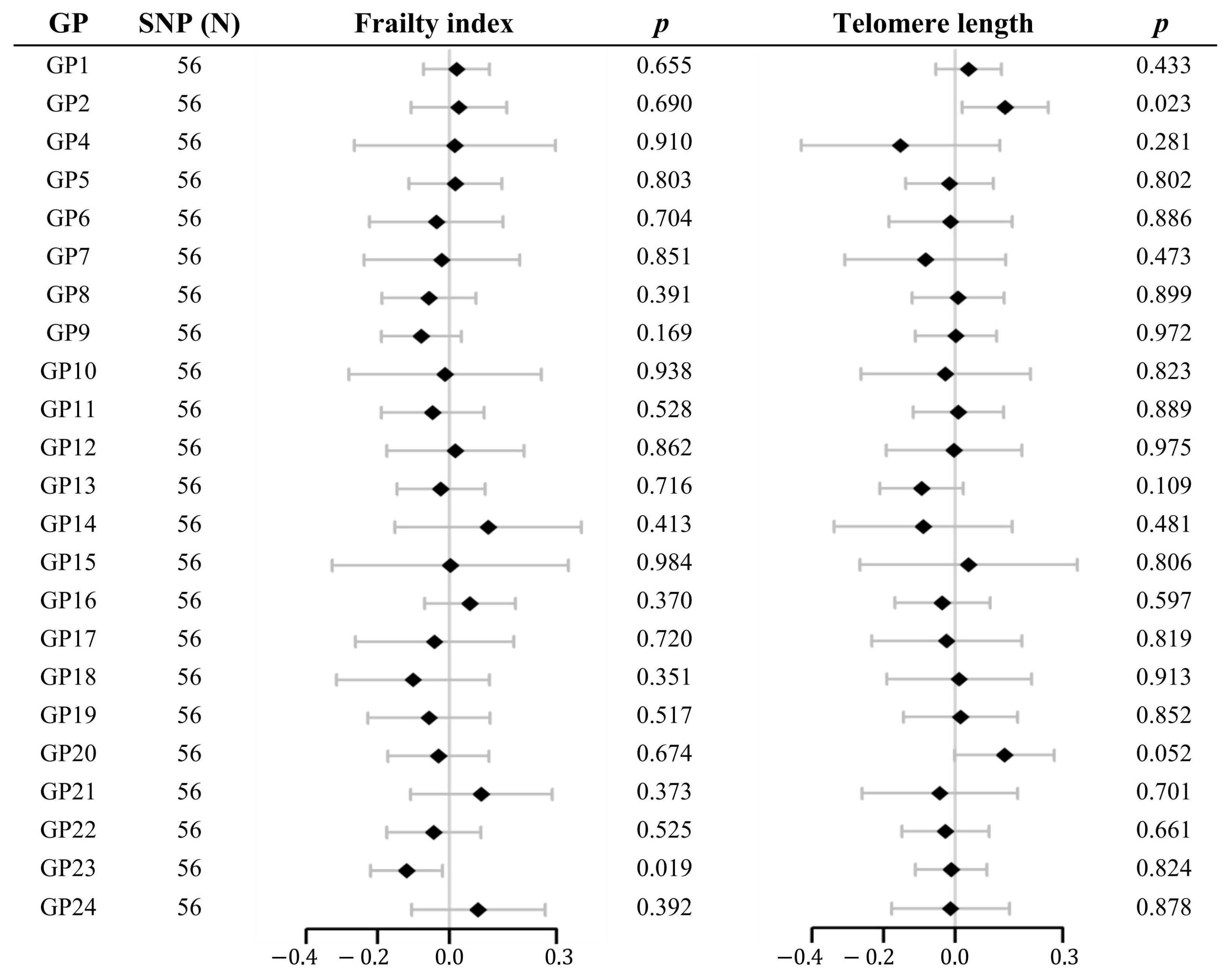
2.2. Multivariable MR Analysis
3. Discussion
4. Materials and Methods
4.1. Study Design
4.2. Data Source
4.3. Isolation of IgG and Glycan Analysis
4.4. Instrumental Variable Selection Criteria
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Opdenakker, G.; Rudd, P.M.; Ponting, C.P.; Dwek, R.A. Concepts and principles of glycobiology. FASEB J. 1993, 7, 1330–1337. [Google Scholar] [CrossRef]
- Varki, A. Biological roles of glycans. Glycobiology 2017, 27, 3–49. [Google Scholar] [CrossRef]
- Zhao, Y.Y.; Takahashi, M.; Gu, J.G.; Miyoshi, E.; Matsumoto, A.; Kitazume, S.; Taniguchi, N. Functional roles of N-glycans in cell signaling and cell adhesion in cancer. Cancer Sci. 2008, 99, 1304–1310. [Google Scholar] [CrossRef]
- Pucic, M.; Knezevic, A.; Vidic, J.; Adamczyk, B.; Novokmet, M.; Polasek, O.; Gornik, O.; Šupraha-Goreta, S.; Wormald, M.R.; Redžić, I.; et al. High throughput isolation and glycosylation analysis of IgG-variability and heritability of the IgG glycome in three isolated human populations. Mol. Cell. Proteom. 2011, 10, M111.010090. [Google Scholar] [CrossRef] [PubMed]
- Lauc, G. Precision medicine that transcends genomics: Glycans as integrators of genes and environment. Biochim. Biophys. Acta 2016, 1860, 1571–1573. [Google Scholar] [CrossRef] [PubMed]
- Pothukuchi, P.; Agliarulo, I.; Russo, D.; Rizzo, R.; Russo, F.; Parashuraman, S. Translation of genome to glycome: Role of the Golgi apparatus. FEBS Lett. 2019, 593, 2390–2411. [Google Scholar] [CrossRef] [PubMed]
- Ohtsubo, K.; Marth, J.D. Glycosylation in cellular mechanisms of health and disease. Cell 2006, 126, 855–867. [Google Scholar] [CrossRef]
- Titorenko, V.I. Molecular and Cellular Mechanisms of Aging and Age-related Disorders. Int. J. Mol. Sci. 2018, 19, 2049. [Google Scholar] [CrossRef] [PubMed]
- Dall’Olio, F.; Malagolini, N. Immunoglobulin G Glycosylation Changes in Aging and Other Inflammatory Conditions. Exp. Suppl. 2021, 112, 303–340. [Google Scholar] [CrossRef]
- Meng, X.; Wang, B.; Xu, X.; Song, M.; Hou, H.; Wang, W.; Wang, Y. Glycomic biomarkers are instrumental for suboptimal health status management in the context of predictive, preventive, and personalized medicine. EPMA J. 2022, 13, 195–207. [Google Scholar] [CrossRef]
- Liu, D.; Chu, X.; Wang, H.; Dong, J.; Ge, S.Q.; Zhao, Z.Y.; Peng, H.-L.; Sun, M.; Wu, L.-J.; Song, M.-S.; et al. The changes of immunoglobulin G N-glycosylation in blood lipids and dyslipidaemia. J. Transl. Med. 2018, 16, 235. [Google Scholar] [CrossRef]
- Hafkenscheid, L.; de Moel, E.; Smolik, I.; Tanner, S.; Meng, X.; Jansen, B.C.; Bondt, A.; Wuhrer, M.; Huizinga, T.W.J.; Toes, R.E.M.; et al. N-Linked Glycans in the Variable Domain of IgG Anti-Citrullinated Protein Antibodies Predict the Development of Rheumatoid Arthritis. Arthritis Rheumatol. 2019, 71, 1626–1633. [Google Scholar] [CrossRef]
- Verhelst, X.; Dias, A.M.; Colombel, J.F.; Vermeire, S.; Van Vlierberghe, H.; Callewaert, N.; Pinho, S.S. Protein Glycosylation as a Diagnostic and Prognostic Marker of Chronic Inflammatory Gastrointestinal and Liver Diseases. Gastroenterology 2020, 158, 95–110. [Google Scholar] [CrossRef] [PubMed]
- Wittenbecher, C.; Stambuk, T.; Kuxhaus, O.; Rudman, N.; Vuckovic, F.; Stambuk, J.; Schiborn, C.; Rahelić, D.; Dietrich, S.; Gornik, O.; et al. Plasma N-Glycans as Emerging Biomarkers of Cardiometabolic Risk: A Prospective Investigation in the EPIC-Potsdam Cohort Study. Diabetes Care 2020, 43, 661–668. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Klaric, L.; Yu, X.; Thaqi, K.; Dong, J.; Novokmet, M.; Wilson, J.; Polasek, O.; Liu, Y.; Krištić, J.; et al. The Association Between Glycosylation of Immunoglobulin G and Hypertension: A Multiple Ethnic Cross-Sectional Study. Medicine 2016, 95, e3379. [Google Scholar] [CrossRef]
- Zhang, X.; Yuan, H.; Lyu, J.; Meng, X.; Tian, Q.; Li, Y.; Zhang, J.; Xu, X.; Su, J.; Hou, H.; et al. Association of dementia with immunoglobulin G N-glycans in a Chinese Han Population. NPJ Aging Mech. Dis. 2021, 7, 3. [Google Scholar] [CrossRef]
- Dall’Olio, F.; Vanhooren, V.; Chen, C.C.; Slagboom, P.E.; Wuhrer, M.; Franceschi, C. N-glycomic biomarkers of biological aging and longevity: A link with inflammaging. Ageing Res. Rev. 2013, 12, 685–698. [Google Scholar] [CrossRef]
- Gudelj, I.; Lauc, G.; Pezer, M. Immunoglobulin G glycosylation in aging and diseases. Cell. Immunol. 2018, 333, 65–79. [Google Scholar] [CrossRef]
- Kristic, J.; Vuckovic, F.; Menni, C.; Klaric, L.; Keser, T.; Beceheli, I.; Pučić-Baković, M.; Novokmet, M.; Mangino, M.; Thaqi, K.; et al. Glycans are a novel biomarker of chronological and biological ages. J. Gerontol. A Biol. Sci. Med. Sci. 2014, 69, 779–789. [Google Scholar] [CrossRef]
- Lado-Baleato, O.; Torre, J.; O’Flaherty, R.; Alonso-Sampedro, M.; Carballo, I.; Fernandez-Merino, C.; Vidal, C.; Gude, F.; Saldova, R.; González-Quintela, A. Age-Related Changes in Serum N-Glycome in Men and Women-Clusters Associated with Comorbidity. Biomolecules 2023, 14, 17. [Google Scholar] [CrossRef]
- Zaytseva, O.O.; Sharapov, S.Z.; Perola, M.; Esko, T.; Landini, A.; Hayward, C.; Wilson, J.F.; Lauc, G.; Aulchenko, Y.S.; Klarić, L.; et al. Investigation of the causal relationships between human IgG N-glycosylation and 12 common diseases associated with changes in the IgG N-glycome. Hum. Mol. Genet. 2022, 31, 1545–1559. [Google Scholar] [CrossRef]
- Liu, D.; Dong, J.; Zhang, J.; Xu, X.; Tian, Q.; Meng, X.; Wu, L.; Zheng, D.; Chu, X.; Wang, W.; et al. Genome-Wide Mapping of Plasma IgG N-Glycan Quantitative Trait Loci Identifies a Potentially Causal Association between IgG N-Glycans and Rheumatoid Arthritis. J. Immunol. 2022, 208, 2508–2514. [Google Scholar] [CrossRef]
- Zhang, X.; Cong, R.; Geng, T.; Zhang, J.; Liu, D.; Tian, Q.; Meng, X.; Song, M.; Wu, L.; Zheng, D.; et al. Assessment of the Causal Effect of IgG N-Glycosylation Level on Risk of Dementia: A 2-Sample Mendelian Randomization Study. J. Alzheimer’s Dis. 2022, 88, 1435–1441. [Google Scholar] [CrossRef] [PubMed]
- Yavorska, O.O.; Burgess, S. MendelianRandomization: An R package for performing Mendelian randomization analyses using summarized data. Int. J. Epidemiol. 2017, 46, 1734–1739. [Google Scholar] [CrossRef]
- Lawlor, D.A.; Harbord, R.M.; Sterne, J.A.; Timpson, N.; Davey Smith, G. Mendelian randomization: Using genes as instruments for making causal inferences in epidemiology. Stat. Med. 2008, 27, 1133–1163. [Google Scholar] [CrossRef]
- Muezzinler, A.; Zaineddin, A.K.; Brenner, H. A systematic review of leukocyte telomere length and age in adults. Ageing Res. Rev. 2013, 12, 509–519. [Google Scholar] [CrossRef] [PubMed]
- Alzain, M.A.; Asweto, C.O.; Zhang, J.; Fang, H.; Zhao, Z.; Guo, X.; Song, M.; Zhou, Y.; Chang, N.; Wang, Y.; et al. Telomere Length and Accelerated Biological Aging in the China Suboptimal Health Cohort: A Case-Control Study. OMICS 2017, 21, 333–339. [Google Scholar] [CrossRef]
- Parekh, R.; Roitt, I.; Isenberg, D.; Dwek, R.; Rademacher, T. Age-related galactosylation of the N-linked oligosaccharides of human serum IgG. J. Exp. Med. 1988, 167, 1731–1736. [Google Scholar] [CrossRef] [PubMed]
- Cobb, B.A. The history of IgG glycosylation and where we are now. Glycobiology 2020, 30, 202–213. [Google Scholar] [CrossRef]
- de Haan, N.; Falck, D.; Wuhrer, M. Monitoring of immunoglobulin N- and O-glycosylation in health and disease. Glycobiology 2020, 30, 226–240. [Google Scholar] [CrossRef]
- De Martinis, M.; Franceschi, C.; Monti, D.; Ginaldi, L. Inflamm-ageing and lifelong antigenic load as major determinants of ageing rate and longevity. FEBS Lett. 2005, 579, 2035–2039. [Google Scholar] [CrossRef]
- Franceschi, C.; Capri, M.; Monti, D.; Giunta, S.; Olivieri, F.; Sevini, F.; Panourgia, M.P.; Invidia, L.; Celani, L.; Scurti, M.; et al. Inflammaging and anti-inflammaging: A systemic perspective on aging and longevity emerged from studies in humans. Mech. Ageing Dev. 2007, 128, 92–105. [Google Scholar] [CrossRef]
- Malhotra, R.; Wormald, M.R.; Rudd, P.M.; Fischer, P.B.; Dwek, R.A.; Sim, R.B. Glycosylation changes of IgG associated with rheumatoid arthritis can activate complement via the mannose-binding protein. Nat. Med. 1995, 1, 237–243. [Google Scholar] [CrossRef]
- Arnold, J.N.; Dwek, R.A.; Rudd, P.M.; Sim, R.B. Mannan binding lectin and its interaction with immunoglobulins in health and in disease. Immunol. Lett. 2006, 106, 103–110. [Google Scholar] [CrossRef]
- Karsten, C.M.; Pandey, M.K.; Figge, J.; Kilchenstein, R.; Taylor, P.R.; Rosas, M.; McDonald, J.U.; Orr, S.J.; Berger, M.; Petzold, D.; et al. Anti-inflammatory activity of IgG1 mediated by Fc galactosylation and association of FcgammaRIIB and dectin-1. Nat. Med. 2012, 18, 1401–1406. [Google Scholar] [CrossRef] [PubMed]
- Mihai, S.; Nimmerjahn, F. The role of Fc receptors and complement in autoimmunity. Autoimmun. Rev. 2013, 12, 657–660. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Klaric, L.; Sharapov, S.; Mangino, M.; Ning, Z.; Wu, D.; Trbojević-Akmačić, I.; Pučić-Baković, M.; Rudan, I.; Polašek, O.; et al. Multivariate discovery and replication of five novel loci associated with Immunoglobulin G N-glycosylation. Nat. Commun. 2017, 8, 447. [Google Scholar] [CrossRef] [PubMed]
- Boyd, P.N.; Lines, A.C.; Patel, A.K. The effect of the removal of sialic acid, galactose and total carbohydrate on the functional activity of Campath-1H. Mol. Immunol. 1995, 32, 1311–1318. [Google Scholar] [CrossRef]
- Peschke, B.; Keller, C.W.; Weber, P.; Quast, I.; Lunemann, J.D. Fc-Galactosylation of Human Immunoglobulin Gamma Isotypes Improves C1q Binding and Enhances Complement-Dependent Cytotoxicity. Front. Immunol. 2017, 8, 646. [Google Scholar] [CrossRef]
- Kumpel, B.M.; Rademacher, T.W.; Rook, G.A.; Williams, P.J.; Wilson, I.B. Galactosylation of human IgG monoclonal anti-D produced by EBV-transformed B-lymphoblastoid cell lines is dependent on culture method and affects Fc receptor-mediated functional activity. Hum Antibodies Hybrid. 1994, 5, 143–151. [Google Scholar]
- Dekkers, G.; Treffers, L.; Plomp, R.; Bentlage, A.E.H.; de Boer, M.; Koeleman, C.A.M.; Lissenberg-Thunnissen, S.N.; Visser, R.; Brouwer, M.; Mok, J.Y.; et al. Decoding the Human Immunoglobulin G-Glycan Repertoire Reveals a Spectrum of Fc-Receptor- and Complement-Mediated-Effector Activities. Front. Immunol. 2017, 8, 877. [Google Scholar] [CrossRef] [PubMed]
- Bakovic, M.P.; Selman, M.H.; Hoffmann, M.; Rudan, I.; Campbell, H.; Deelder, A.M.; Lauc, G.; Wuhrer, M. High-throughput IgG Fc N-glycosylation profiling by mass spectrometry of glycopeptides. J. Proteome Res. 2013, 12, 821–831. [Google Scholar] [CrossRef] [PubMed]
- Scallon, B.J.; Tam, S.H.; McCarthy, S.G.; Cai, A.N.; Raju, T.S. Higher levels of sialylated Fc glycans in immunoglobulin G molecules can adversely impact functionality. Mol. Immunol. 2007, 44, 1524–1534. [Google Scholar] [CrossRef]
- Maverakis, E.; Kim, K.; Shimoda, M.; Gershwin, M.E.; Patel, F.; Wilken, R.; Raychaudhuri, S.; Ruhaak, L.R.; Lebrilla, C.B. Glycans in the immune system and the Altered Glycan Theory of Autoimmunity: A critical review. J. Autoimmun. 2015, 57, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Zabczynska, M.; Polak, K.; Kozlowska, K.; Sokolowski, G.; Pochec, E. The Contribution of IgG Glycosylation to Antibody-Dependent Cell-Mediated Cytotoxicity (ADCC) and Complement-Dependent Cytotoxicity (CDC) in Hashimoto’s Thyroiditis: An in Vitro Model of Thyroid Autoimmunity. Biomolecules 2020, 10, 171. [Google Scholar] [CrossRef]
- Hess, C.; Winkler, A.; Lorenz, A.K.; Holecska, V.; Blanchard, V.; Eiglmeier, S.; Schoen, A.-L.; Bitterling, J.; Stoehr, A.D.; Petzold, D.; et al. T cell-independent B cell activation induces immunosuppressive sialylated IgG antibodies. J. Clin. Investig. 2013, 123, 3788–3796. [Google Scholar] [CrossRef]
- Schwab, I.; Mihai, S.; Seeling, M.; Kasperkiewicz, M.; Ludwig, R.J.; Nimmerjahn, F. Broad requirement for terminal sialic acid residues and FcgammaRIIB for the preventive and therapeutic activity of intravenous immunoglobulins in vivo. Eur. J. Immunol. 2014, 44, 1444–1453. [Google Scholar] [CrossRef]
- Anthony, R.M.; Kobayashi, T.; Wermeling, F.; Ravetch, J.V. Intravenous gammaglobulin suppresses inflammation through a novel T(H)2 pathway. Nature 2011, 475, 110–113. [Google Scholar] [CrossRef]
- Arai, Y.; Martin-Ruiz, C.M.; Takayama, M.; Abe, Y.; Takebayashi, T.; Koyasu, S.; Suematsu, M.; Hirose, N.; von Zglinicki, T. Inflammation, But Not Telomere Length, Predicts Successful Ageing at Extreme Old Age: A Longitudinal Study of Semi-supercentenarians. EBioMedicine 2015, 2, 1549–1558. [Google Scholar] [CrossRef]
- Holland, M.; Yagi, H.; Takahashi, N.; Kato, K.; Savage, C.O.; Goodall, D.M.; Jefferis, R. Differential glycosylation of polyclonal IgG, IgG-Fc and IgG-Fab isolated from the sera of patients with ANCA-associated systemic vasculitis. Biochim. Biophys. Acta 2006, 1760, 669–677. [Google Scholar] [CrossRef]
- Burgess, S.; Thompson, S.G. Multivariable Mendelian randomization: The use of pleiotropic genetic variants to estimate causal effects. Am. J. Epidemiol. 2015, 181, 251–260. [Google Scholar] [CrossRef]
- Klaric, L.; Tsepilov, Y.A.; Stanton, C.M.; Mangino, M.; Sikka, T.T.; Esko, T.; Pakhomov, E.; Salo, P.; Deelen, J.; McGurnaghan, S.J.; et al. Glycosylation of immunoglobulin G is regulated by a large network of genes pleiotropic with inflammatory diseases. Sci. Adv. 2020, 6, eaax0301. [Google Scholar] [CrossRef] [PubMed]
- Atkins, J.L.; Jylhava, J.; Pedersen, N.L.; Magnusson, P.K.; Lu, Y.; Wang, Y.; Hägg, S.; Melzer, D.; Williams, D.M.; Pilling, L.C. A genome-wide association study of the frailty index highlights brain pathways in ageing. Aging Cell 2021, 20, e13459. [Google Scholar] [CrossRef]
- Codd, V.; Wang, Q.; Allara, E.; Musicha, C.; Kaptoge, S.; Stoma, S.; Jiang, T.; Hamby, S.E.; Braund, P.S.; Bountziouka, V.; et al. Polygenic basis and biomedical consequences of telomere length variation. Nat. Genet. 2021, 53, 1425–1433. [Google Scholar] [CrossRef]
- Lauc, G.; Huffman, J.E.; Pucic, M.; Zgaga, L.; Adamczyk, B.; Muzinic, A.; Novokmet, M.; Polašek, O.; Gornik, O.; Krištić, J.; et al. Loci associated with N-glycosylation of human immunoglobulin G show pleiotropy with autoimmune diseases and haematological cancers. PLoS Genet. 2013, 9, e1003225. [Google Scholar] [CrossRef]
- Hemani, G.; Zheng, J.; Elsworth, B.; Wade, K.H.; Haberland, V.; Baird, D.; Laurin, C.; Burgess, S.; Bowden, J.; Langdon, R.; et al. The MR-Base platform supports systematic causal inference across the human phenome. eLife 2018, 7, e34408. [Google Scholar] [CrossRef] [PubMed]
- Palmer, T.M.; Lawlor, D.A.; Harbord, R.M.; Sheehan, N.A.; Tobias, J.H.; Timpson, N.J.; Smith, G.D.; Sterne, J.A. Using multiple genetic variants as instrumental variables for modifiable risk factors. Stat. Methods Med. Res. 2012, 21, 223–242. [Google Scholar] [CrossRef]
- Burgess, S.; Butterworth, A.; Thompson, S.G. Mendelian randomization analysis with multiple genetic variants using summarized data. Genet. Epidemiol. 2013, 37, 658–665. [Google Scholar] [CrossRef]
- Bowden, J.; Davey Smith, G.; Haycock, P.C.; Burgess, S. Consistent Estimation in Mendelian Randomization with Some Invalid Instruments Using a Weighted Median Estimator. Genet. Epidemiol. 2016, 40, 304–314. [Google Scholar] [CrossRef]
- Verbanck, M.; Chen, C.Y.; Neale, B.; Do, R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat. Genet. 2018, 50, 693–698. [Google Scholar] [CrossRef] [PubMed]
- Bowden, J.; Davey Smith, G.; Burgess, S. Mendelian randomization with invalid instruments: Effect estimation and bias detection through Egger regression. Int. J. Epidemiol. 2015, 44, 512–525. [Google Scholar] [CrossRef] [PubMed]
- Burgess, S.; Bowden, J.; Fall, T.; Ingelsson, E.; Thompson, S.G. Sensitivity Analyses for Robust Causal Inference from Mendelian Randomization Analyses with Multiple Genetic Variants. Epidemiology 2017, 28, 30–42. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | Resource | Sample Size | Population Ancestry | Data Download |
|---|---|---|---|---|
| Exposure | ||||
| IgG N-glycosylation | CROATIA-Korcula (recruited from the town of Korčula and the villages of Lumbarda, Žrnovo and Račišće.), CROATIA-Vis (recruited from the villages of Vis and Komiža on the Dalmatian island of Vis), ORCADES (recruited from the isolated Scottish archipelago of Orkney), and TwinsUK (recruited from the United Kingdom female adult twin) | 8090 | European | https://doi.org/10.7488/ds/2481 (accessed on 15 December 2022) |
| Outcome | ||||
| Frailty index | UK Biobank (recruited from the England, Scotland and Wales), and TwinGene (recruited from the Sweden adult twin) | 175,226 | European | https://doi.org/10.6084/m9.figshare.9204998.v4 (accessed on 15 December 2022) |
| Telomere length | UK Biobank | 472,174 | European | https://figshare.com/s/caa99dc0f76d62990195 (accessed on 15 December 2022) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, W.; Jian, X.; Zhang, J.; Meng, X.; Wang, H.; Zheng, D.; Wu, L.; Wang, Y. The Causality between Human Immunoglobulin G (IgG) N-Glycosylation and Aging: A Mendelian Randomization Study. Molecules 2024, 29, 1281. https://doi.org/10.3390/molecules29061281
Sun W, Jian X, Zhang J, Meng X, Wang H, Zheng D, Wu L, Wang Y. The Causality between Human Immunoglobulin G (IgG) N-Glycosylation and Aging: A Mendelian Randomization Study. Molecules. 2024; 29(6):1281. https://doi.org/10.3390/molecules29061281
Chicago/Turabian StyleSun, Wenxin, Xuening Jian, Jie Zhang, Xiaoni Meng, Haotian Wang, Deqiang Zheng, Lijuan Wu, and Youxin Wang. 2024. "The Causality between Human Immunoglobulin G (IgG) N-Glycosylation and Aging: A Mendelian Randomization Study" Molecules 29, no. 6: 1281. https://doi.org/10.3390/molecules29061281
APA StyleSun, W., Jian, X., Zhang, J., Meng, X., Wang, H., Zheng, D., Wu, L., & Wang, Y. (2024). The Causality between Human Immunoglobulin G (IgG) N-Glycosylation and Aging: A Mendelian Randomization Study. Molecules, 29(6), 1281. https://doi.org/10.3390/molecules29061281







