Comparative Pharmacokinetics Research of 13 Bioactive Components of Jieyu Pills in Control and Attention Deficit Hyperactivity Disorder Model Rats Based on UPLC-Orbitrap Fusion MS
Abstract
1. Introduction
2. Experimental Results
2.1. Method Validation
2.1.1. Specificity Test
2.1.2. Calibration Curve and Linear Range
2.1.3. Precision and Accuracy
2.1.4. Substrate Effect and Extraction Recoveries
2.1.5. Stability Test
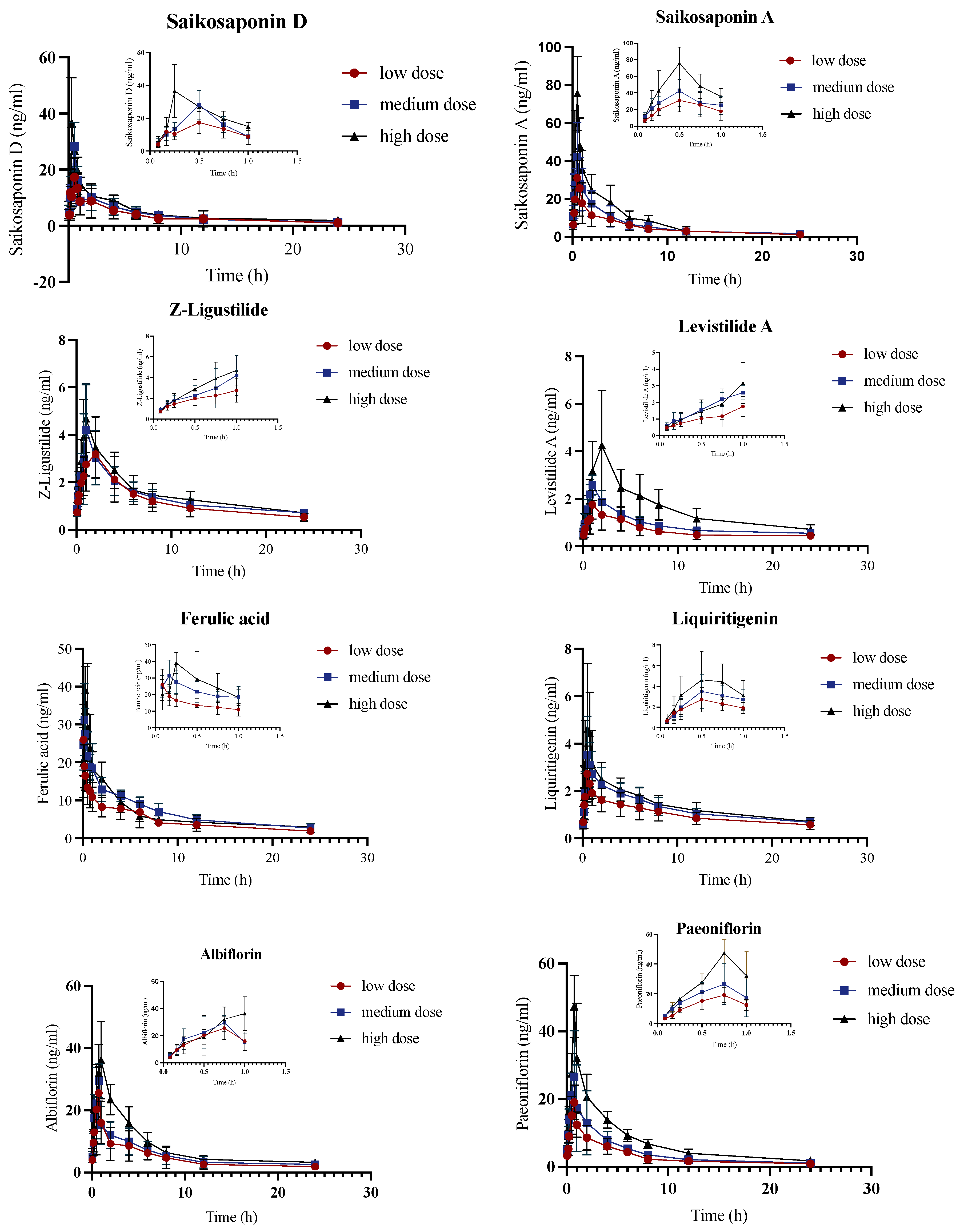
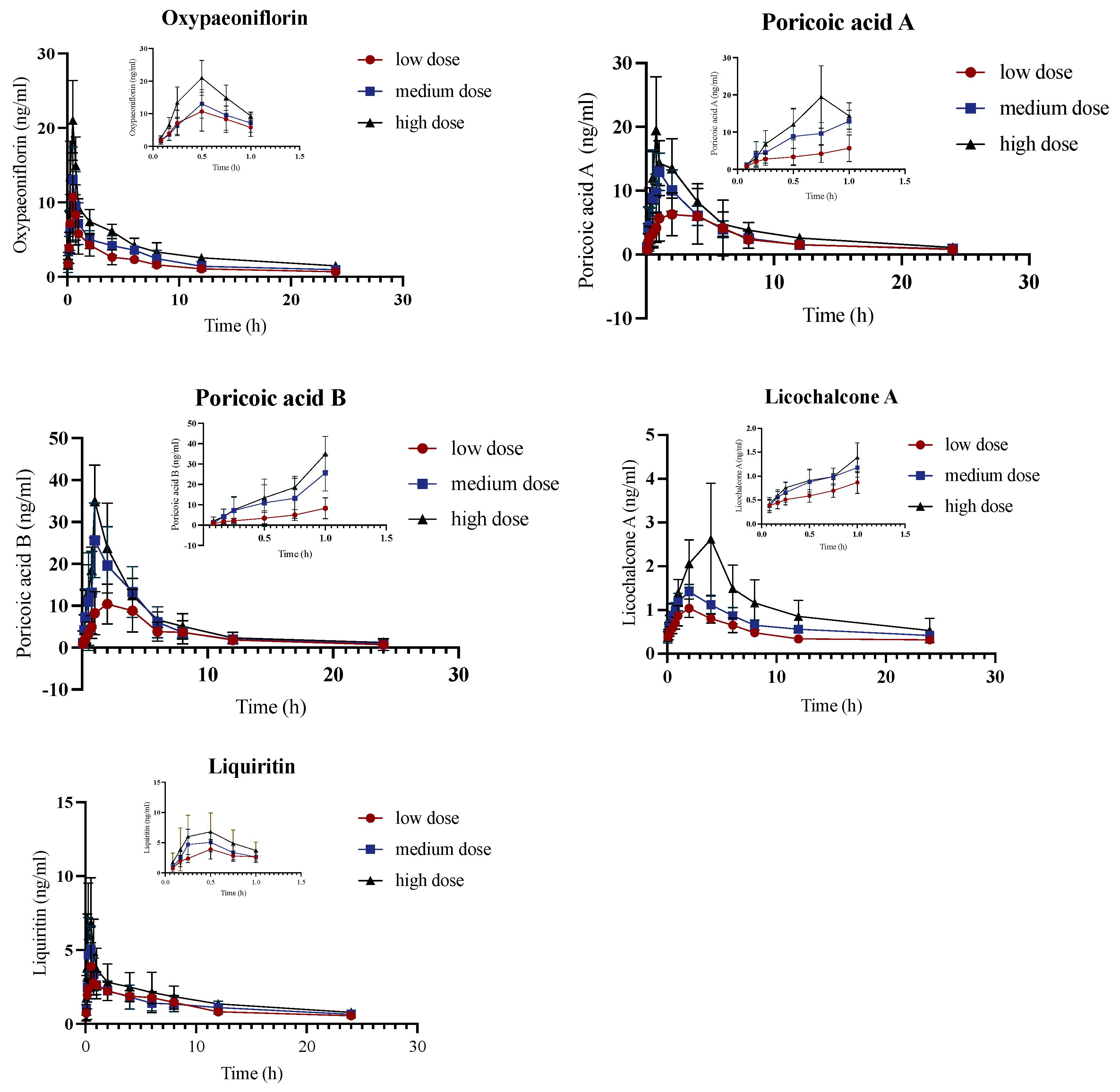
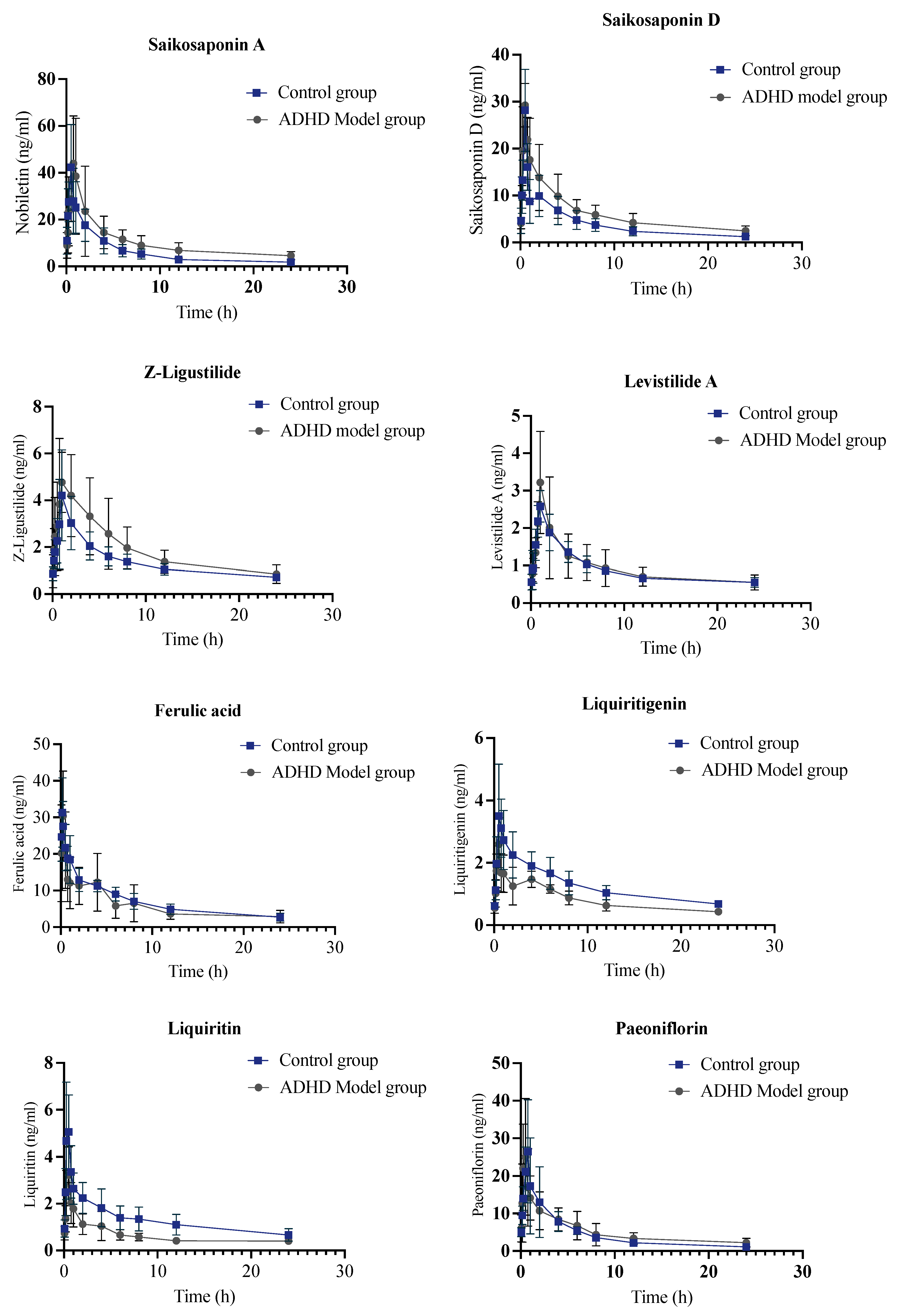
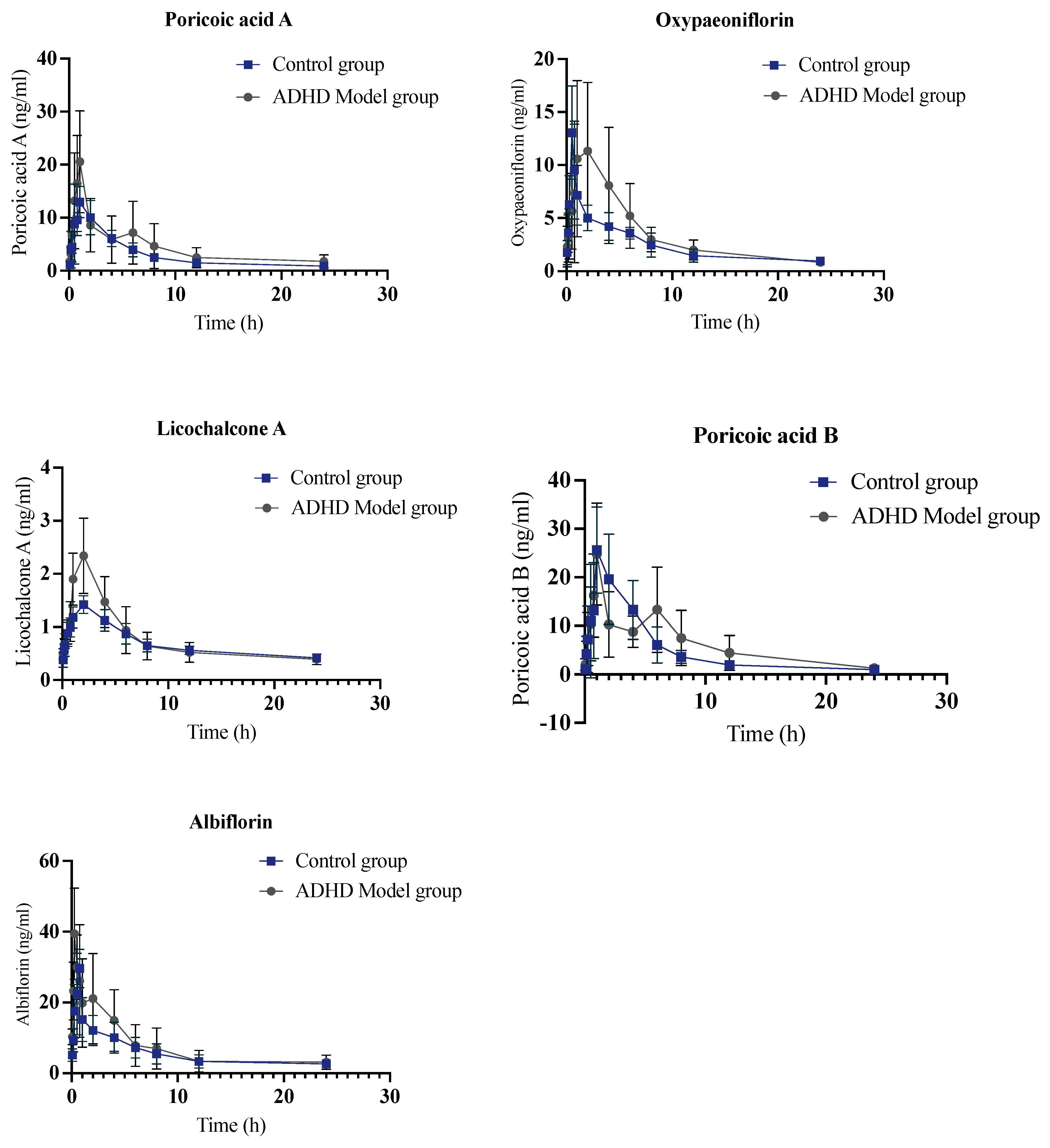
2.2. Pharmacokinetic Analysis of Control Group
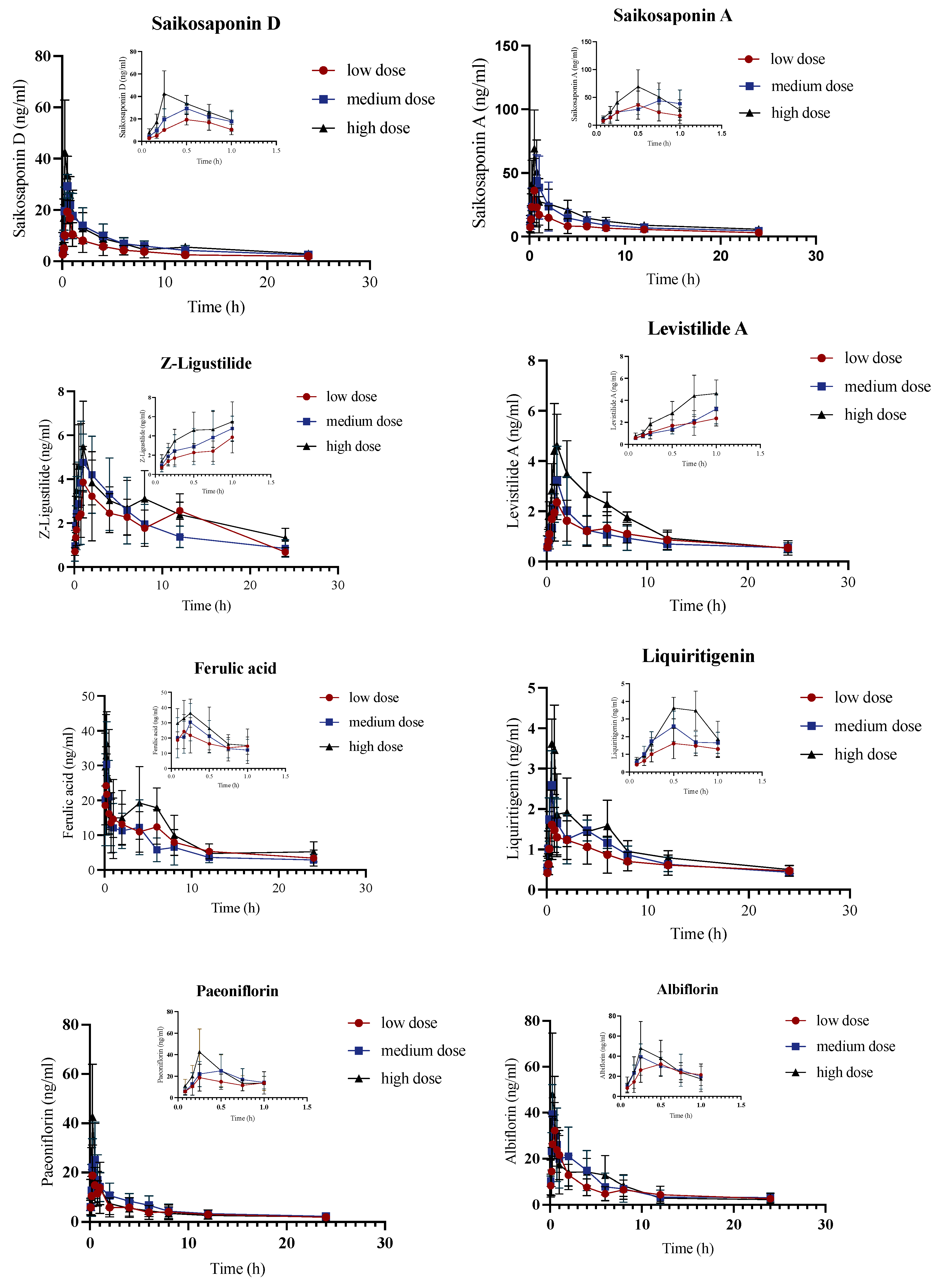
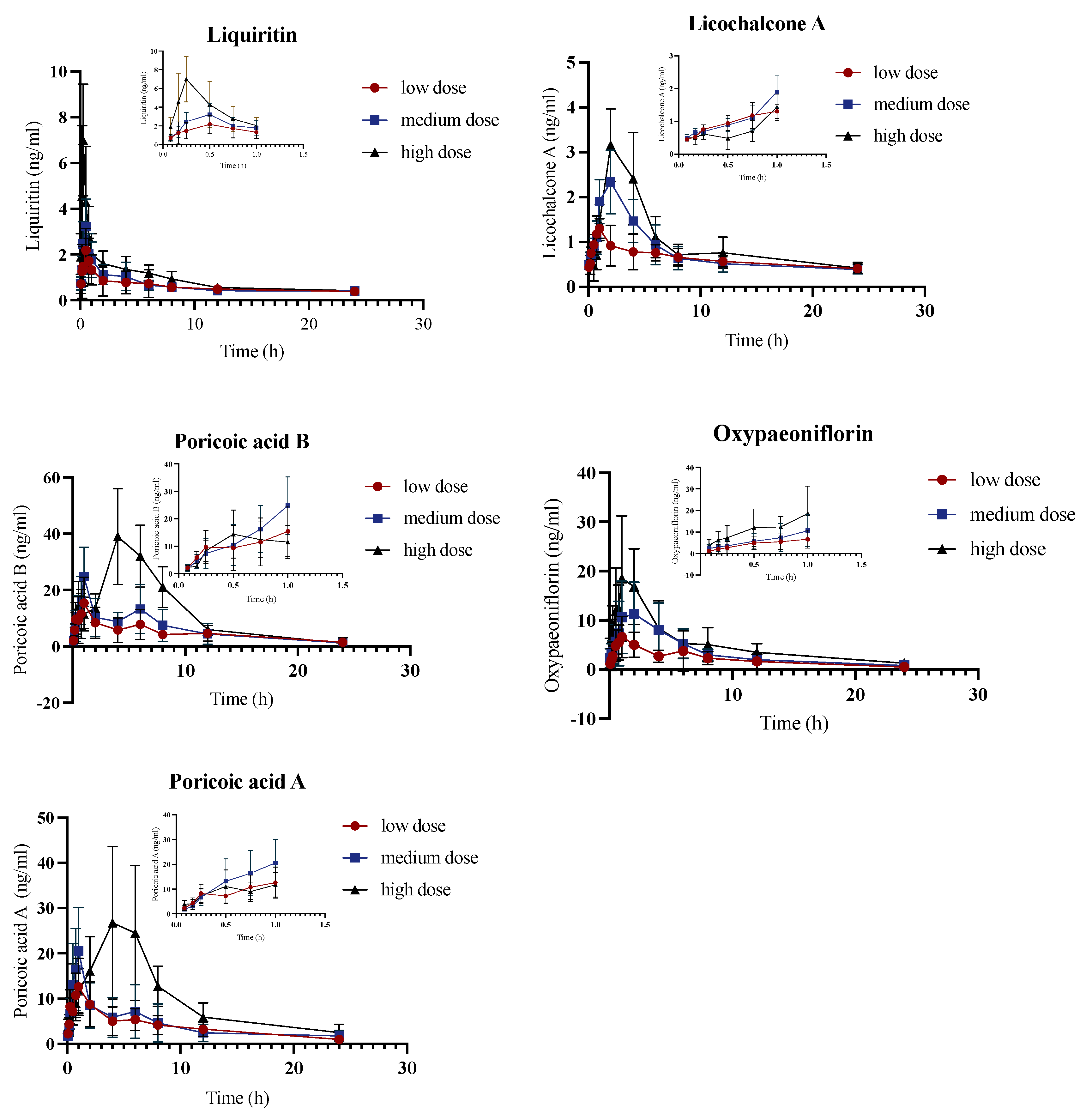
2.3. Pharmacokinetic Study of JYPs in ADHD Model Group
2.4. Pharmacokinetic Comparison of JYPs in Control Rats and ADHD Model Rats
3. Materials and Methods
3.1. Chemical and Reagents
3.2. Instruments
3.3. Experimental Animals
3.4. Preparation of JYP Gavage Solution
3.5. Preparation of Control Solution
3.6. Drug Administration
3.7. Pretreatment of Biological Samples
3.8. Chromatography–Mass Spectrometry Detection Conditions
3.9. Method Validation
3.10. Statistical Analyses and Data Processing
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Saketkoo, L.A.; Escorpizo, R.; Varga, J.; Keen, K.J.; Fligelstone, K.; Birring, S.S.; Alexanderson, H.; Pettersson, H.; Chaudhry, H.A.; Poole, J.L.; et al. World Health Organization (WHO) International Classification of Functioning, Disability and Health (ICF) Core Set Development for Interstitial Lung Disease. Front. Pharmacol. 2022, 13, 979788. [Google Scholar] [CrossRef]
- World Health Organization. The Global Burden of Disease: 2004 Update; World Health Organization: Geneva, Switzerland, 2008; p. 146. [Google Scholar]
- Chen, F.; Zheng, D.; Liu, J.; Gong, Y.; Guan, Z.; Lou, D. Depression and Anxiety among Adolescents during COVID-19: A Cross-Sectional Study. Brain Behav. Immun. 2020, 88, 36–38. [Google Scholar] [CrossRef]
- Malhi, G.S.; Mann, J.J. Depression. Lancet 2018, 392, 2299–2312. [Google Scholar] [CrossRef]
- Gold, S.M.; Köhler-Forsberg, O.; Moss-Morris, R.; Mehnert, A.; Miranda, J.J.; Bullinger, M.; Steptoe, A.; Whooley, M.A.; Otte, C. Comorbid Depression in Medical Diseases. Nat. Rev. Dis. Prim. 2020, 6, 69. [Google Scholar] [CrossRef]
- Yu, Y.; Zhang, G.; Han, T.; Huang, H. Efficacy and Safety of Oral Traditional Chinese Patent Medicine in Treatment of Liver Stagnation and Spleen Deficiency of Depression: A Protocol for Systematic Review. Medicine 2020, 99, e19142. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Ming, S.; Chen, X.; Zhang, T.; Qian, H.; Peng, S.; Ding, Y. Herbal Medicine as Adjunctive Therapy with Antidepressants for Post-Stroke Depression: A Systematic Review and Network Meta-Analysis of Randomized Controlled Trials. Front. Pharmacol. 2023, 14, 1180071. [Google Scholar] [CrossRef]
- Wang, Y.-S.; Shen, C.-Y.; Jiang, J.-G. Antidepressant Active Ingredients from Herbs and Nutraceuticals Used in TCM: Pharmacological Mechanisms and Prospects for Drug Discovery. Pharmacol. Res. 2019, 150, 104520. [Google Scholar] [CrossRef]
- Wu, T.; Yue, T.; Yang, P.; Jia, Y. Notable Efficacy of Shugan Jieyu Capsule in Treating Adult with Post-Stroke Depression: A PRISMA-Compliant Meta-Analysis of Randomized Controlled Trials. J. Ethnopharmacol. 2022, 294, 115367. [Google Scholar] [CrossRef] [PubMed]
- Yao, G.; Zhang, X.; Li, J.; Liu, S.; Li, X.; Liu, P.; Xu, Y. Improving Depressive Symptoms of Post-Stroke Depression Using the Shugan Jieyu Capsule: A Resting-State Functional Magnetic Resonance Imaging Study. Front. Neurol. 2022, 13, 860290. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zheng, S.; Feng, S.; Ma, R.; Jia, Y.; Zhao, A.; Wei, D.; Guo, H.; Duan, N.; Ding, Y.; et al. Effects of Jie Yu Wan on Generalized Anxiety Disorder: A Randomized Clinical Trial. Evid.-Based Complement. Altern. Med. 2022, 2022, 9951693. [Google Scholar] [CrossRef]
- Liu, W.; Qin, J. Clinical Efficacy and Safety of the Shugan Jieyu Capsule in Patients with Acute Myocardial Infarction and Depression. Int. J. Psychiatry Med. 2016, 51, 534–543. [Google Scholar] [CrossRef]
- Tan, J.; Li, X.; Zhu, Y.; Sullivan, M.A.; Deng, B.; Zhai, X.; Lu, Y. Antidepressant Shugan Jieyu Capsule Alters Gut Microbiota and Intestinal Microbiome Function in Rats with Chronic Unpredictable Mild Stress-Induced Depression. Front. Pharmacol. 2022, 13, 828595. [Google Scholar] [CrossRef]
- Zhang, M.; Bai, X. Shugan Jieyu Capsule in Post-Stroke Depression Treatment: From Molecules to Systems. Front. Pharmacol. 2022, 13, 821270. [Google Scholar] [CrossRef]
- Hohmann, N.; Kocheise, F.; Carls, A.; Burhenne, J.; Weiss, J.; Haefeli, W.E.; Mikus, G. Dose-Dependent Bioavailability and CYP3A Inhibition Contribute to Non-Linear Pharmacokinetics of Voriconazole. Clin. Pharmacokinet. 2016, 55, 1535–1545. [Google Scholar] [CrossRef]
- Yang, S.; Qu, Y.; Wang, J.; Gao, F.; Ji, M.; Xie, P.; Zhu, A.; Tan, B.; Wang, X.; Zhu, G. Anshen Dingzhi Prescription in the Treatment of PTSD in Mice: Investigation of the Underlying Mechanism from the Perspective of Hippocampal Synaptic Function. Phytomedicine 2022, 101, 154139. [Google Scholar] [CrossRef]
- Li, D.; Rui, Y.; Guo, S.; Luan, F.; Liu, R.; Zeng, N. Ferulic Acid: A Review of Its Pharmacology, Pharmacokinetics and Derivatives. Life Sci. 2021, 284, 119921. [Google Scholar] [CrossRef] [PubMed]
- Yan, N.; Tang, Z.; Xu, Y.; Li, X.; Wang, Q. Pharmacokinetic Study of Ferulic Acid Following Transdermal or Intragastric Administration in Rats. AAPS PharmSciTech 2020, 21, 169. [Google Scholar] [CrossRef]
- Guo, J.; Zhang, F.; Gao, J.; Guan, X.; Liu, B.; Wang, X.; Qin, Z.; Tang, K.; Liu, S. Proteomics-based Screening of the Target Proteins Associated with Antidepressant-like Effect and Mechanism of Saikosaponin A. J. Cell. Mol. Med. 2020, 24, 174–188. [Google Scholar] [CrossRef] [PubMed]
- Chao, B.; Huang, S.; Pan, J.; Zhang, Y.; Wang, Y. Saikosaponin d Downregulates microRNA-155 and Upregulates FGF2 to Improve Depression-like Behaviors in Rats Induced by Unpredictable Chronic Mild Stress by Negatively Regulating NF-κB. Brain Res. Bull. 2020, 157, 69–76. [Google Scholar] [CrossRef]
- Han, Y.J.; Kang, B.; Yang, E.-J.; Choi, M.-K.; Song, I.-S. Simultaneous Determination and Pharmacokinetic Characterization of Glycyrrhizin, Isoliquiritigenin, Liquiritigenin, and Liquiritin in Rat Plasma Following Oral Administration of Glycyrrhizae Radix Extract. Molecules 2019, 24, 1816. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Chen, J.; Peng, F.; Sun, C.; Lei, Y.; Chen, G.; Li, G.; Yin, Y.; Lin, Z.; Wu, L.; et al. Pharmacological Activities and Pharmacokinetics of Liquiritin: A Review. J. Ethnopharmacol. 2022, 293, 115257. [Google Scholar] [CrossRef]
- Shi, C.-C.; Qin, K.-M.; Xu, K.; Chen, A.; Cai, T.; Cai, B.-C. Development of Liquiritigenin-Phospholipid Complex with the Enhanced Oral Bioavailability. Chin. J. Nat. Med. 2020, 18, 916–921. [Google Scholar] [CrossRef]
- Zhao, D.; Zhang, J.; Zhu, Y.; He, C.; Fei, W.; Yue, N.; Wang, C.; Wang, L. Study of Antidepressant-Like Effects of Albiflorin and Paeoniflorin Through Metabolomics From the Perspective of Cancer-Related Depression. Front. Neurol. 2022, 13, 828612. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Qiao, T.; Wang, Y.; Wang, Q.-S.; Cui, Y.-L. Alginate Nanogels-Based Thermosensitive Hydrogel to Improve Antidepressant-like Effects of Albiflorin via Intranasal Delivery. Drug Deliv. 2021, 28, 2137–2149. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.-S.; Yan, K.; Li, K.-D.; Gao, L.-N.; Wang, X.; Liu, H.; Zhang, Z.; Li, K.; Cui, Y.-L. Targeting Hippocampal Phospholipid and Tryptophan Metabolism for Antidepressant-like Effects of Albiflorin. Phytomedicine 2021, 92, 153735. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Hou, X.; Hu, X.; Lu, C.; Liu, C.; Wang, J.; Liu, W.; Teng, L.; Wang, D. Not Only Serotonergic System, but Also Dopaminergic System Involved in Albiflorin against Chronic Unpredictable Mild Stress-Induced Depression-like Behavior in Rats. Chem.-Biol. Interact. 2015, 242, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.-X.; Fu, J.; Ma, S.-R.; Peng, R.; Yu, J.-B.; Cong, L.; Pan, L.-B.; Zhang, Z.-G.; Tian, H.; Che, C.-T.; et al. Gut-Brain Axis Metabolic Pathway Regulates Antidepressant Efficacy of Albiflorin. Theranostics 2018, 8, 5945–5959. [Google Scholar] [CrossRef] [PubMed]
- Ni, H.; Liao, Y.; Zhang, Y.; Lu, H.; Huang, Z.; Huang, F.; Zhang, Z.; Dong, Y.; Wang, Z.; Huang, Y. Levistilide A Ameliorates Neuroinflammation via Inhibiting JAK2/STAT3 Signaling for Neuroprotection and Cognitive Improvement in Scopolamine-Induced Alzheimer’s Disease Mouse Model. Int. Immunopharmacol. 2023, 124, 110783. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-M.; Shen, J.-D.; Xu, L.-P.; Li, H.-B.; Li, Y.-C.; Yi, L.-T. Ferulic Acid Inhibits Neuro-Inflammation in Mice Exposed to Chronic Unpredictable Mild Stress. Int. Immunopharmacol. 2017, 45, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Cheng, Y.; Chen, Y.; Yue, Y.; Li, Y.; Xia, S.; Li, Y.; Deng, H.; Zhang, J.; Cao, Y. Ferulic Acid Improves Depressive-Like Behavior in Prenatally-Stressed Offspring Rats via Anti-Inflammatory Activity and HPA Axis. Int. J. Mol. Sci. 2019, 20, 493. [Google Scholar] [CrossRef]
- Pathak, L.; Agrawal, Y.; Dhir, A. Natural Polyphenols in the Management of Major Depression. Expert Opin. Investig. Drugs 2013, 22, 863–880. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Zhao, D. Ferulic Acid as a Therapeutic Agent in Depression: Evidence from Preclinical Studies. CNS Neurosci. Ther. 2023, 29, 2397–2412. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Qiu, J.; Wei, C.; Wu, Y.; Li, Y.; Hu, H.; Wang, Z. Comprehensive Quality Evaluation of Two Different Geography Originated Angelica Sinensis Radix Based on Potential Production Area Development and Resource Protection. Plant Physiol. Biochem. 2023, 201, 107878. [Google Scholar] [CrossRef] [PubMed]
- Xie, Q.; Zhang, L.; Xie, L.; Zheng, Y.; Liu, K.; Tang, H.; Liao, Y.; Li, X. Z-ligustilide: A Review of Its Pharmacokinetics and Pharmacology. Phytother. Res. 2020, 34, 1966–1991. [Google Scholar] [CrossRef]

| Compound | Calibration Curve | Linear Range (ng/mL) | r |
|---|---|---|---|
| Saikosaponin A | y = 0.0039x + 0.0527 | 0.53–334.12 | 0.9944 |
| Saikosaponin D | y = 0.0039x + 0.0172 | 0.47–298.65 | 0.9979 |
| Z-Ligustilide | y = 0.0164x + 0.3872 | 0.39–99.72 | 0.9977 |
| Levistilide A | y = 0.0249x + 0.5986 | 0.26–133.65 | 0.9954 |
| Ferulic acid | y = 0.0017x + 0.0083 | 0.44–275.83 | 0.9993 |
| Liquiritigenin | y = 0.0265x + 0.1190 | 0.22–109.96 | 0.9992 |
| Liquiritin | y = 0.018x + 0.0918 | 0.39–99.8 | 0.9993 |
| Paeoniflorin | y = 0.0001x + 0.0038 | 0.37–231.77 | 0.9944 |
| Albiflorin | y = 0.0003x − 0.0012 | 0.37–236.9 | 0.9980 |
| Oxypaeoniflorin | y = 0.0012x + 0.0076 | 0.44–110.4 | 0.9986 |
| Poricoic acid A | y = 0.0105x + 0.2400 | 0.42–107.04 | 0.9959 |
| Poricoic acid B | y = 0.0045x + 0.0166 | 0.45–114.7 | 0.9986 |
| Licochalcone A | y = 0.1271x + 0.2547 | 0.21–107.91 | 0.9987 |
| Compound | Concentration | Intra-Day | Inter-Day | ||||
|---|---|---|---|---|---|---|---|
| Mean ± SD | Accuracy | Precision | Mean ± SD | Accuracy | Precision | ||
| ng/mL | ng/mL | RE, % | RSD, % | ng/mL | RE, % | RSD, % | |
| Saikosaponin A | 334.12 | 338.83 ± 10.69 | 1.41 | 3.16 | 336.59 ± 25.59 | 6.66 | 1.46 |
| 167.06 | 167.65 ± 5.61 | 0.35 | 3.35 | 163.66 ± 4.12 | 1.42 | 4.16 | |
| 0.53 | 0.50 ± 0.02 | −6.17 | 3.58 | 0.52 ± 0.03 | −2.64 | 6.19 | |
| Saikosaponin D | 298.65 | 286.23 ± 7.83 | −4.16 | 2.74 | 283.88 ± 11.11 | −4.94 | 3.91 |
| 149.32 | 136.60 ± 6.17 | 0.32 | 4.52 | 143.01 ± 7.40 | 0.32 | 5.17 | |
| 0.48 | 0.42 ± 0.02 | 1.71 | 4.82 | 0.44 ± 0.03 | 1.71 | 6.68 | |
| Z-Ligustilide | 99.72 | 103.01 ± 4.68 | 3.29 | 4.54 | 99.43 ± 4.54 | 3.30 | 2.46 |
| 49.86 | 50.42 ± 1.64 | 1.13 | 3.25 | 45.16 ± 3.20 | 0.83 | 2.58 | |
| 0.20 | 0.20 ± 0.01 | −2.22 | 5.45 | 0.19 ± 0.01 | −4.13 | 3.27 | |
| Levistilide A | 133.65 | 137.57 ± 4.37 | 2.93 | 3.18 | 133.81 ± 5.30 | 0.12 | 3.96 |
| 66.82 | 64.34 ± 2.92 | −3.72 | 4.54 | 61.59 ± 3.90 | −7.82 | 6.32 | |
| 0.26 | 0.24 ± 0.01 | −7.88 | 3.87 | 0.24 ± 0.02 | −4.43 | 5.88 | |
| Ferulic acid | 275.83 | 280.29 ± 5.39 | 1.62 | 1.92 | 270.57 ± 16.60 | −1.91 | 6.14 |
| 137.92 | 135.87 ± 7.97 | −1.49 | 5.86 | 135.87 ± 7.97 | −5.61 | 8.78 | |
| 0.44 | 0.43 ± 0.02 | −2.96 | 4.65 | 0.41 ± 0.06 | −3.93 | 5.65 | |
| Liquiritigenin | 109.96 | 111.12 ± 2.31 | 1.05 | 2.08 | 106.17 ± 4.74 | −2.73 | 4.67 |
| 54.98 | 54.13 ± 2.46 | −1.54 | 4.54 | 52.07 ± 4.78 | −1.87 | 4.31 | |
| 0.22 | 0.21 ± 0.00 | −6.13 | 1.30 | 0.21 ± 0.02 | −4.39 | 5.98 | |
| Liquiritin | 99.80 | 104.48 ± 1.90 | 4.69 | 1.82 | 99.16 ± 3.72 | −0.64 | 3.75 |
| 49.90 | 48.44 ± 3.11 | −2.92 | 6.42 | 46.29 ± 1.78 | −7.24 | 3.84 | |
| 0.39 | 0.36 ± 0.01 | −7.90 | 3.24 | 0.37 ± 0.02 | −4.45 | 5.63 | |
| Paeoniflorin | 237.77 | 230.19 ± 13.02 | −3.19 | 5.66 | 236.63 ± 16.46 | −0.48 | 6.96 |
| 115.89 | 106.94 ± 4.70 | −7.72 | 4.39 | 102.51 ± 8.40 | 6.05 | 2.40 | |
| 0.37 | 0.40 ± 0.01 | 8.98 | 3.70 | 0.35 ± 0.02 | −0.68 | 4.73 | |
| Albiflorin | 236.90 | 230.32 ± 8.11 | −2.78 | 3.52 | 236.71 ± 11.63 | −0.08 | 4.91 |
| 118.45 | 122.04 ± 2.41 | 3.03 | 1.98 | 105.95 ± 6.58 | 1.56 | 3.60 | |
| 0.37 | 0.36 ± 0.02 | −2.48 | 4.44 | 0.35 ± 0.02 | −4.98 | 4.65 | |
| Oxypaeoniflorin | 110.40 | 109.05 ± 1.12 | −1.22 | 1.03 | 106.90 ± 5.92 | 2.95 | 2.42 |
| 55.20 | 53.77 ± 2.82 | −2.59 | 5.25 | 48.93 ± 2.48 | −0.71 | 3.55 | |
| 0.44 | 0.42 ± 0.01 | −4.23 | 2.52 | 0.44 ± 0.02 | −0.68 | 4.17 | |
| Poricoic acid A | 107.04 | 104.64 ± 5.37 | −2.25 | 5.14 | 105.15 ± 2.17 | −1.77 | 2.07 |
| 53.52 | 56.00 ± 2.52 | 4.63 | 4.50 | 47.39 ± 2.61 | −0.96 | 4.86 | |
| 0.42 | 0.41 ± 0.01 | −2.79 | 2.81 | 0.42 ± 0.02 | 0.88 | 5.87 | |
| Poricoic acid B | 114.70 | 113.56 ± 2.46 | −0.99 | 2.17 | 104.84 ± 4.25 | −0.32 | 2.48 |
| 57.35 | 56.44 ± 1.24 | −1.59 | 2.20 | 48.90 ± 2.67 | −0.98 | 3.05 | |
| 0.45 | 0.45 ± 0.02 | −0.40 | 4.41 | 0.45 ± 0.02 | 0.26 | 4.61 | |
| Licochalcone A | 107.91 | 104.80 ± 3.22 | −2.88 | 3.08 | 103.65 ± 2.58 | −0.23 | 2.33 |
| 53.96 | 53.95 ± 2.21 | 1.06 | 3.53 | 48.21 ± 2.24 | 1.21 | 5.36 | |
| 0.21 | 0.20 ± 0.01 | −3.30 | 2.71 | 0.20 ± 0.01 | 1.61 | 3.63 | |
| Compound | Concentration Added | Matrix Effect | Extraction Recovery | ||
|---|---|---|---|---|---|
| (ng/mL) | Mean ± SD (%) | RSD (%) | Mean ± SD (%) | RSD (%) | |
| Saikosaponin A | 334.12 | 92.43 ± 3.31 | 3.59 | 90.12 ± 2.48 | 2.75 |
| 167.06 | 94.11 ± 5.35 | 5.69 | 92.17 ± 4.31 | 4.68 | |
| 0.53 | 90.93 ± 6.05 | 6.65 | 88.33 ± 6.94 | 7.86 | |
| Saikosaponin D | 298.65 | 95.28 ± 4.82 | 5.06 | 91.15 ± 6.00 | 6.58 |
| 149.32 | 92.86 ± 5.38 | 5.79 | 88.44 ± 4.48 | 5.07 | |
| 0.48 | 96.43 ± 7.63 | 7.91 | 90.67 ± 6.57 | 7.25 | |
| Z-Ligustilide | 99.72 | 91.10 ± 5.95 | 6.53 | 90.07 ± 3.35 | 3.72 |
| 49.86 | 97.15 ± 4.82 | 4.96 | 92.93 ± 4.39 | 4.73 | |
| 0.20 | 87.78 ± 6.41 | 7.30 | 93.24 ± 3.07 | 3.30 | |
| Levistilide A | 133.65 | 88.60 ± 3.82 | 4.31 | 88.52 ± 3.73 | 4.22 |
| 66.82 | 97.45 ± 4.89 | 5.02 | 90.58 ± 7.33 | 8.10 | |
| 0.26 | 94.08 ± 8.04 | 8.55 | 92.43 ± 5.81 | 6.29 | |
| Ferulic acid | 275.83 | 95.58 ± 5.90 | 6.18 | 94.51 ± 2.97 | 3.14 |
| 137.92 | 92.03 ± 7.10 | 7.72 | 91.20 ± 3.98 | 4.36 | |
| 0.44 | 90.01 ± 6.50 | 7.22 | 89.99 ± 4.23 | 4.70 | |
| Liquiritigenin | 99.80 | 94.15 ± 7.07 | 7.51 | 94.04 ± 3.48 | 3.70 |
| 49.90 | 87.59 ± 4.98 | 5.69 | 87.83 ± 7.88 | 8.97 | |
| 0.39 | 96.11 ± 4.96 | 4.88 | 90.97 ± 4.30 | 4.73 | |
| Liquiritin | 109.96 | 91.46 ± 3.43 | 3.75 | 88.13 ± 7.37 | 8.37 |
| 54.98 | 101.24 ± 4.44 | 4.39 | 92.31 ± 4.86 | 5.27 | |
| 0.22 | 98.45 ± 6.07 | 6.17 | 90.99 ± 3.16 | 3.47 | |
| Paeoniflorin | 231.77 | 93.78 ± 5.31 | 5.66 | 93.15 ± 6.20 | 6.65 |
| 115.89 | 94.40 ± 6.66 | 7.06 | 89.84 ± 5.46 | 6.08 | |
| 0.37 | 91.78 ± 6.73 | 7.34 | 102.23 ± 8.76 | 8.57 | |
| Albiflorin | 236.90 | 93.91 ± 5.66 | 6.02 | 91.87 ± 4.18 | 4.54 |
| 118.45 | 92.65 ± 3.65 | 3.93 | 88.66 ± 4.16 | 4.70 | |
| 0.37 | 89.92 ± 5.60 | 6.23 | 93.31 ± 3.22 | 3.45 | |
| Oxypaeoniflorin | 110.40 | 94.84 ± 4.28 | 4.51 | 93.85 ± 2.92 | 3.12 |
| 55.20 | 93.51 ± 7.92 | 8.47 | 89.35 ± 5.93 | 6.64 | |
| 0.44 | 89.59 ± 5.92 | 6.61 | 90.80 ± 7.28 | 8.01 | |
| Poricoic acid A | 107.04 | 100.13 ± 3.67 | 3.67 | 94.65 ± 3.58 | 3.78 |
| 53.52 | 91.97 ± 4.20 | 4.57 | 86.48 ± 4.09 | 4.73 | |
| 0.42 | 89.52 ± 4.07 | 4.55 | 92.88 ± 4.48 | 4.82 | |
| Poricoic acid B | 114.70 | 88.84 ± 5.55 | 6.18 | 87.96 ± 4.64 | 5.27 |
| 57.35 | 100.83 ± 2.96 | 2.94 | 89.46 ± 4.66 | 5.21 | |
| 0.45 | 94.04 ± 3.90 | 4.15 | 90.74 ± 5.53 | 6.10 | |
| Licochalcone A | 107.91 | 94.24 ± 5.22 | 5.54 | 93.04 ± 5.80 | 6.23 |
| 53.96 | 99.05 ± 5.16 | 5.21 | 94.65 ± 1.15 | 1.22 | |
| 0.21 | 97.01 ± 4.95 | 5.10 | 90.59 ± 6.99 | 7.71 | |
| Compound | Concentration | Short-Term Stability | Long-Term Stability | Freeze–Thaw Stability | |||
|---|---|---|---|---|---|---|---|
| Mean ± SD | RSD | Mean ± SD | RSD | Mean ± SD | RSD | ||
| ng/mL | ng/mL | % | ng/mL | % | ng/mL | % | |
| Saikosaponin A | 334.12 | 333.79 ± 5.23 | 1.57 | 333.87 ± 8.64 | 2.59 | 329.84 ± 6.72 | 2.04 |
| 167.06 | 163.03 ± 6.85 | 4.20 | 157.57 ± 7.50 | 4.76 | 162.70 ± 2.48 | 1.52 | |
| 0.53 | 0.52 ± 0.02 | 4.10 | 0.53 ± 0.02 | 4.42 | 0.54 ± 0.02 | 3.10 | |
| Saikosaponin D | 298.65 | 292.71 ± 9.07 | 3.10 | 286.73 ± 7.86 | 2.74 | 297.24 ± 6.14 | 2.06 |
| 149.32 | 149.80 ± 6.27 | 4.19 | 144.97 ± 6.67 | 4.60 | 148.89 ± 2.57 | 1.72 | |
| 0.48 | 0.49 ± 0.03 | 6.27 | 0.48 ± 0.02 | 4.53 | 0.47 ± 0.02 | 4.53 | |
| Z-Ligustilide | 99.72 | 98.66 ± 3.18 | 3.22 | 95.84 ± 3.73 | 3.89 | 94.19 ± 7.09 | 7.53 |
| 49.86 | 49.75 ± 2.30 | 4.62 | 45.73 ± 2.98 | 6.53 | 45.33 ± 5.63 | 12.42 | |
| 0.20 | 0.20 ± 0.01 | 6.98 | 0.20 ± 0.01 | 5.86 | 0.18 ± 0.02 | 12.39 | |
| Levistilide A | 133.65 | 130.75 ± 10.35 | 7.92 | 121.87 ± 2.34 | 1.92 | 134.59 ± 5.82 | 4.32 |
| 66.82 | 66.05 ± 2.50 | 3.78 | 60.04 ± 2.90 | 4.84 | 59.51 ± 3.13 | 5.26 | |
| 0.26 | 0.24 ± 0.02 | 6.59 | 0.23 ± 0.01 | 4.83 | 0.24 ± 0.01 | 5.53 | |
| Ferulic acid | 275.83 | 270.59 ± 11.52 | 4.25 | 273.01 ± 7.25 | 2.65 | 267.03 ± 12.54 | 4.69 |
| 137.92 | 135.24 ± 2.82 | 2.08 | 130.44 ± 5.80 | 4.45 | 130.24 ± 5.58 | 4.29 | |
| 0.44 | 0.46 ± 0.02 | 3.30 | 0.40 ± 0.02 | 5.72 | 0.38 ± 0.02 | 5.72 | |
| Liquiritigenin | 109.96 | 109.52 ± 5.78 | 5.28 | 100.24 ± 4.19 | 4.18 | 103.39 ± 4.89 | 4.73 |
| 54.98 | 54.93 ± 2.87 | 5.22 | 52.98 ± 2.55 | 4.82 | 52.83 ± 2.92 | 5.53 | |
| 0.22 | 0.21 ± 0.02 | 7.80 | 0.21 ± 0.01 | 4.29 | 0.23 ± 0.01 | 3.28 | |
| Liquiritin | 99.80 | 96.45 ± 2.60 | 2.69 | 95.60 ± 3.67 | 3.84 | 95.10 ± 3.05 | 3.20 |
| 49.90 | 48.26 ± 3.11 | 6.44 | 47.24 ± 1.59 | 3.37 | 48.52 ± 1.78 | 3.67 | |
| 0.39 | 0.37 ± 0.02 | 5.06 | 0.40 ± 0.01 | 3.60 | 0.40 ± 0.01 | 3.67 | |
| Paeoniflorin | 237.77 | 237.17 ± 6.60 | 2.78 | 231.08 ± 10.05 | 4.35 | 230.52 ± 6.66 | 2.89 |
| 115.89 | 111.05 ± 5.21 | 4.74 | 112.57 ± 4.52 | 4.01 | 111.74 ± 4.34 | 3.89 | |
| 0.37 | 0.37 ± 0.02 | 5.22 | 0.35 ± 0.01 | 2.67 | 0.33 ± 0.01 | 3.00 | |
| Albiflorin | 236.90 | 230.34 ± 9.76 | 4.24 | 234.12 ± 5.07 | 2.17 | 225.62 ± 4.52 | 2.00 |
| 118.45 | 115.59 ± 4.47 | 3.87 | 1140.07 ± 4.96 | 4.35 | 113.38 ± 3.35 | 2.95 | |
| 0.37 | 0.37 ± 0.02 | 5.65 | 0.35 ± 0.01 | 3.57 | 0.35 ± 0.02 | 5.27 | |
| Oxypaeoniflorin | 110.40 | 106.80 ± 3.52 | 3.30 | 108.06 ± 4.11 | 3.80 | 105.05 ± 2.64 | 2.52 |
| 55.20 | 54.62 ± 1.43 | 2.62 | 52.95 ± 1.78 | 3.37 | 53.32 ± 2.50 | 4.68 | |
| 0.44 | 0.43 ± 0.02 | 3.61 | 0.42 ± 0.02 | 4.77 | 0.44 ± 0.03 | 6.27 | |
| Poricoic acid A | 107.04 | 107.66 ± 3.53 | 3.28 | 107.10 ± 3.59 | 3.35 | 105.14 ± 1.60 | 1.52 |
| 53.52 | 51.71 ± 1.40 | 2.72 | 51.89 ± 1.82 | 3.50 | 51.34 ± 1.91 | 3.72 | |
| 0.42 | 0.42 ± 0.02 | 4.50 | 0.38 ± 0.02 | 5.18 | 0.44 ± 0.02 | 5.57 | |
| Poricoic acid B | 114.70 | 113.41 ± 4.31 | 3.80 | 111.42 ± 2.38 | 2.13 | 109.52 ± 3.94 | 3.60 |
| 57.35 | 53.19 ± 4.00 | 7.52 | 53.53 ± 2.25 | 4.21 | 52.46 ± 1.27 | 2.43 | |
| 0.45 | 0.43 ± 0.03 | 6.80 | 0.42 ± 0.02 | 4.70 | 0.44 ± 0.03 | 7.34 | |
| Licochalcone A | 107.91 | 107.24 ± 2.63 | 2.46 | 105.15 ± 2.91 | 2.77 | 105.15 ± 2.91 | 2.77 |
| 53.96 | 53.79 ± 0.97 | 1.81 | 52.17 ± 3.64 | 6.97 | 50.15 ± 1.82 | 3.62 | |
| 0.21 | 0.21 ± 0.01 | 4.17 | 0.20 ± 0.01 | 5.35 | 0.21 ± 0.01 | 5.77 | |
| Compound | Low Dose | Medium Dose | High Dose |
|---|---|---|---|
| μg/kg/d | μg/kg/d | μg/kg/d | |
| Saikosaponin A | 877.72 | 1775.44 | 3550.88 |
| Saikosaponin D | 557.39 | 1114.78 | 2229.56 |
| Z-Ligustilide | 904.50 | 1809.00 | 3618.00 |
| Levistilide A | 62.96 | 125.92 | 251.84 |
| Ferulic acid | 28.73 | 57.46 | 114.92 |
| Liquiritigenin | 16.31 | 32.62 | 65.24 |
| Liquiritin | 163.40 | 326.80 | 653.60 |
| Paeoniflorin | 2080.62 | 4161.24 | 8322.48 |
| Albiflorin | 1994.65 | 3989.30 | 7978.60 |
| Oxypaeoniflorin | 61.78 | 123.56 | 247.12 |
| Poricoic acid A | 23.65 | 47.30 | 94.60 |
| Poricoic acid B | 11.34 | 22.68 | 45.36 |
| Licochalcone A | 5.29 | 10.58 | 21.16 |
| Compound | Precursor Ion | Daughter Ion | Collision Voltage | RT (min) | ESI |
|---|---|---|---|---|---|
| Saikosaponin A | 779.41 | 144.88 | 45 | 10.89 | - |
| Saikosaponin D | 779.43 | 439.13 | 65 | 12.36 | - |
| Z-Ligustilide | 190.98 | 76.88 | 40 | 15.75 | + |
| Levistilide A | 381.11 | 78.88 | 47 | 15.76 | + |
| Ferulic acid | 192.96 | 133.96 | 15 | 7.44 | - |
| Liquiritigenin | 254.98 | 90.80 | 27 | 8.78 | - |
| Liquiritin | 416.98 | 118.88 | 45 | 6.96 | - |
| Paeoniflorin | 479.01 | 120.88 | 20 | 7.87 | - |
| Albiflorin | 479.01 | 76.88 | 42 | 6.48 | - |
| Oxypaeoniflorin | 495.05 | 136.88 | 29 | 5.17 | - |
| Poricoic acid A | 497.30 | 211.05 | 43 | 14.83 | - |
| Poricoic acid B | 483.31 | 409.13 | 30 | 14.27 | - |
| Licochalcone A | 339.03 | 92.88 | 44 | 12.47 | + |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Wan, Y.; Sun, S.; Wang, T.; Li, T.; Sun, Q.; Zhang, W.; Zhao, D.; Tian, Y.; Feng, S. Comparative Pharmacokinetics Research of 13 Bioactive Components of Jieyu Pills in Control and Attention Deficit Hyperactivity Disorder Model Rats Based on UPLC-Orbitrap Fusion MS. Molecules 2024, 29, 1230. https://doi.org/10.3390/molecules29061230
Liu X, Wan Y, Sun S, Wang T, Li T, Sun Q, Zhang W, Zhao D, Tian Y, Feng S. Comparative Pharmacokinetics Research of 13 Bioactive Components of Jieyu Pills in Control and Attention Deficit Hyperactivity Disorder Model Rats Based on UPLC-Orbitrap Fusion MS. Molecules. 2024; 29(6):1230. https://doi.org/10.3390/molecules29061230
Chicago/Turabian StyleLiu, Xuefang, Yan Wan, Shuding Sun, Ting Wang, Ting Li, Qi Sun, Weiwei Zhang, Di Zhao, Yange Tian, and Suxiang Feng. 2024. "Comparative Pharmacokinetics Research of 13 Bioactive Components of Jieyu Pills in Control and Attention Deficit Hyperactivity Disorder Model Rats Based on UPLC-Orbitrap Fusion MS" Molecules 29, no. 6: 1230. https://doi.org/10.3390/molecules29061230
APA StyleLiu, X., Wan, Y., Sun, S., Wang, T., Li, T., Sun, Q., Zhang, W., Zhao, D., Tian, Y., & Feng, S. (2024). Comparative Pharmacokinetics Research of 13 Bioactive Components of Jieyu Pills in Control and Attention Deficit Hyperactivity Disorder Model Rats Based on UPLC-Orbitrap Fusion MS. Molecules, 29(6), 1230. https://doi.org/10.3390/molecules29061230





