Theoretical Insights into Different Complexation Modes of Dioxovanadium(V) Compounds with Pyridoxal Semicarbazone/Thiosemicarbazone/S-Methyl-iso-thiosemicarbazone Ligands
Abstract
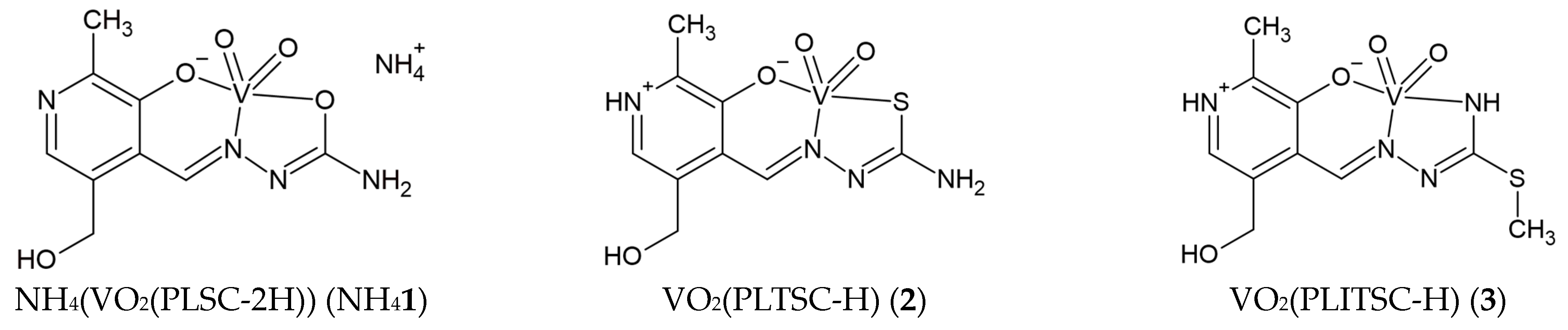
1. Introduction
2. Results and Discussion
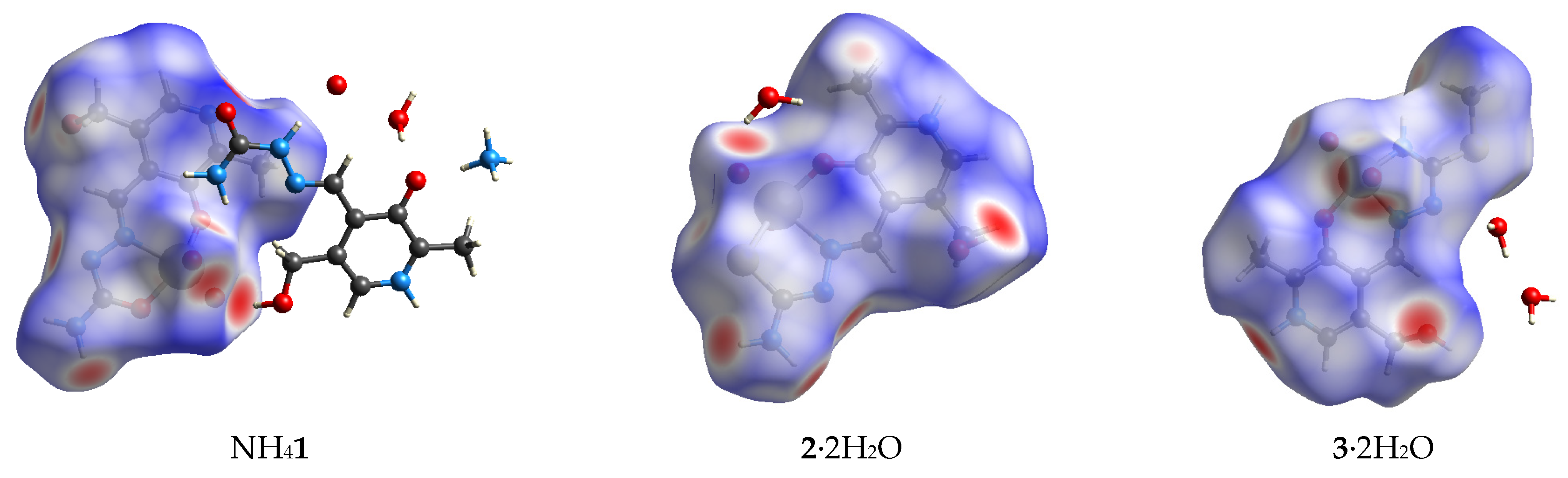
2.1. Hirshfeld Surface Analysis of Crystallographic Data
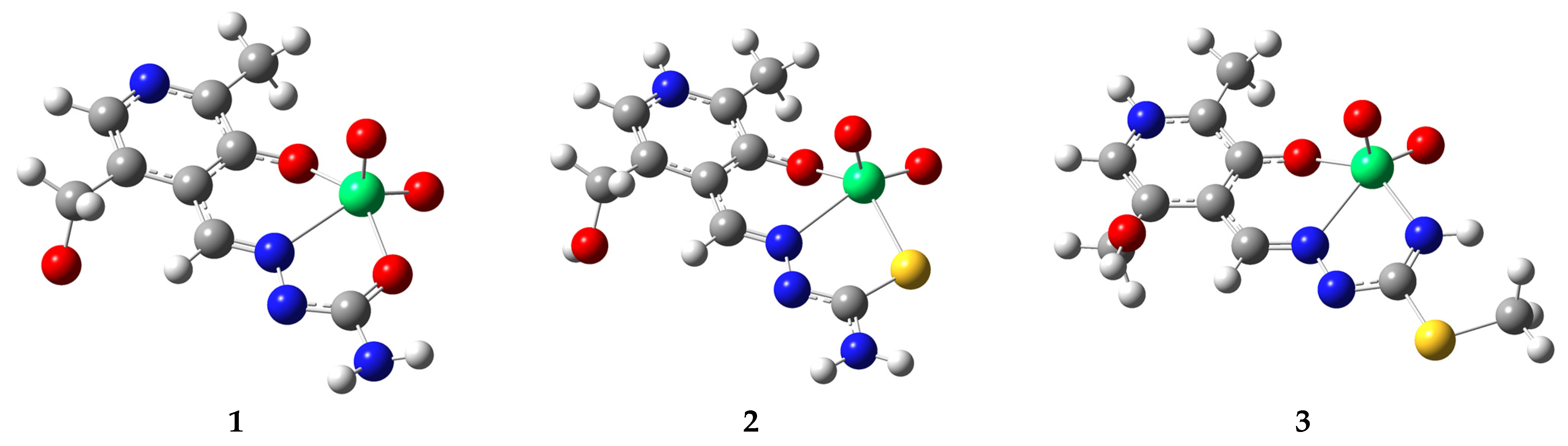
2.2. Optimization of Structures and Comparison with Crystalographic Data
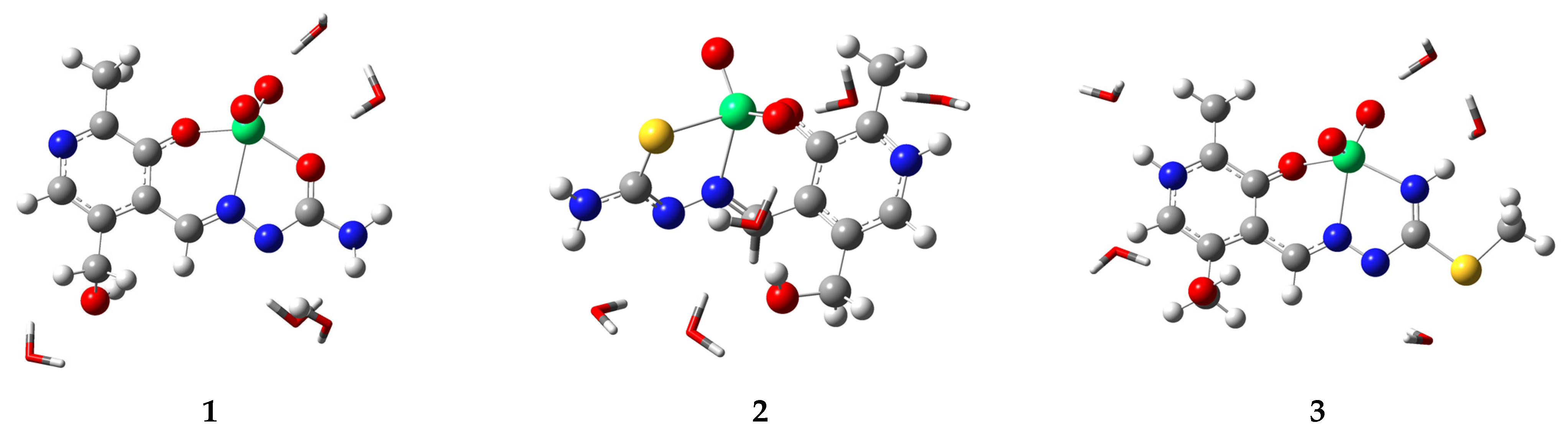
2.3. Explicit Solvent Effect Investigation
2.4. QTAIM Analysis of Structures
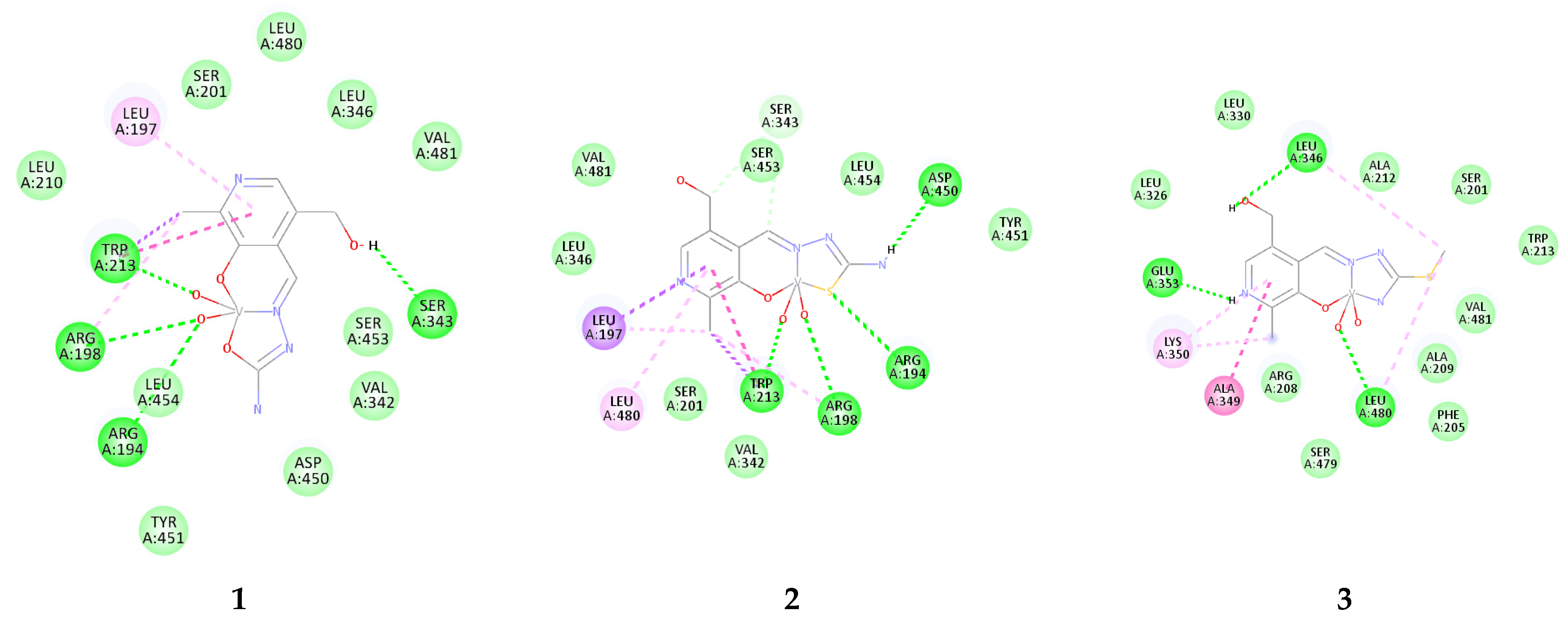
2.5. Molecular Docking Study of Interactions with BSA
3. Materials and Methods
3.1. Hirshfeld Surface Analysis
3.2. Theoretical Analysis
3.3. Molecular Docking Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sharfalddin, A.A.; Al-Younis, I.M.; Mohammed, H.A.; Dhahri, M.; Mouffouk, F.; Abu Ali, H.; Anwar, M.J.; Qureshi, K.A.; Hussien, M.A.; Alghrably, M.; et al. Therapeutic Properties of Vanadium Complexes. Inorganics 2022, 10, 244. [Google Scholar] [CrossRef]
- Crans, D.C.; Smee, J.J.; Gaidamauskas, E.; Yang, L. The Chemistry and Biochemistry of Vanadium and the Biological Activities Exerted by Vanadium Compounds. Chem. Rev. 2004, 104, 849–902. [Google Scholar] [CrossRef] [PubMed]
- Goc, A. Biological activity of vanadium compounds. Open Life Sci. 2006, 1, 314–332. [Google Scholar] [CrossRef]
- Domingo, J.L. Vanadium and Tungsten Derivatives as Antidiabetic Agents. Biol. Trace Elem. Res. 2002, 88, 97–112. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, H. A New Concept: The Use of Vanadium Complexes in the Treatment of Diabetes Mellitus. Chem. Rec. 2002, 2, 237–248. [Google Scholar] [CrossRef] [PubMed]
- Kostova, I. Titanium and Vanadium Complexes as Anticancer Agents. Anticancer Agents Med. Chem. 2009, 9, 827–842. [Google Scholar] [CrossRef] [PubMed]
- Maia, P.I.d.S.; Pavan, F.R.; Leite, C.Q.F.; Lemos, S.S.; de Sousa, G.F.; Batista, A.A.; Nascimento, O.R.; Ellena, J.; Castellano, E.E.; Niquet, E.; et al. Vanadium complexes with thiosemicarbazones: Synthesis, characterization, crystal structures and anti-Mycobacterium tuberculosis activity. Polyhedron 2009, 28, 398–406. [Google Scholar] [CrossRef]
- Barrio, D.A.; Etcheverry, S.B. Potential Use of Vanadium Compounds in Therapeutics. Curr. Med. Chem. 2010, 17, 3632–3642. [Google Scholar] [CrossRef]
- Evangelou, A.M. Vanadium in cancer treatment. Crit. Rev. Oncol. Hematol. 2002, 42, 249–265. [Google Scholar] [CrossRef]
- Ferretti, V.; León, I. An Overview of Vanadium and Cell Signaling in Potential Cancer Treatments. Inorganics 2022, 10, 47. [Google Scholar] [CrossRef]
- Pessoa, J.C.; Etcheverry, S.; Gambino, D. Vanadium compounds in medicine. Coord. Chem. Rev. 2015, 301–302, 24–48. [Google Scholar] [CrossRef] [PubMed]
- Shahzadi, S.; Jabeen, M.; Ali, S.; Fu, L.; Shahid, M.; Ahmad, M. Synthesis, characterization, anticancer activity and biological activities of vanadium complexes of 2-mercapto-5-methyl-benzimidazole as sulphur donor ligand. J. Anal. Pharm. Res. 2020, 9, 48–54. [Google Scholar] [CrossRef][Green Version]
- Damena, T.; Zeleke, D.; Desalegn, T.; Demissie, T.B.; Eswaramoorthy, R. Synthesis, Characterization, and Biological Activities of Novel Vanadium(IV) and Cobalt(II) Complexes. ACS Omega 2022, 7, 4389–4404. [Google Scholar] [CrossRef] [PubMed]
- Jevtović, V.S.; Pelosi, G.; Ianelli, S.; Kovačević, R.Z.; Kaišarević, S.N. Synthesis, structural studies and Biological activity of a dioxovanadium(V) complex with pyridoxal semicarbazone. Acta Chim. Slov. 2010, 57, 363–369. [Google Scholar] [PubMed]
- Shidhani, S.A.; Al Bouromi, M.; Al Ameri, S.; Al Ghawi, S.; Jevtovic, V. Synthesis and Structural Analysis of a Dioxovanadium (V) Complex Incorporating Pyridoxal-Thiosemicarbazone (PLTSC) Ligand. Am. J. Chem. 2016, 6, 8–11. [Google Scholar] [CrossRef]
- Leovac, V.; Divjakovic, V.; Joksovic, M.; Jovanovic, L.; Vojinovic-Jesic, L.; Cesljevic, V.; Mlinar, M. Transition metal complexes with thiosemicarbazide-based ligands. Part 57: Synthesis, spectral and structural characterization of dioxovanadium(V) and dioxomolybdenum(VI) complexes with pyridoxal s-methylisothiosemicarbazone. J. Serbian Chem. Soc. 2010, 75, 1063–1074. [Google Scholar] [CrossRef]
- Kargar, H.; Moghimi, A.; Fallah-Mehrjardi, M.; Behjatmanesh-Ardakani, R.; Rudbari, H.A.; Munawar, K.S. New oxovanadium and dioxomolybdenum complexes as catalysts for sulfoxidation: Experimental and theoretical investigations of E and Z isomers of ONO tridentate Schiff base ligand. J. Sulfur Chem. 2022, 43, 22–36. [Google Scholar] [CrossRef]
- Kargar, H.; Fallah-Mehrjardi, M.; Behjatmanesh-Ardakani, R.; Munawar, K.S.; Ashfaq, M.; Tahir, M.N. Synthesis, spectral characterization, SC-XRD, HSA, DFT and catalytic activity of novel dioxovanadium(V) complex with aminobenzohydrazone Schiff base ligand: An experimental and theoretical approach. Inorganica Chim. Acta 2021, 526, 120535. [Google Scholar] [CrossRef]
- Kargar, H.; Bazrafshan, M.; Fallah-Mehrjardi, M.; Behjatmanesh-Ardakani, R.; Rudbari, H.A.; Munawar, K.S.; Ashfaq, M.; Tahir, M.N. Synthesis, characterization, crystal structures, Hirshfeld surface analysis, DFT computational studies and catalytic activity of novel oxovanadium and dioxomolybdenum complexes with ONO tridentate Schiff base ligand. Polyhedron 2021, 202, 115194. [Google Scholar] [CrossRef]
- Jevtović, V.; Hamoud, H.; Al-Zahrani, S.; Alenezi, K.; Latif, S.; Alanazi, T.; Abdulaziz, F.; Dimić, D. Synthesis, Crystal Structure, Quantum Chemical Analysis, Electrochemical Behavior, and Antibacterial and Photocatalytic Activity of Co Complex with Pyridoxal-(S-Methyl)-isothiosemicarbazone Ligand. Molecules 2022, 27, 4809. [Google Scholar] [CrossRef]
- Jevtovic, V.; Alshammari, N.; Latif, S.; Alsukaibi, A.K.D.; Humaidi, J.; Alanazi, T.Y.A.; Abdulaziz, F.; Matalka, S.I.; Pantelić, N.Đ.; Marković, M.; et al. Synthesis, Crystal Structure, Theoretical Calculations, Antibacterial Activity, Electrochemical Behavior, and Molecular Docking of Ni(II) and Cu(II) Complexes with Pyridoxal-Semicarbazone. Molecules 2022, 27, 6322. [Google Scholar] [CrossRef] [PubMed]
- Jevtovic, V.; Alshamari, A.K.; Milenković, D.; Dimitrić Marković, J.; Marković, Z.; Dimić, D. The Effect of Metal Ions (Fe, Co, Ni, and Cu) on the Molecular-Structural, Protein Binding, and Cytotoxic Properties of Metal Pyridoxal-Thiosemicarbazone Complexes. Int. J. Mol. Sci. 2023, 24, 11910. [Google Scholar] [CrossRef]
- Abdulaziz, F.; Alabbosh, K.F.; Alshammari, O.A.O.; Tuwalah, W.M.B.; Alanazi, T.Y.A.; Rakić, A.; Barić, M.; Marković, M.; Jevtovic, V.; Dimić, D. Crystallographic Structure and Quantum-Chemical Analysis of Biologically Active Co(III)-Pyridoxal–Isothiosemicarbazone Complex. Inorganics 2023, 11, 466. [Google Scholar] [CrossRef]
- Soliman, S.M.; Albering, J.; Abu-Youssef, M.A.M. Structural analyses of two new highly distorted octahedral copper(II) complexes with quinoline-type ligands; Hirshfeld, AIM and NBO studies. Polyhedron 2017, 127, 36–50. [Google Scholar] [CrossRef]
- Bianchi, R.; Gervasio, G.; Marabello, D. Experimental Electron Density Analysis of Mn2(CO)10: Metal−Metal and Metal−Ligand Bond Characterization. Inorg. Chem. 2000, 39, 2360–2366. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Liu, M.; Guo, Q.; Liu, Y.; Zhao, Y.; Wu, Y.; Sun, B.; Wang, Q.; Liu, J.; Han, J. Investigation of binary and ternary systems of human serum albumin with oxyresveratrol/piceatannol and/or mitoxantrone by multipectroscopy, molecular docking and cytotoxicity evaluation. J. Mol. Liq. 2020, 311, 113364. [Google Scholar] [CrossRef]
- Chugh, H.; Kumar, P.; Tomar, V.; Kaur, N.; Sood, D.; Chandra, R. Interaction of noscapine with human serum albumin (HSA): A spectroscopic and molecular modelling approach. J. Photochem. Photobiol. A Chem. 2019, 372, 168–176. [Google Scholar] [CrossRef]
- Bagheri, M.; Fatemi, M.H. Fluorescence spectroscopy, molecular docking and molecular dynamic simulation studies of HSA-Aflatoxin B1 and G1 interactions. J. Lumin. 2018, 202, 345–353. [Google Scholar] [CrossRef]
- Zahirović, A.; Hadžalić, S.; Višnjevac, A.; Fočak, M.; Tüzün, B.; Žilić, D.; Roca, S.; Jurec, J.; Topčagić, A.; Osmanković, I. Vanadium(IV) complexes of salicylaldehyde-based furoic acid hydrazones: Synthesis, BSA binding and in vivo antidiabetic potential. J. Inorg. Biochem. 2023, 244, 112232. [Google Scholar] [CrossRef]
- Guo, Q.; Li, L.; Dong, J.; Liu, H.; Xu, T.; Li, J. Synthesis, crystal structure and interaction of l-valine Schiff base divanadium(V) complex containing a V2O3 core with DNA and BSA. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013, 106, 155–162. [Google Scholar] [CrossRef]
- Turner, M.J.; McKinnon, J.J.; Wolff, S.K.; Grimwood, D.J.; Spackman, P.R.; Jayatilaka, D.; Spackman, M.A. CrystalExplorer17; University of Western Australia: Perth, Australia, 2017. [Google Scholar]
- Spackman, M.A.; Byrom, P.G. A novel definition of a molecule in a crystal. Chem. Phys. Lett. 1997, 267, 215–220. [Google Scholar] [CrossRef]
- Spackman, M.A.; Jayatilaka, D. Hirshfeld surface analysis. CrystEngComm 2009, 11, 19–32. [Google Scholar] [CrossRef]
- Jevtovic, V.; Alhar, M.S.O.; Milenković, D.; Marković, Z.; Dimitrić Marković, J.; Dimić, D. Synthesis, Structural Characterization, Cytotoxicity, and Protein/DNA Binding Properties of Pyridoxylidene-Aminoguanidine-Metal (Fe, Co, Zn, Cu) Complexes. Int. J. Mol. Sci. 2023, 24, 14745. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Scuseria, G.E.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; et al. Gaussian 09, Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648. [Google Scholar] [CrossRef]
- Dunning, T.H. Gaussian basis sets for use in correlated molecular calculations. I. The atoms boron through neon and hydrogen. J. Chem. Phys. 1989, 90, 1007. [Google Scholar] [CrossRef]
- Weigend, F.; Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297. [Google Scholar] [CrossRef]
- Weigend, F. Accurate Coulomb-fitting basis sets for H to Rn. Phys. Chem. Chem. Phys. 2006, 8, 1057. [Google Scholar] [CrossRef] [PubMed]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem. 2011, 32, 1456–1465. [Google Scholar] [CrossRef]
- King, A.E.; Nippe, M.; Atanasov, M.; Chantarojsiri, T.; Wray, C.A.; Bill, E.; Neese, F.; Long, J.R.; Chang, C.J. A Well-Defined Terminal Vanadium(III) Oxo Complex. Inorg. Chem. 2014, 53, 11388–11395. [Google Scholar] [CrossRef]
- Kargar, H.; Forootan, P.; Fallah-Mehrjardi, M.; Behjatmanesh-Ardakani, R.; Amiri Rudbari, H.; Shahzad Munawar, K.; Ashfaq, M.; Nawaz Tahir, M. Novel oxovanadium and dioxomolybdenum complexes of tridentate ONO-donor Schiff base ligand: Synthesis, characterization, crystal structures, Hirshfeld surface analysis, DFT computational studies and catalytic activity for the selective oxidation of benzyl. Inorganica Chim. Acta 2021, 523, 120414. [Google Scholar] [CrossRef]
- Stewart, J.J.P. Optimization of parameters for semi-empirical methods V: Modification of NDDO approximations and application to 70 elements. J. Mol. Model. 2007, 13, 1173–1213. [Google Scholar] [CrossRef] [PubMed]
- Reed, A.E.; Weinstock, R.B.; Weinhold, F. Natural population analysis. J. Chem. Phys. 1985, 83, 735–746. [Google Scholar] [CrossRef]
- Bader, R.F.W. Atoms in molecules. Acc. Chem. Res. 1985, 18, 9–15. [Google Scholar] [CrossRef]
- Grabowski, S.J. Hydrogen bonding strength—Measures based on geometric and topological parameters. J. Phys. Org. Chem. 2004, 17, 18–31. [Google Scholar] [CrossRef]
- Keith, T.A. TK Gristmill Software; (Version 19.10.12); AIMAll: Overland Park, KS, USA, 2019. [Google Scholar]
- Dimic, D.; Petkovic, M. Control of a photoswitching chelator by metal ions: DFT, NBO, and QTAIM analysis. Int. J. Quantum Chem. 2016, 116, 27–34. [Google Scholar] [CrossRef]
- Morris, G.M.; Goodsell, D.S.; Halliday, R.S.; Huey, R.; Hart, W.E.; Belew, R.K.; Olson, A.J. Automated docking using a Lamarckian genetic algorithm and an empirical binding free energy function. J. Comput. Chem. 1998, 19, 1639–1662. [Google Scholar] [CrossRef]
- Bujacz, A.; Zielinski, K.; Sekula, B. Structural studies of bovine, equine, and leporine serum albumin complexes with naproxen. Proteins Struct. Funct. Bioinform. 2014, 82, 2199–2208. [Google Scholar] [CrossRef]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef]
- Fuhrmann, J.; Rurainski, A.; Lenhof, H.-P.; Neumann, D. A new Lamarckian genetic algorithm for flexible ligand-receptor docking. J. Comput. Chem. 2010, 31, 1911–1918. [Google Scholar] [CrossRef]
- BIOVIA. Dassault Systèmes, Discovery Studio 2016; Dassault Systèmes: San Diego, CA, USA, 2016. [Google Scholar]
| Contact | 1 | 2 | 3 |
|---|---|---|---|
| V∙∙∙C | 0.4 | / | 0.4 |
| V∙∙∙H | / | 0.5 | / |
| H∙∙∙H | 30.6 | 25.6 | 33.9 |
| H∙∙∙O | 40.0 | 33.8 | 30.2 |
| H∙∙∙N | 11.3 | 11.5 | 10.1 |
| H∙∙∙C | 5.8 | 9.7 | 5.7 |
| H∙∙∙S | / | 9.7 | / |
| O∙∙∙N | 0.8 | / | 0.6 |
| O∙∙∙C | 3.2 | / | 3.4 |
| O∙∙∙O | / | 1.1 | / |
| O∙∙∙S | / | 1.9 | 1.5 |
| N∙∙∙N | 0.9 | 1.0 | / |
| N∙∙∙C | 5.5 | 2.2 | 4.0 |
| C∙∙∙C | / | 1.9 | / |
| C∙∙∙S | / | / | 1.1 |
| Complex | Title 2 | V=O (Plane/Top) | V–Oaromatic | V–Nhydrazine | V–X (O, S, N for 1, 2, and 3) |
|---|---|---|---|---|---|
| 1 | Electron Density [a.u.] | 0.265/0.267 | 0.100 | 0.054 | 0.080 |
| Laplacian [a.u.] | 0.925/0.912 | 0.482 | 0.196 | 0.380 | |
| Distance [Å] | 1.61/1.61 | 1.93 | 2.26 | 2.03 | |
| 2 | Electron Density [a.u.] | 0.269/0.272 | 0.095 | 0.043 | 0.066 |
| Laplacian [a.u.] | 0.942/0.921 | 0.474 | 0.162 | 0.118 | |
| Distance [Å] | 1.60/1.60 | 1.94 | 2.35 | 2.41 | |
| 3 | Electron Density [a.u.] | 0.267/0.271 | 0.094 | 0.049 | 0.090 |
| Laplacian [a.u.] | 0.935/0.922 | 0.470 | 0.183 | 0.299 | |
| Distance [Å] | 1.61/1.60 | 1.95 | 2.29 | 2.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alshammari, O.A.O.; Maisara, S.; Alshammari, B.; Alshammari, M.R.; Rakic, V.; Dimitrić Marković, J.; Jevtovic, V.; Dimić, D. Theoretical Insights into Different Complexation Modes of Dioxovanadium(V) Compounds with Pyridoxal Semicarbazone/Thiosemicarbazone/S-Methyl-iso-thiosemicarbazone Ligands. Molecules 2024, 29, 1213. https://doi.org/10.3390/molecules29061213
Alshammari OAO, Maisara S, Alshammari B, Alshammari MR, Rakic V, Dimitrić Marković J, Jevtovic V, Dimić D. Theoretical Insights into Different Complexation Modes of Dioxovanadium(V) Compounds with Pyridoxal Semicarbazone/Thiosemicarbazone/S-Methyl-iso-thiosemicarbazone Ligands. Molecules. 2024; 29(6):1213. https://doi.org/10.3390/molecules29061213
Chicago/Turabian StyleAlshammari, Odeh Abdullah Odeh, Sawsan Maisara, Badriah Alshammari, Maha Raghyan Alshammari, Violeta Rakic, Jasmina Dimitrić Marković, Violeta Jevtovic, and Dušan Dimić. 2024. "Theoretical Insights into Different Complexation Modes of Dioxovanadium(V) Compounds with Pyridoxal Semicarbazone/Thiosemicarbazone/S-Methyl-iso-thiosemicarbazone Ligands" Molecules 29, no. 6: 1213. https://doi.org/10.3390/molecules29061213
APA StyleAlshammari, O. A. O., Maisara, S., Alshammari, B., Alshammari, M. R., Rakic, V., Dimitrić Marković, J., Jevtovic, V., & Dimić, D. (2024). Theoretical Insights into Different Complexation Modes of Dioxovanadium(V) Compounds with Pyridoxal Semicarbazone/Thiosemicarbazone/S-Methyl-iso-thiosemicarbazone Ligands. Molecules, 29(6), 1213. https://doi.org/10.3390/molecules29061213







