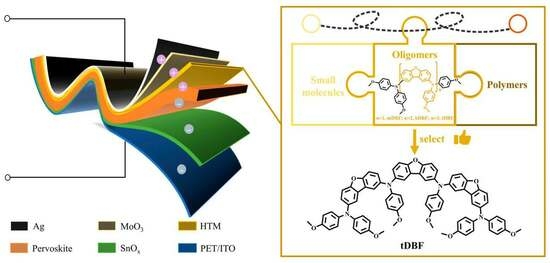
Improvement in Dibenzofuran-Based Hole Transport Materials for Flexible Perovskite Solar Cells
Abstract
1. Introduction
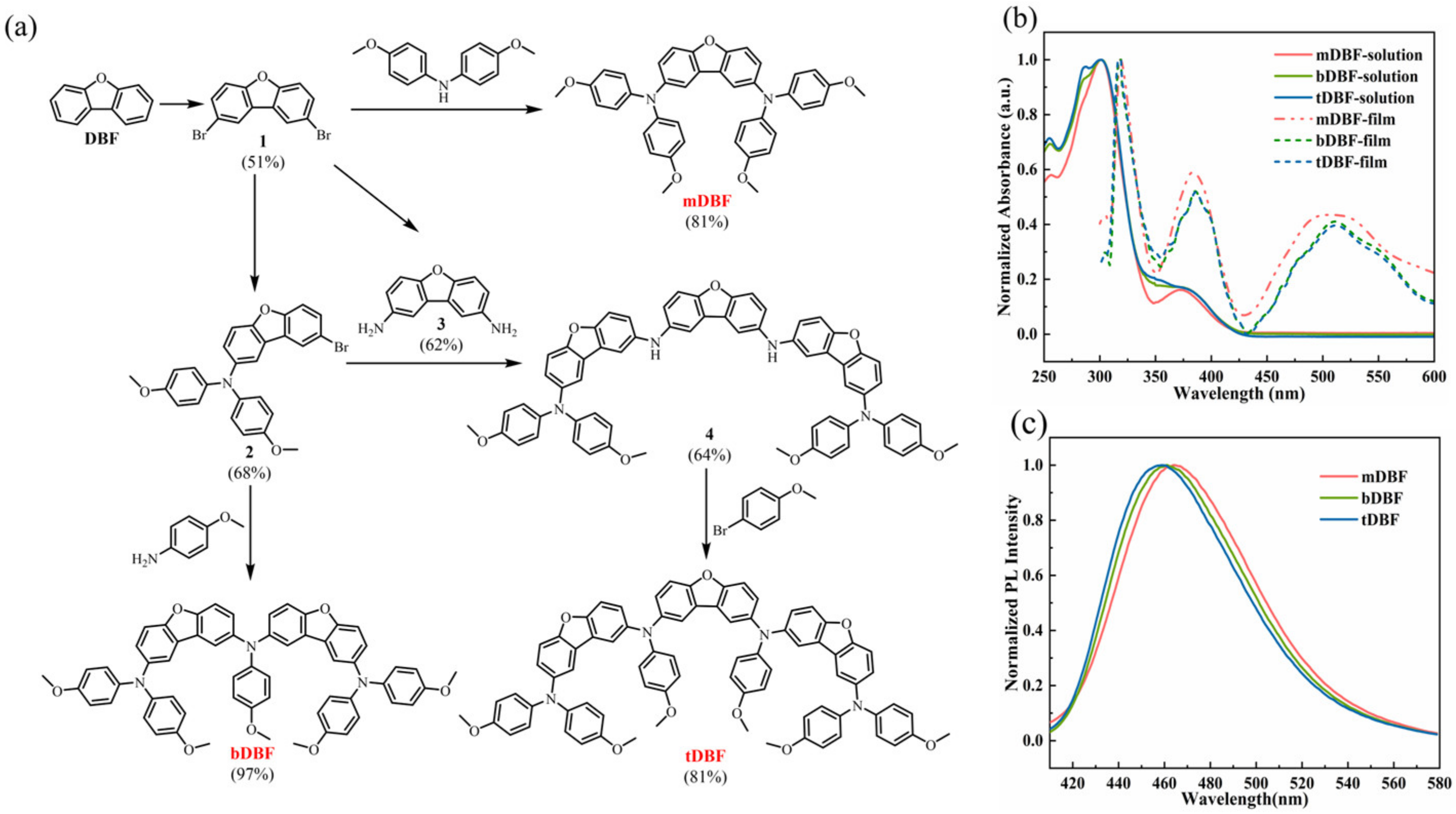
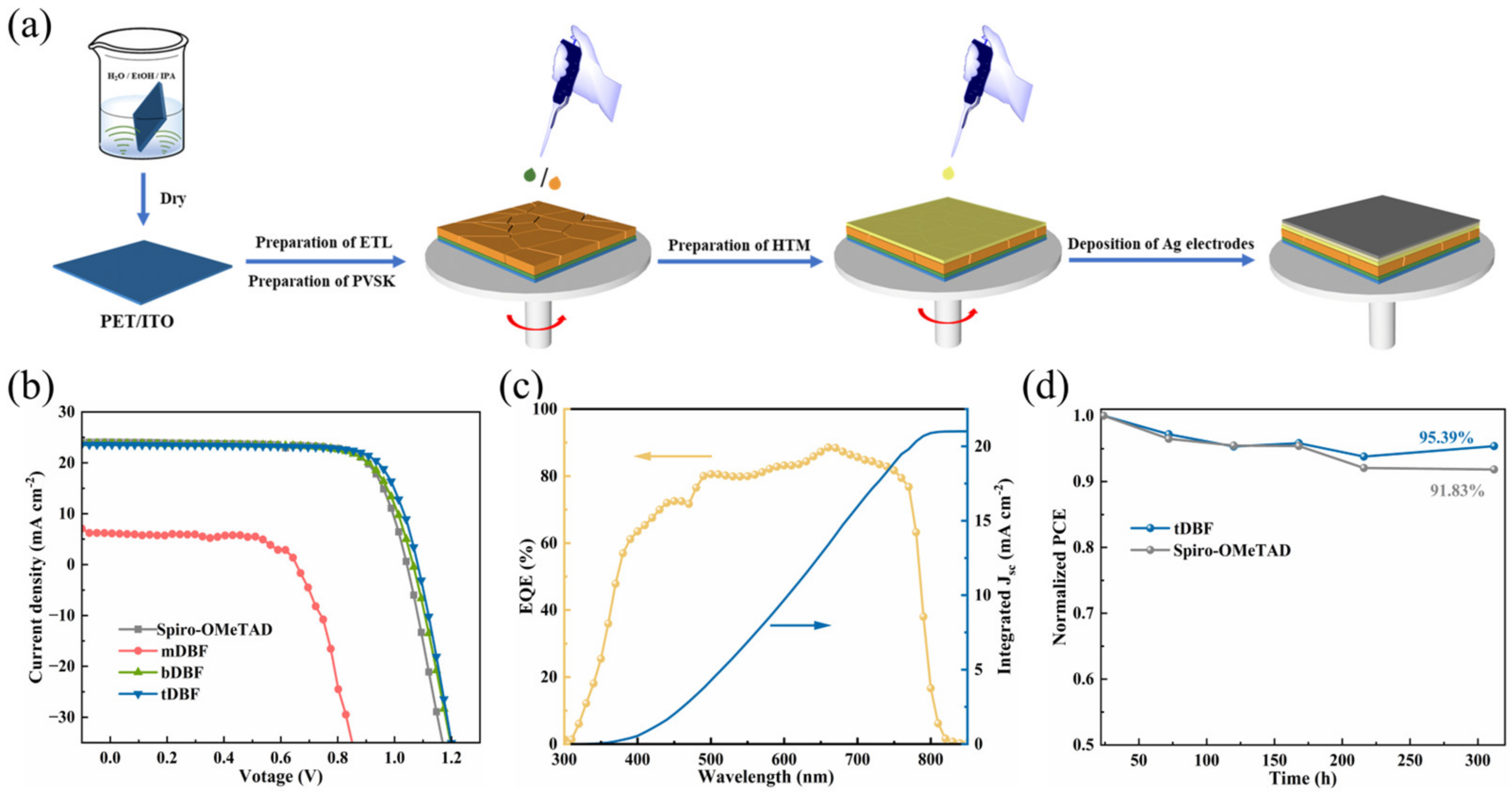
2. Result and Discussion
3. Materials and Methods
3.1. Synthesis of mDBF, bDBF and tDBF
3.2. Characterization and Analysis Methods
3.3. Device Fabrication
3.4. Device Performance Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhou, Z.; Pang, S. Highly efficient inverted hole-transport-layer-free perovskite solar cells. J. Mater. Chem. A 2020, 8, 503–512. [Google Scholar] [CrossRef]
- Ling, W.; Liu, F.; Li, Q.; Li, Z. The crucial roles of the configurations and electronic properties of organic hole-transporting molecules to the photovoltaic performance of perovskite solar cells. J. Mater. Chem. A 2021, 9, 18148–18163. [Google Scholar] [CrossRef]
- Kojima, A.; Teshima, K.; Shirai, Y.; Miyasaka, T. Organometal halide perovskites as visible-light sensitizers for photovoltaic cells. J. Am. Chem. Soc. 2009, 131, 6050–6051. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Lee, C.R.; Im, J.H.; Lee, K.B.; Moehl, T.; Marchioro, A.; Moon, S.J.; Humphry-Baker, R.; Yum, J.H.; Moser, J.E.; et al. Lead iodide perovskite sensitized all-solid-state submicron thin film mesoscopic solar cell with efficiency exceeding 9%. Sci. Rep. 2012, 2, 591. [Google Scholar] [CrossRef] [PubMed]
- NREL. Best Research-Cell Efficiency Chart. Available online: https://www.nrel.gov/pv/interactive-cell-efficiency.html (accessed on 4 February 2024).
- Gnida, P.; Amin, M.F.; Pajak, A.K.; Jarzabek, B. Polymers in High-efficiency solar cells: The latest reports. Polymers 2022, 14, 1946. [Google Scholar] [CrossRef] [PubMed]
- Rombach, F.M.; Haque, S.A.; Macdonald, T.J. Lessons learned from spiro-OMeTAD and PTAA in perovskite solar cells. Energy Environ. Sci. 2021, 14, 5161–5190. [Google Scholar] [CrossRef]
- Magomedov, A.; Al-Ashouri, A.; Kasparavičius, E.; Strazdaite, S.; Niaura, G.; Jošt, M.; Malinauskas, T.; Albrecht, S.; Getautis, V. Self-assembled hole transporting monolayer for highly efficient perovskite solar cells. Adv. Energy Mater. 2018, 8, 1801892. [Google Scholar] [CrossRef]
- Yasuda, T.; Fujita, K.; Tsutsui, T.; Geng, Y.H.; Culligan, S.W.; Chen, S.H. Carrier transport properties of monodisperse glassy-nematic oligofluorenes in organic field-effect transistors. Chem. Mater. 2005, 17, 264–268. [Google Scholar] [CrossRef]
- del Barrio, J.; Chinelatto, L.S.; Serrano, J.L.; Oriol, L.; Piñol, M.; Bolink, H.J. Extended liquid-crystalline oligofluorenes with photo- and electroluminescence. New J. Chem. 2010, 34, 2785–2795. [Google Scholar] [CrossRef]
- Li, E.; Li, W.; Li, L.; Zhang, H.; Shen, C.; Wu, Z.; Zhang, W.; Xu, X.; Tian, H.; Zhu, W.-H.; et al. Efficient p-i-n structured perovskite solar cells employing low-cost and highly reproducible oligomers as hole transporting materials. Sci. China Chem. 2019, 62, 767–774. [Google Scholar] [CrossRef]
- Wang, Q.; Mosconi, E.; Wolff, C.; Li, J.; Neher, D.; De Angelis, F.; Suranna, G.P.; Grisorio, R.; Abate, A. Rationalizing the molecular design of hole-selective contacts to improve charge extraction in perovskite solar cells. Adv. Energy Mater. 2019, 9, 1900990. [Google Scholar] [CrossRef]
- Xia, J.X.; Luizys, P.; Daskeviciene, M.; Xiao, C.X.; Kantminiene, K.; Jankauskas, V.; Rakstys, K.; Kreiza, G.; Gao, X.X.; Kanda, H.; et al. Foldable hole-transporting materials for merging electronic states between defective and perfect perovskite sites. Adv. Mater. (Weinh. Ger.) 2023, 35, 2300720. [Google Scholar] [CrossRef]
- Wang, H.; Wu, C.; Zhai, M.; Chen, C.; Tao, L.; Ding, X.; Miao, Y.; Cheng, M. Constructing Efficient hole transport material through π-Conjunction extension for perovskite solar cell. ACS Appl. Energy Mater. 2022, 5, 13261–13268. [Google Scholar] [CrossRef]
- Liu, L.; Miao, Y.; Zhai, M.; Wang, H.; Ding, X.; Guo, L.; Chen, C.; Cheng, M. Molecular engineering of peripheral substitutions to construct efficient acridine core-based hole transport materials for perovskite solar cells. ACS Appl. Mater. Interfaces 2022, 14, 44450–44459. [Google Scholar] [CrossRef]
- Liu, X.; Ding, B.; Han, M.; Yang, Z.; Chen, J.; Shi, P.; Xue, X.; Ghadari, R.; Zhang, X.; Wang, R.; et al. Extending the π-conjugated system in spiro-type hole transport material enhances the efficiency and stability of perovskite solar modules. Angew. Chem. Int. Edit. 2023, 135, e202304350. [Google Scholar] [CrossRef]
- Shi, Y.; Hou, K.; Wang, Y.; Wang, K.; Ren, H.; Pang, M.; Chen, F.; Zhang, S. Two methoxyaniline-substituted dibenzofuran derivatives as hole-transport materials for perovskite solar cells. J. Mater. Chem. A 2016, 4, 5415–5422. [Google Scholar] [CrossRef]
- Sun, Q.; Zhang, J.; Chen, Q.; Wang, Y.; Zhou, Y.; Song, B.; Jia, X.; Yuan, N.; Ding, J.; Li, Y. High-efficiency planar p-i-n perovskite solar cells based on dopant-free dibenzo[b,d]furan-centred linear hole transporting material. J. Power Sources 2020, 449, 227488. [Google Scholar] [CrossRef]
- Prasad, J.; Shao, Z.; Machhi, H.K.; Sharma, D.S.; Patel, V.K.; Pang, S.; Cui, G.; Soni, S.S. ‘V’ shape A–D–A-type designed small hole conductors for efficient indoor and outdoor staging from solid dye-sensitized solar cells and perovskite solar cells. Sol. RRL 2021, 5, 2100206. [Google Scholar] [CrossRef]
- Cheng, Q.; Chen, H.; Yang, F.; Chen, Z.; Chen, W.; Yang, H.; Shen, Y.; Ou, X.M.; Wu, Y.; Li, Y.; et al. Molecular self-assembly regulated dopant-free hole transport materials for efficient and stable n-i-p perovskite solar cells and scalable modules. Angew. Chem. Int. Edit. 2022, 61, e202210613. [Google Scholar] [CrossRef]
- Yao, Y.; Cheng, C.; Zhang, C.; Hu, H.; Wang, K.; De Wolf, S. Organic hole-transport layers for efficient, stable, and scalable inverted perovskite solar cells. Adv. Mater. (Weinh. Ger.) 2022, 34, 2203794. [Google Scholar] [CrossRef]
- Osedach, T.P.; Andrew, T.L.; Bulovi´c, V. Effect of synthetic accessibility on the commercial viability of organic photovoltaics. Energy Environ. Sci. 2013, 6, 711. [Google Scholar] [CrossRef]
- Lee, W.; Cha, H.; Kim, Y.J.; Jeong, J.-E.; Hwang, S.; Park, C.E.; Woo, H.Y. Amorphous Thieno[3,2-b]thiophene and benzothiadiazole based copolymers for organic photovoltaics. ACS Appl. Mater. Interfaces 2014, 6, 20510–20518. [Google Scholar] [CrossRef]
- Budiawan, W.; Lai, K.W.; Karuppuswamy, P.; Jadhav, T.S.; Lu, Y.A.; Ho, K.C.; Wang, P.C.; Chang, C.C.; Chu, C.W. Asymmetric benzotrithiophene-based hole transporting materials provide high-efficiency perovskite solar cells. ACS Appl. Mater. Interfaces 2020, 12, 29143–29152. [Google Scholar] [CrossRef]
- Guo, H.X.; Liu, C.; Hu, H.L.; Zhang, S.; Ji, X.Y.; Cao, X.M.; Ning, Z.J.; Zhu, W.H.; Tian, H.; Wu, Y.Z. Neglected acidity pitfall: Boric acid-anchoring hole-selective contact for perovskite solar cells. Natl. Sci. Rev. 2023, 10, nwad057. [Google Scholar] [CrossRef]
- Choi, H.; Park, S.; Kang, M.-S.; Ko, J. Efficient, symmetric oligomer hole transporting materials with different cores for high performance perovskite solar cells. Chem. Commun. 2015, 51, 15506–15509. [Google Scholar] [CrossRef] [PubMed]
- Kazim, S.; Ramos, F.J.; Gao, P.; Nazeeruddin, M.K.; Grätzel, M.; Ahmad, S. A dopant free linear acene derivative as a hole transport material for perovskite pigmented solar cells. Energy Environ. Sci. 2015, 8, 1816–1823. [Google Scholar] [CrossRef]
- Yin, C.R.; Lu, J.F.; Xu, Y.C.; Yun, Y.K.; Wang, K.; Li, J.W.; Jiang, L.C.; Sun, J.S.; Scully, A.D.; Huang, F.Z.; et al. Low-Cost N,N′-Bicarbazole-Based Dopant-free hole-transporting materials for large-area perovskite solar cells. Adv. Energy Mater. 2018, 8, 1800538. [Google Scholar] [CrossRef]
- Schwenzer, J.A.; Hellmann, T.; Nejand, B.A.; Hu, H.; Abzieher, T.; Schackmar, F.; Hossain, I.M.; Fassl, P.; Mayer, T.; Jaegermann, W.; et al. Thermal stability and cation composition of hybrid organic–inorganic perovskites. ACS Appl. Mater. Interfaces 2021, 13, 15292–15304. [Google Scholar] [CrossRef]
- Chen, Y.; Wei, X.; Cao, J.; Huang, J.; Gao, L.; Zhang, J.; Su, J.; Tian, H. Novel Bipolar Indole-Based Solution-processed host material for efficient green and red phosphorescent OLEDs. ACS Appl. Mater. Interfaces 2017, 9, 14112–14119. [Google Scholar] [CrossRef]
- Huang, J.C.; Yang, J.; Li, D.; Sun, H.L.; Su, M.Y.; Ji, X.F.; Li, B.L.; Li, B.B.; Liao, Q.G.; Han, D.X.; et al. A low-cost and green-solvent-processable hole-transport material enabled by a traditional bidentate ligand for highly efficient inverted perovskite solar cells. J. Mater. Chem. C 2021, 9, 8930–8938. [Google Scholar] [CrossRef]
- Farokhi, A.; Shahroosvand, H.; Monache, G.D.; Pilkington, M.; Nazeeruddin, M.K. The evolution of triphenylamine hole transport materials for efficient perovskite solar cells. Chem. Soc. Rev. 2022, 51, 5974–6064. [Google Scholar] [CrossRef]
- Liu, X.; Kong, F.; Guo, F.; Cheng, T.; Chen, W.; Yu, T.; Chen, J.; Tan, Z.A.; Dai, S. Influence of π-linker on triphenylamine-based hole transporting materials in perovskite solar cells. Dyes Pigment. 2017, 139, 129–135. [Google Scholar] [CrossRef]
- Krishna, A.; Sabba, D.; Li, H.R.; Yin, J.; Boix, P.P.; Soci, C.; Mhaisalkar, S.G.; Grimsdale, A.C. Novel hole transporting materials based on triptycene core for high efficiency mesoscopic perovskite solar cells. Chem. Sci. 2014, 5, 2702–2709. [Google Scholar] [CrossRef]
- Jeong, M.J.; Yeom, K.M.; Kim, S.J.; Jung, E.H.; Noh, J.H. Spontaneous interface engineering for dopant-free poly(3-hexylthiophene) perovskite solar cells with efficiency over 24%. Energy Environ. Sci. 2021, 14, 2419. [Google Scholar] [CrossRef]
- Kim, H.; Lee, K.S.; Paik, M.J.; Lee, D.Y.; Lee, S.U.; Choi, E.; Yun, J.S.; Seok, S.I. Polymethyl methacrylate as an interlayer between the halide perovskite and copper phthalocyanine layers for stable and efficient perovskite solar cells. Adv. Funct. Mater. 2022, 32, 2110473. [Google Scholar] [CrossRef]
- Zhang, C.P.; Liao, Q.G.; Chen, J.Y.; Li, B.L.; Xu, C.Y.; Wei, K.; Du, G.Z.; Wang, Y.; Liu, D.C.; Deng, J.D.; et al. Thermally crosslinked hole conductor enables stable inverted perovskite solar cells with 23.9% efficiency. Adv. Mater. 2023, 35, 2209422. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.S.; Du, S.Y.; Li, J.; Liu, C.; Pu, Z.W.; Tong, X.Y.; Liu, J.; Wang, Y.H.; Meng, Y.Y.; Yang, M.J.; et al. Molecular dipole engineering-assisted strain release for mechanically robust flexible perovskite solar cells. Energy Environ. Sci. 2023, 16, 5423–5433. [Google Scholar] [CrossRef]
- Liao, Q.; Wang, Y.; Yao, X.; Su, M.; Li, B.; Sun, H.; Huang, J.; Guo, X.A. Dual-functional conjugated polymer as an efficient hole-transporting layer for high-performance inverted perovskite solar cells. ACS Appl. Mater. Interfaces 2021, 13, 16744–16753. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Kim, J.; Yun, H.-S.; Paik, M.J.; Noh, E.; Mun, H.J.; Kim, M.G.; Shin, T.J.; Seok, S.I. Controlled growth of perovskite layers with volatile alkylammonium chlorides. Nature 2023, 616, 724. [Google Scholar] [CrossRef] [PubMed]



| HTM | Experiment Data | Calculation Data e | |||||||
|---|---|---|---|---|---|---|---|---|---|
| λmax a (nm) | λstokes a (nm) | Eg b (eV) | Td (°C) | Tg (°C) | HOMO c (eV) | LUMO d (eV) | HOMO (eV) | LUMO (eV) | |
| mDBF | 301 | 464 | 2.17 | 380 | - | −5.27 | −3.10 | −4.64 | −1.14 |
| bDBF | 301 | 461 | 2.12 | 372 | 98 | −5.29 | −3.17 | −4.69 | −1.27 |
| tDBF | 301 | 459 | 2.11 | 418 | 129 | −5.30 | −3.19 | −4.71 | −1.35 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, Y.; Zhang, X.; Lu, J.; Lin, X.; Lu, Y.; Li, X.; Tu, S. Improvement in Dibenzofuran-Based Hole Transport Materials for Flexible Perovskite Solar Cells. Molecules 2024, 29, 1208. https://doi.org/10.3390/molecules29061208
Lin Y, Zhang X, Lu J, Lin X, Lu Y, Li X, Tu S. Improvement in Dibenzofuran-Based Hole Transport Materials for Flexible Perovskite Solar Cells. Molecules. 2024; 29(6):1208. https://doi.org/10.3390/molecules29061208
Chicago/Turabian StyleLin, Yuanqiong, Xiao Zhang, Jinchuan Lu, Xiaohan Lin, Yinghua Lu, Xin Li, and Song Tu. 2024. "Improvement in Dibenzofuran-Based Hole Transport Materials for Flexible Perovskite Solar Cells" Molecules 29, no. 6: 1208. https://doi.org/10.3390/molecules29061208
APA StyleLin, Y., Zhang, X., Lu, J., Lin, X., Lu, Y., Li, X., & Tu, S. (2024). Improvement in Dibenzofuran-Based Hole Transport Materials for Flexible Perovskite Solar Cells. Molecules, 29(6), 1208. https://doi.org/10.3390/molecules29061208