Abstract
A method was developed for the determination of 26 drugs of abuse from different classes, including illicit drugs in quantitative dried blood spots (qDBSs), with the aim to provide a convenient method for drug testing by using only 10 μL of capillary blood. A satisfactory limit of quantification (LOQ) of 2.5 ng/mL for 9 of the compounds and 5 ng/mL for 17 of the compounds and a limit of detection (LOD) of 0.75 ng/mL for 9 of the compounds and 1.5 ng/mL for 17 of the compounds were achieved for all analytes. Reversed-phase liquid chromatography was applied on a C18 column coupled to MS, providing selective detections with both +ESI and -ESI modes. Extraction from the qDBS was performed using AcN-MeOH, 1:1 (v/v), with recovery ranging from 84.6% to 106%, while no significant effect of the hematocrit was observed. The studied drugs of abuse were found to be stable over five days under three different storage conditions (at ambient temperature 21 °C, at −20 °C, and at 35 °C), thus offering a highly attractive approach for drug screening by minimally invasive sampling for individuals that could find application in forensic toxicology analysis.
1. Introduction
Dried blood spots (DBSs) represent a minimally invasive sample collection approach that can find applications in various fields. From its introduction in bioanalysis a century ago [1,2,3,4], applications have been increasing over the years, including newborn screening, analysis of small molecules, DNA, proteins for diagnostics [5], therapeutic drug monitoring [6], preclinical drug development, and forensic toxicology [7] among others. The cost-effectiveness, facilitated storage, and shipment conditions of the DBSs in combination with decreased biohazard risk support their potential in the area of toxicological analysis.
Whole blood, plasma, and urine are the typical specimens used for drug analysis in forensic toxicology [8,9,10,11]. However, shipment, sample storage, and handling of these types of biological samples may bring limitations. DBSs offer numerous benefits, the most important being the ability to collect blood without venipuncture. Reduced invasiveness and low biohazard risk, for instance, for HIV or other infectious pathogens, during sample shipment, are two additional advantages of DBS sampling [12,13]. Moreover, many reports have already focused on the enhanced stability of the analytes in these types of samples at room temperature, without the need for refrigeration [14,15].
A crucial challenge, however, is the need for highly sensitive instrumentation and methodologies capable of detecting low levels of drugs in such small blood volumes collected in a DBS. With regard to quantification, the accurate and reproducible collection of blood is often difficult; thus, valid and accurate quantitative data are not feasible. Quantitative dried blood spot (qDBS) analysis overcomes this limitation, offering the advantage of collecting small but precise predefined blood volume, e.g., 10, 20, 50 μL. Thus, it can be used for accurate determination [16,17] besides the screening applications.
To date, many analytical protocols have been developed to determine/quantify drugs of abuse in DBSs by modern analytical techniques, including liquid chromatography tandem–mass spectrometry (LC–MS/MS) [16,18,19,20,21,22,23,24,25,26] mainly for quantification purposes; liquid chromatography quadrupole time-of-flight mass spectrometry (LC-QTOF-MS) [17,27] and gas chromatography–mass spectrometry (GC–MS) [11,28] for drug screening. Nowadays, various methodologies have been developed for DBSs for both quantification and screening purposes, offering precise analyte level measurements and efficiency of detection in biological samples. Most of these focus on the detection or/and quantification of a specific category of drugs, such as cocaine and its metabolites, benzodiazepines, new psychoactive substances (NPSs), amphetamines, and cannabinoids [14,16,29]. There are few protocols that enable the simultaneous determination of a plethora of illicit drugs belonging to various categories in DBSs but require a minimum blood volume of 15 μL [17,20,21,30,31,32,33]. In all these cases, spots in a simple filter paper, such as Whatman protein saver cards, are used, which presents various limitations, especially in terms of accurate quantification.
The current study aimed to highlight the opportunities arising from the implementation of qDBSs in drug screening for forensic toxicology purposes. The focus was set on the development of a valuable method for the detection of 26 drugs of abuse and metabolites (both illicit drugs and others) in a 10 μL qDBS that offers high precision in sampling [34,35,36,37,38] and has the potential to be applied for quantitative purposes. The studied substances are those frequently abused. To represent these, we chose the following drugs: benzodiazepines and metabolites (diazepam, bromazepam, temazepam, oxazepam, alprazolam, and 7-aminflunitrazepam), synthetic opioids (methadone and AH-7921), cannabinoids (tetrahydrocannabinol (THC), cannabinol (CBN), cannabidiol (CBD), and synthetic cannabinoids (JWH-018)), cocaine and metabolites (benzoylecgonine, methylecgonine, and cocaethylene), amphetamines analogues (3,4-methylenedioxymethamphetamine (MDMA), 25B-NB2OMe, 25C-NB2Ome, and 25I-NB2OMe), and other stimulants (cathine, mescaline, mephedrone, methylone, 3,4-methylenedioxypyrovalerone (MDPV), and 1-benzylpiperazine). A sensitive qualitative method was developed and evaluated for the detection of the target analytes from qDBSs using liquid chromatography coupled to high tandem mass spectrometry.
To our knowledge, this is the first method developed for drug abuse screening utilizing a commercially available qDBS device. The proposed protocol delivers efficient and reliable results by using only 10 μL of blood, obtained in a minimally invasive way by finger pricking.
2. Results
2.1. LC–MS/MS Optimization
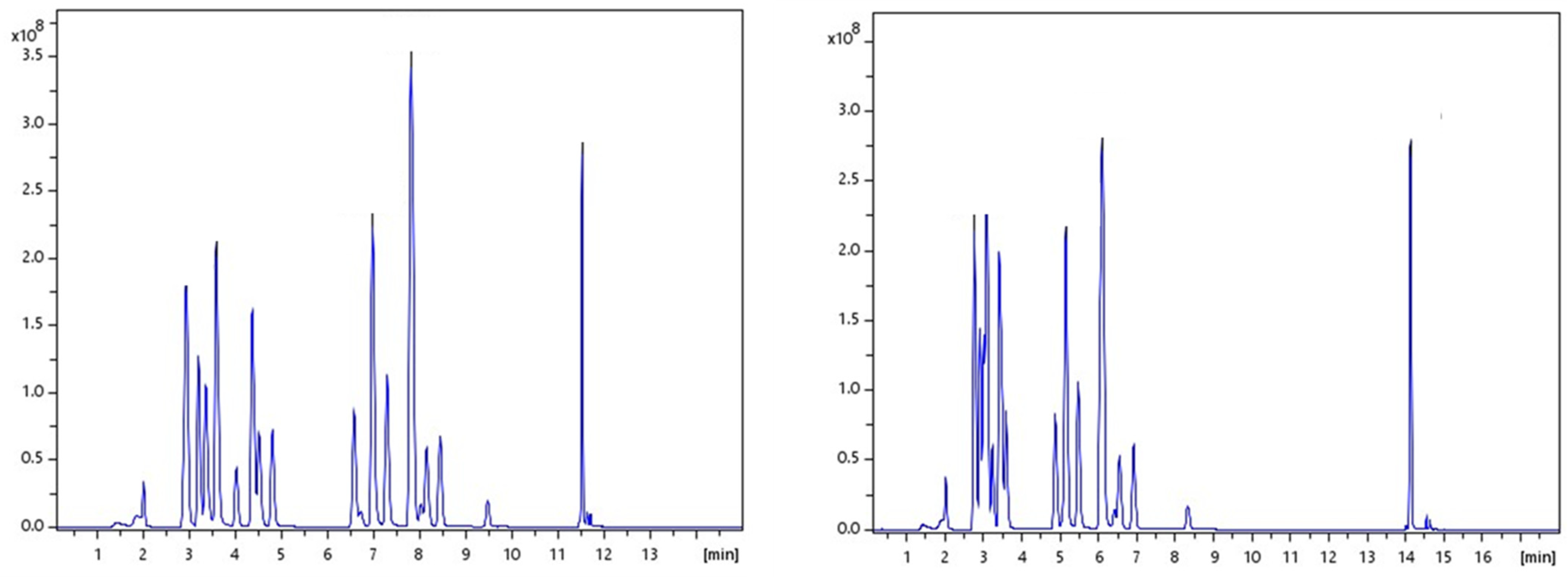
For the efficient separation of twenty-six (26) drugs and metabolites, some of which have similar structures, various chromatographic systems were tested. Two different C18 columns, an Intensity Solo 2 C18 column (2.1 mm × 100 mm, 2.0 μm) and an Acquity BEH C18 (2.1 mm × 100 mm, 1.7 μm), were used. Mobile phase systems applied included the following: (a) A: H2O-MeOH, 90:10 (v/v), 0.01% FA and B: MeOH, 0.01% FA and (b) A: 5 mM AF in H2O-MeOH, 90:10 (v/v) acidified with 0.01% FA and B: 5 mM AF in MeOH acidified with 0.01% FA. Different gradient elution profiles were tested, starting with up to 95% aqueous phase. Based on the literature, retention of more hydrophilic analytes on RPLC assays requires initial mobile phase conditions of 80–90% water [39]. The optimum selected conditions were finally obtained with A: 5 mM aq. AF-MeOH, 90:10 (v/v), 0.01% FA and B: 5 mM AF in MeOH, 0.01% FA on an Intensity Solo 2 C18 column. The system demonstrated satisfactory chromatographic performance under all test conditions. However, further improved peak shapes and signals of higher intensity were achieved under these conditions. The chromatographic traces of the analytes obtained on the two columns can be seen in Figure 1.
Figure 1.
Total ion chromatograms (TICs) of the elution of all 26 illicit drugs in both stationary phases under the same mobile phases and gradient program. The left chromatogram corresponds to Intensity Solo 2 C18 column and the right chromatogram corresponds to Acquity BEH C18.
Furthermore, to achieve the highest level of sensitivity, detection parameters in the mass spectrometer were optimized. Different MS parameters were tested to achieve the highest intensities and signals for all compounds. Specifically, ion spray voltage for +ESI was set at 5000, while for -ESI at −4500. Cone temperature was set at 300 °C, heated probe temperature at 250 °C, while curtain gas was set at 20 psi. Multiple reaction monitoring (MRM) mode was employed to monitor selective transitions for the target analytes. Two daughter ions for each analyte were selected for detection confirmation. The MRM transitions, retention times, and collision energies for every analyte are listed in Table 1.

Table 1.
MRM transitions, retention times, detection parameters, LOD, and LOQ for all analytes.
2.2. Optimization of qDBS Sample Treatment
In the analysis of DBS samples, the analytes extraction is a crucial step. The applied extraction protocol should be carefully designed to achieve the maximum recovery and sufficient sensitivity levels [40], as this can be challenging due to the small sample volume. During this process, several factors should be considered, including the type of paper used in the device, as there is a possibility of substances being released from its materials during sample extraction [41]. Also, blood hematocrit should be considered, as it has multiple effects on the analyte’s extraction from DBSs. Blood hematocrit determines blood viscosity, which can cause varying DBS homogeneity on the filter paper [42]. In addition, in the field of toxicological analyses and drug screening, several other factors should be taken into account, such as the characteristics of the blood (postmortem or in vivo) and the age of the bloodstains, as they might have a substantial impact on the extraction efficiency [21]. Hence, the validity of the extraction system should be assessed in relation to the aforementioned factors [6,7,12].
Despite the fact that there are numerous methods reported in the literature for the quantitative measuring of drugs in DBS, no data exist yet on the extraction of drugs of abuse from qDBSs. Different paper substrates have been tested, aiming to determine illicit drugs, with Whatman 903 protein saver card being the most commonly used [16,17,18,19,22,23,24,26]. Moreover, Whatman BFC 180 [21], Bond Elute Dried matrix spotting cards (Agilent) [25], FTA DMPK cards [43], and Sartorius Stedim Biotech Sample carrier paper [20] have been examined and proved to be adequately efficient for drug extraction purposes. However, these approaches do not offer the possibility for accurate collection of a specific volume of blood; thus, they have some accuracy limitations in drug analysis.
The Capitainer qDBS device allows for the accurate collection of an exact volume of blood as it is transferred through a capillary to a precut 6 mm paper disc. A more detailed description of the device can be found in a previous work of the authors [38].
In the present study, a Capitainer qDBS with two collecting discs (10 μL each) was used. In previously reported studies on the determination of drugs, a variety of samples volume have been used on DBS, in all cases larger than 10 μL. More specifically, either 25 μL [16,17], 30 μL [23,24,43], 50 μL [21,22,26], or 85 μL [18,19] of blood were spotted on paper cards. Hence, the Capitainer qDBS (10 μL of whole blood spotted) is the smallest volume of blood used for such analyses.
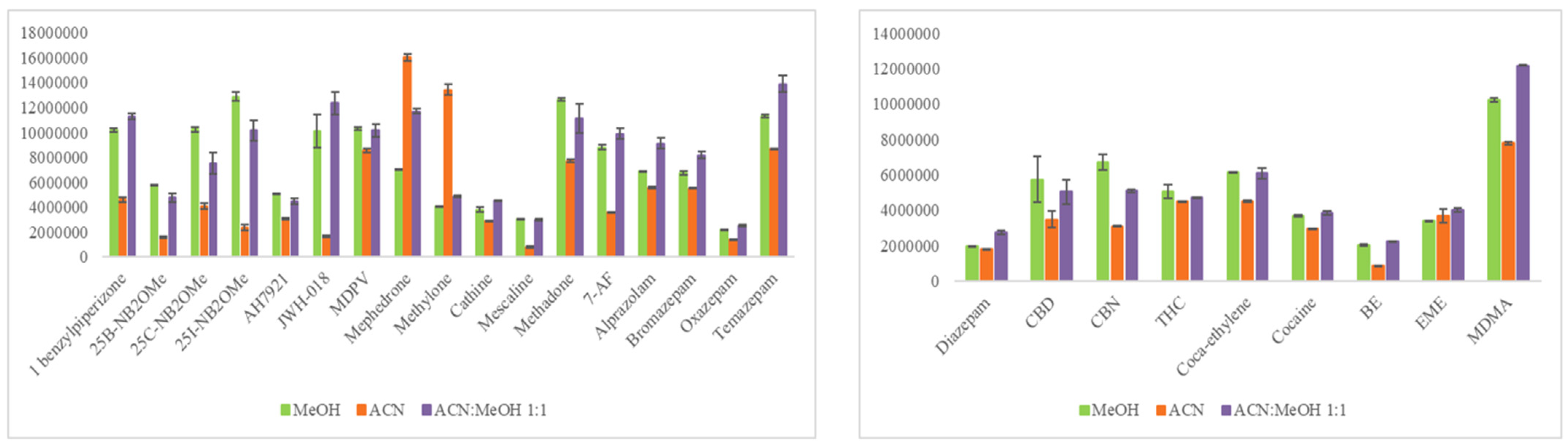
The initial step was to design a comprehensive experimental approach to identify the optimum extraction protocol for the analytes of interest from the qDBS sample. Based on our literature findings, several studies in DBS determining either benzodiazepines or cocaine and metabolites, or both, performed SPE [14,18,19]. Extraction solvents tested include MeOH-AcN 3:1 (v/v) [21,22], pure H2O [23], 0.1% FA in MeOH [25], 1% FA in H2O [26], MeOH [24], AcN-H2O 8:2 (v/v) [17], and AcN-MeOH 1:1 (v/v) [43]. Herein, AcN, MeOH, and a mixture of AcN-MeOH, 1:1 (v/v) were evaluated. An MQC sample (fortified with 50 ng/mL for bromazepam, temazepam, oxazepam, alprazolam, 7-AF, methadone, cathine, mescaline, 25B-NB2OMe, 25C-NB2OMe, 25I-NB2OMe, mephedrone, AH-7921, 1-benzylpiperazine, methylone, MDPV, JWH-018, and with 25 ng/mL for diazepam, cocaine, benzoylecgonine, methylecgonine, cocaethylene, MDMA, THC, CBN, CBD) was used to investigate which would be the most efficient extraction system for all the studied analytes from qDBSs. As can be seen in Figure 2, with the exception of methylone and mephedrone, which were extracted more efficiently with AcN, pure MeOH or mixture of MeOH with AcN provided better extraction recoveries. AcN-MeOH, 1:1 (v/v) was selected as the optimal qDBS extraction solvent given the fact that 14 out of the 24 drugs had higher intensity using this solvent (in comparison to the other test solvents). Acidification of the AcN-MeOH, 1:1 (v/v) mixture by adding 0.1% FA, as previously reported [25,26], did not improve recovery and thus was not considered further. One milliliter of solvent provided satisfactory results; higher volumes were tested with no enhanced recoveries.
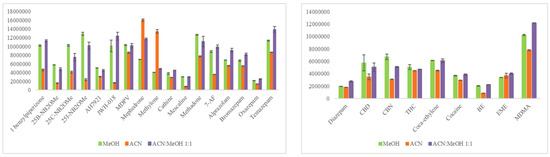
Figure 2.
Bar blots illustrating the efficiency of three different extraction solvents for all analytes (x-axes: analyte’s peak area; y-axes: analyte).
2.3. Drugs Screening
2.3.1. Sensitivity, LOD, and LOQ
The method developed here aims for the application of a minimally invasive sample collection approach for drug screening in blood. Thus, the study was focused on the detection of the drugs of abuse. For this, LOQ and LOD were estimated, as described in Section 3.6.1. Further parameters, such as intra- and interday accuracy and precision related to quantification, were not studied; however, it will be the goal of a future study. For bromazepam, temazepam, oxazepam, alprazolam, 7-AF, methadone, cathine, mescaline, 25B-NB2OMe, 25C-NB2OMe, 25I-NB2OMe, mephedrone, AH-7921, 1-benzylpiperazine, methylone, MDPV, and JWH 018, LOQ was accessed at 5 ng/mL, while for diazepam, cocaine, benzoylecgonine, methylecgonine, cocaethylene, MDMA, THC, CBN, and CBD, at 2.5 ng/mL. Details for LODs are demonstrated in Table 1.
2.3.2. Extraction Recovery (ER%), Hematocrit Effect
Sample volume and hematocrit (Hct) have proved to be two major factors, affecting spot formulation, homogeneity, drying time, and analyte recovery, and are, thus, studied in DBS applications [42,44,45]. In a qDBS device, a precisely measured sample volume is collected on the disc. Nonetheless, the Hct may have an impact on the accuracy of the sampling or, even more so, on the success of the analyte extraction, and, as previously reported [46,47], an independent hematocrit response bias is likely to be observed.
Herein, the impact of Hct on the extraction recovery of all analytes of interest was investigated by estimating the percentage recovery in three different Hct levels. Results of extraction recovery at LH (35%), FH (40%), and HH (50%) in two different fortified levels are illustrated in Table 2A,B. Based on the results, it was concluded that no effect of Hct was observed, given the fact that ER% spans to similar levels (ranging from 84.6% to 106%) and was within the acceptable criteria [48].

Table 2.
(A,B) Extraction recoveries ± sd in two fortified levels, in three hematocrit levels for the listed analytes.
2.3.3. Stability
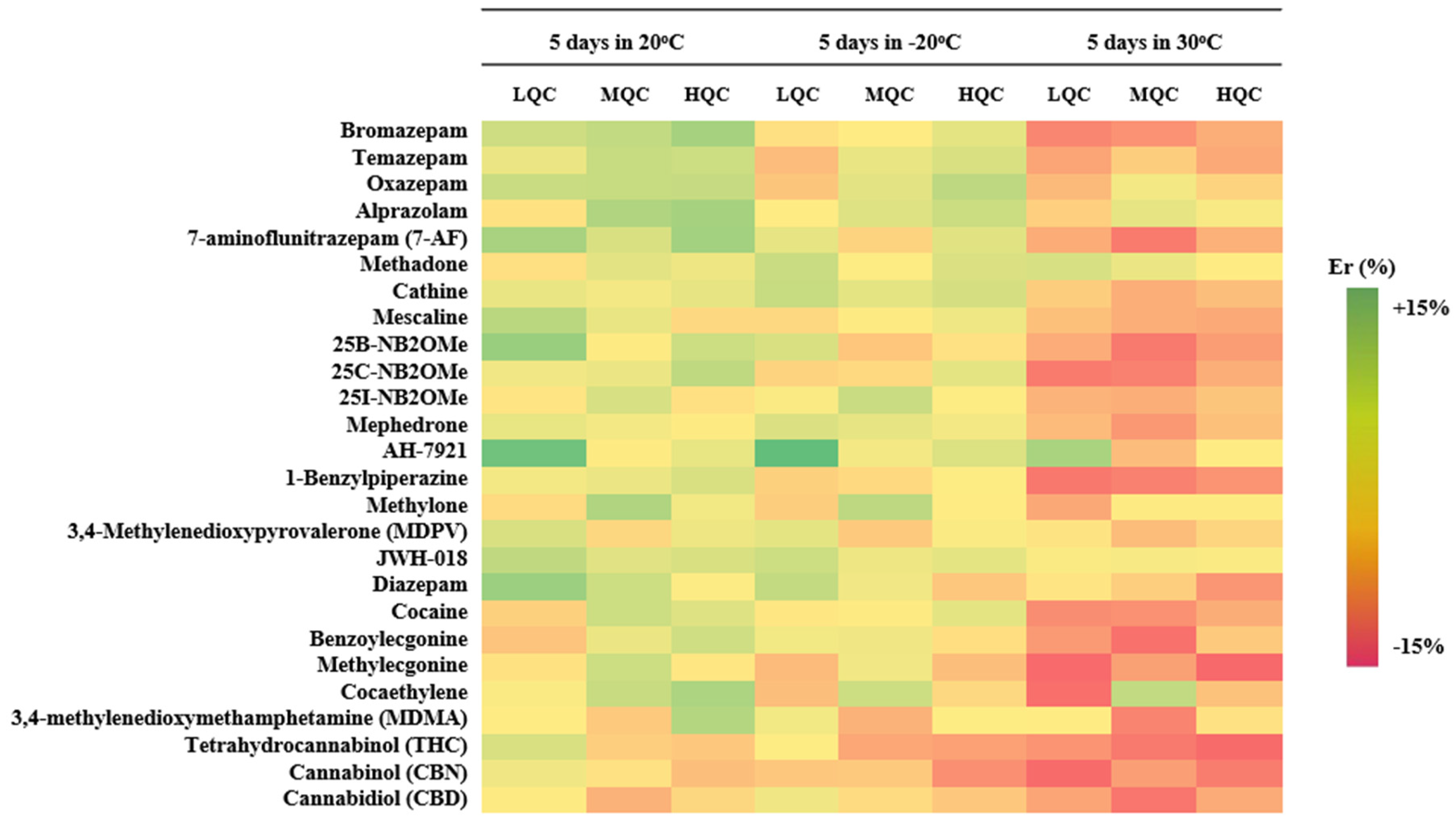
Analyte stability was evaluated by analyzing fortified qDBS samples under three different storage conditions (benchtop 20 °C, freezer −20 °C, and oven 30 °C) over 5 sequential days. Results of stability were expressed as % relative error (% Er), demonstrated in Table 3. As observed in Figure 3, all analytes were estimated to be within +15% Er to −15% Er in all cases, indicating that the analytes are stable under the given conditions for almost a week allowing for a quite adequate time frame for their analysis.

Table 3.
Relative error (%Er) found for all analytes in three different fortified levels, in three different storage conditions over 5 sequential days.
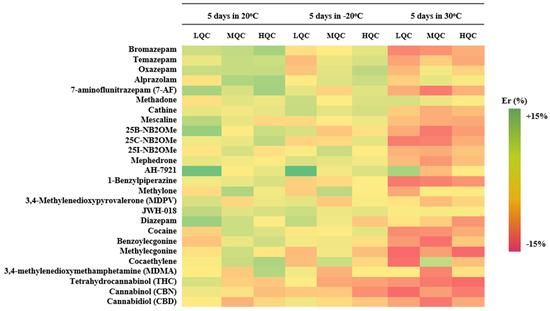
Figure 3.
Heatmap illustrating the % Er for all analytes, in three different levels, in three different storage conditions.
3. Materials and Methods
3.1. Reagents, Materials, and Chemicals
Methanol (MeOH) and acetonitrile (AcN), LC–MS grade, were purchased from HiPerSolv CHROMANORM®. LC–MS grade isopropanol (IPA) was obtained from Fisher Scientific International, Inc., Hampton, NH, USA. A Milli-Q purification system (18.2 MΩ cm−1) was used to provide ultrapure water. Ammonium formate (AF) ≥99% and formic acid (FA) 98–100% mobile phase additives were purchased from Riedel-de Haën® (Sigma-Aldrich, Steinheim, Germany) and ChemLab, Zedelgem Belgium, respectively. Reference standards of diazepam, bromazepam, temazepam, oxazepam, alprazolam, 7-aminoflunitrazepam (7-AF), methadone, 3,4-methylenedioxymethamphetamine (MDMA), cathine, mescaline, cocaine, benzoylecgonine, methylecgonine, cocaethylene, 25B-NB2OMe, 25C-NB2OMe, 25I-NB2OMe, mephedrone, AH-7921, 1-Benzylpiperazine, methylone, 3,4-Methylenedioxypyrovalerone (MDPV), JWH-018, tetrahydrocannabinol (THC), cannabinol (CBN), and cannabidiol (CBD) were of more than 98% purity and were purchased from Lipomed AG (Arlesheim, Switzerland).
Dried blood spots (qDBSs) devices were obtained from Capitainer AB® (Solna, Sweden).
3.2. Working Standard Solutions and Quality Control Samples (QCs)
All analytes’ stock solutions (1 mg/mL) were prepared in methanol by dissolving an appropriate amount of each solid standard. Following dilutions with MeOH, working solutions of 0.1 mg/mL concentration were prepared for each drug. Subsequently, a mixture, containing a concentration of 10 μg/mL bromazepam, temazepam, oxazepam, alprazolam, 7-aminoflunitrazepam (7-AF), methadone, cathine, mescaline, 25B-NB2OMe, 25C-NB2OMe, 25I-NB2OMe, mephedrone, AH-7921, 1-benzylpiperazine, methylone, 3,4-methylenedioxypyrovalerone (MDPV), JWH-018, and at a concentration of 5 μg/mL diazepam, cocaine, benzoylecgonine, methylecgonine, cocaethylene, 3,4-methylenedioxymethamphetamine (MDMA), tetrahydrocannabinol (THC), cannabinol (CBN) and cannabidiol (CBD), was created in H2O-MeOH, 50:50 (v/v). All working and stock solutions were stored at −20 °C.
For validation purposes, a sample prepared by pooling 10 whole blood samples was used (QC). By appropriate spiking of the latter at three different levels of the drugs LQC, MQC, and HQC, samples were prepared, which were then transferred by a syringe onto the qDBS disc. LQC, MQC, and HQC were spiked at 5 ng/mL, 50 ng/mL, and 100 ng/mL, respectively, with bromazepam, temazepam, oxazepam, alprazolam, 7-aminoflunitrazepam (7-AF), methadone, cathine, mescaline, 25B-NB2OMe, 25C-NB2OMe, 25I-NB2OMe, mephedrone, AH-7921, 1-benzylpiperazine, methylone, 3,4-methylenedioxypyrovalerone (MDPV), and JWH-018. For the rest of the drugs, namely, for diazepam, cocaine, benzoylecgonine, methylecgonine, cocaethylene, 3,4-methylenedioxymethamphetamine (MDMA), tetrahydrocannabinol (THC), cannabinol (CBN), cannabidiol (CBD), LQC, MQC, and HQC were prepared by spiking at 2.5 ng/mL, 25 ng/mL, and 50 ng/mL, respectively.
For the evaluation of extraction recovery, venous whole blood collected from three individuals with three different hematocrit levels (low 35%, medium 40%, and high 50%) were used. Spiking at LQC and MQC, as described above for QC, was performed to study the impact of hematocrit in extraction efficiency. The collection of the blood samples was performed under the approval of the Ethical Committee of the Aristotle University of Thessaloniki (protocol number 62883/2023).
3.3. Instrumentation and Analytical Conditions
A reversed-phase liquid chromatography–tandem mass spectrometry (RPLC–MS/MS) method was developed for the determination of the 26 drugs in qDBS extracts using an Elute LC chromatographic system coupled to an EVOQ Elite triple quadrupole mass spectrometer (Bruker Daltonics, Bremen, Germany). Separation was carried out on an Intensity Solo 2 C18 (2.1 × 100 mm, 2 μm) column and the mobile phases consisted of A: H2O-MeOH, 90:10 (v/v), 5 mM ammonium formate, 0.01% formic acid and B: MeOH, 5 mM ammonium formate, 0.01% formic acid. Elution was performed by a 15 min gradient as follows: 0–0.5 min: 15–30% B (flow rate 0.2 mL/min), 0.5–10 min: 30–80% B (flow rate 0.2 mL/min); 10–10.5 min: 80–100% B (flow rate 0.4 mL/min); 10.5–12 min 100% B. At 12.01 min, the composition was returned to the initial conditions and column re-equilibration was applied for 3 min. Column temperature was set at 50 °C, and autosampler’s temperature at 4 °C. Injection volume was 5 μL.
3.4. qDBS Sample Extraction Optimization
Different extraction conditions were tested to examine the extraction efficiency of the analytes from the qDBS disc. Specifically, extraction recovery and repeatability of various solvents or mixtures, including AcN, MeOH, and AcN: MeOH, 1:1 (v/v), were assessed. The extraction procedure started by carefully removing one disc (1 × 10 μL) from the Capitainer device, and then by transferring it into an Eppendorf tube. One milliliter of the extraction solvent was added. Vortex-mixing for 10 min, sonication for 10 min, and/or homogenization by bead beater were tested. For the latter, the disc was placed in a tube that contained approximately 20 ceramic bead media balls, vortex-mixed for 10 min, and then was homogenized with solvent for 30 s at a speed of 6.0 m/s; this was repeated twice. In all cases, centrifugation for 10 min at 6700× g was thereafter held. Finally, 500 μL of the supernatant were transferred to a tube and evaporated until dryness. The dry residue was reconstituted with 50 μL of H2O-MeOH, 85:15 (v/v). The procedure was performed three times for the different extraction conditions. The solvent system that provided better results was also tested at a smaller volume (200 μL); in this condition, one hundred microliters of supernatant were directly transferred to an LC–MS vial and subjected to analysis.
3.5. Final qDBS Sample Treatment Protocol
In a tube that had previously been filled with about 20 ceramic balls (1.4 mm ceramic bead media), one qDBS sample was placed. Then, 1 mL of can-MeOH, 1:1 (v/v) was added. After 10 min of vortex-mixing, two cycles of beat-beater homogenization lasting 30 s each were carried out at a speed of 6.0 m/s. Five hundred microliters of the supernatant was transferred to a 1.5 mL Eppendorf tube after centrifuged at 6700× g for 10 min and evaporated to dryness. The dry residue was reconstituted with 50 mL of H2O-MeOH, 85:15 (v/v).
3.6. qDBS Drugs Screening
3.6.1. Sensitivity, LOD, and LOQ
The sensitivity of the method was estimated through the limits of detection (LODs) for the studied analytes. Limit of quantification (LOQ) values were estimated experimentally by analyzing the spiked qDBS HQC sample after serial dilutions. LODs were established as the concentration where the chromatographic peaks-to-noise ratio was 3:1, whereas for LOQ, a 10:1 ratio was considered.
3.6.2. Extraction Recovery (ER%) and Hematocrit Effect
Extraction recovery (ER%) and hematocrit effect were evaluated for the employed extraction protocol. Three blood samples with different hematocrit spanning from low to high levels (low hematocrit, LH 35%; fixed hematocrit, FH 40%; and high hematocrit, HH 50%) were obtained from volunteers to assess the impact of the hematocrit on the extraction efficiency. Two different levels of the analytes standard mixture were added in qDBS samples (LQC, MQC) before and after extraction. Extraction recovery, ER%, was determined based on Equation (1). Hematocrit effect was evaluated as part of extraction recovery efficiency at the three different samples of different hematocrit levels (LH, FH, HH).
3.6.3. Stability of qDBS Samples
Stability of the analytes in the qDBS samples was studied under three different storage conditions: at benchtop (20 °C), in the oven (30 °C), and in the freezer (−20 °C). Three concentrations (LQC, MQC, and HQC) were examined. Evaluation of short-term stability was carried out by analyzing the spiked qDBS samples (LQC, MQC, HQC) stored under three different conditions for 5 days. The same spiked qDBS samples were analyzed after being freshly prepared to estimate the % relative error (%Er). The three different freshly prepared spiked qDBS QC samples were used to plot a calibration curve, aiding in generating concentration data.
4. Conclusions
The applied UHPLC–MS/MS method was developed with the aim to detect 26 illicit drugs in qDBS samples by analyzing only 10 μL of capillary blood. Herein, a simple, rapid, and trustworthy extraction protocol was achieved, reaching high sensitivity levels for all analytes. This is the first approach reported for the detection of frequently screened drugs utilizing a qDBS device, using just a small drop of blood. Stability experiments showed negligible bias that suggests the validity of the method within a 5-day time period, even at RT storage conditions. Therefore, the method offers a great promise for future applications in drug screening for toxicological and forensic analysis purposes.
Author Contributions
Conceptualization, H.G. and G.T.; methodology, T.M.; software, T.M.; validation, T.M.; formal analysis, T.M.; investigation, T.M. and H.G.; resources, H.G., G.T. and O.B.; data curation, T.M.; writing—original draft preparation, T.M. and H.G.; writing—review and editing, T.M., H.G., G.T. and O.B.; visualization, T.M.; supervision, H.G.; project administration, H.G.; funding acquisition, H.G. and G.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The collection of the blood samples was under the approval of the Ethical Committee of the Aristotle University of Thessaloniki (protocol number 62883/2023, 7 March 2023).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. Written informed consent has been obtained from the patient(s) to publish this paper.
Data Availability Statement
Data are contained within the article.
Acknowledgments
All authors would like to acknowledge Bruker Daltonics (Germany) and Capitainer® AB (Sweden) for the collaboration and technical guidance. The authors also acknowledge Aristea Papaioannou for her technical assistance in the laboratory.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Schmidt, V. Ivar Christian Bang (1869–1918), Founder of Modern Clinical Microchemistry. Clin. Chem. 1986, 32, 213–215. [Google Scholar] [CrossRef] [PubMed]
- Guthrie, R.; Susi, A. A Simple Phenylalanine Method for Detecting Phenylketonuria in Large Populations of Newborn Infants. Pediatrics 1963, 32, 338–343. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Tse, F.L.S. Dried Blood Spot Sampling in Combination with LC-MS/MS for Quantitative Analysis of Small Molecules. Biomed. Chromatogr. 2010, 24, 49–65. [Google Scholar] [CrossRef] [PubMed]
- Hannon, W.H.; Therrell, B.L. Overview of the History and Applications of Dried Blood Samples; Li, W., Lee, M.S., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2014; pp. 1–15. [Google Scholar]
- Eshghi, A.; Pistawka, A.J.; Liu, J.; Chen, M.; Sinclair, N.J.T.; Hardie, D.B.; Elliott, M.; Chen, L.; Newman, R.; Mohammed, Y.; et al. Concentration Determination of >200 Proteins in Dried Blood Spots for Biomarker Discovery and Validation. Mol. Cell. Proteom. 2020, 19, 540–553. [Google Scholar] [CrossRef] [PubMed]
- Edelbroek, P.M.; van der Heijden, J.; Stolk, L.M.L. Dried Blood Spot Methods in Therapeutic Drug Monitoring: Methods, Assays, and Pitfalls. Ther. Drug Monit. 2009, 31, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Stove, C.P.; Ingels, A.-S.M.E.; De Kesel, P.M.M.; Lambert, W.E. Dried Blood Spots in Toxicology: From the Cradle to the Grave? Crit. Rev. Toxicol. 2012, 42, 230–243. [Google Scholar] [CrossRef] [PubMed]
- Kolmonen, M.; Leinonen, A.; Pelander, A.; Ojanperä, I. A General Screening Method for Doping Agents in Human Urine by Solid Phase Extraction and Liquid Chromatography/Time-of-Flight Mass Spectrometry. Anal. Chim. Acta 2007, 585, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.K.; Ho, C.S.; Iu, Y.P.H.; Lai, P.S.J.; Shek, C.C.; Lo, Y.-C.; Klinke, H.B.; Wood, M. Development of a Broad Toxicological Screening Technique for Urine Using Ultra-Performance Liquid Chromatography and Time-of-Flight Mass Spectrometry. Anal. Chim. Acta 2009, 649, 80–90. [Google Scholar] [CrossRef]
- Bjørk, M.K.; Simonsen, K.W.; Andersen, D.W.; Dalsgaard, P.W.; Sigurðardóttir, S.R.; Linnet, K.; Rasmussen, B.S. Quantification of 31 Illicit and Medicinal Drugs and Metabolites in Whole Blood by Fully Automated Solid-Phase Extraction and Ultra-Performance Liquid Chromatography–Tandem Mass Spectrometry. Anal. Bioanal. Chem. 2013, 405, 2607–2617. [Google Scholar] [CrossRef]
- Gomes, D.; de Pinho, P.G.; Pontes, H.; Ferreira, L.; Branco, P.; Remião, F.; Carvalho, F.; Bastos, M.L.; Carmo, H. Gas Chromatography–Ion Trap Mass Spectrometry Method for the Simultaneous Measurement of MDMA (Ecstasy) and Its Metabolites, MDA, HMA, and HMMA in Plasma and Urine. J. Chromatogr. B 2010, 878, 815–822. [Google Scholar] [CrossRef]
- Spooner, N.; Lad, R.; Barfield, M. Dried Blood Spots as a Sample Collection Technique for the Determination of Pharmacokinetics in Clinical Studies: Considerations for the Validation of a Quantitative Bioanalytical Method. Anal. Chem. 2009, 81, 1557–1563. [Google Scholar] [CrossRef] [PubMed]
- Barfield, M.; Spooner, N.; Lad, R.; Parry, S.; Fowles, S. Application of Dried Blood Spots Combined with HPLC-MS/MS for the Quantification of Acetaminophen in Toxicokinetic Studies. J. Chromatogr. B 2008, 870, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Alfazil, A.A.; Anderson, R.A. Stability of Benzodiazepines and Cocaine in Blood Spots Stored on Filter Paper. J. Anal. Toxicol. 2008, 32, 511–515. [Google Scholar] [CrossRef] [PubMed]
- Jantos, R.; Vermeeren, A.; Sabljic, D.; Ramaekers, J.G.; Skopp, G. Degradation of Zopiclone during Storage of Spiked and Authentic Whole Blood and Matching Dried Blood Spots. Int. J. Leg. Med. 2013, 127, 69–76. [Google Scholar] [CrossRef]
- Ambach, L.; Menzies, E.; Parkin, M.C.; Kicman, A.; Archer, J.R.H.; Wood, D.M.; Dargan, P.I.; Stove, C. Quantification of Cocaine and Cocaine Metabolites in Dried Blood Spots from a Controlled Administration Study Using Liquid Chromatography-Tandem Mass Spectrometry. Drug Test. Anal. 2019, 11, 709–720. [Google Scholar] [CrossRef]
- Chepyala, D.; Tsai, I.-L.; Liao, H.-W.; Chen, G.-Y.; Chao, H.-C.; Kuo, C.-H. Sensitive Screening of Abused Drugs in Dried Blood Samples Using Ultra-High-Performance Liquid Chromatography-Ion Booster-Quadrupole Time-of-Flight Mass Spectrometry. J. Chromatogr. A 2017, 1491, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Moretti, M.; Visonà, S.D.; Freni, F.; Tomaciello, I.; Vignali, C.; Groppi, A.; Tajana, L.; Osculati, A.M.M.; Morini, L. A Liquid Chromatography-Tandem Mass Spectrometry Method for the Determination of Cocaine and Metabolites in Blood and in Dried Blood Spots Collected from Postmortem Samples and Evaluation of the Stability over a 3-Month Period. Drug Test. Anal. 2018, 10, 1430–1437. [Google Scholar] [CrossRef]
- Moretti, M.; Freni, F.; Tomaciello, I.; Vignali, C.; Groppi, A.; Visonà, S.D.; Tajana, L.; Osculati, A.M.M.; Morini, L. Determination of Benzodiazepines in Blood and in Dried Blood Spots Collected from Post-Mortem Samples and Evaluation of the Stability over a Three-Month Period. Drug Test. Anal. 2019, 11, 1403–1411. [Google Scholar] [CrossRef]
- Kacargil, C.U.; Daglioglu, N.; Goren, I.E. Determination of Illicit Drugs in Dried Blood Spots by LC–MS/MS Method: Validation and Application to Real Samples. Chromatographia 2020, 83, 885–892. [Google Scholar] [CrossRef]
- Sadler Simões, S.; Castañera Ajenjo, A.; Dias, M.J. Dried Blood Spots Combined to an UPLC-MS/MS Method for the Simultaneous Determination of Drugs of Abuse in Forensic Toxicology. J. Pharm. Biomed. Anal. 2018, 147, 634–644. [Google Scholar] [CrossRef]
- de Lima Feltraco Lizot, L.; da Silva, A.C.C.; Bastiani, M.F.; Hahn, R.Z.; Bulcão, R.; Perassolo, M.S.; Antunes, M.V.; Linden, R. Simultaneous Determination of Cocaine, Ecgonine Methyl Ester, Benzoylecgonine, Cocaethylene and Norcocaine in Dried Blood Spots by Ultra-Performance Liquid Chromatography Coupled to Tandem Mass Spectrometry. Forensic Sci. Int. 2019, 298, 408–416. [Google Scholar] [CrossRef] [PubMed]
- Saussereau, E.; Lacroix, C.; Gaulier, J.M.; Goulle, J.P. On-Line Liquid Chromatography/Tandem Mass Spectrometry Simultaneous Determination of Opiates, Cocainics and Amphetamines in Dried Blood Spots. J. Chromatogr. B 2012, 885–886, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Kyriakou, C.; Marchei, E.; Scaravelli, G.; García-Algar, O.; Supervía, A.; Graziano, S. Identification and Quantification of Psychoactive Drugs in Whole Blood Using Dried Blood Spot (DBS) by Ultra-Performance Liquid Chromatography Tandem Mass Spectrometry. J. Pharm. Biomed. Anal. 2016, 128, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Odoardi, S.; Anzillotti, L.; Strano-Rossi, S. Simplifying Sample Pretreatment: Application of Dried Blood Spot (DBS) Method to Blood Samples, Including Postmortem, for UHPLC-MS/MS Analysis of Drugs of Abuse. Forensic Sci. Int. 2014, 243, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Ellefsen, K.N.; da Costa, J.L.; Concheiro, M.; Anizan, S.; Barnes, A.J.; Pirard, S.; Gorelick, D.A.; Huestis, M.A. Cocaine and Metabolite Concentrations in DBS and Venous Blood after Controlled Intravenous Cocaine Administration. Bioanalysis 2015, 7, 2041–2056. [Google Scholar] [CrossRef] [PubMed]
- Stöth, F.; Martin Fabritius, M.; Weinmann, W.; Luginbühl, M.; Gaugler, S.; König, S. Application of Dried Urine Spots for Non-Targeted Quadrupole Time-of-Flight Drug Screening. J. Anal. Toxicol. 2023, 47, 332–337. [Google Scholar] [CrossRef]
- Scheidweiler, K.B.; Barnes, A.J.; Huestis, M.A. A Validated Gas Chromatographic–Electron Impact Ionization Mass Spectrometric Method for Methamphetamine, Methylenedioxymethamphetamine (MDMA), and Metabolites in Mouse Plasma and Brain. J. Chromatogr. B 2008, 876, 266–276. [Google Scholar] [CrossRef]
- Abarca, R.; Gerona, R. Development and Validation of an LC-MS/MS Assay for the Quantitative Analysis of Alprazolam, α-Hydroxyalprazolam and Hydrocodone in Dried Blood Spots. J. Chromatogr. B 2023, 1220, 123639. [Google Scholar] [CrossRef]
- Gaugler, S.; Al-Mazroua, M.K.; Issa, S.Y.; Rykl, J.; Grill, M.; Qanair, A.; Cebolla, V.L. Fully Automated Forensic Routine Dried Blood Spot Screening for Workplace Testing. J. Anal. Toxicol. 2019, 43, 212–220. [Google Scholar] [CrossRef]
- Joye, T.; Sidibé, J.; Déglon, J.; Karmime, A.; Sporkert, F.; Widmer, C.; Favrat, B.; Lescuyer, P.; Augsburger, M.; Thomas, A. Liquid Chromatography-High Resolution Mass Spectrometry for Broad-Spectrum Drug Screening of Dried Blood Spot as Microsampling Procedure. Anal. Chim. Acta 2019, 1063, 110–116. [Google Scholar] [CrossRef]
- Gaugler, S.; Rykl, J.; Grill, M.; Cebolla, V. Fully Automated Drug Screening of Dried Blood Spots Using Online LC-MS/MS Analysis. J. Appl. Bioanal. 2018, 4, 7–15. [Google Scholar] [CrossRef]
- Stelmaszczyk, P.; Gacek, E.; Wietecha-Posłuszny, R. Optimized and Validated DBS/MAE/LC-MS Method for Rapid Determination of Date-Rape Drugs and Cocaine in Human Blood Samples-A New Tool in Forensic Analysis. Separations 2021, 8, 249. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, L.; Yuan, Y.; Xie, F. A Validated UHPLC–MS/MS Method to Quantify Eight Antibiotics in Quantitative Dried Blood Spots in Support of Pharmacokinetic Studies in Neonates. Antibiotics 2023, 12, 199. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Chace, D.H.; Garrett, T.J. Quantitation of Phenylalanine and Tyrosine from Dried Blood/Plasma Spots with Impregnated Stable Isotope Internal Standards (SIIS) by FIA-SRM. Clin. Chim. Acta 2023, 549, 117551. [Google Scholar] [CrossRef]
- Deprez, S.; Van Uytfanghe, K.; Stove, C.P. Liquid Chromatography-Tandem Mass Spectrometry for Therapeutic Drug Monitoring of Immunosuppressants and Creatinine from a Single Dried Blood Spot Using the Capitainer® qDBS Device. Anal. Chim. Acta 2023, 1242, 340797. [Google Scholar] [CrossRef]
- Carling, R.S.; Barclay, Z.; Cantley, N.; Emmett, E.C.; Hogg, S.L.; Finezilber, Y.; Schulenburg-Brand, D.; Murphy, E.; Moat, S.J. Investigation of the Relationship between Phenylalanine in Venous Plasma and Capillary Blood Using Volumetric Blood Collection Devices. JIMD Rep. 2023, 64, 468–476. [Google Scholar] [CrossRef] [PubMed]
- Meikopoulos, T.; Begou, O.; Theodoridis, G.; Gika, H. Ceramides Biomarkers Determination in Quantitative Dried Blood Spots by UHPLC-MS/MS. Anal. Chim. Acta 2023, 1255, 341131. [Google Scholar] [CrossRef]
- Orfanidis, A.; Gika, H.G.; Theodoridis, G.; Mastrogianni, O.; Raikos, N. A UHPLC-MS-MS Method for the Determination of 84 Drugs of Abuse and Pharmaceuticals in Blood. J. Anal. Toxicol. 2021, 45, 28–43. [Google Scholar] [CrossRef]
- Sadones, N.; Capiau, S.; De Kesel, P.M.; Lambert, W.E.; Stove, C.P. Spot Them in the Spot: Analysis of Abused Substances Using Dried Blood Spots. Bioanalysis 2014, 6, 2211–2227. [Google Scholar] [CrossRef]
- Pablo, A.; Breaud, A.R.; Clarke, W. Automated Analysis of Dried Urine Spot (DUS) Samples for Toxicology Screening. Clin. Biochem. 2020, 75, 70–77. [Google Scholar] [CrossRef]
- De Kesel, P.M.; Sadones, N.; Capiau, S.; Lambert, W.E.; Stove, C.P. Hemato-Critical Issues in Quantitative Analysis of Dried Blood Spots: Challenges and Solutions. Bioanalysis 2013, 5, 2023–2041. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Park, Y.; Jo, J.; In, S.; Park, Y.; Kim, E.; Pyo, J.; Choe, S. Analysis of Benzodiazepines and Their Metabolites Using DBS Cards and LC-MS/MS. Forensic Sci. Int. 2015, 255, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Timmerman, P.; White, S.; Globig, S.; Lüdtke, S.; Brunet, L.; Smeraglia, J. EBF Recommendation on the Validation of Bioanalytical Methods for Dried Blood Spots. Bioanalysis 2011, 3, 1567–1575. [Google Scholar] [CrossRef] [PubMed]
- Timmerman, P.; White, S.; Cobb, Z.; de Vries, R.; Thomas, E.; van Baar, B. European Bioanalysis Forum Update of the EBF Recommendation for the Use of DBS in Regulated Bioanalysis Integrating the Conclusions from the EBF DBS-Microsampling Consortium. Bioanalysis 2013, 5, 2129–2136. [Google Scholar] [CrossRef]
- Velghe, S.; Stove, C.P. Evaluation of the Capitainer-B Microfluidic Device as a New Hematocrit-Independent Alternative for Dried Blood Spot Collection. Anal. Chem. 2018, 90, 12893–12899. [Google Scholar] [CrossRef]
- Carling, R.S.; Emmett, E.C.; Moat, S.J. Evaluation of Volumetric Blood Collection Devices for the Measurement of Phenylalanine and Tyrosine to Monitor Patients with Phenylketonuria. Clin. Chim. Acta 2022, 535, 157–166. [Google Scholar] [CrossRef]
- Guideline on Bioanalytical Method Validation. EMEA/CHMP/EWP/192217/2009 Rev. 1 Corr. 2** Committee for Medicinal Products for Human Use (CHMP). 2011. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-bioanalytical-method-validation_en.pdf (accessed on 15 February 2024).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).