Biodegradable Metal Complex-Gated Organosilica for Dually Enhanced Chemodynamic Therapy through GSH Depletions and NIR Light-Triggered Photothermal Effects
Abstract
1. Introduction
2. Results and Discussion
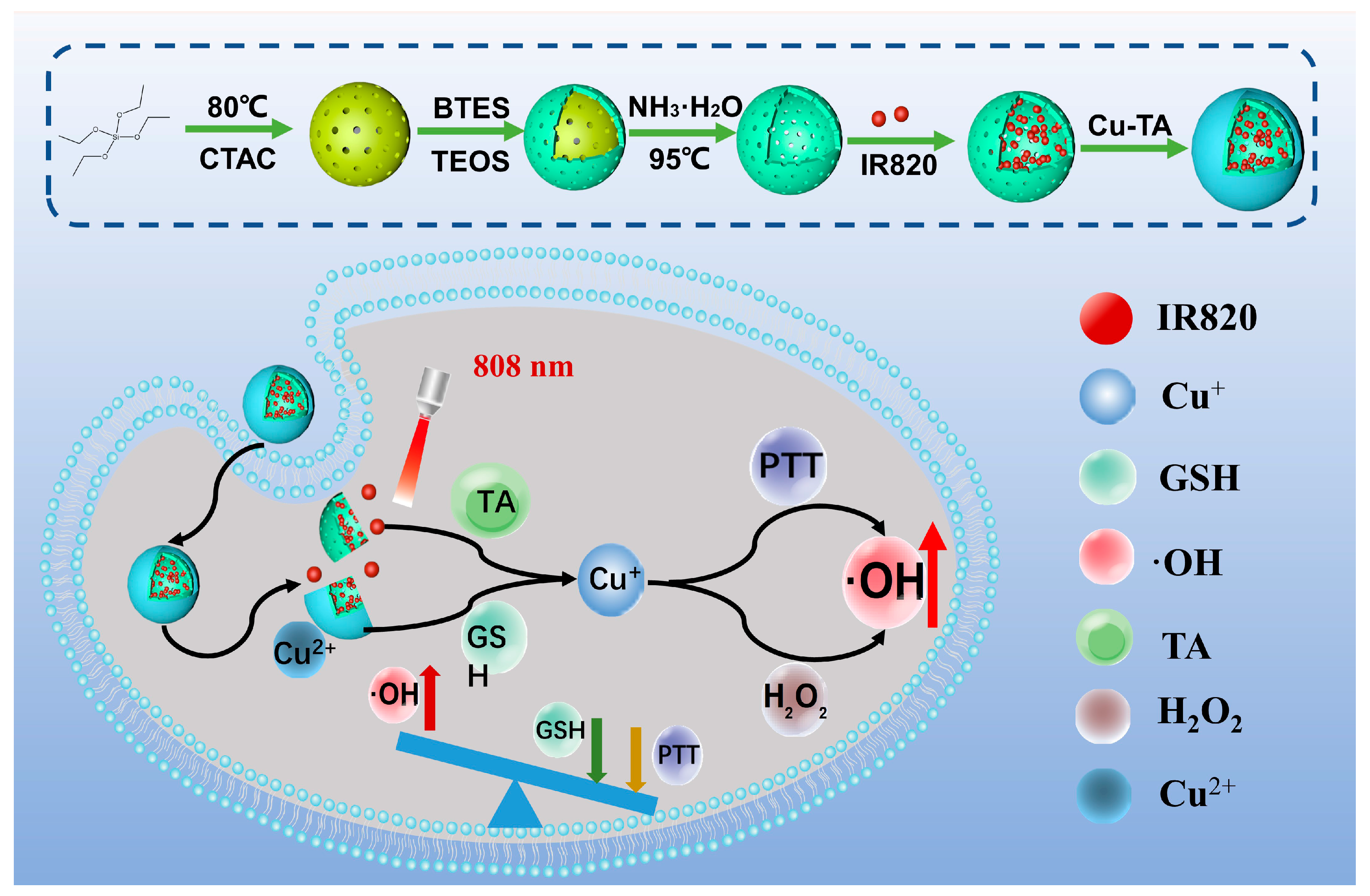
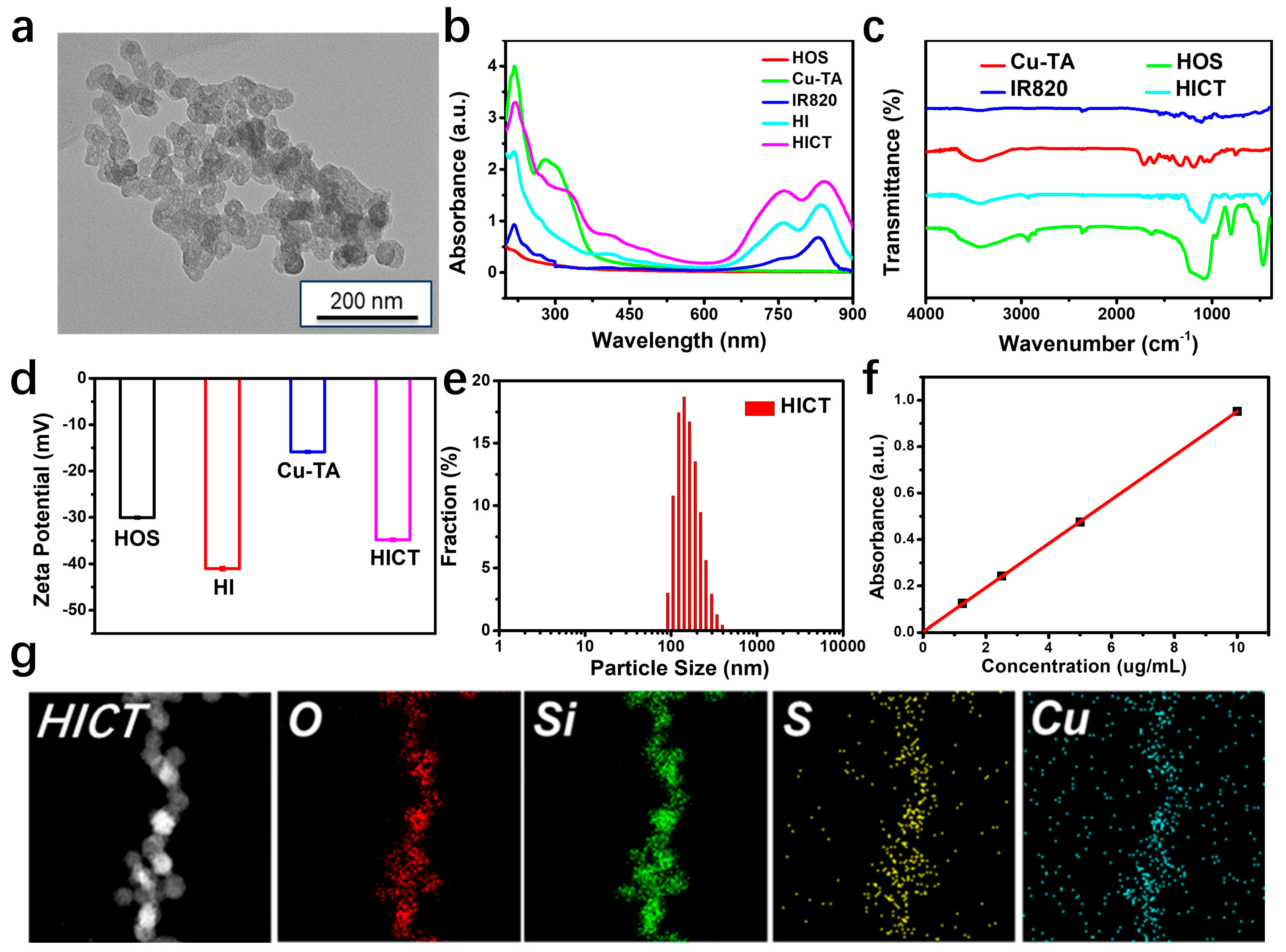
2.1. Preparation of Hollow Organosilica Spheres Loaded with IR820 and Deposited Cu-TA (HICT)
2.2. Photothermal Properties of HICT
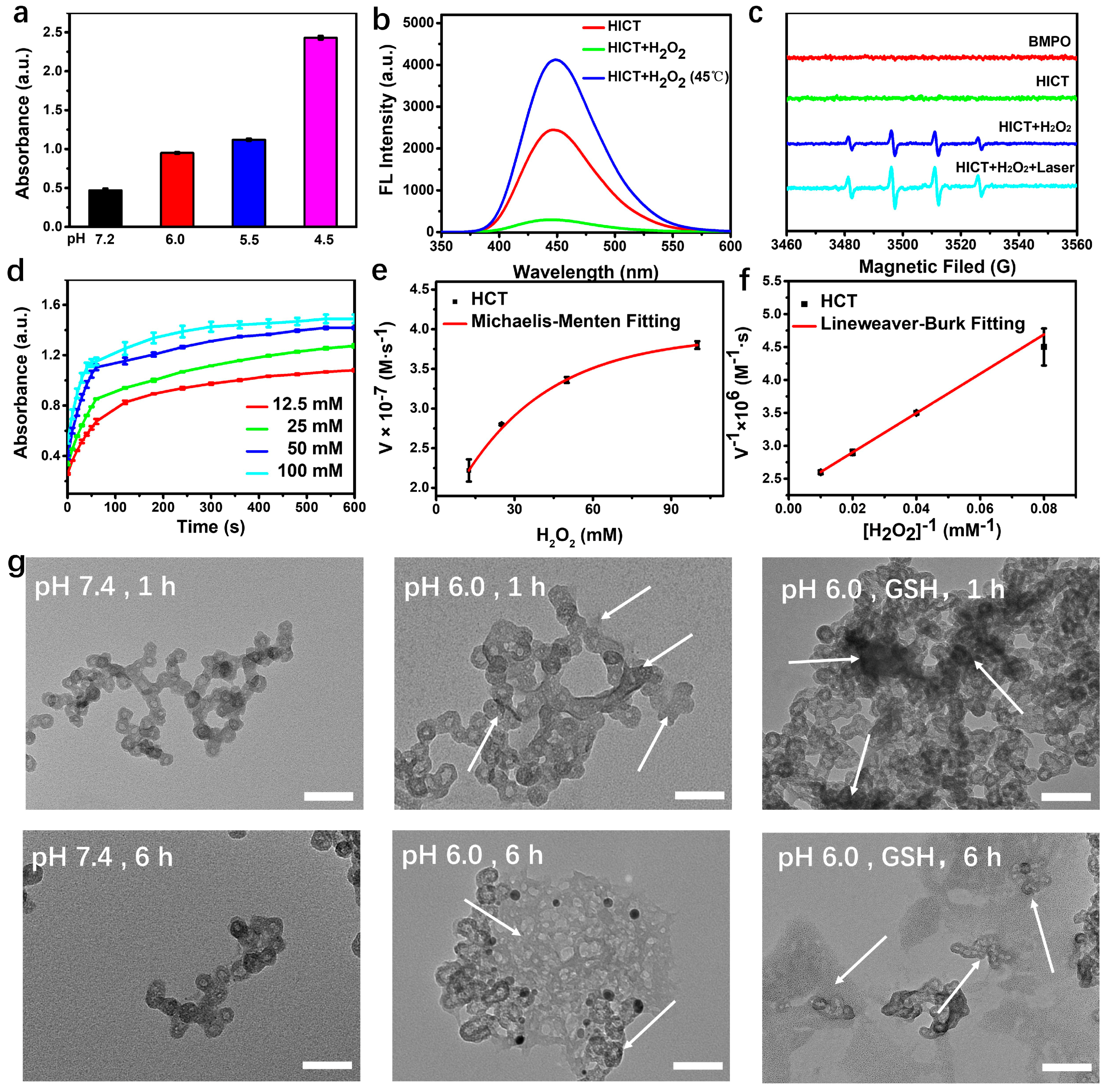
2.3. Characterization of the Chemodynamic Performance
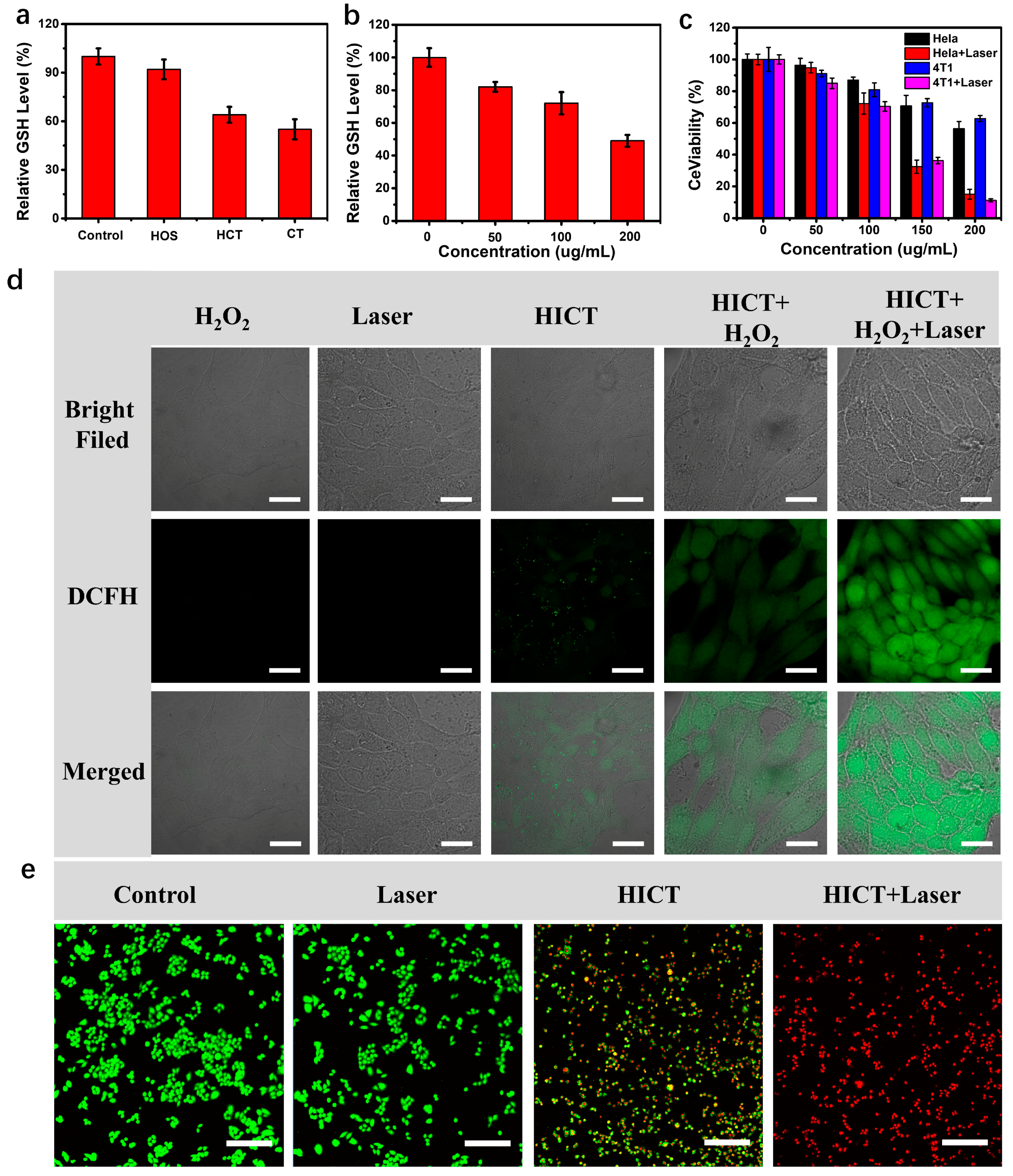
2.4. In Vitro Anticancer Activity
2.5. In Vivo Synergistic Anticancer Activity
3. Materials and Methods
3.1. Materials
3.2. Preparation of the Hollow Organosilica Spheres Loaded with IR820 and Deposited Cu-TA (HICT)
3.3. Measurement of the HICT Assembly
3.4. Detection of GSH Consumption
3.5. Measurement of ·OH Generation
3.6. Photothermal Effect Evaluation
3.7. Intracellular GSH Consumption Detection
3.8. Intracellular ROS Detection
3.9. Cell Viability Evaluation
3.10. Live/Dead Cell Staining Assay
3.11. Animal Experiments
3.12. In Vivo Photothermal Imaging
3.13. In Vivo Anticancer Effect Evaluation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jassim, A.; Rahrmann, E.P.; Simons, B.D.; Gilbertson, R.J. Cancers make their own luck: Theories of cancer origins. Nat. Rev. Cancer 2023, 23, 710–724. [Google Scholar] [CrossRef]
- Jin, M.; Zhao, Y.; Guan, Z.-J.; Fang, Y. Porous Framework Materials for Bioimaging and Cancer Therapy. Molecules 2023, 28, 1360. [Google Scholar] [CrossRef]
- Li, Z.; Di, C.; Li, S.; Yang, X.; Nie, G. Smart Nanotherapeutic Targeting of Tumor Vasculature. Acc. Chem. Res. 2019, 52, 2703–2712. [Google Scholar] [CrossRef]
- Felsher, D.W. Cancer revoked: Oncogenes as therapeutic targets. Nat. Rev. Cancer 2003, 3, 375–380. [Google Scholar] [CrossRef]
- Lima, E.; Reis, L.V. Photodynamic Therapy: From the Basics to the Current Progress of N-Heterocyclic-Bearing Dyes as Effective Photosensitizers. Molecules 2023, 28, 5092. [Google Scholar] [CrossRef]
- Cheng, L.; Wang, X.; Gong, F.; Liu, T.; Liu, Z. 2D Nanomaterials for Cancer Theranostic Applications. Adv. Mater. 2020, 32, e1902333. [Google Scholar] [CrossRef]
- Chen, J.; Fan, T.; Xie, Z.; Zeng, Q.; Xue, P.; Zheng, T.; Chen, Y.; Luo, X.; Zhang, H. Advances in nanomaterials for photodynamic therapy applications: Status and challenges. Biomaterials 2020, 237, 119827. [Google Scholar] [CrossRef]
- Gao, P.; Chen, Y.; Pan, W.; Li, N.; Liu, Z.; Tang, B. Antitumor Agents Based on Metal-Organic Frameworks. Angew. Chem. Int. Ed. 2021, 60, 16763–16776. [Google Scholar] [CrossRef]
- Iranpour, S.; Bahrami, A.R.; Saljooghi, A.S.; Matin, M.M. Application of smart nanoparticles as a potential platform for effective colorectal cancer therapy. Coord. Chem. Rev. 2021, 442, 213949. [Google Scholar] [CrossRef]
- Zhou, G.; Li, M. Near-Infrared-II Plasmonic Trienzyme-Integrated Metal–Organic Frameworks with High-Efficiency Enzyme Cascades for Synergistic Trimodal Oncotherapy. Adv. Mater. 2022, 34, 2200871. [Google Scholar] [CrossRef]
- Wang, X.; Chen, Q.; Lu, C. Aptamer-Functionalized Iron-Based Metal–Organic Frameworks (MOFs) for Synergistic Cascade Cancer Chemotherapy and Chemodynamic Therapy. Molecules 2022, 27, 4247. [Google Scholar] [CrossRef]
- Tang, Z.; Liu, Y.; He, M.; Bu, W. Chemodynamic Therapy: Tumour Microenvironment-Mediated Fenton and Fenton-like Reactions. Angew. Chem. Int. Ed. 2019, 58, 946–956. [Google Scholar] [CrossRef]
- Zhou, Y.; Fan, S.; Feng, L.; Huang, X.; Chen, X. Manipulating Intratumoral Fenton Chemistry for Enhanced Chemodynamic and Chemodynamic-Synergized Multimodal Therapy. Adv. Mater. 2021, 33, 2104223. [Google Scholar] [CrossRef]
- López-Lázaro, M. Dual role of hydrogen peroxide in cancer: Possible relevance to cancer chemoprevention and therapy. Cancer Lett. 2007, 252, 1–8. [Google Scholar] [CrossRef]
- Zhang, L.; Li, C.-X.; Wan, S.-S.; Zhang, X.-Z. Nanocatalyst-Mediated Chemodynamic Tumor Therapy. Adv. Healthc. Mater. 2022, 11, 101971. [Google Scholar] [CrossRef]
- Xiang, H.; Feng, W.; Chen, Y. Single-Atom Catalysts in Catalytic Biomedicine. Adv. Mater. 2020, 32, e1905994. [Google Scholar] [CrossRef] [PubMed]
- Tian, Q.; Xue, F.; Wang, Y.; Cheng, Y.; An, L.; Yang, S.; Chen, X.; Huang, G. Recent advances in enhanced chemodynamic therapy strategies. Nano Today 2021, 39, 101162. [Google Scholar] [CrossRef]
- Bian, Y.; Liu, B.; Liang, S.; Ding, B.; Zhao, Y.; Jiang, F.; Cheng, Z.; Al Kheraif, A.A.; Ma, P.; Lin, J. Cu-based MOFs decorated dendritic mesoporous silica as tumor microenvironment responsive nanoreactor for enhanced tumor multimodal therapy. Chem. Eng. J. 2022, 435, 135046. [Google Scholar] [CrossRef]
- Li, S.-L.; Jiang, P.; Jiang, F.-L.; Liu, Y. Recent Advances in Nanomaterial-Based Nanoplatforms for Chemodynamic Cancer Therapy. Adv. Funct. Mater. 2021, 31, 2100243. [Google Scholar] [CrossRef]
- Chen, F.; Zhang, X.; Wang, Z.; Xu, C.; Hu, J.; Liu, L.; Zhou, J.; Sun, B. Dual-responsive and NIR-driven free radical nanoamplifier with glutathione depletion for enhanced tumor-specific photothermal/thermodynamic/chemodynamic synergistic Therapy. Biomater. Sci. 2022, 10, 5912–5924. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Jia, K.; Sun, K.; Zhang, L.; Wang, Z. Smart responsive nanoplatform via in situ forming disulfiram-copper ion chelation complex for cancer combination chemotherapy. Chem. Eng. J. 2021, 415, 128947. [Google Scholar] [CrossRef]
- Yang, L.; Dong, S.; Gai, S.; Yang, D.; Ding, H.; Feng, L.; Yang, G.; Rehman, Z.; Yang, P. Deep Insight of Design, Mechanism, and Cancer Theranostic Strategy of Nanozymes. Nano-Micro Lett. 2023, 16, 28. [Google Scholar] [CrossRef]
- Huang, Y.; Shen, K.; Si, Y.; Shan, C.; Guo, H.; Chen, M.; Wu, L. Dendritic organosilica nanospheres with large mesopores as multi-guests vehicle for photoacoustic/ultrasound imaging-guided photodynamic therapy. J. Colloid Interface Sci. 2021, 583, 166–177. [Google Scholar] [CrossRef]
- Cheng, Y.; Hu, J.; Qin, S.; Zhang, A.; Zhang, X. Recent advances in functional mesoporous silica-based nanoplatforms for combinational photo-chemotherapy of cancer. Biomaterials 2020, 232, 119738. [Google Scholar] [CrossRef] [PubMed]
- Guimaraes, R.S.; Rodrigues, C.F.; Moreira, A.F.; Correia, I.J. Overview of stimuli-responsive mesoporous organosilica nanocarriers for drug delivery. Pharmacol. Res. 2020, 155, 104742. [Google Scholar] [CrossRef]
- Luo, L.; Bock, L.; Liang, Y.; Anwander, R. Gold-Loaded Mesoporous Organosilica-Silica Core-Shell Nanoparticles as Catalytic Nanoreactors. Eur. J. Inorg. Chem. 2020, 2020, 3967–3976. [Google Scholar] [CrossRef]
- Chen, Y.; Meng, Q.; Wu, M.; Wang, S.; Xu, P.; Chen, H.; Li, Y.; Zhang, L.; Wang, L.; Shi, J. Hollow Mesoporous Organosilica Nanoparticles: A Generic Intelligent Framework-Hybridization Approach for Biomedicine. J. Am. Chem. Soc. 2014, 136, 16326–16334. [Google Scholar] [CrossRef]
- Yang, B.; Yao, H.; Yang, J.; Chen, C.; Guo, Y.; Shi, J. In Situ Synthesis of Natural Antioxidase Mimics for Catalytic Anti-Inflammatory Treatments: Rheumatoid Arthritis as an Example. J. Am. Chem. Soc. 2022, 144, 314–330. [Google Scholar] [CrossRef]
- Shahzad, K.; Imran Khan, M.; Elboughdiri, N.; Ghernaout, D.; ur Rehman, A. Energizing periodic mesoporous organosilica (PMOS) with bismuth and cerium for photo-degrading methylene blue and methyl orange in water. Water Environ. Res. 2021, 93, 1116–1125. [Google Scholar] [CrossRef] [PubMed]
- Shan, L.; Fan, W.; Wang, W.; Tang, W.; Yang, Z.; Wang, Z.; Liu, Y.; Shen, Z.; Dai, Y.; Cheng, S.; et al. Organosilica-Based Hollow Mesoporous Bilirubin Nanoparticles for Antioxidation-Activated Self-Protection and Tumor-Specific Deoxygenation-Driven Synergistic Therapy. ACS Nano 2019, 13, 8903–8916. [Google Scholar] [CrossRef]
- Lu, N.; Fan, W.; Yi, X.; Wang, S.; Wang, Z.; Tian, R.; Jacobson, O.; Liu, Y.; Yung, B.C.; Zhang, G.; et al. Biodegradable Hollow Mesoporous Organosilica Nanotheranostics for Mild Hyperthermia-Induced Bubble-Enhanced Oxygen-Sensitized Radiotherapy. ACS Nano 2018, 12, 1580–1591. [Google Scholar] [CrossRef]
- Tang, W.; Fan, W.; Wang, Z.; Zhang, W.; Zhou, S.; Liu, Y.; Yang, Z.; Shao, E.; Zhang, G.; Jacobson, O.; et al. Acidity/Reducibility Dual-Responsive Hollow Mesoporous Organosilica Nanoplatforms for Tumor-Specific Self-Assembly and Synergistic Therapy. ACS Nano 2018, 12, 12269–12283. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, B.; Sun, Q.; Dong, S.; Kuang, Y.; Dong, Y.; He, F.; Gai, S.; Yang, P. Fusiform-Like Copper(II)-Based Metal-Organic Framework through Relief Hypoxia and GSH-Depletion Co-Enhanced Starvation and Chemodynamic Synergetic Cancer Therapy. ACS Appl. Mater. Interfaces 2020, 12, 17254–17267. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Meng, X.; Liu, M.; Lv, R.; Cai, B.; Wang, Z. Biodegradable Mesoporous Organosilica Nanosheets for Chemotherapy/Mild Thermotherapy of Cancer: Fast Internalization, High Cellular Uptake, and High Drug Loading. ACS Appl. Mater. Interfaces 2020, 12, 30234–30246. [Google Scholar] [CrossRef] [PubMed]
- You, C.; Wu, H.; Zhang, R.; Liu, Y.; Ning, L.; Gao, Z.; Sun, B.; Wang, F. Dendritic Mesoporous Organosilica Nanoparticles: A pH-Triggered Autocatalytic Fenton Reaction System with Self-supplied H2O2 for Generation of High Levels of Reactive Oxygen Species. Langmuir 2020, 36, 5262–5270. [Google Scholar] [CrossRef] [PubMed]
- Shao, D.; Zhang, F.; Chen, F.; Zheng, X.; Hu, H.; Yang, C.; Tu, Z.; Wang, Z.; Chang, Z.; Lu, J.; et al. Biomimetic Diselenide-Bridged Mesoporous Organosilica Nanoparticles as an X-ray-Responsive Biodegradable Carrier for Chemo-Immunotherapy. Adv. Mater. 2020, 32, 2004385. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Zhao, Y.; Wang, L.; Hu, M.; Wu, Z.; Liu, L.; Zhu, W.; Pei, R. Synthesis of Au@MOF core-shell hybrids for enhanced photodynamic/photothermal therapy. J. Mater. Chem. B 2021, 9, 6646–6657. [Google Scholar] [CrossRef]
- Liu, C.; Liu, B.; Zhao, J.; Di, Z.; Chen, D.; Gu, Z.; Li, L.; Zhao, Y. Nd3+-Sensitized Upconversion Metal-Organic Frameworks for Mitochondria-Targeted Amplified Photodynamic Therapy. Angew. Chem. Int. Ed. 2020, 59, 2634–2638. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kong, L.; Li, J.; Zhang, Y.; Wang, J.; Liang, K.; Xue, X.; Chen, T.; Hao, Y.; Ren, H.; Wang, P.; et al. Biodegradable Metal Complex-Gated Organosilica for Dually Enhanced Chemodynamic Therapy through GSH Depletions and NIR Light-Triggered Photothermal Effects. Molecules 2024, 29, 1177. https://doi.org/10.3390/molecules29051177
Kong L, Li J, Zhang Y, Wang J, Liang K, Xue X, Chen T, Hao Y, Ren H, Wang P, et al. Biodegradable Metal Complex-Gated Organosilica for Dually Enhanced Chemodynamic Therapy through GSH Depletions and NIR Light-Triggered Photothermal Effects. Molecules. 2024; 29(5):1177. https://doi.org/10.3390/molecules29051177
Chicago/Turabian StyleKong, Lin, Jian Li, Yunxiu Zhang, Jian Wang, Ke Liang, Xiaokuang Xue, Tiejin Chen, Yongliang Hao, Haohui Ren, Pengfei Wang, and et al. 2024. "Biodegradable Metal Complex-Gated Organosilica for Dually Enhanced Chemodynamic Therapy through GSH Depletions and NIR Light-Triggered Photothermal Effects" Molecules 29, no. 5: 1177. https://doi.org/10.3390/molecules29051177
APA StyleKong, L., Li, J., Zhang, Y., Wang, J., Liang, K., Xue, X., Chen, T., Hao, Y., Ren, H., Wang, P., & Ge, J. (2024). Biodegradable Metal Complex-Gated Organosilica for Dually Enhanced Chemodynamic Therapy through GSH Depletions and NIR Light-Triggered Photothermal Effects. Molecules, 29(5), 1177. https://doi.org/10.3390/molecules29051177




