Investigation of Flame Structures of Double-Base Propellant and Modified Double-Base Propellant Containing Nitramine Using OH-PLIF and Kinetic Simulation
Abstract
1. Introduction
2. Results and Discussion
2.1. OH-PLIF Signals of Propellants
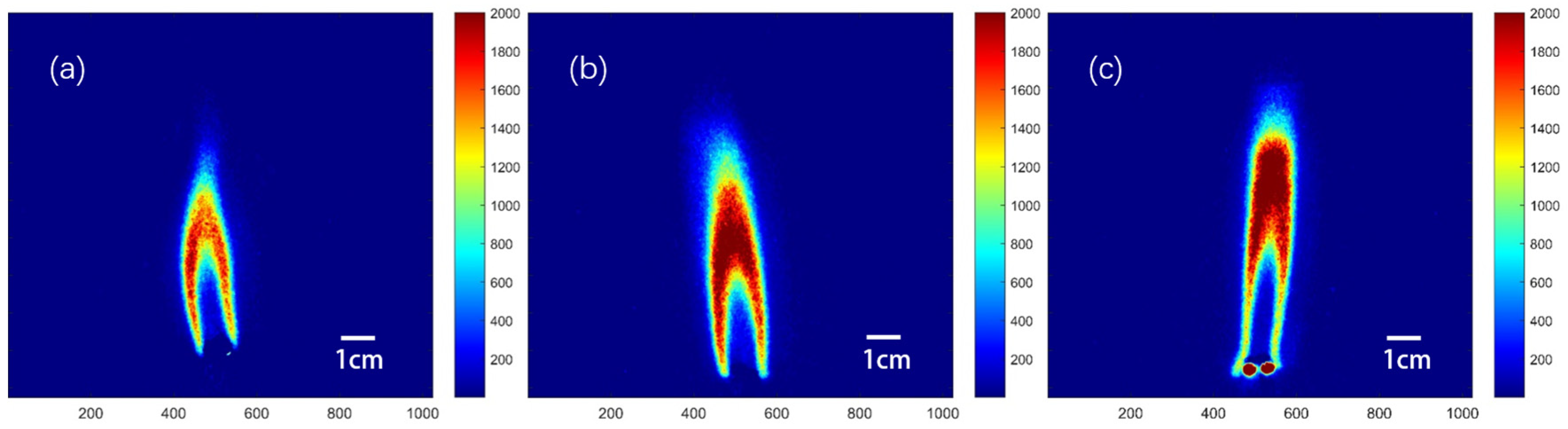
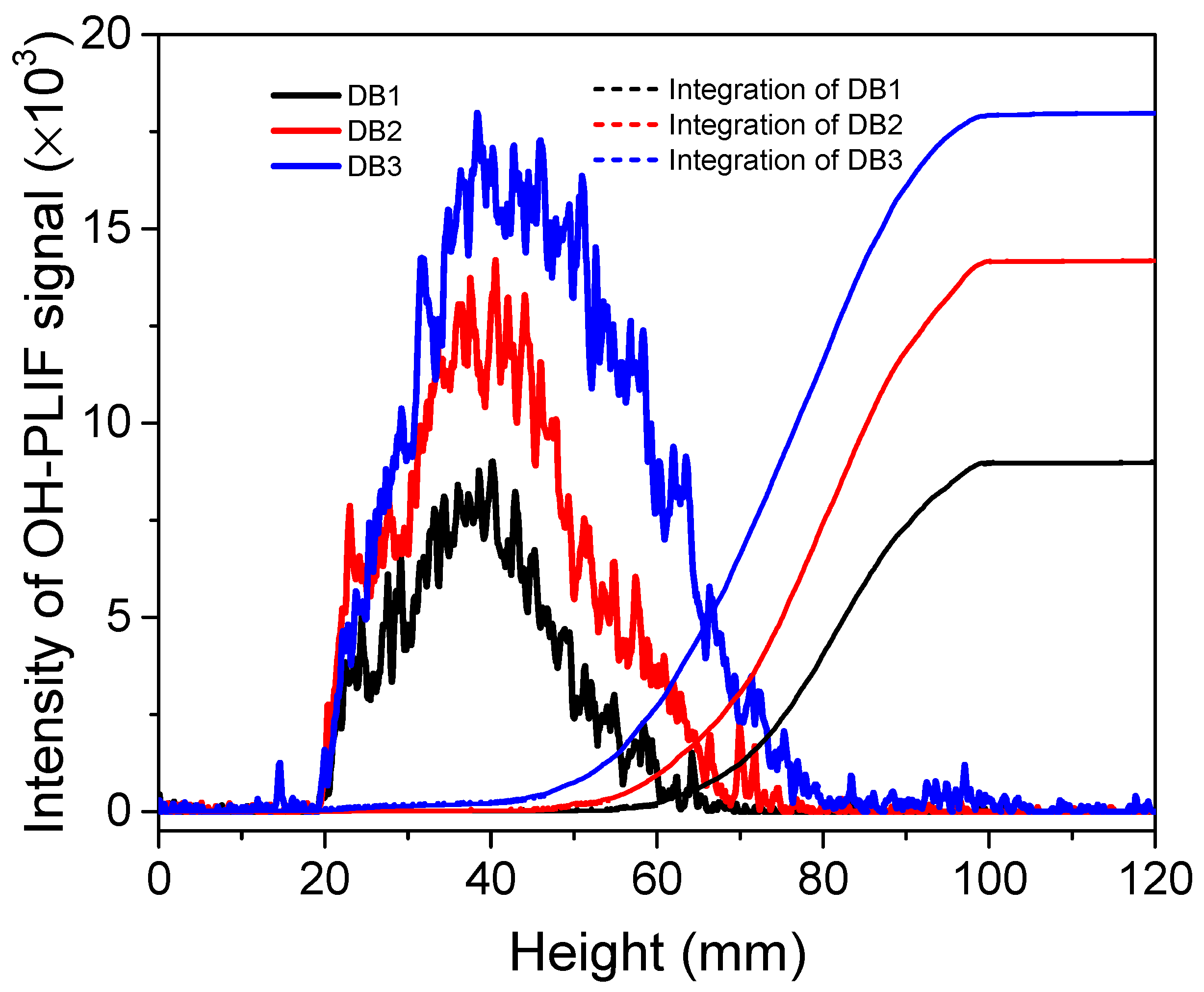
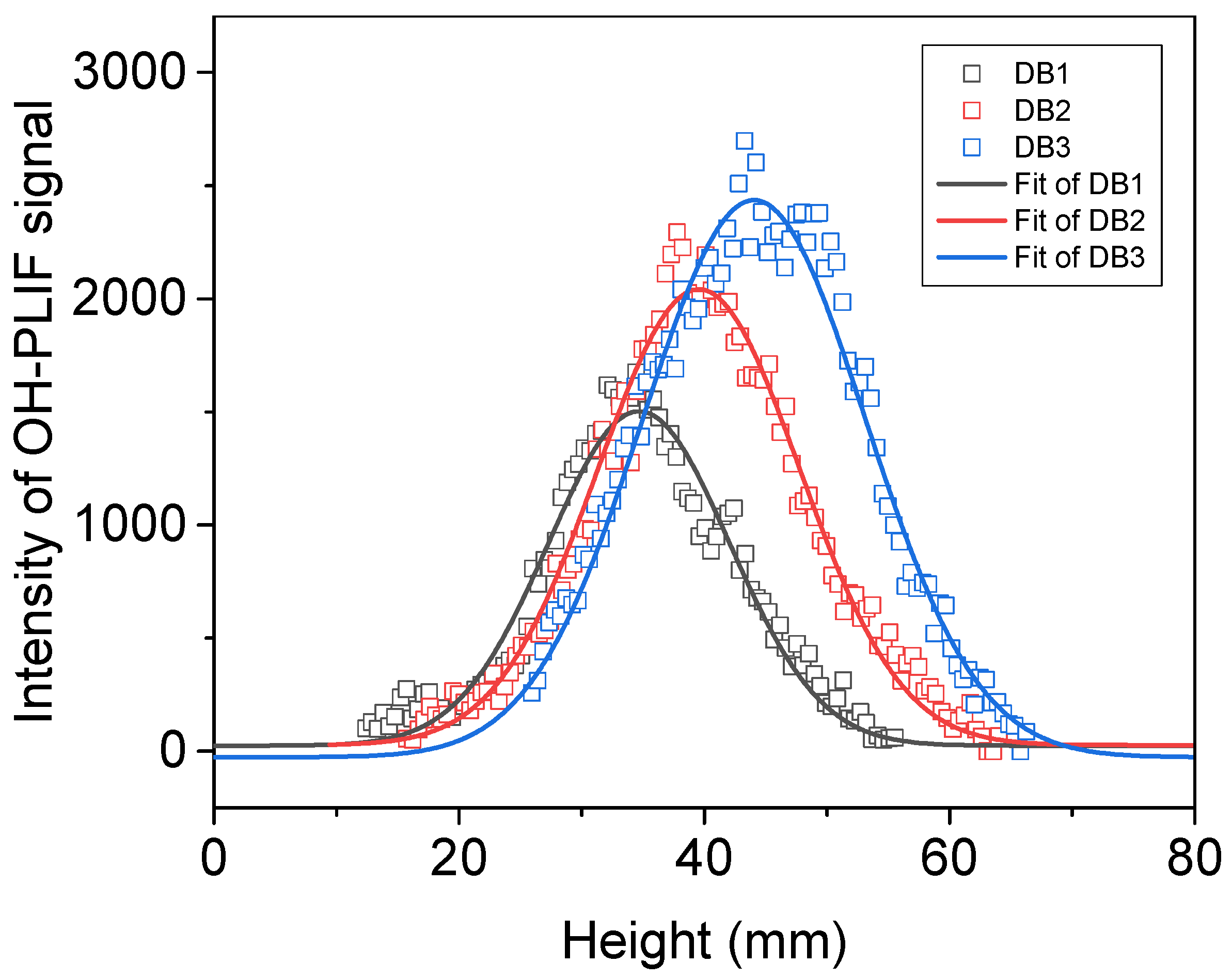
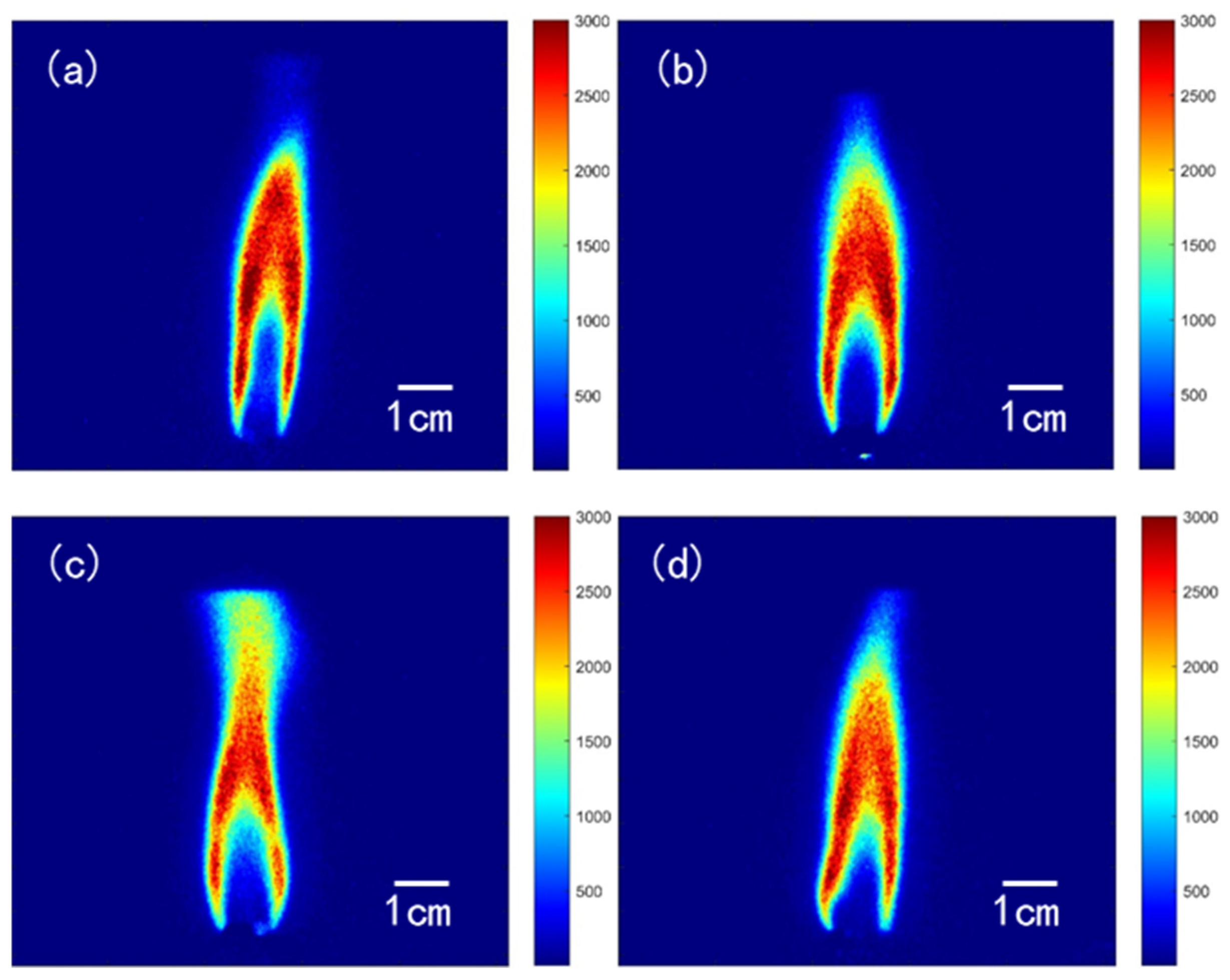
2.1.1. Double-Base Propellant
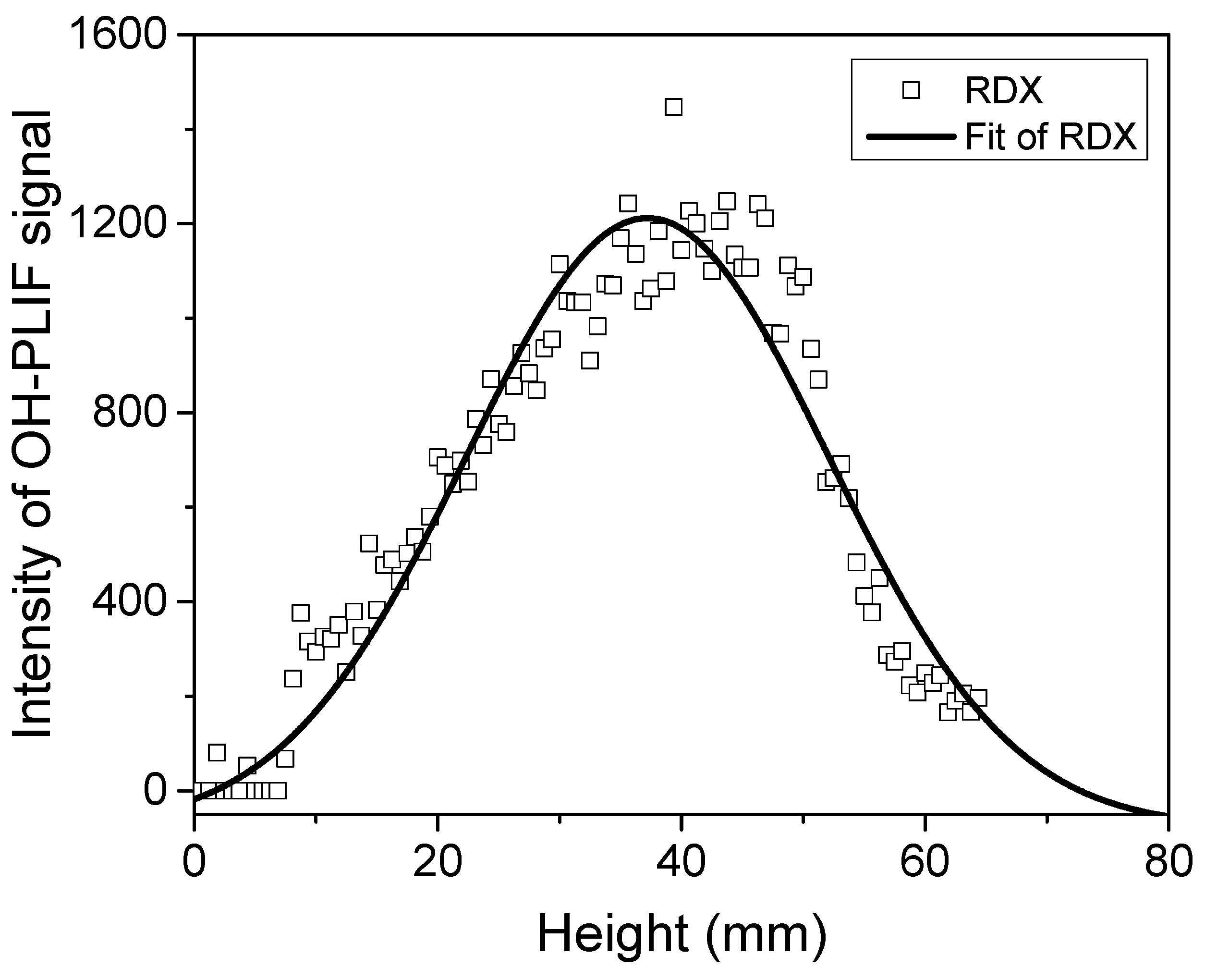
2.1.2. RDX Specimen
2.1.3. Double-Base Propellant Containing RDX
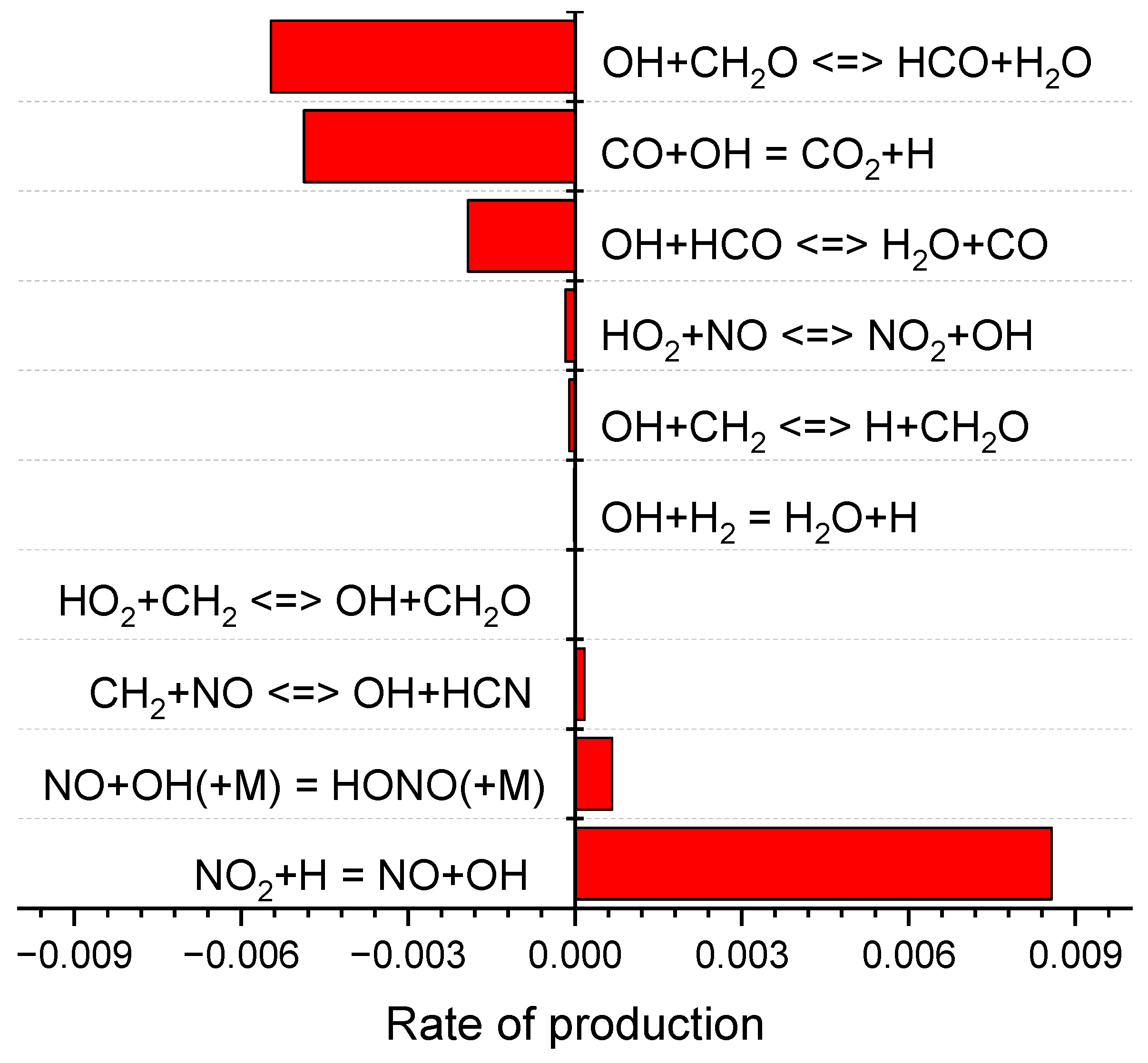
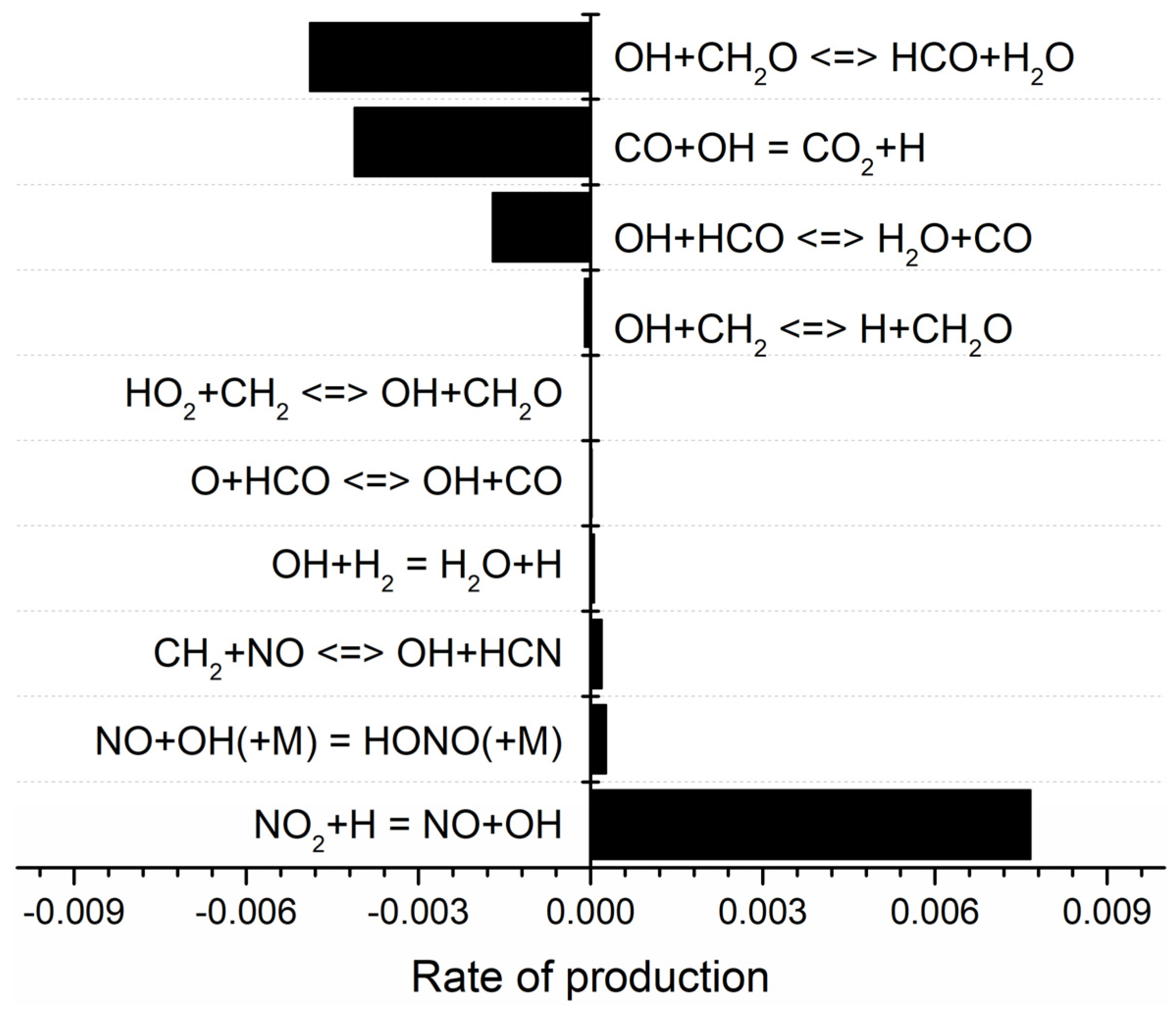
2.2. Detailed Mechanism Analysis Using CHEMKIN
3. Materials and Methods
3.1. Materials and Specimens
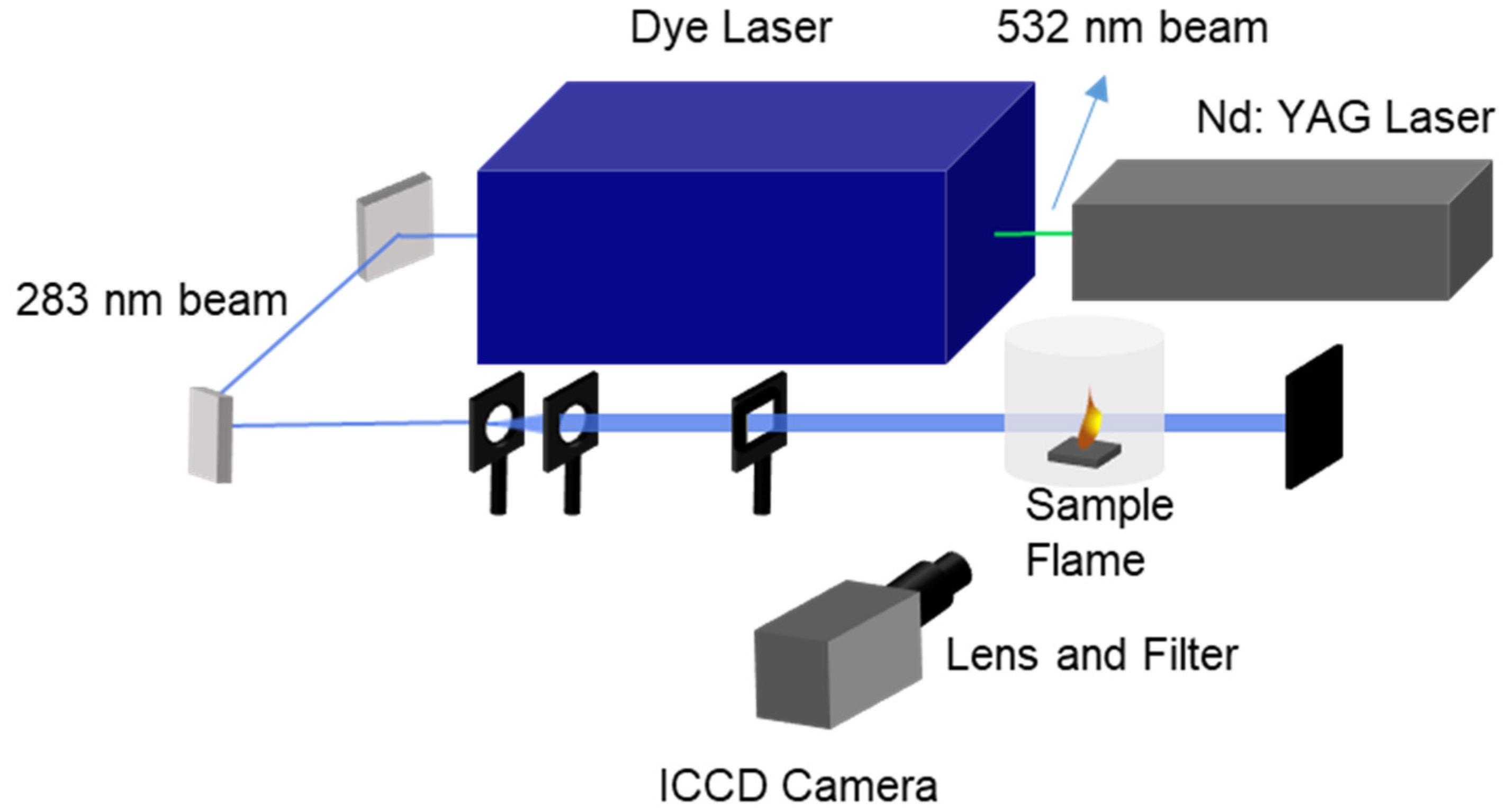
3.2. Equipment and Experimentation
3.2.1. Combustion and Ignition Systems
3.2.2. PLIF System
4. Conclusions
- (1)
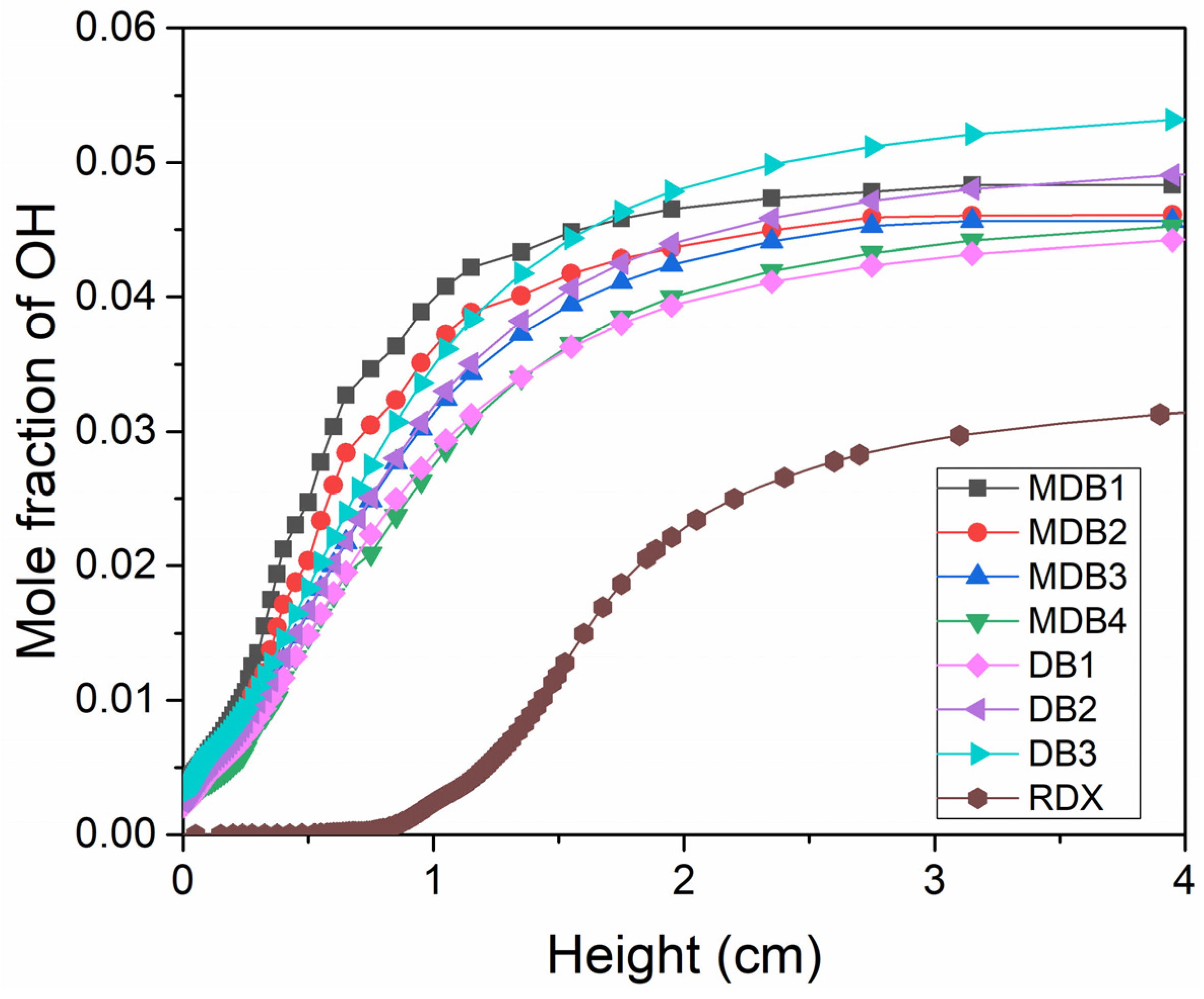
- In the combustion flame of double-base propellant, OH radicals are predominantly found on both sides and at the downstream center. As the content of NG increases, the maximum signal intensity and overall signal intensity of OH-PLIF also rise, indicating an increase in the concentration of OH radicals. The OH flame brush was utilized to compare the trends in experimental and modeled OH radical generation accumulation values. This observation supports the predictions made by Yetter about the M2 and M9 double-base propellant models.
- (2)
- OH-PLIF experiments were performed to analyze the combustion behavior of RDX powder-pressed pillars and modified double-base propellants containing RDX. Observations revealed that during the combustion of RDX powder, OH radicals were mainly concentrated at the central region of the flame’s distal end. In contrast, in the case of modified double-base propellant containing RDX, OH radicals were predominantly located along the flanks of the luminous flame zone and at the core of the distal flame end, displaying a distinct distribution pattern for OH.
- (3)
- As the RDX content in the modified double-base propellant increased, a reduction in the generation of OH radicals was noted. This phenomenon was hypothesized, drawing on ROP analyses, to potentially stem from a concomitant rise in RDX concentration and a decline in the NG component. The combustion of NG yields higher production of NO2 compared with RDX, and NO2 plays a pivotal role as a reactant in the generation of OH radicals.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Elghafour, A.M.A.; Radwan, M.A.; Mostafa, H.E.; Fahd, A.; Elbasuney, S. Highly energetic nitramines: A novel platonizing agent for double-base propellants with superior combustion characteristics. Fuel 2018, 227, 478–484. [Google Scholar] [CrossRef]
- Homan, B.E.; Miller, M.S.; Vanderhoff, J.A. Absorption diagnostics and modeling investigations of RDX flame structure. Combust. Flame 2000, 120, 301–317. [Google Scholar] [CrossRef]
- Yan, Q.L.; Song, Z.W.; Shi, X.B.; Yang, Z.Y.; Zhang, X.H. Combustion mechanism of double-base propellant containing nitrogen heterocyclic nitroamines (II): The temperature distribution of the flame and its chemical structure. Acta Astronaut. 2009, 64, 602–614. [Google Scholar] [CrossRef]
- Miller, M.S.; Anderson, W.R. Burning-Rate Predictor for Multi-Ingredient Propellants: Nitrate–Ester Propellants. J. Propuls. Power 2004, 20, 440–454. [Google Scholar] [CrossRef]
- Elbasuney, S.; Fahd, A.; Mostafa, H.E. Combustion characteristics of extruded double base propellant based on ammonium perchlorate/aluminum binary mixture. Fuel 2017, 208, 296–304. [Google Scholar] [CrossRef]
- Zenin, A. HMX and RDX—Combustion mechanism and influence on modern double-base propellant combustion. J. Propuls. Power 1995, 11, 752–758. [Google Scholar] [CrossRef]
- Volkov, E.N.; Paletsky, A.A.; Korobeinichev, O.P. RDX and HMX flame structure at a pressure of 0.1 MPa. Int. J. Energetic Mater. Chem. Propuls. 2009, 8, 183–198. [Google Scholar] [CrossRef]
- Paletsky, A.A.; Volkov, E.N.; Korobeinichev, O.P.; Tereshchenko, A.G. Flame structure of composite pseudo-propellants based on nitramines and azide polymers at high pressure. Proc. Combust. Inst. 2007, 31, 2079–2087. [Google Scholar] [CrossRef]
- Yan, Q.L.; Li, X.J.; Wang, Y.; Zhang, W.H.; Zhao, F.Q. Combustion Mechanism of Double-Base Propellant Containing Nitrogen Heterocyclic Nitroamines (I): The Effect of Heat and Mass Transfer to the Burning Characteristics. Combust. Flame 2009, 156, 633–641. [Google Scholar] [CrossRef]
- Parr, T.P.; Hanson-Parr, D.M. Solid propellant diffusion flame structure. Symp. Combust. 1996, 26, 1981–1987. [Google Scholar] [CrossRef]
- Parr, T.; Hanson-Parr, D. Cyclotetramethylene tetranitramine/glycidyl azide polymer/butanetriol trinitrate propellant flame structure. Combust. Flame 2004, 137, 38–49. [Google Scholar] [CrossRef]
- Ruesch, M.; Powell, M.S.; Satija, A.; Ruesch, J.P.; Son, S.F. Burning rate and flame structure of cocrystals of CL-20 and a polycrystalline composite crystal of HMX/AP. Combust. Flame 2020, 219, 129–135. [Google Scholar] [CrossRef]
- Chevalier, P.-H.; Dorval, N.; Devillers, R.; Davidenko, D.; Mercier, X.; Pichillou, J. Investigation of aluminum droplet combustion in solid propellant flames: Al-PLIF experiments and numerical simulation. In Proceedings of the AIAA Propulsion and Energy 2021 Forum, Online, 9–11 August 2021. [Google Scholar]
- Xin, S.; Wang, W.; He, Y.; Zhu, Y.; Wang, Z. Effect of low fuel temperature on combustion deterioration of kerosene swirling spray flames using OH-PLIF. Fuel 2024, 358, 130098. [Google Scholar] [CrossRef]
- Yang, Y.; Zhu, T.; Yan, Z.; Li, Q.; Liu, B.; Wang, J.; Huang, Z. A Study on Combustion Characteristics of Insensitive Triple-Base Propellant. Appl. Sci. 2023, 13, 5462. [Google Scholar] [CrossRef]
- Yan, Z.; Cao, X.; Tao, J.; Zhang, K.; Li, Q.; Wang, J.; Huang, Z. The Influence of AP Addition on the Combustion Characteristics of TKX-50 with Concentrated Ignition. Propellants Explos. Pyrotech. 2022, 47, e202200050. [Google Scholar] [CrossRef]
- Tang, Y.; Li, S.; Yao, Z.; Huang, B.; Li, S. Ignition of an ionic liquid dual-mode monopropellant using a microwave plasma torch. Proc. Combust. Inst. 2023, 39, 5063–5071. [Google Scholar] [CrossRef]
- Kim, Y.H.; Kim, J.H.; Kwon, O.C. Combustion characteristics of O2/CH4 coaxial jet flames in a model combustor through their visualization and the statistical analysis. Energy 2023, 275, 127548. [Google Scholar] [CrossRef]
- Sui, M.; Zhu, Z.; Li, F.; Wang, H. Effect of ferrocene as a combustion catalyst on the premixed combustion flame characteristics of Jatropha biodiesel. Combust. Flame 2024, 259, 113180. [Google Scholar] [CrossRef]
- Lutz, A.E.; Kee, R.J.; Grcar, J.F.; Rupley, F.M. OPPDIF: A Fortran program for computing opposed-flow diffusion flames. In Office of Scientific & Technical Information Technical Reports; Sandia National Lab. (SNL-CA): Livermore, CA, USA, 1997. [Google Scholar]
- Fox, J.; Gaston, M.; Danehy, P.; Mudford, N.; Houwing, A.; Gai, S. Instantaneous mole-fraction PLIF imaging of mixing layers behind hypermixing injectors. In Proceedings of the 37th Aerospace Sciences Meeting and Exhibit, Reno, NV, USA, 11–14 January 1999. [Google Scholar]
- Yetter, R.A.; Dryer, F.L.; Allen, M.T.; Gatto, J.L. Development of gas-phase reaction mechanisms for nitramine combustion. J. Propuls. Power 1995, 11, 683–697. [Google Scholar] [CrossRef]
- Mashruk, S. Nitric Oxide Formation Analysis Using Chemical Reactor Modelling and Laser Induced Fluorescence Measurements on Industrial Swirl Flames; Cardiff University: Cardiff, UK, 2020. [Google Scholar]
- Zenin, A.A.; Finjakov, S.V. Studying RDX and HMX combustion mechanisms by various experimental techniques. Combust. Explos. Shock Waves 2009, 45, 559–578. [Google Scholar] [CrossRef]
- Parr, T.; Hanson-Parr, D. Optical diagnostics of solid-propellant flame structures. Prog. Astronaut. Aeronaut. 2000, 185, 381–412. [Google Scholar]
- Anderson, W.R.; Conner, C.B. Comparison of gas-phase mechanisms applied to RDX combustion model(Article). Proc. Combust. Inst. 2009, 32, 2123–2130. [Google Scholar] [CrossRef]
- Cornell, R.E.; Barbet, M.C.; Burke, M.P. Automated discovery of influential chemically termolecular reactions in energetic material combustion: A case study for RDX. Proc. Combust. Inst. 2021, 38, 787–794. [Google Scholar] [CrossRef]
- Glorian, J.; Ehrhardt, J.; Baschung, B. Estimating ignition delay times of nitroglycerin: A chemical kinetic modeling study. Combust. Sci. Technol. 2024, 196, 406–420. [Google Scholar] [CrossRef]


| Samples | Percent of Component/% | |||||
|---|---|---|---|---|---|---|
| NC (12.0) | NG | RDX | DBP | C2 | V | |
| MDB1 | 49.52 | 36.48 | 6.00 | 5.00 | 2.50 | 0.50 |
| MDB2 | 43.76 | 32.24 | 16.00 | 5.00 | 2.50 | 0.50 |
| MDB3 | 38.00 | 28.00 | 26.00 | 5.00 | 2.50 | 0.50 |
| MDB4 | 32.24 | 23.76 | 36.00 | 5.00 | 2.50 | 0.50 |
| DB1 | 72.00 | 20.00 | 0 | 5.00 | 2.50 | 0.50 |
| DB2 | 62.00 | 30.00 | 0 | 5.00 | 2.50 | 0.50 |
| DB3 | 52.00 | 40.00 | 0 | 5.00 | 2.50 | 0.50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Zhang, Y.; Li, H.; Yao, E.; Yu, J.; Zhao, F.; Xu, S. Investigation of Flame Structures of Double-Base Propellant and Modified Double-Base Propellant Containing Nitramine Using OH-PLIF and Kinetic Simulation. Molecules 2024, 29, 1175. https://doi.org/10.3390/molecules29051175
Wang Y, Zhang Y, Li H, Yao E, Yu J, Zhao F, Xu S. Investigation of Flame Structures of Double-Base Propellant and Modified Double-Base Propellant Containing Nitramine Using OH-PLIF and Kinetic Simulation. Molecules. 2024; 29(5):1175. https://doi.org/10.3390/molecules29051175
Chicago/Turabian StyleWang, Yiping, Yan Zhang, Heng Li, Ergang Yao, Jin Yu, Fengqi Zhao, and Siyu Xu. 2024. "Investigation of Flame Structures of Double-Base Propellant and Modified Double-Base Propellant Containing Nitramine Using OH-PLIF and Kinetic Simulation" Molecules 29, no. 5: 1175. https://doi.org/10.3390/molecules29051175
APA StyleWang, Y., Zhang, Y., Li, H., Yao, E., Yu, J., Zhao, F., & Xu, S. (2024). Investigation of Flame Structures of Double-Base Propellant and Modified Double-Base Propellant Containing Nitramine Using OH-PLIF and Kinetic Simulation. Molecules, 29(5), 1175. https://doi.org/10.3390/molecules29051175





