An Exceptionally Active and Highly Selective Perchlorate Transporter Containing a Trimesic Amide Scaffold
Abstract
1. Introduction
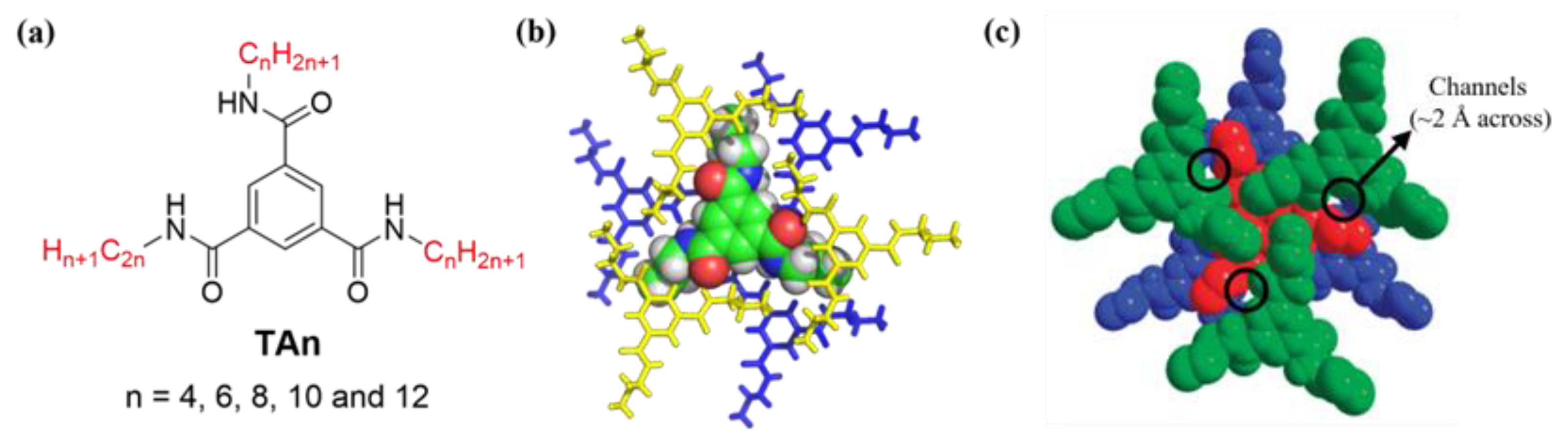
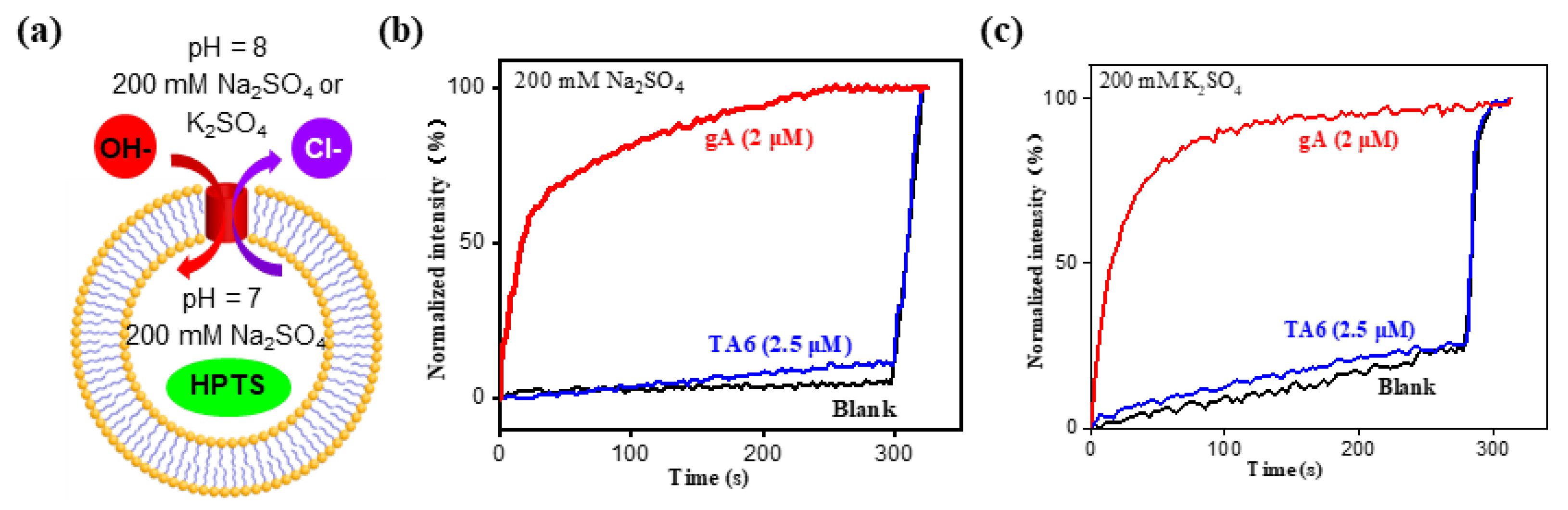
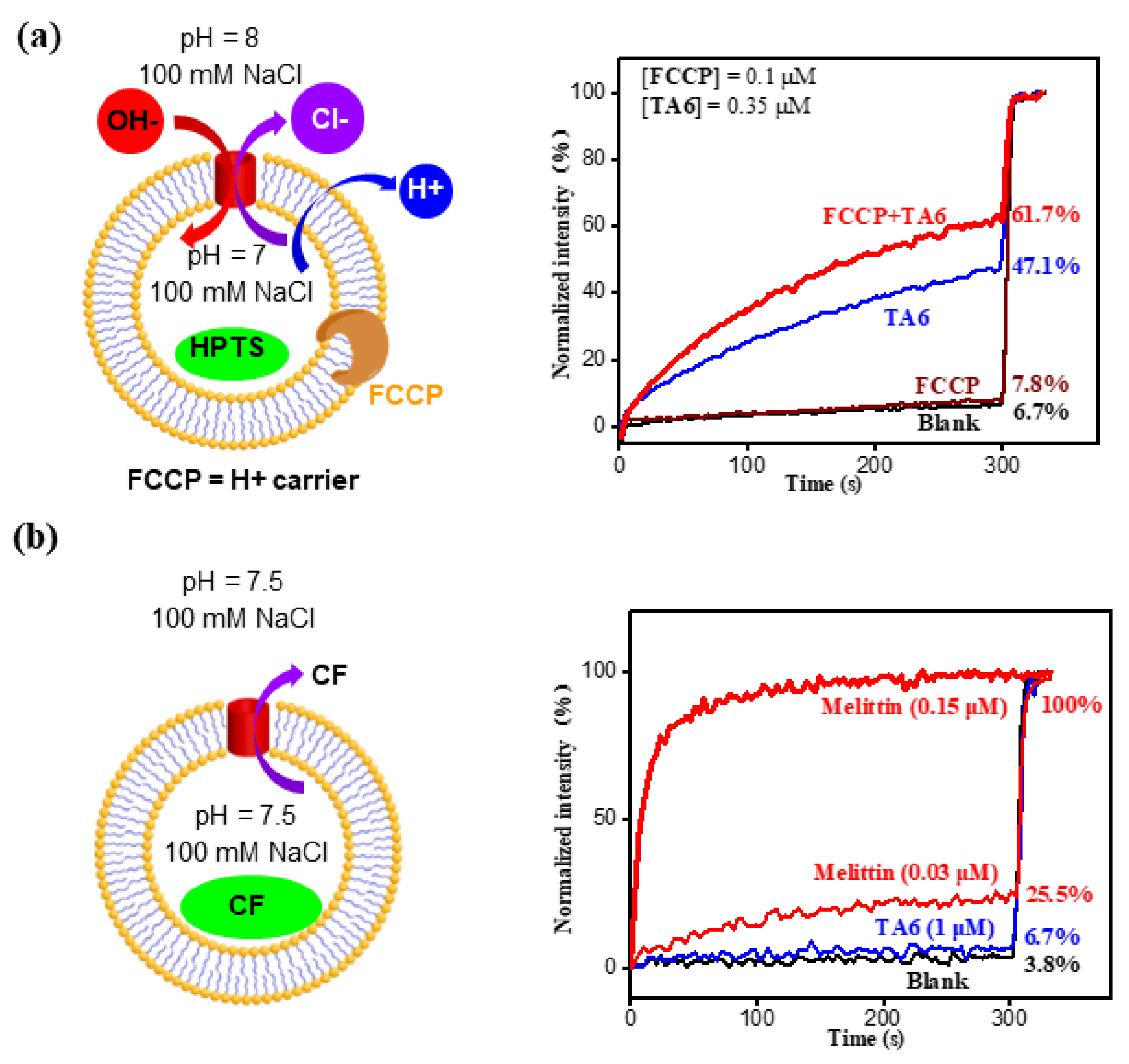
2. Results and Discussion
3. Materials and Methods
3.1. General Considerations
3.2. General Procedure for the Synthesis of TAn Molecules as Typified Using TA6
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, J.; Berg, A.P.; Wang, Y.; Jantarajit, W.; Sutcliffe, K.J.; Stevens, E.B.; Sheppard, D.N. A small molecule CFTR potentiator restores ATP-dependent channel gating to the cystic fibrosis mutant G551D-CFTR. Br. J. Pharmacol. 2022, 179, 1319–1337. [Google Scholar] [CrossRef] [PubMed]
- Noel, S.; Servel, N.; Hatton, A.; Golec, A.; Rodrat, M.; Ng, D.R.; Sermet-Gaudelus, I. Correlating genotype with phenotype using CFTR-mediated whole-cell Cl− currents in human nasal epithelial cells. J. Physiol. 2022, 600, 1515–1531. [Google Scholar] [CrossRef]
- Salomon, J.J.; Albrecht, T.; Graeber, S.Y.; Scheuermann, H.; Butz, S.; Schatterny, J.; Mall, M.A. Chronic rhinosinusitis with nasal polyps is associated with impaired TMEM16A-mediated epithelial chloride secretion. J. Allergy Clin. Immunol. 2021, 147, 2191–2201.e2. [Google Scholar] [CrossRef] [PubMed]
- Duan, R.; Li, H.M.; Hu, W.B.; Hong, C.G.; Chen, M.L.; Cao, J.; Liu, Z.Z. Recurrent de novo single point variant on the gene encoding Na+/K+ pump results in epilepsy. Prog. Neurobiol. 2022, 216, 102310. [Google Scholar] [CrossRef]
- Barreto, R.F.; de Mello Prado, R.; Bodelão, N.C.; Teixeira, G.C.M.; Teixeira, G.C.M. Na improves the growth of K-deficient but not K-sufficient kale. Food Chem. 2022, 370, 131017. [Google Scholar] [CrossRef]
- Li, Y.; Tang, W.; Kang, L.; Kong, S.; Dong, Z.; Zhao, D.; Yu, S. Functional correlation of ATP1A2 mutations with phenotypic spectrum: From pure hemiplegic migraine to its variant forms. J. Headache Pain. 2021, 22, 92. [Google Scholar] [CrossRef]
- Li, Y.; Chang, C.; Zhu, Z.; Sun, L.; Yu, S. Terahertz wave enhances permeability of the voltage-gated calcium channel. J. Am. Chem. Soc. 2021, 143, 4311–4318. [Google Scholar] [CrossRef] [PubMed]
- Miziak, B.; Czuczwar, S.J. Approaches for the discovery of drugs that target K Na 1.1 channels in KCNT1-associated epilepsy. Expert Opin. Drug Discov. 2022, 17, 1313–1328. [Google Scholar] [CrossRef]
- Vanden Abeele, F.; Lotteau, S.; Ducreux, S.; Dubois, C.; Monnier, N.; Hanna, A.; Prevarskaya, N. TRPV1 variants impair intracellular Ca2+ signaling and may confer susceptibility to malignant hyperthermia. Genet. Med. 2019, 21, 441–450. [Google Scholar] [CrossRef]
- Wylde, J.; Hopkins, P.; Kaura, V. Elevated resting extracellular Ca2+ entry is conserved in the CaV1.1 p. T 1009K variant that is associated with malignant hyperthermia. Br. J. Anaesth. 2023, 131, e98. [Google Scholar] [CrossRef]
- Davis, J.T.; Okunola, O.; Quesada, R. Recent advances in the transmembrane transport of anions. Chem. Soc. Rev. 2010, 39, 3843–3862. [Google Scholar] [CrossRef] [PubMed]
- Brotherhood, P.R.; Davis, A.P. Steroid-based anion receptors and transporters. Chem. Soc. Rev. 2010, 39, 3633–3647. [Google Scholar] [CrossRef] [PubMed]
- Matile, S.; Jentzsch, A.V.; Montenegro, J.; Fin, A. Recent synthetic transport systems. Chem. Soc. Rev. 2011, 40, 2453–2474. [Google Scholar] [CrossRef]
- Montenegro, J.; Ghadiri, M.R.; Granja, J.R. Ion channel models based on self-assembling cyclic peptide nanotubes. Acc. Chem. Res. 2013, 46, 2955–2965. [Google Scholar] [CrossRef] [PubMed]
- Fyles, T.M. How do amphiphiles form ion-conducting channels in membranes? Lessons from linear oligoesters. Acc. Chem. Res. 2013, 46, 2847–2855. [Google Scholar] [CrossRef] [PubMed]
- Otis, F.; Auger, M.; Voyer, N. Exploiting Peptide Nanostructures to Construct Functional Artificial Ion Channels. Acc. Chem. Res. 2013, 46, 2934–2943. [Google Scholar] [CrossRef] [PubMed]
- De Riccardis, F.; Izzo, I.; Montesarchio, D.; Tecilla, P. Ion transport through lipid bilayers by synthetic ionophores: Modulation of activity and selectivity. Acc. Chem. Res. 2013, 46, 2781–2790. [Google Scholar] [CrossRef]
- Mosgaard, L.D.; Heimburg, T. Lipid ion channels and the role of proteins. Acc. Chem. Res. 2013, 46, 2966–2976. [Google Scholar] [CrossRef]
- Gong, B.; Shao, Z. Self-assembling organic nanotubes with precisely defined, sub-nanometer pores: Formation and mass transport characteristics. Acc. Chem. Res. 2013, 46, 2856–2866. [Google Scholar] [CrossRef]
- Kim, D.S.; Sessler, J.L. Calix [4] pyrroles: Versatile molecular containers with ion transport, recognition, and molecular switching functions. Chem. Soc. Rev. 2015, 44, 532–546. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.Y.; Hou, J.L. Controllable synthetic ion channels. Org. Chem. Front. 2018, 5, 1728–1736. [Google Scholar] [CrossRef]
- Wu, X.; Gilchrist, A.M.; Gale, P.A. Prospects and challenges in anion recognition and transport. Chem 2020, 6, 1296–1309. [Google Scholar] [CrossRef]
- Zheng, S.P.; Huang, L.B.; Sun, Z.; Barboiu, M. Self-assembled artificial ion-channels toward natural selection of functions. Angew. Chem. Int. Ed. 2021, 60, 566–597. [Google Scholar] [CrossRef]
- Yang, J.; Yu, G.; Sessler, J.L.; Shin, I.; Gale, P.A.; Huang, F. Artificial transmembrane ion transporters as potential therapeutics. Chem 2021, 7, 3256–3291. [Google Scholar] [CrossRef]
- Roy, A.; Talukdar, P. Recent advances in bioactive artificial ionophores. ChemBioChem 2021, 22, 2925–2940. [Google Scholar] [CrossRef]
- Vargas Jentzsch, A.; Matile, S. Transmembrane halogen-bonding cascades. J. Am. Chem. Soc. 2013, 135, 5302–5303. [Google Scholar] [CrossRef] [PubMed]
- Gilles, A.; Barboiu, M. Highly selective artificial K+ channels: An example of selectivity-induced transmembrane potential. J. Am. Chem. Soc. 2016, 138, 426–432. [Google Scholar] [CrossRef] [PubMed]
- Ren, C.; Shen, J.; Zeng, H. Combinatorial evolution of fast-conducting highly selective K+-channels via modularly tunable directional assembly of crown ethers. J. Am. Chem. Soc. 2017, 139, 12338–12341. [Google Scholar] [CrossRef] [PubMed]
- Ren, C.; Ding, X.; Roy, A.; Shen, J.; Zhou, S.; Chen, F.; Zeng, H. A halogen bond-mediated highly active artificial chloride channel with high anticancer activity. Chem. Sci. 2018, 9, 4044–4051. [Google Scholar] [CrossRef]
- Xin, P.; Kong, H.; Sun, Y.; Zhao, L.; Fang, H.; Zhu, H.; Chen, C.P. Artificial K+ channels formed by pillararene-cyclodextrin hybrid molecules: Tuning cation selectivity and generating membrane potential. Angew. Chem. Int. Ed. 2019, 131, 2805–2810. [Google Scholar] [CrossRef]
- Huang, W.L.; Wang, X.D.; Ao, Y.F.; Wang, Q.Q.; Wang, D.X. Artificial chloride-selective channel: Shape and function mimic of the clc channel selective pore. J. Am. Chem. Soc. 2020, 142, 13273–13277. [Google Scholar] [CrossRef] [PubMed]
- Mondal, A.; Save, S.N.; Sarkar, S.; Mondal, D.; Mondal, J.; Sharma, S.; Talukdar, P. A Benzohydrazide-Based Artificial Ion Channel that Modulates Chloride Ion Concentration in Cancer Cells and Induces Apoptosis by Disruption of Autophagy. J. Am. Chem. Soc. 2023, 145, 9737–9745. [Google Scholar] [CrossRef] [PubMed]
- Mandal, S.M.; Sharma, S.; Pinnaka, A.K.; Kumari, A.; Korpole, S. Isolation and characterization of diverse antimicrobial lipopeptides produced by Citrobacter and Enterobacter. BMC Microbiol. 2013, 13, 152. [Google Scholar] [CrossRef] [PubMed]
- Tareq, F.S.; Lee, M.A.; Lee, H.S.; Lee, Y.J.; Lee, J.S.; Hasan, C.M.; Shin, H.J. Gageotetrins A–C, noncytotoxic antimicrobial linear lipopeptides from a marine bacterium Bacillus subtilis. Org. Lett. 2014, 16, 928–931. [Google Scholar] [CrossRef] [PubMed]
- Peraro, M.D.; Van Der Goot, F.G. Pore-forming toxins: Ancient, but never really out of fashion. Nat. Rev. Microbiol. 2015, 14, 77–92. [Google Scholar] [CrossRef]
- Lau, S.Y.; Taneja, A.K.; Hodges, R.S. Synthesis of a model protein of defined secondary and quaternary structure. Effect of chain length on the stabilization and formation of two-stranded alpha-helical coiled-coils. J. Biol. Chem. 1984, 259, 13253–13261. [Google Scholar] [CrossRef]
- Sasaki, T.; Kaiser, E.T. Helichrome: Synthesis and enzymic activity of a designed hemeprotein. J. Am. Chem. Soc. 1989, 111, 380–381. [Google Scholar] [CrossRef]
- Hill, C.P.; Anderson, D.H.; Wesson, L.; DeGrado, W.F.; Eisenberg, D. Crystal structure of α1: Implications for protein design. Science 1990, 249, 543–546. [Google Scholar] [CrossRef]
- Schafmeister, C.E.; LaPorte, S.L.; Miercke, L.J.; Stroud, R.M. A designed four helix bundle protein with native-like structure. Nat. Struct. Biol. 1997, 4, 1039. [Google Scholar] [CrossRef]
- Hibbs, R.E.; Gouaux, E. Principles of activation and permeation in an anion-selective Cys-loop receptor. Nature 2011, 474, 54–60. [Google Scholar] [CrossRef]
- Hou, X.; Pedi, L.; Diver, M.M.; Long, S.B. Crystal structure of the calcium release–activated calcium channel Orai. Science 2012, 338, 1308–1313. [Google Scholar] [CrossRef]
- Jones, J.E.; Diemer, V.; Adam, C.; Raftery, J.; Ruscoe, R.E.; Sengel, J.T.; Webb, S.J. Length-dependent formation of transmembrane pores by 310-helical α-aminoisobutyric acid foldamers. J. Am. Chem. Soc. 2016, 138, 688–695. [Google Scholar] [CrossRef]
- Ren, C.; Zeng, F.; Shen, J.; Chen, F.; Roy, A.; Zhou, S.; Zeng, H. Pore-forming monopeptides as exceptionally active anion channels. J. Am. Chem. Soc. 2018, 140, 8817–8826. [Google Scholar] [CrossRef] [PubMed]
- Zeng, F.; Liu, F.; Yuan, L.; Zhou, S.; Shen, J.; Li, N.; Ren, H.; Zeng, H.Q. A pore-forming tripeptide as an extraordinarily active anion channel. Org. Lett. 2019, 21, 4826–4830. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Shen, J.; Ye, R.; Chen, F.; Zeng, H. Structurally simple trimesic amides as highly selective anion channels. Chem. Commun. 2019, 55, 4797–4800. [Google Scholar] [CrossRef] [PubMed]
- Dutta, C.; Krishnamurthy, P.; Su, D.; Yoo, S.H.; Collie, G.W.; Pasco, M.; Kumar, P.P. Nature-inspired synthetic oligourea foldamer channels allow water transport with high salt rejection. Chem 2023, 9, 2237–2254. [Google Scholar] [CrossRef]
- Matsunaga, Y.; Nakayasu, Y.; Sakai, S.; Yonenaga, M. Liquid crystal phases exhibited by N,N′,N″-trialkyl-1,3,5-benzenetricaboxamides. Mol. Cryst. Liq. Cryst. 1986, 141, 327–333. [Google Scholar] [CrossRef]
- Van Bommel, K.J.C.; ven der Pol, C.; Muizebelt, I.; Friggeri, A.; Heeres, A.; Meetsma, A.; van Esch BL Feringa, J. Responsive cyclohexane-based low-molecular-weight hydrogelators with modular architecture. Angew. Chem. Int. Ed. 2004, 43, 1663. [Google Scholar] [CrossRef] [PubMed]
- Rao, J.; Lahiri, J.; Isaacs, L.; Weis, R.M.; Whitesides, G.M. A trivalent system from vancomycin d-Ala-d-Ala with higher affinity than avidin-biotin. Science 1998, 280, 708–711. [Google Scholar] [CrossRef]
- Mazik, M.; Bandmann, H.; Sicking, W. Molecular recognition of carbohydrates by artificial polypyridine and polypyrimidine receptors. Angew. Chem. Int. Ed. 2000, 39, 511–554. [Google Scholar] [CrossRef]
- Jang, W.D.; Aida, T. Supramolecular nanofiber formation of macrocyclic dendrimer. Macromolecules 2004, 37, 7325–7330. [Google Scholar] [CrossRef]
- Hasegawa, S.; Horike, S.; Matsuda, R.; Furukawa, S.; Mochizuki, K.; Kinoshita, Y.; Kitagawa, S. Three-dimensional porous coordination polymer functionalized with amide groups based on tridentate ligand: Selective sorption and catalysis. J. Am. Chem. Soc. 2007, 129, 2607–2614. [Google Scholar] [CrossRef] [PubMed]
- De Greef, T.F.; Smulders, M.M.; Wolffs, M.; Schenning, A.P.; Sijbesma, R.P.; Meijer, E.W. Supramolecular Polymerization. Chem. Rev. 2009, 109, 5687–5754. [Google Scholar] [CrossRef] [PubMed]
- Fitié, C.F.; Roelofs, W.C.; Kemerink, M.; Sijbesma, R.P. Remnant polarization in thin films from a columnar liquid crystal. J. Am. Chem. Soc. 2010, 132, 6892–6893. [Google Scholar] [CrossRef] [PubMed]
- Cantekin, S.; Balkenende, D.W.; Smulders, M.M.; Palmans, A.R.; Meijer, E.W. The effect of isotopic substitution on the chirality of a self-assembled helix. Nat. Chem. 2010, 3, 42–46. [Google Scholar] [CrossRef]
- Roosma, J.; Mes, T.; Leclère, P.; Palmans, A.R.; Meijer, E.W. Supramolecular materials from benzene-1,3,5-tricarboxamide-based nanorods. J. Am. Chem. Soc. 2008, 130, 1120–1121. [Google Scholar] [CrossRef]
- Jimenez, C.A.; Belmar, J.B.; Ortiz, L.; Hidalgo, P.; Fabelo, O.; Pasán, J.; Ruiz-Perez, C. Influence of the aliphatic wrapping in the crystal structure of benzene tricarboxamide supramolecular polymers. Cryst. Growth Des. 2009, 9, 4987–4989. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, S.; Li, Z.; Yuan, L.; Zeng, H. An Exceptionally Active and Highly Selective Perchlorate Transporter Containing a Trimesic Amide Scaffold. Molecules 2024, 29, 1118. https://doi.org/10.3390/molecules29051118
Deng S, Li Z, Yuan L, Zeng H. An Exceptionally Active and Highly Selective Perchlorate Transporter Containing a Trimesic Amide Scaffold. Molecules. 2024; 29(5):1118. https://doi.org/10.3390/molecules29051118
Chicago/Turabian StyleDeng, Shaowen, Zhongyan Li, Lin Yuan, and Huaqiang Zeng. 2024. "An Exceptionally Active and Highly Selective Perchlorate Transporter Containing a Trimesic Amide Scaffold" Molecules 29, no. 5: 1118. https://doi.org/10.3390/molecules29051118
APA StyleDeng, S., Li, Z., Yuan, L., & Zeng, H. (2024). An Exceptionally Active and Highly Selective Perchlorate Transporter Containing a Trimesic Amide Scaffold. Molecules, 29(5), 1118. https://doi.org/10.3390/molecules29051118






