Anti-Inflammatory Effect of Chamaecyparis obtusa (Siebold & Zucc.) Endl. Leaf Essential Oil
Abstract
1. Introduction
2. Results
2.1. GC-MS Analysis of COD
2.2. Cell Viability of COD- and Sabinene-Pretreated RAW 264.7 Cells
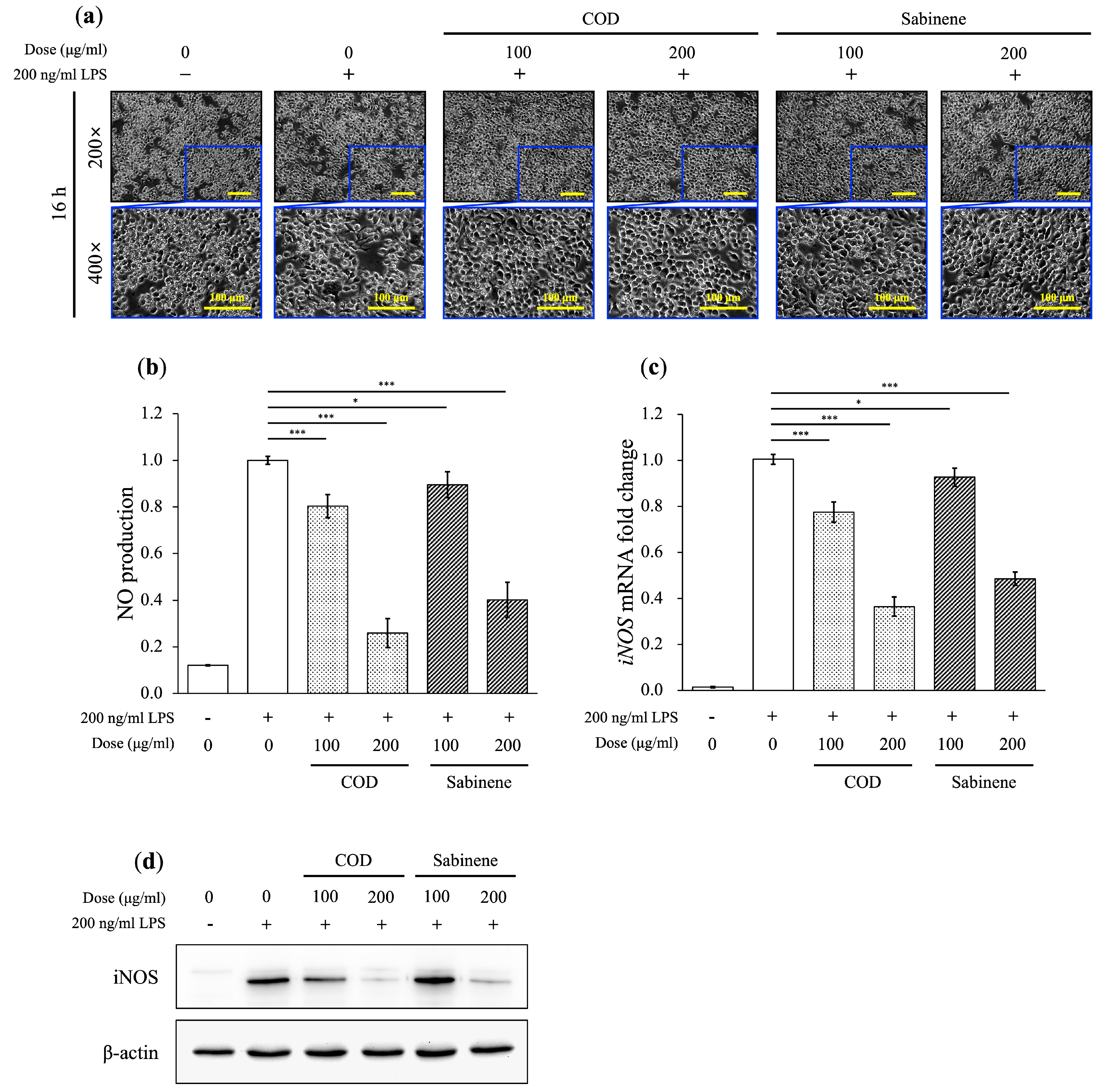
2.3. Anti-Inflammatory Properties of COD and Sabinene in LPS-Induced RAW 264.7 Cells
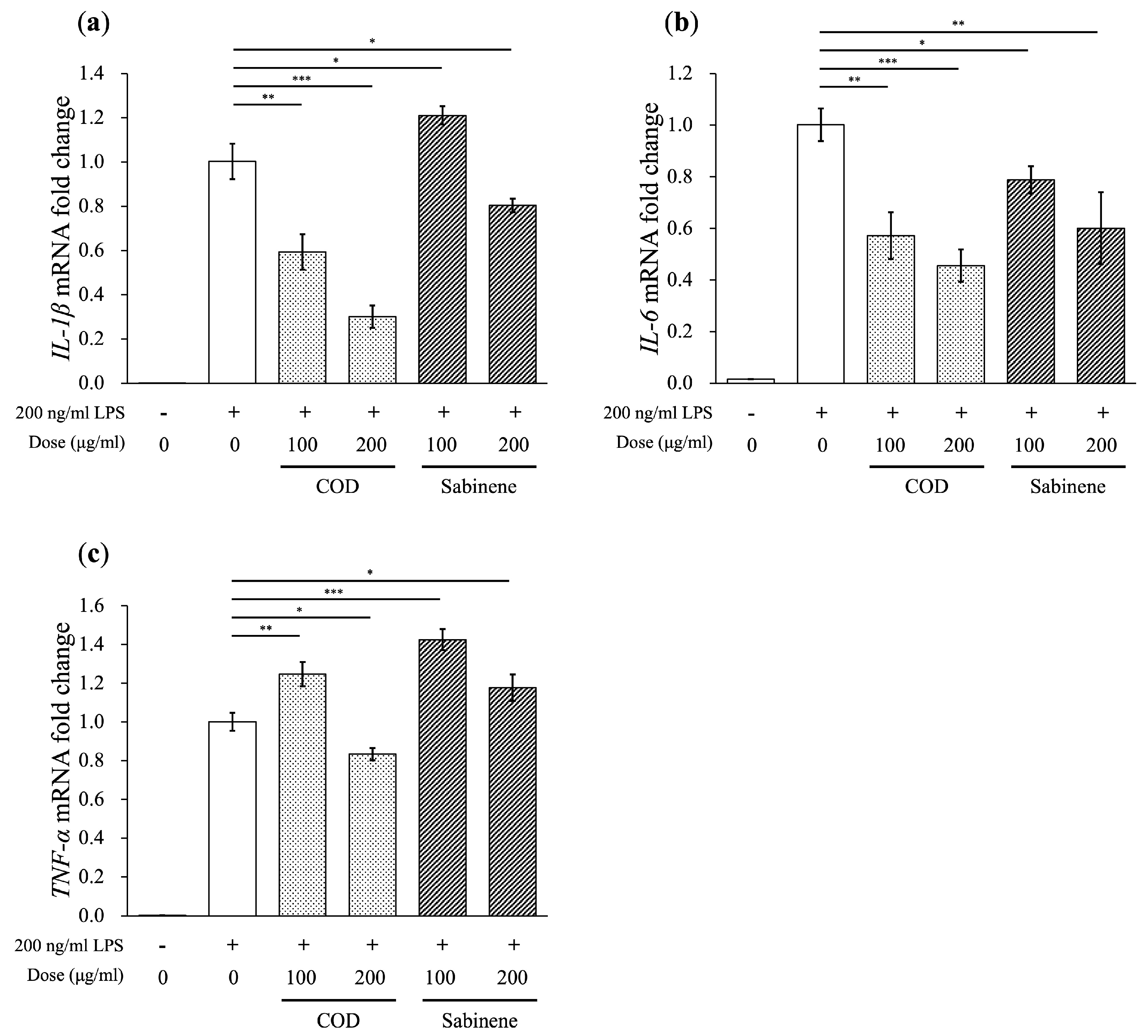
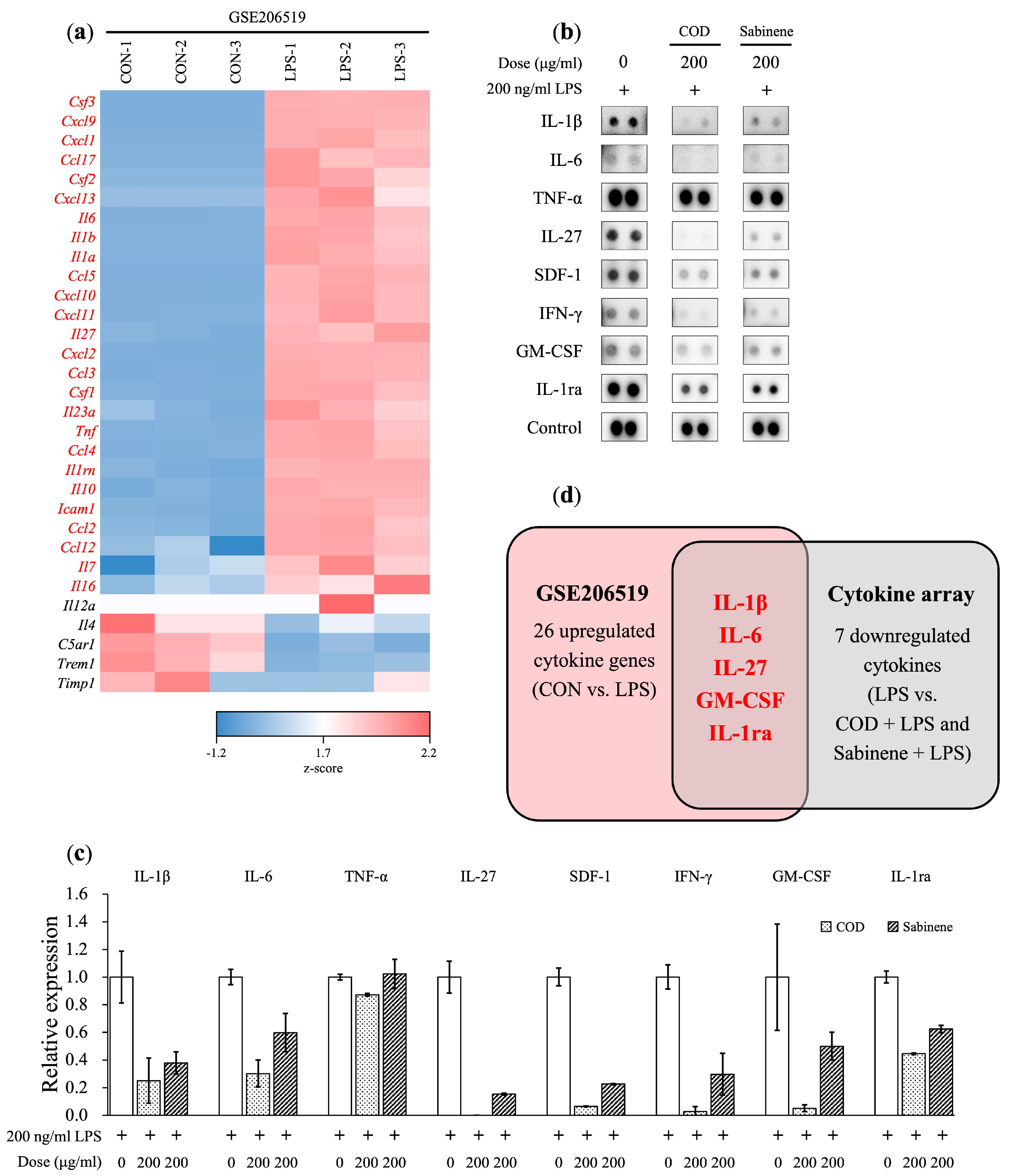
2.4. Reductions in Pro-Inflammatory Cytokine Levels by COD and Sabinene in LPS-Induced RAW 264.7 Cells
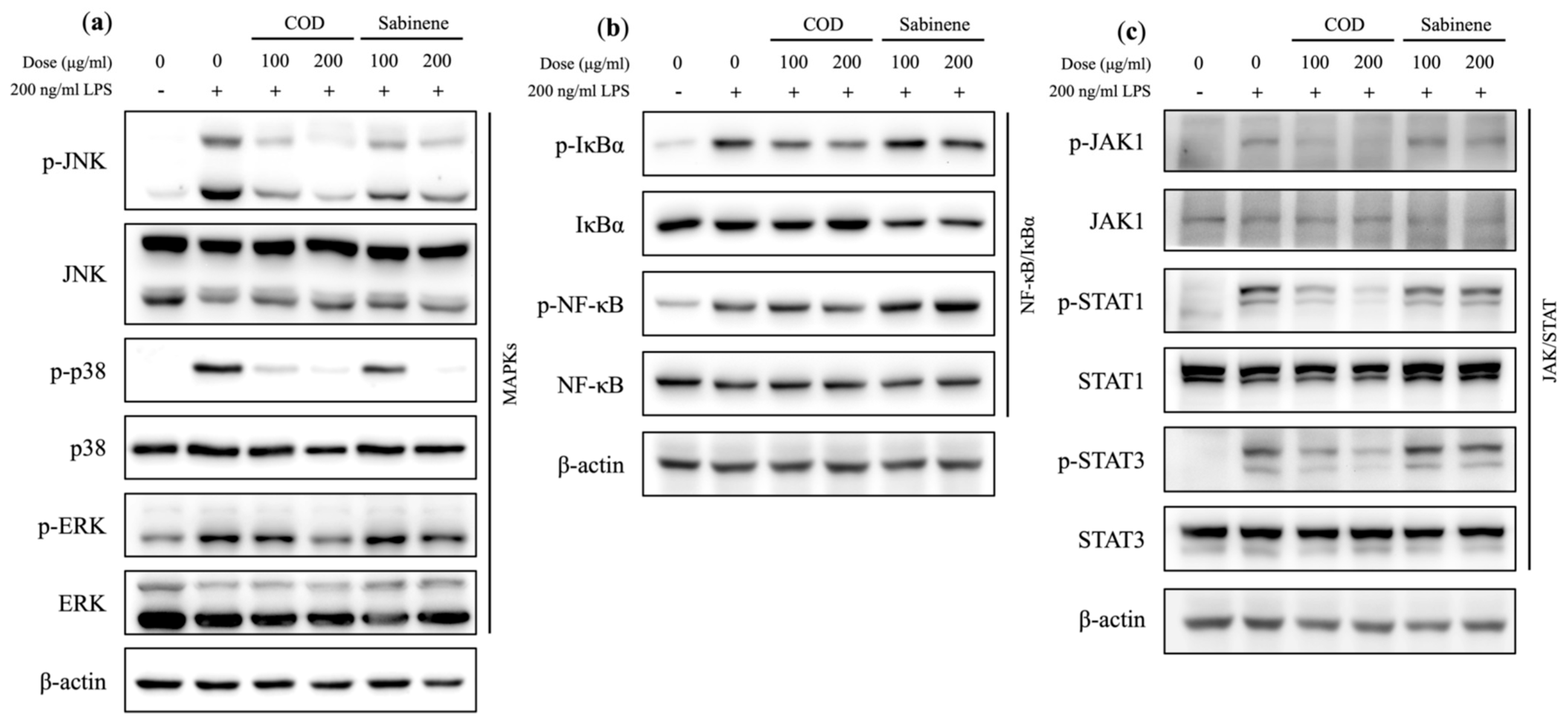
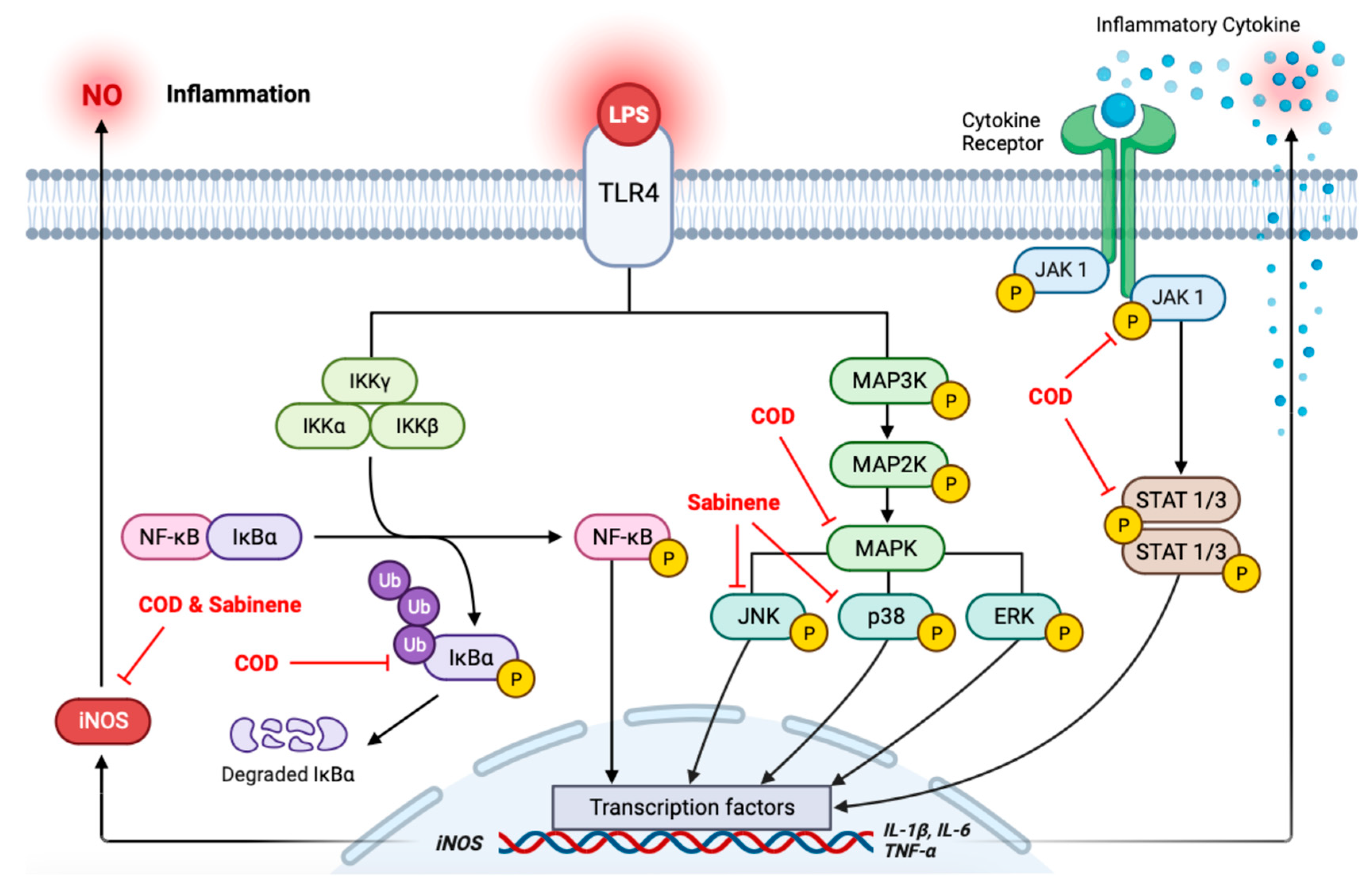
2.5. Evaluation of MAPKs, NF-κB/IκBα, and the JAK/STAT Axis in COD- and Sabinene-Pretreated LPS-Induced RAW 264.7 Cells
2.6. Cytokine Array Analysis in COD- and Sabinene-Pretreated LPS-Induced RAW 264.7 Cells
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Antibodies
4.3. Plant Material and Extraction of C. obtusa Essential Oil
4.4. Gas Chromatography-Mass Spectrometry (GC-MS) Analysis
4.5. Cell line and Culture
4.6. Cell Viability Assay
4.7. Nitric Oxide (NO) Assay
4.8. Immunoblotting
4.9. RNA Isolation and Quantitative Real-Time PCR (RT-qPCR)
4.10. Cytokine Array
4.11. Dataset Analysis
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2018, 9, 7204. [Google Scholar] [CrossRef]
- Medzhitov, R. Inflammation 2010: New adventures of an old flame. Cell 2010, 140, 771–776. [Google Scholar] [CrossRef]
- Sherwood, E.R.; Toliver-Kinsky, T. Mechanisms of the inflammatory response. Best Pract. Res. Clin. Anaesthesiol. 2004, 18, 385–405. [Google Scholar] [CrossRef] [PubMed]
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G.W. Chronic inflammation in the etiology of disease across the life span. Nat. Med. 2019, 25, 1822–1832. [Google Scholar] [CrossRef] [PubMed]
- Ko, W.; Sohn, J.H.; Jang, J.; Ahn, J.S.; Kang, D.G.; Lee, H.S.; Kim, J.; Kim, Y.; Oh, H. Inhibitory effects of alternaramide on inflammatory mediator expression through TLR4-MyD88-mediated inhibition of NF-кB and MAPK pathway signaling in lipopolysaccharide-stimulated RAW264. 7 and BV2 cells. Chem. Biol. Interact. 2016, 244, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, M.; Hart, L.; Lindsay, M.; Barnes, P.J.; Newton, R. IκBα Degradation and Nuclear Factor-κB DNA Binding Are Insufficient for Interleukin-1β and Tumor Necrosis Factor-α-induced κB-dependent Transcription*: Requirement for an Additional Activation Pathway. J. Biol. Chem. 1998, 273, 6607–6610. [Google Scholar] [CrossRef] [PubMed]
- Marrero, M.B.; Venema, V.J.; He, H.; Caldwell, R.B.; Venema, R.C. Inhibition by the JAK/STAT pathway of IFNγ-and LPS-stimulated nitric oxide synthase induction in vascular smooth muscle cells. Biochem. Biophys. Res. Commun. 1998, 252, 508–512. [Google Scholar] [CrossRef] [PubMed]
- Bennett, B.L.; Sasaki, D.T.; Murray, B.W.; O’Leary, E.C.; Sakata, S.T.; Xu, W.; Leisten, J.C.; Motiwala, A.; Pierce, S.; Satoh, Y. SP600125, an anthrapyrazolone inhibitor of Jun N-terminal kinase. Proc. Natl. Acad. Sci. USA 2001, 98, 13681–13686. [Google Scholar] [CrossRef]
- Di Paola, R.; Galuppo, M.; Mazzon, E.; Paterniti, I.; Bramanti, P.; Cuzzocrea, S. PD98059, a specific MAP kinase inhibitor, attenuates multiple organ dysfunction syndrome/failure (MODS) induced by zymosan in mice. Pharmacol. Res. 2010, 61, 175–187. [Google Scholar] [CrossRef] [PubMed]
- Barančík, M.; Boháčová, V.; Kvačkajová, J.; Hudecová, S.; Križanová, O.; Breier, A. SB203580, a specific inhibitor of p38-MAPK pathway, is a new reversal agent of P-glycoprotein-mediated multidrug resistance. Eur. J. Pharm. Sci. 2001, 14, 29–36. [Google Scholar] [CrossRef]
- Gurbuz, V.; Konac, E.; Varol, N.; Yilmaz, A.; Gurocak, S.; Menevse, S.; Sozen, S. Effects of AG490 and S3I-201 on regulation of the JAK/STAT3 signaling pathway in relation to angiogenesis in TRAIL-resistant prostate cancer cells in vitro. Oncol. Lett. 2014, 7, 755–763. [Google Scholar] [CrossRef]
- Al-Khayri, J.M.; Rashmi, R.; Toppo, V.; Chole, P.B.; Banadka, A.; Sudheer, W.N.; Nagella, P.; Shehata, W.F.; Al-Mssallem, M.Q.; Alessa, F.M. Plant Secondary Metabolites: The Weapons for Biotic Stress Management. Metabolites 2023, 13, 716. [Google Scholar] [CrossRef]
- Herman, A.; Herman, A.P. Essential oils and their constituents as skin penetration enhancer for transdermal drug delivery: A review. J. Pharm. Pharmacol. 2015, 67, 473–485. [Google Scholar] [CrossRef]
- Raha, S.; Kim, S.M.; Lee, H.J.; Lee, S.J.; Heo, J.D.; Venkatarame Gowda Saralamma, V.; Ha, S.E.; Kim, E.H.; Mun, S.P.; Kim, G.S. Essential oil from Korean Chamaecyparis obtusa leaf ameliorates respiratory activity in Sprague-Dawley rats and exhibits protection from NF-κB-induced inflammation in WI38 fibroblast cells. Int. J. Mol. Med. 2019, 43, 393–403. [Google Scholar] [CrossRef]
- Kwon, Y.; Seo, E.; Kim, S.; Noh, K.H.; Lee, H.; Joung, Y.; Shin, H.M.; Jang, Y.; Kim, Y.M.; Lee, J. Chamaecyparis obtusa (Siebold & Zucc.) Endl. leaf extracts prevent inflammatory responses via inhibition of the JAK/STAT axis in RAW264. 7 cells. J. Ethnopharmacol. 2022, 282, 114493. [Google Scholar]
- Bayazid, A.B.; Jang, Y.A.; Jeong, S.A.; Lim, B.O. Cypress tree (Chamaecyparis obtusa) Bark extract inhibits melanogenesis through repressing CREB and MITF signalling pathways in α-MSH-stimulated B16F10 cells. Food Agric. Immunol. 2022, 33, 498–510. [Google Scholar] [CrossRef]
- Lee, G.; Hong, E.; Gwak, K.; Park, M.; Choi, K.; Choi, I.; Jang, J.; Jeung, E. The essential oils of Chamaecyparis obtusa promote hair growth through the induction of vascular endothelial growth factor gene. Fitoterapia 2010, 81, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Mockute, D.; Nivinskiene, O. The sabinene chemotype of essential oil of seeds of Daucus carota L. ssp. carota growing wild in Lithuania. J. Essent. Oil Res. 2004, 16, 277–281. [Google Scholar] [CrossRef]
- Asili, J.; Emami, S.A.; Rahimizadeh, M.; Fazly-Bazzaz, B.S.; Hassanzadeh, M.K. Chemical and antimicrobial studies of Juniperus sabina L. and Juniperus foetidissima Willd. essential oils. J. Essent. Oil Bear. Plants 2010, 13, 25–36. [Google Scholar] [CrossRef]
- Valente, J.; Zuzarte, M.; Gonçalves, M.J.; Lopes, M.C.; Cavaleiro, C.; Salgueiro, L.; Cruz, M.T. Antifungal, antioxidant and anti-inflammatory activities of Oenanthe crocata L. essential oil. Food Chem. Toxicol. 2013, 62, 349–354. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Zhang, H.; Liu, H.; Liu, W.; Zhang, R.; Xian, M.; Liu, H. Biosynthesis and production of sabinene: Current state and perspectives. Appl. Microbiol. Biotechnol. 2018, 102, 1535–1544. [Google Scholar] [CrossRef]
- Fujiwara, N.; Kobayashi, K. Macrophages in inflammation. Curr. Drug Targets Inflamm. Allergy 2005, 4, 281–286. [Google Scholar] [CrossRef]
- Kany, S.; Vollrath, J.T.; Relja, B. Cytokines in inflammatory disease. Int. J. Mol. Sci. 2019, 20, 6008. [Google Scholar] [CrossRef]
- Liu, J.; Poojary, M.M.; Zhu, L.; Williams, A.R.; Lund, M.N. Phenolic Acid–Amino Acid Adducts Exert Distinct Immunomodulatory Effects in Macrophages Compared to Parent Phenolic Acids. J. Agric. Food Chem. 2023, 71, 2344–2355. [Google Scholar] [CrossRef]
- Shimizu, M.; Ogura, K.; Mizoguchi, I.; Chiba, Y.; Higuchi, K.; Ohtsuka, H.; Mizuguchi, J.; Yoshimoto, T. IL-27 promotes nitric oxide production induced by LPS through STAT1, NF-κB and MAPKs. Immunobiology 2013, 218, 628–634. [Google Scholar] [CrossRef]
- Becher, B.; Tugues, S.; Greter, M. GM-CSF: From growth factor to central mediator of tissue inflammation. Immunity 2016, 45, 963–973. [Google Scholar] [CrossRef]
- Volarevic, V.; Al-Qahtani, A.; Arsenijevic, N.; Pajovic, S.; Lukic, M.L. Interleukin-1 receptor antagonist (IL-1Ra) and IL-1Ra producing mesenchymal stem cells as modulators of diabetogenesis. Autoimmunity 2010, 43, 255–263. [Google Scholar] [CrossRef]
- Campbell, I.K.; Gerondakis, S.; O’Donnell, K.; Wicks, I.P. Distinct roles for the NF-κB1 (p50) and c-Rel transcription factors in inflammatory arthritis. J. Clin. Investig. 2000, 105, 1799–1806. [Google Scholar] [CrossRef] [PubMed]
- Majdalawieh, A.; Ro, H. Regulation of IκBα function and NF-κB signaling: AEBP1 is a novel proinflammatory mediator in macrophages. Mediat. Inflamm. 2010, 2010, 823821. [Google Scholar] [CrossRef] [PubMed]
- Israël, A. The IKK complex, a central regulator of NF-κB activation. Cold Spring Harb. Perspect. Biol. 2010, 2, a000158. [Google Scholar] [CrossRef] [PubMed]
- Yue, J.; López, J.M. Understanding MAPK signaling pathways in apoptosis. Int. J. Mol. Sci. 2020, 21, 2346. [Google Scholar] [CrossRef]
- Santarpia, L.; Lippman, S.M.; El-Naggar, A.K. Targeting the MAPK–RAS–RAF signaling pathway in cancer therapy. Expert Opin. Ther. Targets 2012, 16, 103–119. [Google Scholar] [CrossRef]
- Son, Y.; Cheong, Y.; Kim, N.; Chung, H.; Kang, D.G.; Pae, H. Mitogen-activated protein kinases and reactive oxygen species: How can ROS activate MAPK pathways? J. Signal Transduct. 2011, 2011, 792639. [Google Scholar] [CrossRef]
- Kang, J.S.; Yoon, Y.D.; Lee, K.H.; Park, S.; Kim, H.M. Costunolide inhibits interleukin-1β expression by down-regulation of AP-1 and MAPK activity in LPS-stimulated RAW 264.7 cells. Biochem. Biophys. Res. Commun. 2004, 313, 171–177. [Google Scholar] [CrossRef]
- Wang, Y.; van Boxel-Dezaire, A.H.; Cheon, H.; Yang, J.; Stark, G.R. STAT3 activation in response to IL-6 is prolonged by the binding of IL-6 receptor to EGF receptor. Proc. Natl. Acad. Sci. USA 2013, 110, 16975–16980. [Google Scholar] [CrossRef]
- Tian, B.; Nowak, D.E.; Brasier, A.R. A TNF-induced gene expression program under oscillatory NF-κB control. BMC Genom. 2005, 6, 137. [Google Scholar] [CrossRef]
- An, B.; Kang, J.; Yang, H.; Jung, E.; Kang, H.; Choi, I.; Park, M.; Jeung, E. Anti-inflammatory effects of essential oils from Chamaecyparis obtusa via the cyclooxygenase-2 pathway in rats. Mol. Med. Rep. 2013, 8, 255–259. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Yang, K.; Kim, S.S.; Park, S.M.; Park, K.J.; Kim, K.S.; Choi, Y.H.; Cho, K.K.; Lee, N.H.; Hyun, C. Chemical composition and anti-inflammatory effects of essential oil from Hallabong flower. EXCLI J. 2013, 12, 933. [Google Scholar] [PubMed]
- Arunkumar, R.; Nair, S.A.; Rameshkumar, K.B.; Subramoniam, A. The essential oil constituents of Zornia diphylla (L.) Pers, and anti-inflammatory and antimicrobial activities of the oil. Rec. Nat. Prod. 2014, 8, 385. [Google Scholar]
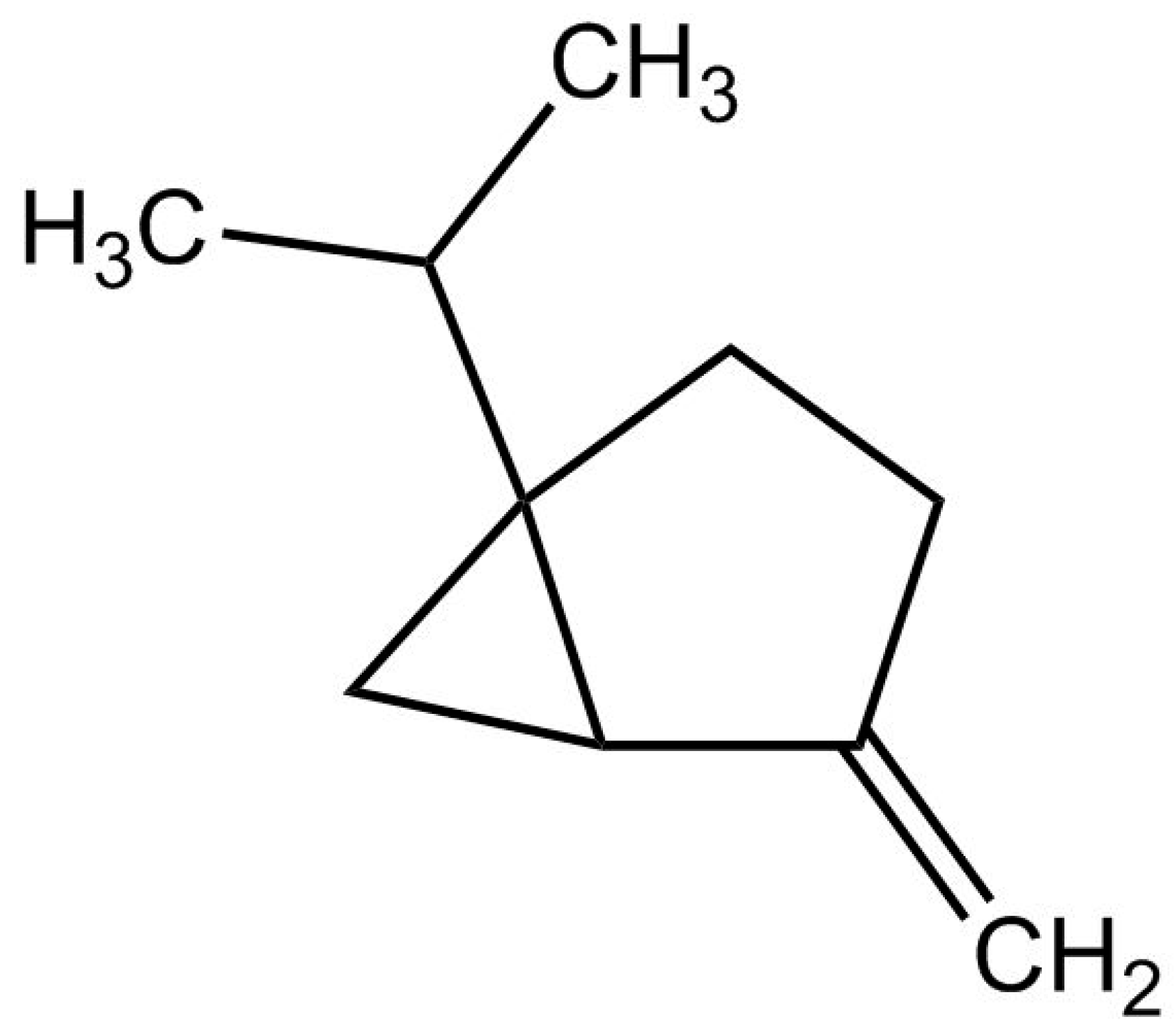

| Chemotype | Compound | R. Time (min) | Relative Abundance |
|---|---|---|---|
| Monoterpene | 3-Thujene | 10.693 | 0.53% |
| α-Pinene | 10.963 | 1.67% | |
| Camphene | 11.613 | 0.32% | |
| Sabinene | 12.875 | 12.34% | |
| β-Myrcene | 13.835 | 3.62% | |
| α-Phellandrene | 14.371 | 0.08% | |
| α-Terpinene | 14.973 | 1.10% | |
| O-Cymene | 15.374 | 0.87% | |
| D-Limonene | 15.559 | 6.91% | |
| γ-Terpinene | 17.089 | 3.96% | |
| α-Terpinolene | 18.543 | 1.09% | |
| Trans sabinene hydrate | 20.15 | 0.05% | |
| Terpinen-4-ol | 22.838 | 2.77% | |
| α-Terpinenol | 23.497 | 0.35% | |
| δ 3-Carene | 26.658 | 0.03% | |
| Bornyl acetate | 27.961 | 10.78% | |
| Isoterpinolene | 28.47 | 0.07% | |
| α-Terpyl acetate | 30.814 | 18.78% | |
| Sesquiterpene | β-Elemene | 32.642 | 0.12% |
| Trans-α-Bergamotene | 33.031 | 0.08% | |
| α-Cedrene | 33.405 | 0.06% | |
| (+)-Epi-Bicyclosesquiphellandrene | 33.544 | 0.20% | |
| Trans-caryophyllene | 33.729 | 0.44% | |
| Widdrene | 34.218 | 3.77% | |
| δ-EIemene | 34.434 | 0.08% | |
| α-Cubebene | 34.905 | 0.92% | |
| Humulene | 35.144 | 0.09% | |
| β-Cubebene | 35.579 | 2.94% | |
| α-Himachalene | 36.131 | 0.24% | |
| Germacrene-D | 36.331 | 0.74% | |
| α-Gurjunene | 37.079 | 2.94% | |
| Cuparene | 37.318 | 0.26% | |
| γ-Muurolene | 37.673 | 0.16% | |
| δ-Cadinene | 38.07 | 1.91% | |
| α-Muurolene | 38.606 | 0.03% | |
| Santalene | 38.725 | 6.28% | |
| Elemol | 39.068 | 0.06% | |
| γ-Gurjunene | 39.338 | 0.25% | |
| α-Cedrol | 41.023 | 1.44% | |
| Cubenol | 41.593 | 0.20% | |
| 8-Epi-γ-eudesmol | 41.752 | 0.17% | |
| γ-Eudesmol | 42.218 | 2.71% | |
| τ-Cadinol | 42.577 | 0.31% | |
| β-Eudesmol | 42.899 | 1.78% | |
| α-Eudesmol | 43.005 | 2.90% | |
| Hedycaryol | 43.548 | 0.11% | |
| β-Elemene | 43.914 | 0.09% | |
| (+)-α-Bisabolol | 44.157 | 0.11% | |
| α-Farnesene | 45.098 | 0.05% | |
| Diterpene | Stachen | 52.412 | 2.06% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.-H.; Jang, Y.-A.; Kwon, Y.-J. Anti-Inflammatory Effect of Chamaecyparis obtusa (Siebold & Zucc.) Endl. Leaf Essential Oil. Molecules 2024, 29, 1117. https://doi.org/10.3390/molecules29051117
Kim S-H, Jang Y-A, Kwon Y-J. Anti-Inflammatory Effect of Chamaecyparis obtusa (Siebold & Zucc.) Endl. Leaf Essential Oil. Molecules. 2024; 29(5):1117. https://doi.org/10.3390/molecules29051117
Chicago/Turabian StyleKim, Sung-Hee, Young-Ah Jang, and Yong-Jin Kwon. 2024. "Anti-Inflammatory Effect of Chamaecyparis obtusa (Siebold & Zucc.) Endl. Leaf Essential Oil" Molecules 29, no. 5: 1117. https://doi.org/10.3390/molecules29051117
APA StyleKim, S.-H., Jang, Y.-A., & Kwon, Y.-J. (2024). Anti-Inflammatory Effect of Chamaecyparis obtusa (Siebold & Zucc.) Endl. Leaf Essential Oil. Molecules, 29(5), 1117. https://doi.org/10.3390/molecules29051117







