Toxicity Study on Crude Alkaloid Extracts of Houttuyniae herba Based on Zebrafish and Mice
Abstract
1. Introduction
2. Results
2.1. Zebrafish Experiment
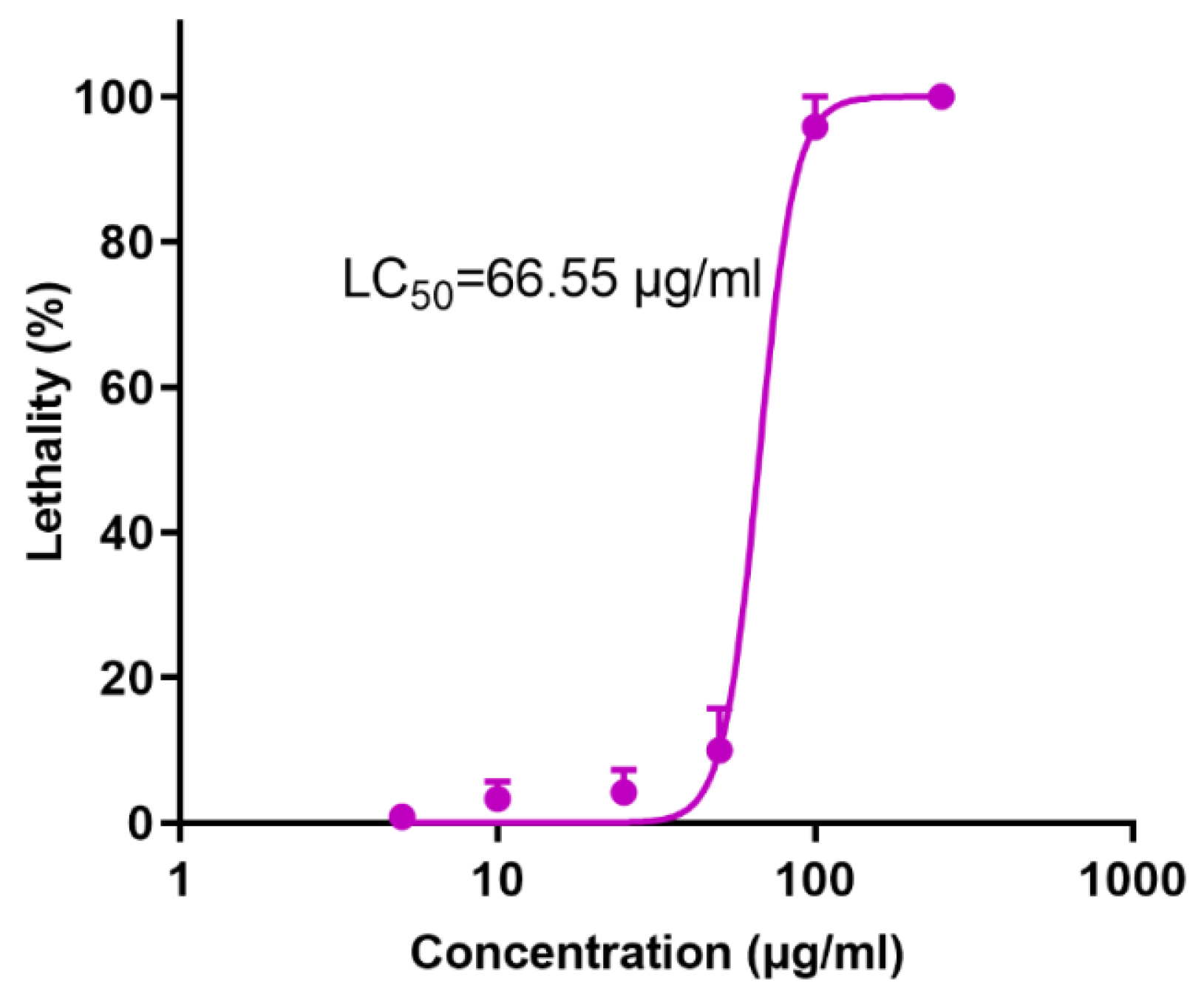
2.1.1. Embryo Toxicity
2.1.2. Phenotypic Effects of Zebrafish Embryo Development
2.1.3. Effects of HHTAE on the Body Length of Zebrafish
2.2. Mouse Experiment
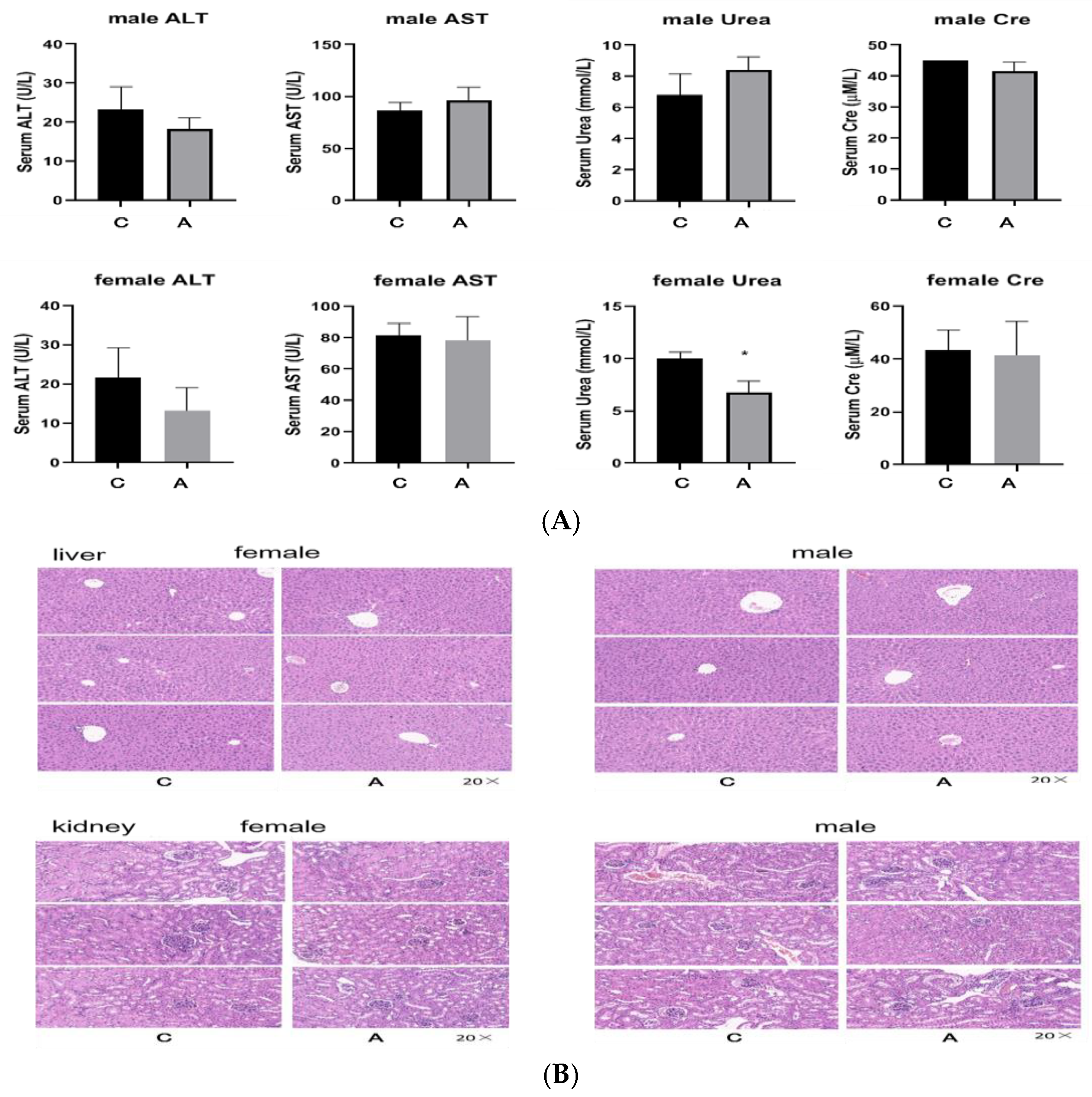
2.2.1. Single Dose of 500 mg/kg and 2000 mg/kg
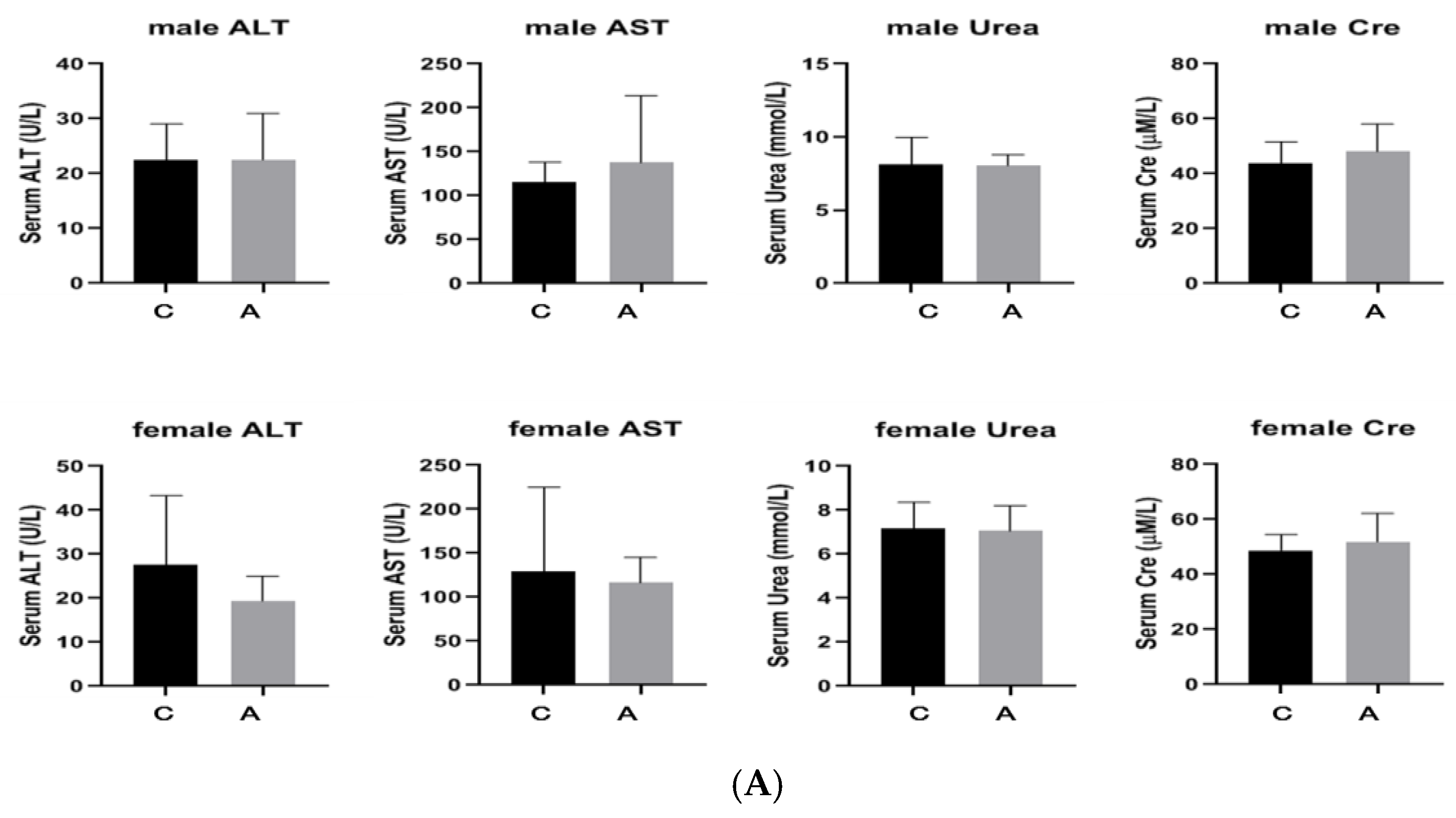
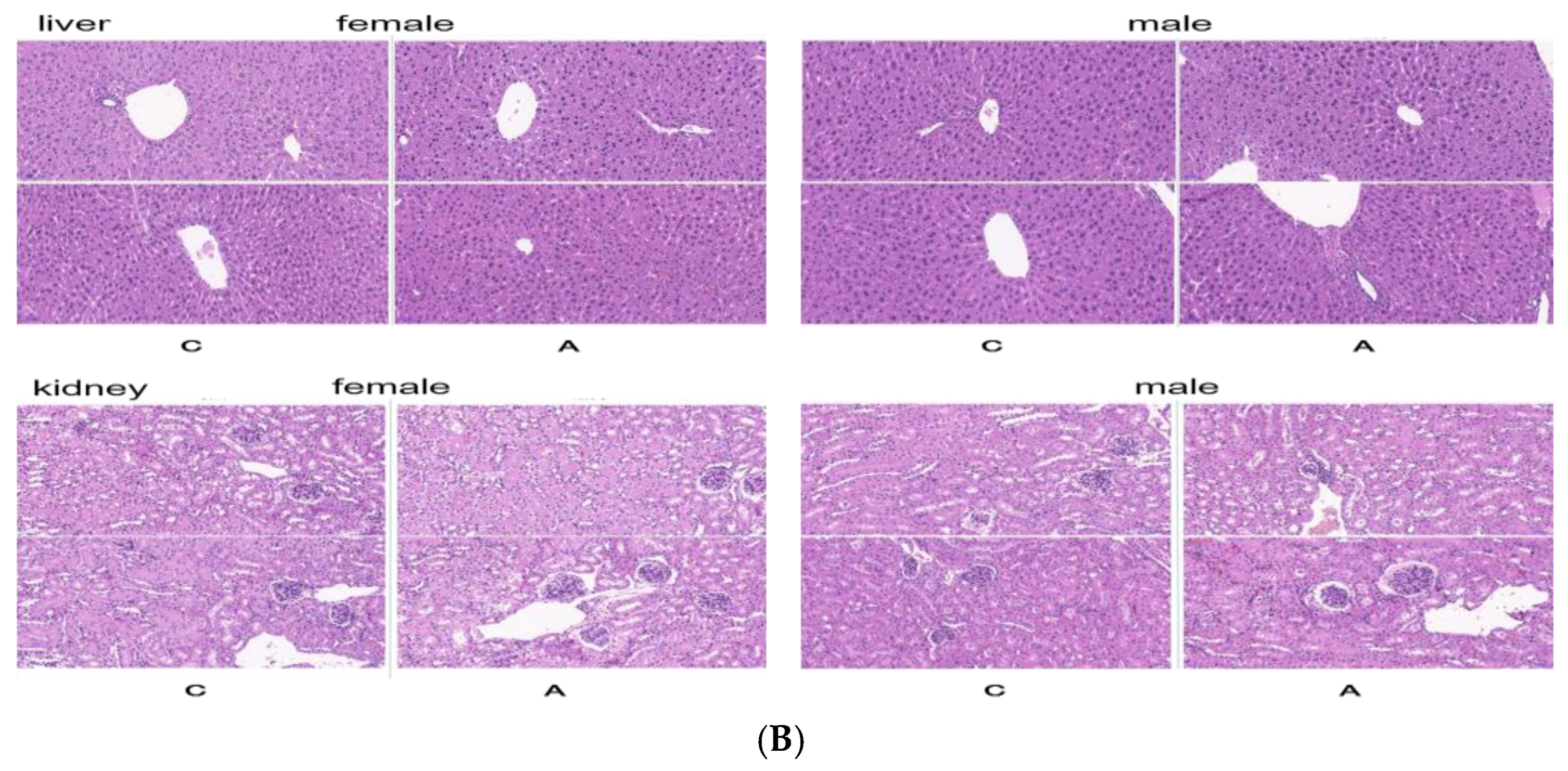
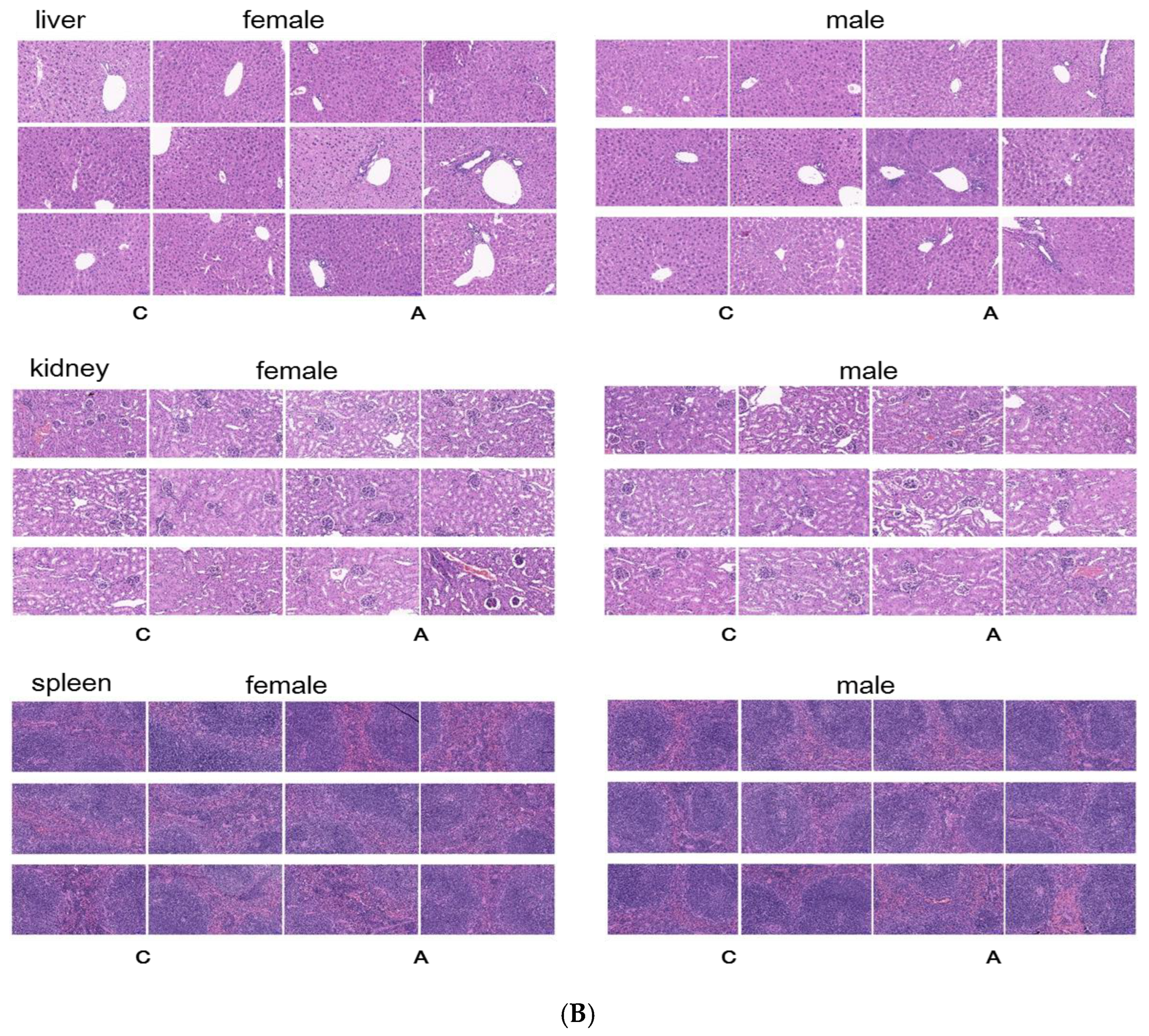
2.2.2. Continuous Administration of 2000 mg/kg, Twice a Day for 7 Days
3. Discussion
3.1. Necessity of Zebrafish Toxicity Experiment
3.2. Solubility of Aristolactam Components
3.3. Use of a Single Extract
3.4. Differences between Animal Models
4. Materials and Methods
4.1. Plant Materials and Preparation
4.2. Instruments and Reagents
4.3. Animals
4.4. Zebrafish Experiment
4.4.1. Administration Dose
4.4.2. Acute Toxicity Test
- (1)
- Grouping and administration: Half-encapsulated embryos (6 hpf under standard conditions) were taken, and the administration group was treated with a series of concentrated HHTAE solutions of 2 mL on zebrafish embryos. Embryos of the same batch of zebrafish developed in normal feeding liquid were the normal control group. Each group of 30 embryos was developed under standard conditions.
- (2)
- Observation: Embryos of both groups were observed and recorded daily under a microscope, including the brain, eyes, heart, blood flow, trunk (notochord/neural tube, somite), embryo growth rate, and so on (photos were taken and recorded at any time when necessary). At the third day of development (3 dpf), the administration group embryos were washed with normal culture medium 3 times, and the normal culture medium was replaced. The phenotypes of zebrafish (after membrane removal) were photographed, and the malformations (including body length) and the number of dead/surviving embryos of the embryos were counted. Then, they continued to develop until the embryo reached the fifth day, and they were recorded every day until the end of the experiment. The experiment was repeated at least three times.
- (3)
- Data processing: Mortality and body length were expressed as mean ± standard deviation (Mean ± SD). The data of each administration group were compared with the normal control group, the statistical data were plotted, and the median lethal concentration (LC50) was calculated.
4.5. Mouse Experiment
4.5.1. Administration Dose
4.5.2. Acute Toxicity Test
- (1)
- Grouping and administration: A certain number of KM mice weighing approximately 22 g, half females and half males, were selected and divided into four groups: male administration group, male control group, female administration group, and female control group. The administration group and the control group were kept in separate cages. Both groups were administered HHTAE at 500 mg/kg and 2000 mg/kg, with 0.5% CMC-Na by gavage, separately.
- (2)
- Toxic reaction observation: Within 14 days of administration, the mice were observed for any toxic reactions. This included general indicators such as animal appearance, behavior, secretions, excreta, etc., animal mortality (time of death, pre-death reactions, etc.), and changes in animal weight (weighed once before administration and before being killed at the end of the experiment). All deaths and symptoms, and the starting time, severity, and duration time were recorded. The end point time was 14 days later, and the mice were euthanized.
- (3)
- Pathological examination: All animals underwent gross dissection at the end of the experiment, including those euthanized during the experiment, those who died, and those still alive at the end of the experiment. The samples of plasma, heart, liver, spleen, and kidney were taken for biochemical and histopathology examination.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wu, Z.; Deng, X.; Hu, Q.; Xiao, X.; Jiang, W.; Ma, X.; Wu, M. Houttuynia cordata Thunb: An ethnopharmacological review. Front. Pharmacol. 2021, 12, 714694. [Google Scholar] [CrossRef] [PubMed]
- Rafiq, S.; Hao, H.; Ijaz, M.; Raza, A. Pharmacological effects of Houttuynia cordata Thunb (H. cordata): A comprehensive review. Pharmaceuticals 2022, 15, 1079. [Google Scholar] [CrossRef] [PubMed]
- Chinese Phamacopoeia Commission. Chinese Phamacopoeia (VolI); The Medicine Science and Technology Press of China: Beijing, China, 2020; pp. 234–235. [Google Scholar]
- Wu, Y.; Ding, Q.; Liu, J.; Dai, Z.; Ma, S. Research progress on chemical components, pharmacology and quality control of Houttuyniae herba. Chin. J. Pharm. Anal. 2022, 42, 108–120. [Google Scholar]
- Cai, H.; Liu, J.; Chen, S.; Cao, G.; Chen, H. Research progress on chemical consituents, bioactivities and clinical application of Houttuynia cordata. Chin. Tradit. Pat. Med. 2019, 41, 2719–2728. [Google Scholar]
- Ahn, J.; Chae, H.; Chin, Y.; Kin, J. Alkaloids from the aerial parts of Houttuynia cordata and their anti-inflammatory activity. Bioorg. Med. Chem. Lett. 2017, 27, 2807–2811. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Ahn, J.; Kim, J.; Lee, S.; Kim, H. Flavonoids from the aerial parts of Houttuynia cordata attenuate lung inflammation in mice. Arch. Pharm. Res. 2015, 38, 1304–1311. [Google Scholar] [CrossRef] [PubMed]
- Yanarojana, M.; Nararatwanchai, T.; Thairat, S.; Tancharoen, S. Antiproliferative activity and induction of apoptosis in human melanoma cells by Houttuynia cordata Thunb Extract. Anticancer. Res. 2017, 37, 6619–6628. [Google Scholar] [PubMed]
- Chen, H.; Sha, X.; Luo, Y.; Chen, J.; Li, X.; Wang, J.; Cao, G.; Peng, X. Acute and subacute toxicity evaluation of Houttuynia cordata ethanol extract and plasma metabolic profiling analysis in both male and female rats. J. Appl. Toxicol. 2021, 41, 2068–2082. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, G.; Yang, C.; Shi, Z.; Ru, Y.; Shen, N.; Xiao, C.; Wang, Y.; Gao, Y. The mechanism of Houttuynia cordata embryotoxicity was explored in combination with an experimental model and network phamacology. Toxins 2023, 15, 73. [Google Scholar] [CrossRef]
- Wu, Y.; Kang, S.; Li, Y.; Liu, J.; Dai, Z.; Ma, S. Isolation and identification of lactams from Houttuynia cordata Thunb. under guidance of hepatorenal cytotoxicity. Mod. Chin. Med. 2023, 25, 544–548. [Google Scholar]
- Wu, Y.; Liu, J.; Kang, S.; Dai, Z.; Ma, S. Rapid analysis of aristolochic acids and aristolactams in Houttuyniae herba by LC–MS/MS. Molecules 2022, 27, 8969. [Google Scholar] [CrossRef] [PubMed]
- Surjya, N.; Lipika, P. Flight for fish in drug discovery: A review of zebrafish-based screening of molecules. Biol. Lett. 2023, 19, 20220541. [Google Scholar]
- Yuvendran, M. The use of larval zebrafish (Danio rerio) model for identifying new anxiolytic drugs from herbal medicine. Zebrafish 2018, 15, 321–339. [Google Scholar]
- Arie, O.; Ann, R. Zebrafish: A novel research tool for cardiac (patho)electrophysiology and ion channel disorders. Front. Physiol. 2012, 3, 255. [Google Scholar]
- Katharine, A.; Jennifer, L. Making waves: New developments in toxicology with the zebrafish. Toxicol. Sci. 2018, 163, 5–12. [Google Scholar]
- Mektrirat, R.; Yano, T.; Okonongi, S.; Katip, W.; Pikulkaew, S. Phytochemical and safety evaluation of volatile tepenoids from Zingiber cassumunar Roxb. on mature carp peripheral blood mononuclear cells and embryonic zebrafish. Molecules 2020, 25, 613. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Wu, Y.; Xu, Y.; Han, Y.; Kang, S.; Dai, Z.; Jin, H.; Wei, F.; Ma, S. Toxicity Study on Crude Alkaloid Extracts of Houttuyniae herba Based on Zebrafish and Mice. Molecules 2024, 29, 1107. https://doi.org/10.3390/molecules29051107
Liu J, Wu Y, Xu Y, Han Y, Kang S, Dai Z, Jin H, Wei F, Ma S. Toxicity Study on Crude Alkaloid Extracts of Houttuyniae herba Based on Zebrafish and Mice. Molecules. 2024; 29(5):1107. https://doi.org/10.3390/molecules29051107
Chicago/Turabian StyleLiu, Jing, Yingxue Wu, Yanni Xu, Ying Han, Shuai Kang, Zhong Dai, Hongyu Jin, Feng Wei, and Shuangcheng Ma. 2024. "Toxicity Study on Crude Alkaloid Extracts of Houttuyniae herba Based on Zebrafish and Mice" Molecules 29, no. 5: 1107. https://doi.org/10.3390/molecules29051107
APA StyleLiu, J., Wu, Y., Xu, Y., Han, Y., Kang, S., Dai, Z., Jin, H., Wei, F., & Ma, S. (2024). Toxicity Study on Crude Alkaloid Extracts of Houttuyniae herba Based on Zebrafish and Mice. Molecules, 29(5), 1107. https://doi.org/10.3390/molecules29051107





