Constituents of Chimaphila japonica and Their Diuretic Activity
Abstract
1. Introduction
2. Results and Discussion
2.1. Structure Elucidation
2.2. In Vitro Cytotoxicity
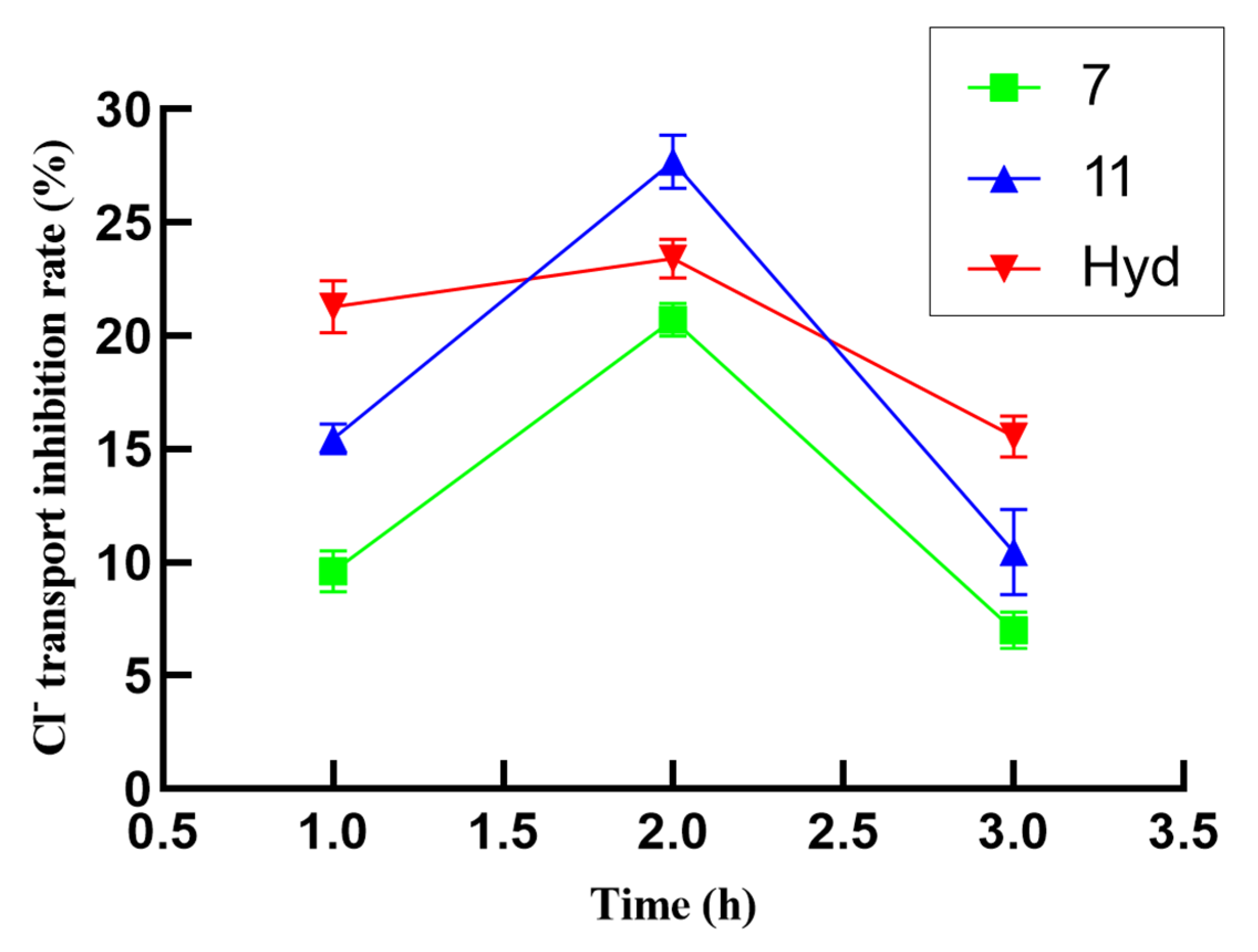
2.3. In Vitro Diuretic Activity
2.4. Molecular Docking
3. Materials and Methods
3.1. Chemistry
3.2. Plant Material and Identification
3.3. Extraction and Isolation
3.4. Characterization of the Isolates
3.5. In Vitro Cytotoxicity Assays
3.6. In Vitro Diuretic Activity Assay
3.7. Molecular Docking Study
3.8. Statistical Analyses
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ngamlai, E.V.; Pradhan, R.B.; Lalbiaknii, P.C.; Ralte, V.; Lalnunmawia, F.; Vanlalhluna, P.C.; Mehta, S.K. Diuretic activity evaluation and chemical composition analysis of Hedyotis scandens extract from Mizoram, India, in rat models. J. Ethnopharmacol. 2024, 319, 117079. [Google Scholar] [CrossRef]
- Li, X.X.; Liao, J.; Jiang, Z.L.; Liu, X.Y.; Chen, S.; He, X.; Zhu, L.; Duan, X.M.; Xu, Z.Y.; Qi, B.W.; et al. A concise review of recent advances in anti-heart failure targets and its small molecules inhibitors in recent years. Eur. J. Med. Chem. 2020, 186, 111852. [Google Scholar] [CrossRef]
- Kehrenberg, M.C.A.; Bachmann, H.S. Diuretics: A contemporary pharmacological classifcation? Naunyn-Schmiedeberg’s Arch. Pharmacol. 2022, 395, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Tetiana, T.; Lina, P.; Iryna, D.; Yevgen, T. Modern trends in diuretics development. Eur. J. Med. Chem. 2020, 208, 112855. [Google Scholar]
- He, H.; Sui, Y.; Yu, X.; Luo, G.; Xue, J.; Yang, W.; Long, Y. Potential low toxic alternative for Na-Cl cotransporter inhibition: A diuretic effect and mechanism study of Pyrrosia petiolosa. Ann. Pharm. Fr. 2024, 82, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Lang, T.Q.; Zhang, Y.; Chen, F.; Luo, G.Y.; Yang, W.D. Characterization of chemical components with diuretic potential from Pyrrosia petiolosa. J. Asian Nat. Prod. Res. 2021, 23, 764–771. [Google Scholar] [CrossRef] [PubMed]
- Saha, B.; Leite-Dellova, D.C.A.; Demko, J.; Soerensen, M.V.; Takagi, E.; Gleason, C.E.; Shabbir, W.; Pearce, D. WNK1 is chloride-stimulated scaffold that regulates mTORC2 activity and ion transport. J. Cell Sci. 2022, 135, jcs260313. [Google Scholar] [CrossRef] [PubMed]
- Rodan, A.R.; Jenny, A. WNK Kinases in Development and Disease. Curr. Top. Dev. Biol. 2017, 123, 1–47. [Google Scholar] [PubMed]
- Argaiz, E.R.; Chavez-Canales, M.; Ostrosky-Frid, M.; Rodriguez-Gama, A.; Vazquez, N.; Gonzalez-Rodriguez, X.; Garcia-Valdes, J.; Hadchouel, J.; Ellison, D.; Gamba, G. Kidney-specific WNK1 isoform (KS-WNK1) is a potent activator of WNK4 and NCC. Am. J. Physiol. 2018, 315, F734–F745. [Google Scholar] [CrossRef]
- Sui, Y.; Teng, M.G.; Chai, H.F.; Yu, X.; Long, Y.; Luo, G.Y.; Yang, W.D. Leaching effect of babaric acid in Pyrrosia petiolosa. Chin. Tradit. Herb. Drugs 2021, 52, 1026–1030. [Google Scholar]
- Yu, Y.; Liu, D.; Elshafei, A.; Lang, M.Y.; Hu, D.R.; Sun, Y.H.; Kang, D.Z.; Zheng, M.S. Chemical constituents from Chimaphila japonica Miq. and their chemotaxonomic significance. Biochem. Syst. Ecol. 2023, 109, 104664. [Google Scholar] [CrossRef]
- Yu, Y.; Elshafei, A.; Zheng, X.D.; Cheng, S.Y.; Wang, Y.X.; Piao, M.H.; Wang, Y.M.; Jin, M.; Li, G.; Zheng, M.S. Chemical constituents of Chimaphila japonica Miq. Biochem. Syst. Ecol. 2021, 95, 104219. [Google Scholar] [CrossRef]
- Yu, Y.; Cheng, S.Y.; Alaa, E.; Zheng, X.D.; Piao, M.H.; Zheng, M.S. Chemical constituents of Chimaphila japonica herb. J. Chin. Med. Mater. 2021, 44, 1124–1127. [Google Scholar]
- Ren, F.X.; Zhao, Y.M.; Zhang, A.J.; Yang, Y.; Zhang, Y. Extraction Method of Effective Components from Chinese Medicine Pyrola and Its Application for Treating Hemorrhage and/or Pain-Related Disease. CN102204937A, 5 October 2011. [Google Scholar]
- Raja, S.; Ravindranadh, K.; Keerthi, T. A complete profile on Chimphila umbellata-traditional uses, pharmacological activites and phytoconstituents. Int. J. Phytomed. 2014, 6, 464–470. [Google Scholar]
- Dang, Q.L.; Vu, H.D.; Nguyen, V.M.; Choi, G.J.; Hoa, L.T.P.; Dung, D.T.; Kiem, P.V.; Nhiem, N.X.; Tran, Q.D.; Nguyen, Q.C.; et al. Desmodinosides A-E: New flavonoid C-glycosides from Desmodium heterocarpon var. stigosum with hepatoprotective and antifungal activity. Fitoterapia 2023, 169, 10609. [Google Scholar] [CrossRef]
- Qin, Y.Q.; Liu, W.; Yin, R.; Xiao, P.T.; Wang, Z.Y.; Huang, T.Q.; Liu, E.H. New 4′,5′-methylenedioxyflavone derivatives from the whole plant of Sarcandra glabra. Nat. Prod. Res. 2022, 38, 177–185. [Google Scholar] [CrossRef]
- Zedet, A.; Kanga, Y.; Pudlo, M.; Senejoux, F.; Girard, C. Arginase inhibitory activities of chemical constituents from Macaranga hurifolia Beille leaves. Nat. Prod. Res. 2023, 1, 1–6. [Google Scholar] [CrossRef]
- Yu, Y.; Liu, G.J.; Piao, M.H.; Lang, M.Y.; Wang, Y.M.; Jin, M.; Li, G.; Zheng, M.S. Chemical constituents of Polygonum aviculare L. and their chemotaxonomic significance. Biochem. Syst. Ecol. 2022, 105, 104529. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, B.; Yang, J.; Zhang, R.; Liu, N.N.; Wang, X.; Yu, C.P.; Rong, Z.J.; Zhang, H.L.; Long, Q.Z. Chemical investigation and anti-inflammatory activities of the aerial part of Filipendula palmata. J. Ethnopharmacol. 2022, 287, 114959. [Google Scholar] [CrossRef]
- Chang, J.; Inui, T. Novel phenolic glycoside dimer and trimer from the whole herb of Pyrola rotundifolia. Chem. Pharm. Bull. 2005, 53, 1051–1053. [Google Scholar] [CrossRef][Green Version]
- Kasai, Y.; Taji, H.; Fujita, T.; Yamamoto, Y.; Akagi, M.; Sugio, A.; Kuwahara, S.; Watanabe, M.; Harada, N.; Ichikawa, A.; et al. MαNP acid, a powerful chiral molecular tool for preparation of enantiopure alcohols by resolution and determination of their absolute configurations by the 1H NMR anisotropy method. Chirality 2004, 16, 569–585. [Google Scholar] [CrossRef] [PubMed]
- Itano, K.; Yamasaki, K.; Kihara, C.; Tanaka, O. Stereospecific preparation of monoglucosides of optically active trans-1,2-cyclohexanediols by enzymic trans-D-glucosylation and carbon-13NMR spectroscopy of the resulting mono-D-glucopyranosides. Carbohyd. Res. 1980, 87, 27–34. [Google Scholar] [CrossRef]
- Yu, X.; Sui, Y.; Xi, Y.K.; Zhang, Y.; Luo, G.Y.; Long, Y.; Yang, W.D. Semisynthesis, biological evaluation and molecular docking studies of barbatic acid derivatives as novel diuretic candidates. Molecules 2023, 28, 4010. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Han, W.; Chen, X.; Lu, K. A study on the mechanism of aloperine on breast cancer based on network pharmacology and molecular docking. Clin. J. Chin. Med. 2023, 15, 28–34. [Google Scholar]




| Position | 1 | 2 | ||
|---|---|---|---|---|
| δH, Mult (J in Hz) | δC | δH, Mult (J in Hz) | δC | |
| 1 | - | 150.6 | - | 150.5 |
| 2 | - | 130.6 | - | 130.7 |
| 3 | 6.57 (d, 2.7) | 118.1 | 6.57 (d, 2.8) | 118.2 |
| 4 | - | 153.6 | - | 153.6 |
| 5 | 6.52 (dd, 8.6, 3.0) | 113.8 | 6.52 (dd, 8.6, 2.9) | 113.8 |
| 6 | 6.98 (d, 8.7) | 118.7 | 6.96 (d, 8.7) | 118.7 |
| 7 | 2.21 (s) | 16.6 | 2.21 (s) | 16.7 |
| 1′ | 4.74 (d, 7.6) | 103.8 | 4.76 (d, 7.0) | 103.8 |
| 2′ | 3.60 (m) | 74.4 | 3.22 (m) | 74.9 |
| 3′ | 3.49 (m) | 88.0 | 3.62 (m) | 76.5 |
| 4′ | 3.49 (m) | 69.9 | 3.64 (m) | 80.4 |
| 5′ | 3.32 (m) | 77.8 | 3.64 (m) | 76.5 |
| 6′ | 3.88 (d, 12.0) 3.71 (dd, 12.0, 5.2) | 62.5 | 3.89 (dd, 11.2, 3.2) 3.73 (dd, 12.0, 5.2) | 61.7 |
| 1″ | 4.59 (d, 7.7) | 105.3 | 4.60 (d, 7.7) | 104.6 |
| 2″ | 3.30 (1H, m) | 75.5 | 3.54 (m) | 74.8 |
| 3″ | 3.34 (1H, m) | 78.2 | 3.35 (m) | 78.1 |
| 4″ | 3.44 (1H, m) | 71.6 | 3.35 (m) | 71.4 |
| 5″ | 3.34 (1H, m) | 77.7 | 3.35 (m) | 77.8 |
| 6″ | 3.88 (d, 12.0) 3.71 (dd, 12.0, 5.2) | 62.6 | 3.89 (dd, 11.2, 3.2) 3.73 (dd, 12.0, 5.2) | 62.4 |
| Position | δH, Mult (J in Hz) | δC | Position | δH, Mult (J in Hz) | δC |
|---|---|---|---|---|---|
| 1 (1′) | - | 151.3 | Glc-1 (1′) | 4.80 (d, 7.4) | 104.4 |
| 2 (2′) | - | 130.4 | Glc-2 (2′) | 3.44 (m) | 75.1 |
| 3 (3′) | 6.70 (s) | 119.4 | Glc-3 (3′) | 3.39 (m) | 78.1 |
| 4 (4′) | - | 149.8 | Glc-4 (4′) | 3.37 (m) | 71.6 |
| 5 (5′) | - | 125.4 | Glc-5 (5′) | 3.39 (m) | 78.0 |
| 6 (6′) | 7.12 (s) | 120.9 | Glc-6 (6′) | 3.86 (d, 11.8) 3.66 (d, 10.5) | 62.6 |
| 7 (7′) | 2.26 (s) | 16.4 |
| Position | δH, Mult (J in Hz) | δC | Position | δH, Mult (J in Hz) | δC |
|---|---|---|---|---|---|
| 1 | 3.78 (m) | 78.4 | 1′ | 4.45 (d, 7.8) | 102.3 |
| 2 | 1.77 (d, 11.7) 1.51 (m) | 36.5 | 2′ | 3.60 (m) | 75.1 |
| 3 | 1.64 (m) | 36.5 | 3′ | 3.39 (m) | 77.9 |
| 4 | 3.71 (m) | 69.8 | 4′ | 3.37 (m) | 71.7 |
| 5 | 1.91 (d, 8.5) 1.61 (m) | 26.3 | 5′ | 3.39 (m) | 78.0 |
| 6 | 1.90 (m), 1.55 (m) | 32.3 | 6′ | 3.86 (d, 11.8) 3.66 (d, 10.5) | 62.8 |
| 7 | 1.03 (d, 6.7) | 18.7 |
| Position | δH, Mult (J in Hz) | δC | Position | δH, Mult (J in Hz) | δC |
|---|---|---|---|---|---|
| 1 | - | 150.6 | 3′ | - | 135.3 |
| 2 | - | 129.1 | 4′ | 3.89 (s) | 69.0 |
| 3 | - | 128.3 | 5′ | 1.80 (s) | 14.0 |
| 4 | - | 151.5 | 1″ | 4.66 (d, 7.4) | 104.3 |
| 5 | 6.55 (d, 8.8) | 113.3 | 2″ | 3.41 (m) | 75.1 |
| 6 | 6.87 (d, 8.8) | 116.2 | 3″ | 3.30 (m) | 77.9 |
| 7 | 2.20 (s) | 12.6 | 4″ | 3.38 (m) | 71.5 |
| 1′ | 3.40 (m) | 26.2 | 5″ | 3.40 (m) | 78.2 |
| 2′ | 5.33 (dd, 6.8, 5.6) | 125.4 | 6″ | 3.68 (dd, 12.0, 5.1) 3.89 (m) | 62.6 |
| Compound | Growth Inhibition Rate (%) a | Compound | Growth Inhibition Rate (%) a |
|---|---|---|---|
| 1 | 4.89 ± 0.05 | 7 | / |
| 2 | 29.48 ± 0.12 | 8 | / |
| 3 | 23.31 ± 0.13 | 9 | / |
| 4 | 4.89 ± 0.05 | 10 | / |
| 5 | 4.08 ± 0.03 | 11 | / |
| 6 | / b | 12 | / |
| Hyd c | / |
| Compound | Transport Inhibition Rate (%) a | Compound | Transport Inhibition Rate (%) a | ||
|---|---|---|---|---|---|
| Na+ | Cl− | Na+ | Cl− | ||
| 1 | −11.70 ± 1.14 e | 9.44 ± 0.78 e | 8 | 18.52 ± 1.02 e | 0.65 ± 1.45 |
| 4 | 21.13 ± 0.89 e | 12.23 ± 0.18 e | 9 | 6.22 ± 0.53 e | −8.36 ± 2.60 e |
| 5 | 7.61 ± 0.71 e | 4.57 ± 0.65 d | 10 | 1.82 ±0.81 | −13.16 ± 1.89 e |
| 6 | 10.22 ± 1.24 e | 12.17 ± 1.33 e | 11 | 28.81± 1.04 e | 27.68 ± 1.18 e |
| 7 | 35.95 ± 1.42 e | 20.72 ± 0.74 e | 12 | 14.18 ± 0.69 e | 3.27 ± 0.31 c |
| Hyd b | 39.74 ± 0.64 e | 23.42 ± 0.87 e | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, Y.; Hu, D.; Liu, J.; Wu, C.; Sun, Y.; Lang, M.; Han, X.; Kang, D.; Min, J.Z.; Cui, H.; et al. Constituents of Chimaphila japonica and Their Diuretic Activity. Molecules 2024, 29, 1092. https://doi.org/10.3390/molecules29051092
Yu Y, Hu D, Liu J, Wu C, Sun Y, Lang M, Han X, Kang D, Min JZ, Cui H, et al. Constituents of Chimaphila japonica and Their Diuretic Activity. Molecules. 2024; 29(5):1092. https://doi.org/10.3390/molecules29051092
Chicago/Turabian StyleYu, Yue, Deri Hu, Jinze Liu, Chenghao Wu, Yuhong Sun, Mingyue Lang, Xuan Han, Dongzhou Kang, Jun Zhe Min, Hong Cui, and et al. 2024. "Constituents of Chimaphila japonica and Their Diuretic Activity" Molecules 29, no. 5: 1092. https://doi.org/10.3390/molecules29051092
APA StyleYu, Y., Hu, D., Liu, J., Wu, C., Sun, Y., Lang, M., Han, X., Kang, D., Min, J. Z., Cui, H., & Zheng, M. (2024). Constituents of Chimaphila japonica and Their Diuretic Activity. Molecules, 29(5), 1092. https://doi.org/10.3390/molecules29051092






