Quantum Dots Mediated Heterojunction Coupling MoSe2 Photoanode for Photoelectrochemical Water Splitting
Abstract
1. Introduction
2. Results
2.1. Synthesis and Characterization
2.1.1. Preparation Process
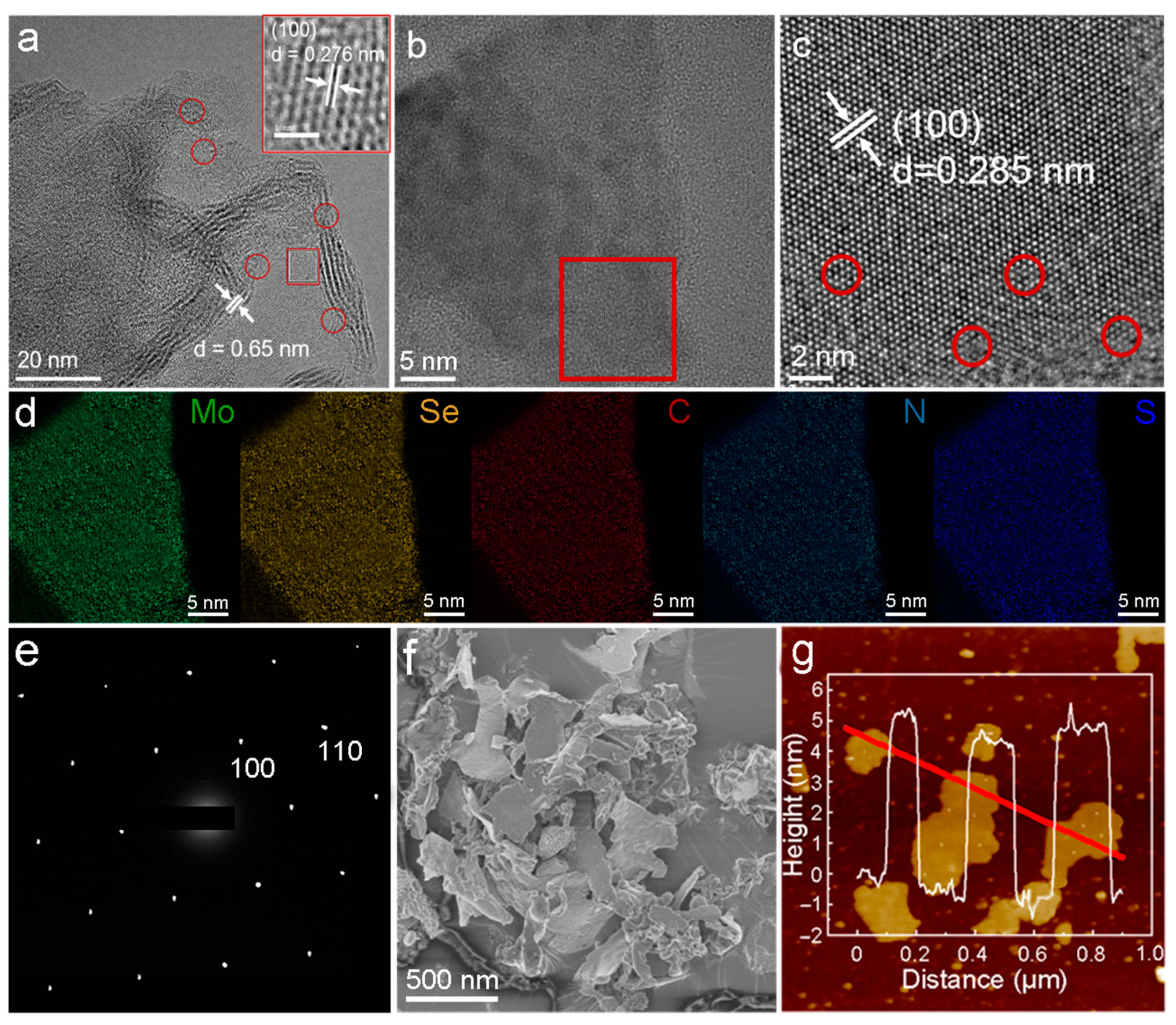
2.1.2. Morphology Characterizations
2.1.3. Properties Characterizations
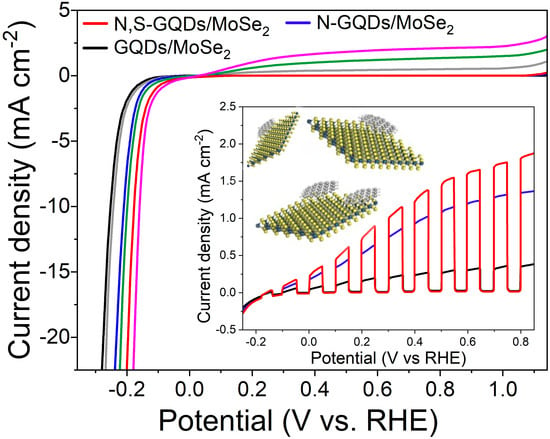
2.2. PEC Studies
2.2.1. PEC HER Performance
2.2.2. Photodegradation Performance
3. Discussion
4. Materials and Methods
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, M.; Sun, L.; Lin, Z.; Cai, J.; Xie, K.; Lin, C. p-n Heterojunction photoelectrodes composed of Cu2O-loaded TiO2 nanotube arrays with enhanced photoelectrochemical and photoelectrocatalytic activities. Energy Environ. Sci. 2013, 6, 1211–1220. [Google Scholar] [CrossRef]
- Su, J.; Zhu, L.; Chen, G. Ultrasmall graphitic carbon nitride quantum dots decorated self-organized TiO2 nanotube arrays with highly efficient photoelectrochemical activity. Appl. Catal. B-Environ. 2016, 186, 127–135. [Google Scholar] [CrossRef]
- Tang, Z.K.; Tao, Y.; Wang, K.H.; Bao, D.Q.; Gao, Z.Q.; Zhao, H.G.; Zhang, H.; Wen, Z.; Sun, X.H. Lattice Mn2+ doped CdSe/CdS quantum dots for high-performance photoelectrochemical hydrogen evolution. Nano Energy 2023, 113, 108533. [Google Scholar] [CrossRef]
- Wang, W.H.; Jin, C.; Qi, L.M. Hierarchical CdS Nanorod@SnO2 Nanobowl Arrays for Efficient and Stable Photoelectrochemical Hydrogen Generation. Small 2018, 14, 1801352. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.N.; Chen, S.B.; Ng, Y.H.; Gao, Q.Z.; Yang, S.Y.; Zhang, S.Q.; Peng, F.; Fang, Y.P.; Zhang, S.S. ZnO/CdS/PbS nanotube arrays with multi-heterojunctions for efficient visible-light-driven photoelectrochemical hydrogen evolution. Chem. Eng. J. 2019, 362, 658–666. [Google Scholar] [CrossRef]
- Iqbal, N.; Khan, M.S.; Zubair, M.; Khan, S.A.; Ali, A.; Aldhafeeri, N.; Alsahli, S.; Alanzi, M.; Enazi, A.; Alroyle, T.; et al. Advanced Photoelectrochemical Hydrogen Generation by CdO-g-C3N4 in Aqueous Medium under Visible Light. Molecules 2022, 27, 8646. [Google Scholar] [CrossRef]
- Mo, Z.; Xu, H.; Chen, Z.G.; She, X.J.; Song, Y.H.; Wu, J.J.; Yan, P.C.; Xu, L.; Leia, Y.C.; Yuan, S.Q.; et al. Self-assembled synthesis of defect-engineered graphitic carbon nitride nanotubes for efficient conversion of solar energy. Appl. Catal. B-Environ. 2018, 225, 154–161. [Google Scholar] [CrossRef]
- Yan, Y.; Zhai, D.; Liu, Y.; Gong, J.; Chen, J.; Zan, P.; Zeng, Z.; Li, S.; Huang, W.; Chen, P. van der Waals Heterojunction between a Bottom-Up Grown Doped Graphene Quantum Dot and Graphene for Photoelectrochemical Water Splitting. ACS Nano 2020, 14, 1185–1195. [Google Scholar] [CrossRef]
- Wang, R.; Su, W.; Zhang, S.; Jin, L.; Zhang, J.; Bian, H.; Zhang, Y. Application of Lignin-Derived Graphene Quantum Dots in Visible Light-Driven Photoelectrochemical Photodetector. Adv. Opt. Mater. 2023, 11, 2202944. [Google Scholar] [CrossRef]
- Yan, Y.; Chen, J.; Li, N.; Tian, J.; Li, K.; Jiang, J.; Liu, J.; Tian, Q.; Chen, P. Systematic Bandgap Engineering of Graphene Quantum Dots and Applications for Photocatalytic Water Splitting and CO2 Reduction. ACS Nano 2018, 12, 3523–3532. [Google Scholar] [CrossRef]
- Zheng, L.; Zhang, H.; Won, M.; Kim, E.; Li, M.; Kim, J.S. Codoping g-C3N4 with boron and graphene quantum dots: Enhancement of charge transfer for ultrasensitive and selective photoelectrochemical detection of dopamine. Biosens. Bioelectron. 2023, 224, 115050. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.H.; Huang, Y.Y.; Cheng, X.L.; Geng, X.S.; Tang, Q.; You, Y.; Yu, Y.Q.; Zhou, R.; Xu, J. Arrays of ZnSe/MoSe2 Nanotubes with Electronic Modulation as Efficient Electrocatalysts for Hydrogen Evolution Reaction. Adv. Mater. Interfaces 2017, 4, 1700948. [Google Scholar] [CrossRef]
- Yang, C.M.; Zhou, L.H.; Wang, C.T.; Duan, W.; Zhang, L.; Zhang, F.C.; Zhang, J.J.; Zhen, Y.Z.; Gao, L.J.; Fu, F.; et al. Large-scale synthetic Mo@(2H-1T)-MoSe2 monolithic electrode for efficient hydrogen evolution in all pH scale ranges and seawater. Appl. Catal. B-Environ. 2022, 304, 120993. [Google Scholar] [CrossRef]
- Huang, L.; Wei, G.; Wang, J.; Li, D.; Jia, S.; Wu, S.; Jiang, T.; Guo, Y.; Liu, Y.; Ren, F. Ion Irradiation Activated Catalytic Activity of MoSe2 Nanosheet for High-Efficiency Hydrogen Evolution Reaction. Adv. Energy Mater. 2023, 13, 2300651. [Google Scholar] [CrossRef]
- Nie, Z.; Xu, F.; Liu, H.; Wang, H.; Jiao, Y.; Zhu, W.; Wang, Q.; Yan, Y.; Zhu, J. Hierarchical heterostructure of vacant carbon-box erected MoSe2−x nanosheets as a functional mediator for high-performance lithium-sulfur batteries. Chem. Eng. J. 2024, 481, 148433. [Google Scholar] [CrossRef]
- Song, Z.; Yi, J.; Qi, J.; Zheng, Q.; Zhu, Z.; Tao, L.; Cao, Y.; Li, Y.; Gao, Z.; Zhang, R.; et al. Line defects in monolayer TiSe2 with adsorption of Pt atoms potentially enable excellent catalytic activity. Nano Res. 2022, 15, 4687–4692. [Google Scholar] [CrossRef]
- Roy, K.; Maitra, S.; Ghosh, D.; Kumar, P.; Devi, P. 2D-Heterostructure assisted activation of MoS2 basal plane for enhanced photoelectrochemical hydrogen evolution reaction. Chem. Eng. J. 2022, 435, 134963. [Google Scholar] [CrossRef]
- Kwon, I.S.; Kwak, I.H.; Kim, J.Y.; Debela, T.T.; Park, Y.C.; Park, J.; Kang, H.S. Concurrent Vacancy and Adatom Defects of Mo1−xNbxSe2 Alloy Nanosheets Enhance Electrochemical Performance of Hydrogen Evolution Reaction. ACS Nano 2021, 15, 5467–5477. [Google Scholar] [CrossRef]
- Zhu, C.; He, Q.; Sun, T.; Xu, M.; Wang, J.; Jin, Q.; Chen, C.; Duan, X.; Xu, H.; Wang, S. Edge-enriched laminar hexagonal (2H) MoSe2-anchored sulfur vacancies-rich ReS2 nanoflowers for boosted light-to-hydrogen conversion. Chem. Eng. J. 2023, 464, 142704. [Google Scholar] [CrossRef]
- Chung, Y.-J.; Yang, C.-S.; Lee, J.-T.; Wu, G.H.; Wu, J.M. Coupling Effect of Piezo-Flexocatalytic Hydrogen Evolution with Hybrid 1T-and 2H-Phase Few-Layered MoSe2 Nanosheets. Adv. Energy Mater. 2020, 10, 2002082. [Google Scholar] [CrossRef]
- Li, Y.; Wang, M.; Sun, J. Molecular Engineering Strategies toward Molybdenum Diselenide Design for Energy Storage and Conversion. Adv. Energy Mater. 2022, 12, 2202600. [Google Scholar] [CrossRef]
- Deng, S.; Yang, F.; Zhang, Q.; Zhong, Y.; Zeng, Y.; Lin, S.; Wang, X.; Lu, X.; Wang, C.-Z.; Gu, L.; et al. Phase Modulation of (1T-2H)-MoSe2/TiC-C Shell/Core Arrays via Nitrogen Doping for Highly Efficient Hydrogen Evolution Reaction. Adv. Mater. 2018, 30, 1802223. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.; Zhong, Y.; Zeng, Y.; Wang, Y.; Yao, Z.; Yang, F.; Lin, S.; Wang, X.; Lu, X.; Xia, X.; et al. Directional Construction of Vertical Nitrogen-Doped 1T-2H MoSe2/Graphene Shell/Core Nanoflake Arrays for Efficient Hydrogen Evolution Reaction. Adv. Mater. 2017, 29, 1700748. [Google Scholar] [CrossRef] [PubMed]
- Mushtaq, M.A.; Arif, M.; Fang, X.; Yasin, G.; Ye, W.; Basharat, M.; Zhou, B.; Yang, S.; Ji, S.; Yan, D. Photoelectrochemical reduction of N2 to NH3 under ambient conditions through hierarchical MoSe2@g-C3N4 heterojunctions. J. Mater. Chem. A 2021, 9, 2742–2753. [Google Scholar] [CrossRef]
- Li, L.; Shi, H.; Yu, H.; Tan, X.; Wang, Y.; Ge, S.; Wang, A.; Cui, K.; Zhang, L.; Yu, J. Ultrathin MoSe2 nanosheet anchored CdS-ZnO functional paper chip as a highly efficient tandem Z-scheme heterojunction photoanode for scalable photoelectrochemical water splitting. Appl. Catal. B Environ. 2021, 292, 120184. [Google Scholar] [CrossRef]
- Zhang, X.; Zeng, Y.; Shi, W.; Tao, Z.; Liao, J.; Ai, C.; Si, H.; Wang, Z.; Fisher, A.C.; Lin, S. S-scheme heterojunction of core–shell biphase (1T-2H)-MoSe2/TiO2 nanorod arrays for enhanced photoelectrocatalytic production of hydrogen peroxide. Chem. Eng. J. 2022, 429, 131312. [Google Scholar] [CrossRef]
- Ji, Q.; Zhang, Y.; Gao, T.; Zhang, Y.; Ma, D.; Liu, M.; Chen, Y.; Qiao, X.; Tan, P.-H.; Kan, M.; et al. Epitaxial Monolayer MoS2 on Mica with Novel Photoluminescence. Nano Lett. 2013, 13, 3870–3877. [Google Scholar] [CrossRef]
- Lee, H.; Park, J.Y. Height determination of single-layer graphene on mica at controlled humidity using atomic force microscopy. Rev. Sci. Instrum. 2019, 90, 103702. [Google Scholar] [CrossRef]
- Liu, D.; Wang, D.; Jing, X.; Zhao, X.; Xi, D.; Dang, D.; Meng, L. Continuous phase regulation of MoSe2 from 2H to 1T for the optimization of peroxidase-like catalysis. J. Mater. Chem. B 2020, 8, 6451–6458. [Google Scholar] [CrossRef]
- Wang, H.; Wang, X.; Wang, L.; Wang, J.; Jiang, D.; Li, G.; Zhang, Y.; Zhong, H.; Jiang, Y. Phase Transition Mechanism and Electrochemical Properties of Nanocrystalline MoSe2 as Anode Materials for the High Performance Lithium-Ion Battery. J. Phys. Chem. C 2015, 119, 10197–10205. [Google Scholar] [CrossRef]
- Jiang, M.; Zhang, J.; Wu, M.; Jian, W.; Xue, H.; Ng, T.-W.; Lee, C.-S.; Xu, J. Synthesis of 1T-MoSe2 ultrathin nanosheets with an expanded interlayer spacing of 1.17 nm for efficient hydrogen evolution reaction. J. Mater. Chem. A 2016, 4, 14949–14953. [Google Scholar] [CrossRef]
- Setayeshgar, S.; Karimipour, M.; Molaei, M.; Moghadam, M.R.; Khazraei, S. Synthesis of scalable 1T/2H–MoSe2 nanosheets with a new source of Se in basic media and study of their HER activity. Int. J. Hydrogen Energy 2020, 45, 6090–6101. [Google Scholar] [CrossRef]
- Zhao, Z.; Hu, Z.; Liang, H.; Li, S.; Wang, H.; Gao, F.; Sang, X.; Li, H. Nanosized MoSe2@Carbon Matrix: A Stable Host Material for the Highly Reversible Storage of Potassium and Aluminum Ions. ACS Appl. Mater. Interfaces 2019, 11, 44333–44341. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Nam, G.-H.; He, Q.; Wu, X.-J.; Zhang, K.; Yang, Z.; Chen, J.; Ma, Q.; Zhao, M.; Liu, Z.; et al. High phase-purity 1T′-MoS2- and 1T′-MoSe2-layered crystals. Nat. Chem. 2018, 10, 638–643. [Google Scholar] [CrossRef]
- Dai, C.; Qing, E.; Li, Y.; Zhou, Z.; Yang, C.; Tian, X.; Wang, Y. Novel MoSe2 hierarchical microspheres for applications in visible-light-driven advanced oxidation processes. Nanoscale 2015, 7, 19970–19976. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.; Wang, L.; Sun, S.; Wang, Y.; Chen, X.; Zhang, K.; Zhang, D.; Xue, Z.; Zhou, X. Layered MoS2@graphene functionalized with nitrogen-doped graphene quantum dots as an enhanced electrochemical hydrogen evolution catalyst. Chin. Chem. Lett. 2019, 30, 1253–1260. [Google Scholar] [CrossRef]
- Zhu, H.; Du, M.; Zhang, M.; Zou, M.; Yang, T.; Wang, S.; Yao, J.; Guo, B. S-rich single-layered MoS2 nanoplates embedded in N-doped carbon nanofibers: Efficient co-electrocatalysts for the hydrogen evolution reaction. Chem. Commun. 2014, 50, 15435–15438. [Google Scholar] [CrossRef] [PubMed]
- Krysa, J.; Zlamal, M.; Kment, S.; Brunclikova, M.; Hubicka, Z. TiO2 and Fe2O3 Films for Photoelectrochemical Water Splitting. Molecules 2015, 20, 1046–1058. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, D.; Li, B.; Zhang, C.; Du, Z.; Yin, H.; Bi, X.; Yang, S. Ultrastable In-Plane 1T–2H MoS2 Heterostructures for Enhanced Hydrogen Evolution Reaction. Adv. Energy Mater. 2018, 8, 1801345. [Google Scholar] [CrossRef]
- Wu, H.; Chen, Z.; Xiao, F.; Wang, Y.; Cao, E.; Chen, S.; Du, S.; Wu, Y.; Ren, Z. Tunable doping of N and S in carbon nanotubes by retarding pyrolysis-gas diffusion to promote electrocatalytic hydrogen evolution. Chem. Commun. 2019, 55, 10011–10014. [Google Scholar] [CrossRef]
- Cheon, J.Y.; Kim, J.H.; Kim, J.H.; Goddeti, K.C.; Park, J.Y.; Joo, S.H. Intrinsic Relationship between Enhanced Oxygen Reduction Reaction Activity and Nanoscale Work Function of Doped Carbons. J. Am. Chem. Soc. 2014, 136, 8875–8878. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Yan, Y.; Lee, J.-M. Recent progress on transition metal diselenides from formation and modification to applications. Nanoscale 2022, 14, 1075–1095. [Google Scholar] [CrossRef] [PubMed]
- Gupta, U.; Naidu, B.; Maitra, U.; Singh, A.; Shirodkar, S.N.; Waghmare, U.V.; Rao, C. Characterization of few-layer 1T-MoSe2 and its superior performance in the visible-light induced hydrogen evolution reaction. APL Mater. 2014, 2, 092802. [Google Scholar] [CrossRef]
- Lei, Z.; Xu, S.; Wu, P. Ultra-thin and porous MoSe2 nanosheets: Facile preparation and enhanced electrocatalytic activity towards the hydrogen evolution reaction. Phys. Chem. Chem. Phys. 2016, 18, 70–74. [Google Scholar] [CrossRef]
- Singh, H.; Lalla, N.; Deshpande, U.; Arora, S.K. Engineered MoSe2/WSe2 based heterostructures for efficient hydrogen evolution reaction. Mater. Today Proc. 2021, 45, 4787–4791. [Google Scholar]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Sun, J.; Zhao, M.; Wei, Y.; Luo, T.; Zhao, Z.; Yan, Y. Quantum Dots Mediated Heterojunction Coupling MoSe2 Photoanode for Photoelectrochemical Water Splitting. Molecules 2024, 29, 1070. https://doi.org/10.3390/molecules29051070
Zhang L, Sun J, Zhao M, Wei Y, Luo T, Zhao Z, Yan Y. Quantum Dots Mediated Heterojunction Coupling MoSe2 Photoanode for Photoelectrochemical Water Splitting. Molecules. 2024; 29(5):1070. https://doi.org/10.3390/molecules29051070
Chicago/Turabian StyleZhang, Lin, Jiana Sun, Mengmeng Zhao, Yuxuan Wei, Taigang Luo, Zhengping Zhao, and Yibo Yan. 2024. "Quantum Dots Mediated Heterojunction Coupling MoSe2 Photoanode for Photoelectrochemical Water Splitting" Molecules 29, no. 5: 1070. https://doi.org/10.3390/molecules29051070
APA StyleZhang, L., Sun, J., Zhao, M., Wei, Y., Luo, T., Zhao, Z., & Yan, Y. (2024). Quantum Dots Mediated Heterojunction Coupling MoSe2 Photoanode for Photoelectrochemical Water Splitting. Molecules, 29(5), 1070. https://doi.org/10.3390/molecules29051070