Efficient Adsorption of Nitrogen and Phosphorus in Wastewater by Biochar
Abstract
1. Introduction
2. Preparation of Biochar
2.1. Preparation of Original Biochar
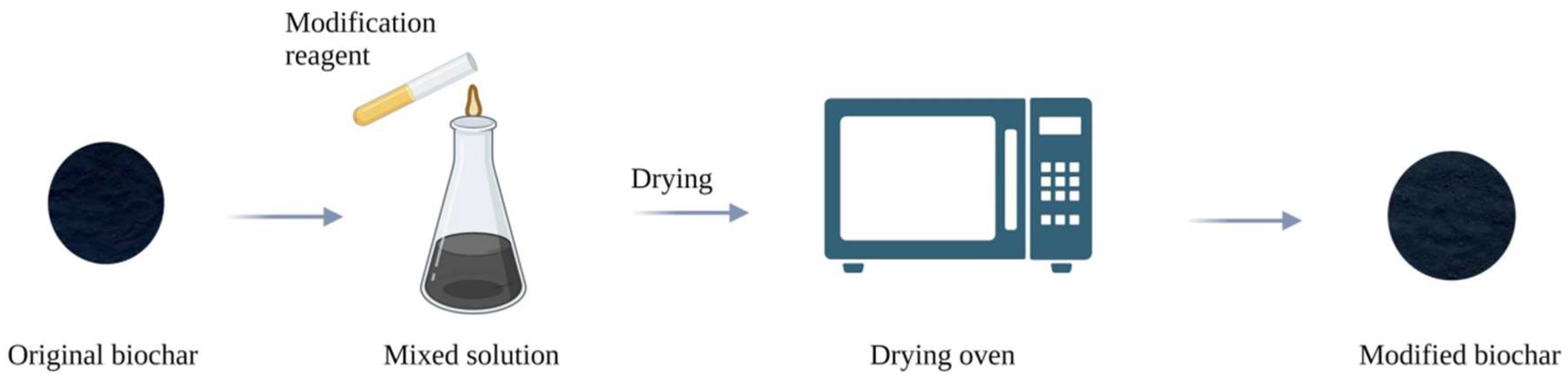
2.2. Preparation of Modified Biochar
2.2.1. Preparation of Biochar by Modification before Pyrolysis
2.2.2. Preparation of Biochar by Modification after Pyrolysis
3. Modification Methods of Biochar
3.1. Chemical Modification
3.2. Physical Modification
3.3. Biological Modification
4. Study on the Adsorption of Nitrogen and Phosphorus by Biochar
4.1. Adsorption of Nitrogen by Biochar
4.2. Adsorption of Phosphorus by Biochar
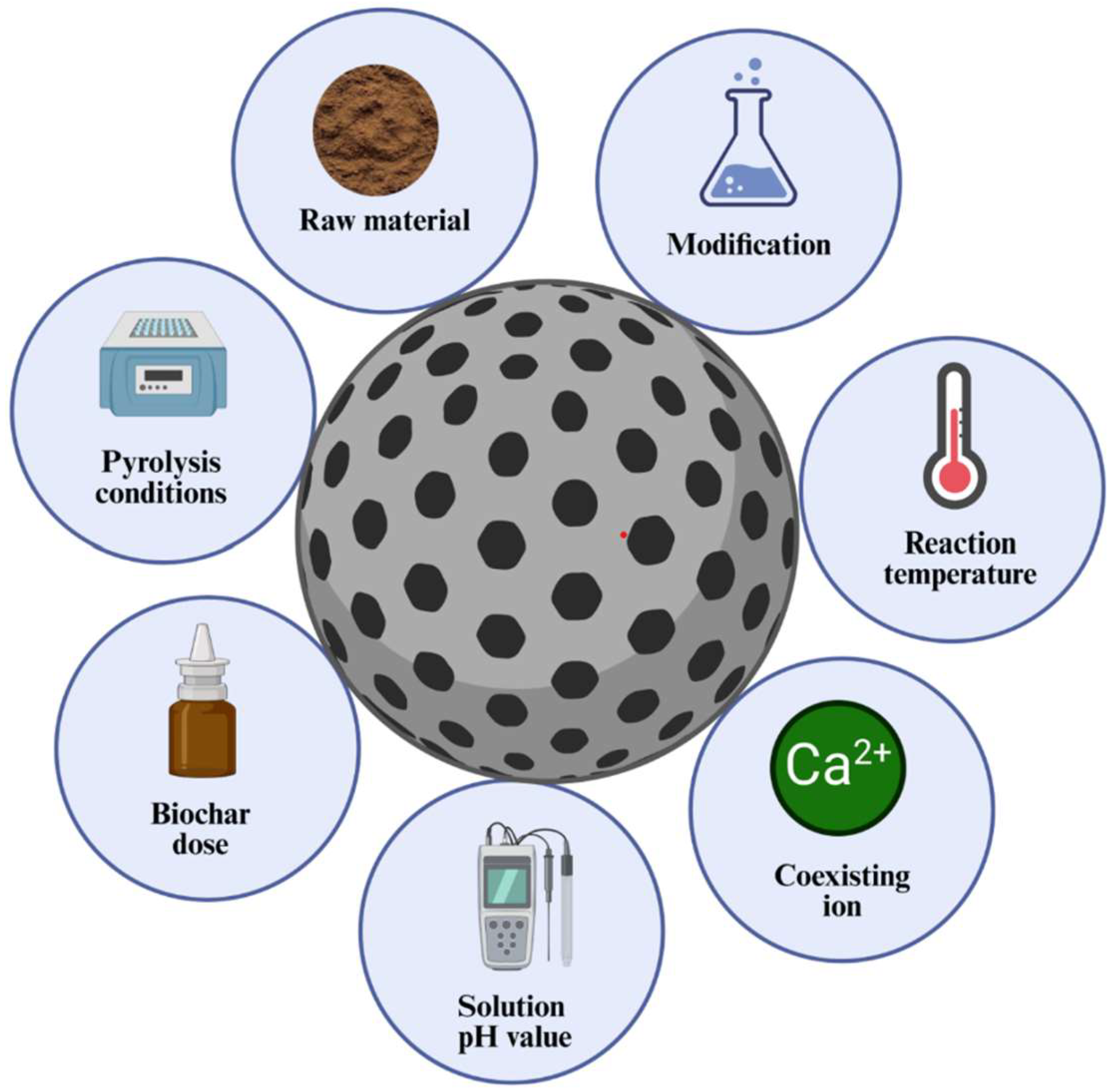
5. Factors Affecting Adsorption of Nitrogen and Phosphorus by Biochar
5.1. Influence of Preparation Conditions of Biochar
5.1.1. Influence of Raw Materials
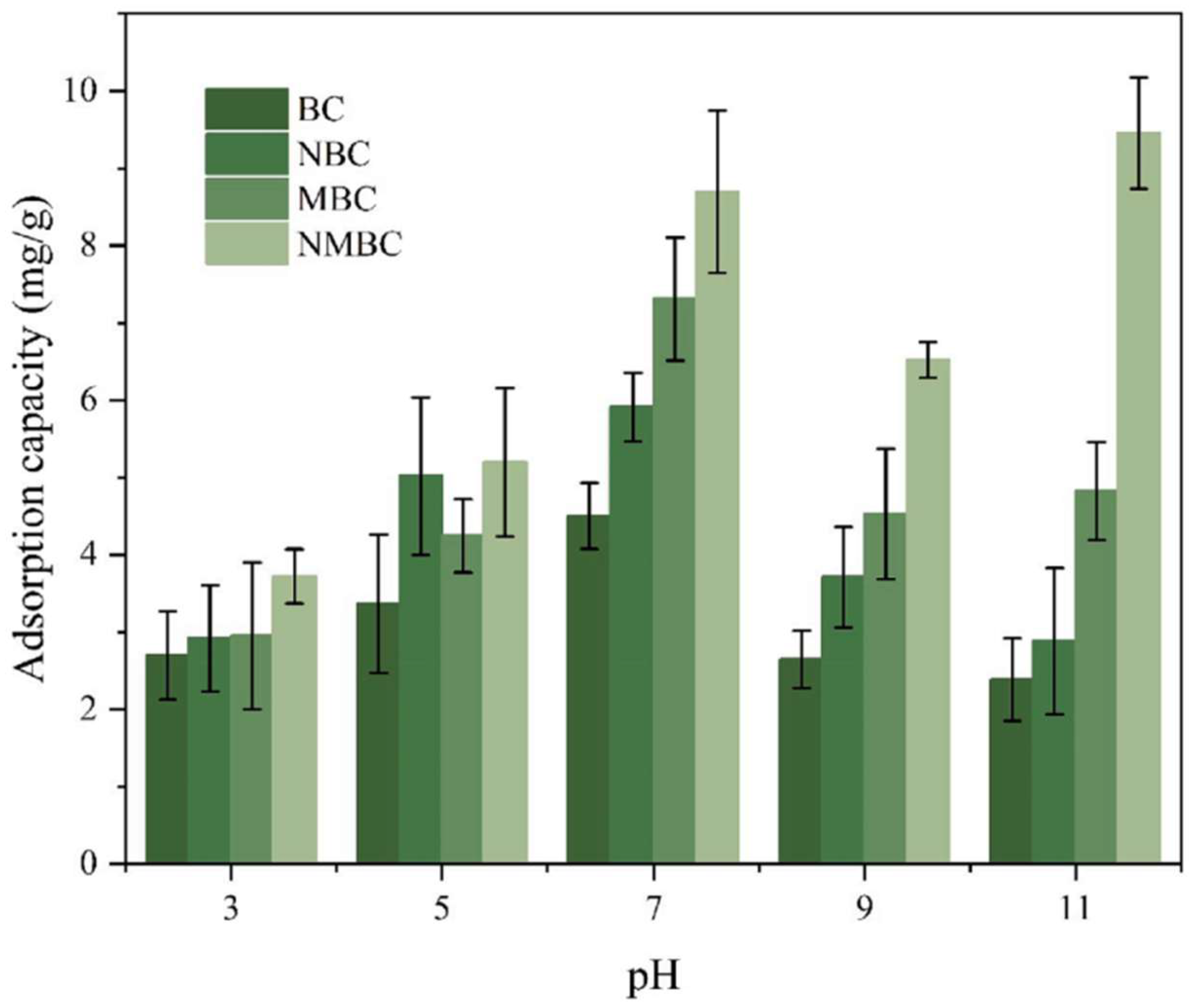
5.1.2. Influence of Chemical Modification
5.1.3. Influence of Pyrolysis Conditions
5.2. Environmental Factors Affecting Nitrogen and Phosphorus Adsorption by Biochar
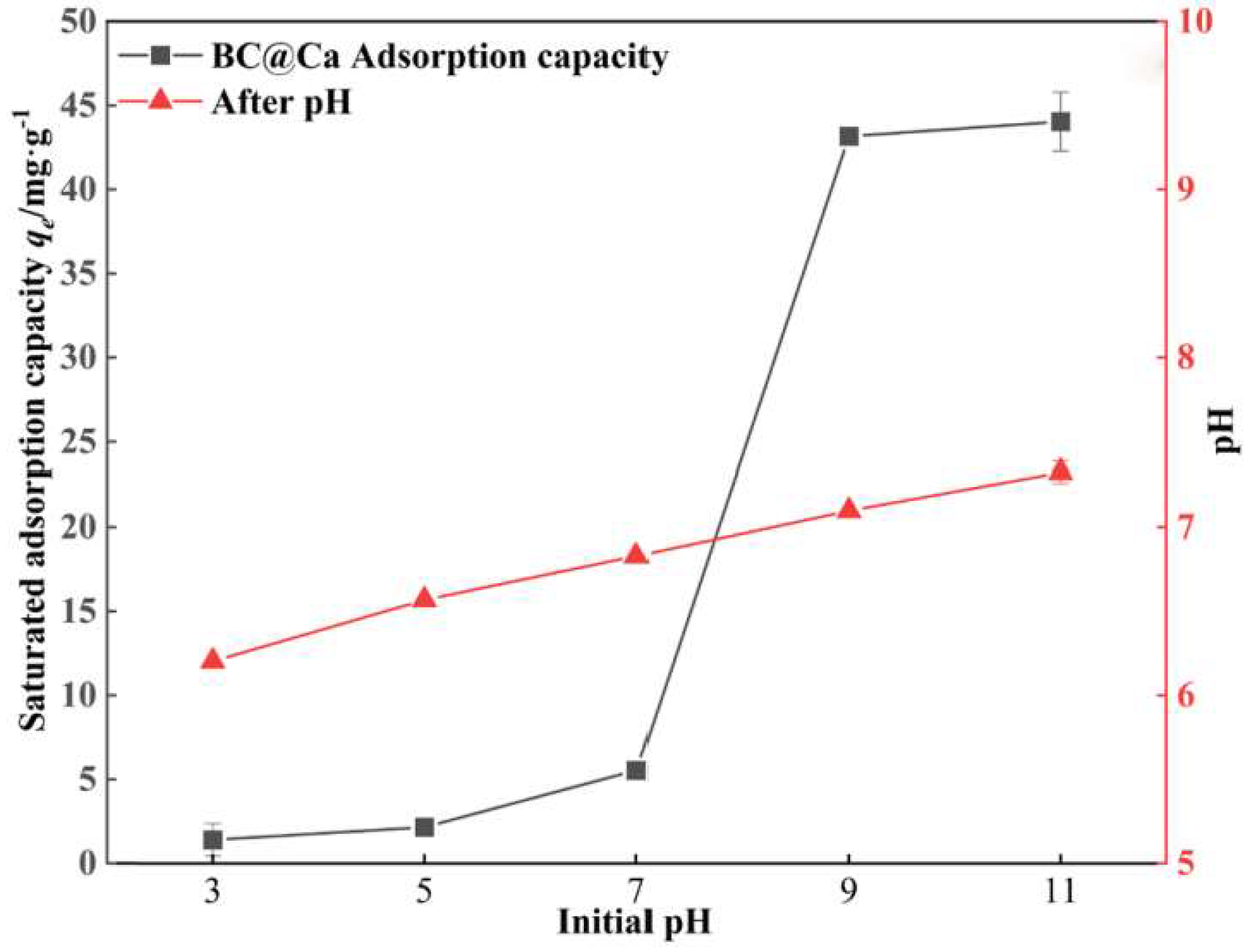
5.2.1. pH of the Solution
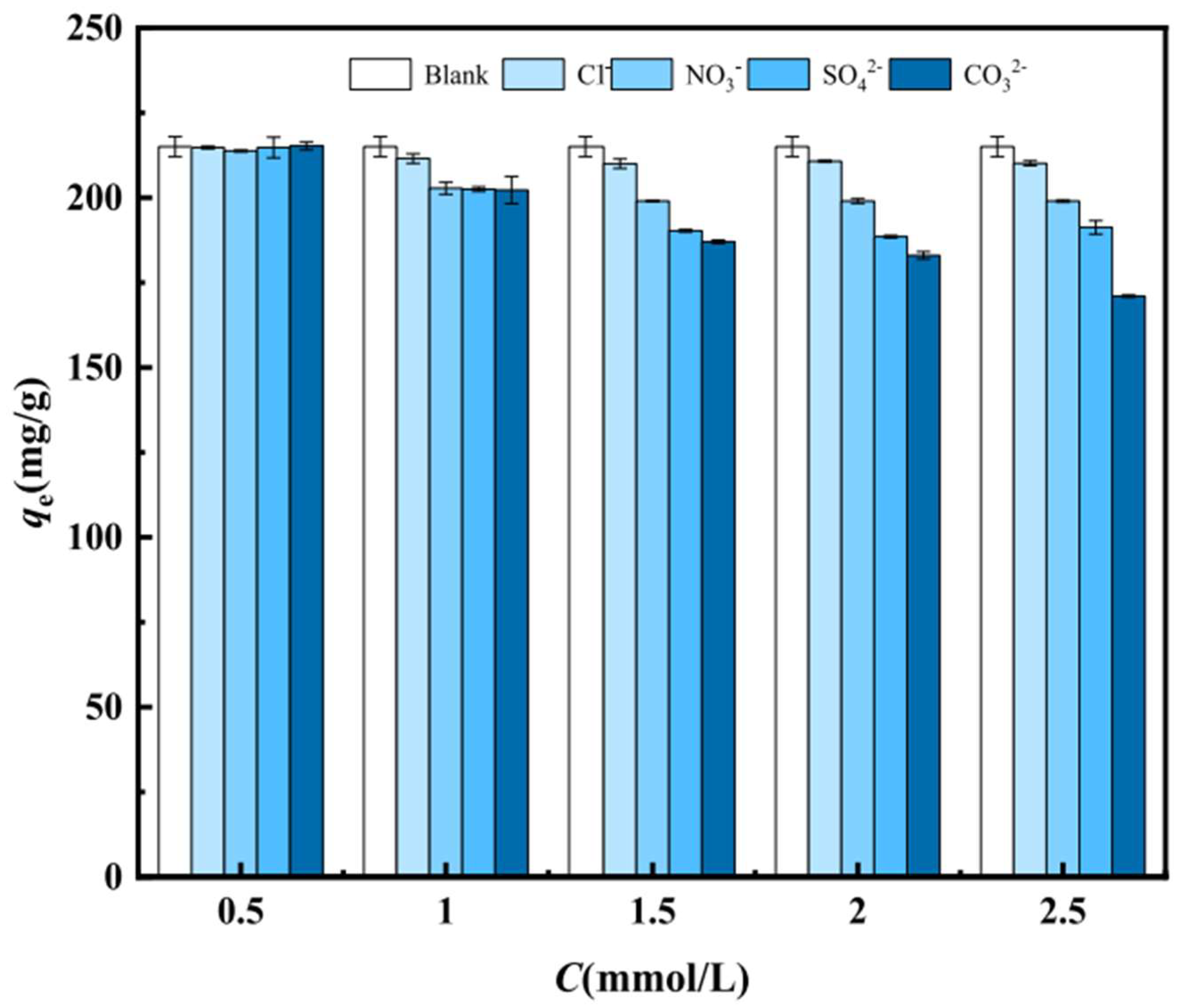
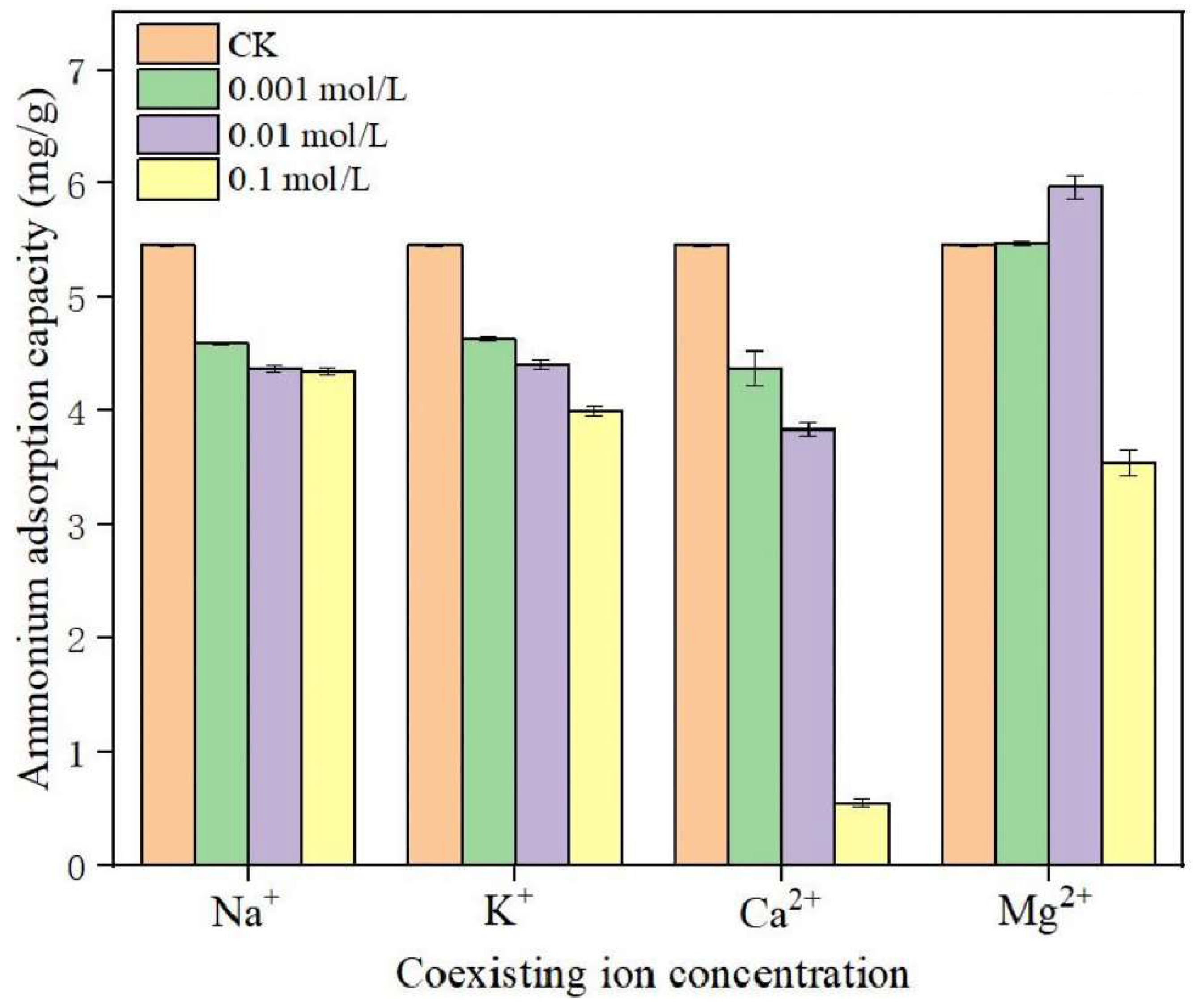
5.2.2. Coexisting Ions
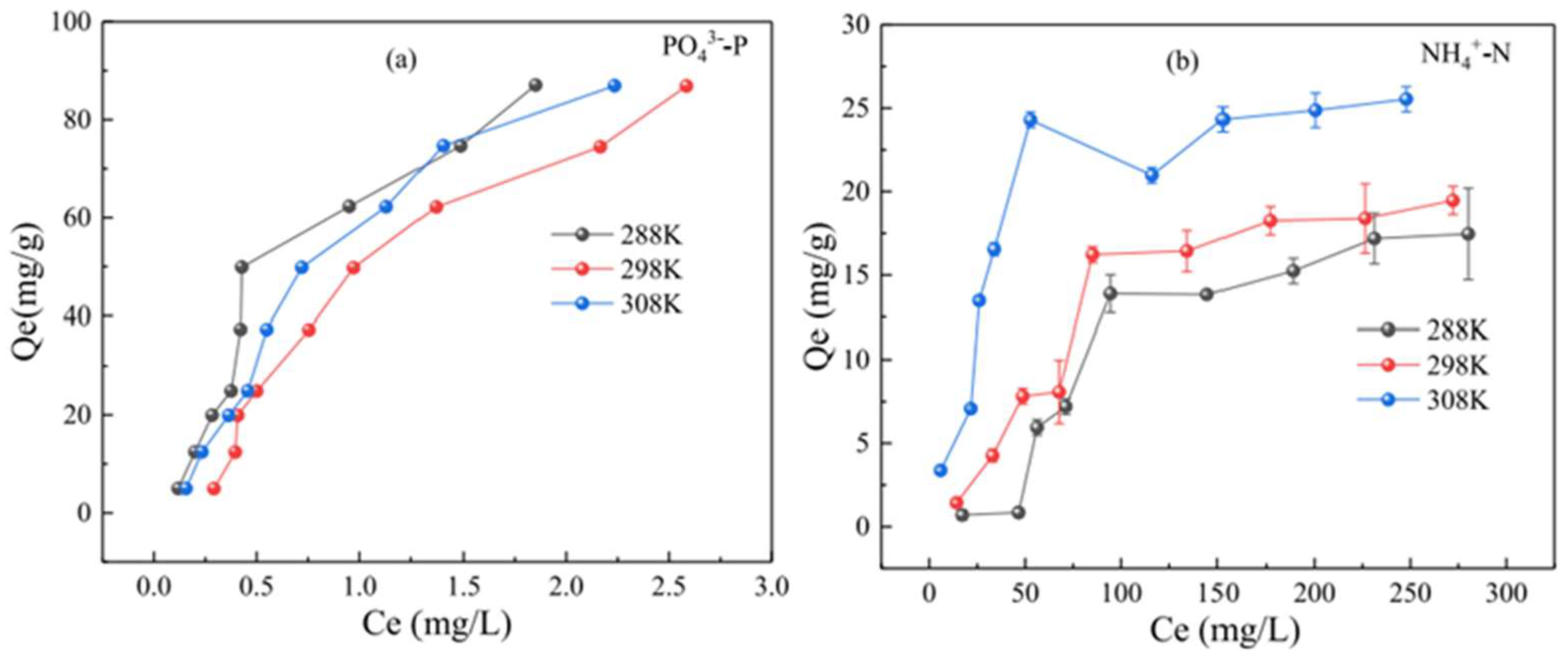
5.2.3. Effect of Reaction Temperature
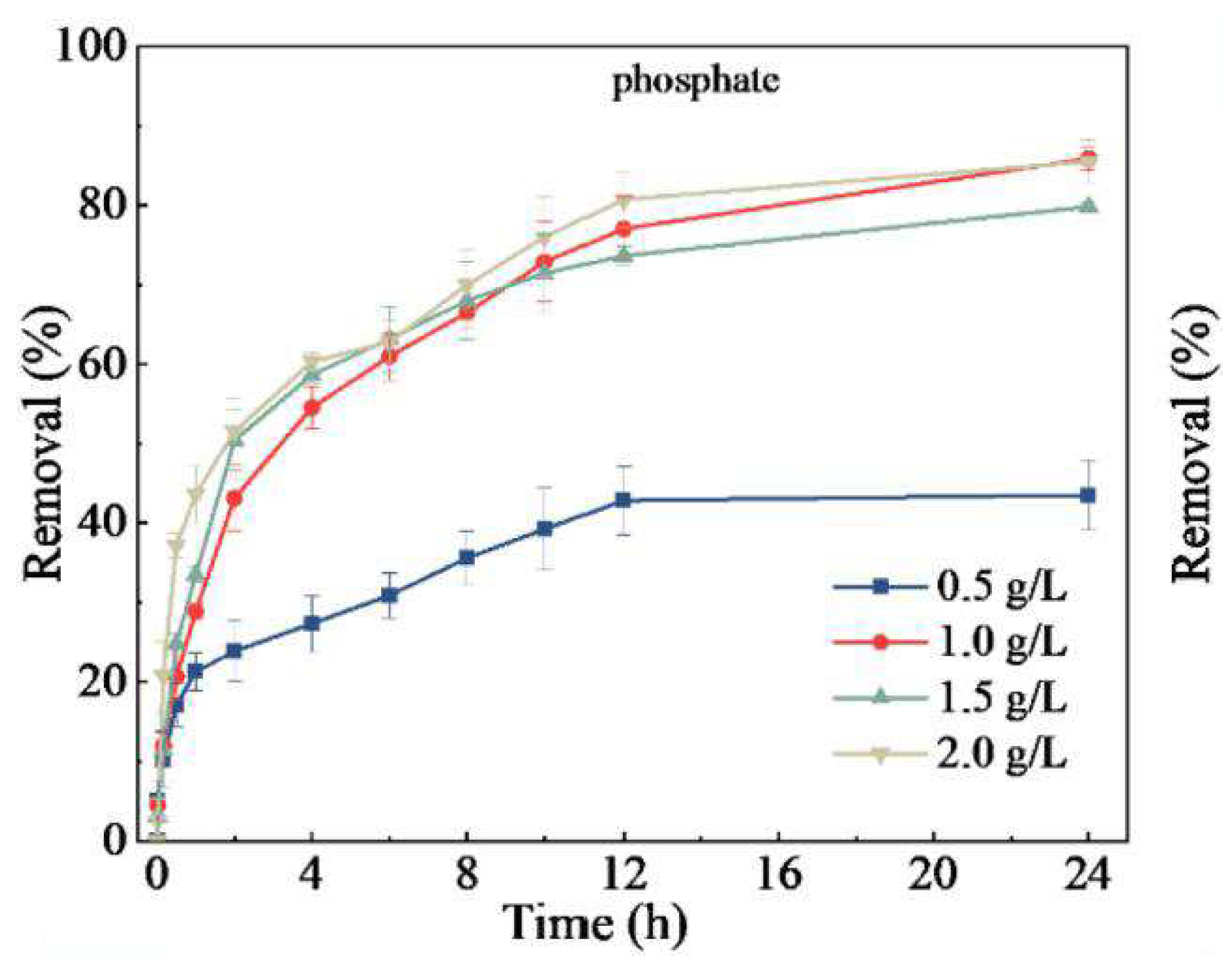
5.2.4. Influence of Biochar Dosage
6. Reusability of Biochar
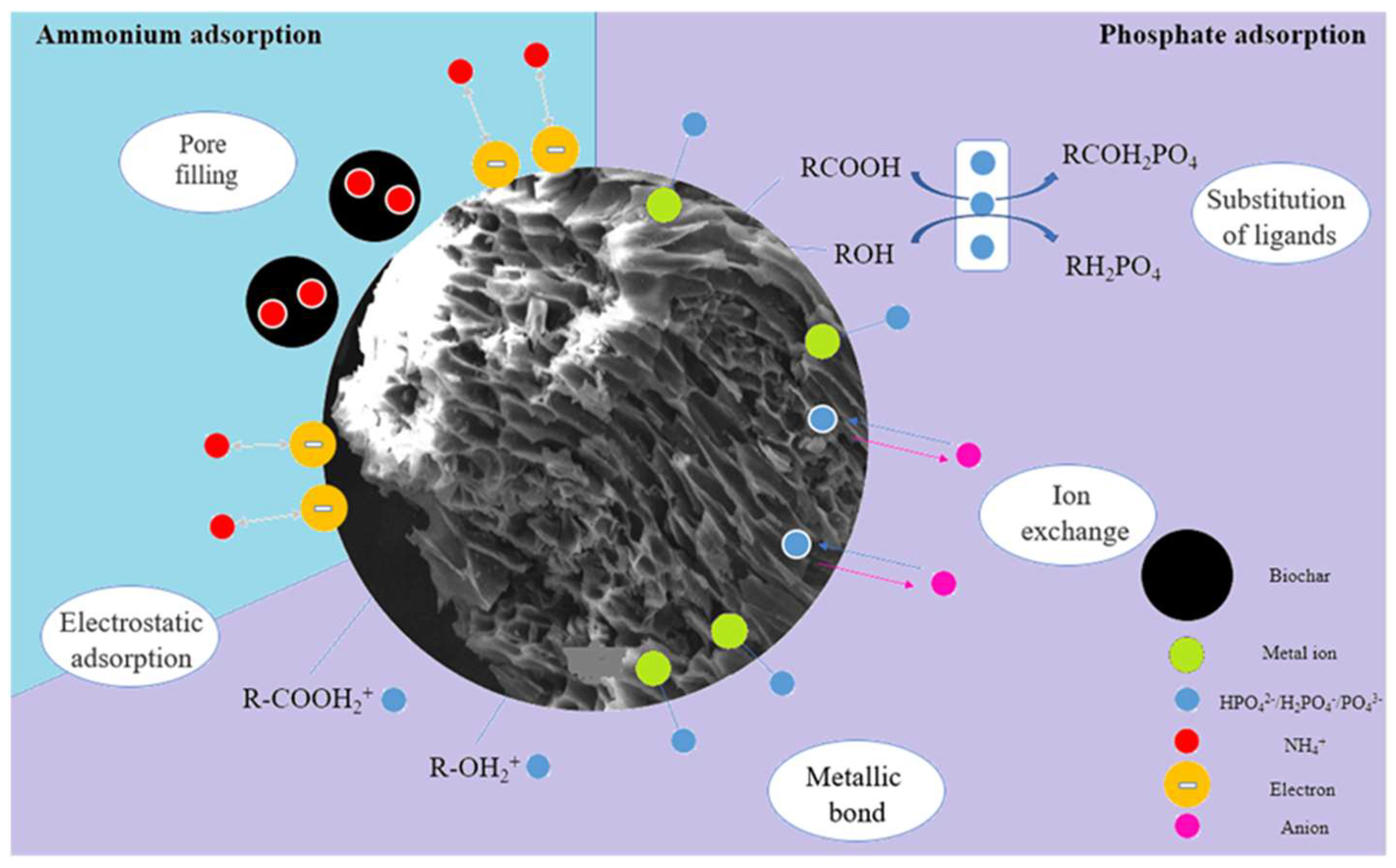
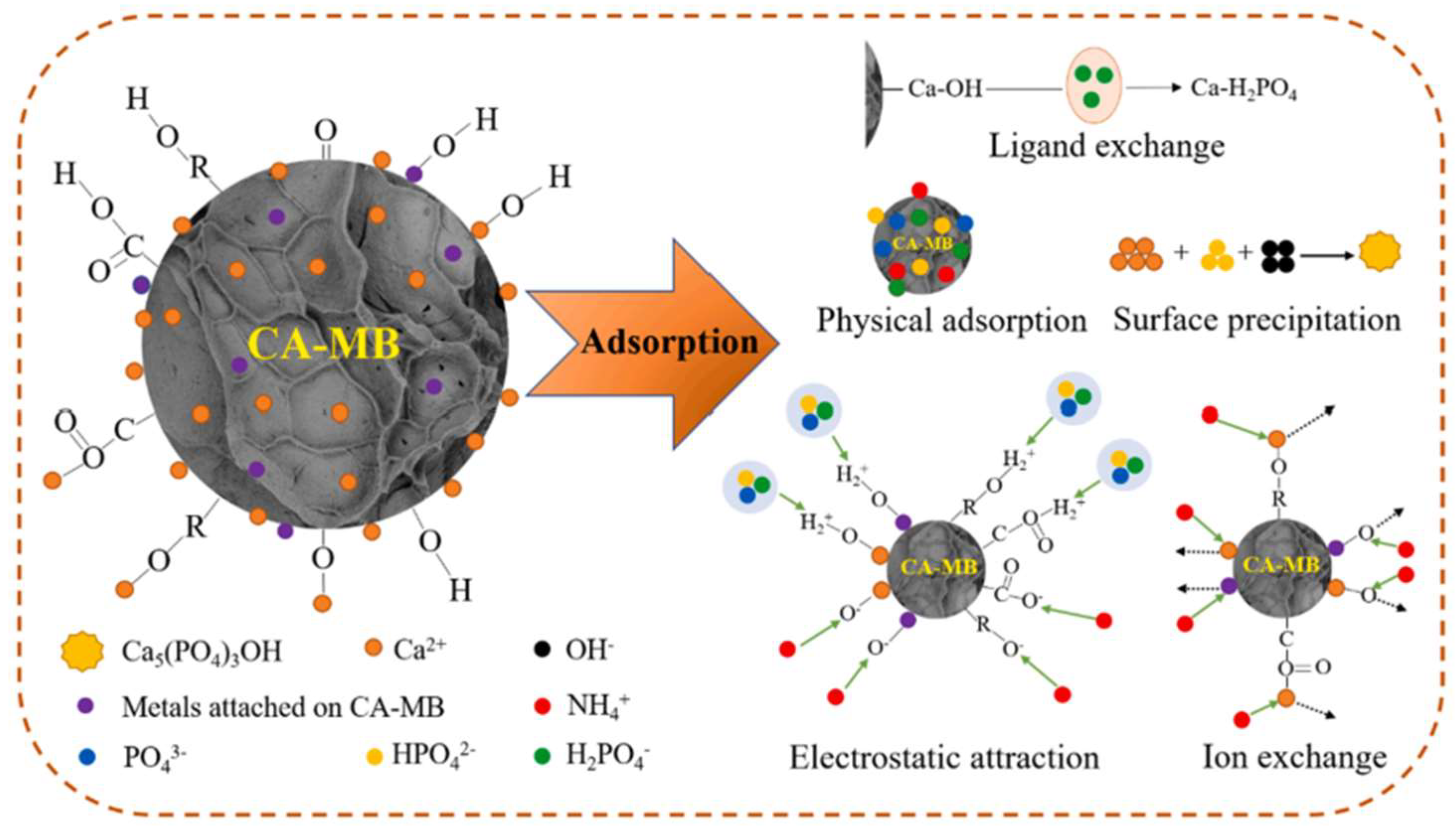
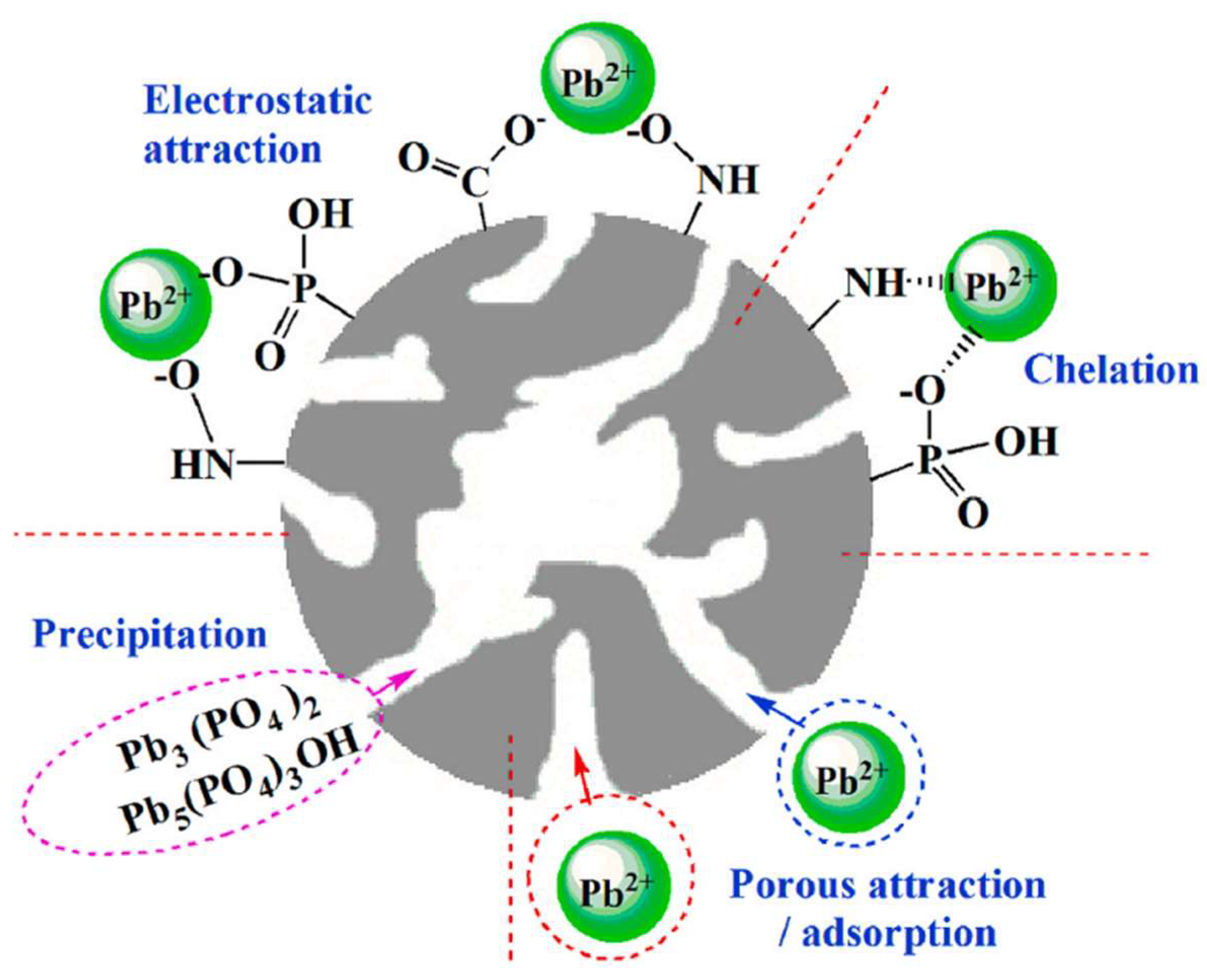
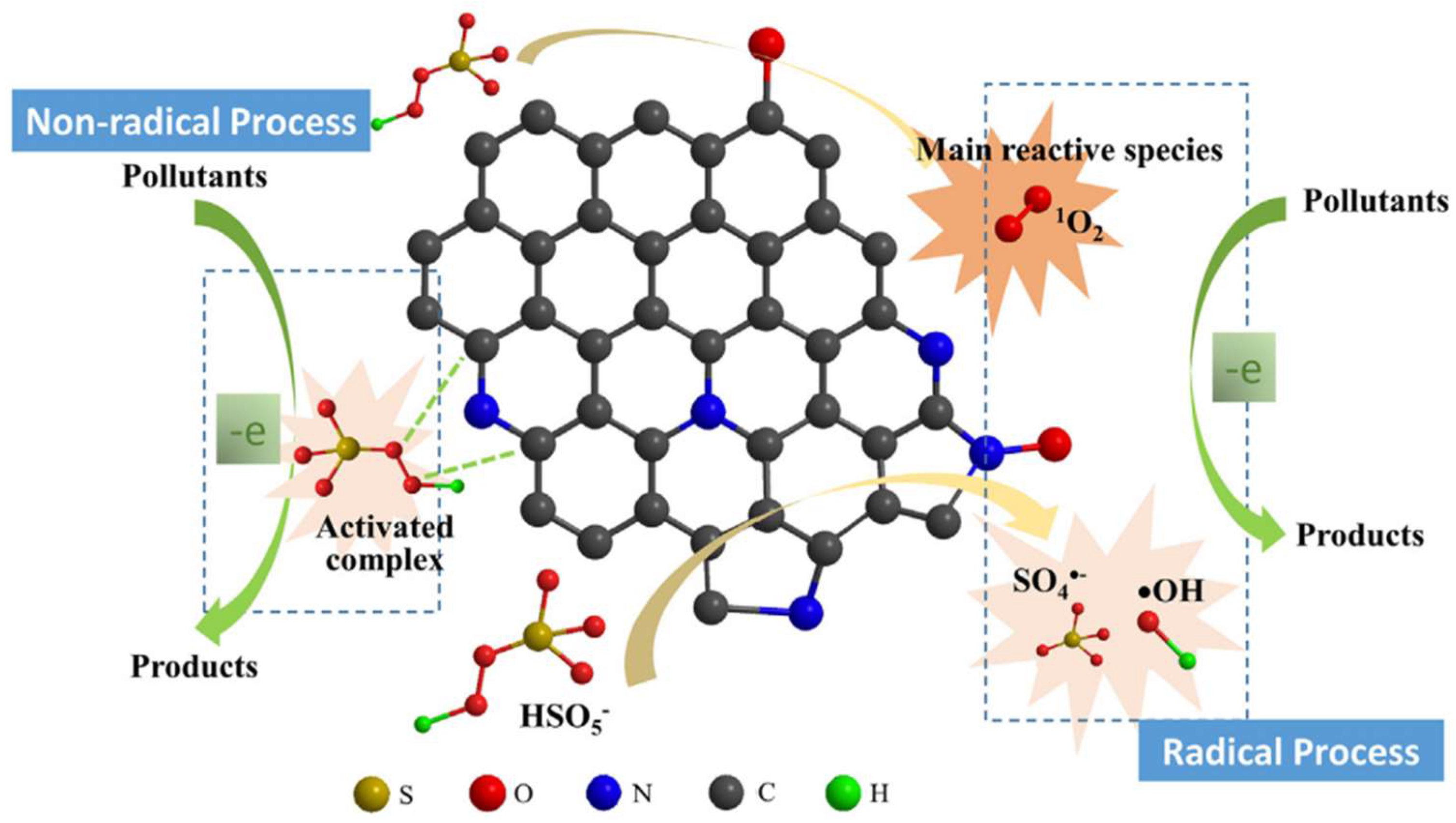
7. Adsorption Mechanism
8. The Resource Utilization of Biochar Loaded with Nitrogen and Phosphorus
9. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Qin, B.; Zhang, Y.; Zhu, G.; Gao, G. Eutrophication control of large shallow lakes in China. Sci. Total Environ. 2023, 881, 163494. [Google Scholar] [CrossRef]
- Akinnawo, S. Eutrophication: Causes, consequences, physical, chemical and biological techniques for mitigation strategies. Environ. Chall. 2023, 12, 100733. [Google Scholar] [CrossRef]
- Cheng, N.; Wang, B.; Feng, Q.; Zhang, X.; Chen, M. Co-adsorption performance and mechanism of nitrogen and phosphorus onto eupatorium adenophorum biochar in water. Bioresour. Technol. 2021, 340, 125696. [Google Scholar] [CrossRef]
- Fernández, F.J.; Martínez, A.; Alvarez-Vázquez, L.J. Controlling eutrophication by means of water recirculation: An optimal control perspective. J. Comput. Appl. Math. 2023, 421, 114886. [Google Scholar] [CrossRef]
- Zhang, M.; Gao, B.; Yao, Y.; Xue, Y.; Inyang, M. Synthesis of porous MgO-biochar nanocomposites for removal of phosphate and nitrate from aqueous solutions. Chem. Eng. J. 2012, 210, 26–32. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. Preparation, modification and environmental application of biochar: A review. J. Clean. Prod. 2019, 227, 1002–1022. [Google Scholar] [CrossRef]
- Vithanage, M.; Herath, I.; Joseph, S.; Bundschuh, J.; Bolan, N.; Ok, Y.S.; Kirkham, M.; Rinklebe, J. Interaction of arsenic with biochar in soil and water: A critical review. Carbon 2017, 113, 219–230. [Google Scholar] [CrossRef]
- Li, M.; Zhang, Z.; Li, Z.; Wu, H. Removal of nitrogen and phosphorus pollutants from water by FeCl3-impregnated biochar. Ecol. Eng. 2020, 149, 105792. [Google Scholar]
- Li, H.; Wang, Y.; Zhao, Y.; Wang, L.; Feng, J.; Sun, F. Efficient simultaneous phosphate and ammonia adsorption using magnesium-modified biochar beads and their recovery performance. J. Environ. Chem. Eng. 2023, 11, 110875. [Google Scholar] [CrossRef]
- Sun, C.; Cao, H.; Huang, C.; Wang, P.; Yin, J.; Liu, H.; Tian, H.; Xu, H.; Zhu, J.; Liu, Z. Eggshell based biochar for highly efficient adsorption and recovery of phosphorus from aqueous solution: Kinetics, mechanism and potential as phosphorus fertilizer. Bioresour. Technol. 2022, 362, 127851. [Google Scholar] [CrossRef] [PubMed]
- Van Truong, T.; Kim, Y.; Kim, D. Study of biochar impregnated with Al recovered from water sludge for phosphate adsorption/desorption. J. Clean. Prod. 2023, 383, 135507. [Google Scholar] [CrossRef]
- Zhao, Z.; Wang, B.; Feng, Q.; Chen, M.; Zhang, X.; Zhao, R. Recovery of nitrogen and phosphorus in wastewater by red mud-modified biochar and its potential application. Sci. Total Environ. 2023, 860, 160289. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Shi, Y.; Mohammed, A.; Liu, Y. Wastewater ammonia removal using an integrated fixed-film activated sludge-sequencing batch biofilm reactor (IFAS-SBR): Comparison of suspended flocs and attached biofilm. Int. Biodeterior. Biodegrad. 2017, 116, 38–47. [Google Scholar] [CrossRef]
- Li, N.; Zhou, L.; Jin, X.; Owens, G.; Chen, Z. Simultaneous removal of tetracycline and oxytetracycline antibiotics from wastewater using a ZIF-8 metal organic-framework. J. Hazard. Mater. 2019, 366, 563–572. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Xu, Z.; Hou, D.; Gao, B.; Cao, X.; Ok, Y.S.; Rinklebe, J.; Bolan, N.S.; Tsang, D.C.W. Waste-derived biochar for water pollution control and sustainable development. Nat. Rev. Earth Environ. 2019, 3, 444–460. [Google Scholar] [CrossRef]
- Zeng, Z.; Ye, S.; Wu, H.; Xiao, R.; Zeng, G.; Liang, J.; Zhang, C.; Yu, J.; Fang, Y.; Song, B. Research on the sustainable efficacy of g-MoS2 decorated biochar nanocomposites for removing tetracycline hydrochloride from antibiotic-polluted aqueous solution. Sci. Total Environ. 2019, 648, 206–217. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, Y.; Li, Y.; Chen, L.; Jiang, H.; Jiang, L.; Yan, H.; Zhao, M.; Hou, S.; Zhao, C.; et al. Elaborating the mechanism of lead adsorption by biochar: Considering the impacts of water-washing and freeze-drying in preparing biochar. Bioresour. Technol. 2023, 386, 129447. [Google Scholar] [CrossRef]
- Chen, X.; Zhao, Y.; Yang, L.; Yang, Y.; Wang, L.; Wei, Z.; Song, C. Identifying the specific pathways to improve nitrogen fixation of different straw biochar during chicken manure composting based on its impact on the microbial community. Waste Manag. 2023, 170, 8–16. [Google Scholar] [CrossRef]
- Li, X.; Liu, T.; Han, X.; Li, Y.; Ma, X. Removal of heavy metals lead and ciprofloxacin from farm wastewater using peanut shell biochar. Environ. Technol. Innov. 2023, 30, 103121. [Google Scholar] [CrossRef]
- Moradi, M.; Sadani, M.; Shahsavani, A.; Bakhshoodeh, R.; Alavi, N. Enhancing anaerobic digestion of automotive paint sludge through biochar addition. Heliyon 2023, 9, e17640. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Li, Z.; Qi, X.; Chen, Z.; Ni, H.; Gao, Y.; Liu, X. Improvement of cow manure anaerobic digestion performance by three different crop straw biochars. Environ. Technol. Innov. 2023, 31, 103233. [Google Scholar] [CrossRef]
- Dong, S.; Shen, X.; Guo, Q.; Cheng, H.; Giannakis, S.; He, Z.; Wang, L.; Wang, D.; Song, S.; Ma, J. Valorization of soybean plant wastes in preparation of N-doped biochar for catalytic ozonation of organic contaminants: Atrazine degradation performance and mechanistic considerations. Chem. Eng. J. 2023, 472, 145153. [Google Scholar] [CrossRef]
- Iamsaard, K.; Weng, C.; Tzeng, J.; Anotai, J.; Jacobson, A.R.; Lin, Y. Systematic optimization of biochars derived from corn wastes, pineapple leaf, and sugarcane bagasse for Cu(II) adsorption through response surface methodology. Bioresour. Technol. 2023, 382, 129131. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Cheng, S.; Xing, B.; Qu, X.; Shi, C.; Meng, W.; Zhang, C.; Xia, H. Preparation of pyrolysis products by catalytic pyrolysis of poplar: Application of biochar in antibiotic wastewater treatment. Chemosphere 2023, 338, 139519. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Sun, P.; Chen, Y.; Li, X.; Zheng, X. Distinct chromium removal mechanisms by iron-modified biochar under varying pH: Role of iron and chromium speciation. Chemosphere 2023, 331, 138796. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Al-Kurdhani, J.M.H.; Ma, J.; Wang, Y. Adsorption of Zn2+ ion by macadamia nut shell biochar modified with carboxymethyl chitosan and potassium ferrate. J. Environ. Chem. Eng. 2023, 11, 110150. [Google Scholar] [CrossRef]
- Jiang, W.; Cai, Y.; Liu, D.; Shi, Q.; Wang, Q. Adsorption properties and mechanism of Suaeda biochar and modified materials for tetracycline. Environ. Res. 2023, 235, 116549. [Google Scholar] [CrossRef]
- Huang, W.; Wu, R.; Chang, J.; Juang, S.; Lee, D. Pristine and manganese ferrite modified biochars for copper ion adsorption: Type-wide comparison. Bioresour. Technol. 2023, 360, 127529. [Google Scholar] [CrossRef]
- Xiao, J.; Zhang, Y.; Zhang, T.C.; Yuan, S. Prussian blue-impregnated waste pomelo peels-derived biochar for enhanced adsorption of NH3. J. Clean. Prod. 2023, 382, 135393. [Google Scholar] [CrossRef]
- Ji, X.; Liu, Y.; Gao, Z.; Lin, H.; Xu, X.; Zhang, Y.; Zhu, K.; Zhang, Y.; Sun, H.; Duan, J. Efficiency and mechanism of adsorption for imidacloprid removal from water by Fe-Mg co-modified water hyacinth-based biochar: Batch adsorption, fixed-bed adsorption, and DFT calculation. Sep. Purif. Technol. 2024, 330, 125235. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, Y.; Liu, S.; Tan, X.; Zeng, G.; Zeng, W.; Ding, Y.; Cao, W.; Zheng, B. Enhanced adsorption of methylene blue by citric acid modification of biochar derived from water hyacinth (Eichornia crassipes). Environ. Sci. Pollut. Res. 2016, 23, 23606–23618. [Google Scholar] [CrossRef] [PubMed]
- Tomczyk, A.; Kondracki, B.; Szewczuk-Karpisz, K. Chemical modification of biochars as a method to improve its surface properties and efficiency in removing xenobiotics from aqueous media. Chemosphere 2023, 312, 137238. [Google Scholar] [CrossRef] [PubMed]
- Kasozi, G.N.; Zimmerman, A.R.; Nkedi-Kizza, P.; Gao, B. Catechol and humic acid sorption onto a range of laboratory-produced black carbons (biochars). Environ. Sci. Technol. 2010, 44, 6189–6195. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.; Fan, X.; Wang, S.; Li, B.; Zhou, N.; Xu, H. Effect of chitosan modification on the properties of magnetic porous biochar and its adsorption performance towards tetracycline and Cu2+. Sustain. Chem. Pharm. 2023, 33, 101057. [Google Scholar] [CrossRef]
- Xu, Z.; Zhang, C.; Zhang, C.; Chen, Z. Quantitative evaluation on phosphate adsorption by modified biochar: A meta-analysis. Process Saf. Environ. Prot. 2023, 177, 42–51. [Google Scholar] [CrossRef]
- Gong, Y.; Hou, R.; Fu, Q.; Li, T.; Wang, J.; Su, Z.; Shen, W.; Zhou, W.; Wang, Y.; Li, M. Modified biochar reduces the greenhouse gas emission intensity and enhances the net ecosystem economic budget in black soil soybean fields. Soil Tillage Res. 2024, 237, 105978. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Z.; Chen, C.; Li, F.; Shen, K. Effects of UV-modified biochar derived from phytoremediation residue on Cd bioavailability and uptake in Coriandrum sativum L. in a Cd-contaminated soil. Environ. Sci. Pollut. Res. 2021, 28, 17395–17404. [Google Scholar] [CrossRef] [PubMed]
- Tomin, O.; Vahala, R.; Yazdani, M.R. Synthesis and efficiency comparison of reed straw-based biochar as a mesoporous adsorbent for ionic dyes removal. Heliyon 2024, 10, e24722. [Google Scholar] [CrossRef]
- Zhou, Y.; Leong, S.Y.; Li, Q. Modified biochar for removal of antibiotics and antibiotic resistance genes in the aqueous environment: A review. J. Water Process Eng. 2023, 55, 104222. [Google Scholar] [CrossRef]
- Schommer, V.A.; Nazari, M.T.; Melara, F.; Braun, J.C.A.; Rempel, A.; Dos Santos, L.F.; Ferrari, V.; Colla, L.M.; Dettmer, A.; Piccin, J.S. Techniques and mechanisms of bacteria immobilization on biochar for further environmental and agricultural applications. Microbiol. Res. 2023, 278, 127534. [Google Scholar] [CrossRef]
- Yu, T.; Wang, L.; Ma, F.; Wang, Y.; Bai, S. A bio-functions integration microcosm: Self-immobilized biochar-pellets combined with two strains of bacteria to remove atrazine in water and mechanisms. J. Hazard. Mater. 2020, 384, 121326. [Google Scholar] [CrossRef]
- Tu, C.; Wei, J.; Guan, F.; Liu, Y.; Sun, Y.; Luo, Y. Biochar and bacteria inoculated biochar enhanced Cd and Cu immobilization and enzymatic activity in a polluted soil. Environ. Int. 2020, 137, 105576. [Google Scholar] [CrossRef]
- Peng, J.; Zhang, Z.; Wang, Z.; Zhou, F.; Yu, J.; Chi, R.; Xiao, C. Adsorption of Pb2+ in solution by phosphate-solubilizing microbially modified biochar loaded with Fe3O4. J. Taiwan Inst. Chem. Eng. 2024, 156, 105363. [Google Scholar] [CrossRef]
- Chang, J.; Sivasubramanian, P.; Dong, C.; Kumar, M. Study on adsorption of ammonium and nitrate in wastewater by modified biochar. Bioresour. Technol. Rep. 2023, 21, 101346. [Google Scholar] [CrossRef]
- Premarathna, K.S.D.; Rajapaksha, A.U.; Sarkar, B.; Kwon, E.E.; Bhatnagar, A.; Ok, Y.S.; Vithanage, M. Biochar-based engineered composites for sorptive decontamination of water: A review. Chem. Eng. J. 2019, 372, 536–550. [Google Scholar] [CrossRef]
- Nguyen, V.; Vo, T.; Tran, T.; Nguyen, T.; Le, T.; Bui, X.; Bach, L. Biochar derived from the spent coffee ground for ammonium adsorption from aqueous solution. Case Stud. Chem. Environ. Eng. 2021, 4, 100141. [Google Scholar] [CrossRef]
- Li, X.; Shi, J. Simultaneous adsorption of tetracycline, ammonium and phosphate from wastewater by iron and nitrogen modified biochar: Kinetics, isotherm, thermodynamic and mechanism. Chemosphere 2022, 293, 133574. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.; Li, Y.; Chi, D.; Wu, Q. Efficient removal of ammonium in aqueous solution by ultrasonic magnesium-modified biochar. Chem. Eng. J. 2023, 461, 142072. [Google Scholar] [CrossRef]
- Bian, H.; Wang, M.; Huang, J.; Liang, R.; Du, J.; Fang, C.; Shen, C.; Man, Y.B.; Wong, M.H.; Shan, S.; et al. Large particle size boosting the engineering application potential of functional biochar in ammonia nitrogen and phosphorus removal from biogas slurry. J. Water Process Eng. 2024, 57, 104640. [Google Scholar] [CrossRef]
- Wu, L.; Xu, D.; Li, B.; Wu, D.; Yang, H. Enhanced removal efficiency of nitrogen and phosphorus from swine wastewater using MgO modified pig manure biochar. J. Environ. Chem. Eng. 2024, 12, 111793. [Google Scholar] [CrossRef]
- Bian, H.; Wang, M.; Han, J.; Hu, X.; Xia, H.; Wang, L.; Fang, C.; Shen, C.; Man, Y.B.; Wong, M.H.; et al. MgFe-LDH@biochars for removing ammonia nitrogen and phosphorus from biogas slurry: Synthesis routes, composite performance, and adsorption mechanisms. Chemosphere 2023, 324, 138333. [Google Scholar] [CrossRef]
- Cao, J.; Wang, R.; Zhu, H.; Cao, S.; Duan, Z. Effect of Fenton pre-oxidation on the physicochemical properties of sludge-based biochar and its adsorption mechanisms for ammonia nitrogen removal. J. Environ. Chem. Eng. 2023, 11, 110689. [Google Scholar] [CrossRef]
- Wang, M.; Hu, S.; Wang, Q.; Liang, Y.; Liu, C.; Xu, H.; Ye, Q. Enhanced nitrogen and phosphorus adsorption performance and stabilization by novel panda manure biochar modified by CMC stabilized nZVZ composite in aqueous solution: Mechanisms and application potential. J. Clean. Prod. 2021, 291, 125221. [Google Scholar] [CrossRef]
- Wang, Z.; Guo, H.; Shen, F.; Yang, G.; Zhang, Y.; Zeng, Y.; Wang, L.; Xiao, H.; Deng, S. Biochar produced from oak sawdust by Lanthanum (La)-involved pyrolysis for adsorption of ammonium (NH4+), nitrate (NO3−), and phosphate (PO43−). Chemosphere 2015, 119, 646–653. [Google Scholar] [CrossRef] [PubMed]
- Gai, X.; Wang, H.; Liu, J.; Zhai, L.; Liu, S.; Ren, T.; Liu, H. Effects of Feedstock and Pyrolysis Temperature on Biochar Adsorption of Ammonium and Nitrate. PLoS ONE 2014, 9, e113888. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Chen, Q.; Zhao, C.; Fu, Y.; Li, J.; Feng, Y.; Li, L. A new approach to removing and recovering phosphorus from livestock wastewater using dolomite. Chemosphere 2020, 255, 127005. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Wu, Y.; He, Y.; Liu, H.; Guo, J.; Li, B.; Peng, H. Efficient removal of phosphorus by adsorption. Phosphorus Sulfur Silicon Relat. Elem. 2023, 198, 375–384. [Google Scholar] [CrossRef]
- Akindolie, M.S.; Choi, H.J. Fe12LaO19 fabricated biochar for removal of phosphorus in water and exploration of its adsorption mechanism. J. Environ. Manag. 2023, 329, 117053. [Google Scholar] [CrossRef]
- Ai, D.; Ma, H.; Meng, Y.; Wei, T.; Wang, B. Phosphorus recovery and reuse in water bodies with simple ball-milled Ca-loaded biochar. Sci. Total Environ. 2023, 860, 160502. [Google Scholar] [CrossRef] [PubMed]
- Oginni, O.; Yakaboylu, G.A.; Singh, K.; Sabolsky, E.M.; Unal-Tosun, G.; Jaisi, D.; Khanal, S.; Shah, A. Phosphorus adsorption behaviors of MgO modified biochars derived from waste woody biomass resources. J. Environ. Chem. Eng. 2020, 8, 103723. [Google Scholar] [CrossRef]
- Wang, Z.; Miao, R.; Ning, P.; He, L.; Guan, Q. From wastes to functions: A paper mill sludge-based calcium-containing porous biochar adsorbent for phosphorus removal. J. Colloid Interface Sci. 2021, 593, 434–446. [Google Scholar] [CrossRef]
- Zhang, L.; Deng, F.; Liu, Z.; Ai, L. Removal of ammonia nitrogen and phosphorus by biochar prepared from sludge residue after rusty scrap iron and reduced iron powder enhanced fermentation. J. Environ. Manag. 2021, 282, 111970. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, J.; Chen, D.; Xiao, W.; Zhao, S.; Ye, X.; Li, H. In situ formation of Ca(OH)2 coating shell to extend the longevity of zero-valent iron biochar composite derived from Fe-rich sludge for aqueous phosphorus removal. Sci. Total Environ. 2023, 854, 158794. [Google Scholar] [CrossRef]
- Chen, D.; Yin, Y.; Xu, Y.; Liu, C. Adsorptive recycle of phosphate by MgO-biochar from wastewater: Adsorbent fabrication, adsorption site energy analysis and long-term column experiments. J. Water Process Eng. 2023, 51, 103445. [Google Scholar] [CrossRef]
- Feng, Y.; Luo, Y.; He, Q.; Zhao, D.; Zhang, K.; Shen, S.; Wang, F. Performance and mechanism of a biochar-based Ca-La composite for the adsorption of phosphate from water. J. Environ. Chem. Eng. 2021, 9, 105267. [Google Scholar] [CrossRef]
- Zeng, S.; Kan, E. Sustainable use of Ca(OH)2 modified biochar for phosphorus recovery and tetracycline removal from water. Sci. Total Environ. 2022, 839, 156159. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Li, Y.; Wang, K.; Ding, K.; Sha, M.; Cao, Y.; Wang, S. Recovery of phosphorus from eutrophic water using nano zero-valent iron-modified biochar and its utilization. Chemosphere 2021, 284, 131391. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; He, Y.; Zhang, H.; Wang, H.; Li, W.; Li, Y.; Xu, J.; Wang, B.; Hu, G. Selective adsorption behavior and mechanism of phosphate in water by different lanthanum modified biochar. J. Environ. Chem. Eng. 2022, 10, 107476. [Google Scholar] [CrossRef]
- Huang, W.; Zhang, X.; Tang, Y.; Luo, J.; Chen, J.; Lu, Y.; Wang, L.; Luo, Z.; Zhang, J. ZVI/biochar derived from amino-modified corncob and its phosphate removal properties in aqueous solution. J. Water Process Eng. 2022, 49, 103026. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, A.; Deng, H.; Ye, C.; Wu, Y.; Linmu, Y.; Hang, H. Characteristics of nitrogen and phosphorus adsorption by Mg-loaded biochar from different feedstocks. Bioresour. Technol. 2019, 276, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, S.; Dai, T.; Ren, H.; Liu, B. Simultaneous adsorption of phosphate and tetracycline by calcium modified corn stover biochar: Performance and mechanism. Bioresour. Technol. 2022, 359, 127477. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.; Zhang, D.; Zheng, X.; Ye, X.; Niu, X.; Lin, Z.; Fu, M.; Zhou, S. Adsorption recovery of phosphate from waste streams by Ca/Mg-biochar synthesis from marble waste, calcium-rich sepiolite and bagasse. J. Clean. Prod. 2021, 288, 125638. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, C.; Chen, S.; Ma, L.; Li, Y.; Lu, Y. Phosphate adsorption characteristics of La(OH)3-modified, canna-derived biochar. Chemosphere 2022, 286, 131773. [Google Scholar] [CrossRef] [PubMed]
- Konneh, M.; Wandera, S.M.; Murunga, S.I.; Raude, J.M. Adsorption and desorption of nutrients from abattoir wastewater: Modelling and comparison of rice, coconut and coffee husk biochar. Heliyon 2021, 7, e8458. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Zhang, X.; Xu, Y.; Xiang, W.; Wang, R.; Ding, F.; Hong, P.; Gao, B. Straw and wood based biochar for CO2 capture: Adsorption performance and governing mechanisms. Sep. Purif. Technol. 2022, 287, 120592. [Google Scholar] [CrossRef]
- Kizito, S.; Wu, S.; Kipkemoi Kirui, W.; Lei, M.; Lu, Q.; Bah, H.; Dong, R. Evaluation of slow pyrolyzed wood and rice husks biochar for adsorption of ammonium nitrogen from piggery manure anaerobic digestate slurry. Sci. Total Environ. 2015, 505, 102–112. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Luo, J.; Qin, J.; Huang, Y.; Ke, T.; Luo, Y.; Yang, M. Efficient removal of phytochrome using rice straw-derived biochar: Adsorption performance, mechanisms, and practical applications. Bioresour. Technol. 2023, 376, 128918. [Google Scholar] [CrossRef]
- Sudan, S.; Khajuria, A.; Kaushal, J. Adsorption potential of pristine biochar synthesized from rice husk waste for the removal of Eriochrome black azo dye. Mater. Today Proc. 2023. [Google Scholar] [CrossRef]
- He, L.; Wang, D.; Zhu, T.; Lv, Y.; Li, S. Pyrolysis recycling of pig manure biochar adsorption material for decreasing ammonia nitrogen in biogas slurry. Sci. Total Environ. 2023, 881, 163315. [Google Scholar] [CrossRef]
- Xu, Q.; Jin, Y.; Zheng, F.; Lu, J. Exploitation of pomelo peel developing porous biochar by N, P co-doping and KOH activation for efficient CO2 adsorption. Sep. Purif. Technol. 2023, 324, 124595. [Google Scholar] [CrossRef]
- Li, X.; Li, R.; Wang, K.; Feng, X. Highly synergic adsorption and photocatalytic degradation of walnut shell biochar/NiCr-layered double hydroxides composite for Methyl orange. J. Ind. Eng. Chem. 2023, 126, 270–282. [Google Scholar] [CrossRef]
- Schmidt, M.P.; Ashworth, D.J.; Celis, N.; Ibekwe, A.M. Optimizing date palm leaf and pistachio shell biochar properties for antibiotic adsorption by varying pyrolysis temperature. Bioresour. Technol. Rep. 2023, 21, 101325. [Google Scholar] [CrossRef]
- Yin, Q.; Liu, M.; Ren, H. Biochar produced from the co-pyrolysis of sewage sludge and walnut shell for ammonium and phosphate adsorption from water. J. Environ. Manag. 2019, 249, 109410. [Google Scholar] [CrossRef]
- Phuong Tran, T.C.; Nguyen, T.P.; Nguyen Nguyen, T.T.; Thao Tran, T.N.; Hang Nguyen, T.A.; Tran, Q.B.; Nguyen, X.C. Enhancement of phosphate adsorption by chemically modified biochars derived from Mimosa pigra invasive plant. Case Stud. Chem. Environ. Eng. 2021, 4, 100117. [Google Scholar] [CrossRef]
- Wang, K.; Peng, N.; Zhang, D.; Zhou, H.; Gu, J.; Huang, J.; Liu, C.; Chen, Y.; Liu, Y.; Sun, J. Efficient removal of methylene blue using Ca(OH)2 modified biochar derived from rice straw. Environ. Technol. Innov. 2023, 31, 103145. [Google Scholar] [CrossRef]
- Qin, X.; Cheng, S.; Xing, B.; Xiong, C.; Yi, G.; Shi, C.; Xia, H.; Zhang, C. Preparation of high-efficient MgCl2 modified biochar toward Cd(II) and tetracycline removal from wastewater. Sep. Purif. Technol. 2023, 325, 124625. [Google Scholar] [CrossRef]
- Hu, S.; Liu, C.; Bu, H.; Chen, M.; Fei, Y. Efficient reduction and adsorption of Cr(VI) using FeCl3-modified biochar: Synergistic roles of persistent free radicals and Fe(II). J. Environ. Sci. 2024, 137, 626–638. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Feng, X.; Zuo, X.; Zhu, Z.; Yang, S.; Zhu, B.; Wang, W.; Zhang, J.; Li, G. Facile fabrication of highly porous MgO-modified biochar derived from agricultural residue for efficient Cd(II) removal from wastewater. Inorg. Chem. Commun. 2023, 154, 110900. [Google Scholar] [CrossRef]
- Gao, Z.; Shan, D.; He, J.; Huang, T.; Mao, Y.; Tan, H.; Shi, H.; Li, T.; Xie, T. Effects and mechanism on cadmium adsorption removal by CaCl2-modified biochar from selenium-rich straw. Bioresour. Technol. 2023, 370, 128563. [Google Scholar] [CrossRef] [PubMed]
- Ding, E.; Jiang, J.; Lan, Y.; Zhang, L.; Gao, C.; Jiang, K.; Qi, X.; Fan, X. Optimizing Cd2+ adsorption performance of KOH modified biochar adopting response surface methodology. J. Anal. Appl. Pyrolysis 2023, 169, 105788. [Google Scholar] [CrossRef]
- Hu, H.; Gao, M.; Wang, T.; Jiang, L. Efficient uranium adsorption and mineralization recycle by nano-MgO biochar with super-hydrophilic surface. J. Environ. Chem. Eng. 2023, 11, 110542. [Google Scholar] [CrossRef]
- Huang, Z.; Fang, X.; Wang, S.; Zhou, N.; Fan, S. Effects of KMnO4 pre- and post-treatments on biochar properties and its adsorption of tetracycline. J. Mol. Liq. 2023, 373, 121257. [Google Scholar] [CrossRef]
- Chen, M.; Wang, F.; Zhang, D.; Yi, W.; Liu, Y. Effects of acid modification on the structure and adsorption NH4+-N properties of biochar. Renew. Energy 2021, 169, 1343–1350. [Google Scholar] [CrossRef]
- Dai, Y.; Wang, W.; Lu, L.; Yan, L.; Yu, D. Utilization of biochar for the removal of nitrogen and phosphorus. J. Clean. Prod. 2020, 257, 120573. [Google Scholar] [CrossRef]
- An, Q.; Li, Z.; Zhou, Y.; Meng, F.; Zhao, B.; Miao, Y.; Deng, S. Ammonium removal from groundwater using peanut shell based modified biochar: Mechanism analysis and column experiments. J. Water Process Eng. 2021, 43, 102219. [Google Scholar] [CrossRef]
- Zheng, Q.; Yang, L.; Song, D.; Zhang, S.; Wu, H.; Li, S.; Wang, X. High adsorption capacity of Mg–Al-modified biochar for phosphate and its potential for phosphate interception in soil. Chemosphere 2020, 259, 127469. [Google Scholar] [CrossRef]
- Wang, S.; Kong, L.; Long, J.; Su, M.; Diao, Z.; Chang, X.; Chen, D.; Song, G.; Shih, K. Adsorption of phosphorus by calcium-flour biochar: Isotherm, kinetic and transformation studies. Chemosphere 2018, 195, 666–672. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Jia, M.; Li, Q.; Lu, S.; Zhou, D.; Feng, L.; Hou, Z.; Yu, J. Comparative analysis of the properties of biochars produced from different pecan feedstocks and pyrolysis temperatures. Ind. Crops Prod. 2023, 197, 116638. [Google Scholar] [CrossRef]
- Cong, P.; Song, S.; Song, W.; Dong, J.; Zheng, X. Biochars prepared from biogas residues: Temperature is a crucial factor that determines their physicochemical properties. Biomass Convers. Biorefinery 2022. [Google Scholar] [CrossRef]
- Fan, X.; Wang, X.; Zhao, B.; Wan, J.; Tang, J.; Guo, X. Sorption mechanisms of diethyl phthalate by nutshell biochar derived at different pyrolysis temperature. J. Environ. Chem. Eng. 2022, 10, 107328. [Google Scholar] [CrossRef]
- Das, S.K.; Ghosh, G.K.; Avasthe, R.K.; Sinha, K. Compositional heterogeneity of different biochar: Effect of pyrolysis temperature and feedstocks. J. Environ. Manag. 2021, 278, 111501. [Google Scholar] [CrossRef]
- Chun, Y.; Sheng, G.; Chiou, C.T.; Xing, B. Compositions and Sorptive Properties of Crop Residue-Derived Chars. Environ. Sci. Technol. 2004, 38, 4649–4655. [Google Scholar] [CrossRef]
- Liao, W.; Zhang, X.; Ke, S.; Shao, J.; Yang, H.; Zhang, S.; Chen, H. Effect of different biomass species and pyrolysis temperatures on heavy metal adsorption, stability and economy of biochar. Ind. Crops Prod. 2022, 186, 115238. [Google Scholar] [CrossRef]
- Xu, D.; Cao, J.; Li, Y.; Howard, A.; Yu, K. Effect of pyrolysis temperature on characteristics of biochars derived from different feedstocks: A case study on ammonium adsorption capacity. Waste Manag. 2019, 87, 652–660. [Google Scholar] [CrossRef]
- Yuan, J.; Xu, R.; Zhang, H. The forms of alkalis in the biochar produced from crop residues at different temperatures. Bioresour. Technol. 2011, 102, 3488–3497. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Ye, J.; Shi, C.; Zhang, P.; Guo, J.; Zubair, M.; Chang, J.; Zhang, L. Pyrolysis temperature regulates sludge-derived biochar production, phosphate adsorption and phosphate retention in soil. J. Environ. Chem. Eng. 2022, 10, 107744. [Google Scholar] [CrossRef]
- Leng, L.; Huang, H. An overview of the effect of pyrolysis process parameters on biochar stability. Bioresour. Technol. 2018, 270, 627–642. [Google Scholar] [CrossRef] [PubMed]
- Cross, A.; Sohi, S.P. A method for screening the relative long-term stability of biochar. Gcb Bioenergy 2013, 5, 215–220. [Google Scholar] [CrossRef]
- Zornoza, R.; Moreno-Barriga, F.; Acosta, J.A.; Muñoz, M.A.; Faz, A. Stability, nutrient availability and hydrophobicity of biochars derived from manure, crop residues, and municipal solid waste for their use as soil amendments. Chemosphere 2016, 144, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Yang, D.; Liu, W.; Dong, Y.; Zhang, L.; Lin, H. Insight into the impacts of pyrolysis time on adsorption behavior of Pb2+ and Cd2+ by Mg modified biochar: Performance and modification mechanism. Environ. Res. 2023, 239, 117215. [Google Scholar] [CrossRef]
- Shagali, A.A.; Hu, S.; Wang, Y.; Li, H.; Wang, Y.; Su, S.; Xiang, J. Comparative study on one-step pyrolysis activation of walnut shells to biochar at different heating rates. Energy Rep. 2021, 7, 388–396. [Google Scholar] [CrossRef]
- Angın, D. Effect of pyrolysis temperature and heating rate on biochar obtained from pyrolysis of safflower seed press cake. Bioresour. Technol. 2013, 128, 593–597. [Google Scholar] [CrossRef] [PubMed]
- Cai, G.; Ye, Z. Concentration-dependent adsorption behaviors and mechanisms for ammonium and phosphate removal by optimized Mg-impregnated biochar. J. Clean. Prod. 2022, 349, 131453. [Google Scholar] [CrossRef]
- Li, J.; Cao, L.; Li, B.; Huang, H.; Yu, W.; Sun, C.; Long, K.; Young, B. Utilization of activated sludge and shell wastes for the preparation of Ca-loaded biochar for phosphate removal and recovery. J. Clean. Prod. 2023, 382, 135395. [Google Scholar] [CrossRef]
- Haddad, K.; Jellali, S.; Jeguirim, M.; Ben Hassen Trabelsi, A.; Limousy, L. Investigations on phosphorus recovery from aqueous solutions by biochars derived from magnesium-pretreated cypress sawdust. J. Environ. Manag. 2018, 216, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Seo, E.; Lee, C.; Park, S. Fe-loaded biochar obtained from food waste for enhanced phosphate adsorption and its adsorption mechanism study via spectroscopic and experimental approach. J. Environ. Chem. Eng. 2021, 9, 105751. [Google Scholar] [CrossRef]
- Chen, Z.; Wu, Y.; Huang, Y.; Song, L.; Chen, H.; Zhu, S.; Tang, C. Enhanced adsorption of phosphate on orange peel-based biochar activated by Ca/Zn composite: Adsorption efficiency and mechanisms. Colloids Surf. A Physicochem. Eng. Asp. 2022, 651, 129728. [Google Scholar] [CrossRef]
- Wang, T.; Li, G.; Yang, K.; Zhang, X.; Wang, K.; Cai, J.; Zheng, J. Enhanced ammonium removal on biochar from a new forestry waste by ultrasonic activation: Characteristics, mechanisms and evaluation. Sci. Total Environ. 2021, 778, 146295. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Alam, M.S.; Konhauser, K.O.; Alessi, D.S.; Xu, S.; Tian, W.; Liu, Y. Influence of pyrolysis temperature on production of digested sludge biochar and its application for ammonium removal from municipal wastewater. J. Clean. Prod. 2019, 209, 927–936. [Google Scholar] [CrossRef]
- Wang, H.; Liu, R.; Chen, Q.; Mo, Y.; Zhang, Y. Biochar-supported starch/chitosan-stabilized nano-iron sulfide composites for the removal of lead ions and nitrogen from aqueous solutions. Bioresour. Technol. 2022, 347, 126700. [Google Scholar] [CrossRef]
- Liu, N.; Charrua, A.B.; Weng, C.; Yuan, X.; Ding, F. Characterization of biochars derived from agriculture wastes and their adsorptive removal of atrazine from aqueous solution: A comparative study. Bioresour. Technol. 2015, 198, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Chen, S.; Zheng, Q.; Huang, B.; Zhang, J.; Fu, H.; Gao, H. Removal and recovery of phosphorus from solution by bifunctional biochar. Inorg. Chem. Commun. 2022, 139, 109341. [Google Scholar] [CrossRef]
- Li, L.; Chen, Q.; Zhao, C.; Guo, B.; Xu, X.; Liu, T.; Zhao, L. A novel chitosan modified magnesium impregnated corn straw biochar for ammonium and phosphate removal from simulated livestock wastewater. Environ. Technol. Innov. 2022, 26, 102519. [Google Scholar] [CrossRef]
- Wang, T.; Zheng, J.; Liu, H.; Peng, Q.; Zhou, H.; Zhang, X. Adsorption characteristics and mechanisms of Pb2+ and Cd2+ by a new agricultural waste–Caragana korshinskii biomass derived biochar. Environ. Sci. Pollut. Res. 2021, 28, 13800–13818. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.; Chen, M.; Wu, P.; Zhang, X.; Wang, S.; Yu, Z.; Wang, B. Simultaneous reclaiming phosphate and ammonium from aqueous solutions by calcium alginate-biochar composite: Sorption performance and governing mechanisms. Chem. Eng. J. 2022, 429, 132166. [Google Scholar] [CrossRef]
- Wang, B.; Ma, Y.; Lee, X.; Wu, P.; Liu, F.; Zhang, X.; Chen, M. Environmental-friendly coal gangue-biochar composites reclaiming phosphate from water as a slow-release fertilizer. Sci. Total Environ. 2021, 758, 143664. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Ma, X.; Xiong, Q.; Zhou, X.; Wu, H.; Yan, S.; Zhang, Z. Functional biochar fabricated from red mud and walnut shell for phosphorus wastewater treatment: Role of minerals. Environ. Res. 2023, 232, 116348. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Zhang, X.; Ngo, H.H.; Guo, W.; Wen, H.; Li, C.; Ma, C. Comparison study on the ammonium adsorption of the biochars derived from different kinds of fruit peel. Sci. Total Environ. 2020, 707, 135544. [Google Scholar] [CrossRef]
- Liang, Y.; Li, F.; Li, Q. WITHDRAWN Study on the adsorption of phosphate by composite biochar of phosphogypsum and rape straw. Bioresour. Technol. Rep. 2023, 101536. [Google Scholar] [CrossRef]
- Antunes, E.; Vuppaladadiyam, A.K.; Kumar, R.; Vuppaladadiyam, V.S.S.; Sarmah, A.; Anwarul Islam, M.; Dada, T. A circular economy approach for phosphorus removal using algae biochar. Clean. Circ. Bioecon. 2022, 1, 100005. [Google Scholar] [CrossRef]
- Wu, R.; Zhai, X.; Dai, K.; Lian, J.; Cheng, L.; Wang, G.; Li, J.; Yang, C.; Yin, Z.; Li, H.; et al. Synthesis of acidified magnetic sludge-biochar and its role in ammonium nitrogen removal: Perception on Effect and mechanism. Sci. Total Environ. 2022, 832, 154780. [Google Scholar] [CrossRef]
- Carey, D.E.; McNamara, P.J.; Zitomer, D.H. Biochar from Pyrolysis of Biosolids for Nutrient Adsorption and Turfgrass Cultivation. Water Environ. Res. 2015, 87, 2098–2106. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Wang, J.J.; Zhou, B.; Awasthi, M.K.; Ali, A.; Zhang, Z.; Lahori, A.H.; Mahar, A. Recovery of phosphate from aqueous solution by magnesium oxide decorated magnetic biochar and its potential as phosphate-based fertilizer substitute. Bioresour. Technol. 2016, 215, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Rui, T.; You, Y.; Shen, D.; Liu, T. Magnetic biochar with Mg/La modification for highly effective phosphate adsorption and its potential application as an algaecide and fertilizer. Environ. Res. 2023, 231, 116252. [Google Scholar] [CrossRef] [PubMed]
- Wan, S.; Wang, S.; Li, Y.; Gao, B. Functionalizing biochar with Mg–Al and Mg–Fe layered double hydroxides for removal of phosphate from aqueous solutions. J. Ind. Eng. Chem. 2017, 47, 246–253. [Google Scholar] [CrossRef]
- Fan, Y.; Wang, H.; Deng, L.; Wang, Y.; Kang, D.; Li, C.; Chen, H. Enhanced adsorption of Pb(II) by nitrogen and phosphorus co-doped biochar derived from Camellia oleifera shells. Environ. Res. 2020, 191, 110030. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; He, F.; Shen, X.; Hu, D.; Huang, Q. Pyrolyzed fabrication of N/P co-doped biochars from (NH4)3PO4−pretreated coffee shells and appraisement for remedying aqueous Cr(VI) contaminants. Bioresour. Technol. 2020, 315, 123840. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Xie, Y.; Lu, S.; Li, P.; Xie, T.; Zhang, Y.; Wang, Y. One-step synthesis of nitrogen-doped sludge carbon as a bifunctional material for the adsorption and catalytic oxidation of organic pollutants. Sci. Total Environ. 2019, 680, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Ding, D.; Yang, S.; Qian, X.; Chen, L.; Cai, T. Nitrogen-doping positively whilst sulfur-doping negatively affect the catalytic activity of biochar for the degradation of organic contaminant. Appl. Catal. B Environ. 2020, 263, 118348. [Google Scholar] [CrossRef]
- Jin, Z.; Wang, B.; Ma, L.; Fu, P.; Xie, L.; Jiang, X.; Jiang, W. Air pre-oxidation induced high yield N-doped porous biochar for improving toluene adsorption. Chem. Eng. J. 2020, 385, 123843. [Google Scholar] [CrossRef]
- Shen, Y.; Zhang, N. A facile synthesis of nitrogen-doped porous carbons from lignocellulose and protein wastes for VOCs sorption. Environ. Res. 2020, 189, 109956. [Google Scholar] [CrossRef] [PubMed]
- Lian, F.; Cui, G.; Liu, Z.; Duo, L.; Zhang, G.; Xing, B. One-step synthesis of a novel N-doped microporous biochar derived from crop straws with high dye adsorption capacity. J. Environ. Manag. 2016, 176, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; Sun, H.; Wang, S. Metal-Free Carbocatalysis in Advanced Oxidation Reactions. Acc. Chem. Res. 2018, 51, 678–687. [Google Scholar] [CrossRef] [PubMed]


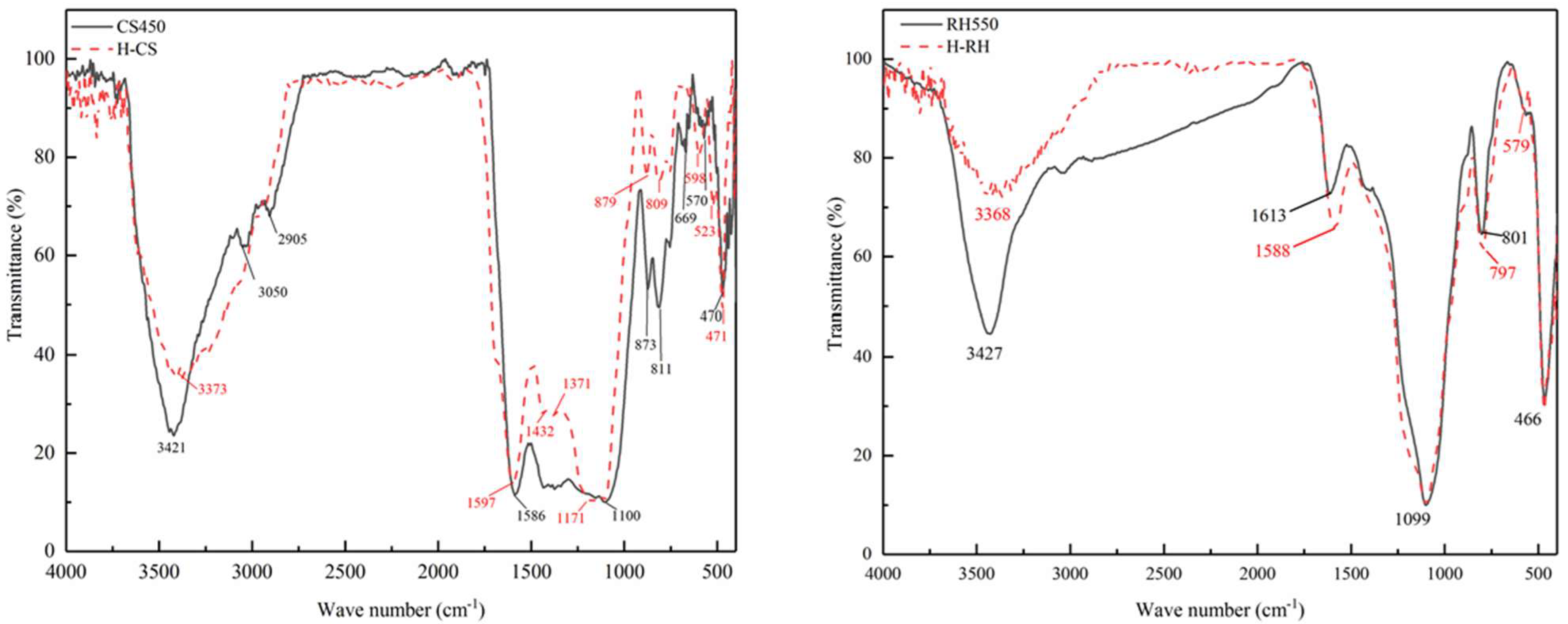


| Raw Material | Adding Reagents or Biomass | Pyrolysis Temperature (°C) | Pyrolysis Time (h) | Name | Specific Surface Area (m2/g) | Pore Volume (cm3/g) | Model Fitting | Phosphorus Adsorption Capacity (mg/g) | Cite |
|---|---|---|---|---|---|---|---|---|---|
| Corn stalk | FeCl3·6H2O, Mg(OH)2 | 500 | 2 | LDH@BCL | 238.71 | 0.154 | Thomas | 105.73 | [49] |
| Pig manure | MgO | 550 | 2 | MgO-PMBC | 144.431 | 0.197 | Langmuir | 122.01 | [50] |
| Corn stalk | Sodium alginate, MgCl2·6H2O, CaCl2 | Ca/MgBC | Langmuir–Freundlich | 177.25 | [9] | ||||
| Sludge | 6H2O, Mg(OH)2 | 500 | 2 | MgFe-LDH@BC | 51.4556 | 0.0463 | Pseudo-second-order | 85.758 | [51] |
| Panda manure | Fenton’s reagent, H2O2, KMnO4 | 400 | FSC-400 | 37.61 | 0.13 | 221.7 | [52] | ||
| Oak sawdust | ZnCl2, NaBH4, Carboxymethyl cellulose | 600 | 2 | nZVZ-CMC-PMBC | 72.9647 | 0.0494 | Langmuir | 45.71 | [53] |
| Oak sawdust | LaCl3·7H2O | 500 | 0.5 | La-500 | 10.39 ± 0.12 | Langmuir | 32.0 | [54] | |
| Peanut shell | 400 | 2 | C-BC400 | Langmuir | 25.4560 | [55] |
| Raw Material | Adding Reagents or Biomass | Pyrolysis Temperature (°C) | Pyrolysis Time (h) | Name | Specific Surface Area (m2/g) | Pore Volume (cm3/g) | Isotherm | Phosphorus Adsorption Capacity (mg/g) | Cite |
|---|---|---|---|---|---|---|---|---|---|
| Coffee grounds | KOH, MgCl2, NaOH | 500 | 2 | MgSCG-500 | 107.3 | Langmuir–Freundlich | 112.20 | [64] | |
| Sheep manure | Tested oyster | 800 | 2 | BC-Ca5 | 5.09 | 0.04 | Langmuir | 79.33 | [65] |
| Sheep manure, Tested oyster shells | LaCl3·7H2O | 800 | 2 | BC-La4 | 12.04 | 0.06 | Langmuir | 92.67 | [65] |
| Yellow Pine wood | Ca(OH)2 | 100 | 2 | Ca-BC100 | 120.26 | Langmuir | 125.60 | [66] | |
| Reed straw | FeSO4·7H2O, NaBH4 | 700 | 2 | Fe-700-BC | 53.25 | Langmuir | 95.20 | [67] | |
| Wheat straw | LaCl3·7H2O, Na2CO3 | 300 | 2 | LCB300 | 64.30 | [68] | |||
| Wheat straw | LaCl3·7H2O, NaOH | 800 | 2 | LHB800 | 65.00 | [68] | |||
| Corn cob | FeCl3·6H2O, DETA | 900 | 1 | ZVI/BC-N | 379.63 | 0.31 | Freundlich | 82.78 | [69] |
| Corn cob | FeCl3·6H2O | 900 | 1 | ZVI/BC | 215.33 | 0.14 | Langmuir | 17.93 | [69] |
| Banana straw | MgCl2 | 430 | 4 | BSB | 6.6839 | 0.0267 | Langmuir | 31.15 | [70] |
| Corn stover | CaCl2 | 800 | 1 | CaBC800 | 0.02383 | 0.1326 | Langmuir | 33.944 | [71] |
| Bagasse powder | Marble waste | 800 | 2 | Mar-BC800 | 92.81 | 265,000 | Langmuir | 263.17 | [72] |
| Bagasse powder | Calcium-rich sepiolite | 800 | 2 | Sep-BC800 | 106.67 | 397,000 | Langmuir | 128.21 | [72] |
| Canna | La(NO3)3·6H2O | 800 | 1 | CBC-La | Langmuir | 37.37 | [73] | ||
| Canna | 800 | 1 | CBC | 193.1461 | 0.1228 | Langmuir | 9.47 | [73] |
| Raw Material | Modification Reagent | Pyrolysis Temperature (°C) | Pyrolysis Time (h) | Name | Specific Surface Area (m2/g) | Total Pore Volume (cm3/g) | Model Fitting | Nitrogen Adsorption Capacity (mg/g) | Cite |
|---|---|---|---|---|---|---|---|---|---|
| Walnut shell | 600 | 2 | BC | 285.4958 | 0.1156 | Sips | 60.82 | [47] | |
| Walnut shell | C2H4N4 | 600 | 2 | N@BC | 456.2524 | 0.3425 | Sips | 73.51 | [47] |
| Walnut shell | FeCl3·6H2O | 600 | 2 | Fe@BC | 536.4587 | 0.3625 | Sips | 83.42 | [47] |
| Walnut shell | C2H4N4, FeCl3·6H2O | 600 | 2 | Fe/N@BC | 967.1084 | 0.7425 | Sips | 111.87 | [47] |
| Maize straw | 450 | 2 | BC | 162.50 | 0.20 | Langmuir | 10.379 | [48] | |
| Maize straw | MgCl2 | 450 | 2 | MBC | 152.00 | 0.31 | Langmuir | 18.335 | [48] |
| Oak sawdust | 500 | 0.5 | CK-300 | 0.050 ± 0.02 | Langmuir | 5.31 | [54] | ||
| Oak sawdust | LaCl3·7H2O | 500 | 0.5 | La-300 | 1.57 ± 0.12 | Langmuir | 10.1 | [54] | |
| Peanut shell | 500 | 2 | BC | Sips | 3.83 | [95] | |||
| Peanut shell | KMnO4, KOH | 500 | 2 | MBC | Sips | 6.92 | [95] |
| Raw Material | Modification Reagent | Pyrolysis Temperature (°C) | Pyrolysis Time (h) | Name | Specific Surface Area (m2/g) | Total Pore Volume (cm3/g) | Isotherm | Phosphorus Adsorption Capacity (mg/g) | Cite |
|---|---|---|---|---|---|---|---|---|---|
| Oak sawdust | 500 | 0.5 | CK-500 | 7.72 ± 0.19 | Langmuir | 142.7 | [54] | ||
| Oak sawdust | LaCl3·7H2O | 500 | 0.5 | La-500 | 10.39 ± 0.12 | Langmuir | 32.0 | [54] | |
| Yellow Pine wood | 500 | 2 | BC | 260.50 | 4.00 | [66] | |||
| Yellow Pine wood | Ca(OH)2 | 500 | 2 | Ca-BC100 | 120.26 | 138.70 | [66] | ||
| Canna | 800 | 1 | CBC | 193.1461 | 0.1228 | Langmuir | 9.47 | [73] | |
| Canna | La(OH)3 | 800 | 1 | CBC-La | Langmuir | 37.37 | [73] | ||
| Mimosa pudica | 500 | 2 | BC | 285.53 | 0.15 | 5.1 | [84] | ||
| Mimosa pudica | AlCl3·6H2O | 500 | 2 | BAl1 | 130.48 | 0.12 | 65.6 | [84] | |
| Mimosa pudica | AlCl3·6H2O | 500 | 2 | BAl2 | 255.85 | 0.28 | 70.6 | [84] | |
| Wheat straw | 600 | 2 | BC | 227.12 | Langmuir | 1.64 | [96] | ||
| Wheat straw | MgCl2, AlCl3 | 600 | 2 | MABC | 268.50 | Langmuir | 153.40 | [96] | |
| Flour | 600 | 2 | BC | Langmuir | 48.44 | [97] | |||
| Flour | Ca(OH)2 | 600 | 2 | Ca-BC (2:1) | Langmuir | 314.22 | [97] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, X.; Quan, W.; Chen, Q.; Gong, W.; Wang, A. Efficient Adsorption of Nitrogen and Phosphorus in Wastewater by Biochar. Molecules 2024, 29, 1005. https://doi.org/10.3390/molecules29051005
Wu X, Quan W, Chen Q, Gong W, Wang A. Efficient Adsorption of Nitrogen and Phosphorus in Wastewater by Biochar. Molecules. 2024; 29(5):1005. https://doi.org/10.3390/molecules29051005
Chicago/Turabian StyleWu, Xichang, Wenxuan Quan, Qi Chen, Wei Gong, and Anping Wang. 2024. "Efficient Adsorption of Nitrogen and Phosphorus in Wastewater by Biochar" Molecules 29, no. 5: 1005. https://doi.org/10.3390/molecules29051005
APA StyleWu, X., Quan, W., Chen, Q., Gong, W., & Wang, A. (2024). Efficient Adsorption of Nitrogen and Phosphorus in Wastewater by Biochar. Molecules, 29(5), 1005. https://doi.org/10.3390/molecules29051005







