3.1. Characterization and Screening of Supports for Nickel-Based Catalysts
Nickel-based catalysts are efficient for degradation of biomass in SCWG. Nickel promotes the reforming and water–gas shift reactions to enhance hydrogen yield. However, using only nickel metal catalysts for SCWG resulted in a marginal improvement of 9.4% from 8.1 mmol/g of non-catalytic run. This is due to low availability of metal surface area due to low Ni dispersion, as the activity of catalysts is proportional to the available surface area of active metal. Therefore, catalyst supports are used to enhance the dispersion of nickel metal to significantly increase the active metal surface area, which results in improved performance of the catalyst for the same amount of active metal loading. Furthermore, catalyst support also plays a key role in the activity, stability, and durability of the metal catalysts, especially for the SCWG reaction, which employs severe reaction conditions. Therefore, in this study, six different supports, namely zirconium dioxide (ZrO2), carbon nanotubes (CNTs), activated carbon (AC), aluminum oxide (Al2O3), hydrothermal liquefaction-hydrochar (HTL-HC), and hydrothermal carbonization-hydrochar (HTC-HC), were synthesized for nickel-based catalysts. The physical properties and activity of these supported catalysts for SCWG were evaluated for the selection of the most suitable support for further modification.
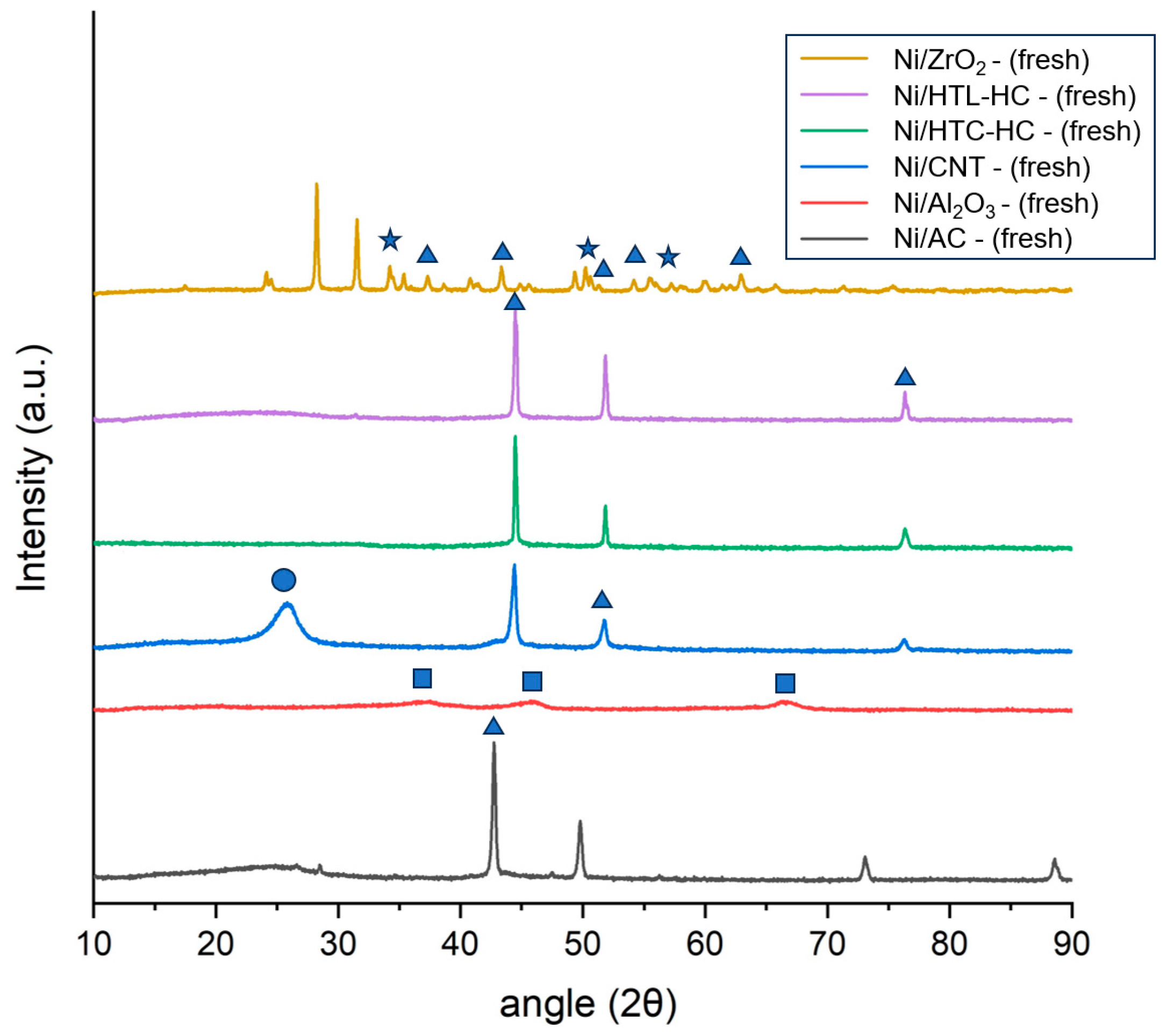
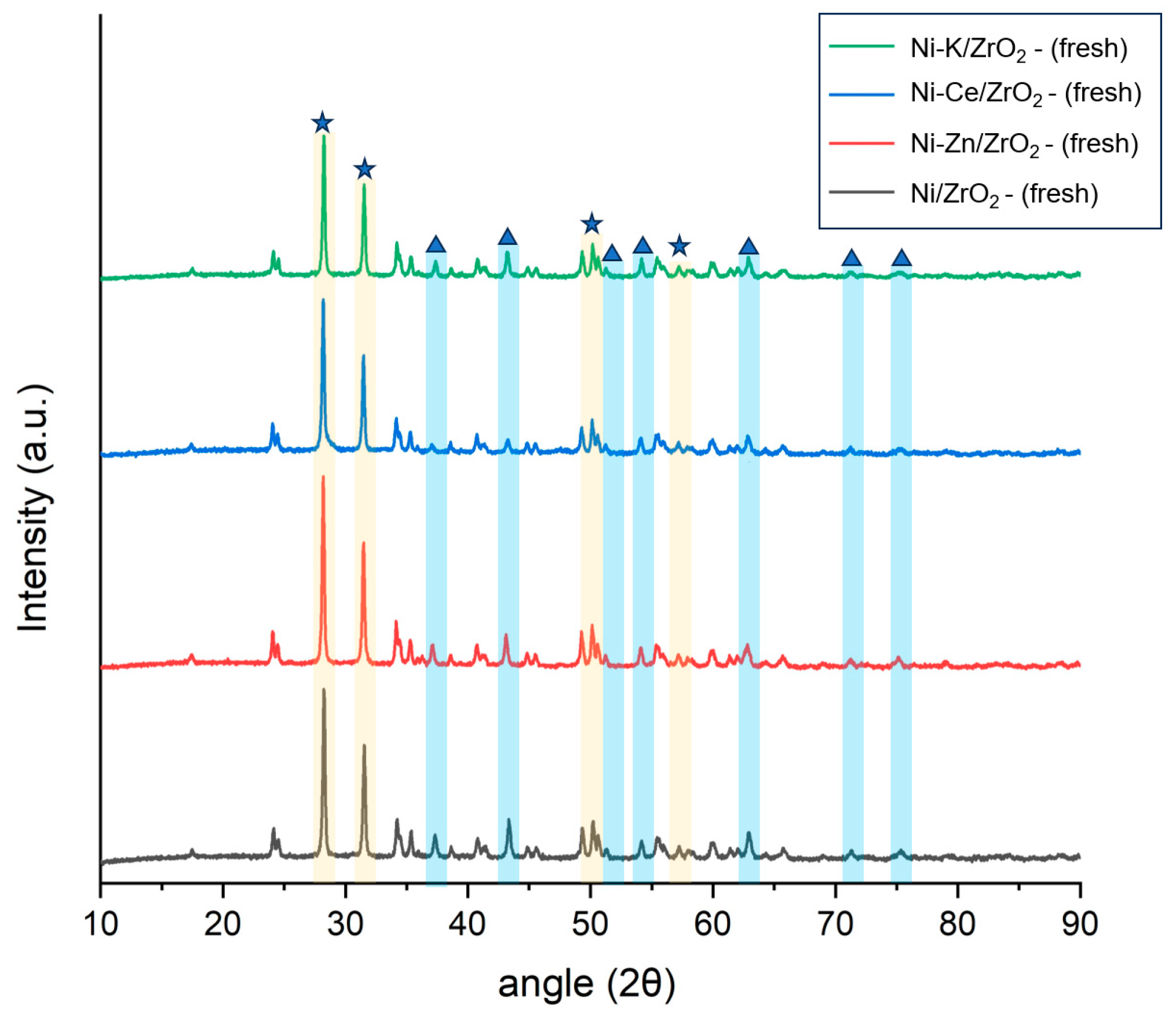
Crystallographic phases of fresh catalysts were studied using powdered X-ray diffraction analysis (XRD).
Figure 1 represents the XRD pattern of the fresh Ni catalysts supported on AC, CNT, HTC-HC, HTL-HC, Al
2O
3, and ZrO
2. It can be identified that nickel in all catalysts was present in the oxide form and identified by the peaks of NiO observed at the 2θ angle of 37°, 44°, 52°, 62.5°, 67°, and 76° [
28,
34]. Small shifts in catalysts can be assigned to the difference in the metal–support interactions of catalysts. For Ni/Al
2O
3, a broad peak was observed at the 2θ angle of 36°, 46°, and 67° in the XRD pattern, representing the crystalline phase of Al
2O
3. The XRD pattern of Ni/ZrO
2 showed a distinct crystalline structure with its sharp peaks. Peaks observed at the 2θ angle of 28°, 31°, 34°, and 56° represent the (111), (111), (020), and (130) planes of monoclinic ZrO
2. However, other peaks observed at the 2θ angle of 30°, 35°, 51°, and 60° represent the (101), (110), (200), and (211) planes of tetragonal ZrO
2. This indicates that the ZrO
2 present in the catalyst support was a mixture of both tetragonal and monoclinic ZrO
2. However, it can be identified that the intensity of planes of monoclinic ZrO
2 was higher than the intensity of planes of tetragonal ZrO
2. This represents the fact that the proportion of the monoclinic phase of ZrO
2 was higher in catalyst support than the tetragonal ZrO
2. For CNT catalysts, a distinct peak observed at 2θ of 25° represents the crystalline carbon peak of the CNT. Among all the catalysts, the intensity of the NiO peak at 2θ of 44° was the minimum for ZrO
2- and Al
2O
3-supported catalysts. This represents the higher dispersion of Ni on these catalyst supports, whereas the intensity of NiO peaks was highest in both Ni/AC and Ni/CNT catalysts. Additionally, in all carbon supports, a distinct peak of NiO at 2θ of 76° was visible as compared to other non-carbon supports. The intensity of this peak was highest in Ni/CNT, closely followed by Ni/AC. This corresponds to the larger particle size of nickel in both catalysts. This also explains the relatively superior performance of the ZrO
2 catalyst and poor performance of Ni/CNT and Ni/AC.
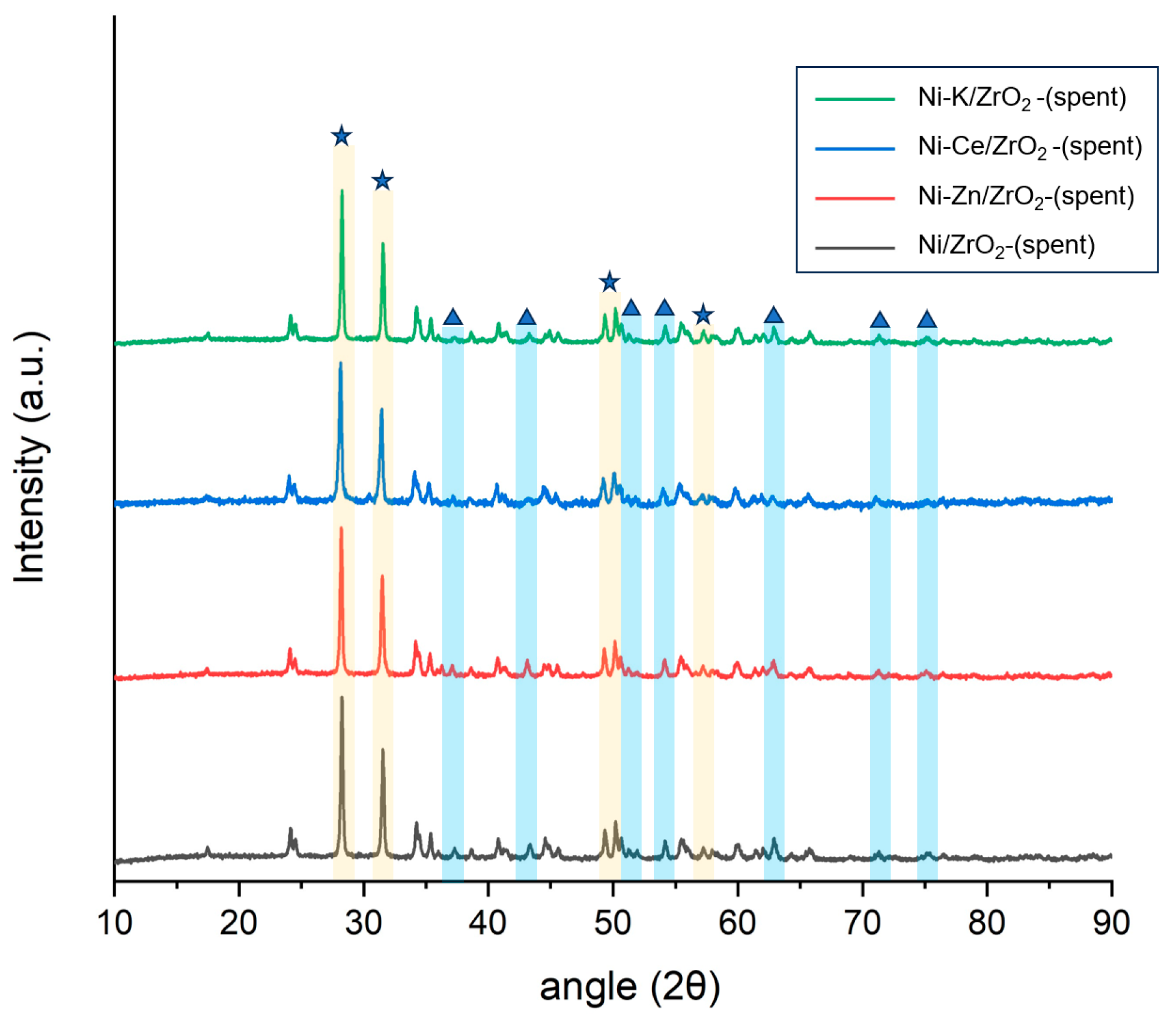
In XRD diffraction analysis of spent Ni-supported catalysts in SCWG of canola after they were used for gasification of canola straw in SCW, a broad peak in the region of 13–26° was observed (
Figure 2). This peak was not identified in the fresh catalysts, and it represents the amorphous carbon possibly originating through coke formation in the spent catalyst. In all spent catalysts, a significant rise in NiO peaks was observed compared with those of the fresh catalyst. This shows that during the gasification of the canola straw in SCW, the catalyst suffers from the growth in the particle size of Ni particles. Among all spent catalysts, the intensity of the NiO peak for the Ni/CNT catalyst compared to the fresh catalyst increased quite significantly, followed by the Ni/AC catalyst. This represents the fact that the growth in Ni particles on the CNT surface was higher during SCWG than for other catalysts, which significantly reduced the activity of the catalysts in gasification. For the Ni/ZrO
2 catalyst, however, the increase in the intensity of the NiO peak compared to the fresh catalyst was small. This shows that the Ni particles were still dispersed at the ZrO
2 support surface and did not agglomerate, thus retaining higher catalytic activity. A probable reason for this behavior might be the difference in metal–support interaction in various nickel-supported catalysts, which can affect the growth of NiO particles during the gasification reaction in SCW.
Brunauer–Emmett–Teller (BET) analysis of fresh catalysts was conducted to evaluate the surface properties of supported nickel catalysts, and results are presented in
Table 1. The BET surface area of 590 m
2/g of Ni/AC was the highest, followed by 291 m
2/g of Ni/CNT, 280 m
2/g of Ni/Al
2O
3, 59 m
2/g of Ni/HTL-HC, 45 m
2/g of Ni/HTC-HC, and 6 m
2/g of Ni/ZrO
2. Meanwhile, the total pore volume of (0.93 cm
3/g) Ni/CNT was the highest. This is due to the use of multi-walled CNT as a support in the synthesis of Ni/CNT. The order of total pore volume was (0.93 cm
3/g) Ni/CNT > (0.68 cm
3/g) Ni/AC > (0.58 cm
3/g) Ni/Al
2O
3 > (0.07 cm
3/g) Ni/HTL-HC > (0.03 cm
3/g) Ni/HTC-HC > (0.02 m
2/g) Ni/ZrO
2. However, the pore size of (12.8 nm) Ni/ZrO
2 was very high and comparable to the pore size of 17.1 nm of Ni/CNT. BET analysis of spent catalysts post SCWG of canola straw was also conducted to study the changes in surface properties of supported nickel catalysts during gasification in SCW.
From
Table 1, it can also be identified that for all spent catalysts, the BET surface area decreased compared to the BET surface area of fresh catalysts. Similarly, the pore volume of spent catalysts was lower than that of the fresh counterpart. This can be explained by the fact that during gasification in SCW, pores of catalysts start to clog as the size of nickel crystals starts to grow in catalysts due to sintering, as indicated by an increase in the intensity of NiO peaks in XRD analysis of spent catalysts as compared to their fresh counterparts. This leads to lower pore volume and lower BET surface area of the spent catalysts. The order of the BET surface areas of spent catalysts followed a similar trend to the BET surface areas of fresh catalysts. The extent of drop in the BET surface area of spent Ni/AC catalysts as compared to fresh Ni/AC catalysts was very drastic and highest compared to other catalysts. This was due to severe coking in the Ni/AC catalyst which decreased the surface area of the spent catalyst. This was also accompanied by its drastic drop in pore volume. Change in the BET surface area and pore volume of spent catalysts compared to fresh catalysts was lowest in the case of Ni/ZrO
2 catalysts. This highlights the excellent structural stability of the Ni/ZrO
2 catalyst during the gasification of canola straw in SCW.
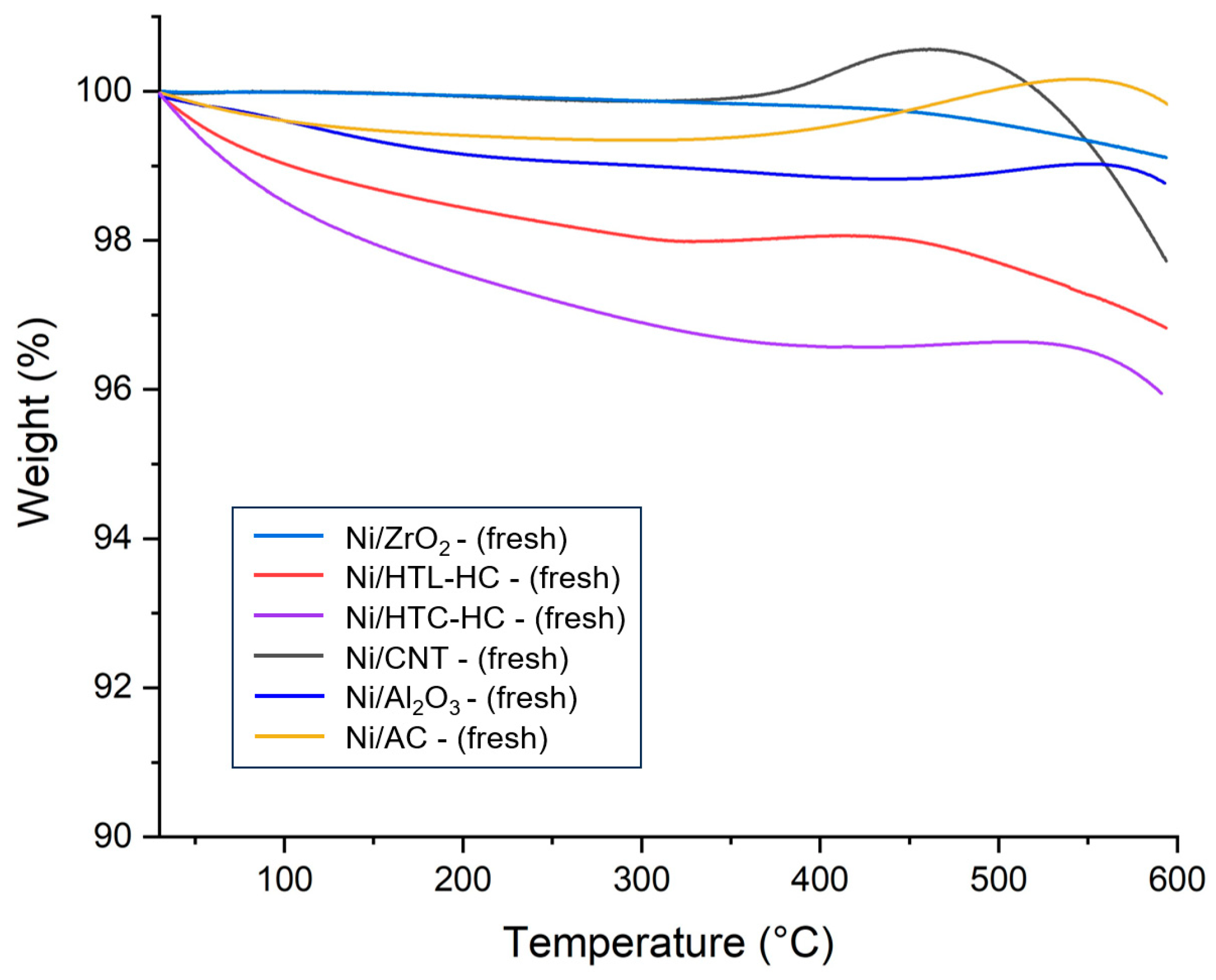
The catalytic stability is a key parameter for selecting a suitable catalyst for the SCWG reaction. To determine the stability of supported nickel catalysts, thermogravimetric analysis (TGA) of fresh supported nickel catalysts was performed.
Figure 3 represents the TGA curves for all fresh catalysts. From
Figure 3, it can be observed that all catalysts suffered a small amount of weight loss up to 200 °C. This can be attributed to the desorption and removal of any adsorbed gases and moisture from the environment. Interestingly, the weight of catalysts seems to start increasing at temperatures near 450–600 °C. This weight gain can be assigned to the oxidation of nickel metal present in the catalysts. Weight gain in Ni/ZrO
2 was lowest in this range, indicating that the Ni/ZrO
2 has strong metal–support interaction, preventing the oxidation of metallic nickel.
In SCWG, catalyst deactivation could be attributed to active metal sintering, phase transformation of support, and coking. Deactivation of nickel catalysts occurs primarily due to coke deposition. Coke deposition tendency of catalysts reduces its activity in reaction and impacts its reusability. Catalysts with less coke deposition usually have better activity and stability in the reaction. The TGA of spent catalysts also represents the coke deposition on the surface of the catalyst. To evaluate the coke deposition behavior of Ni-based catalysts, TGA analysis of spent catalysts recovered from SCWG of canola straw was performed, and mass loss was observed.
Figure 4 represents the TGA curves of spent catalysts used in the gasification of canola straw in SCW. From
Figure 4, it can be seen that in all catalysts, a mass loss was majorly observed in two regions. In the first region, up to a temperature of 200 °C, weight loss could be assigned to the desorption of moisture on the catalytic surface [
29]. In the second region, beyond 350 °C, major mass loss was ascribed to the oxidation of deposited coke on the catalysts’ surface [
29]. In spent catalysts, the Ni/ZrO
2 catalyst demonstrated the least amount of mass loss, followed by the Ni/Al
2O
3 catalyst. Interestingly, all carbon supports suffered a drastic mass loss beyond 550 °C except the Ni/CNT catalyst. This was due to the oxidation of carbon supports resulting in the loss of catalyst mass.
Results of catalytic gasification of canola straw with nickel catalysts supported on AC, CNT, HTL-HC, HTC-HC, ZrO
2, and Al
2O
3 at 500 °C, 10 wt%, and 1 h are presented in
Table 2. It can be identified that the Ni/Al
2O
3 catalyst showed the highest total gas yield of 35.3 mmol/g, followed by 33.0 mmol/g for Ni/ZrO
2. The higher gas yield of Ni/Al
2O
3 can be attributed to its ability to enhance the reforming and hydrolysis of canola straw, which promoted the gasification of canola straw for the production of gaseous products. In SCWG, usually, for liquid feedstock, catalysts with higher surface areas show better catalytic activity as compared to catalysts with lower surface areas. However, despite having a high surface area and the highest pore volume, the gas yield obtained with the use of the Ni/CNT catalyst was the lowest at 27.1 mmol/g, which was even lower than the total gas yield obtained in the non-catalytic run. Similarly, the Ni/AC catalyst also had the highest surface area and very high pore volume, but it too performed poorly, with a total gas yield of 30.9 mmol/g. A possible reason for the poor performance of Ni/AC with a higher surface area compared to Ni/Al
2O
3 can be assigned to coke deposition identified via BET surface area, as well as lower Ni dispersion and metal sintering during the SCWG reaction, as indicated by XRD analysis. Among all the carbon-based nickel-supported catalysts, Ni/HTC-HC had the highest total gas yield of 32 mmol/g. This shows that even though higher surface area catalysts are beneficial for SCWG of lignocellulosic biomass, other factors such as metal dispersion and catalyst deactivation via coking and sintering play an important role in deciding the catalytic activity of catalysts during gasification in SCW.
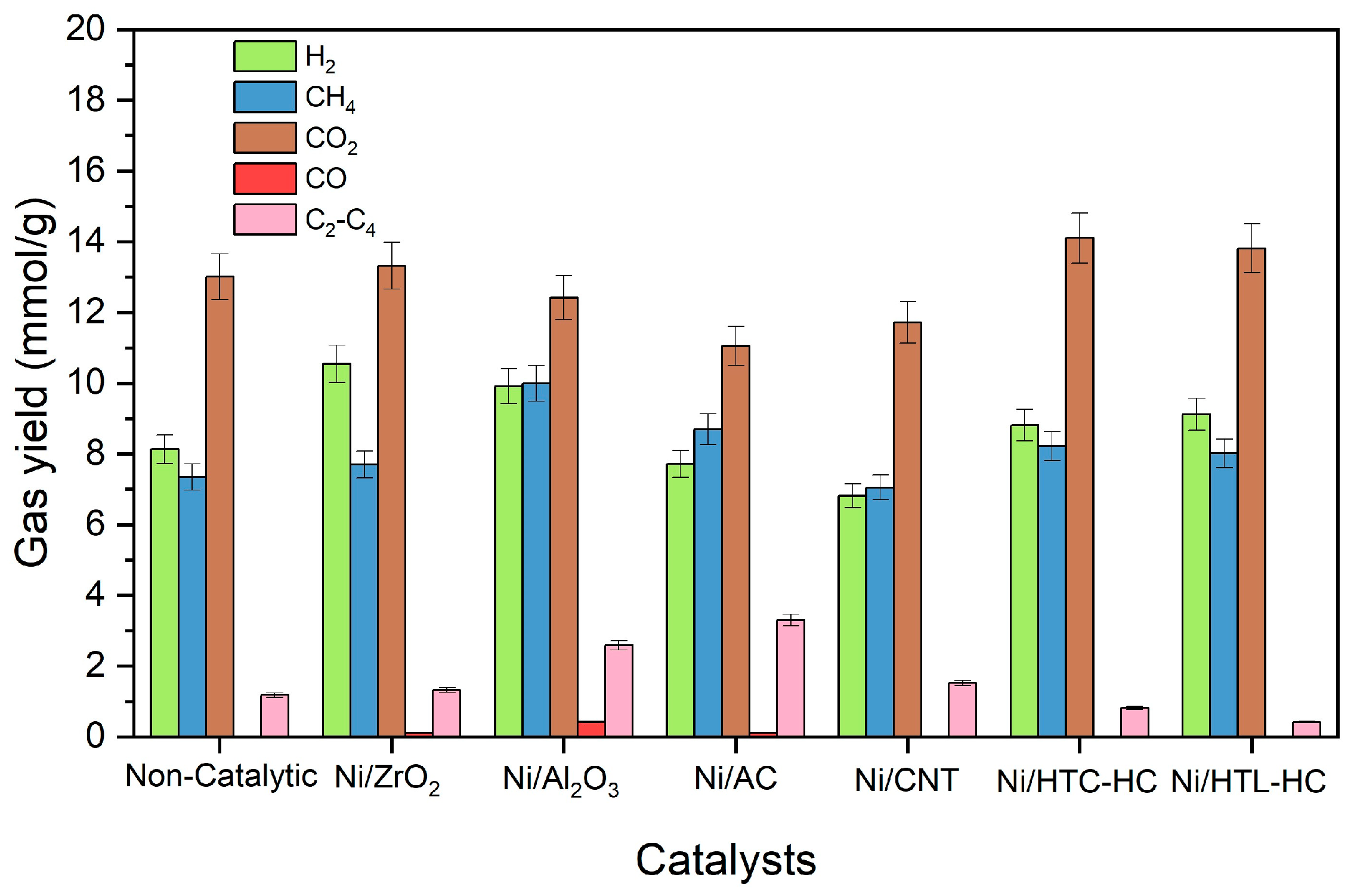
Individual gas yields of SCWG of canola straw with supported nickel catalysts are presented in
Figure 5. It can be observed that the Ni/ZrO
2 catalyst showed the highest hydrogen yield of 10.5 mmol/g. This is due to the basic nature of Ni/ZrO
2, which promotes reforming and water–gas shift reactions and enhances the hydrogen yield. Kou et al. [
35] also observed an increase in hydrogen yield with the use of a Ni/ZrO
2 catalyst for SCWG of oil-containing wastewater. They reported approximately 360% rise in hydrogen yield with the use of the Ni/ZrO
2 catalyst compared to a non-catalytic run. They concluded that the Ni/ZrO
2 favored the reforming and water–gas shift reaction, which facilitated the cleavage of C=C bonds, resulting in a high yield of hydrogen and gaseous products. Statistical significance of the results was analyzed using ANOVA to measure any statistically significant differences between the means of the gas yields. Unequal variance
t-tests (Welch’s
t-tests) were used to compare the difference in mean hydrogen yield from the catalytic run with those from the non-catalytic run. ANOVA analysis confirmed the difference in the means of all gas yields with very high significance (
p-values < 0.05) and with high values of F-Statistics (25.5–365.9). Furthermore, a
t-test showed a significant difference in hydrogen yield of catalysts compared to the non-catalytic run, with high significance. It also showed that the mean hydrogen yield using Ni/AC and Ni/CNT was lower compared to that from the non-catalytic run.
Interestingly, despite showing the highest total gas yield, the Ni/Al
2O
3 catalyst showed a relatively lower hydrogen yield. This might be due to the acidic nature of Ni/Al
2O
3 catalysts compared to Ni/ZrO
2 [
32]. The acidic nature of Ni/Al
2O
3 retards the water–gas shift reaction while enhancing the methanation reaction [
22]. This limits the formation of hydrogen, and the produced hydrogen is also consumed for the production of methane via the methanation reaction. This was also made evident by the higher methane and CO yield and lower CO
2 yield of the Ni/Al
2O
3 catalyst. Additionally, the Ni/ZrO
2 catalyst was more stable compared to Ni/Al
2O
3, as demonstrated by the TGA analysis. The lowest hydrogen yield was demonstrated by Ni/CNT and Ni/AC catalysts with hydrogen yields of 6.8 mmol/g and 7.7 mmol/g, respectively. Poor hydrogen yield of Ni/CNT and Ni/AC catalysts can be attributed to diminishing reforming and WGS reactions while favoring other side reactions, such as methanation and hydrogenation reactions. These reactions increase methane yield while decreasing the hydrogen yield.
Overall, the Ni/ZrO2 catalyst demonstrated high activity for the gasification of canola straw in SCW, with the highest hydrogen yield and hydrogen selectivity. Due to its superior performance and stability among all supported catalysts, Ni/ZrO2 was further modified with the promotors and evaluated for SCWG of canola straw.
3.2. SCWG Gas Yields of Promoted Ni/ZrO2 Catalysts
Due to the promising results of Ni/ZrO
2, it was modified with the addition of potassium (K), zinc (Zn), and cerium (Ce) promotor. Promotors are used to improve the hydrogen yield and selectivity while also providing increased stability to the catalyst. Potassium was selected because it is an alkali earth metal that enhances the WGS reaction to improve the hydrogen yield. Tavasoli et al. [
33] used potassium as a promotor and witnessed its promoting effect in SCWG of sugarcane bagasse with Cu supported on Al
2O
3 catalysts. Zn promotors have demonstrated a high effectivity in decreasing methane and hydrogen-consuming reactions [
36]. Zn blocks the adsorption of H
2 and CO on the active sites of the catalyst [
37]. For Ni-based catalysts, Ce proved to be an effective promotor in methane reforming processes [
38] and for SCWG of glucose [
39].
Promoted Ni/ZrO
2 catalysts were utilized in catalytic SCWG of canola straw at reaction conditions of 500 °C, 10 wt%, 23–25 MPa, and 60 min to evaluate the comparative performance of the promotors for Ni/ZrO
2 catalysts. Results of individual gas yield, total gas yield, H
2 selectivity, and LHV are presented in
Table 2 and
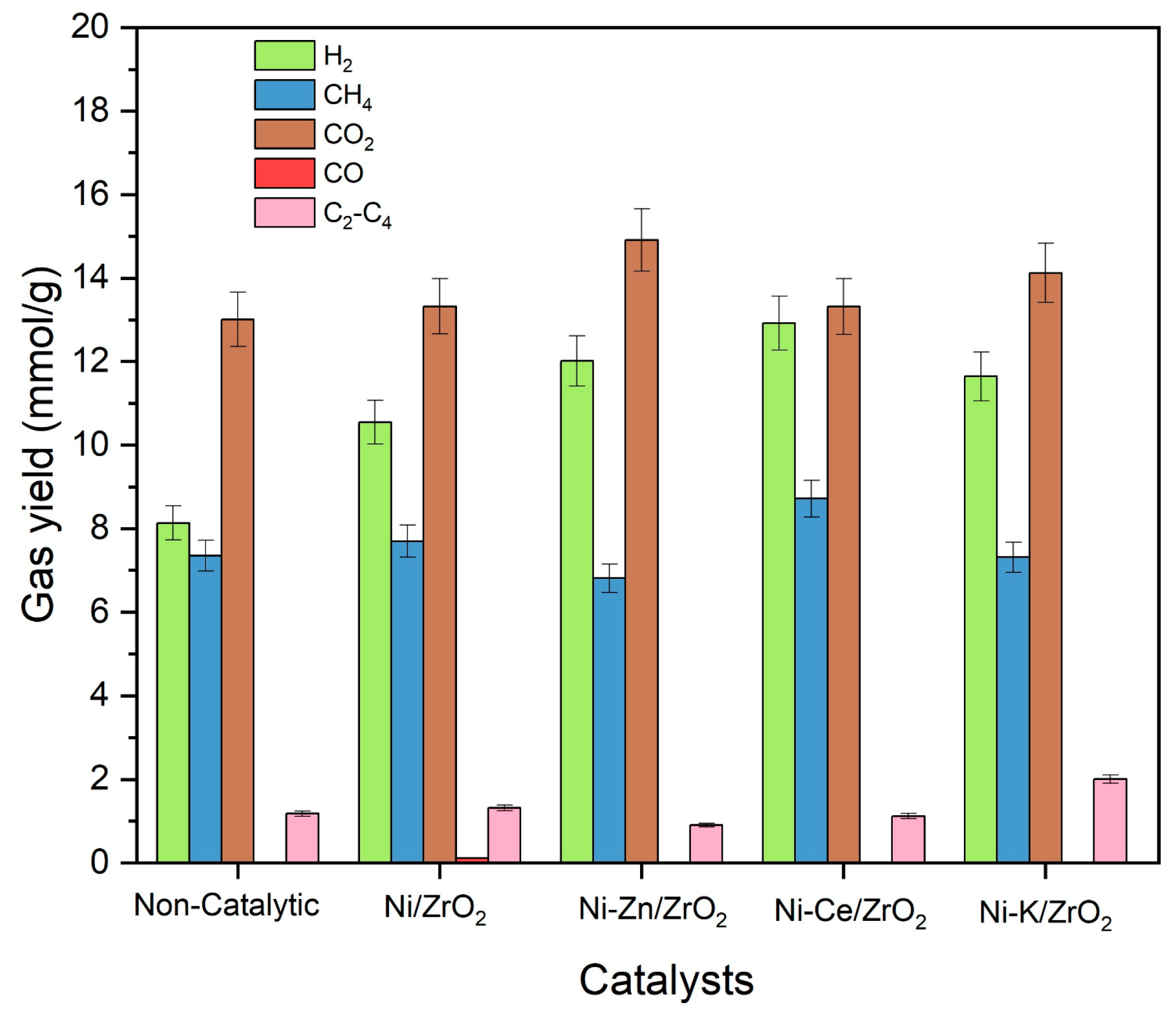
Figure 6. It can be observed from
Table 2 that the total gas yield improved from 29.7 in a non-catalytic run to 33.0 mmol/g with the addition of Ni/ZrO
2. However, the addition of promotors significantly increased the total gas yield to 36.1 mmol/g with the use of Ni-Ce/ZrO
2, followed by (35.1 mmol/g) Ni-K/ZrO
2 and (34.7 mmol/g) Ni-Zn/ZrO
2. The addition of Ni-Ce/ZrO
2 also increased the LHV of gaseous products to 5243 kJ/Nm
3, compared to 4761 kJ/Nm
3 of the unpromoted Ni/ZrO
2 catalyst and 4271 kJ/Nm
3 of the non-catalytic run. Similarly, hydrogen yields in the non-catalytic run and with the unpromoted Ni/ZrO
2 catalyst were 8.1 mmol/g and 10.5 mmol/g, respectively (
Figure 6). Addition of promotors significantly further increased the hydrogen yield to 12.9 mmol/g with Ni-Ce/ZrO
2, followed by 12.0 mmol/g with Ni-Zn/ZrO
2 and 11.6 mmol/g with Ni-K/ZrO
2 catalysts. ANOVA analysis confirmed the difference in the means of all gas yields for different promoted catalysts with high F-statistics (13.3–142.7) and high significance, and Welch’s
t-tests showed an increase in the means of hydrogen yields with use of promotors compared to those using the unpromoted Ni/ZrO
2 catalyst, with high significance.
Canola straw is a lignocellulosic biomass composed of complex structures of cellulose, hemicellulose, and lignin. The presence of lignin in biomass limits the degradation of biomass during gasification in SCW. Lignin also influences the decomposition of cellulose and hemicellulose, resulting in an overall lower yield of gaseous compounds. This results in lower total gas yield and subsequently lower hydrogen yield in non-catalytic runs. Since lignin has ester bonds joining its constituting phenyl propane molecules [
40], this makes the hydrolysis of lignin in SCW difficult. The addition of promoted Ni-based catalysts enhances the hydrolysis and reforming of lignin by facilitating the cleavage of ester bonds and ring-opening reactions. This leads to improved overall gasification of lignocellulosic biomass, as well as higher total gas yield and hydrogen yield with the use of promoted nickel-based catalysts.
Figure 6 represents the individual gas yields of canola straw in the presence of promoted Ni-based catalysts. The nickel-based catalysts improved the yield of hydrogen, CO
2 and CH
4. The addition of an alkali metal promotor to the Ni/ZrO
2 catalyst improved the hydrogen yield at the expense of the CO yield compared to the unpromoted Ni/ZrO
2 catalysts. This is due to the catalytic action of alkali metal in promoting the water–gas shift reaction by forming a formate intermediate compound increasing the conversion of CO for the production of hydrogen. This limits the methanation of CO, which reduces methane yield, resulting in a higher yield of hydrogen and a lower yield of methane.
The addition of Ce promotors to the Ni/ZrO2 catalyst significantly enhanced the yield of hydrogen and CH4 gases while reducing the yield of CO compared to the unprompted Ni/ZrO2 catalytic run. High yields with Ni-Ce/ZrO2 can be assigned to its ability to promote the reforming reactions to enhance the gasification of canola straw in SCW. This also led to the highest LHV of 5243 kJ/Nm3 of gaseous products obtained with the use of Ce-promoted Ni/ZrO2 catalyst. Additionally, the Ce promotor facilitates the oxidation of the coke and char, which minimizes the coke deposition on the catalyst’s surface. This results in high stability and activity of Ce-promoted Ni/ZrO2 catalysts, resulting in a high yield of gaseous products.
Interestingly, the addition of Zn promotor enhanced the hydrogen and CO2 gas yields while showing the lowest CO yield. This is due to the enhanced reforming and water–gas shift reactions, which enhanced the yield of H2 and CO2 at the expense of CO. However, it resulted in the lowest yield of methane (6.8 mmol/g) among all runs, including unpromoted and non-catalytic runs. It also showed the lowest yield of C2-C4 hydrocarbons compared to all promoted and unpromoted Ni/ZrO2 catalytic runs. This can be explained by the fact that the addition of Zn inhibited the hydrogenation of CO to minimize the formation of methane and heavy hydrocarbons in the reaction. Zn forms a layer on the Ni surface, which restricts the adsorption of CO and H2 on its catalytic surface. This results in the diminution of methanation and hydrogenation reactions, while the rate of water–gas shift reaction remains unaffected. Thus, it results in an increased yield of H2 and CO2 while reducing the methane yield. This was further substantiated by its high hydrogen selectivity of 34.7%.
3.4. BET Analysis of Promoted Ni/ZrO2 Catalysts
Results of BET analysis of freshly promoted Ni/ZrO
2 catalysts are shown in
Table 3. It can be observed that the addition of promotors to Ni/ZrO
2 catalysts decreased the surface area of the catalysts. The order of surface area of catalysts was (5.1 m
2/g) Ni-K/ZrO
2 > (4.4 m
2/g) Ni-Zn/ZrO
2 > (3.9 m
2/g) Ni-Ce/ZrO
2 compared to the BET surface area of 6.0 m
2/g of unpromoted Ni/ZrO
2 catalyst. Similarly, the addition of promotors also decreased the pore volume of the catalysts. This decrease in BET surface area and pore volume with the addition of promotors is due to the introduction of the metal particles of promotors in the catalyst pore. This blocks the pores and reduces the available surface area and pore volume of the catalysts. Su et al. [
23] also made similar observations for promoted Ni catalysts. They reported a decrease in the pore volume and surface area of the Ni/Al
2O
3 catalysts with the introduction of the La promotors. They also observed that an increase in La promotor loading further decreases the pore volume and surface area of the catalyst.
Even though the addition of promotors resulted in a decrement in the surface area, it does not necessarily translate into the poor gasification of biomass in the SCW. Promotors prevent the active nickel particles from coagulating, which reduces the metal sintering by preventing the growth of nickel particles during the gasification of biomass in SCW. Additionally, due to the hydrogen splitting ability of the promotors, split hydrogen ions can spill over to nickel active metal to enhance the reduction in nickel particles and result in the sustained high performance of the catalysts during the reaction. This also prevents the deactivation of catalysts and improves the gas yields of the SCWG process.
Furthermore, the analysis of the spent promoted Ni/ZrO2 catalysts after use in SCWG of canola straw revealed that, similar to unpromoted catalysts, promoted catalysts also suffered a loss in surface area and pore volume. However, the extent of the reduction in surface area and pore volume in spent catalysts compared to fresh catalysts was low in promoted catalysts compared to unpromoted catalysts. This can be attributed to the textural stability provided by the promotors to catalysts, which limited the sintering of catalysts by restricting the growth of Ni particles during the gasification. The lowest change in surface area and pore volume in spent catalysts compared to fresh catalysts was observed in the Ce-promoted Ni/ZrO2 catalysts, followed by Zn- and K-promoted Ni/ZrO2 catalysts. This is due to the enhanced dispersion of nickel particles due to the addition of the Ce promotor, as identified by the XRD analysis of the Ni-Ce/ZrO2 catalyst. Additionally, Ce promotor could also oxidize the coke deposited on the catalyst. This reduces the blockage of the pores of the catalysts caused by coke and Ni particles, resulting in stable surface area and pore volume of the catalysts over their use in SCWG. It also explains the highest total gas yield and hydrogen yield obtained with the Ni-Ce/ZrO2 catalyst.
BET adsorption and desorption isotherms of fresh and spent Ni-Ce/ZrO
2 catalysts revealed a type IV isotherm (
Figure 9). According to the International Union of Pure and Applied Chemistry (IUPAC), type IV isotherm is indicative of the presence of mesopores, where the adsorption and desorption curves do not overlap, indicating the presence of capillary condensation [
35]. It also showed the type H3 hysteresis loop, which does not exhibit any limiting adsorption at high values, and the lack of a plateau at high pressures suggests that the material does not have a uniform pore structure.
3.5. TGA Analysis of Promoted Ni/ZrO2 Catalysts
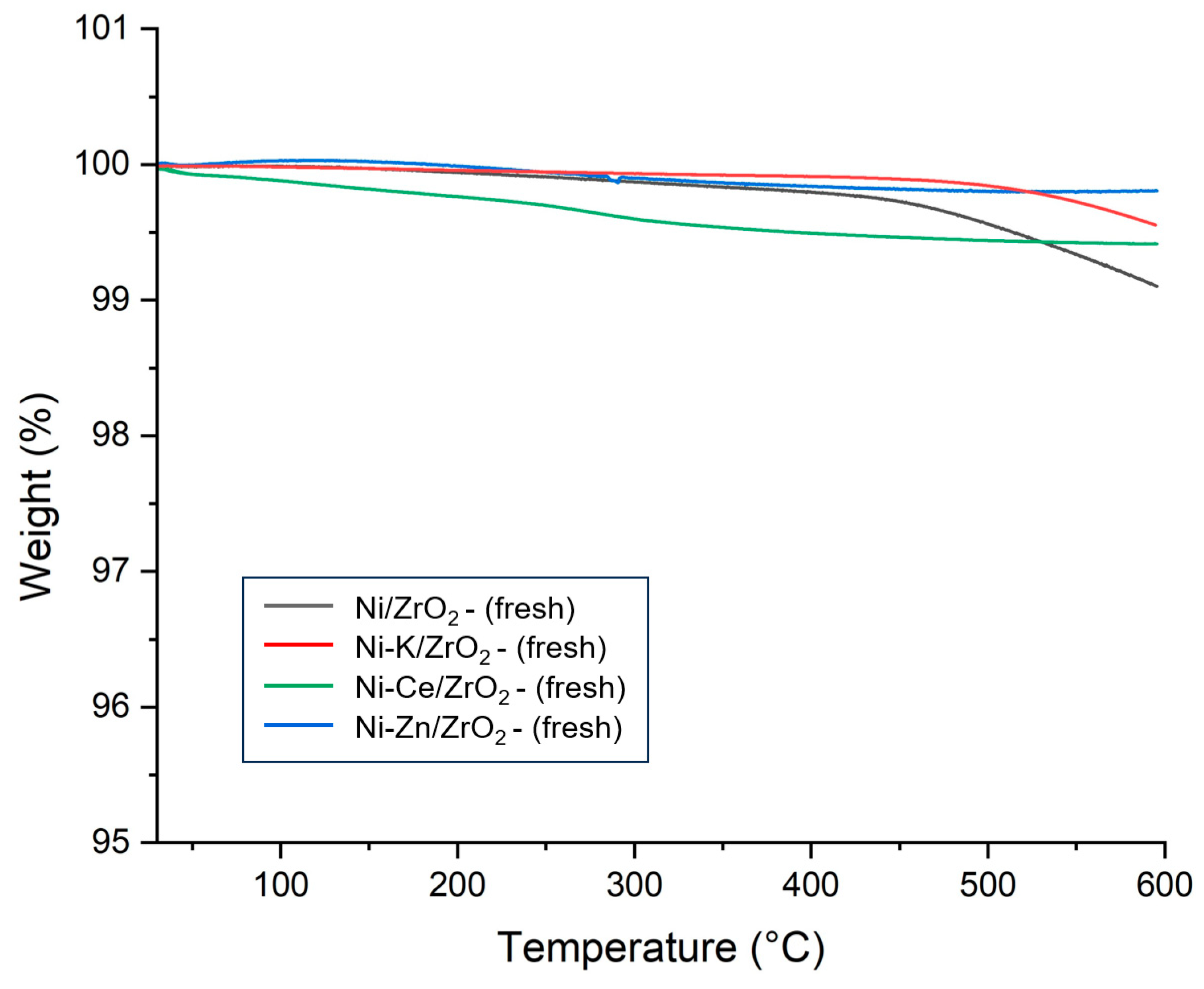
The thermal stability of promoted Ni/ZrO
2 catalysts was determined using the TGA, and results are presented in
Figure 10. It can be observed that all promoted Ni/ZrO
2 catalysts suffered a very minimum mass loss similar to unpromoted fresh catalysts. A very small weight loss was observed in the range of 200 °C due to the desorption of adsorbed gas and moisture. A very marginal hike in catalyst weight was noticed in the range of 450–600 °C due to the oxidation of nickel. However, total mass change was low in promoted Ni/ZrO
2 catalysts compared to unpromoted Ni/ZrO
2 catalysts. Minimum weight change was observed in the Ni-Ce/ZrO
2 catalyst. This is due to the improved strength of interaction of nickel metal with support with the addition of Ce promotor, which prevented the oxidation of the catalyst and enhanced the thermal stability of the Ni-Ce/ZrO
2 catalyst.
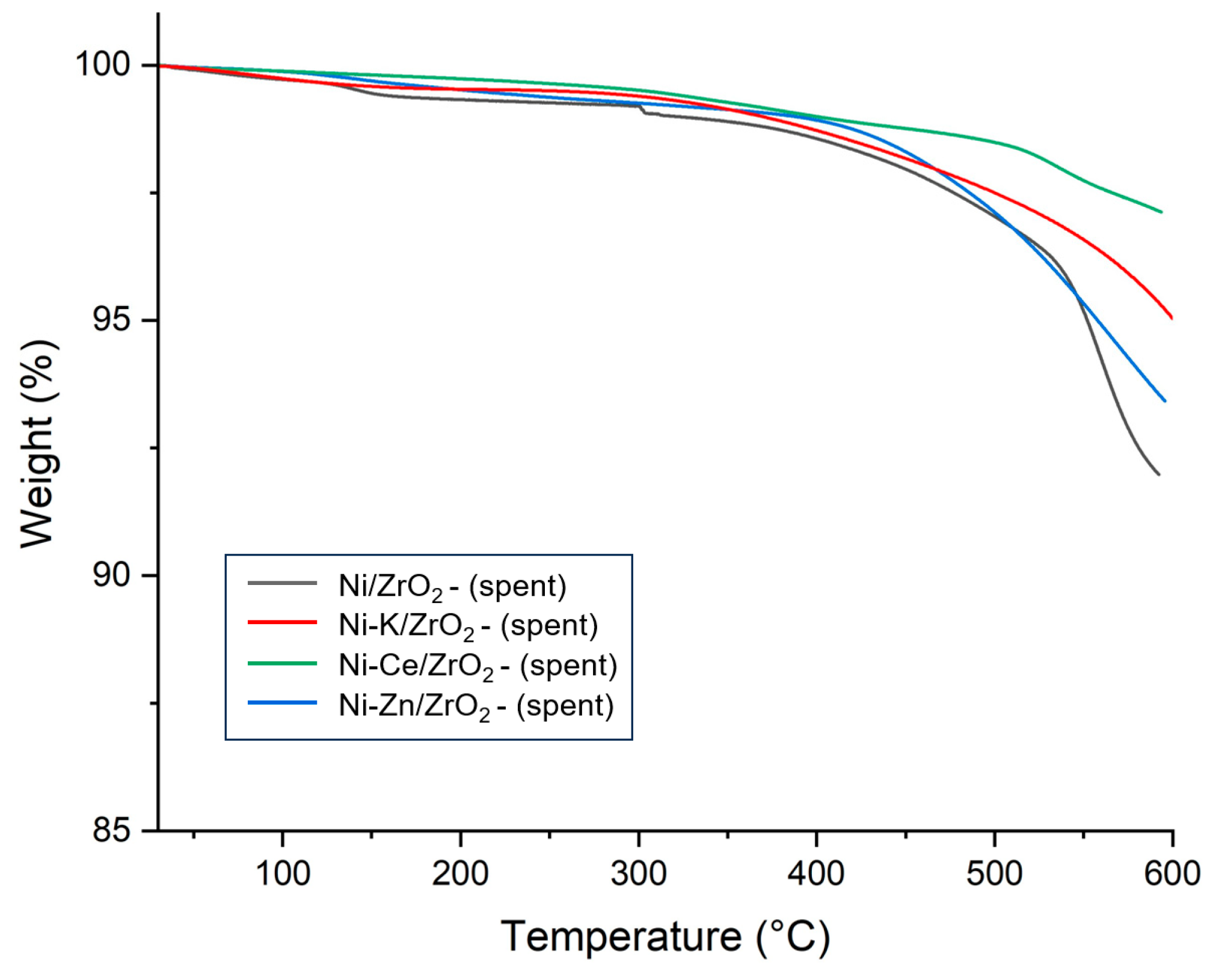
To analyze the ability of promoters to prevent coke deposition during the SCWG reaction, TGA of the spent catalysts of the SCWG reaction of canola straw was performed. Results of the TGA of the spent promoted catalysts are presented in
Figure 11. In the TGA analysis of spent catalysts, two counter effects were taking place which influenced the change in the spent catalysts’ weight. First, catalysts suffered mass loss due to the oxidization of the coke deposited on the surface of the catalysts. However, the oxidation of active metal also increased the weight of the catalysts by forming a metal oxide on the surface of the catalysts. Oxidation of carbon occurred in two phases; at a low-temperature range, mostly oxidation of amorphous carbon which was easier to oxidize took place [
41]. However, at higher temperature ranges, there were mostly graphitized carbon oxides, depending on their crystallization [
42]. It can be identified that the addition of the promotors reduced the percentage of mass loss of the catalysts compared to the 10% mass loss observed for unpromoted Ni/ZrO
2 catalysts. This represents the fact that the addition of the promotor reduced the carbon deposition on the catalyst and improved the stability of the catalyst in the reaction.
The Ce-promoted Ni-Ce/ZrO
2 catalyst demonstrated the lowest mass loss of 3%, followed by the 5% mass loss of the Ni-K/ZrO
2 and the 7% mass loss of the Ni-Zn/ZrO
2 catalyst. This shows that the coke deposition in Ni-Ce/ZrO
2 was minimal, which can be attributed to strong metal–promotor interactions and higher dispersion of the nickel in the Ni-Ce/ZrO
2 catalyst. The ability of Ce promotors to prevent the deactivation of Ni-Ce/ZrO
2 catalysts from coke deposition contributed to their having the highest hydrogen and total gas yield among all catalysts. Thus, the ability to restrict the coke formation via a catalyst is an important parameter and strongly influences the performance of the catalyst in the SCWG of biomass. Kang et al. [
28] also observed that the addition of Ce promotor to Ni supported by Al
2O
3 catalyst for SCWG of lignin decreased the coke deposition and enhanced the gas yields. The addition of Ce promotor improved the hydrogen yield by nearly 175% and the total gas yield by approximately 57% compared to unpromoted catalysts.
The high stability of Ni-Ce/ZrO
2 against coke formation and its high activity during gasification in SCW are due to the redox ability of the Ce promotor. Ce has two stable oxidation states of Ce
3+ and Ce
4+ [
43]. This gives Ce the ability to store and release oxygen via redox shift between its two oxidation states [
44]. It allows Ce to rapidly mobilize oxygen over the catalyst via its oxygen uptake and release it in a reversible redox reaction [
43,
45].
This lattice oxygen produced at the Ce surface can partially oxidize solid coke adsorbed on the surface of the catalyst to form CO [
46].
Ol−1 results in the formation of the reduced site on the surface of Ce.
Lattice oxygen can also react with produced CO on the catalyst’s surface for oxidation of CO into CO
2. Increased formation of CO
2 instead of CO can restrict the coke deposition.
This imparts high coke resistance to the Ni-Ce/ZrO
2 catalyst and enhances the stability of the catalyst. Furthermore, nickel oxide present in Ni-Ce/ZrO
2 catalyst can also react with the lattice oxygen of Ce to be reduced in the metallic form. It increases the dispersion of the nickel metal and increases active sites in the Ni-Ce/ZrO
2 catalyst. In the case of Ni-K/ZrO
2-promoted catalyst, alkali metals are known to increase the alkalinity of the catalysts and enable CO
2 chemisorption [
47]. This increases the number of oxygen vacancies and improves the dispersion of Ni metal, whereas for the Ni-Zn/ZrO
2 catalyst, the Zn promotor also increases the alkalinity of the catalysts and has strong synergetic effects with nickel metal, which imparts high thermal stability with high metal dispersion to the catalyst [
48]. To further assess the morphology of the Ni-Ce/ZrO
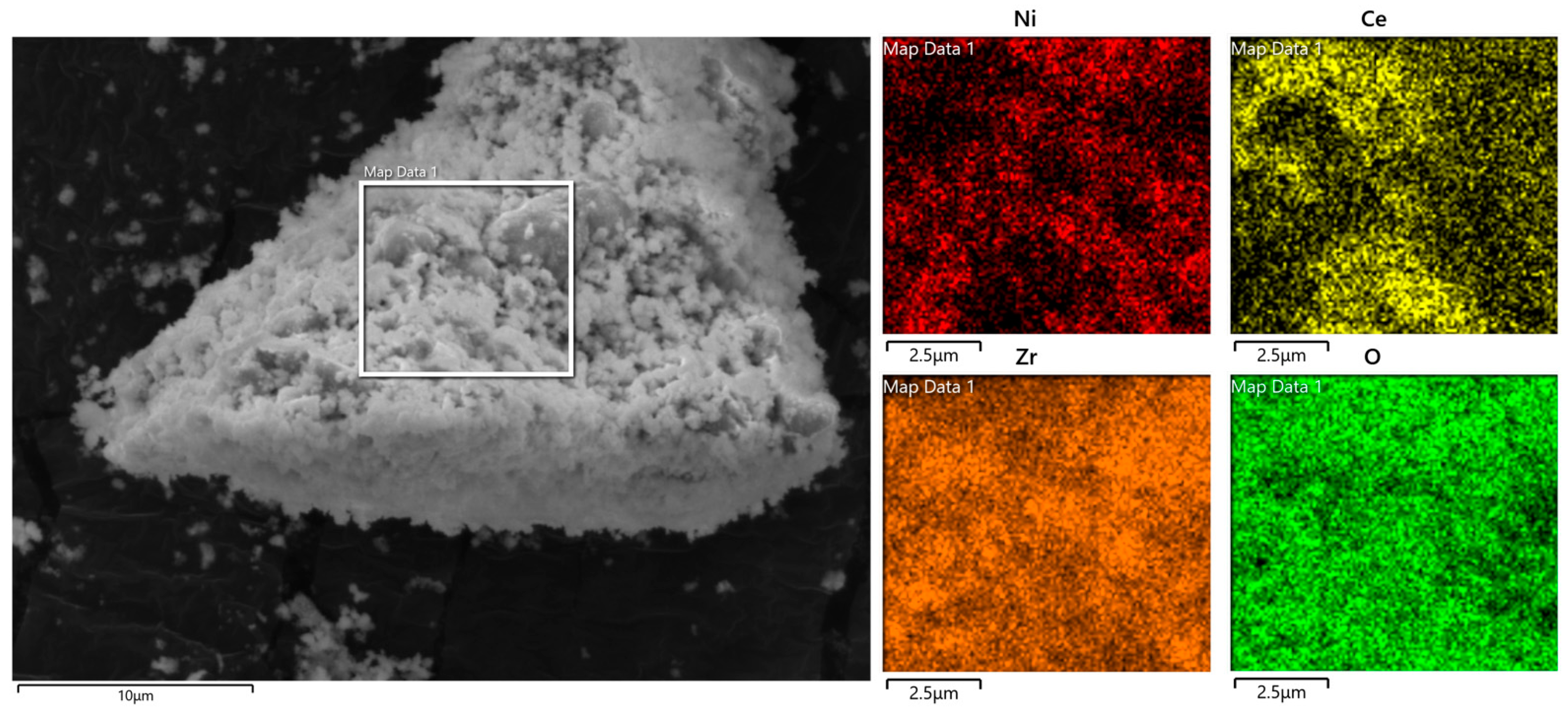
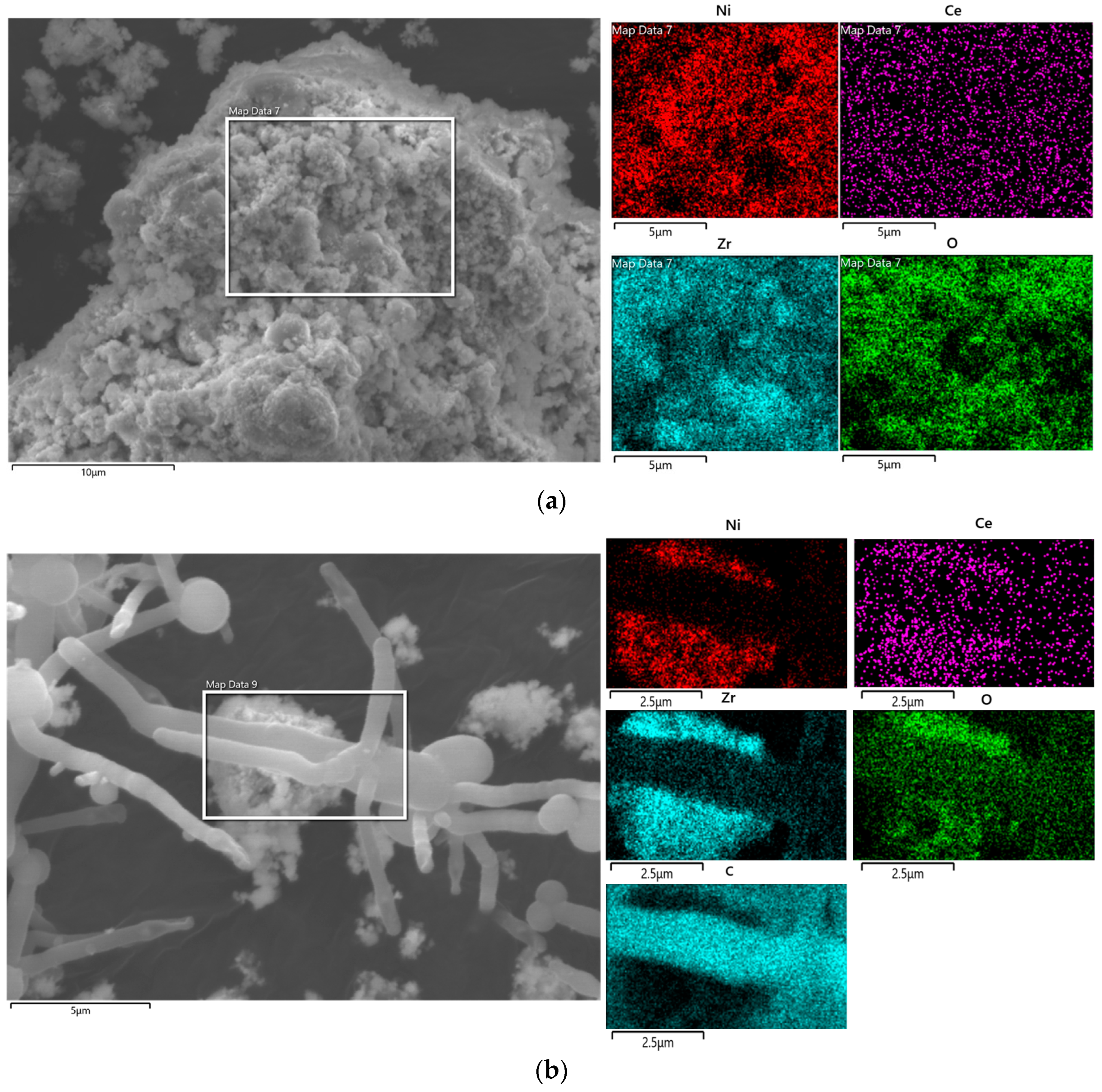
2 catalyst, SEM analysis of Ni-Ce/ZrO
2 catalyst was performed.
3.7. Reusability of Ni-Ce/ZrO2 Catalysts
Owing to the ability to recover the heterogeneous catalysts after the completion of the reaction, the reusability of the catalysts plays an important role in the selection of the suitable catalysts. The reusability of catalysts not only reduces the amount of fresh catalyst required but also improves the economics of the process. The reusability of Ni-Ce/ZrO2 catalyst over its repeated use for gasification of canola straw in SCW was tested to evaluate its catalytic stability. It was then compared with the reusability of Ni/ZrO2 catalyst to evaluate the effect of Ce promotor on minimizing the deactivation of the Ni-Ce/ZrO2 catalyst. Catalysts were recovered from the SCWG reactor after the completion of the SCWG reaction. Two catalytic re-runs were performed without regeneration to test coke deposition and sintering of catalysts. All the catalytic runs were performed at 500 °C, 23–25 MPa, 10 wt%, and 60 min.
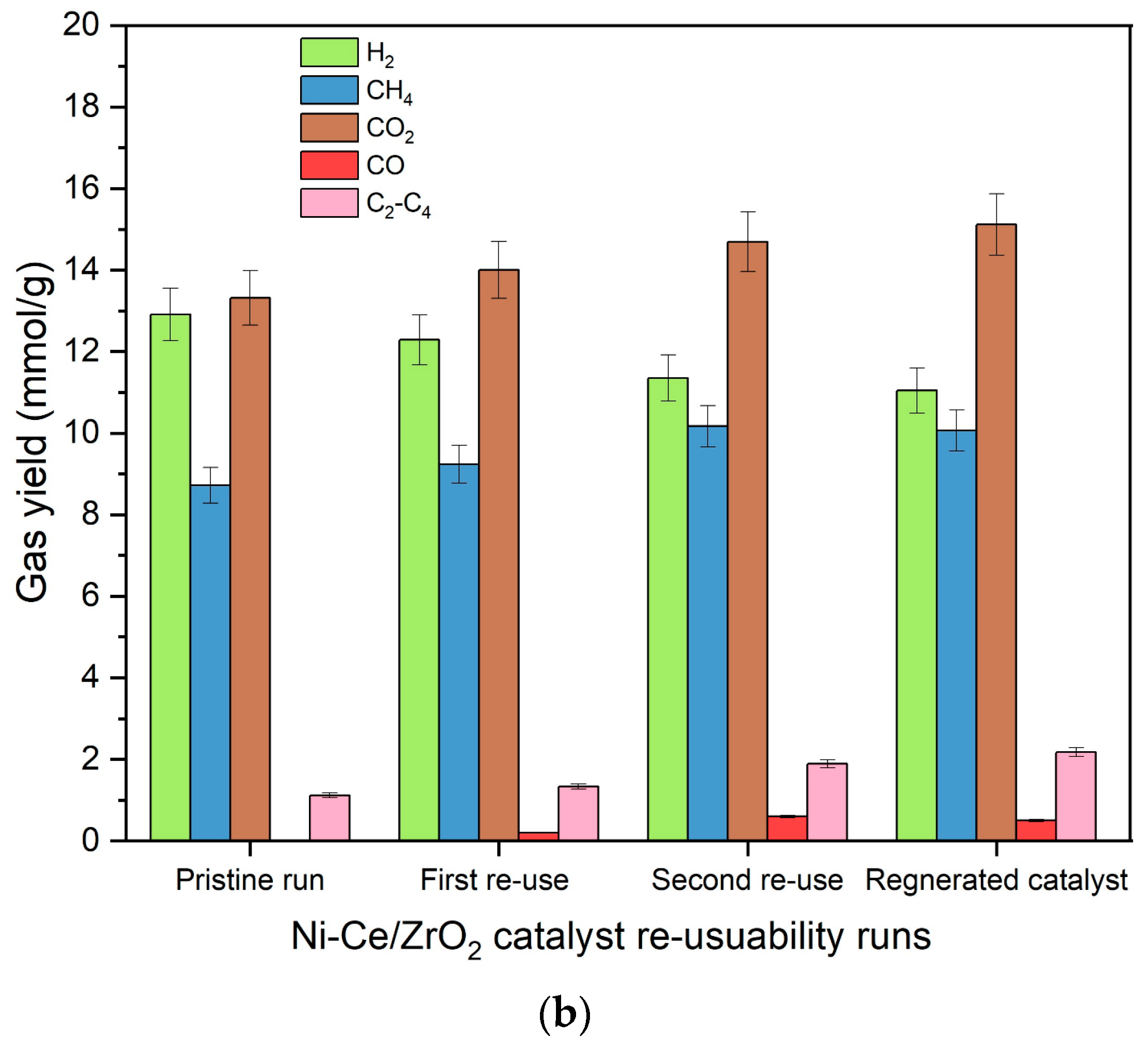
Results for the reusability of Ni/ZrO
2 and Ni-Ce/ZrO
2 catalysts are presented in
Figure 14. It can be observed from
Figure 14 that reusing both Ni/ZrO
2 and Ni-Ce/ZrO
2 catalysts after the first run caused them to suffer decrements in the hydrogen yield. For Ni/ZrO
2, hydrogen yield decreased to 9.6 mmol/g in the first reuse run, compared to the yield of 10.5 mmol/g for pristine Ni/ZrO
2 catalysts. Ni-Ce/ZrO
2 catalysts also suffered a decrement in hydrogen yield to 12.3 mmol/g in the first reuse from 12.9 mmol/g from the pristine Ni-Ce/ZrO
2 catalytic run. A similar trend was observed for the second reuse of the Ni/ZrO
2 catalyst, in which hydrogen yield further decreased to 8.3 mmol/g. For the Ni-Ce/ZrO
2 catalyst, too, hydrogen yield further decreased to 11.4 mmol/g in the second reuse of the Ni-Ce/ZrO
2 catalyst. Ni/ZrO
2 catalyst suffered a loss of 8% in the first reuse cycle and 21% in the second reuse cycle compared to the pristine Ni/ZrO
2 catalyst run for gasification of canola straw in SCW. Even though the Ni-Ce/ZrO
2 catalyst also suffered a loss in hydrogen yield, the addition of the Ce promotor reduced the extent of hydrogen yield in its reuse cycles. Only 5% and 12% decrements were observed in hydrogen yield for the first reuse and second reuse cycle, respectively, of the Ni-Ce/ZrO
2 catalyst, compared to the pristine Ni-Ce/ZrO
2 catalytic run. This shows the superior thermal stability of the Ni-Ce/ZrO
2 catalyst compared to the unpromoted Ni/ZrO
2 catalyst. Su et al. [
23] also observed a similar decrement in hydrogen yield for La-promoted Ni/Al
2O
3 catalysts for gasification of food waste. They reported an 87% drop in hydrogen production in the third run cycle for unpromoted Ni/Al
2O
3 catalysts. This drop in hydrogen yield was minimized by the addition of La promotor, and a 65% drop in hydrogen production was observed in the third run cycle for the La-promoted Ni/Al
2O
3 catalyst.
On the contrary, the yield of methane and C2-C4 hydrocarbons increased from 8.7 and 1.1 mmol/g in the pristine Ni-Ce/ZrO2 catalytic run to 9.2 and 1.3 mmol/g in the first reuse of the catalyst. Similarly, the yield of methane and C2-C4 hydrocarbons further increased to 10.2 and 1.9 mmol/g in the second reuse of the Ni-Ce/ZrO2 catalyst. Ni/ZrO2 catalyst also observed a similar rise in yield of methane and C2-C4 hydrocarbons over its reuse cycles. This is due to the enhancement of methanation and secondary reactions over reforming and water–gas shift reactions during the reuse of catalysts, which is due to the reduced activity of catalysts over their repeated use. This enhanced the yield of methane and heavy molecular hydrocarbon gases while decreasing the hydrogen yield. Interestingly, despite the diminution of reforming and water–gas shift reactions, yields of CO and CO2 witnessed a continuous rise over repeated use of the catalysts. However, this rise in yield of CO and CO2 is due to the oxidation of the coke deposited on the surface of the catalysts.
A major reason for decrement in the catalytic activity was due to the coke deposition and active metal sintering. Loss of activity of catalysts due to coke deposition is reversible where regeneration of catalysts in the presence of oxygen will restore the catalytic activity of the catalyst. However, metal sintering is an irreversible process that results in permanent loss of catalytic activity. To test the cause of the loss of activity of the catalysts, catalysts were also regenerated by performing calcination and reduction of the recovered used catalysts after the second rerun, and then they were utilized to conduct the gasification of canola straw in SCW at 500 °C, 23–25 MPa, 10 wt%, and 60 min. Results of the activity of regenerated catalysts for gasification of canola straw in SCW are also presented in
Figure 14. From
Figure 14, it can be observed that the regeneration of catalysts did not improve catalytic activity and still resulted in a decrement to 11.0 mmol/g in hydrogen yield, compared to 11.4 mmol/g for the second reuse run and 12.9 mmol/g for the pristine Ni-Ce/ZrO
2 catalytic run. This shows that the loss of the catalytic activity of Ni-Ce/ZrO
2 is primarily due to sintering of nickel metal. Statistical analysis using ANOVA also confirmed the differences in the means of all gas yields for reused and regenerated catalysts for both Ni-Ce/ZrO
2 and Ni/ZrO
2 catalysts, with high F-statistics ((11.7–553.1) and (17.58–730.2), respectively), and with high significance. Welch’s
t-tests also confirmed a decrease in the mean of hydrogen yield in reused and regenerated catalysts compared to that from the pristine run for both Ni-Ce/ZrO
2 and Ni/ZrO
2 catalysts.
Overall, the addition of Ce promotor to Ni/ZrO
2 also enhanced the reusability of the Ni-Ce/ZrO
2 catalyst. Comparison of performance of the Ni-Ce/ZrO
2 catalyst with reported modified nickel-based catalysts for SCWG of lignocellulosic biomass also demonstrated its superior catalytic activity for SCWG (
Table 4). Therefore, due to high catalytic activity, thermal stability, reusability, metal dispersion, and lower sintering and coking of Ni-Ce/ZrO
2 catalyst, the addition of Ce promotor successfully improved the performance of the Ni/ZrO
2 catalyst. Ni-Ce/ZrO
2 proved to be the most suitable catalyst for the gasification of canola straw in SCW.