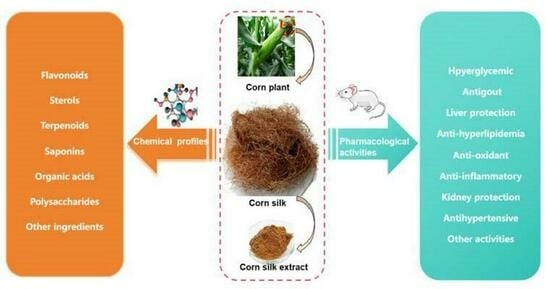
An Umbrella Insight into the Phytochemistry Features and Biological Activities of Corn Silk: A Narrative Review
Abstract
1. Introduction
2. Chemical Classification and Structures
2.1. Flavonoids
2.2. Sterols, Terpenoids and Saponins
2.3. Organic Acids
2.4. Polysaccharides and Other Ingredients
3. Pharmacological Actions
3.1. Hyperglycemic Effect
3.2. Antigout Action
3.3. Liver Protection
3.4. Anti-Hyperlipidemia Action
3.5. Anti-Oxidant Activity
3.6. Anti-Inflammatory Effect
3.7. Kidney Protection
3.8. Antihypertensive Activity
3.9. Other Activities
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Colombo, R.; Ferron, L.; Papetti, A. Colored corn: An Up-Date on metabolites extraction, health implication, and potential use. Molecules 2021, 26, 199. [Google Scholar] [CrossRef]
- Ikpeazu, V.O.; Ugbogu, E.A.; Emmanuel, O.; Uche-Ikonne, C.; Okoro, B.; Nnaemeka, J. Evaluation of the safety of oral intake of aqueous extract of Stigma maydis (corn silk) in rats. Acta Sci. Pol. Technol. Aliment. 2018, 17, 387–397. [Google Scholar]
- Wang, B.; Xiao, T.; Ruan, J.; Liu, W. Beneficial effects of corn silk on metabolic syndrome. Curr. Pharm. Des. 2017, 23, 5097–5103. [Google Scholar] [CrossRef]
- Dong, W.; Zhao, Y.; Li, X.; Huo, J.; Wang, W. Corn silk polysaccharides attenuate diabetic nephropathy through restoration of the gut microbial ecosystem and metabolic homeostasis. Front. Endocrinol. 2023, 14, 1232132. [Google Scholar] [CrossRef]
- Singh, J.; Rasane, P.; Nanda, V.; Kaur, S. Bioactive compounds of corn silk and their role in management of glycaemic response. J. Food Sci. Technol. 2023, 60, 1695–1710. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, X. Progress in modern pharmacological research and clinical application of traditional Chinese medicine corn silk. Acad. J. Shanghai Univ. Tradit. Chin. Med. 2022, 36, 287–290. [Google Scholar]
- Hsiang, C.Y.; Lo, H.Y.; Lu, G.L.; Liao, P.Y.; Ho, T.Y. A novel heat-stable angiotensin-converting enzyme zinc-binding motif inhibitory peptide identified from corn silk. J. Ethnopharmacol. 2024, 320, 117435. [Google Scholar] [CrossRef] [PubMed]
- Cho, I.J.; Shin, J.H.; Choi, B.R.; Park, H.R.; Park, J.E.; Hong, S.H.; Kwon, Y.S.; Oh, W.S.; Ku, S.K. Lemon balm and corn silk mixture alleviates metabolic disorders caused by a high-fat diet. Antioxidants 2022, 11, 730. [Google Scholar] [CrossRef] [PubMed]
- Sarfare, S.; Khan, S.I.; Zulfiqar, F.; Radhakrishnan, S.; Ali, Z.; Khan, I.A. Undescribed C-Glycosylflavones from corn silk and potential anti-inflammatory activity evaluation of isolates. Planta Med. 2022, 88, 745–752. [Google Scholar] [CrossRef] [PubMed]
- Gumaih, H.S.; Alasbahy, A.; Alharethi, S.H.; Al-Asmari, S.M.; Al-Khulaidi, A. Antiurolithiasis activities of Zea mays extract and its mechanism as antiurolithiasis remedy. Am. J. Clin. Exp. Urol. 2023, 11, 443–451. [Google Scholar] [PubMed]
- Khan, U.; Hayat, F.; Khanum, F.; Shao, Y.; Iqbal, S.; Munir, S.; Abdin, M.; Li, L.; Ahmad, R.M.; Qiu, J.; et al. Optimizing extraction conditions and isolation of bound phenolic compounds from corn silk (Stigma maydis) and their antioxidant effects. J. Food Sci. 2023, 88, 3341–3356. [Google Scholar] [CrossRef]
- Zhang, Y.; Yao, L.; Liu, Y.; Chen, B.; Wang, C.; Gong, K.; Wang, F.; Qiao, Y. Acidic polysaccharide from corn silk: Structural & conformational properties and hepatoprotective activity. Int. J. Biol. Macromol. 2023, 236, 123851. [Google Scholar]
- Yucharoen, R.; Srisuksomwong, P.; Julsrigival, J.; Mungmai, L.; Kaewkod, T.; Tragoolpua, Y. Antioxidant, anti-tyrosinase, and anti-skin pathogenic bacterial activities and phytochemical compositions of corn silk extracts, and stability of corn silk facial cream product. Antibiotics 2023, 12, 1443. [Google Scholar] [CrossRef]
- Natan, R.; Lucia, F.; Victor, D.; Silvia, L.; Leonardo, Z.; Pio, C.; Caline, G.; Paulo, R. Identification of bioactive metabolites from corn silk extracts by a combination of metabolite profiling, univariate statistical analysis and chemometrics. Food Chem. 2021, 365, 130479. [Google Scholar]
- Li, P.; Huang, Y.; Zhu, H.; Chen, J.; Ren, G.; Jiang, D.; Liu, C. Authentication, chemical profiles analysis, and quality evaluation of corn silk via DNA barcoding and UPLC-LTQ/Orbitrap MS chemical profiling. Food Res. Int. 2023, 167, 112667. [Google Scholar] [CrossRef]
- Wang, Y.; Gu, M.; Mao, J.; Liu, J.; Fan, S.; Zhang, H.; Bu, H.; Liu, Q. Phytochemical study: Fragmentation patterns of flavonoid-C-glycosides in the enriched flavonoids from corn silk using high-efficiency ultra-high-performance liquid chromatography combined with quadrupole time-of-flight mass spectrometry. Sep. Sci. Plus 2023, 7, 2300156. [Google Scholar] [CrossRef]
- Yi, T.; Zhao, Z.; Liu, M. Analysis of the chemical constituents of flavonoids in corn stigama by HPLC-Q-TOF-MS. China Pharm. 2019, 22, 1776–1780. [Google Scholar]
- Li, Q.; Li, T.; Wang, D.; Ren, T. Analysis of flavonoid glycosides derived from corn silk by HPLC-MS. J. Food Sci. Technol. 2016, 34, 56–61. [Google Scholar]
- Zhang, P.; Zhuang, Y.; Huo, J.; Wang, W. Overview of the effective chemical constituents and pharmacological effects of corn whiskers. Heilongjiang J. Tradit. Chin. Med. 2017, 46, 74–75. [Google Scholar]
- Pan, W.; Deng, J.; Hou, X.; Qin, J.; Hao, E.; Du, Z.; Xie, J.; Wei, W.; Chen, M. Chemical comstituents of agricultural residues producing from 4 kinds of gramineous crops and their pharmacological effects. Chin. J. Exp. Tradit. Med. Formulae 2019, 25, 214–225. [Google Scholar]
- Snook, M.E.; Widstrom, N.W.; Wiseman, B.R.; Byrne, P.F.; Harwood, J.S.; Costello, C.E. New C—4″-hydroxy derivatives of maysin and 3′-methoxymaysin isolated from corn silks (Zea mays). J. Agr. Food Chem. 1995, 43, 2740–2745. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, D. Study on the chemical constituents of flavones from corn silk. J. Chin. Med. Mater. 2007, 164–166. [Google Scholar]
- Li, Z.; Zhang, J.; Guo, L.; Ma, Y.; Wang, L.; Liu, J. Research Progress on Chemical Constituents of Stigma maydis. Chem. Ind. Times 2022, 36, 23–31. [Google Scholar]
- Wang, H.; Du, Y.; Song, H. α-Glucosidase and α-amylase inhibitory activities of guava leaves. Food Chem. 2010, 123, 6–13. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, J.; Fan, S.; Yang, D.; Wang, H.; Wang, Y. Rapid discovery and global characterization of multiple components in corn silk using a multivariate data processing approach based on UHPLC coupled with electrospray ionization/quadrupole time-of-flight mass spectrometry. J. Sep. Sci. 2018, 41, 4022–4030. [Google Scholar] [CrossRef]
- Xue, X. Study on Chemical Constituents of Zea mays L. with Non-enzymatic glycation inhibitory activity. Master’s Thesis, Yanbian University, Jilin, China, 2009; pp. 1–128. [Google Scholar]
- Xu, Y.; Liang, J.; Zou, Z.; Yang, J. A Novel Flavone and Two Urea Glycosides from the Style of Zea mays L. Acta Chim Sin. 2008, 66, 1235–1238. [Google Scholar]
- Zhang, J.; He, Z.; Wang, X.; Fan, H. Research progress on pharmacological action of corn silk polysaccharide. J. Jilin Med. Univ. 2021, 42, 64–66. [Google Scholar]
- Wang, H.; Liu, Q.; Dong, Y. Research progress of corn whisker polysaccharide and its hypoglycemic function. J. Beijing Vocat. Coll. Agric. 2018, 32, 30–34. [Google Scholar]
- Li, X.; Zhao, M.; Ma, Y.; Wang, D.; Shi, Z.; Li, J.; Wang, J.; Wang, W.; Zhang, S. Chemical consituents from style and stigma of Zea mays. Chin. Tradit. Herb. Drugs 2021, 52, 3480–3484. [Google Scholar]
- Yang, Q.; Zhi, H.; Zhang, H.; Sun, J. Research Progress on Chemical Constituents, Pharmacological Activity and Utilization Status of Different Parts of Corn. Jilin J. Chin. Med. 2019, 39, 837–840. [Google Scholar]
- Fougere, L.; Rhino, B.; Elfakir, C.; Destandau, E. Comparison of the flavonoid profiles of corn silks to select efficient varieties as trap plants for Helicoverpa zea. J. Agric. Food Chem. 2020, 68, 5356–5364. [Google Scholar] [CrossRef]
- Liu, J.; Wang, C.; Wang, Z.; Zhang, C.; Lu, S.; Liu, J. The antioxidant and free-radical scavenging activities of extract and fractions from corn silk (Zea mays L.) and related flavone glycosides. Food Chem. 2011, 126, 261–269. [Google Scholar] [CrossRef]
- Liu, C.; Tai, Z.; Li, A.; Cai, L.; Ding, Z. Chemical constituents of the style of Zea mays L. Nat. Prod. Res. Dev. 2011, 23, 1041–1044. [Google Scholar]
- Suzuki, R.; Okada, Y.; Okuyama, T. A new flavone C-glycoside from the style of Zea mays L. with glycation inhibitory activity. Chem. Pharm. Bull. 2003, 51, 1186–1188. [Google Scholar] [CrossRef]
- Ren, S.; Ding, X. Extraction, separation and structural identification of flavonoids from corn silk (I). Chin. Tradit. Herb. Drugs 2004, 35, 21–22. [Google Scholar]
- Tian, J.; Chen, H.; Chen, S.; Xing, L.; Wang, Y.; Wang, J. Comparative studies on the constituents, antioxidant and anticancer activities of extracts from different varieties of corn silk. Food Funct. 2013, 4, 1526–1534. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Y.; Sun, G.; Tan, B.; Wang, W. Analysis of chemical constituents of Yumixu(Stigma maydis) based on UPLC-Q-TOF/MS. Chin. J. Tradit. Med. Sci. Technol. 2023, 30, 239–247. [Google Scholar]
- Zhang, D.; Wang, Y.; Liu, H. Corn silk extract inhibit the formation of Nε-carboxymethyllysine by scavenging glyoxal/methyl glyoxal in a casein glucose-fatty acid model system. Food Chem. 2020, 309, 125708. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Li, Q. Determination of β-sitosterol in corn silk of different terms by HPLC-ELSD. J. Henan Univ. (Med. Sci.) 2006, 4, 53–55. [Google Scholar]
- Tian, J. Studies on Liposoluble Constituents of Corn Silk; Tianjin University: Tianjin, China, 2014. [Google Scholar]
- Zhao, M.; Liu, C.; Yin, T. Chemical constituents of the style of Zea mays L. from Yunnan. Chem. Res. Appl. 2013, 25, 846–850. [Google Scholar]
- Suzuki, R.; Iijima, M.; Okada, Y.; Okuyama, T. Chemical constituents of the style of Zea mays L. with glycation inhibitory activity. Chem. Pharm. Bull. 2007, 55, 153–155. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Zhang, Y.; Zhao, P.; Zhou, L.; Wang, X.; Huang, X.; Lin, B.; Song, S. Ent-kaurane diterpenoids with neuroprotective properties from Corn Silk (Zea mays). J. Nat. Prod. 2018, 81, 1225–1234. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Liang, J. Studies on the chemical composition of corn whiskers. Chin. Tradit. Herb. Drugs 2006, 6, 831–833. [Google Scholar]
- Kim, D.Y.; Won, K.J.; Hwang, D.I.; Kim, H.B.; Li, Y.; Lee, H.M. Migration-and proliferation-promoting activities in human keratinocytes of Zea mays flower absolute and its chemical composition. Chem. Biodivers. 2020, 17, e2000227. [Google Scholar] [CrossRef] [PubMed]
- Hasanudin, K.; Hashim, P.; Mustafa, S. Corn silk (Stigma maydis) in healthcare: A phytochemical and pharmacological review. Molecules 2012, 17, 9697–9715. [Google Scholar] [CrossRef] [PubMed]
- Widstrom, N.W.; Snook, M.E. A gene controlling biosynthesis of isoorientin, a compound in corn silks antibiotic to the corn earworm. Entomol. Exp. Appl. 2010, 89, 119–124. [Google Scholar] [CrossRef]
- Zhou, W.; Lv, T.; Hou, Z.; Bai, M.; Lin, B.; Huang, X.; Song, S.J. A new monoterpene-lactone with neuroprotective activity from corn silk. Nat. Prod. Res. 2021, 35, 3142–3145. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Zhao, P.; Zhang, Y.; Bai, M.; Lin, B.; Huang, X.; Song, S. Sesquiterpenes from Stigma maydis (Zea mays) as a crop by-product and their potential neuroprotection and inhibitory activities of Aβ aggregation. Ind. Crops Prod. 2018, 121, 411–417. [Google Scholar] [CrossRef]
- Song, X.; Guo, R.; Qi, X.; Han, F.; Lin, B.; Huang, X.; Yao, G.; Song, S. Terpenoids from Stigma maydis (Zea mays L.) alleviate hydrogen peroxide-induced SH-SY5Y cell injury by activating Nrf2. Bioorg. Chem. 2020, 102, 104131. [Google Scholar] [CrossRef]
- Chaudhary, R.K.; Karoli, S.S.; Dwivedi, P.; Bhandari, R. Anti-diabetic potential of Corn silk (Stigma maydis): An in-silico approach. J. Diabetes Metab. Disord. 2022, 21, 445–454. [Google Scholar] [CrossRef]
- Yang, L.; Zi, C.; Li, Y.; Huang, J.; Gu, Z.; Wang, C.; Hu, J.M.; Jiang, Z.; Zhang, W. An in-depth investigation of molecular interaction in zeaxanthin/corn silk glycan complexes and its positive role in hypoglycemic activity. Food Chem. 2024, 438, 137986. [Google Scholar] [CrossRef]
- Wang, B.Z.; Ding, C.; Liu, L. Determination of amino acid content in corn silks. Ginseng Res. 2000, 3, 35–36. [Google Scholar]
- Chaiittianan, R.; Sutthanut, K.; Rattanathongkom, A. Purple corn silk: A potential anti-obesity agent with inhibition on adipogenesis and induction on lipolysis and apoptosis in adipocytes. J. Ethnopharmacol. 2017, 201, 9–16. [Google Scholar] [CrossRef]
- Wang, P. Chemical Components and Their Antioxidant Activities Studies on Maize Silk (Stigma maydis). Master’s Thesis, Jilin Agricultural University, Jinlin, China, 2004; pp. 1–145. [Google Scholar]
- Wang, H.Y.; Luo, Y.F.; Jiang, J.; Ding, K.; Dong, Y. Extraction, purfication and structural characterization analysis of stigma maydis polysacchsride. J. Cap. Norm. Univ. (Nat. Sci. Ed.) 2019, 40, 44–49. [Google Scholar]
- Ren, S.; Ding, X. Determination of organic acids in corn silk with GC-MS. J. Wuxi Univ. Light Ind. 2003, 6, 89–91. [Google Scholar]
- Zhai, X.; Ma, M.; Zhang, D.; Wu, D.; Zhou, H. Study on fat-soluble chemical constituents of Stigma Maydis. J. Jilin Inst. Chem. Technol. 2010, 27, 33–35. [Google Scholar]
- Li, X.; Ma, Y.; Wang, D.; Shi, Z.; Li, J.; Wang, J.; Wang, W.; Zhang, S.; Zhao, M. Chemical constituents of ethyl acetate extract of Stigma maydis. J. Qiqihar Univ. (Nat. Sci. Ed.) 2020, 36, 54–56. [Google Scholar]
- Wang, Y.; Liu, Q.; Fan, S.; Yang, X.; Ming, L.; Wang, H.; Liu, J. Rapid analysis and characterization of multiple constituents of corn silk aqueous extract using ultra-high-performance liquid chromatography combined with quadrupole time-of-flight mass spectrometry. J. Sep. Sci. 2019, 42, 3054–3066. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zou, Z.; Liang, J. Chemical Constituents from the style of Zea mays L. Chin. J. Nat. Med. 2008, 3, 237–238. [Google Scholar] [CrossRef]
- Haghi, G.; Arshi, R.; Safaei, A. Improved high-performance liquid chromatography (HPLC) method for qualitative and quantitative analysis of allantoin in Zea mays. J. Agric. Food Chem. 2008, 56, 1205–1209. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Lou, L.; Guo, R.; Xi, Y.; Lin, B.; Huang, X.; Song, S. Diverse metabolites from corn silk with anti-Aβ1–42 aggregation activity. Fitoterapia 2019, 138, 104356. [Google Scholar] [CrossRef]
- Jin, D.; Chen, Y.; Chai, T.; Ren, S.; Yuan, Y.; Cheng, Y. Hypoglycemic and Hepatoprotective Effects of Corn Silk Extract on Type 2 Diabetic Mice. J. Chin. Inst. Food Sci. Technol. 2022, 22, 101–108. [Google Scholar]
- Zhang, Y.; Wang, J.; Wang, L.; Zhen, L.; Zhu, Q.; Chen, X. A study on hypoglycaemic health care function of Stigma maydis polysaccharides. Afr. J. Tradit. Complement. Altern. Med. 2013, 10, 401–407. [Google Scholar] [PubMed]
- Zhang, X.; Pan, Y.; Wang, J.; Zhang, T.; Chen, H. Active components analysis and hypoglycemic potential of N-Butanol fraction from corn silk. Food Res. Dev. 2022, 43, 52–61. [Google Scholar]
- Li, M.; Liu, C. Effects of different probiotics fermented corn whisker products on in vivo and in vitro hypoglycemic. Anhui Agric. Sci. Bull. 2021, 27, 16–17. [Google Scholar]
- Jing, Y.; Jing, R.; Ren, Y.; Hu, T. Effects of flavones from Zea Mays L (ZMLF) on blood lipid and hemorheologic parameters in hyperlipemic rats. Chin. J. New Drugs 2010, 19, 797–800. [Google Scholar]
- Zhang, H.; Jiang, H.; Zhao, M.; Xu, Y.; Liang, J.; Ye, Y.; Chen, H. Treatment of gout with TCM using turmeric and corn silk: A concise review article and pharmacology network analysis. Evid. Based Complement. Altern. Med. 2022, 2022, 3143733. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Song, J.S.; Li, Q.; Zhang, Q.; Cui, H.; Guan, B.; Zhao, Y.; Song, Z. Curative effect analysis of flavone extract from Stigma Maydis on rats of modified acute gouty arthritis model. China Mod. Med. 2018, 25, 8–11. [Google Scholar]
- Lv, G.; Qiu, Z.; Chang, S.; Guo, J.; Lin, H.; Ye, D.; Li, P.; Lu, J.; Lin, Z. Effect of cornbeard total flavonoids extract on renal uric acid excretion in rats with gouty nephropathy. Chin. Tradit. Pat. Med. 2018, 40, 1373–1376. [Google Scholar]
- Zhe, L. Study on the Mechanism of Anti-Gouty Nephropathy Effect of Corn Beard Total Flavonoids; Changchun University of Chinese Medicine: Changchun, China, 2014; pp. 1–5. [Google Scholar]
- Chen, Y.; Gao, X.; Guan, D.; Geng, D.; Zhang, J. Study on the pharmacodynamic of ethanol extracts from lonicera japonica in rats with hepatic fibrosis. Chin. J. Exp. Tradit. Med. Formulae 2012, 18, 195–199. [Google Scholar]
- Jing, Y.; Hu, T. Preventive effect of corn silk total flavonoid (CSTF) on treating rats with chronic liver injury caused by CCl4. J. Anhui Agric. Sci. 2011, 39, 17148–17149. [Google Scholar]
- Reamy, B.V.; Ford, B.; Goodman, C. Novel pharmacotherapies for hyperlipidemia. Prim Care 2024, 51, 27–40. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Xi, Y.; Feng, Y. Ovarian cancer risk in relation to blood lipid levels and hyperlipidemia: A systematic review and meta-analysis of observational epidemiologic studies. Eur. J. Cancer Prev. 2021, 30, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Zhao, Z.; Guan, Y.; Zhang, C. Effect of corn silk polysaccharides with differert molecular weight on hypolipidemic and its mechanism. China Food Addit. 2023, 34, 241–248. [Google Scholar]
- Zhang, Y.; Wu, L.; Ma, Z.; Cheng, J.; Liu, J. Anti-diabetic, anti-oxidant and anti-hyperlipidemic activities of flavonoids from corn silk on STZ-induced diabetic mice. Molecules 2015, 21, E7. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Liu, J.; Guan, H.; Hou, H. The preventive effect of stigma maydis concentrate on reducing the hyperlipemia. Med. Innov. China 2015, 12, 112–115. [Google Scholar]
- Lapcik, L.; Repka, D.; Lapcikova, B.; Sumczynski, D.; Gautam, S.; Li, P.; Valenta, T. A physicochemical study of the antioxidant activity of corn silk extracts. Foods 2023, 12, 2159. [Google Scholar] [CrossRef] [PubMed]
- El-Ghorab, A.; El-Massry, K.F.; Shibamoto, T. Chemical composition of the volatile extract and antioxidant activities of the volatile and nonvolatile extracts of Egyptian corn silk (Zea mays L.). J. Agric. Food Chem. 2007, 55, 9124–9127. [Google Scholar] [CrossRef]
- Ren, S.C.; Qiao, Q.Q.; Ding, X.L. Antioxidative activity of five flavones glycosides from corn silk (Stigma maydis). Czech J. Food Sci. 2013, 31, 148–155. [Google Scholar] [CrossRef]
- Maksimovi, Z.; Maleni, O.; Kovaevi, N. Polyphenol contents and antioxidant activity of Maydis stigma extracts. Bioresour. Technol. 2005, 96, 873–877. [Google Scholar] [CrossRef]
- Wang, G.; Xu, T.; Bu, X.; Liu, B. Anti-inflammation effects of corn silk in a rat model of carrageenin-induced pleurisy. Inflammation 2012, 35, 822–827. [Google Scholar] [CrossRef]
- Tao, T.Z.; Wang, L.; Liu, J. Role of corn silk for the treatment of Alzheimer’s disease: A mechanism research based on network pharmacology combined with molecular docking and experimental validation. Chem. Biol. Drug Des. 2023, 102, 1231–1247. [Google Scholar] [CrossRef]
- Jin, J.; Yang, Y.; Gong, Q.; Wang, J.; Ni, W.; Wen, J.; Meng, X. Role of epigenetically regulated inflammation in renal diseases. Semin. Cell Dev. Biol. 2024, 154, 295–304. [Google Scholar] [CrossRef]
- Yu, J.; Long, T.; Zhang, P.; Mo, Y.; Li, J.; Li, Y. Study on the mechanism of action of corn silk active ingredient in the treatment of chronic glomerulonephritis based on network pharmacology research. Guangdong Chem. Ind. 2020, 47, 25–27. [Google Scholar]
- Shi, S.; Li, S.; Li, W.; Xu, H. Corn silk tea for hypertension: A systematic review and Meta-Analysis of randomized controlled Trials. Evid. Based Complement. Altern. Med. 2019, 2019, 2915498. [Google Scholar] [CrossRef]
- George, G.O.; Idu, F.K. Corn silk aqueous extracts and intraocular pressure of systemic and non-systemic hypertensive subjects. Clin. Exp. Optom. 2015, 98, 138–149. [Google Scholar] [CrossRef]
- Li, C.; Lee, Y.; Lo, H.; Huang, Y.; Hsiang, C.; Ho, T. Antihypertensive effects of corn silk extract and its novel bioactive constituent in spontaneously hypertensive rats: The involvement of Angiotensin-Converting enzyme inhibition. Molecules 2019, 24, 1886. [Google Scholar] [CrossRef] [PubMed]
- Gulati, A.; Singh, J.; Rasane, P.; Kaur, S.; Kaur, J.; Nanda, V. Anti-cancerous effect of corn silk: A critical review on its mechanism of action and safety evaluation. 3 Biotech 2023, 13, 246. [Google Scholar] [CrossRef]
- Sa’Adatzadeh, M.; Oroojan, A.A.; Behmanesh, M.A.; Mard-Soltani, M. Protective effect of aqueous and methanolic extracts of corn silk on nicotine-induced reproductive system disorders in male mice. JBRA Assist. Reprod. 2023, 27, 644–650. [Google Scholar] [CrossRef] [PubMed]
- Amani, A.; Montazer, M.; Mahmoudirad, M. Synthesis of applicable hydrogel corn silk/ZnO nanocomposites on polyester fabric with antimicrobial properties and low cytotoxicity. Int. J. Biol. Macromol. 2019, 123, 1079–1090. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Dong, W.; Huo, J.; Wang, W. Study on action mechanism of sorn silk in treating type II diabetic rats based on urine metabonomics. Chin. Pharmacol. Bull. 2019, 35, 265–272. [Google Scholar]
- Hu, Q.; Deng, Z. Protective effects of flavonoids from corn silk on oxidative stress induced by exhaustive exercise in mice. Afr. J. Biotechnol. 2011, 10, 3163–3167. [Google Scholar]
- Bai, H.; Hai, C.; Xi, M.; Liang, X.; Liu, R. Protective effect of maize silks (Maydis stigma) ethanol extract on radiation-induced oxidative stress in mice. Plant Foods Hum. Nutr. 2010, 65, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Guan, J.; Chen, Z.; Mo, J.; Wu, K.; Hu, X.; Lan, T.; Guo, J. Fufang Zhenzhu Tiaozhi capsule ameliorates hyperuricemic nephropathy by inhibition of PI3K/AKT/NF-κB pathway. J. Ethnopharmacol. 2022, 298, 115644. [Google Scholar] [CrossRef]
- Tao, H.; Chen, X.; Du, Z.; Ding, K. Corn silk crude polysaccharide exerts anti-pancreatic cancer activity by blocking the EGFR/PI3K/AKT/CREB signaling pathway. Food Funct. 2020, 11, 6961–6970. [Google Scholar] [CrossRef]
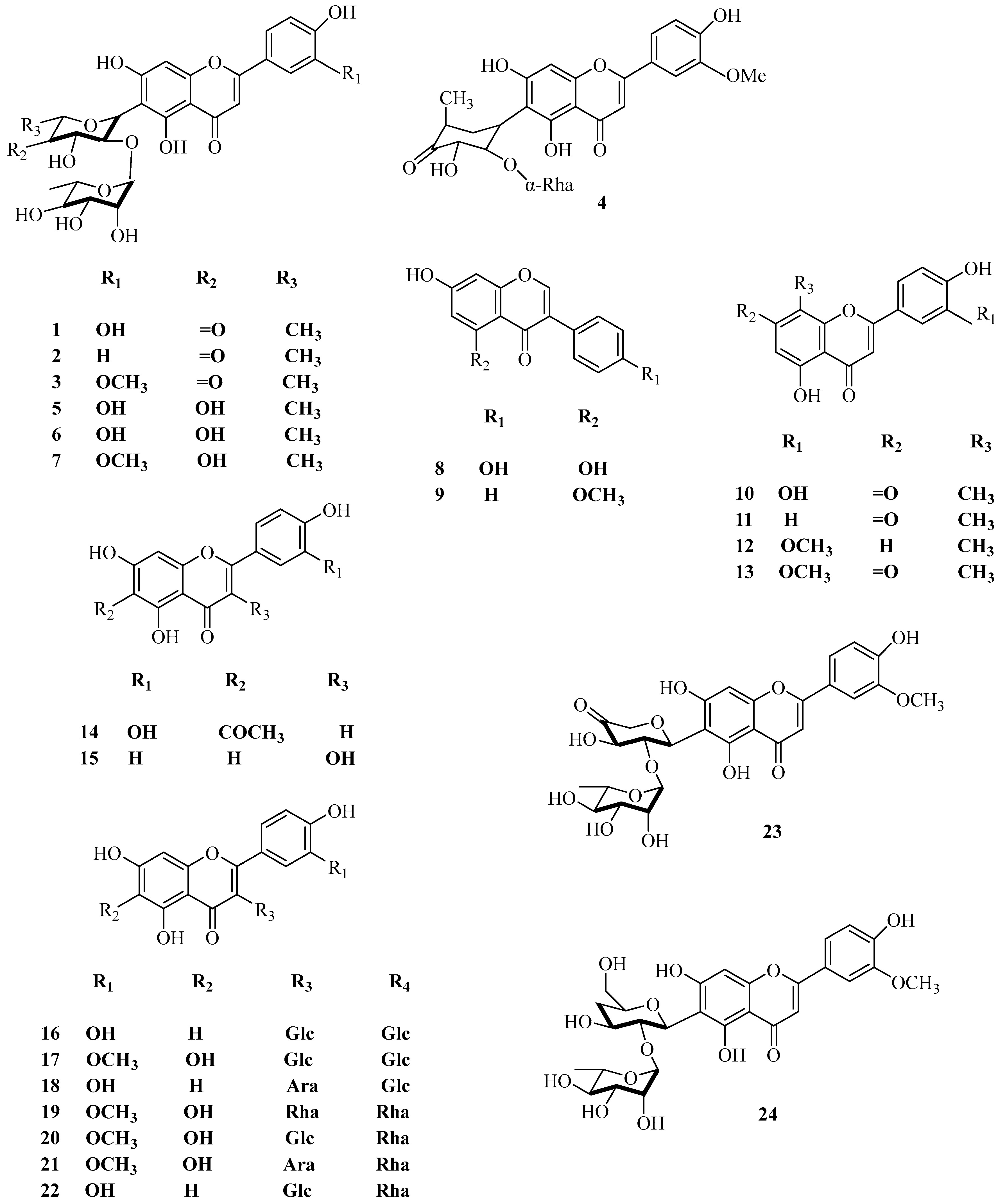
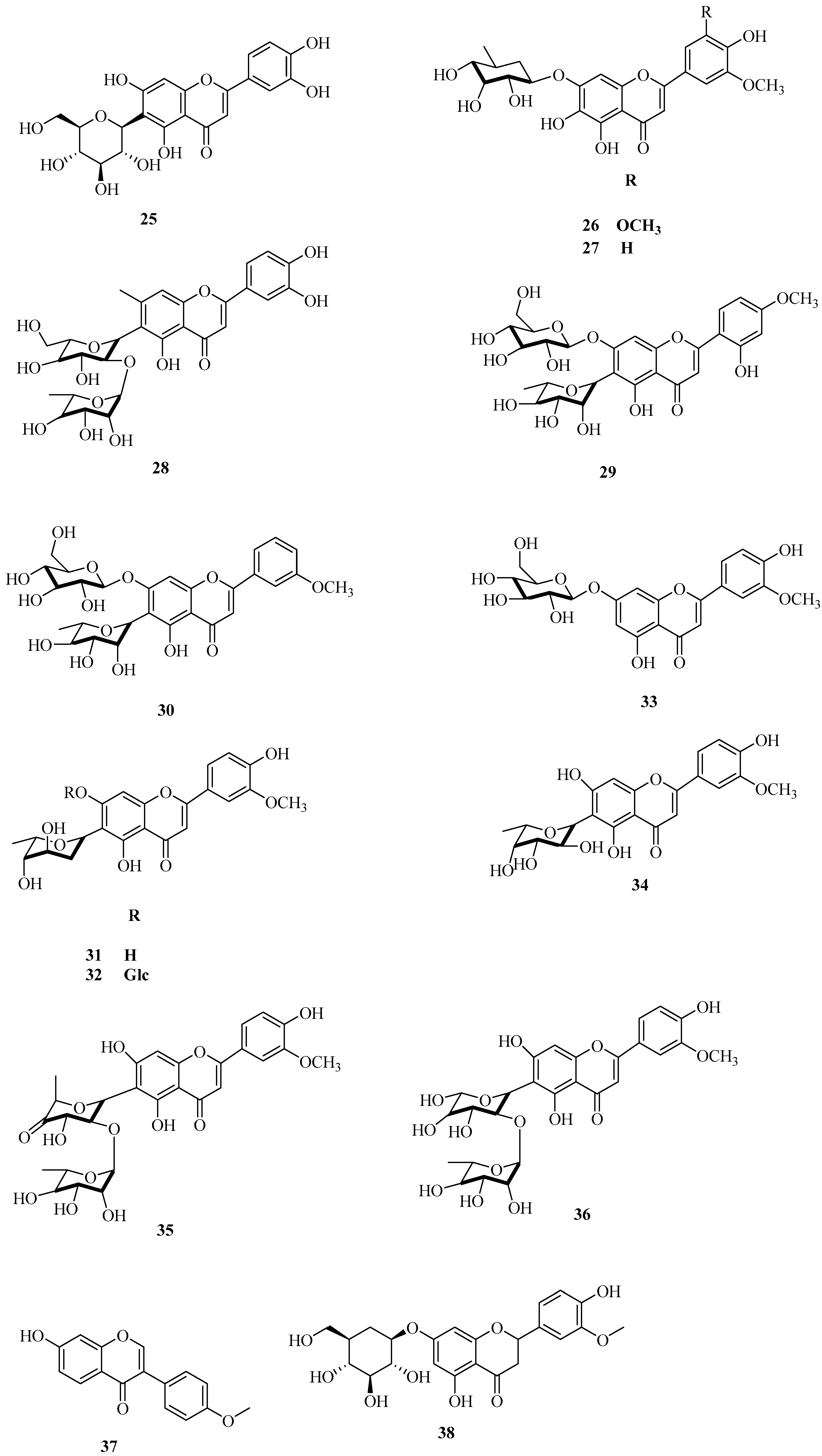
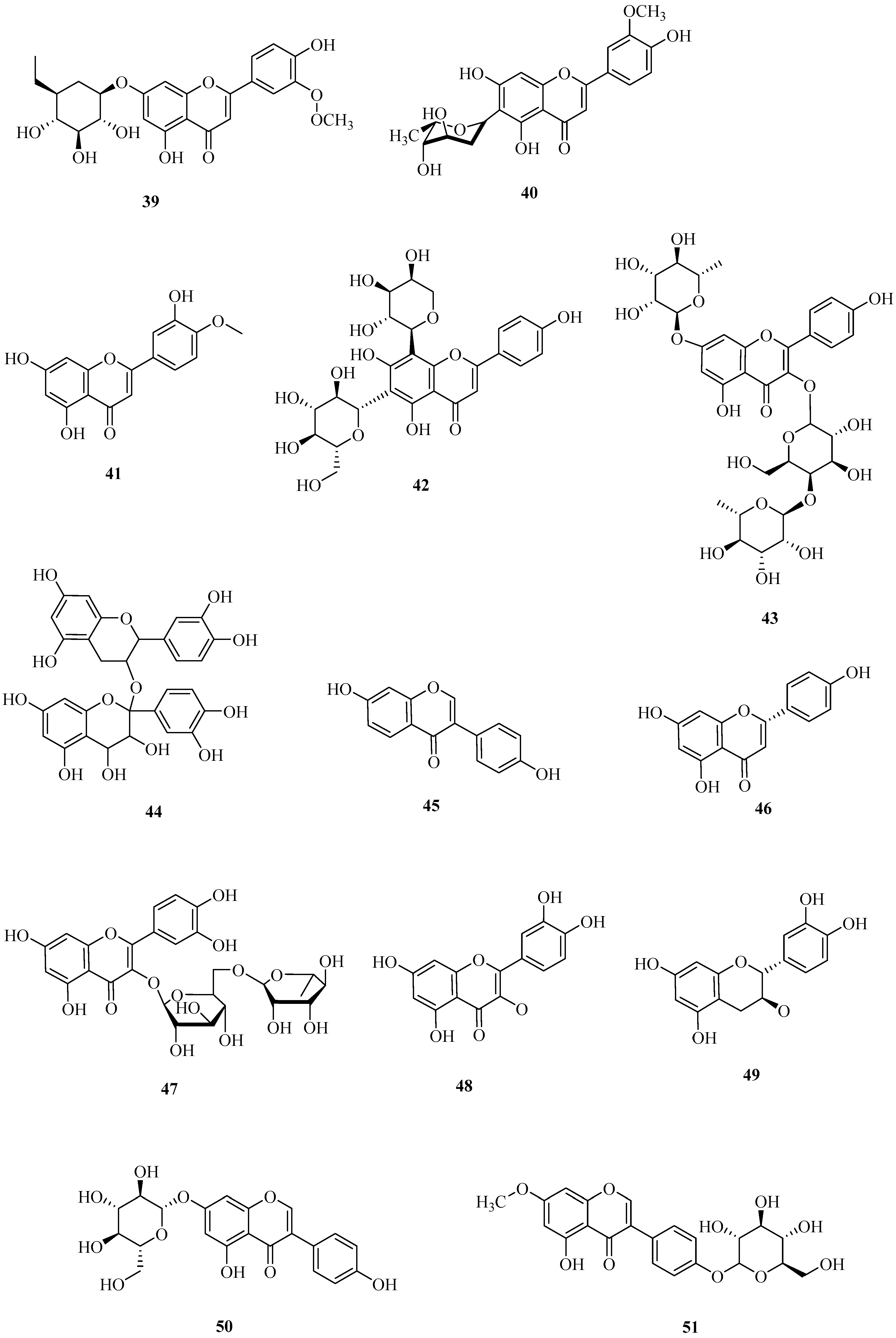


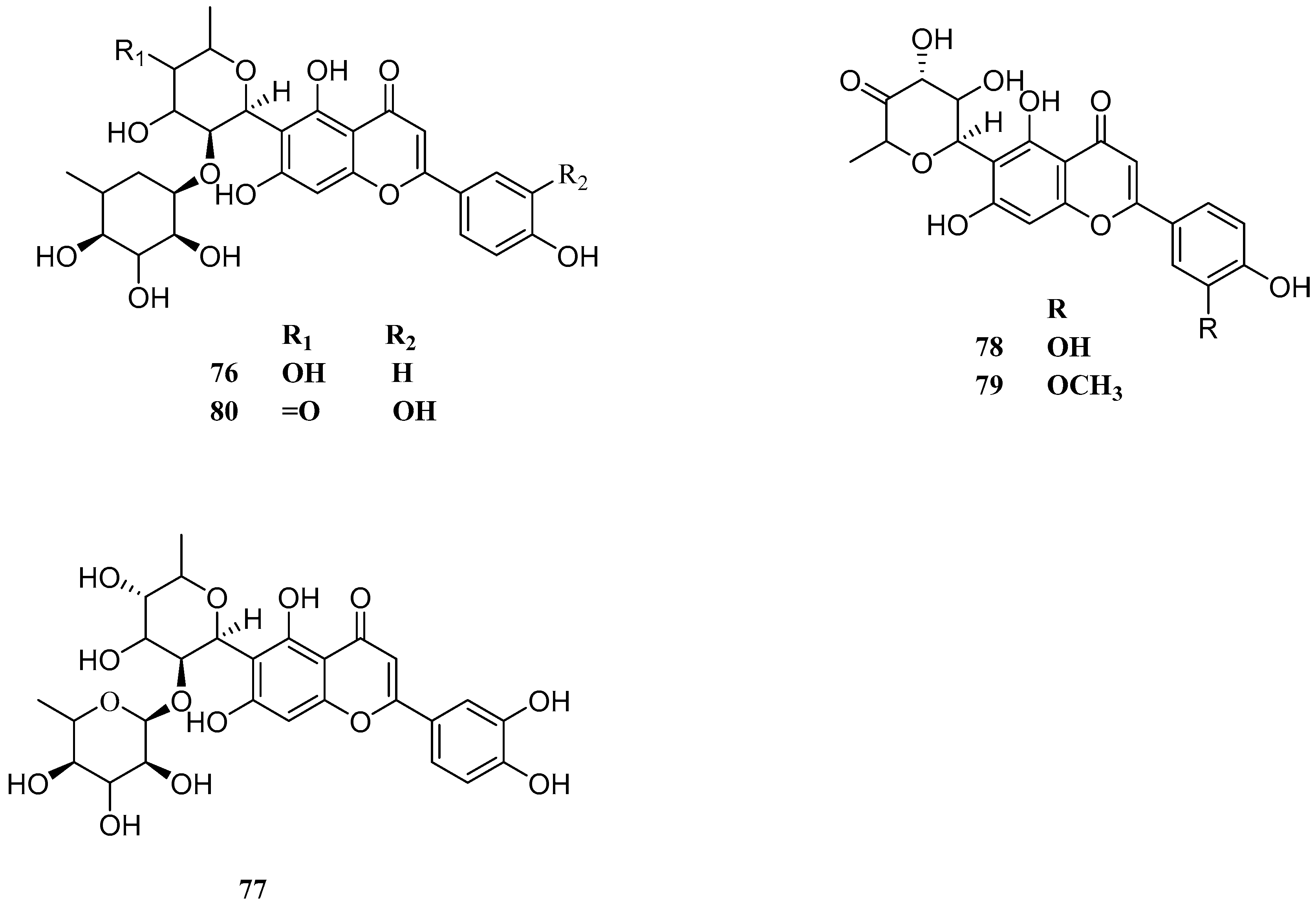


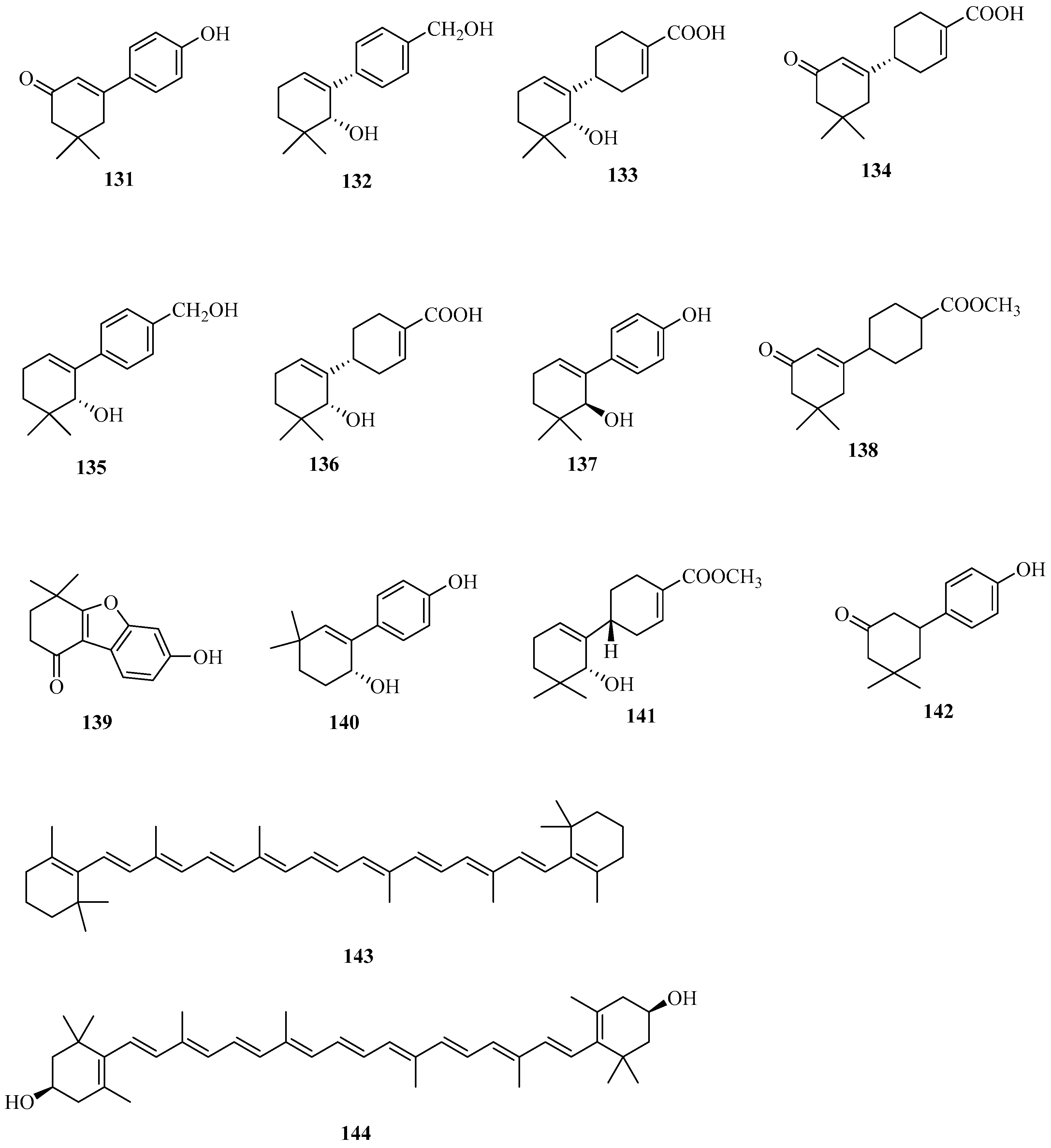
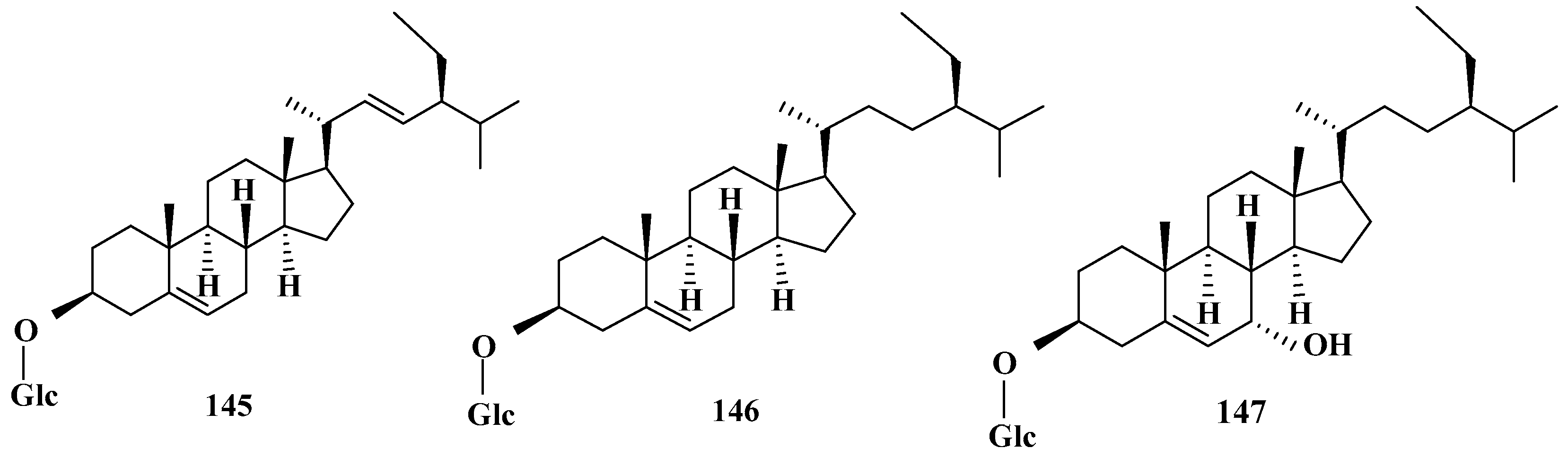
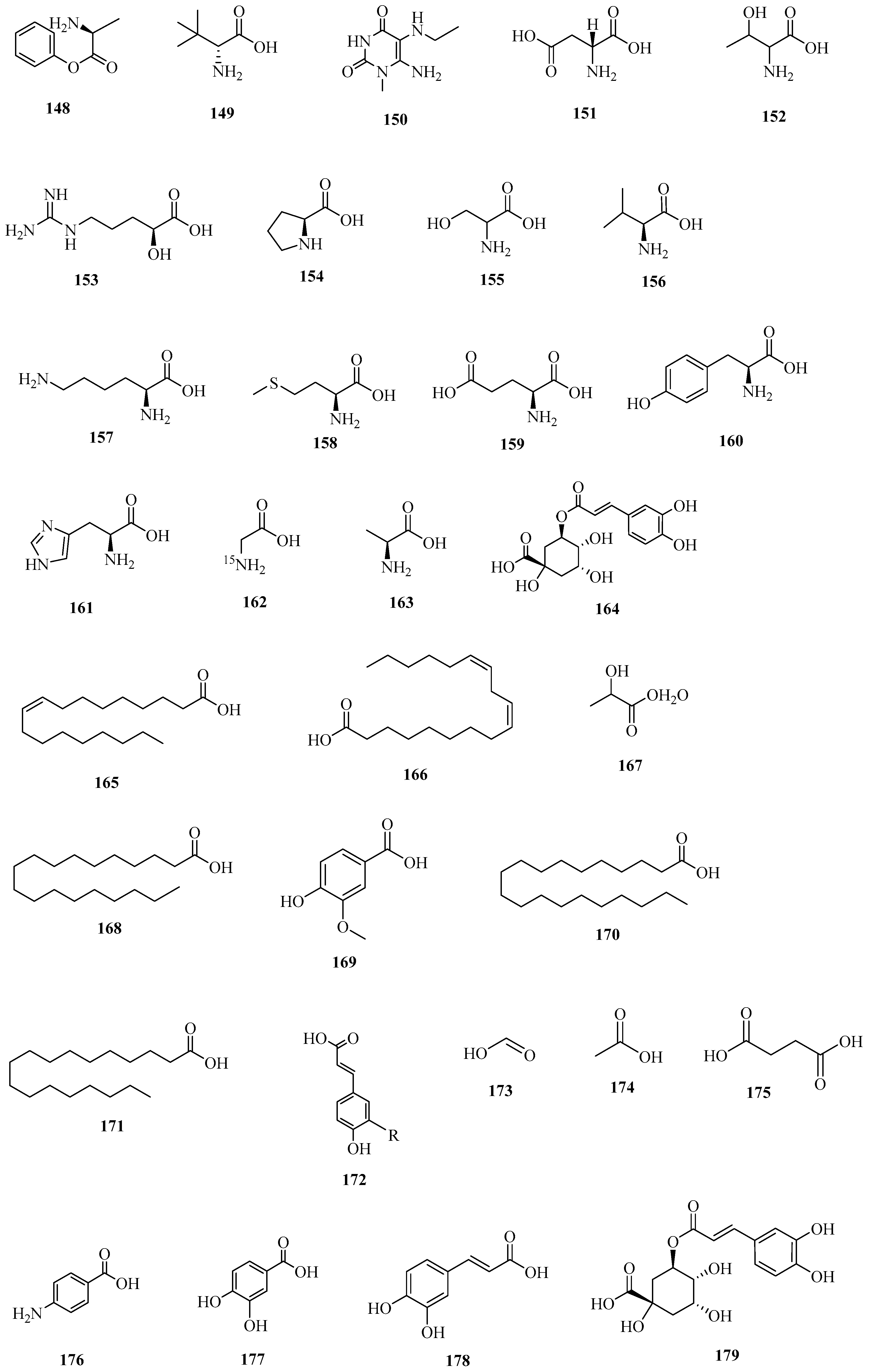
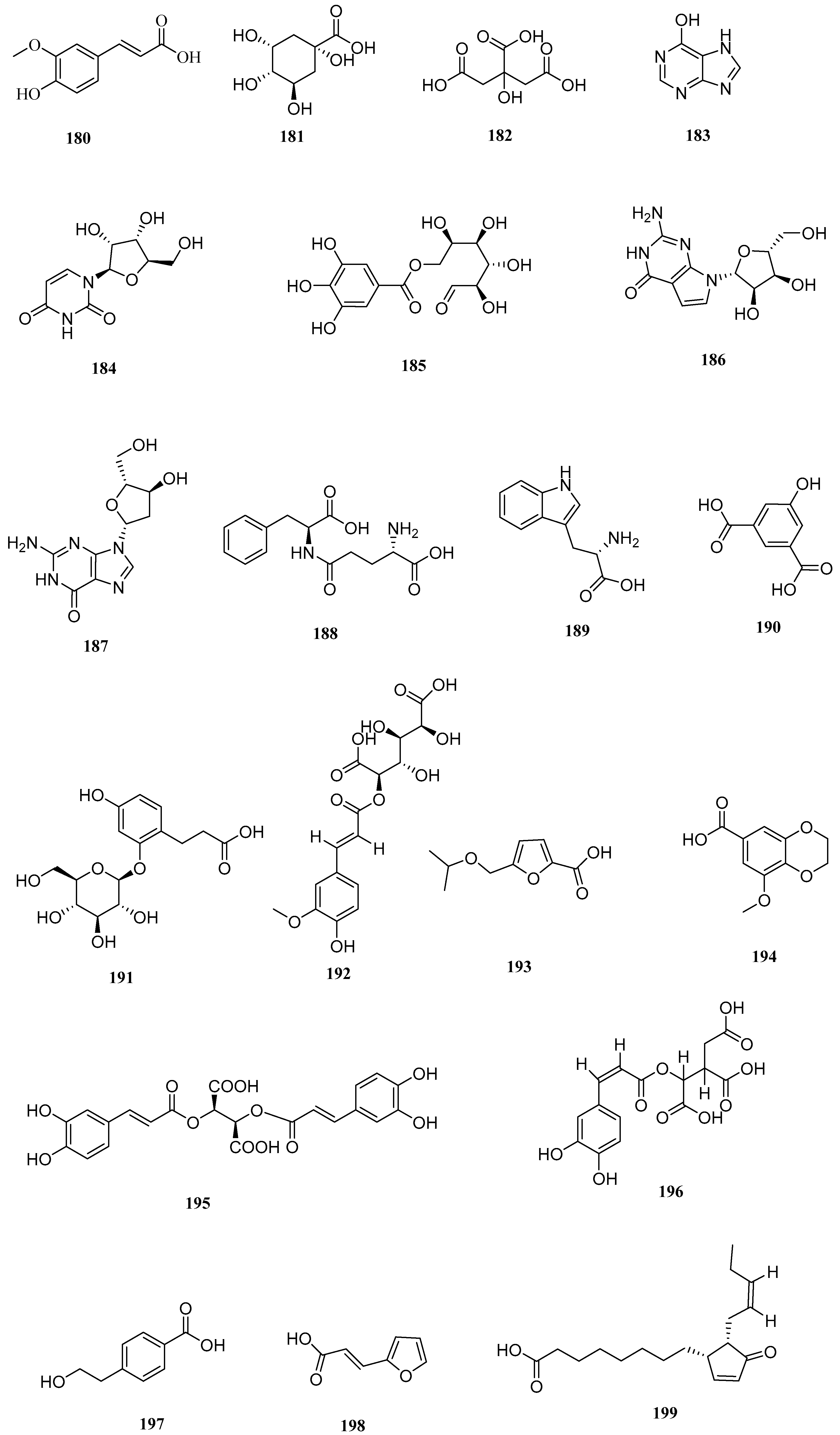
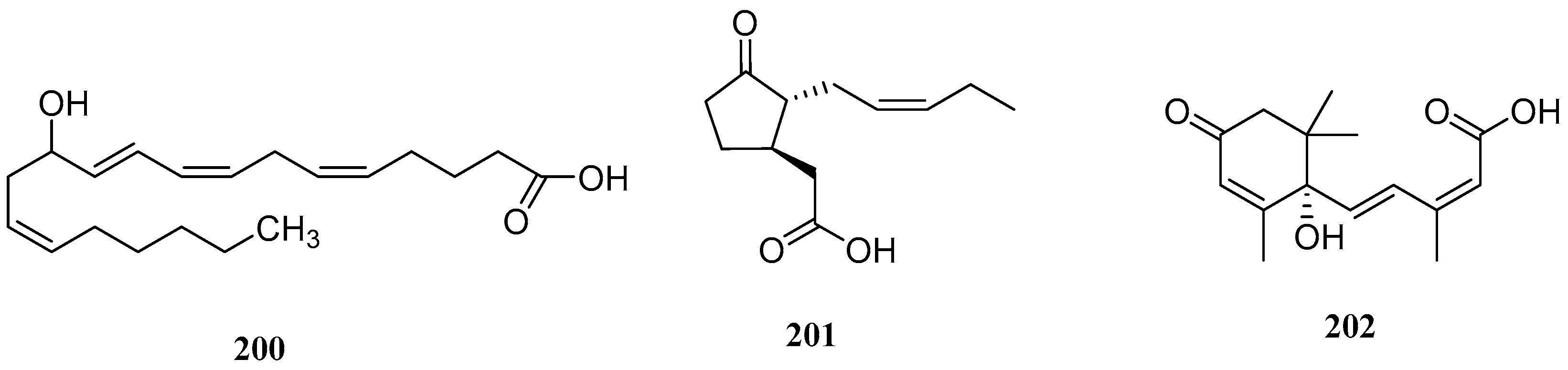

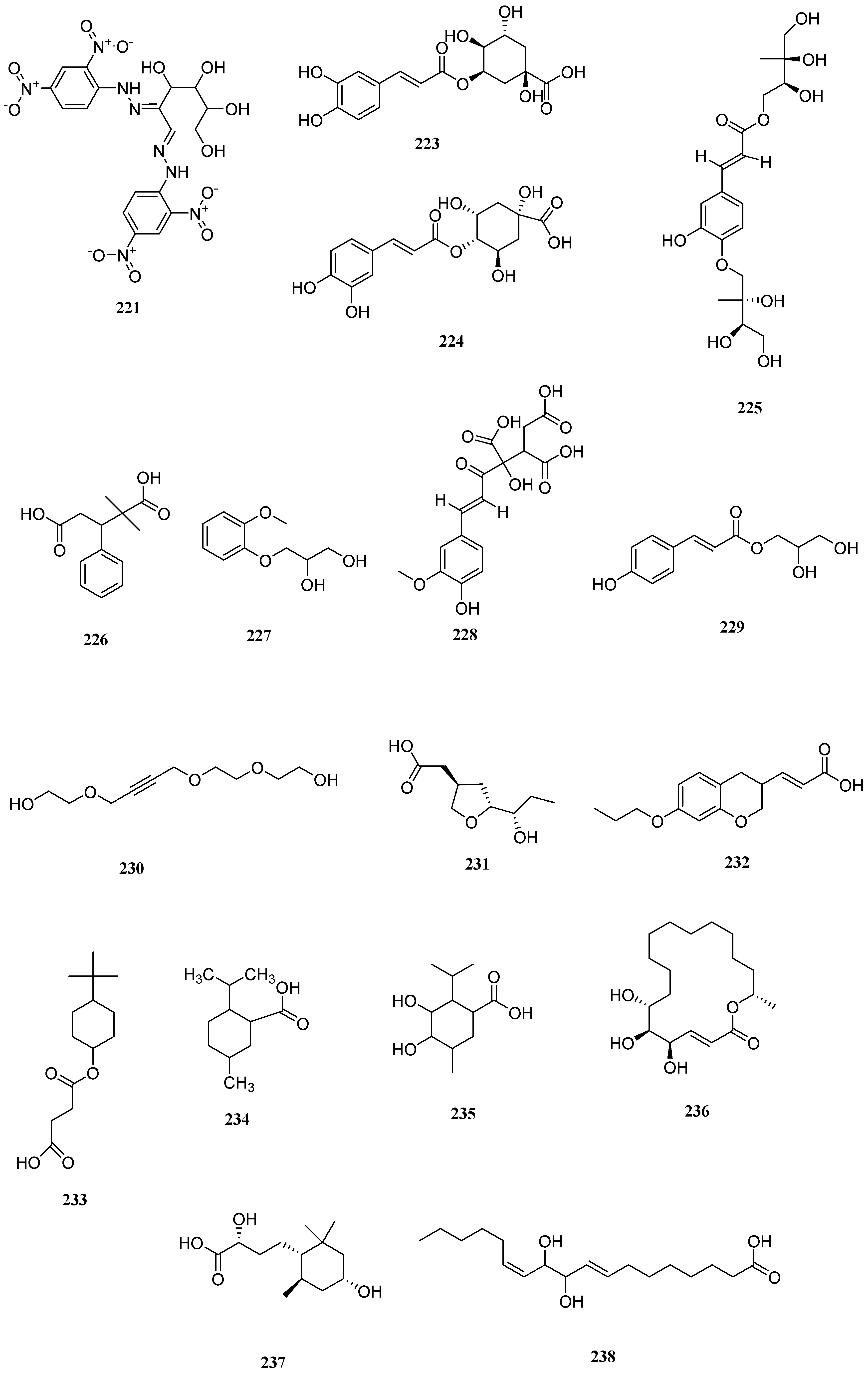
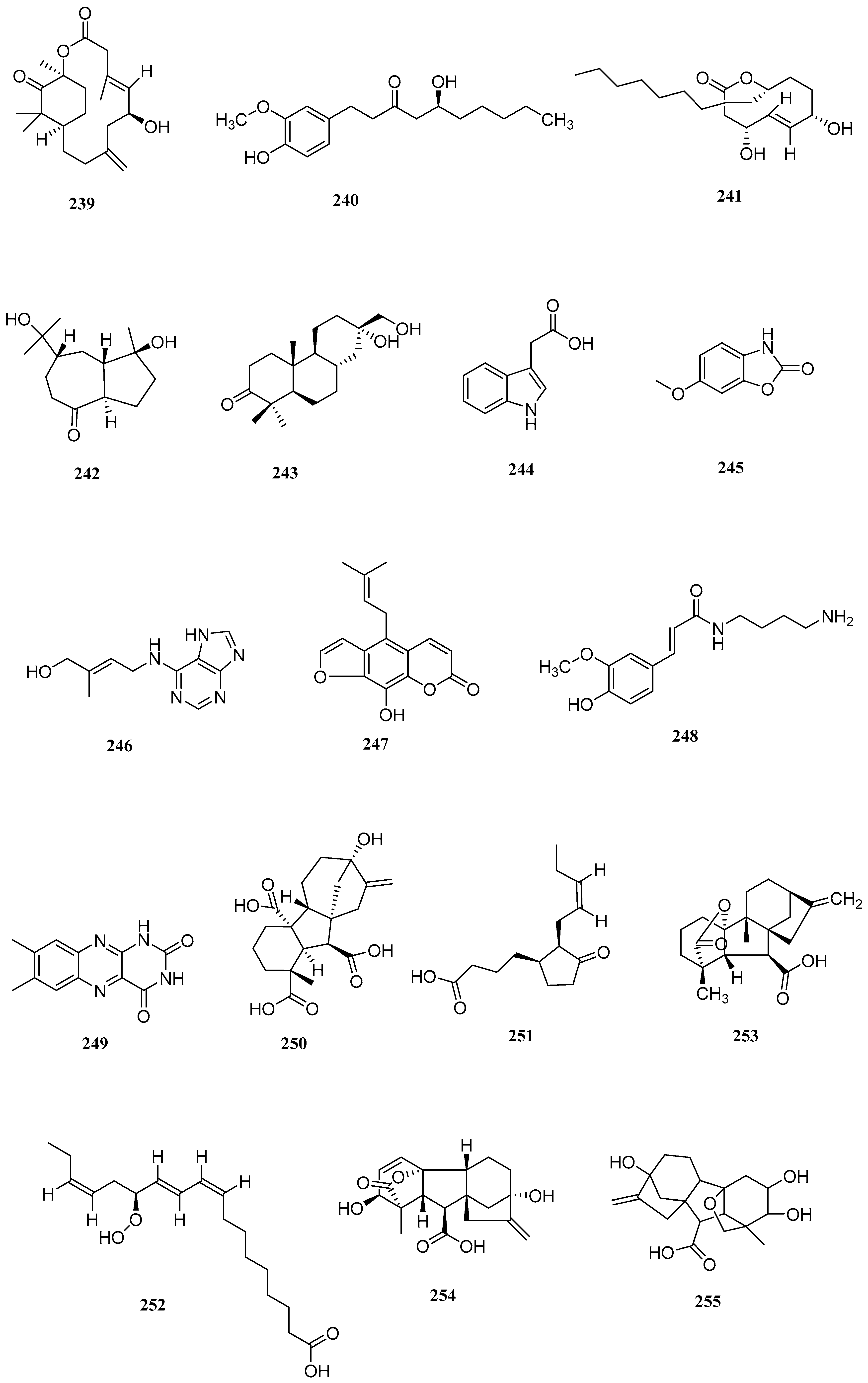
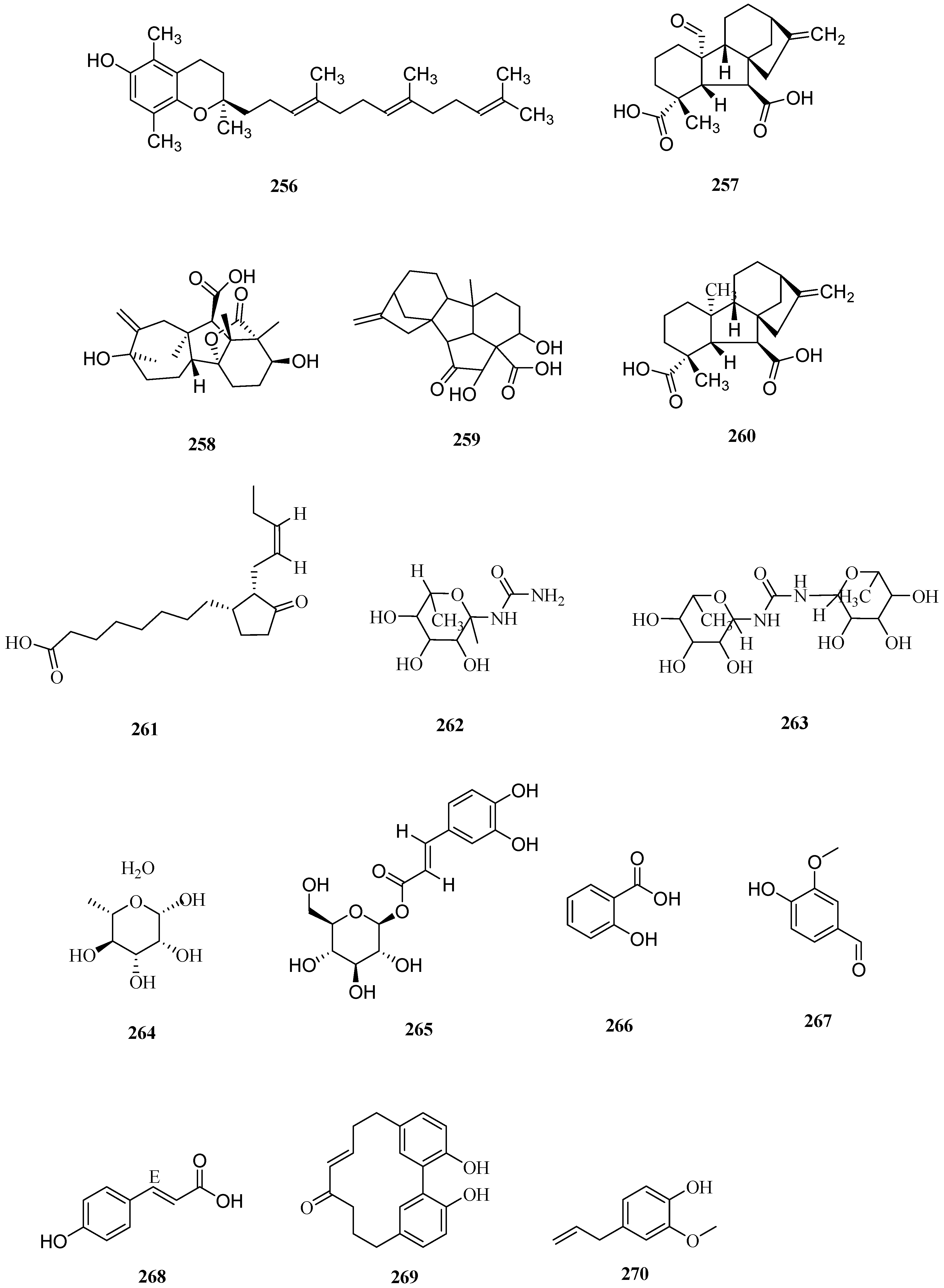
| No. | Name | CAS | Formula | Reference |
|---|---|---|---|---|
| 1 | maysin | 70255-49-1 | C27H28O14 | [20] |
| 2 | apimaysin | 74158-04-6 | C23H46N4O2 | [20] |
| 3 | 3′-methaxymaysin | 101920255 | C28H30O14 | [20] |
| 4 | ax-5″-methane-3′-methoxymaysin | 74977694 | C28H32O14 | [21] |
| 5 | 2″-O-α-l-rhamnose-6-C-quinose-Luteolin | — — | C28H34O14 | [21] |
| 6 | 2″-O-α-l-rhamnose-6-C-fucose-Luteolin | — — | C28H34O14 | [21] |
| 7 | 2″-O-α-l-rhamnoside-6-C-fucoside-3′-methoxyluteolin | — — | C29H36O14 | [22] |
| 8 | genistein | 446-72-0 | C15H10O5 | [23] |
| 9 | 7-hydroxy-4′-methoxyflavone | 487-17-2 | C16H12O4 | [22] |
| 10 | apigenin | 520-36-5 | C15H10O5 | [24] |
| 11 | luteolin | 491-70-3 | C15H10O6 | [25] |
| 12 | chrysoeriol | 491-71-4 | C16H12O6 | [23] |
| 13 | 5,8,4′-trihydroxy-7-methoxyflavanone | — — | C17H13O6 | [26] |
| 14 | 6-acetyl-luteolin | 122377901 | C29H32O16 | [27] |
| 15 | isorhamnetin | 480-19-3 | C16H12O7 | [28] |
| 16 | 5,7,4′-trihydroxyflavone-3,6-C-diglucoside | — — | C27H33O18 | [29] |
| 17 | 5,7,3′-trihydroxy-4′-methoxyflavanone-3,6-C-diglucoside | — — | C18H35O19 | [29] |
| 18 | 5,7,4′-trihydroxyflavone-3-C-Arabinose, 6-C-glucoside | — — | C27H31O17 | [30] |
| 19 | 5,7,3′-trihydroxy-4′-methoxyflavanone-3,6-C-dirhamnoside | — — | C28H35O17 | [30] |
| 20 | 5,7,3′-trihydroxy-4′-methoxyflavanone-3-C-glucose, 6-C-rhamnoside | — — | C28H35O18 | [30] |
| 21 | 5,7,3′-trihydroxy-4′-methoxyflavanone-3-C-rhamnose, 6-C-Arabinoside | — — | C27H33O17 | [30] |
| 22 | 5,7,4′-trihydroxyflavone-3-C-glucose, 6-C-rhamnoside | — — | C27H33O17 | [30] |
| 23 | 2″-O-α-l-rhamnoside-6-C-(6-C-Deoxy-ax-5-Methyl-xyl-hexan-4-carbonyl)-3′-methoxyluteolin | — — | C27H28O14 | [17] |
| 24 | 2″-O-α-l-rhamnoside-6-C-(3-Deoxyglucoside)-3′-methoxyluteolin | — — | C28H32O14 | [22] |
| 25 | luteolin-6-C-glucoside | 4261-42-1 | C21H20O11 | [17] |
| 26 | 6,4′-dihydroxy-3′,5′-dimethoxyflavanone-7-O-glucoside | — — | C24H26O11 | [31] |
| 27 | 6,4′-dihydroxy-3′-methoxyflavanone-7-O-glucoside | — — | C23H24O10 | [32] |
| 28 | isoorientin-2″-O-α-l-rhamnoglucoside | 50980-94-4 | C27H30O15 | [33] |
| 29 | 5,7-dihydroxy-3′-methoxyflavanone-6-C-diglucoside | — — | C28H32O15 | [34] |
| 30 | 5-hydroxy-4′-methoxyflavanone-6-C-rhamnose-7-O-glucoside | — — | C28H32O14 | [34] |
| 31 | chrysoeriol-6-C-β-boywinoside | — — | C22H22O9 | [34] |
| 32 | homoeriodictyol-6-C-β-boivinose-7-O-β-glucopyranoside | — — | C28H43O18 | [34] |
| 33 | homoeriodictyol-7-O-β-d-glucopyranoside | — — | C22H22O11 | [34] |
| 34 | homoeriodictyol-6-C-β-fucoside | — — | C22H22O10 | [35] |
| 35 | 2″-O-α-l-rhamnose-6-C-(trans-5″-methyl-xyl-hexan-4-glucoside)-3′-methoxyluteolin | — — | C28H30O14 | [22] |
| 36 | 7,4′-dihydroxy-3′-methoxyflavanone-2″-O-α-l-rhamnose-6-C-fucoside | — — | C27H30O15 | [36] |
| 37 | formononetin | 485-72-3 | C16H12O4 | [37] |
| 38 | homoeriodictyol 7-O-glucoside | 14982-11-7 | C22H24O11 | [34] |
| 39 | homoeriodictyol-6-C-β-boivinose-7-O-β-glucoside | — — | C24H26O10 | [34] |
| 40 | homoeriodictyol-6-C-β-boivinoside | — — | C22H22O9 | [34] |
| 41 | diosmetin | 520-34-3 | C16H12O6 | [38] |
| 42 | schaftoside | 51938-32-0 | C26H28O14 | [38] |
| 43 | robinin | 301-19-9 | C33H40O19 | [39] |
| 44 | procyanidins | 20347-71-1 | C30H26O13 | [39] |
| 45 | daidzein | 486-66-8 | C15H10O4 | [39] |
| 46 | naringenin | 480-41-1 | C15H12O5 | [39] |
| 47 | rutin | 153-18-4 | C27H30O16 | [39] |
| 48 | quercetin | 117-39-5 | C15H10O7 | [39] |
| 49 | catechin | 154-23-4 | C15H14O6 | [39] |
| 50 | genistin | 529-59-9 | C21H20O10 | [16] |
| 51 | prunetin 5-O-β-d-glucopyranoside | 89595-66-4 | C22H22O10 | [16] |
| 52 | eriodictyol-7-O-glucoside | 38965-51-4 | C21H22O11 | [16] |
| 53 | isovitexin 8-C-β-glucoside | 23666-13-9 | C27H30O15 | [16] |
| 54 | apigenin-6,8-di-glucopyranoside | 73140-47-3 | C25H26O13 | [16] |
| 55 | homoeriodictyol | 69097-98-9 | C16H14O6 | [16] |
| 56 | violanthin | 40581-17-7 | C27H30O14 | [16] |
| 57 | kaempferol-3-O-rhamnoside | 83170-31-4 | C33H40O19 | [16] |
| 58 | 8-C-(2-Rhamnosyl-6-deoxyhexopyranosulyl)-luteolin | 933463-03-7 | C27H28O14 | [16] |
| 59 | isorhamnetin 3-O-neohesperidoside | 55033-90-4 | C28H32O16 | [16] |
| 60 | quercetin 3,7-dimethyl ether-5-glucoside | 44259668 | C23H24O12 | [16] |
| 61 | pectolinarigenin | 520-12-7 | C17H14O6 | [16] |
| 62 | isorhamnetin 3,4′-diglucoside | 5901757 | C28H32O17 | [16] |
| 63 | chrysin-7-O-β-d-glucuronide | 35775-49-6 | C21H18O10 | [16] |
| 64 | astilbin | 29838-67-3 | C21H22O11 | [16] |
| 65 | 3′-deoxymaysin | 44257705 | C27H28O13 | [16] |
| 66 | 3,7-dihydroxy-3′,4′-dimethoxyflavone | 5378832 | C17H14O6 | [16] |
| 67 | 3′-deoxyderhamnosylmaysin | 44257654 | C21H18O9 | [16] |
| 68 | quercetin-3,7,3′,4′-tetramethyl ether | 1245-15-4 | C19H18O7 | [16] |
| 69 | kaempferol 3,7,4′-trimethyl ether | 15486-34-7 | C18H16O6 | [16] |
| 70 | alternanthin | 44258156 | C22H22O9 | [16] |
| 71 | engeletin | 572-31-6 | C21H22O10 | [16] |
| 72 | cirsimaritin | 6601-62-3 | C17H14O6 | [16] |
| 73 | cirsilineol | 41365-32-6 | C18H16O7 | [16] |
| 74 | rhoifolin | 17306-46-6 | C27H30O14 | [16] |
| 75 | prunetrin | 154-36-9 | C22H22O10 | [16] |
| 76 | 2′-O-alpha-l-Rhamnosyl-6-C-quinovopyranosyl-luteolin | 44257958 | C27H30O14 | [25] |
| 77 | 2′-O-alpha-l-Rhamnosyl-6-C-fucosyl-luteolin | 44257957 | C27H30O14 | [25] |
| 78 | derhamnosylmaysin | 44257945 | C21H18O10 | [25] |
| 79 | 3′-O-methylderhamnosylmaysin | 44258171 | C22H20O10 | [25] |
| 80 | 3′-Deoxymaysin | 44257705 | C27H28O13 | [16] |
| No. | Serial Number | Name | CAS | Formula | Reference |
|---|---|---|---|---|---|
| 1 | 81 | stigmasterol | 83-48-7 | C29H48O | [42] |
| 2 | 82 | stigmastone | 1058-61-3 | C29H48O | [43] |
| 3 | 83 | sitostenone | 1058-61-3 | C29H48O | [43] |
| 4 | 84 | 7α-Hydroxysitosterol | 34427-61-7 | C29H50O2 | [34] |
| 5 | 85 | 7β-Hydroxysitosterol | 15140-59-7 | C29H50O2 | [34] |
| 6 | 86 | stigmast-5,22-3β,7α-diol | 375649565 | C29H48O2 | [42] |
| 7 | 87 | β-sitosterol | 83-46-5 | C29H50O | [30] |
| 8 | 88 | stigmast-4-ene-3β,6β-diol | 439985368 | C29H50O2 | [41] |
| 9 | 89 | ergosta-7,22-diene-3β,5α,6β-triol | 12302764 | C28H46O3 | [34] |
| 10 | 90 | stigmast-4,22-diene-3β,6β-diol | 167958-89-6 | C29H48O2 | [41] |
| 11 | 91 | stigmast-5-ene-3β,7α-diol | 34427-61-7 | C29H50O2 | [41] |
| 12 | 92 | ergosterol endoperoxide | 2061-64-5 | C28H44O3 | [41] |
| 13 | 93 | daucosterol-palmitate | 542-44-9 | C19H38O4 | [34] |
| 14 | 94 | cholest-5-en-3-yl acetate | 604-35-3 | C29H48O2 | [38] |
| No. | Serial Number | Name | CAS | Formula | Reference |
|---|---|---|---|---|---|
| 1 | 95 | costunolide | 553-21-9 | C15H20O2 | [38] |
| 2 | 96 | friedelin | 559-74-0 | C30H50O12 | [45] |
| 3 | 97 | α-amyrin | 638-95-9 | C30H50O12 | [46] |
| 4 | 98 | α-terpineol | 98-55-5 | C10H18O | [47] |
| 5 | 99 | citronellol | 106-22-9 | C10H20O | [47] |
| 6 | 100 | 6,11-oxidoacor-4-ene | — — | C15H24O | [47] |
| 7 | 101 | trans-pinocamphone | 547-60-4 | C10H16O | [47] |
| 8 | 102 | neo-iso-3-thujanol | — — | C10H18O | [47] |
| 9 | 103 | cis-sabinene hydrate | 7712-82-5 | C10H18O | [47] |
| 10 | 104 | pseudolaric acid E | — — | C21H30O4 | [48] |
| 11 | 105 | 19-hydroxy-R-kaurane-15-ene-17-carboxylic acid | — — | C21H32O3 | [42] |
| 12 | 106 | 17-hydroxy-R-kaurane-15-ene-19-oleic acid | — — | C21H32O3 | [42] |
| 13 | 107 | 3α-hydroxy-R-kaurane-15-ene-17-oleic acid-19-methyl carboxylate | — — | C22H32O5 | [42] |
| 14 | 108 | ursolic acid | 77-52-1 | C30H48O3 | [45] |
| 15 | 109 | 3-O-Lauryl lactone | — — | C22H32O4 | [49] |
| 16 | 110 | R-iosane-5β,15,16-triol | — — | C20H36O3 | [42] |
| 17 | 111–118 | stigmaydene A–H | — — | — — | [48] |
| 18 | 119 | ent-16α,17-Dihydroxy-19-kauranoic acid | 74365-74-5 | C20H32O4 | [48] |
| 19–26 | 120–124 | stigmaydene I–M | — — | — — | [50] |
| 27 | 125–128 | stigmane A–D | — — | — — | [51] |
| 28–32 | 129 | zeamalic acid A | — — | C15H18O3 | [26] |
| 33–36 | 130 | zeamalic acid C | — — | C15H18O3 | [26] |
| 37 | 131 | 3-(4-hydroxyphenyl)-5,5-dimethyl-2-Cyclohexene-1-one | 4045-07-2 | C24H37N3O2 | [51] |
| 38 | 132–136 | stigmene A–E | — — | — — | [50] |
| 39 | 137–140 | stigmene F–I | — — | — — | [50] |
| 40–44 | 141 | zealexin A3 | 134820458 | C15H21O3 | [50] |
| 45–48 | 142 | 3-(4-hydroxyphenyl)-5,5-dimethyl-2-cyclohexen-1-one | — — | C14H18O2 | [51] |
| 49 | 143 | β-carotene | 7235-40-7 | C40H56 | [52] |
| 50 | 144 | zeaxanthin | 144-68-3 | C40H56O2 | [53] |
| No. | Serial Number | Name | CAS | Formular | Reference |
|---|---|---|---|---|---|
| 1 | 145 | 7α-hydroxy stigmast-3-O-β-d-glucopyranoside | 112137-81-2 | C35H60O7 | [34] |
| 2 | 146 | stigmasterol-3-O-β-d-glucopyranoside | 19716-26-8 | C35H58O6 | [45] |
| 3 | 147 | stigmast-3-O-β-d-glucopyranoside | 474-58-8 | C35H60O6 | [45] |
| No. | Serial Number | Name | CAS | Formula | Reference |
|---|---|---|---|---|---|
| 1 | 148 | phenylalanine | 62056-68-2 | C9H11NO2 | [38] |
| 2 | 149 | d-tert-Leucine | 26782-71-8 | C6H13NO2 | [54] |
| 3 | 150 | l-isoleucine | 131598-62-4 | C6H13NO2 | [54] |
| 4 | 151 | l-aspartic acid | 6899-03-2 | C4H7NO4 | [54] |
| 5 | 152 | dl-threonine | 80-68-2 | C4H9NO3 | [54] |
| 6 | 153 | argininic acid | 157-07-3 | C6H13N3O3 | [54] |
| 7 | 154 | proline | 147-85-3 | C5H9NO2 | [54] |
| 8 | 155 | serine | 302-84-1 | C3H7NO3 | [54] |
| 9 | 156 | valine | 7004-03-7 | C5H11NO2 | [26] |
| 10 | 157 | l-lysine | 56-87-1 | C6H14N2O2 | [54] |
| 11 | 158 | l-methionine | 63-68-3 | C5H11NO2S | [54] |
| 12 | 159 | l-glutamic acid | 56-86-0 | C5H9NO4 | [54] |
| 13 | 160 | l-(−)-Tyrosine | 55520-40-6 | C9H11NO3 | [54] |
| 14 | 161 | l-Histidine | 71-00-1 | C6H9N3O2 | [54] |
| 15 | 162 | glycine-15N | 7299-33-4 | C2H5NO2 | [54] |
| 16 | 163 | l-alanine | 6898-94-8 | C3H7NO2 | [54] |
| 17 | 164 | chlorogenic acid | 327-97-9 | C16H18O9 | [55] |
| 18 | 165 | oleic acid | 112-80-1 | C18H34O2 | [56] |
| 19 | 166 | linoleic acid | 60-33-3 | C18H32O2 | [57] |
| 20 | 167 | lactic acid | 50-21-5 | C3H6O3 | [58] |
| 21 | 168 | docosanoic acid | 112-85-6 | C22H44O2 | [58] |
| 22 | 169 | vanillic acid | 121-34-6 | C8H8O4 | [58] |
| 23 | 170 | stearic acid | 57-11-4 | C18H36O2 | [59] |
| 24 | 171 | palmitic acid-13C | 287100-87-2 | C16H32O2 | [34] |
| 25 | 172 | trans-4-Hydroxycinnamic acid | 4501-31-9 | C9H8O3 | [23] |
| 26 | 173 | formic acid | 64-18-6 | CH2O2 | [58] |
| 27 | 174 | acetic acid | 64-19-7 | C2H4O2 | [58] |
| 28 | 175 | succinic acid | 110-15-6 | C4H6O4 | [58] |
| 29 | 176 | para-aminobenzoic acid | 150-13-0 | C7H7NO2 | [60] |
| 30 | 177 | protocatechuic acid | 99-50-3 | C7H6O4 | [60] |
| 31 | 178 | caffeic acid | 501-16-6 | C9H8O4 | [61] |
| 32 | 179 | 3-O-caffeoylquinic acid | 1049703-62-9 | C16H18O9 | [61] |
| 33 | 180 | ferulic acid | 1135-24-6 | C10H10O4 | [61] |
| 34 | 181 | quinic acid | 77-95-2 | C7H12O6 | [61] |
| 35 | 182 | citric acid | 77-92-9 | C6H8O7 | [61] |
| 36 | 183 | 6-Hydroxypurine | 146469-94-5 | C5H4N4O | [61] |
| 37 | 184 | uridine | 58-96-8 | C9H12N2O6 | [61] |
| 38 | 185 | galloylglucose | 13186-19-1 | C13H16O10 | [61] |
| 39 | 186 | guanosine | 85-30-3 | C10H13N5O5 | [61] |
| 40 | 187 | 2-deoxy-d-guanosine monohydrate | 312-693-72-4 | C10H13N5O4 | [61] |
| 41 | 188 | γ-GLU-PHE | 7432-24-8 | C14H18N2O5 | [61] |
| 42 | 189 | l(−)-tryptophan | 73-22-3 | C11H12N2O2 | [61] |
| 43 | 190 | 5-hydroxyiosphthalic acid | 618-83-7 | C8H6O5 | [61] |
| 44 | 191 | 2-(β-d-Glucopyranosyloxy)-3-(4-hydroxyphenyl)propanoic acid | 9602775 | C15H20O9 | [61] |
| 45 | 192 | 2-(E)-O-feruloyl-d-galactaric acid | 14104340 | C16H18O11 | [61] |
| 46 | 193 | 5-(Isopropoxymethyl)-2-furoic acid | 3926408 | C9H12O4 | [61] |
| 47 | 194 | dicaffeoyltartaric acid | 70831-56-0 | C22H18O12 | [61] |
| 48 | 195 | 2-caffeoylcitric acid | 5280552 | C15H14O10 | [61] |
| 49 | 196 | 8-methoxy-2,3-dihydro-1,4-benzodioxine-6-carboxylic acid | 4962316 | C10H10O5 | [61] |
| 50 | 197 | 4-(2-Hydroxyethyl)benzoic acid | 46112-46-3 | C9H10O3 | [61] |
| 51 | 198 | 2-furanacrylic acid | 539-47-9 | C7H6O3 | [61] |
| 52 | 199 | 12-oxo-PDA | 5280411 | C18H28O3 | [61] |
| 53 | 200 | 12-HETE | 71030-37-0 | C20H32O3 | [25] |
| 54 | 201 | (−)-jasmonic acid | 6894-38-8 | C12H18O3 | [25] |
| 55 | 202 | (s)-(+)-abscisic acid | 21293-29-8 | C15H20O4 | [25] |
| No. | Serial Number | Name | CAS | Formula | Reference |
|---|---|---|---|---|---|
| 1 | 203 | allantoin | 97-59-6 | C4H6N4O3 | [63] |
| 2 | 204 | vanillin | 121-33-5 | C8H8O3 | [63] |
| 3 | 205 | 6,10,14-Trimethyl-5,9,13-pentadecatrien-2-one | 762-29-8 | C18H30O | [38] |
| 4 | 206 | 6-methoxybenzo[d]isoxazole-3-carboxylic acid | 28691-48-7 | C9H7NO4 | [58] |
| 5 | 207 | adenosine | 58-61-7 | C10H13N5O4 | [58] |
| 6 | 208 | guanine | 73-40-5 | C5H5N5O | [58] |
| 7 | 209 | uracil | 66-22-8 | C4H4N2O2 | [58] |
| 8 | 210 | dextrose | 492-62-6 | C6H12O6 | [42] |
| 9 | 211 | l-Rhamnose | 6155-35-7 | C6H14O6 | [23] |
| 10 | 212 | d-mannopyranose | 530-26-7 | C6H12O6 | [23] |
| 11 | 213 | d-Galactose | 59-23-4 | C6H12O6 | [23] |
| 12 | 214 | dl-Xylose | 25990-60-7 | C5H10O5 | [23] |
| 13 | 215 | d-(+)-Fucose | 3615-37-0 | C6H12O5 | [23] |
| 14 | 216 | d-Ribose | 50-69-1 | C5H10O5 | [23] |
| 15 | 217 | l-(+)-Ribose | 24259-59-4 | C5H10O5 | [23] |
| 16 | 218 | bovolide | 774-64-1 | C11H16O2 | [64] |
| 17 | 219 | 5,6-Dihydro-1H-imidazo [4,5-d]pyridazine-4,7-dione | 6293-09-0 | C5H4N4O2 | [61] |
| 18 | 220 | (2S)-2-(β-d-Glucopyranosyloxy)-3-hydroxypropyl butyrate | 9089566 | C13H24O9 | [61] |
| 19 | 221 | 5,6-Bis[(2,4-dinitrophenyl)hydrazono]-1,2,3,4-hexanetetrol | 54027-04-2 | C18H18N8O12 | [61] |
| 20 | 222 | 3,4-dihydroxybenzaldehyde | 134998-43-9 | C7H6O3 | [61] |
| 21 | 223 | neochlorogenic acid | 906-33-2 | C16H18O9 | [61] |
| 22 | 224 | cryptochlorogenic acid | 905-99-7 | C16H18O9 | [61] |
| 23 | 225 | Evolvoid A | 16723783 | C19H28O10 | [61] |
| 24 | 226 | 2,2-Dimethyl-3-phenylpentanedioic acid hydrate (1:1) | 2029423 | C13H18O5 | [61] |
| 25 | 227 | guaifenesin | 93-14-1 | C10H14O4 | [61] |
| 26 | 228 | 1-O-p-coumaroylglycerol | 106055-11-2 | C12H14O5 | [61] |
| 27 | 229 | feruloylisocitric acid | 129661569 | C16H16O10 | [61] |
| 28 | 230 | 2-(2-((4-(2-Hydroxyethoxy)-2-butynyl)oxy)ethoxy)ethanol | 84282-21-3 | C10H18O5 | [61] |
| 29 | 231 | {(3R,5R)-5-[(1S)-1-Hydroxypropyl]tetrahydro-3-furanyl}acetic acid | 9681387 | C9H16O4 | [61] |
| 30 | 232 | (2E)-3-(7-Propoxy-3,4-dihydro-2H-chromen-3-yl)acrylic acid | 10826028 | C15H18O4 | [61] |
| 31 | 233 | 4-[(4-tert-butylcyclohexyl)oxy]-4-oxobutanoic acid | 148114-19-6 | C14H24O4 | [61] |
| 32 | 234 | 2-Isopropyl-5-methylhexanedioic acid | 39668-86-5 | C11H20O2 | [61] |
| 33 | 235 | 3,4-Dihydroxy-2-isopropyl-5-methylcyclohexanecarboxylic acid | 28288176 | C11H20O4 | [61] |
| 34 | 236 | (+)-Aspicilin | 52461-05-9 | C18H32O5 | [61] |
| 35 | 237 | (2R)-2-Hydroxy-4-[(1S,4R,6R)-4-hydroxy-2,2,6-trimethylcyclohexyl]butanoic acid | 16216665 | C13H24O4 | [61] |
| 36 | 238 | (8E,12Z)-10,11-Dihydroxy-8,12-octadecadienoic acid | 27025513 | C18H32O4 | [61] |
| 37 | 239 | cespitularin Q | 101408387 | C20H30O4 | [25] |
| 38 | 240 | (+)-Gingerol | 1391-73-7 | C17H26O4 | [61] |
| 39 | 241 | seimatopolide B | 57409556 | C18H3O4 | [61] |
| 40 | 242 | pleocarpenone | 102158596 | C14H24O3 | [61] |
| 41 | 243 | 13-Hydroxy-13-(hydroxymethyl)podocarpan-3-one | 28284391 | C18H30O3 | [61] |
| 42 | 244 | indole-3-acetic acid | 87-51-4 | C10H9NO2 | [61] |
| 43 | 245 | 6-Methoxybenzoxazolin-2(3H)-one | 10772 | C8H7NO3 | [61] |
| 44 | 246 | trans-Zeatin | 1637-39-4 | C10H13N5O | [61] |
| 45 | 247 | alloimperatorin | 642-05-7 | C16H14O4 | [61] |
| 46 | 248 | subaphyllin | 501-13-3 | C14H20N2O3 | [61] |
| 47 | 249 | lumichrome | 1086-80-2 | C12H10N4O2 | [61] |
| 48 | 250 | gibberellin A17 | 5460657 | C20H26O7 | [61] |
| 49 | 251 | OPC-4:0 | 5716900 | C14H22O3 | [61] |
| 50 | 252 | 13(S)-Hydroperoxylinolenic acid | 5497123 | C18H30O4 | [61] |
| 51 | 253 | gibberellin A9 | 427-77-0 | C19H24O4 | [61] |
| 52 | 254 | gibberellin A3 | 77-06-5 | C19H22O6 | [61] |
| 53 | 255 | gibberellin A8 | 7044-72-6 | C19H24O7 | [61] |
| 54 | 256 | β-Tocotrienol | 490-23-3 | C28H42O2 | [61] |
| 55 | 257 | gibberellin A24 | 19427-32-8 | C20H26O5 | [61] |
| 56 | 258 | gibberellin A1 | 545-97-1 | C19H24O6 | [61] |
| 57 | 259 | gibberellin A14 | 429678-85-3 | C20H28O5 | [61] |
| 58 | 260 | gibberellin A12 | 1164-45-0 | C20H28O4 | [61] |
| 59 | 261 | 3-Oxo-2-(2-entenyl)cyclopentaneoctanoic acid | 5280729 | C18H30O3 | [61] |
| 60 | 262 | rhamnosyl urea | — — | C13H24N2O9 | [27] |
| 61 | 263 | 1,3-dirhamnosyl urea | — — | C8H16N2O5 | [27] |
| 62 | 264 | l-mannosehydrat | 10030-85-0 | C6H14O6 | [34] |
| 63 | 265 | caffeoyl glucoside | 5281761 | C15H18O9 | [61] |
| 64 | 266 | salicylic acid | 69-72-7 | C7H6O3 | [61] |
| 65 | 267 | vanillic aldehyde | 8014-42-4 | C8H8O3 | [61] |
| 66 | 268 | (E)-p-coumaric acid | 501-98-4 | C9H8O3 | [61] |
| 67 | 269 | alnusone | 52330-11-7 | C19H18O3 | [45] |
| 68 | 270 | eugenol | 97-53-0 | C10H12O2 | [47] |
| 69 | 271 | (2S,3R,4R,5E)-5-[(2E)-{6-Amino-9-[(2R,3R,4S,5R)-3,4-dihydroxy-5-(hydroxymethyl)tetrahydro-2-furanyl]-1,9-dihydro-2H-purin-2-ylidene}hydrazono]-1,2,3,4-pentanetetrol | — — | C15H23N7O8 | [61] |
| 70 | 272 | 1-(4-Amino-1,2,5-oxadiazol-3-yl)-5-(methoxymethyl)-N’-(2-oxo-2H indol-3-yl)-1H-1,2,3-triazole-4-carbohydrazide | — — | C15H13N9O4 | [61] |
| 71 | 273 | ylamide | — — | C18H24N4O11 | [61] |
| 72 | 274 | 6-O-(2-Hydroxyhexanoyl)-d-glucopyranose | — — | C12H22O8 | [61] |
| 73 | 275 | dimethyl3,3-(2,5-dihydroxy-3,6-dioxo-1,4-cyclohexadiene-1,4-diyl)bis [3-(3-hydroxy-4-methoxyphenyl)propanoate] | — — | C28H28O12 | [61] |
| 74 | 276 | 5-Hydroxy-2-(4-hydroxy-3-methoxyphenyl)-4-oxo-4H-chromen-7-yl2-O-β-d-threo-hexopyranuronosyl-β-d-threo hexopyranosiduronic acid | — — | C28H28O18 | [61] |
| 75 | 277 | 4-[Bis(2-hydroxy-4-oxo-4H-chromen-3-yl)methyl]phenyl(2E)-3-(3,4-dihydroxyphenyl)acrylate | — — | C34H22O10 | [61] |
| 76 | 278 | (1S,3R,4R,5R)-1-{[(3S)-3-(3,4-Dihydroxyphenyl)-3-methoxypropanoyl]oxy}-3-{[(2E)-3-(3,4-dihydroxyphenyl)-2-propenoyl]oxy}-4,5-dihydroxycyclohexanecarboxylic acid | — — | C26H28O13 | [61] |
| 77 | 279 | (1S,3R,4R,5R)-3-{[(3S)-3-(3,4-Dihydroxyphenyl)-3-methoxypropanoyl]oxy}-1-{[(2E)-3-(3,4-dihydroxyphenyl)-2-propenoyl]oxy}-4,5-dihydroxycyclohexanecarboxylic acid | — — | C26H28O13 | [61] |
| 78 | 280 | 5-Hydroxy-2-(4-methoxyphenyl)-4-oxo-4H-chromen-7-yl 2-O-(6-deoxy-α-l-mannopyranosyl)-β-d-glucopyranosiduronic acid | — — | C28H30O15 | [61] |
| 79 | 281 | (3R,4R,5E,9S,10R)-9-Hydroxy-3-methyl-2-oxo-10-pentyl-3,4,7,8,9,10-hexahydro-2H-oxecin-4-yl acetate | — — | C17H28O5 | [61] |
| 80 | 282 | (4S,6S,7Z,9S,10S)-4,6,9-Trihydroxy-10-nonyl-3,4,5,6,9,10-hexahydro-2H-oxecin-2-one | — — | C18H32O5 | [61] |
| 81 | 283 | (3E,5S,6R,7S,18S)-5,6,7-Trihydroxy-18-methyloxacyclooctadec-3-en-2-one | — — | C18H32O5 | [61] |
| 82 | 284 | 5-{(2R,5R)-5-[(1R)-1-Hydroxynonyl]tetrahydro-2-furanyl}pentanoic acid | — — | C18H34O4 | [25] |
| No. | Pharmacological Actions | Mechanisms | References |
|---|---|---|---|
| 1 | Hyperglycemic effect | Callback sugar metabolism, fat metabolism, amino acid metabolic pathways of chenodeoxycholic acid, 5-HIAA, (R)-3-hydroxybutyric acid, argininosuccinic acid, 4,6-dihydroxyquinoline, LTB4, and other sites, improve glucose, lipid, and amino acid metabolism disorders | [95] |
| Repair the pathological changes in the liver, kidney, and pancreas | [67] | ||
| Exhibits good hypoglycemic effect on type II diabetic mice, and has a good inhibitory effect on α-glucosidase and α-amylase activities | [68] | ||
| 2 | Antigout action | Inhibit the expression of IL-1β and decrease serum uric acid level | [71] |
| Reduce the production of UA, BUN, and Cr by reducing the concentration of Xanthine oxidase (XOD) and PRPS | [73] | ||
| 3 | Liver protection | Down-regulation of Smad3 mRNA expression in liver tissue reduces the secretion of ECM and inhibits the development of liver fibrosis | [74] |
| Reduce MDA content in serum and liver and increase SOD activity, the mechanism may be related to anti-lipid peroxidation | [75] | ||
| 4 | Anti-hyperlipidemia | Increase LPL and HL enzyme activities, reduce TC, TG, and LDL-C contents, and increase HDL-C content to regulate blood lipid balance and increase SOD, GSH-px, and CAT anti-oxidant enzyme activities | [78] |
| 5 | Anti-oxidant | Increase anti-oxidant enzyme levels and inhibit lipid peroxidation | [96] |
| Against oxidative stress through the upregulation of Nrf2 | [97] | ||
| 6 | Anti-inflammatory | Enhance T-cell-mediated immune response and decrease inflammatory factors | [47] |
| Reduce the expression of TNF-α and IL-1β | [88] | ||
| 7 | Kidney protection | Decrease UA production by interfering with XOD | [47] |
| PI3K/AKT and NF-κB signaling were the pivotal pathways | [98] | ||
| 8 | Antihypertensive | Vascular expansion in low-concentration | [90] |
| 9 | Anti-cancer | Anti-cancer through the serine/threonine kinases (Akt)/lipid kinases (PI3Ks) pathway | [99] |
| 10 | Protecting the reproductive system | Recover the amounts of sex hormones and sperm count to normal conditions by reducing lipid peroxidation | [93] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Mao, J.; Zhang, M.; Liu, L.; Zhu, Y.; Gu, M.; Zhang, J.; Bu, H.; Sun, Y.; Sun, J.; et al. An Umbrella Insight into the Phytochemistry Features and Biological Activities of Corn Silk: A Narrative Review. Molecules 2024, 29, 891. https://doi.org/10.3390/molecules29040891
Wang Y, Mao J, Zhang M, Liu L, Zhu Y, Gu M, Zhang J, Bu H, Sun Y, Sun J, et al. An Umbrella Insight into the Phytochemistry Features and Biological Activities of Corn Silk: A Narrative Review. Molecules. 2024; 29(4):891. https://doi.org/10.3390/molecules29040891
Chicago/Turabian StyleWang, Yumei, Jialin Mao, Meng Zhang, Lei Liu, Yu Zhu, Meiling Gu, Jinling Zhang, Hongzhou Bu, Yu Sun, Jia Sun, and et al. 2024. "An Umbrella Insight into the Phytochemistry Features and Biological Activities of Corn Silk: A Narrative Review" Molecules 29, no. 4: 891. https://doi.org/10.3390/molecules29040891
APA StyleWang, Y., Mao, J., Zhang, M., Liu, L., Zhu, Y., Gu, M., Zhang, J., Bu, H., Sun, Y., Sun, J., Ma, Y., Guo, L., Zheng, Y., & Liu, Q. (2024). An Umbrella Insight into the Phytochemistry Features and Biological Activities of Corn Silk: A Narrative Review. Molecules, 29(4), 891. https://doi.org/10.3390/molecules29040891