Quantitative Analysis of Cenobamate and Concomitant Anti-Seizure Medications in Human Plasma via Ultra-High Performance Liquid Chromatography–Tandem Mass Spectrometry
Abstract
1. Introduction
2. Results and Discussion
2.1. Comparison of Existing Methods
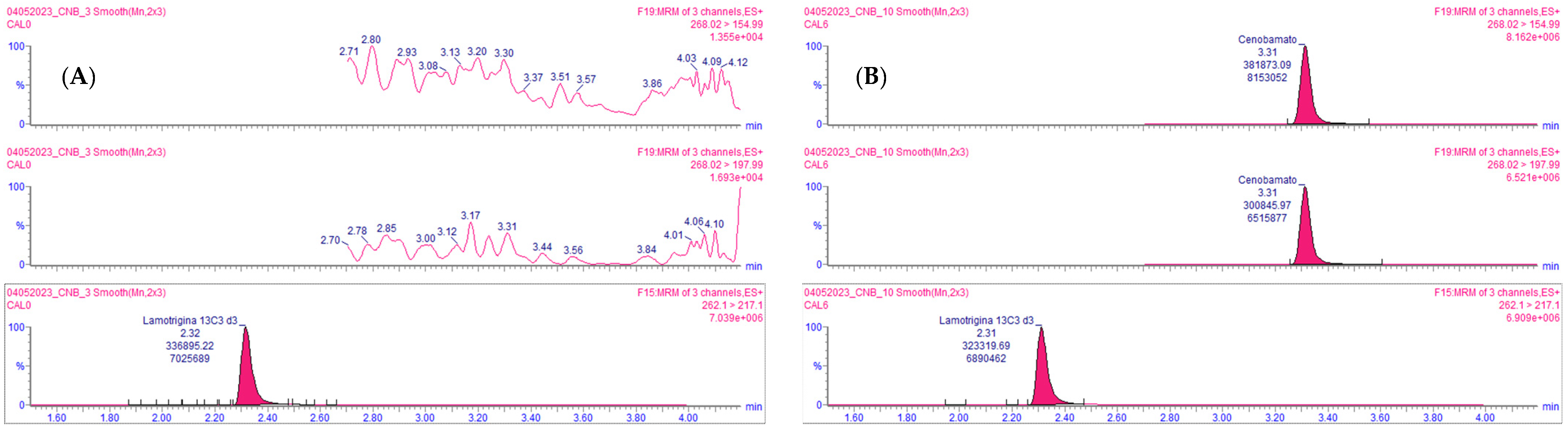
2.2. Method Validation
Automated Sample Extraction Validation
2.3. Analysis of Patient Samples
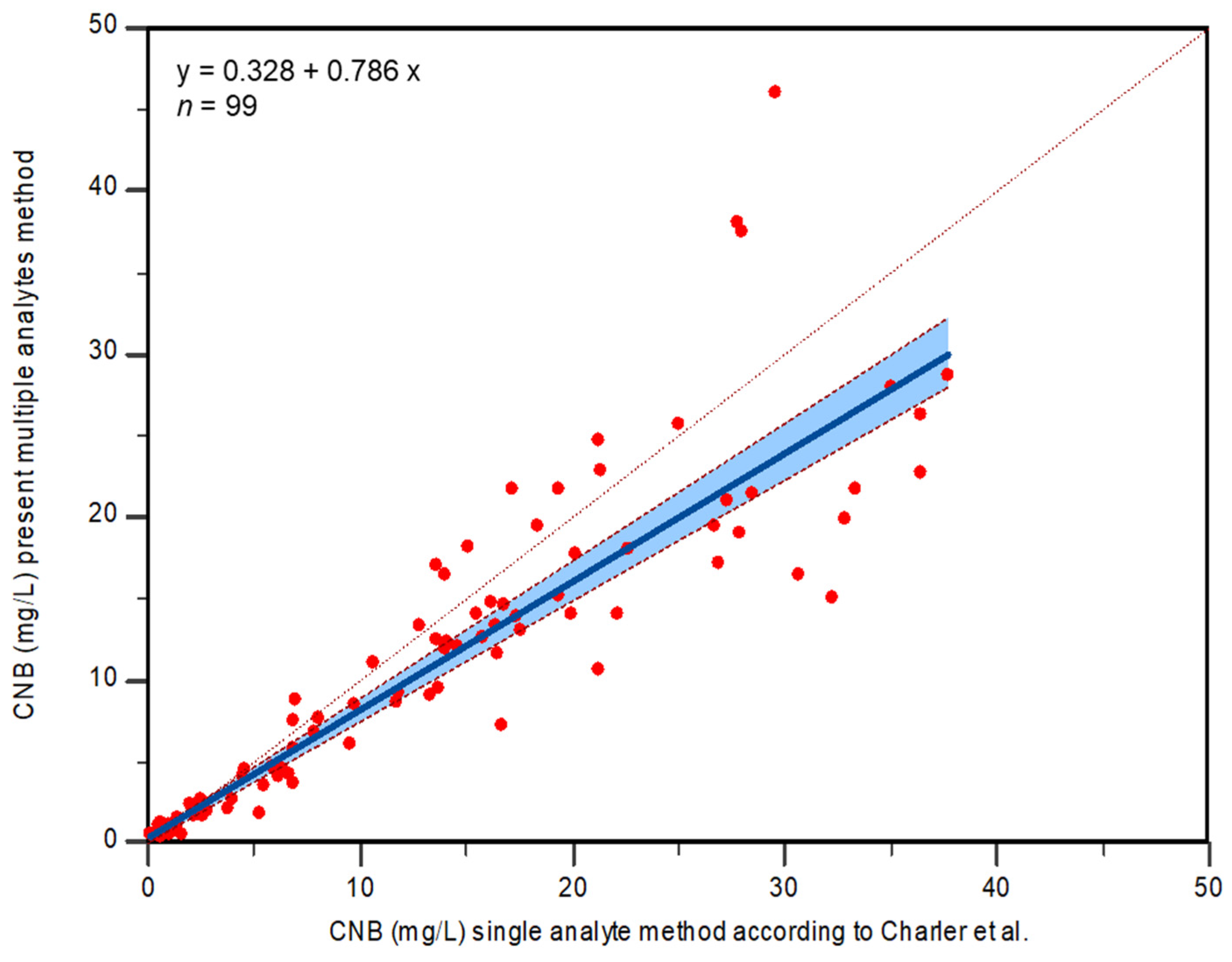
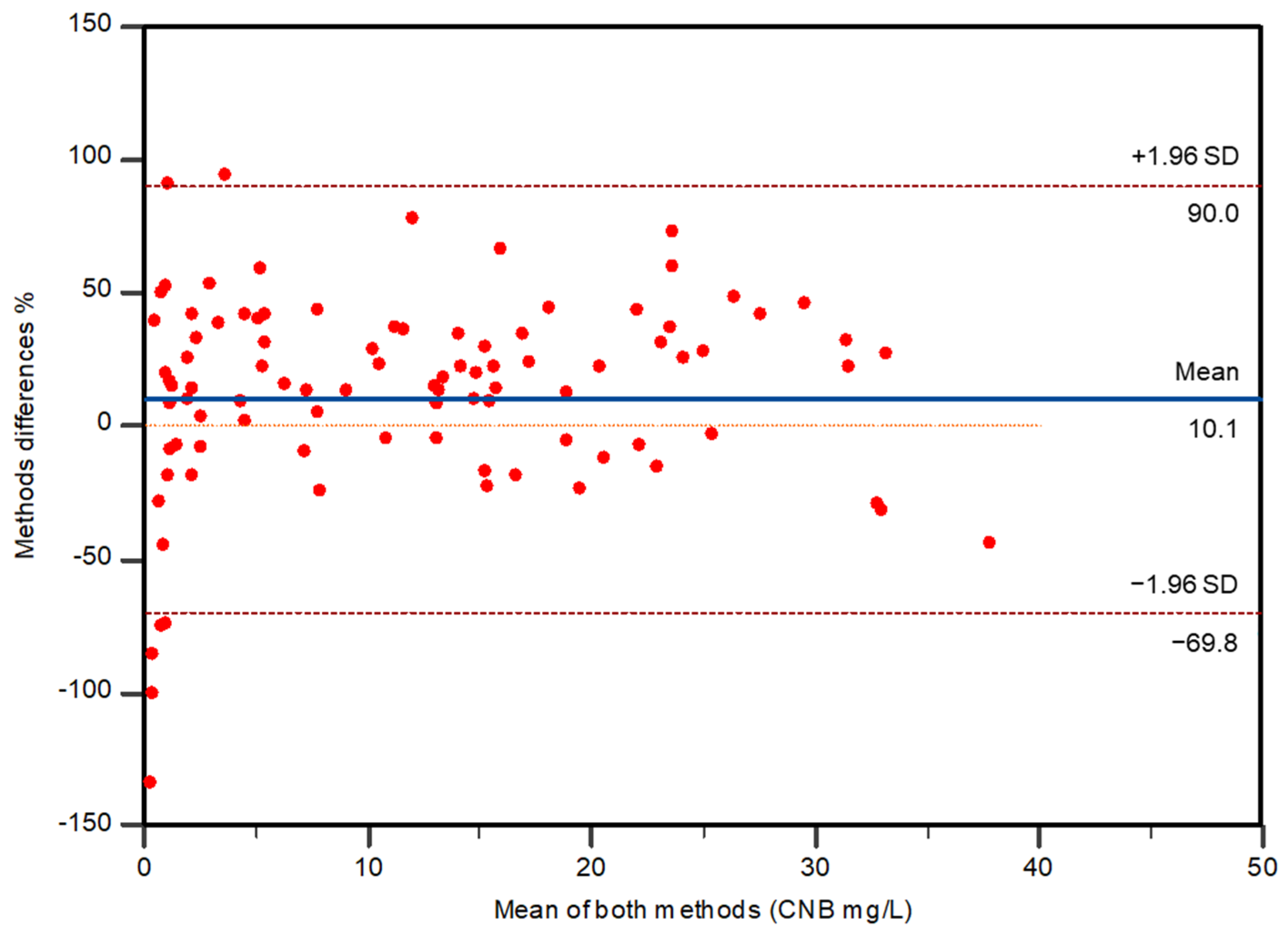
2.4. Method Comparison
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Calibration Curves and Quality Control Samples
3.3. Sample Preparation
3.3.1. Manual Sample Preparation
3.3.2. Automated Sample Preparation
3.4. Instrument Setup and Parameters
3.5. Method Validation
3.5.1. Selectivity and Specificity
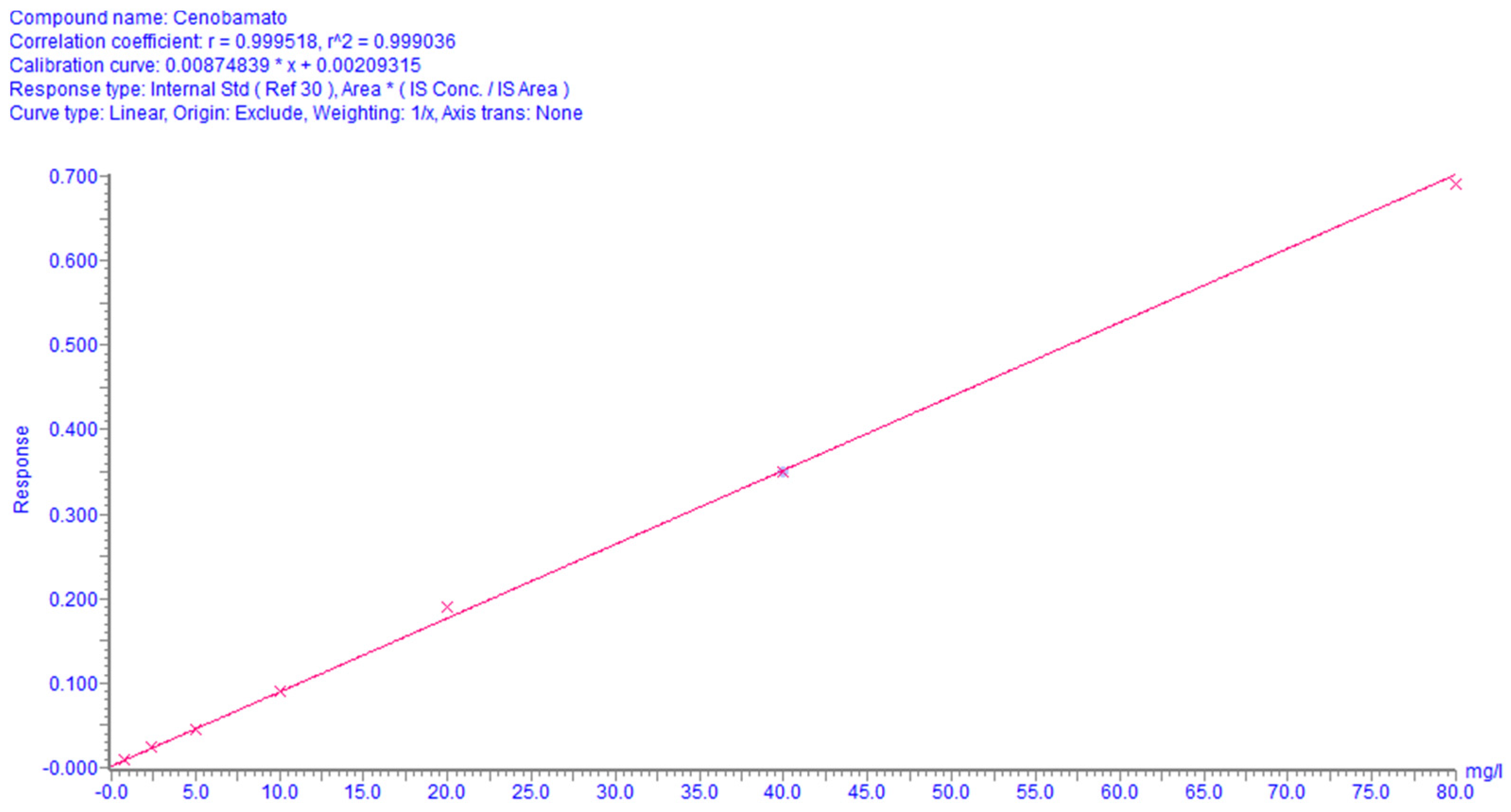
3.5.2. Linearity
3.5.3. Carry-Over
3.5.4. Dilution Integrity
3.5.5. Recovery and Matrix Effect
3.5.6. Accuracy and Precision
3.5.7. Reinjection Reproducibility
3.5.8. Stability
3.5.9. Incurred Sample Reanalysis
3.5.10. Automated Sample Extraction Validation
3.6. Method Comparison
Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Perucca, P.; Scheffer, I.E.; Kiley, M. The management of epilepsy in children and adults. Med. J. Aust. 2018, 208, 226–233. [Google Scholar] [CrossRef]
- Beghi, E. The Epidemiology of Epilepsy. Neuroepidemiology 2019, 54, 185–191. [Google Scholar] [CrossRef]
- Chen, Z.; Brodie, M.J.; Liew, D.; Kwan, P. Treatment Outcomes in Patients with Newly Diagnosed Epilepsy Treated with Established and New Antiepileptic Drugs: A 30-Year Longitudinal Cohort Study. JAMA Neurol. 2018, 75, 279. [Google Scholar] [CrossRef]
- Perucca, E.; Tomson, T. The pharmacological treatment of epilepsy in adults. Lancet Neurol. 2011, 10, 446–456. [Google Scholar] [CrossRef]
- Hauser, W.A. Questioning the Effectiveness of Newer Antiseizure Medications. JAMA Neurol. 2018, 75, 273. [Google Scholar] [CrossRef]
- Kwan, P.; Brodie, M.J. Early Identification of Refractory Epilepsy. N. Engl. J. Med. 2000, 342, 314–319. [Google Scholar] [CrossRef]
- Kwan, P. Drug-Resistant Epilepsy. N. Engl. J. Med. 2011, 365, 919–926. [Google Scholar] [CrossRef]
- Golyala, A.; Kwan, P. Drug development for refractory epilepsy: The past 25 years and beyond. Seizure 2017, 44, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Guignet, M.; Campbell, A.; White, H.S. Cenobamate (XCOPRI): Can preclinical and clinical evidence provide insight into its mechanism of action? Epilepsia 2020, 61, 2329–2339. [Google Scholar] [CrossRef] [PubMed]
- Roberti, R.; De Caro, C.; Iannone, L.F.; Zaccara, G.; Lattanzi, S.; Russo, E. Pharmacology of Cenobamate: Mechanism of Action, Pharmacokinetics, Drug–Drug Interactions and Tolerability. CNS Drugs 2021, 35, 609–618. [Google Scholar] [CrossRef] [PubMed]
- Krauss, G.L.; Klein, P.; Brandt, C.; Lee, S.K.; Milanov, I.; Milovanovic, M.; Steinhoff, B.J.; Kamin, M. Safety and efficacy of adjunctive cenobamate (YKP3089) in patients with uncontrolled focal seizures: A multicentre, double-blind, randomised, placebo-controlled, dose-response trial. Lancet Neurol. 2020, 19, 38–48. [Google Scholar] [CrossRef]
- Sperling, M.R.; Klein, P.; Aboumatar, S.; Gelfand, M.; Halford, J.J.; Krauss, G.L.; Rosenfeld, W.E.; Vossler, D.G.; Wechsler, R.; Borchert, L.; et al. Cenobamate (YKP3089) as adjunctive treatment for uncontrolled focal seizures in a large, phase 3, multicenter, open-label safety study. Epilepsia 2020, 61, 1099–1108. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.S.; French, J.A.; Kowalski, J.; Krauss, G.L.; Lee, S.K.; Maciejowski, M.; Rosenfeld, W.E.; Sperling, M.R.; Mizne, S.; Kamin, M. Randomized phase 2 study of adjunctive cenobamate in patients with uncontrolled focal seizures. Neurology 2020, 94, e2311–e2322. [Google Scholar] [CrossRef]
- Vossler, D.G. Remarkably High Efficacy of Cenobamate in Adults with Focal-Onset Seizures: A Double-Blind, Randomized, Placebo-Controlled Trial. Epilepsy Curr. 2020, 20, 85–87. [Google Scholar] [CrossRef] [PubMed]
- Klein, P.; Aboumatar, S.; Brandt, C.; Dong, F.; Krauss, G.L.; Mizne, S.; Sánchez-Álvarez, J.C.; Steinhoff, B.J.; Villanueva, V. Long-term Efficacy and Safety From an Open-Label Extension of Adjunctive Cenobamate in Patients With Uncontrolled Focal Seizures. Neurology 2022, 99, e989–e998. [Google Scholar] [CrossRef]
- Buckley, C.T.; Waters, O.R.; DeMaagd, G. Cenobamate: A New Adjunctive Agent for Drug-Resistant Focal Onset Epilepsy. Ann. Pharmacother. 2021, 55, 318–329. [Google Scholar] [CrossRef]
- French, J.A. Cenobamate for focal seizures—A game changer? Nat. Rev. Neurol. 2020, 16, 133–134. [Google Scholar] [CrossRef]
- Löscher, W.; Sills, G.J.; White, H.S. The ups and downs of alkyl-carbamates in epilepsy therapy: How does cenobamate differ? Epilepsia 2021, 62, 596–614. [Google Scholar] [CrossRef]
- Nakamura, M.; Cho, J.-H.; Shin, H.; Jang, I.-S. Effects of cenobamate (YKP3089), a newly developed anti-epileptic drug, on voltage-gated sodium channels in rat hippocampal CA3 neurons. Eur. J. Pharmacol. 2019, 855, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Nakamura, M.; Neupane, C.; Jeon, B.H.; Shin, H.; Melnick, S.M.; Glenn, K.J.; Jang, I.-S.; Park, J.B. Positive allosteric modulation of GABAA receptors by a novel antiepileptic drug cenobamate. Eur. J. Pharmacol. 2020, 879, 173117. [Google Scholar] [CrossRef]
- Sills, G.J.; Rogawski, M.A. Mechanisms of action of currently used antiseizure drugs. Neuropharmacology 2020, 168, 107966. [Google Scholar] [CrossRef] [PubMed]
- Drug Approval Package: XCOPRI. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2019/212839Orig1s000TOC.cfm (accessed on 11 July 2023).
- Charlier, B.; Coglianese, A.; Operto, F.F.; Coppola, G.; de Grazia, U.; Menna, P.; Filippelli, A.; Piaz, F.D.; Izzo, V. Development and Validation of a UHPLC–MS/MS-Based Method to Quantify Cenobamate in Human Plasma Samples. Molecules 2022, 27, 7325. [Google Scholar] [CrossRef] [PubMed]
- ICH Guideline M10 on Bioanalytical Method Validation and Study Sample Analysis. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/ich-guideline-m10-bioanalytical-method-validation-step-5_en.pdf (accessed on 10 February 2023).
- Oh, J.-H.; Jeong, J.-W.; Ji, Y.-G.; Shin, Y.-M.; Lee, K.-R.; Cho, K.H.; Koo, T.-S. Development of a liquid chromatography-tandem mass spectrometry method for assaying cenobamate in rat plasma. J. Liq. Chromatogr. Relat. Technol. 2018, 41, 992–997. [Google Scholar] [CrossRef]
- Vernillet, L.; Greene, S.A.; Kamin, M. Pharmacokinetics of Cenobamate: Results From Single and Multiple Oral Ascending-Dose Studies in Healthy Subjects. Clin. Pharmacol. Drug Dev. 2020, 9, 428–443. [Google Scholar] [CrossRef]
- de Grazia, U.; Menna, P.; Cantù, M.; Baldelli, S.; Goffredo, B.M.; Deleo, F.; D’Urso, A. Indicazioni per l’armonizzazione degli intervalli di riferimento nel monitoraggio terapeutico di farmaci antiepilettici. Biochim. Clin. 2023, 47, 82. [Google Scholar] [CrossRef]
- de Grazia, U.; D'Urso, A.; Ranzato, F.; De Riva, V.; Contarato, G.; Billo, G.; Perini, F.; Galloni, E. A Liquid Chromatography-Mass Spectrometry Assay for Determination of Perampanel and Concomitant Antiepileptic Drugs in the Plasma of Patients With Epilepsy Compared with a Fluorescent HPLC Assay. Ther. Drug Monit. 2018, 40, 477–485. [Google Scholar] [CrossRef]
- Matuszewski, B.K.; Constanzer, M.L.; Chavez-Eng, C.M. Strategies for the Assessment of Matrix Effect in Quantitative Bioanalytical Methods Based on HPLC−MS/MS. Anal. Chem. 2003, 75, 3019–3030. [Google Scholar] [CrossRef]
- De Nicolò, A.; Cantù, M.; D'Avolio, A. Matrix effect management in liquid chromatography mass spectrometry: The internal standard normalized matrix effect. Bioanalysis 2017, 9, 1093–1105. [Google Scholar] [CrossRef]
- Vidali, M.; Tronchin, M.; Dittadi, R. Protocollo per la comparazione di due metodi analitici di laboratorio. Biochim. Clin. 2016, 40, 129–142. [Google Scholar] [CrossRef]
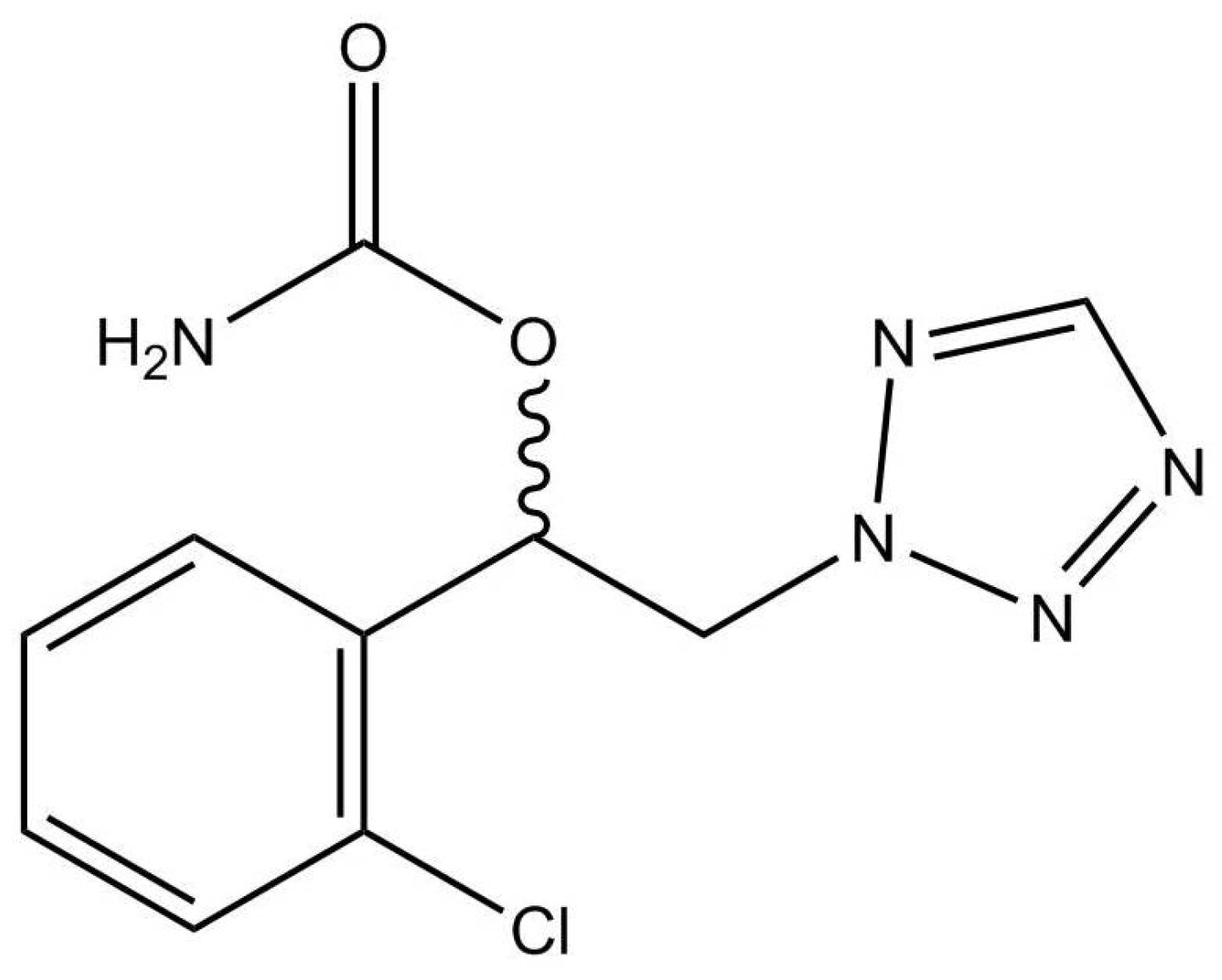

| Methods | Calibration Range for CNB | Co- Detected Compounds | Matrix (Anticoagulant) | Extraction | Internal Standard | Chromatographic Separation | Mass Spectrometry Analysis | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Column | Mobile Phase | Run Time | Instrument | Source | ||||||
| Drug Approval Package: XCOPRI [22] | 0.02–10.00 mg/L | / | Human plasma (heparin) | Protein precipitation | Phenacetin | / | / | / | AB/MDS Sciex API 4000 (triple quadrupole) | / |
| Oh et al., 2019 [25] | 0.01–5.00 mg/L | / | Rat plasma | Protein precipitation (AcN) | Carisbamate (30 mg/L) | C18 column | 60:40 (v/v) ammonium formate (10 mM):AcN | 3 min | AB/MDS Sciex API 4000 (triple quadrupole) | ESI |
| Vernillet et al., 2020 [26] | 0.02–25.00 mg/L | / | Human plasma (sodium heparin) | Protein precipitation (AcN) | Phenacetin (0.01 mg/L) | C18 column | 62:38 (v/v) ammonium acetate (2 mM):MeOH | / | Triple quadrupole mass spectrometer | Heated nebulizer source |
| 0.02–10.00 mg/L | / | Protein precipitation (AcN), evaporation and reconstitution with 40:60 (v/v) AcN:H2O | Phenacetin (0.01 mg/L) | C18 column | 62:38 (v/v) ammonium acetate (2 mM):MeOH | / | Triple quadrupole mass spectrometer | APCI | ||
| 0.05–40.00 mg/L | / | Lipid extraction (methyl tertiary-butyl ether), evaporation and reconstitution with 3:2 (v/v) MeOH:H2O | d4-cenobamate (100 mg/L) | / | A: 1000; 1.00; 0.40 (v/v) H2O:HCOOH:ammonium hydroxide B: 1000; 1.00; 0.40 (v/v) MeOH:HCOOH:ammonium hydroxide | / | Triple quadrupole mass spectrometer | TurboIon Spray® | ||
| Charlier et al., 2022 [23] | 0.05–20.00 mg/L | / | Human plasma (EDTA) | Protein precipitation (AcN) | Lamotrigine-C13-d3 (2 mg/L) | Pentafluoro-phenyl column | A: H2O:0.1% HCOOH B: AcN:0.1% HCOOH | 3.5 min | Endura TSQ (triple quadrupole) | ESI |
| Our Method | 0.80–80.00 mg/L | Other 16 ASMs | Human plasma (lithium heparin) | Protein precipitation (AcN) and dilution with mobile phase | Lamotrigine-C13-d3 | C18 column | M1: H2O:0.1% HCOOH M2: AcN:0.1% HCOOH | 7 min | Xevo TQ-XS (triple quadrupole) | ESI |
| Matrix 1 | Matrix 2 | Matrix 3 | Matrix 4 | Matrix 5 | Matrix 6 | Matrix 7 | ||
|---|---|---|---|---|---|---|---|---|
| ME% | LQC | 107 | 106 | 105 | 112 | 111 | 113 | 107 |
| HQC | 102 | 103 | 109 | 111 | 114 | 111 | 112 | |
| RE% | LQC | 105 | 101 | 106 | 105 | 105 | 99 | 106 |
| HQC | 107 | 98 | 101 | 102 | 98 | 106 | 108 | |
| ISn-ME% | LQC | 99 | 92 | 99 | 101 | 105 | 102 | 92 |
| HQC | 90 | 97 | 98 | 99 | 99 | 101 | 100 | |
| ISn-RE% | LQC | 112 | 112 | 101 | 106 | 99 | 97 | 111 |
| HQC | 111 | 98 | 104 | 111 | 107 | 113 | 114 |
| INTER-DAY | INTRA-DAY | ||||||
|---|---|---|---|---|---|---|---|
| QCs | Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | ||
| LLOQ | Bias% | 7.2 | 18.4 | −11.9 | −16.3 | −6.7 | −1.9 |
| CV% | 4.4 | 2.6 | 10.2 | 9.2 | 3.4 | 14.6 | |
| LQC | Bias% | 5.4 | −1.7 | −0.9 | 4.5 | 12.4 | 3.9 |
| CV% | 3.4 | 3.2 | 5.2 | 7.4 | 6.1 | 5.5 | |
| MQC | Bias% | −0.6 | −5.3 | 10.3 | 1.4 | 4.8 | 2.1 |
| CV% | 6.1 | 5.3 | 5.8 | 9.3 | 7.8 | 5.8 | |
| HQC | Bias% | −0.2 | −13.7 | −0.5 | −1.2 | −1.5 | −3.4 |
| CV% | 3.6 | 5.5 | 6.5 | 3.2 | 7.1 | 6.0 | |
| Storage Conditions | QCs | % Difference from T0 | ||
|---|---|---|---|---|
| 48 h | 72 h | 1 week | ||
| 4 °C | LQC | 10.6 | −14.6 | −11.6 |
| HQC | −0.8 | −11.3 | −13.6 | |
| 72 h | ||||
| RT | LQC | −27.8 | ||
| HQC | −19.6 | |||
| 24 h | ||||
| Autosampler (10 °C) | LQC | −7.9 | ||
| HQC | −0.6 | |||
| 1 month | Freeze-thaw | |||
| −20 °C | LQC | −19.5 | −16.5 | |
| HQC | 5.1 | −9.6 | ||
| 1 month | ||||
| −40 °C | LQC | −11.9 | ||
| HQC | −3.4 | |||
| Gender | ||
|---|---|---|
| Woman | 16 (34%) | |
| Man | 31 (66%) | |
| Age (year), median (range) | 26.21 (18–60) | |
| Body weight (kg), median (range) (n = 37) | 66.62 (54–107) | |
| Cenobamate dose (mg) (n = 86) | ||
| Mean (SD) | 115 (81) | |
| Median (range) | 100 (12.5–250) | |
| Cenobamate plasma concentration (mg/L) | ||
| Mean (SD) | 10 (10) | |
| Median (range) | 7 (0.4–46) | |
| Patients with no data | 7 | |
| Patients with unknown concomitant ASMs | 19 | |
| Patients with 1 concomitant ASM | 4 | |
| Patient with 2 or more concomitant ASMs | 17 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Molteni, L.; Charlier, B.; Coglianese, A.; Izzo, V.; Assenza, G.; Menna, P.; de Grazia, U.; D’Urso, A. Quantitative Analysis of Cenobamate and Concomitant Anti-Seizure Medications in Human Plasma via Ultra-High Performance Liquid Chromatography–Tandem Mass Spectrometry. Molecules 2024, 29, 884. https://doi.org/10.3390/molecules29040884
Molteni L, Charlier B, Coglianese A, Izzo V, Assenza G, Menna P, de Grazia U, D’Urso A. Quantitative Analysis of Cenobamate and Concomitant Anti-Seizure Medications in Human Plasma via Ultra-High Performance Liquid Chromatography–Tandem Mass Spectrometry. Molecules. 2024; 29(4):884. https://doi.org/10.3390/molecules29040884
Chicago/Turabian StyleMolteni, Linda, Bruno Charlier, Albino Coglianese, Viviana Izzo, Giovanni Assenza, Pierantonio Menna, Ugo de Grazia, and Annachiara D’Urso. 2024. "Quantitative Analysis of Cenobamate and Concomitant Anti-Seizure Medications in Human Plasma via Ultra-High Performance Liquid Chromatography–Tandem Mass Spectrometry" Molecules 29, no. 4: 884. https://doi.org/10.3390/molecules29040884
APA StyleMolteni, L., Charlier, B., Coglianese, A., Izzo, V., Assenza, G., Menna, P., de Grazia, U., & D’Urso, A. (2024). Quantitative Analysis of Cenobamate and Concomitant Anti-Seizure Medications in Human Plasma via Ultra-High Performance Liquid Chromatography–Tandem Mass Spectrometry. Molecules, 29(4), 884. https://doi.org/10.3390/molecules29040884












