Pyrrole Compounds from the Two-Step One-Pot Conversion of 2,5-Dimethylfuran for Elastomer Composites with Low Dissipation of Energy
Abstract
1. Introduction
2. Results and Discussion
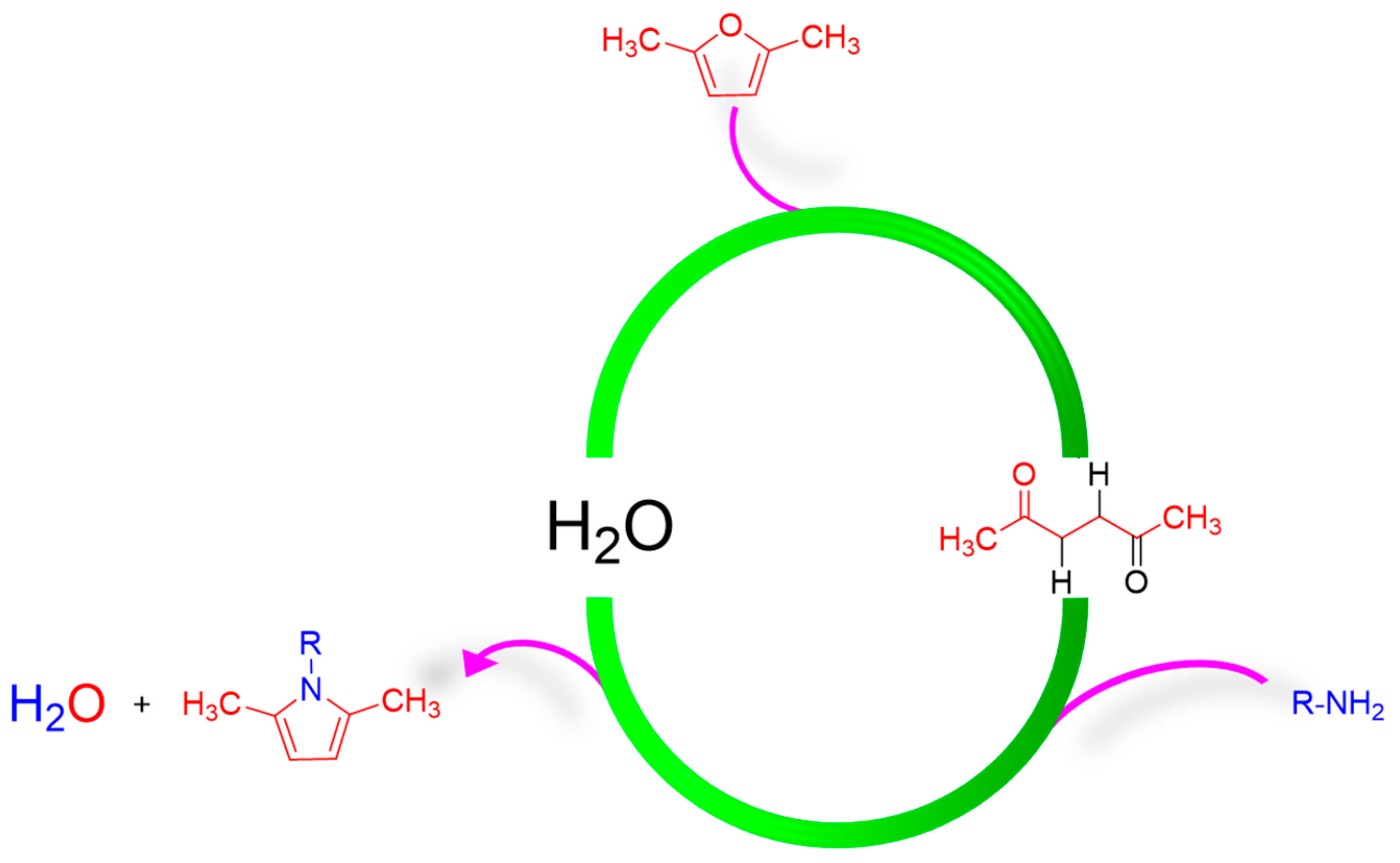
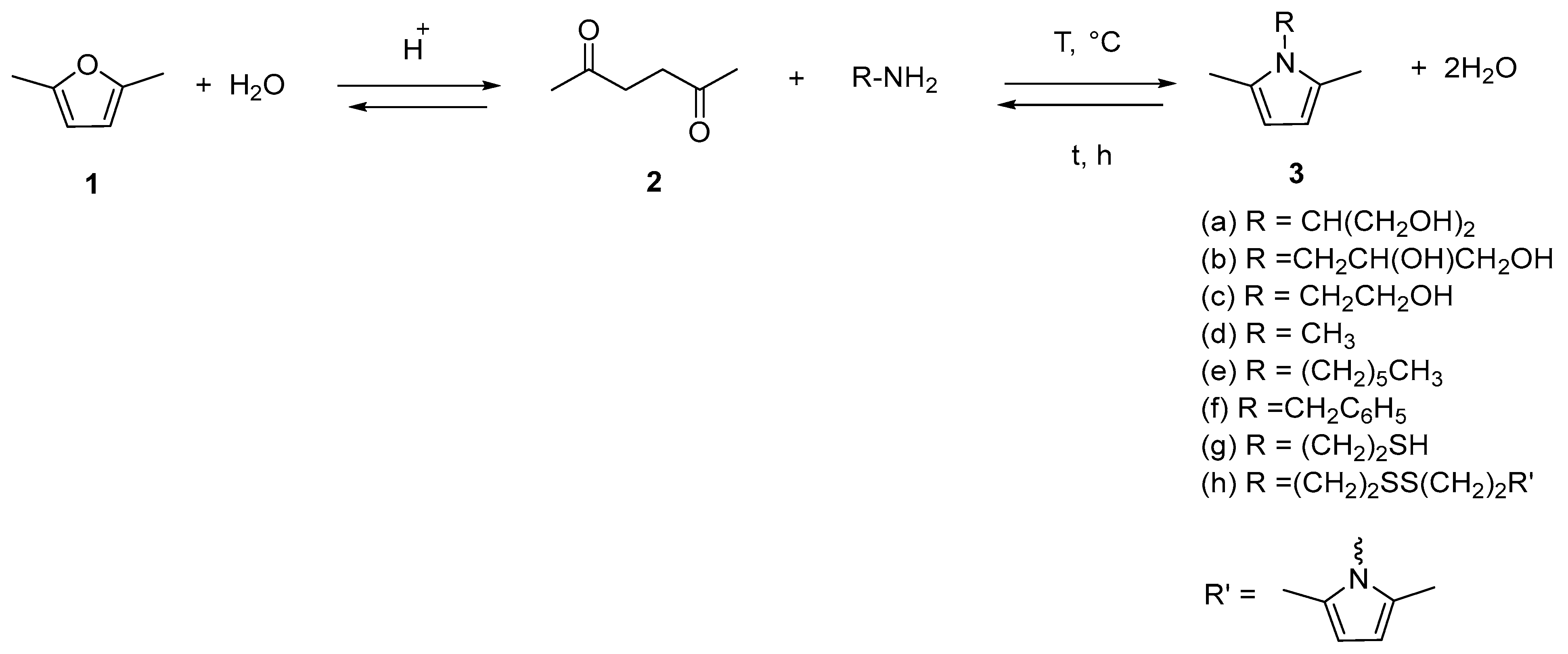
2.1. Synthesis of Pyrrole Compounds
- Step 1. Ring-opening reaction of DF to HD.
- Step 2. Paal–Knorr reaction of primary amines with HD derived from DF.
2.2. Elastomer Composites with CB/SHP as the Reinforcing Filler
Crosslinking
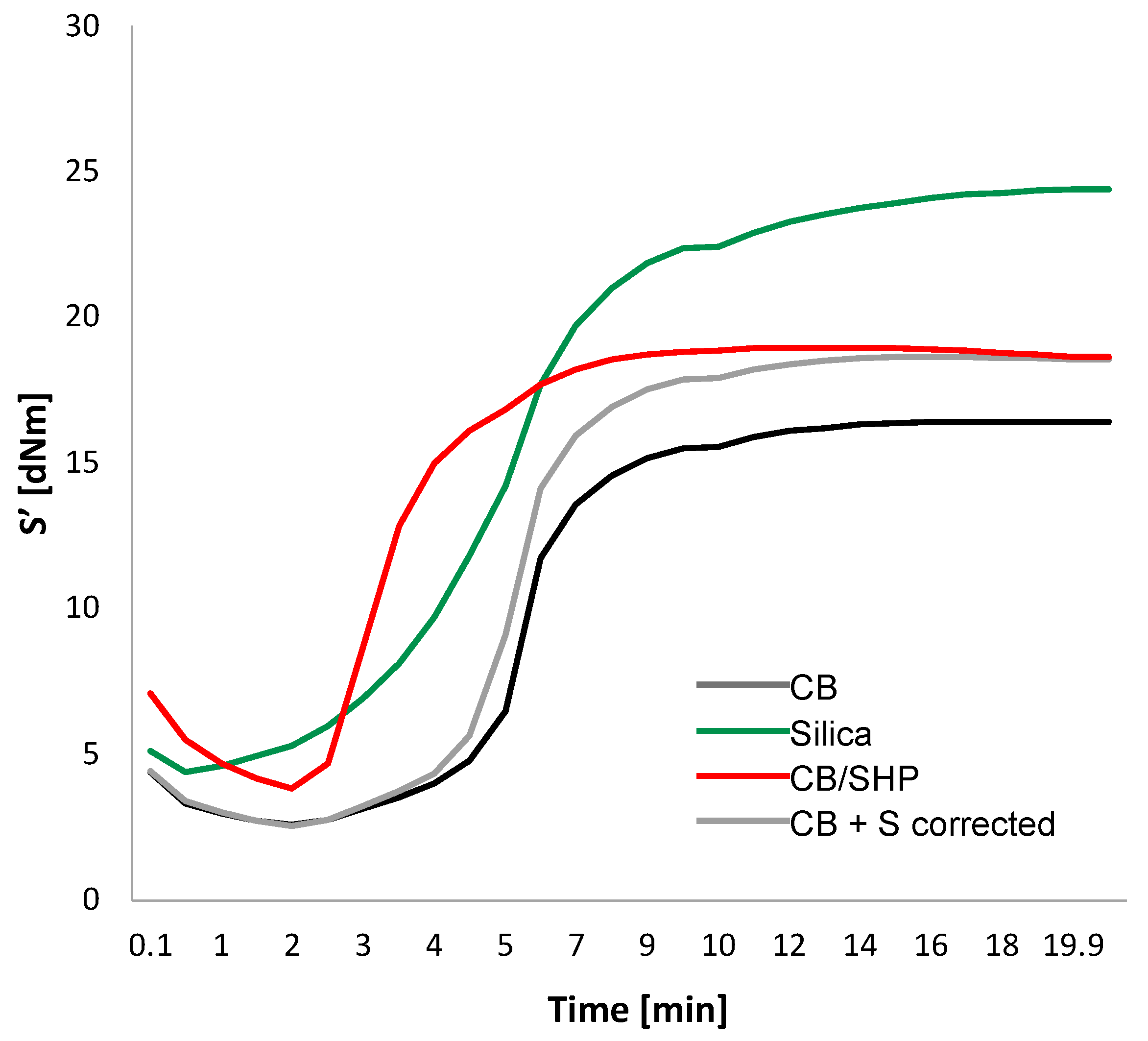
2.3. Dynamic Mechanical Properties in the Shear Mode
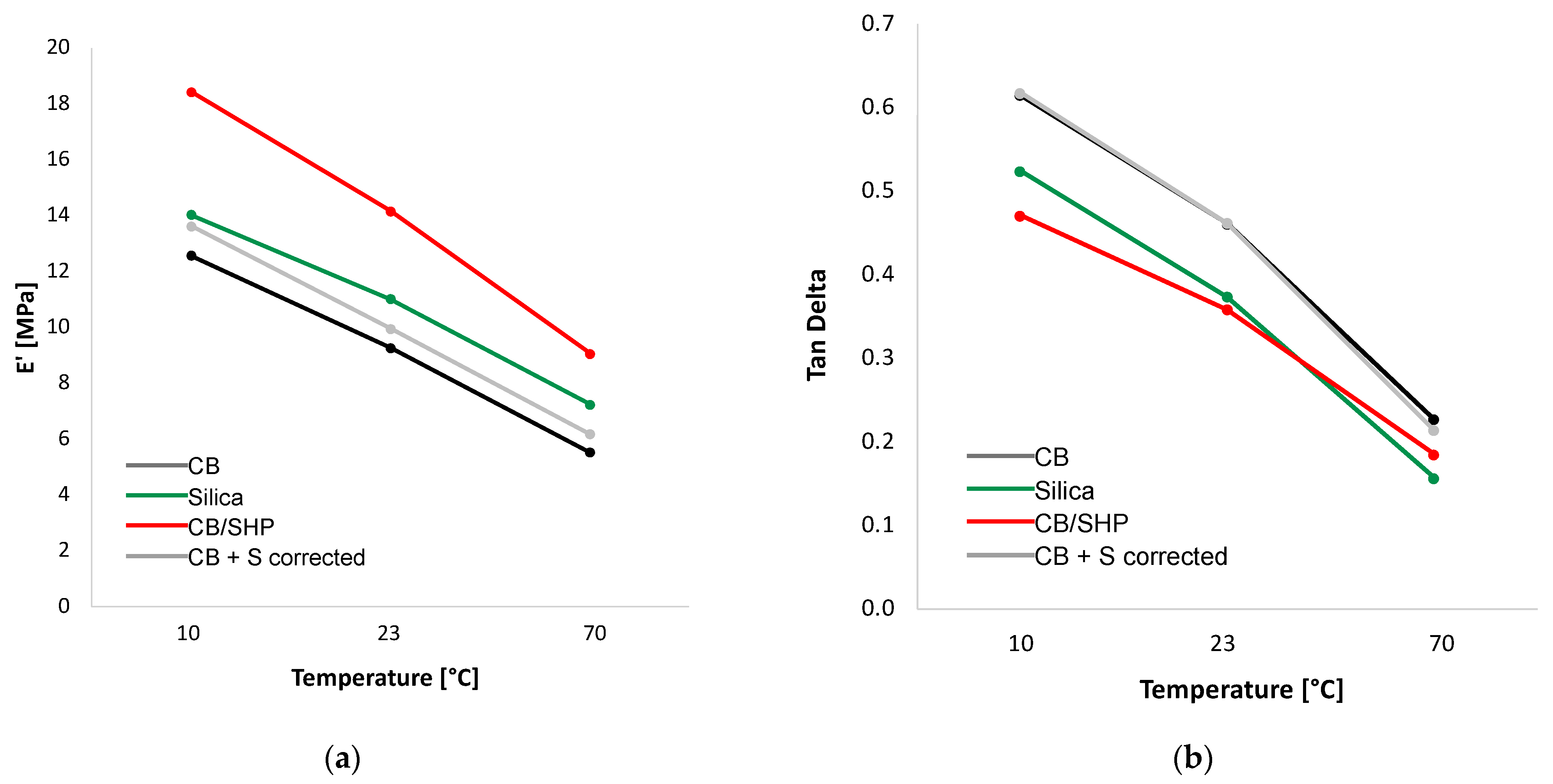
2.4. Dynamic Mechanical Properties in the Axial Mode
3. Experimental Section
3.1. Materials
3.1.1. For the Preparation of Pyrrole Compounds
3.1.2. For the Preparation of CB/SHP Adduct
3.1.3. For the Preparation of Rubber Composites
3.1.4. Synthesis of Pyrrole Derivatives
- Step 1. General procedure for the synthesis of 2,5-hexanedione (2) (STEP 1)
- Step 2. General procedure for the synthesis of pyrrole compounds
3.1.5. Preparation and Characterization of the CB/PyC Adduct
3.2. Preparation of Elastomeric Composites
3.2.1. Recipes
3.2.2. Mixing Procedure
3.3. Characterization Methods
3.3.1. Characterization of Pyrrole Compounds
3.3.2. Characterization of CB/SHP Adduct
3.3.3. Characterization of Elastomer Composites
Crosslinking
3.3.4. Dynamic Mechanical Analyses in Shear Strain Sweep Tests
3.3.5. Dynamic Mechanical Characterization in the Axial Mode
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- The 17 Goals for the Sustainable Development, United Nations, Department of Economic and Social Affairs. Available online: https://sdgs.un.org/goals (accessed on 27 January 2022).
- Ragauskas, A.J.; Williams, C.K.; Davison, B.H.; Britovsek, G.; Cairney, J.; Eckert, C.A.; Frederick, W.J.; Hallet, J.P.; Leak, D.J.; Liotta, C.L.; et al. The path forward for biofuels and biomaterials. Science 2006, 311, 484–489. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.; Peng Xong, L.; Wu, W.; Chen, Y.; Maravelias, C.T. Greenhouse gas emission mitigation potential of chemicals produced from biomass. ACS Sustain. Chem. Eng. 2021, 9, 14480–14487. [Google Scholar] [CrossRef]
- Bozell, J.J.; Petersen, G.R. Technology development for the production of biobased products from biorefinery carbohydrates—The US Department of Energy’s “Top 10” revisited. Green Chem. 2010, 12, 539. [Google Scholar] [CrossRef]
- Varma, R.S. Biomass-derived renewable carbonaceous materials for sustainable chemical and environmental applications. ACS Sustain. Chem. Eng. 2019, 7, 6458–6470. [Google Scholar] [CrossRef]
- Cho, E.J.; Trinh, L.T.P.; Song, Y.; Lee, Y.G.; Bae, H.J. Bioconversion of biomass waste into high value chemicals. Bioresour. Technol. 2020, 298, 122386. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Luo, N.; Xie, S.; Zhang, H.; Zhang, Q.; Wang, F.; Wang, Y. Photocatalytic transformations of lignocellulosic biomass into chemicals. Chem. Soc. Rev. 2020, 49, 6198–6223. [Google Scholar] [CrossRef] [PubMed]
- Newth, F.H. The formation of furan compounds from hexoses. Adv. Carbohydr. Chem. 1951, 6, 83. [Google Scholar]
- Feather, M.S.; Harris, J.F. Dehydration reactions of carbohydrates. Adv. Carbohydr. Chem. 1973, 28, 161. [Google Scholar]
- Kuster, B.F.M. 5-Hydroxymethylfurfural (HMF). A review focussing on its manufacture. Starch/Staerke 1990, 42, 314. [Google Scholar] [CrossRef]
- Moreau, C.; Belgacem, M.N.; Gandini, A. Recent catalytic advances in the chemistry of substituted furans from carbohydrates and in the ensuing polymers. Top. Catal. 2004, 27, 11–30. [Google Scholar] [CrossRef]
- Tong, X.; Ma, Y.; Li, Y. Biomass into chemicals: Conversion of sugars to furan derivatives by catalytic processes. Appl. Catal. A Gen. 2010, 385, 1–13. [Google Scholar] [CrossRef]
- Ståhlberg, T.; Fu, W.; Woodley, J.M.; Riisager, A. Synthesis of 5-(Hydroxymethyl) furfural in ionic liquids: Paving the way to renewable chemicals. ChemSusChem. 2011, 4, 451–458. [Google Scholar] [CrossRef]
- Lima, S.; Antunes, M.M.; Pillinger, M.; Valente, A.A. Ionic liquids as tools for the acid-catalyzed hydrolysis/dehydration of saccharides to furanic aldehydes. ChemCatChem 2011, 3, 1686–1706. [Google Scholar] [CrossRef]
- Román-Leshkov, Y.; Chheda, J.N.; Dumesic, J.A. Phase modifiers promote efficient production of hydroxymethylfurfural from fructose. Science 2006, 312, 1933–1937. [Google Scholar] [CrossRef]
- van Putten, R.J.; Van Der Waal, J.C.; De Jong, E.D.; Rasrendra, C.B.; Heeres, H.J.; de Vries, J.G. Hydroxymethylfurfural, a versatile platform chemical made from renewable resources. Chem. Rev. 2013, 113, 1499–1597. [Google Scholar] [CrossRef] [PubMed]
- Kashparova, V.P.; Chernysheva, D.V.; Klushin, V.A.; Andreeva, V.E.; Kravchenko, O.A.; Smirnova, N.V. Furan monomers and polymers from renewable plant biomass. Russ. Chem. Rev. 2021, 90, 750. [Google Scholar] [CrossRef]
- Dedes, G.; Karnaouri, A.; Topakas, E. Novel routes in transformation of lignocellulosic biomass to furan platform chemicals: From pretreatment to enzyme catalysis. Catalysts 2020, 7, 743. [Google Scholar] [CrossRef]
- Li, J.; Muller, E.; Pera-Titus, M.; Jérôme, F.; Vigier, K.D.O. Synthesis of functionalized tetrahydrofuran derivatives from 2, 5-dimethylfuran through cascade reactions. Green Chem. 2019, 21, 2601–2609. [Google Scholar] [CrossRef]
- Hu, L.; Lin, L.; Liu, S. Chemoselective hydrogenation of biomass-derived 5-hydroxymethylfurfural into the liquid biofuel 2, 5-dimethylfuran. Ind. Eng. Chem. Res. 2014, 53, 9969–9978. [Google Scholar] [CrossRef]
- Román-Leshkov, Y.; Barrett, C.J.; Liu, Z.Y.; Dumesic, J.A. Production of dimethylfuran for liquid fuels from biomass-derived carbohydrates. Nature 2007, 447, 982–985. [Google Scholar] [CrossRef] [PubMed]
- Thananatthanachon, T.; Rauchfuss, T.B. Efficient production of the liquid fuel 2, 5-dimethylfuran from fructose using formic acid as a reagent. Angew. Chem. 2010, 122, 6766–6768. [Google Scholar] [CrossRef]
- Dutta De, S.; Saha, B. One-pot conversions of lignocellulosic and algal biomass into liquid fuels. ChemSusChem. 2012, 5, 1826–1833. [Google Scholar] [PubMed]
- Yin, J.; Shen, C.; Feng, X.; Ji, K.; Du, L. Highly selective production of p-xylene from 2,5-dimethylfuran over hierarchical NbO x-based catalyst. ACS Sustain. Chem. Eng. 2018, 6, 1891–1899. [Google Scholar] [CrossRef]
- Chang, C.C.; Cho, H.J.; Yu, J.; Gorte, R.J.; Gulbinski, J.; Dauenhauer, P.; Fan, W. Lewis acid zeolites for tandem Diels–Alder cycloaddition and dehydration of biomass-derived dimethylfuran and ethylene to renewable p-xylene. Green Chem. 2016, 18, 1368. [Google Scholar] [CrossRef]
- Sullivan, R.J.; Latifi, E.; Chung, B.K.M.; Soldatov, D.V.; Schlaf, M. Hydrodeoxygenation of 2,5-hexanedione and 2,5-dimethylfuran by water-air and acid-stable homogeneous ruthenium and iridium catalysts. ACS Catal. 2014, 4, 4116–4128. [Google Scholar] [CrossRef]
- Xu, L.; Chen, H.; Liu, J.; Zhou, L.; Liu, Q.; Lan, Y.; Xiao, J. Chiral phosphoric acid-catalyzed asymmetric C(sp3)–H functionalization of biomass-derived 2,5-dimethylfuran via two sequential Cope-type rearrangements. Org. Chem. Front. 2019, 6, 1162–1167. [Google Scholar] [CrossRef]
- Liu, F.; Audemar, M.; De Oliveira Vigier, K.; Clacens, J.M.; De Campo, F.; Jérôme, F. Palladium/carbon dioxide cooperative catalysis for the production of diketone derivatives from carbohydrates. ChemSusChem. 2014, 7, 2089–2095. [Google Scholar] [CrossRef]
- Li, H.; Guo, H.; Fang, Z.; Aida, T.M.; Smith, R.L. Cycloamination strategies for renewable N-heterocycles. Green Chem. 2020, 22, 582–611. [Google Scholar] [CrossRef]
- Kaneda, K.; Ueno, S.; Imanaka, T.; Shimotsuma, E.; Nishiyama, Y.; Ishii, Y. Baeyer-Villiger oxidation of ketones using molecular oxygen and benzaldehyde in the absence of metal catalysts. Org. Chem. 1994, 59, 2915–2917. [Google Scholar] [CrossRef]
- Iovel, I.; Goldberg, Y.; Shymanska, M. Hydroxymethylation of furan and its derivatives in the presence of cation-exchange resins. J. Mol. Catal. 1989, 57, 91–103. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, S.; Peng, C.; Chen, Y.; Tang, Z.; Wu, Q. Continuous synthesis of 2,5-hexanedione through direct C–C coupling of acetone in a Hilbert fractal photo microreactor. Adv. Mater. Res. 2012, 518, 3947–3950. [Google Scholar] [CrossRef]
- Waidmann, C.R.; Pierpont, A.W.; Batista, E.R.; Gordon, J.C.; Martin, R.L.; West, R.M.; Wu, R. Functional group dependence of the acid catalyzed ring opening of biomass derived furan rings: An experimental and theoretical study. Catal. Sci. Technol. 2013, 3, 106–115. [Google Scholar] [CrossRef]
- Gilkey, M.J.; Vlachos, D.G.; Xu, B. Poisoning of Ru/C by homogeneous Brønsted acids in hydrodeoxygenation of 2,5-dimethylfuran via catalytic transfer hydrogenation. Appl. Catal. A Gen. 2017, 542, 327–335. [Google Scholar] [CrossRef][Green Version]
- Li, Y.; Lv, G.; Wang, Y.; Deng, T.; Wang, Y.; Hou, X.; Yang, Y. Synthesis of 2,5-Hexanedione from Biomass Resources Using a Highly Efficient Biphasic System. Chem. Select 2016, 6, 1252–1255. [Google Scholar] [CrossRef]
- Vidal, T.; Petit, A.; Loupy, A.; Gedye, R.N. Re-examination of microwave-induced synthesis of phthalimides. Tethraedon Lett. 1990, 40, 3957–3960. [Google Scholar] [CrossRef]
- Balme, G.; Bossharth, E.; Monteiro, N. Pd-assisted multicomponent synthesis of heterocycles. Eur. J. Org. Chem. 2003, 21, 4101–4111. [Google Scholar] [CrossRef]
- Nieuwland, P.J.; Segers, R.; Koch, K.; van Hest, J.C.; Rutjes, F.P. Fast scale-up using microreactors: Pyrrole synthesis from micro to production scale. Org. Process Res. Dev. 2011, 15, 783–787. [Google Scholar] [CrossRef]
- Galimberti, M.; Barbera, V.; Guerra, S.; Conzatti, L.; Castiglioni, C.; Brambilla, L.; Serafini, A. Biobased Janus molecule for the facile preparation of water solutions of few layer graphene sheets. RSC Adv. 2015, 5, 81142–81152. [Google Scholar] [CrossRef]
- Barbera, V.; Bernardi, A.; Palazzolo, A.; Rosengart, A.; Brambilla, L.; Galimberti, M. Facile and sustainable functionalization of graphene layers with pyrrole compounds. Pure Appl. Chem. 2018, 90, 253–270. [Google Scholar] [CrossRef]
- Locatelli, D.; Barbera, V.; Brambilla, L.; Castiglioni, C.; Sironi, A.; Galimberti, M. Tuning the solubility parameters of carbon nanotubes by means of their adducts with Janus pyrrole compounds. Nanomaterials 2020, 10, 1176. [Google Scholar] [CrossRef]
- Locatelli, D.; Bernardi, A.; Rubino, L.; Gallo, S.; Vitale, A.; Bongiovanni, R.; Barbera, V.; Galimberti, M. Biosourced Janus Molecules as Silica Coupling Agents in Elastomer Composites for Tires with Lower Environmental Impact. ACS Sustain. Chem. Eng. 2023, 11, 2713–2726. [Google Scholar] [CrossRef]
- De Gennes, P.-G. Soft matter (nobel lecture). Angew. Chem. Int. Ed. 1992, 31, 842–845. [Google Scholar] [CrossRef]
- Casagrande, C.; Fabre, P.; Raphaël, E.; Veyssié, M. Janus beads: Realization and behaviour at water/oil interfaces. Europhys. Lett. 1989, 9, 251–255. [Google Scholar] [CrossRef]
- Barbera, V.; Brambilla, L.; Milani, A.; Palazzolo, A.; Castiglioni, C.; Vitale, A.; Galimberti, M. Domino reaction for the sustainable functionalization of few-layer graphene. Nanomaterials 2018, 9, 44. [Google Scholar] [CrossRef]
- Prioglio, G.; Naddeo, S.; Giese, U.; Barbera, V.; Galimberti, M. Bio-Based Pyrrole Compounds Containing Sulfur Atoms as Coupling Agents of Carbon Black with Unsaturated Elastomers. Nanomaterials 2023, 13, 2761. [Google Scholar] [CrossRef]
- Magaletti, F.; Margani, F.; Monti, A.; Dezyani, R.; Prioglio, G.; Giese, U.; Barbera, V.; Galimberti, M. Adducts of Carbon Black with a Biosourced Janus Molecule for Elastomeric Composites with Lower Dissipation of Energy. Polymers 2023, 15, 3120. [Google Scholar] [CrossRef] [PubMed]
- Pirelli Tyre; Annual Report: The Human Dimension. 2020, 106. Available online: https://corporate.pirelli.com/var/files2020/EN/PDF/PIRELLI_ANNUAL_REPORT_2020_ENG.pdf (accessed on 4 October 2022).
- Curzons, A.D.; Constable, D.J.; Mortimer, D.N.; Cunningham, V.L. So you think your process is green, how do you know?—Using principles of sustainability to determine what is green—A corporate perspective. Green Chem. 2001, 3, 1–6. [Google Scholar] [CrossRef]
- Sheldon, R.A. The E factor 25 years on: The rise of green chemistry and sustainability. Green Chem. 2017, 19, 18–43. [Google Scholar] [CrossRef]
- Sheldon, R.A. Metrics of green chemistry and sustainability: Past, present, and future. ACS Sustain. Chem. Eng. 2018, 6, 32–48. [Google Scholar] [CrossRef]
- Chevalier, Y.; Morawski, J.C. Precipitated Silica with Morphological Properties, Process for Producing It and Its Application, Especially as a Filler. Legrand. EP0157703 B1, 6 April 1984. [Google Scholar]
- Leblanc, J.L. Rubber-filler interactions and rheological properties in filled compounds. Progr. Polym. Sci. 2002, 27, 627–687. [Google Scholar] [CrossRef]
- Legrand, A.P. On the silica edge. In The Surface Properties of Silicas; Legrand, A.P., Ed.; Wiley and Sons: New York, NY, USA, 1998; pp. 1–20. [Google Scholar]
- Donnet, J.B.; Custodero, E. Reinforcement of Elastomers by Particulate Fillers. In The Science and Technology of Rubber, 3rd ed.; Mark, J.E., Erman, B., Eirich, F.R., Eds.; Elsevier: Amsterdam, The Netherlands, 2005; pp. 367–400. [Google Scholar]
- Fröhlich, J.; Niedermeier, W.; Luginsland, H.D. The effect of filler-filler and filler-elastomer interaction on rubber reinforcement. Compos. Part A 2005, 36, 449–460. [Google Scholar] [CrossRef]
- Hoyle, C.E.; Bowman, C.N. Thiol–ene click chemistry. Angew. Chem. Int. Ed. 2010, 49, 1540–1573. [Google Scholar] [CrossRef]
- Wang, X.; Liang, H.; Jiang, J.; Wang, Q.; Luo, Y.; Feng, P.; Zhang, C.A. Cysteine derivative-enabled ultrafast thiol–ene reaction for scalable synthesis of a fully bio-based internal emulsifier for high-toughness waterborne polyurethanes. Green Chem. 2020, 22, 5722–5729. [Google Scholar] [CrossRef]
- Sato, M.; Mihara, S.; Amino, N.; Dierkes, W.K.; Blume, A. Reactivity study of mercapto–silane and sulfide–silane with polymer. Rubber Chem. Technol. 2020, 93, 319–345. [Google Scholar] [CrossRef]
- Dillon, J.H.; Prettyman, I.B.; Hall, G.L. Hysteretic and elastic properties of rubberlike materials under dynamic shear stresses. J. Appl. Phys. 1944, 15, 309. [Google Scholar] [CrossRef]
- Fletcher, W.P.; Gent, A.N. Nonlinearity in the dynamic properties of vulcanized rubber compounds. Trans. Inst. Rubber Ind. 1953, 29, 266. [Google Scholar] [CrossRef]
- Payne, A.R. The dynamic properties of carbon black-loaded natural rubber vulcanizates. Part I. J. Appl. Polym. Sci. 1962, 6, 57. [Google Scholar] [CrossRef]
- Warasitthinon, N.; Genix, A.C.; Sztucki, M.; Oberdisse, J.; Robertson, C.G. The Payne effect: Primarily polymer-related or filler-related phenomenon? Rubber Chem. Technol. 2019, 92, 599–611. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Z.; Wemyss, A.M.; Wan, C.; Liu, Y.; Song, P.; Wang, S. Effective thermal-oxidative reclamation of waste tire rubbers for producing high-performance rubber composites. ACS Sustain. Chem. Eng. 2020, 8, 9079–9087. [Google Scholar] [CrossRef]
- Jiang, C.; Bo, J.; Xiao, X.; Zhang, S.; Wang, Z.; Yan, G.; Wu, Y.; Wong, C.; He, H. Converting waste lignin into nano-biochar as a renewable substitute of carbon black for reinforcing styrene-butadiene rubber. Waste Manag. 2020, 102, 732–742. [Google Scholar] [CrossRef]
- Vogel, A.I.; Tatchell, A.R.; Furnis, B.S.; Hannaford, A.J.; Smith, P.W.G. (Eds.) Vogel’s Textbook of Practical Organic Chemistry 1996; Prentice Hall: Hoboken, NJ, USA, 1996; ISBN 978-0-582-46236-6. [Google Scholar]
- Anastas, P.; Eghbali, N. Green chemistry: Principles and practice. Chem. Soc. Rev. 2010, 39, 301–312. [Google Scholar] [CrossRef] [PubMed]


| Entry | Ingredient: Type and Amount | Yield (%) | |||
|---|---|---|---|---|---|
| DF (mmol) | H2O (mmol) | Acid Catalyst | |||
| Type | mol % a | ||||
| 1 | 47 | 141 | H2SO4 b | 15 | 95 |
| 2 | 47 | 94 | H2SO4 b | 15 | 97 |
| 3 | 47 | 70 | H2SO4 b | 15 | 99 |
| 4 | 47 | 47 | H2SO4 b | 15 | 98 |
| 5 | 47 | 47 | H2SO4 b | 4 | 95 |
| 6 | 47 | 47 | H2SO4 b | 1.7 | 17 |
| 7 | 47 | 47 | H2SO4 b | 0.1 | 13 |
| 8 | 47 | 47 | HCl c | 4 | 97 |
| 9 | 47 | 47 | HBr d | 4 | 97 |
| 10 | 47 | 47 | HNO3 e | 4 | <5 |
| 11 | 47 | 47 | CH3COOH f | 4 | 0 |
| Entry | Primary Amine a | T (°C) | Time (h) | Pyrrole Compound Yield | Carbon Efficiency b | E-Factor d |
|---|---|---|---|---|---|---|
| 1 a | Serinol | 150 | 2.5 | 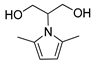 80% | 80% c | 0.097 |
| 2 | Isoserinol | 50 | 2 | 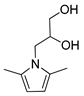 92% | 92% | 0.084 |
| 3 | Ethanolamine | 155 | 2 | 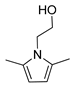 93% | 93% | 0.101 |
| 4 | Methylamine | room temperature | 2 | 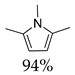 94% | 94% | 0.128 |
| 5 | Hexylamine | 60 | 2 | 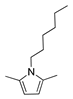 85% | 85% | 0.086 |
| 6 | Benzylamine | 100 | 2 | 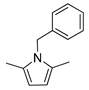 80% | 80% | 0.088 |
| 7 | Cysteamine | Room temperature | 4 | 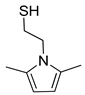 79% | 79% | 0.076 |
| 8 | Cystamine | 80 | 2 |  74% | 74% | 0.080 |
| Composite Based on | |||||
|---|---|---|---|---|---|
| Property | Temperature (°C) | Silica | CB/SHP | CB + S | CB |
| E’ (MPa) | 10 | 14.02 | 18.42 | 13.62 | 12.56 |
| 23 | 11.00 | 14.15 | 9.95 | 9.27 | |
| 70 | 7.23 | 9.05 | 6.18 | 5.52 | |
| E’’ (MPa) | 10 | 7.34 | 8.67 | 8.41 | 7.72 |
| 23 | 4.11 | 5.07 | 4.59 | 4.27 | |
| 70 | 1.13 | 1.67 | 1.32 | 1.25 | |
| Tan Delta | 10 | 0.52 | 0.47 | 0.62 | 0.61 |
| 23 | 0.37 | 0.36 | 0.46 | 0.46 | |
| 70 | 0.16 | 0.18 | 0.21 | 0.23 | |
| ΔE’ (E’70°C − E’10°C) (MPa) | 6.79 | 7.44 | 9.37 | 7.04 | |
| Composite Based on | ||||
|---|---|---|---|---|
| Ingredient | Silica | CB/SHP | CB + S | CB |
| S-SBR 4630 | 70 | 70 | 70 | 70 |
| NR | 30 | 30 | 30 | 30 |
| Silica | 65 | 0 | 0 | 0 |
| Silane TESPT | 5.2 | 0 | 0 | 0 |
| CB N234 | 0 | 0 | 55 | 55 |
| CB N234-SHP | 0 | 58.70 | 0 | 0 |
| Sulphur S8 | 1.80 | 1.80 | 2.57 | 1.80 |
| Sulphur atoms b | 3.04 | 2.57 | 2.57 | 1.80 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naddeo, S.; Gentile, D.; Margani, F.; Prioglio, G.; Magaletti, F.; Galimberti, M.; Barbera, V. Pyrrole Compounds from the Two-Step One-Pot Conversion of 2,5-Dimethylfuran for Elastomer Composites with Low Dissipation of Energy. Molecules 2024, 29, 861. https://doi.org/10.3390/molecules29040861
Naddeo S, Gentile D, Margani F, Prioglio G, Magaletti F, Galimberti M, Barbera V. Pyrrole Compounds from the Two-Step One-Pot Conversion of 2,5-Dimethylfuran for Elastomer Composites with Low Dissipation of Energy. Molecules. 2024; 29(4):861. https://doi.org/10.3390/molecules29040861
Chicago/Turabian StyleNaddeo, Simone, Davide Gentile, Fatima Margani, Gea Prioglio, Federica Magaletti, Maurizio Galimberti, and Vincenzina Barbera. 2024. "Pyrrole Compounds from the Two-Step One-Pot Conversion of 2,5-Dimethylfuran for Elastomer Composites with Low Dissipation of Energy" Molecules 29, no. 4: 861. https://doi.org/10.3390/molecules29040861
APA StyleNaddeo, S., Gentile, D., Margani, F., Prioglio, G., Magaletti, F., Galimberti, M., & Barbera, V. (2024). Pyrrole Compounds from the Two-Step One-Pot Conversion of 2,5-Dimethylfuran for Elastomer Composites with Low Dissipation of Energy. Molecules, 29(4), 861. https://doi.org/10.3390/molecules29040861







