Plant Extracts and Phytochemicals from the Asteraceae Family with Antiviral Properties
Abstract
1. Introduction
2. Results
2.1. Organic Extracts Prepared from Asteraceae Species
2.2. Pure Phytochemicals Isolated from Asteraceae
2.3. Cytotoxicity and Antiviral Activity of Extracts from Asteraceae Species
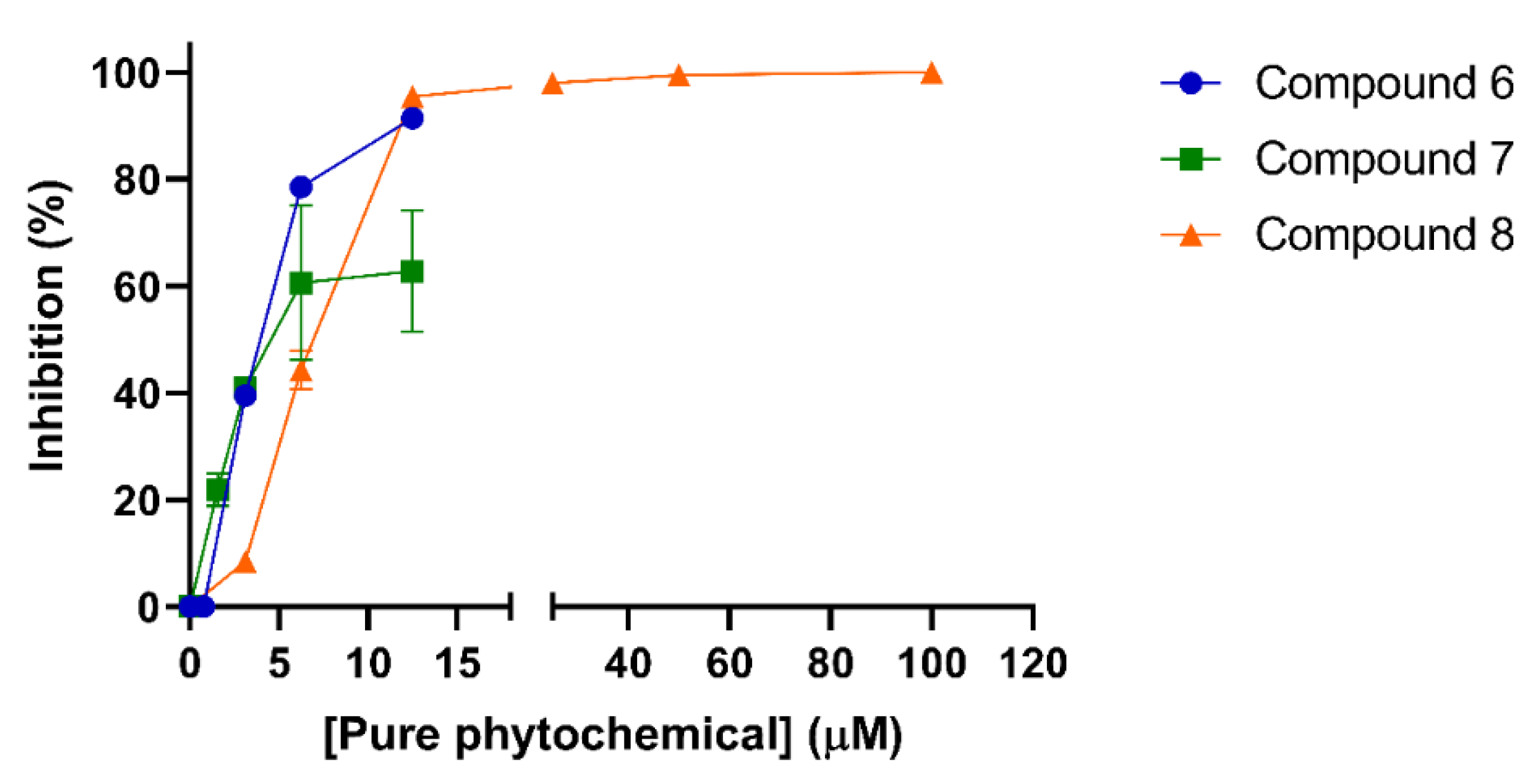
2.4. Cytotoxicity and Ant-Iviral Activity of the Isolated Phytochemicals
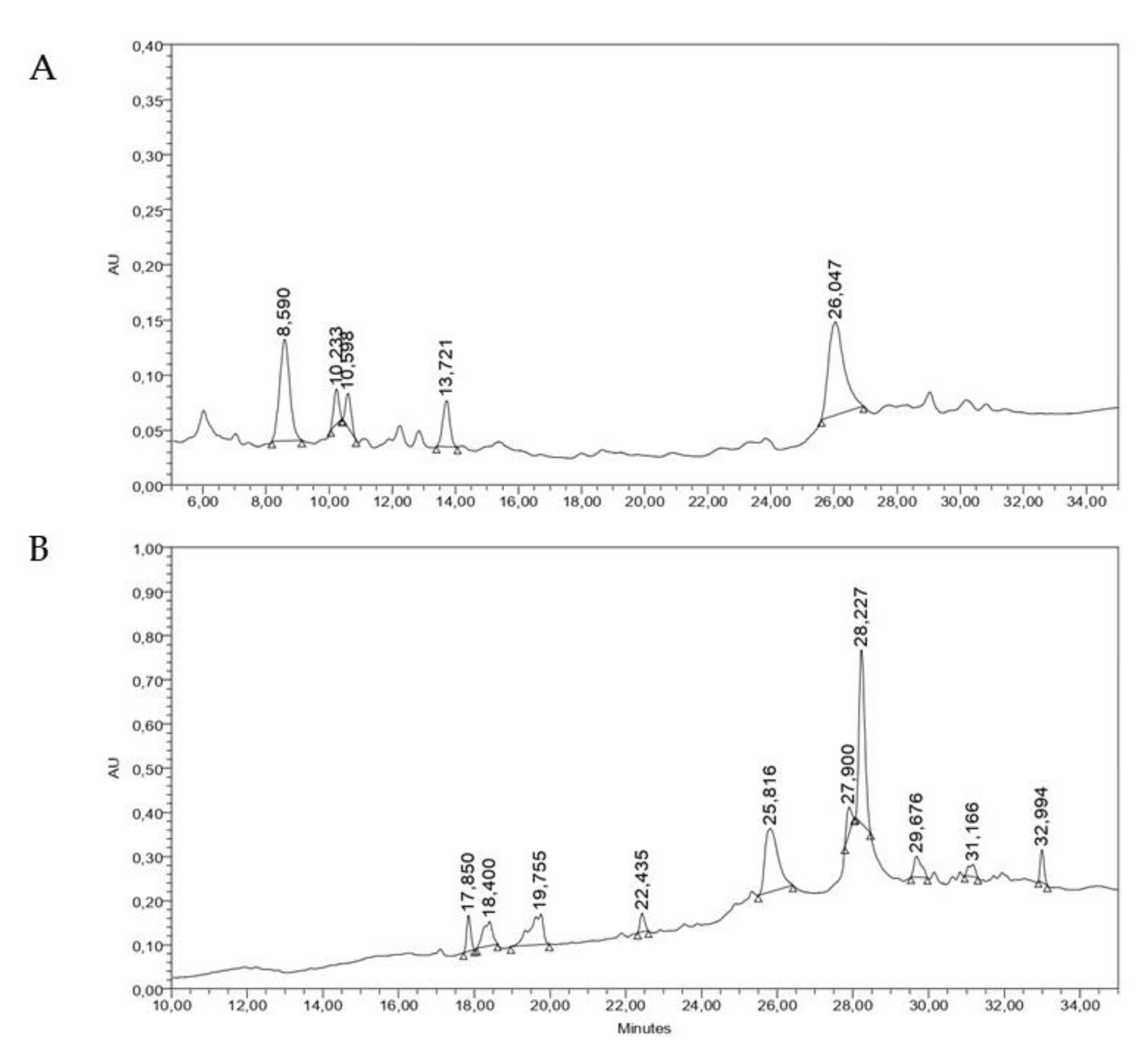
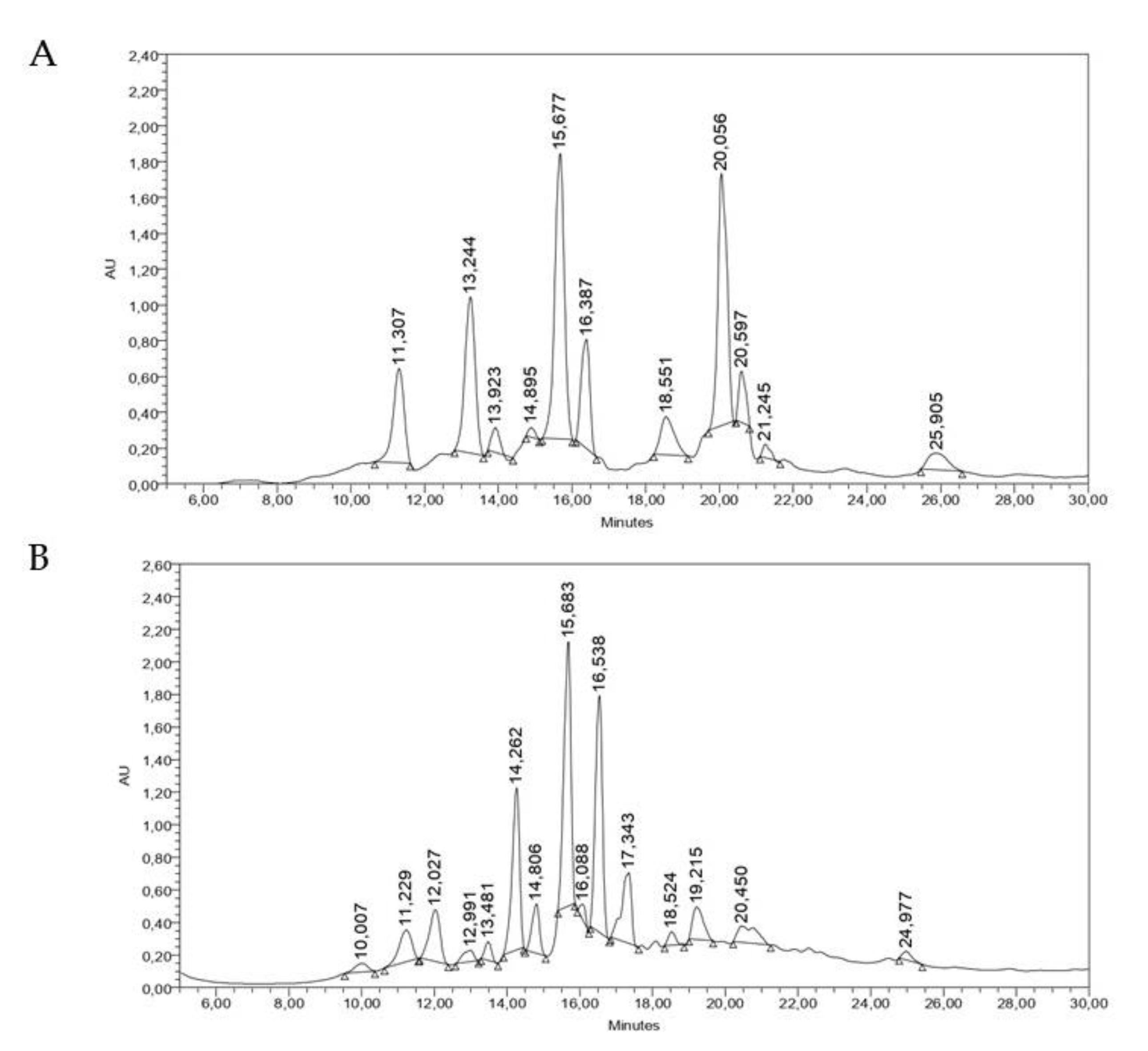
2.5. Chromatographic Analysis of the Active Extracts against DENV-2
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.1.1. Asteraceae Plant Materials Used to Prepare the Organic Extracts Tested against DENV-2
4.1.2. Asteraceae Plant Materials Used to Obtain the Pure Phytochemicals Tested against DEN-2
4.2. Plant Extracts
4.2.1. Plant Extracts Obtention
4.2.2. Chromatographic Analysis of the Active Extracts against DENV-2
Thin Layer Chromatography Analysis (TLC)
High Performance Liquid Chromatography Analysis (HPLC)
4.3. Isolation of Pure Phytochemicals from Asteraceae Species
4.3.1. Previously Reported Compounds from Asteraceae Species
4.3.2. Novel Phytochemicals Isolated from Asteraceae Species
4.4. Identification of Pure Compounds from Asteraceae
4.5. Cells and Virus
4.6. Cytotoxicity Assay
4.7. Antiviral Activity Assay
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rolnik, A.; Olas, B. The Plants of the Asteraceae Family as Agents in the Protection of Human Health. Int. J. Mol. Sci. 2021, 22, 3009. [Google Scholar] [CrossRef] [PubMed]
- Panda, S.K.; da Silva, L.C.N.; Sahal, D.; Leonti, M. Editorial: Ethnopharmacological Studies for the Development of Drugs with Special Reference to Asteraceae. Front. Pharmacol. 2019, 10, 955. [Google Scholar] [CrossRef] [PubMed]
- Moraes Neto, R.N.; Setúbal, R.F.B.; Higino, T.M.M.; Brelaz-de-Castro, M.C.A.; da Silva, L.C.N.; Aliança, A.S.D.S. Asteraceae Plants as Sources of Compounds Against Leishmaniasis and Chagas Disease. Front. Pharmacol. 2019, 10, 477. [Google Scholar] [CrossRef]
- Dengue and Severe Dengue. Available online: https://www.who.int/news-room/fact-sheets/detail/dengue-and-severe-dengue (accessed on 26 July 2023).
- Dengue—OPS/OMS | Organización Panamericana de La Salud. Available online: https://www.paho.org/es/temas/dengue (accessed on 26 July 2023).
- Banco de Recursos de Comunicación Del Ministerio de Salud de La Nación | Boletín Epidemiológico Nacional N 686 SE 1 | 2024. Available online: https://bancos.salud.gob.ar/recurso/boletin-epidemiologico-nacional-n-686-se-1-2024 (accessed on 24 January 2024).
- Dengue | DNDi. Available online: https://dndi.org/diseases/dengue/ (accessed on 26 July 2023).
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef]
- Saqallah, F.G.; Abbas, M.A.; Wahab, H.A. Recent Advances in Natural Products as Potential Inhibitors of Dengue Virus with a Special Emphasis on NS2b/NS3 Protease. Phytochemistry 2022, 202, 113362. [Google Scholar] [CrossRef] [PubMed]
- Chiow, K.H.; Phoon, M.C.; Putti, T.; Tan, B.K.H.; Chow, V.T. Evaluation of Antiviral Activities of Houttuynia Cordata Thunb. Extract, Quercetin, Quercetrin and Cinanserin on Murine Coronavirus and Dengue Virus Infection. Asian Pac. J. Trop. Med. 2016, 9, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Amraiz, D.; Zaidi, N.-S.S.; Fatima, M. Antiviral Evaluation of an Hsp90 Inhibitor, Gedunin, against Dengue Virus. Trop. J. Pharm. Res. 2017, 16, 997–1004. [Google Scholar] [CrossRef]
- Vázquez-Calvo, Á.; Jiménez de Oya, N.; Martín-Acebes, M.A.; Garcia-Moruno, E.; Saiz, J.-C. Antiviral Properties of the Natural Polyphenols Delphinidin and Epigallocatechin Gallate against the Flaviviruses West Nile Virus, Zika Virus, and Dengue Virus. Front. Microb. 2017, 8, 1314. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, L.; Wang, H.; Xiong, Y. Recent Advances in Antiviral Activities of Triterpenoids. Pharmaceuticals 2022, 15, 1169. [Google Scholar] [CrossRef]
- Herz, W.; Santhanam, P.S.; Subramaniam, P.S.; Schmid, J.J. The Structure of Mikanolide, a New Sesquiterpene Dilactone from Mikania Scandens (L.) Willd. Tetrahedron Let. 1967, 8, 3111–3115. [Google Scholar] [CrossRef]
- Ito, K.; Sakakibara, Y.; Haruna, M. Seven Guaianolides from Eupatorium Chinense. Phytochemistry 1982, 21, 715–720. [Google Scholar] [CrossRef]
- Zdero, C.; Bohlmann, F.; King, R.M.; Robinson, H. The First 12.8β-Germacrolide and Other Constituents from Bolivian Stevia Species. Phytochemistry 1988, 27, 2835–2842. [Google Scholar] [CrossRef]
- Vega, M.R.G.; De Carvalho, M.G.; Vieira, I.J.C.; Braz-Filho, R. Chemical Constituents from the Paraguayan Medicinal Plant, Eupatorium Macrocephalum Less. J. Nat. Med. 2008, 62, 122–123. [Google Scholar] [CrossRef] [PubMed]
- Goleniowski, M.E.; Bongiovanni, G.A.; Palacio, L.; Nuñez, C.O.; Cantero, J.J. Medicinal Plants from the “Sierra de Comechingones”, Argentina. J. Ethnopharmacol. 2006, 107, 324–341. [Google Scholar] [CrossRef] [PubMed]
- Clavin, M.L.; Redko, F.; Acevedo, C.; Martino, V.; Gorzalczany, S. In Vivo Anti-Inflammatory Activity and Flavonoid Identification of Medicinal Eupatorium Species. Pharmacogn. J. 2013, 5, 91–93. [Google Scholar] [CrossRef]
- Pereira Cabral, M.R.; Cecchetto, M.; Batista, J.M.; Batista, A.N.L.; Foglio, M.A.; Tasca Gois Ruiz, A.L.; Barrotto Do Carmo, M.R.; Ferreira Da Costa, W.; Baldoqui, D.C.; Sarragiotto, M.H. Cytotoxic Sesquiterpene Lactones from Campuloclinium Macrocephalum (=Eupatorium Macrocephalum). Phytochemistry 2020, 179, 112469. [Google Scholar] [CrossRef] [PubMed]
- Park, J.E.; Kwon, H.J.; Lee, H.J.; Hwang, H.S. Anti-Inflammatory Effect of Taxifolin in TNF-α/IL-17A/IFN-γ Induced HaCaT Human Keratinocytes. Appl. Biol. Chem. 2023, 66, 8. [Google Scholar] [CrossRef]
- Hernandez, X.E.; Orden, A.A.; Giordano, O.S.; Kurina, M. Effects of Elicitor and Copper Sulfate on Grindelic Acid Production in Submerged Cultures of Grindelia Pulchella. Electron. J. Biotechnol. 2005, 8, 276–283. [Google Scholar] [CrossRef][Green Version]
- Gierlikowska, B.; Gierlikowski, W.; Bekier, K.; Skalicka-Woźniak, K.; Czerwińska, M.E.; Kiss, A.K. Inula Helenium and Grindelia Squarrosa as a Source of Compounds with Anti-Inflammatory Activity in Human Neutrophils and Cultured Human Respiratory Epithelium. J. Ethnopharmacol. 2020, 249, 112311. [Google Scholar] [CrossRef]
- Petenatti, E.; Pestchanker, M.J.; Guo, M.; Guerreiro, E. Sesquiterpene Lactones and Flavonoids from Helenium Radiatum. Phytochemistry 1990, 29, 3669–3671. [Google Scholar] [CrossRef]
- Visintini Jaime, M.F.; Campos, R.H.; Martino, V.S.; Cavallaro, L.V.; Muschietti, L.V. Antipoliovirus Activity of the Organic Extract of Eupatorium Buniifolium: Isolation of Euparin as an Active Compound. eCAM 2013, 2013, e402364. [Google Scholar] [CrossRef]
- Choi, J.-G.; Lee, H.; Hwang, Y.-H.; Lee, J.-S.; Cho, W.-K.; Ma, J.Y. Eupatorium Fortunei and Its Components Increase Antiviral Immune Responses against RNA Viruses. Front. Pharmacol. 2017, 8, 511. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Feng, D.; Xiang, Y.; Guo, J.; Huang, N.; Yu, N.; Yang, H.; Liu, C.; Zou, K. Synthesis and Inhibitory Activity of Euparin Derivatives as Potential Dual Inhibitors against α-Glucosidase and Protein Tyrosine Phosphatase 1B (PTP1B). Fitoterapia 2023, 169, 105596. [Google Scholar] [CrossRef]
- Limjindaporn, T.; Panaampon, J.; Malakar, S.; Noisakran, S.; Yenchitsomanus, P. Tyrosine Kinase/Phosphatase Inhibitors Decrease Dengue Virus Production in HepG2 Cells. Biochem. Biophys. Res. Commun. 2017, 483, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Borgo, J.; Elso, O.G.; Gomez, J.; Coll, M.; Catalán, C.A.N.; Mucci, J.; Alvarez, G.; Randall, L.M.; Barrera, P.; Malchiodi, E.L.; et al. Anti-Trypanosoma Cruzi Properties of Sesquiterpene Lactones Isolated from Stevia spp.: In Vitro and In Silico Studies. Pharmaceutics 2023, 15, 647. [Google Scholar] [CrossRef]
- Elso, O.G.; Bivona, A.E.; Sanchez Alberti, A.; Cerny, N.; Fabian, L.; Morales, C.; Catalán, C.A.N.; Malchiodi, E.L.; Cazorla, S.I.; Sülsen, V.P. Trypanocidal Activity of Four Sesquiterpene Lactones Isolated from Asteraceae Species. Molecules 2020, 25, 2014. [Google Scholar] [CrossRef] [PubMed]
- Laurella, L.C.; Cerny, N.; Bivona, A.E.; Alberti, A.S.; Giberti, G.; Malchiodi, E.L.; Martino, V.S.; Catalan, C.A.; Alonso, M.R.; Cazorla, S.I.; et al. Assessment of Sesquiterpene Lactones Isolated from Mikania Plants Species for Their Potential Efficacy against Trypanosoma Cruzi and Leishmania Sp. PLOS Negl. Trop. Dis. 2017, 11, e0005929. [Google Scholar] [CrossRef]
- Elso, O.G.; Clavin, M.; Hernandez, N.; Sgarlata, T.; Bach, H.; Catalan, C.A.N.; Aguilera, E.; Alvarez, G.; Sülsen, V.P. Antiprotozoal Compounds from Urolepis Hecatantha (Asteraceae). eCAM 2021, 2021, e6622894. [Google Scholar] [CrossRef]
- Clavin, M.; Gorzalczany, S.; Macho, A.; Muñoz, E.; Ferraro, G.; Acevedo, C.; Martino, V. Anti-Inflammatory Activity of Flavonoids from Eupatorium Arnottianum. J. Ethnopharmacol. 2007, 112, 585–589. [Google Scholar] [CrossRef]
- Beer, M.F.; Frank, F.M.; Germán Elso, O.; Ernesto Bivona, A.; Cerny, N.; Giberti, G.; Luis Malchiodi, E.; Susana Martino, V.; Alonso, M.R.; Patricia Sülsen, V.; et al. Trypanocidal and Leishmanicidal Activities of Flavonoids Isolated from Stevia Satureiifolia Var. Satureiifolia. Pharm. Biol. 2016, 54, 2188–2195. [Google Scholar] [CrossRef]
- De Heluani, C.S.; de Lampasona, M.P.; Catalán, C.A.N.; Goedken, V.L.; Gutiérrez, A.B.; Herz, W. Guaianolides, Heliangolides and Other Constituents from Stevia Alpina. Phytochemistry 1989, 28, 1931–1935. [Google Scholar] [CrossRef]
- Cuenca, M.D.R.; Bardon, A.; Catalan, C.A.N.; Kokke, W.C.M.C. Sesquiterpene Lactones from Mikania Micrantha. J. Nat. Prod. 1988, 51, 625–626. [Google Scholar] [CrossRef]
- Hernández, L.R.; de Riscala, E.C.; Catalán, C.A.N.; Díaz, J.G.; Herz, W. Sesquiterpene Lactones and Other Constituents of Stevia Maimarensis and Synedrellopsis Grisebachii. Phytochemistry 1996, 42, 681–684. [Google Scholar] [CrossRef]
- Cuenca, M.D.R.; Borkosky, S.; Catalán, C.A.N.; Goedken, V.L.; Diaz, J.G.; Herz, W. Sesquiterpene Lactones of Mikania Minima. Phytochemistry 1993, 32, 1509–1513. [Google Scholar] [CrossRef]
- Díaz, C.E.; Fraga, B.M.; Portero, A.G.; Brito, I.; López-Balboa, C.; Ruiz-Vásquez, L.; González-Coloma, A. Insect Antifeedant Benzofurans from Pericallis Species. Molecules 2023, 28, 975. [Google Scholar] [CrossRef] [PubMed]
- Erdenetsogt, U.; Nadmid, S.; Paulus, C.; Chanagsuren, G.; Dolgor, E.; Gotov, C.; Dahse, H.-M.; Luzhetskyy, A.; Dagvadorj, E. Bioactive Flavonoids from Plant Extract of Pyrethrum Pulchrum and Its Acute Toxicity. Nat. Prod. Res. 2021, 35, 5960–5963. [Google Scholar] [CrossRef]
- Lee, S.-J.; Chung, H.-Y.; Maier, C.G.-A.; Wood, A.R.; Dixon, R.A.; Mabry, T.J. Estrogenic Flavonoids from Artemisia vulgaris L. J. Agric. Food Chem. 1998, 46, 3325–3329. [Google Scholar] [CrossRef]
- Kiene, M.; Blum, S.; Jerz, G.; Winterhalter, P. A Comparison between High-Performance Countercurrent Chromatography and Fast-Centrifugal Partition Chromatography for a One-Step Isolation of Flavonoids from Peanut Hulls Supported by a Conductor like Screening Model for Real Solvents. Molecules 2023, 28, 5111. [Google Scholar] [CrossRef] [PubMed]
- Tastan, P.; Hajdú, Z.; Kúsz, N.; Zupkó, I.; Sinka, I.; Kivcak, B.; Hohmann, J. Sesquiterpene Lactones and Flavonoids from Psephellus Pyrrhoblepharus with Antiproliferative Activity on Human Gynecological Cancer Cell Lines. Molecules 2019, 24, 3165. [Google Scholar] [CrossRef] [PubMed]
- Sahid, E.D.N.; Claudino, J.C.; Oda, F.B.; Carvalho, F.A.; Santos, A.G.D.; Graminha, M.A.S.; Clementino, L.D.C. Baccharis Trimera (Less.) DC Leaf Derivatives and Eupatorin Activities against Leishmania Amazonensis. Nat. Prod. Res. 2022, 36, 1599–1603. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Zhang, L.; Lai, C.; Ouyang, Z.; Zhang, J. Evaluation of Antibacterial Activity of Compounds Isolated from the Peel of Newhall Navel Orange. Nat. Prod. Res. 2023, 37, 2060–2064. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid Colorimetric Assay for Cellular Growth and Survival: Application to Proliferation and Cytotoxicity Assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]



| Species | Dichloromethane Extract | Methanolic Extract | ||
|---|---|---|---|---|
| Abbreviation | Yield (%) | Abbreviation | Yield (%) | |
| Acmella bellidioides | DAB | 0.2 | MAB | 2.7 |
| Campuloclinium macrocephalum | DCM | 1.6 | MCM | 8.7 |
| Grindelia pulchella | DGPU | 11.5 | MGPU | 13.7 |
| Grindelia chiloensis | DGCH | 15.5 | MGCH | 6.8 |
| Helenium radiatum | DHR | 3.5 | MHR | 8.1 |
| Viguiera tuberosa | DVT | 0.3 | MVT | 8.5 |
| Extract | CC50 (µg/mL) | EC50 (µg/mL) | SI |
|---|---|---|---|
| DGPU | ˃125.00 | 26.58 ± 4.80 | ˃4.70 |
| DGCH | 266.50 ± 48.10 | 39.68 ± 1.20 | 6.72 |
| DVT | ˃250.00 | 53.96 ± 1.39 | >4.63 |
| DAB | 230.80 ± 55.25 | 33.15 ± 1.09 | 6.96 |
| DHR | 2.82 ± 1.21 | 0.15 ± 0.06 | 18.80 |
| DCM | 18.82 ± 1.26 | 0.11 ± 0.06 | 171.10 |
| Extract | CC50 (µg/mL) | EC50 (µg/mL) | SI |
|---|---|---|---|
| MGPU | ˃250.00 | 3.85 ± 1.28 | ˃64.93 |
| MGCH | ˃250.00 | 29.96 ± 5.81 | ˃8.34 |
| MVT | ˃31.25 | I | - |
| MAB | ˃15.60 | I | - |
| MHR | ˃62.50 | I | - |
| MCM | ˃31.25 | 1.80 ± 0.09 | ˃17.36 |
| Compound | CC50 (µM) | EC50 (µM) | SI |
|---|---|---|---|
| 1 | 6.69 ± 0.49 | I | - |
| 2 | 10.80 ± 0.88 | I | - |
| 3 | 11.15 ± 0.36 | I | - |
| 4 | 10.63 ± 0.55 | I | - |
| 5 | 23.46 ± 0.99 | I | - |
| 6 | 21.81 ± 4.15 | 3.72 ± 0.28 | 5.86 |
| 7 | 21.04 ± 3.65 | 3.12 ± 1.39 | 6.74 |
| 8 | >500 | 6.81 ± 0.20 | >73.4 |
| 9 | >100 | I | - |
| 10 | >100 | I | - |
| 11 | >100 | I | - |
| 12 | >100 | I | - |
| 13 | >200 | I | - |
| 14 | 183.70 ± 27.80 | I | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borgo, J.; Wagner, M.S.; Laurella, L.C.; Elso, O.G.; Selener, M.G.; Clavin, M.; Bach, H.; Catalán, C.A.N.; Bivona, A.E.; Sepúlveda, C.S.; et al. Plant Extracts and Phytochemicals from the Asteraceae Family with Antiviral Properties. Molecules 2024, 29, 814. https://doi.org/10.3390/molecules29040814
Borgo J, Wagner MS, Laurella LC, Elso OG, Selener MG, Clavin M, Bach H, Catalán CAN, Bivona AE, Sepúlveda CS, et al. Plant Extracts and Phytochemicals from the Asteraceae Family with Antiviral Properties. Molecules. 2024; 29(4):814. https://doi.org/10.3390/molecules29040814
Chicago/Turabian StyleBorgo, Jimena, Mariel S. Wagner, Laura C. Laurella, Orlando G. Elso, Mariana G. Selener, María Clavin, Hernán Bach, César A. N. Catalán, Augusto E. Bivona, Claudia S. Sepúlveda, and et al. 2024. "Plant Extracts and Phytochemicals from the Asteraceae Family with Antiviral Properties" Molecules 29, no. 4: 814. https://doi.org/10.3390/molecules29040814
APA StyleBorgo, J., Wagner, M. S., Laurella, L. C., Elso, O. G., Selener, M. G., Clavin, M., Bach, H., Catalán, C. A. N., Bivona, A. E., Sepúlveda, C. S., & Sülsen, V. P. (2024). Plant Extracts and Phytochemicals from the Asteraceae Family with Antiviral Properties. Molecules, 29(4), 814. https://doi.org/10.3390/molecules29040814






