Use of Novel Homochiral Thioureas Camphor Derived as Asymmetric Organocatalysts in the Stereoselective Formation of Glycosidic Bonds
Abstract
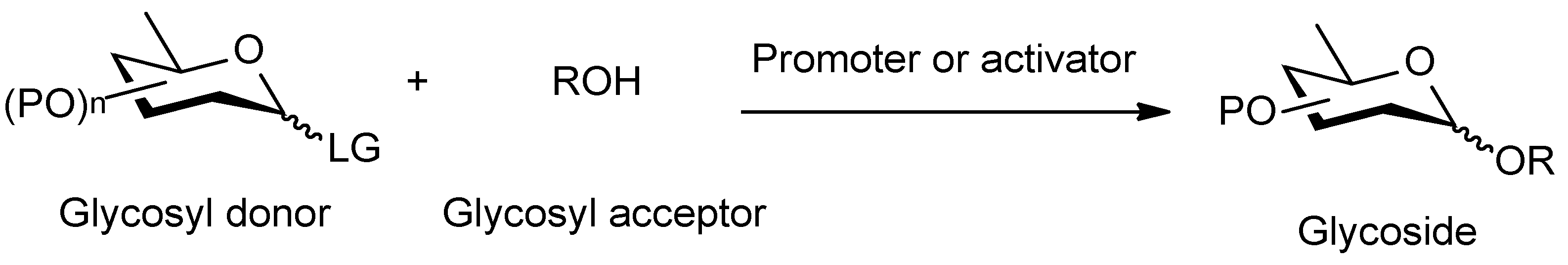
1. Introduction
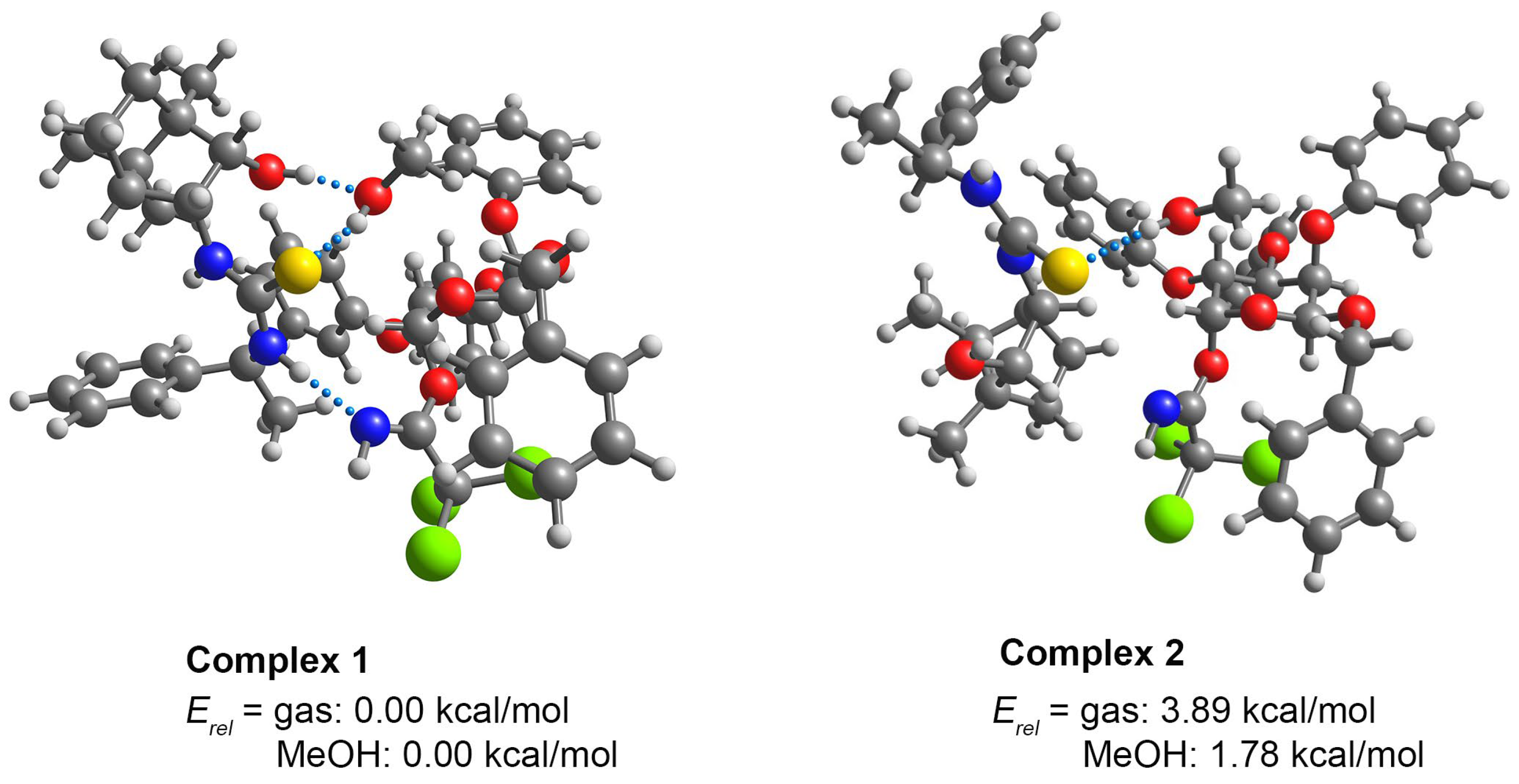
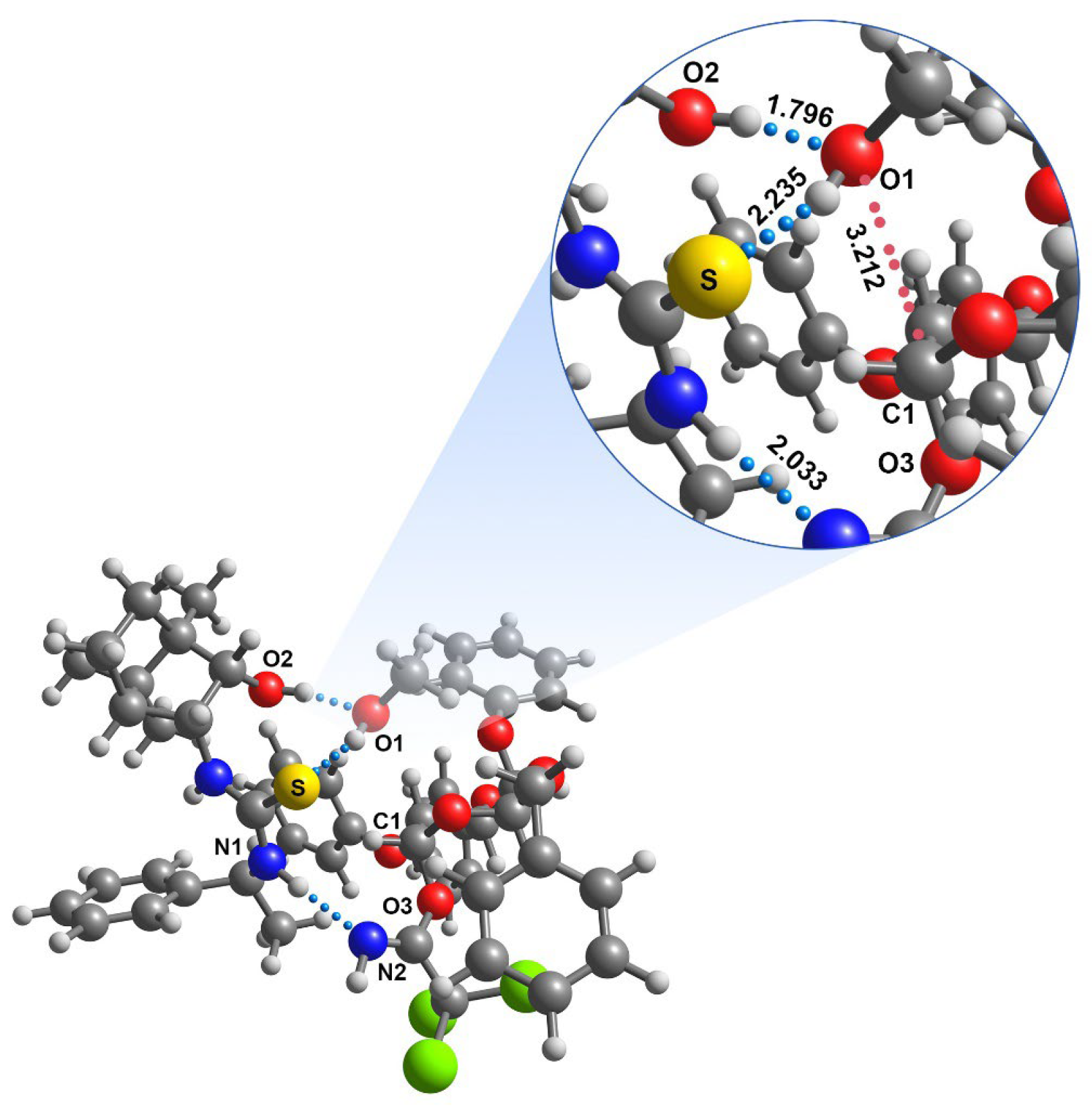
2. Results and Discussion
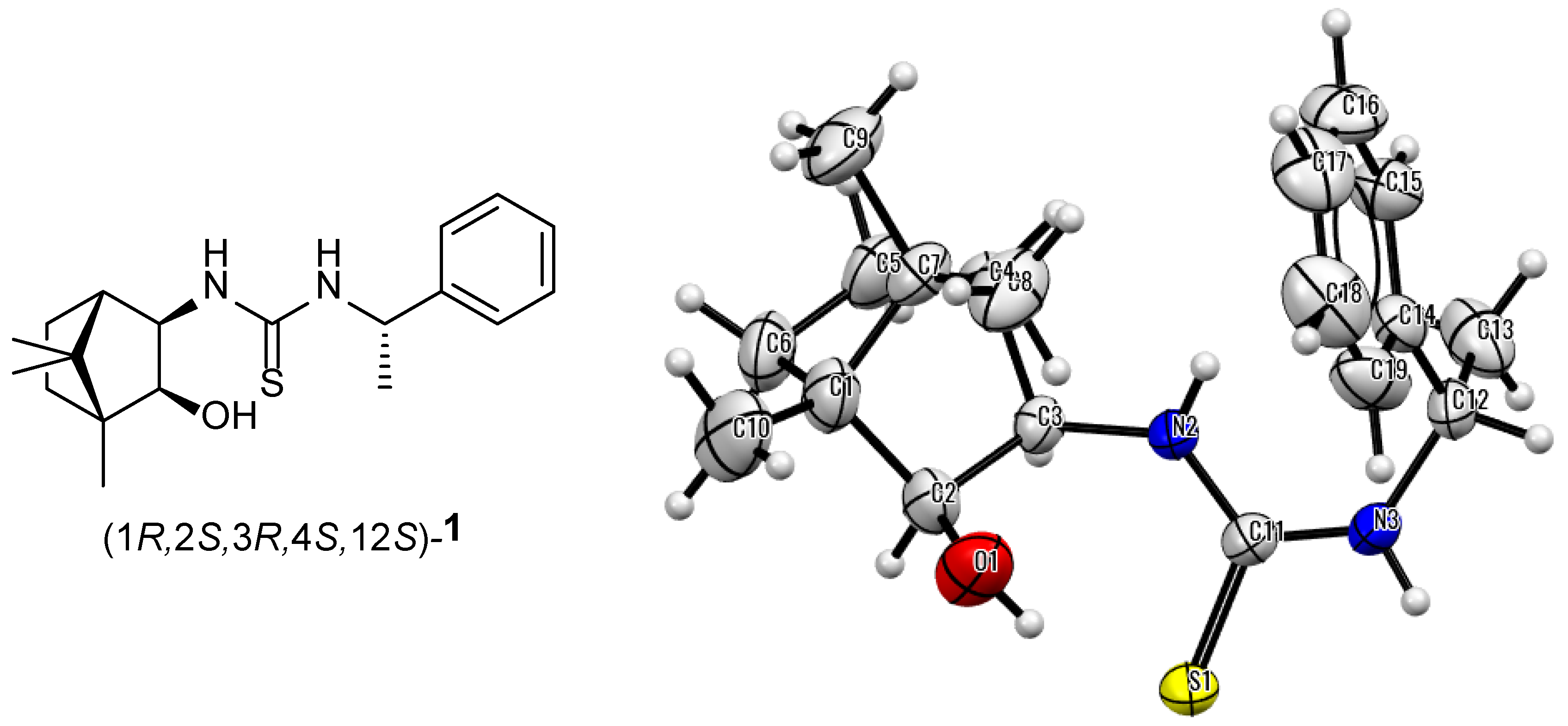
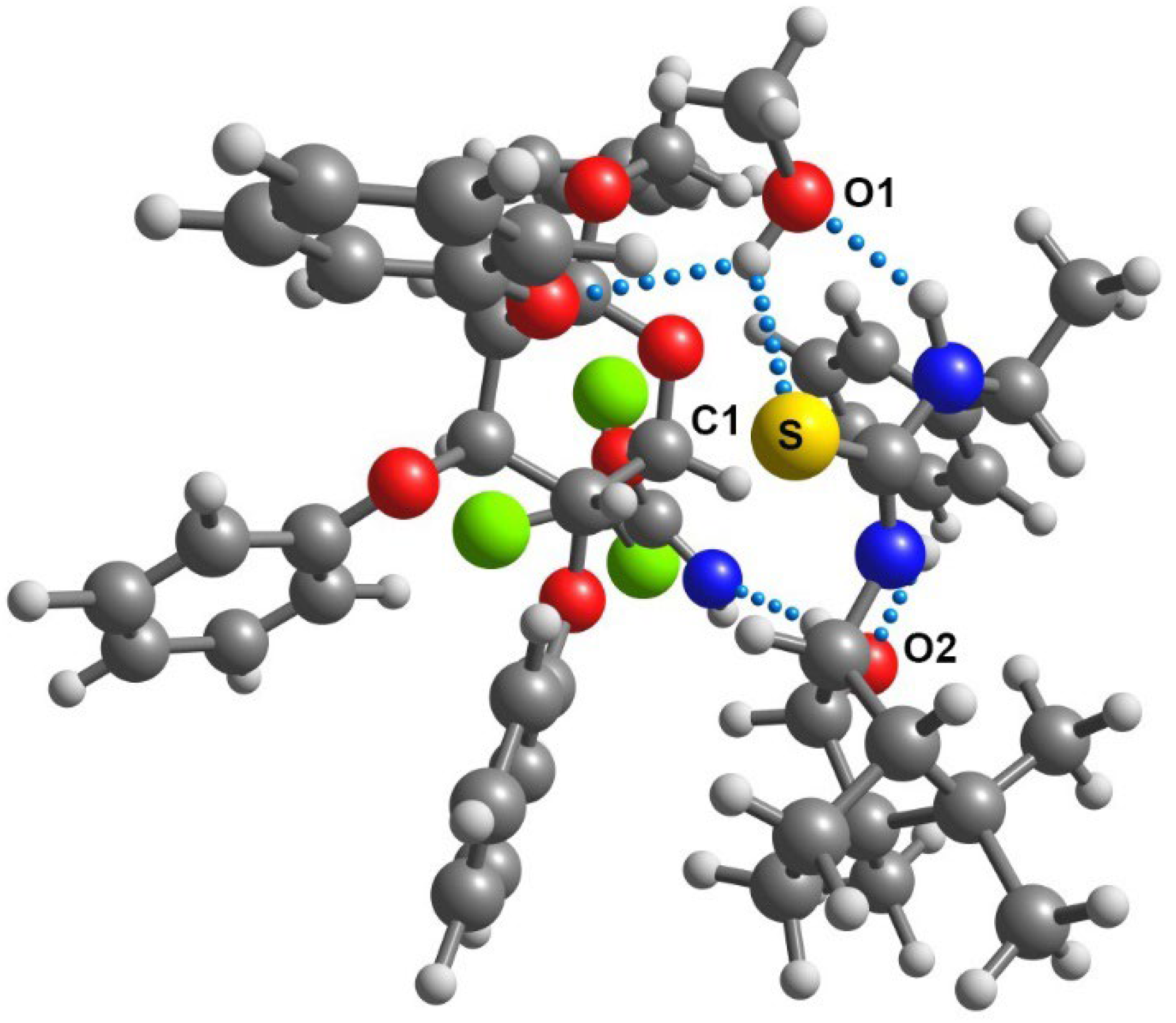
2.1. Synthesis of New Homochiral Thioureas Derived from Camphor as Potential Organocatalysts
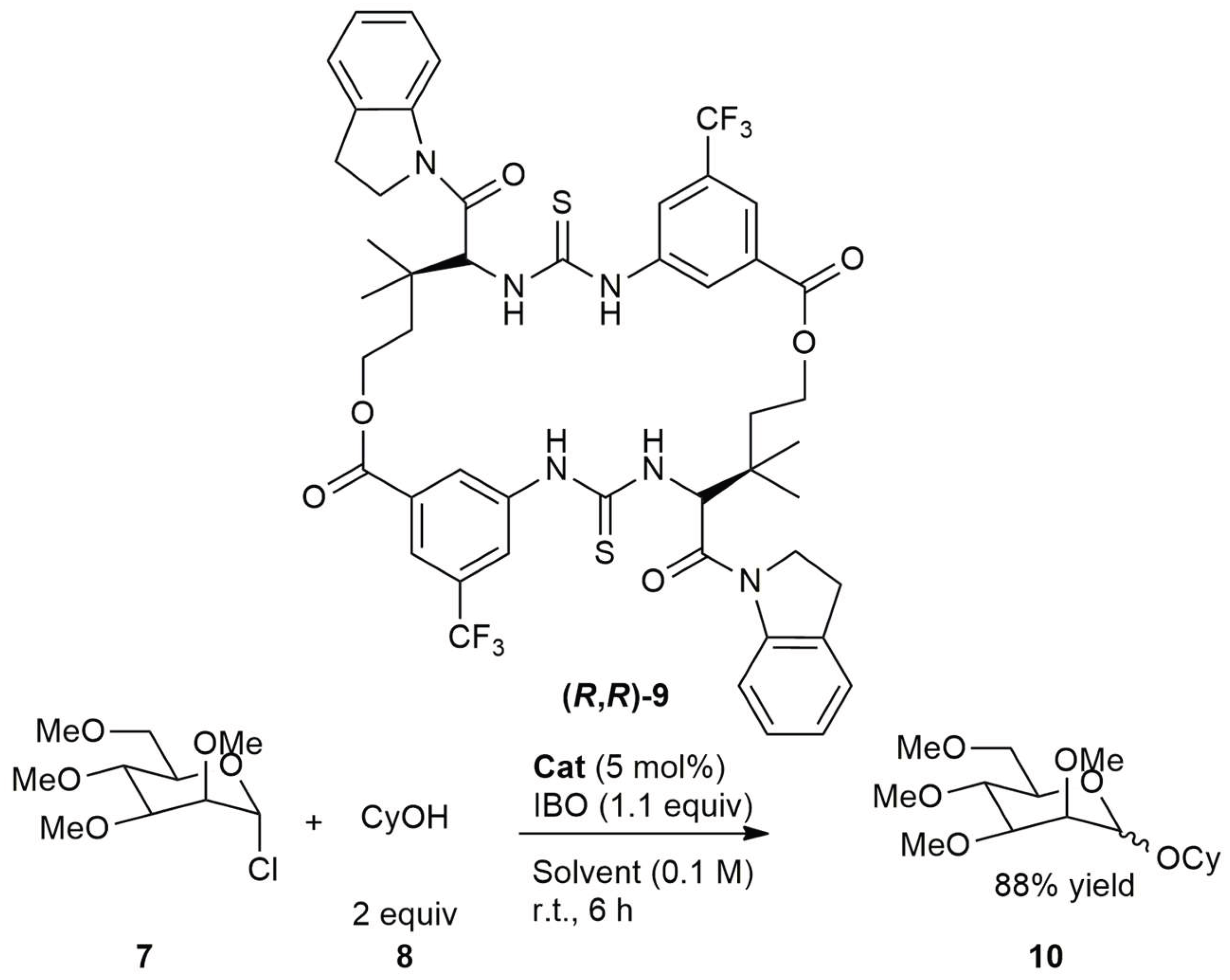
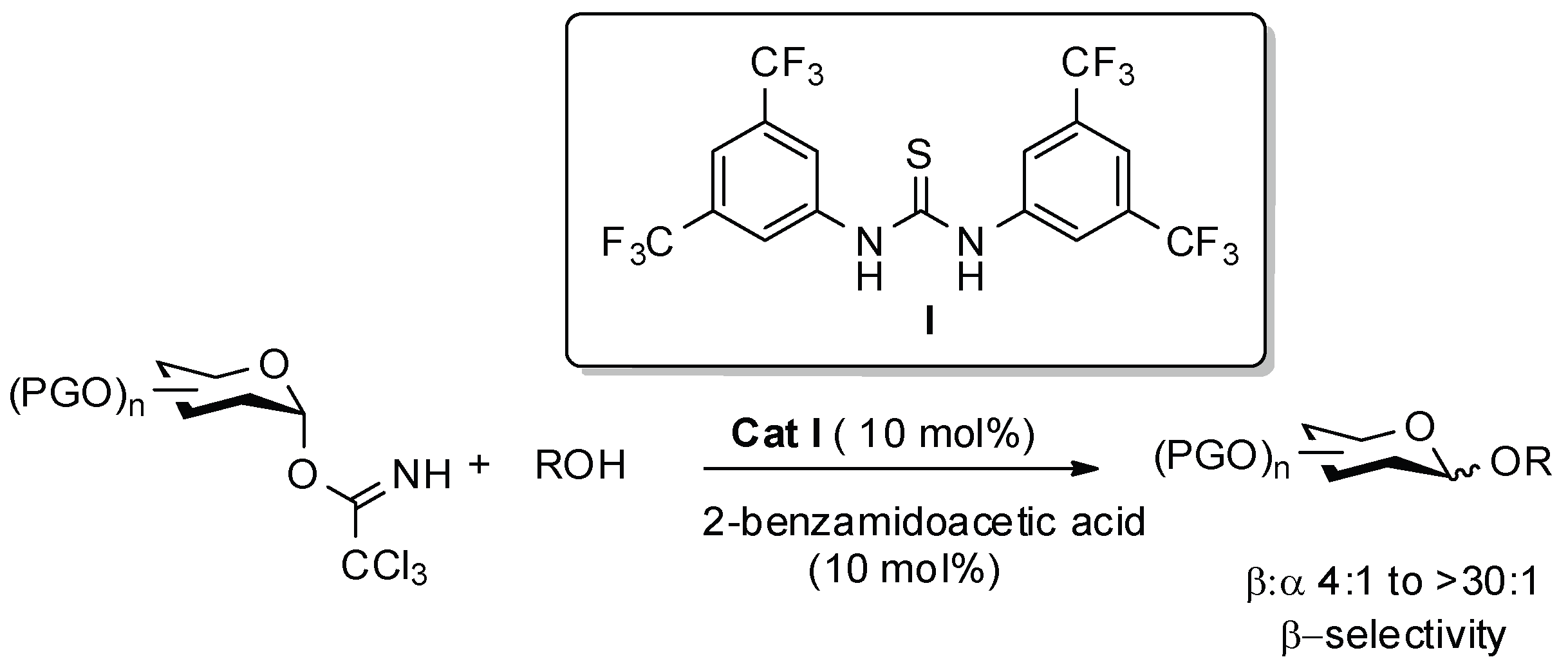
2.2. Homochiral Thioureas 1–6 as Organocatalysts in Glycosylation Reaction
3. Materials and Methods
3.1. General Synthesis of Homochiral Thioureas
3.1.1. Synthesis of 1-((1R,2S,3R,4S)-2-Hydroxy-1,7,7-Trimethylbicyclo[2.2.1]Heptan-3-yl)-3-((S)-1-Phenylethyl)Thiourea-1
3.1.2. Synthesis of 1-((1R,2S,3R,4S)-2-Hydroxy-1,7,7-Trimethylbicyclo[2.2.1]Heptan-3-yl)-3-((R)-1-Phenylethyl)Thiourea-2
3.1.3. Synthesis of 3-[3,5-Bis(Trifluoromethyl)phenyl]-1-[(1R,2S,3R,4S)-2-Hydroxy-1,7,7-Trimethylbicyclo[2.2.1]Heptan-3-yl]Thiourea-3
3.1.4. Synthesis of 1-Benzhydryl-3-((1R,2S,3R,4S)-2-Hydroxy-1,7,7-Trimethylbicyclo[2.2.1]Heptan-3-yl)Thiourea-4
3.1.5. Synthesis of 1-Benzyl-3-((1R,2S,3R,4S)-2-Hydroxy-1,7,7-Trimethylbicyclo[2.2.1]Heptan-3-yl)Thiourea-5
3.1.6. Synthesis of 1-((1R,2S,3R,4S)-2-Hydroxy-1,7,7-Trimethylbicyclo[2.2.1]Heptan-3-yl)-3-Phenylthiourea 6
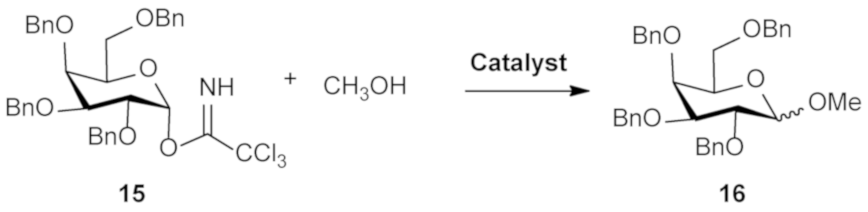
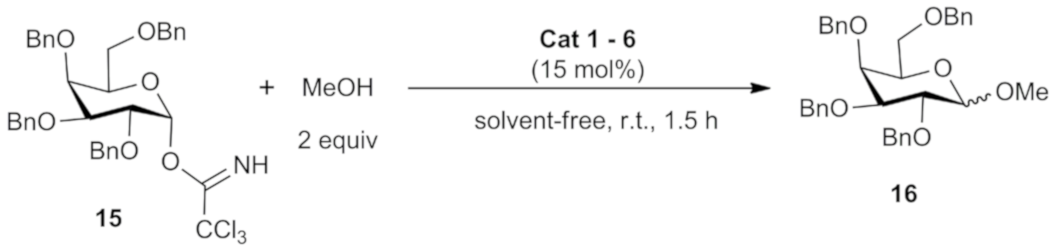
3.2. General Methodology for Glycosylation Reaction
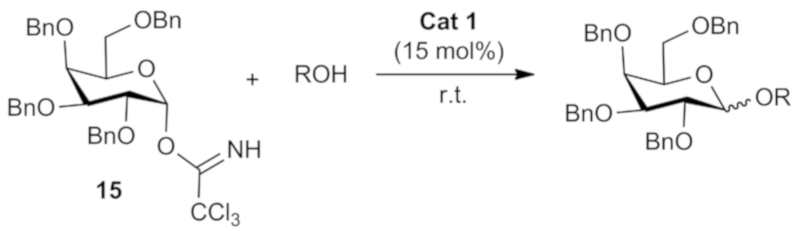
3.3. General Methodology for Solvent-Free Glycosylation Reaction [32]
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sasaki, K.; Hashimoto, Y. 2,6-Lactones as a New Entry in Stereoselective Glycosylations. Synlett 2017, 28, 1121–1126. [Google Scholar] [CrossRef]
- Adero, P.O.; Amarasekara, H.; Wen, P.; Bohé, L.; Crich, D. The Experimental Evidence in Support of Glycosylation Mechanisms at the SN1–SN2 Interface. Chem. Rev. 2018, 118, 8242–8284. [Google Scholar] [CrossRef] [PubMed]
- Codée, J.D.C.; Litjens, R.E.J.N.; van den Bos, L.J.; Overkleeft, H.S.; van der Marel, G.A. Thioglycosides in sequential glycosylation strategies. Chem. Soc. Rev. 2005, 34, 769–782. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Moon, S.; Hentschel, F.; Gilmore, K.; Seeberger, P.H. An Empirical Understanding of the Glycosylation Reaction. J. Am. Chem. Soc. 2018, 140, 11942–11953. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-H.; Yang, H.; Khot, V.; Whitfield, D.; Boons, G.-J. Stereoselective Glycosylations Using (R)- or (S)-(Ethoxycarbonyl)benzyl Chiral Auxiliaries at C-2 of Glycopyranosyl Donors. Eur. J. Org. Chem. 2006, 2006, 440. [Google Scholar] [CrossRef]
- Yang, B.; Yang, W.; Ramadan, S.; Huang, X. Pre-Activation-Based Stereoselective Glycosylations. Eur. J. Org. Chem. 2018, 2018, 1075–1096. [Google Scholar] [CrossRef] [PubMed]
- López, M.; de Parrodi, A.C.; Huelgas, G.; Lozada-Ramírez, D.J. Organocatalyzed Stereoselective Glycosylation: An Overview of the Last Decade. Mini-Rev. Org. Chem. 2024, 21, 318–345. [Google Scholar] [CrossRef]
- El-Badry, M.H.; Gervay-Hague, J. Thermal effect in β-selective glycosylation reactions using glycosyl iodides. Tetrahedron Lett. 2005, 46, 6727–6728. [Google Scholar] [CrossRef]
- Demchenko, A.; Pornsuriyasak, P.; De Meo, C.; Malysheva, N. Potent, Versatile, and Stable: Thiazolyl Thioglycosides as Glycosyl Donors. Angew. Chem. Int. Ed. 2004, 43, 3069–3072. [Google Scholar] [CrossRef]
- Kim, K.S.; Kim, J.H.; Lee, Y.J.; Lee, Y.J.; Park, J. 2-(Hydroxycarbonyl)benzyl Glycosides: A Novel Type of Glycosyl Donors for Highly Efficient β-Mannopyranosylation and Oligosaccharide Synthesis by Latent-Active Glycosylation. J. Am. Chem. Soc. 2001, 123, 8477–8481. [Google Scholar] [CrossRef]
- Lee, Y.J.; Baek, J.Y.; Lee, B.-Y.; Kang, S.S.; Park, H.-S.; Jeon, H.B.; Kim, K.S. 2′-Carboxybenzyl glycosides: Glycosyl donors for C-glycosylation and conversion into other glycosyl donors. Carbohydr. Res. 2006, 341, 1708–1716. [Google Scholar] [CrossRef]
- Zhu, X.; Schmidt, R.R. New Principles for Glycoside-Bond Formation. Angew. Chem. Int. Ed. 2009, 48, 1900–1934. [Google Scholar] [CrossRef]
- Kimura, T.; Eto, T.; Takahashi, D.; Toshima, K. Stereocontrolled Photoinduced Glycosylation Using an Aryl Thiourea as an Organo photoacid. Org. Lett. 2016, 18, 3190–3193. [Google Scholar] [CrossRef]
- Gouliaras, C.; Lee, D.; Chan, L.; Taylor, M.S. Regioselective Activation of Glycosyl Acceptors by a Diarylborinic Acid-Derived Catalyst. J. Am. Chem. Soc. 2011, 133, 13926–13929. [Google Scholar] [CrossRef]
- Tomita, S.; Tanaka, M.; Inoue, M.; Inaba, K.; Takahashi, D.; Toshima, K. Diboron-Catalyzed Regio- and 1,2-cis-α-Stereoselective Glycosylation of trans-1,2-Diols. J. Org. Chem. 2020, 85, 16254–16262. [Google Scholar] [CrossRef] [PubMed]
- Shaw, M.; Kumar, Y.; Thakur, R.; Kumar, A. Electron-deficient pyridinium salts/thiourea cooperative catalyzed O-glycosylation via activation of O-glycosyl trichloroacetimidate donors. Beilstein J. Org. Chem. 2017, 13, 2385–2395. [Google Scholar] [CrossRef] [PubMed]
- Demchenko, A. General Aspects of the Glycosidic Bond Formation. In Handbook of Chemical Glycosylation: Advances in Stereoselectivity and Therapeutic Relevance; Wiley: Hoboken, NJ, USA, 2008; pp. 1–27. [Google Scholar]
- Bohé, L.; Crich, D. Synthesis of Glycosides. In Comprehensive Organic Synthesis; Knochel, P., Ed.; Elsevier: Amsterdam, The Netherlands, 2014; Volume 6. [Google Scholar]
- Morelli, L.; Legnani, L.; Ronchi, S.; Confalonieri, L.; Imperio, D.; Toma, L.; Compostella, F. 2,3-Carbamate mannosamine glycosyl donors in glycosylation reactions of diacetone-d-glucose. An experimental and theoretical study. Carbohydr. Res. 2021, 509, 108421. [Google Scholar] [CrossRef] [PubMed]
- Ling, J.; Bennett, C.S. Recent Developments in Stereoselective Chemical Glycosylation. Asian J. Org. Chem. 2019, 8, 802–813. [Google Scholar] [CrossRef] [PubMed]
- Yu, B. Gold(I)-Catalyzed Glycosylation with Glycosyl o-Alkynylbenzoates as Donors. Acc Chem Res 2018, 51, 507–516. [Google Scholar] [CrossRef] [PubMed]
- Nigudkar, S.S.; Parameswar, A.R.; Pornsuriyasak, P.; Stine, K.J.; Demchenko, A.V. O-Benzoxazolyl imidates as versatile glycosyl donors for chemical glycosylation. Org. Biomol. Chem. 2013, 11, 4068–4076. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Nashida, J.; Takahashi, D.; Toshima, K. Glycosyl-Acceptor-Derived Borinic Ester-Promoted Direct and β-Stereoselective Mannosylation with a 1,2-Anhydromannose Donor. Org. Lett. 2016, 18, 2288–2291. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Bao, Z.; Xing, H. N,N′-Bis[3,5-bis(trifluoromethyl)phenyl]thiourea: A privileged motif for catalyst development. Org. Biomol. Chem. 2014, 12, 3151–3162. [Google Scholar] [CrossRef]
- Geng, Y.; Kumar, A.; Faidallah, H.M.; Albar, H.A.; Mhkalid, I.A.; Schmidt, R.R. Cooperative Catalysis in Glycosidation Reactions with O-Glycosyl Trichloroacetimidates as Glycosyl Donors. Angew. Chem. Int. Ed. 2013, 52, 10089–10092. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Harper, K.C.; Kuhl, N.; Kwan, E.E.; Liu, R.Y.; Jacobsen, E.N. Macrocyclic bis-thioureas catalyze stereospecific glycosylation reactions. Science 2017, 355, 162–166. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Loh, C.C.J. An ultra-low thiourea catalyzed strain-release glycosylation and a multicatalytic diversification strategy. Nat. Commun. 2018, 9, 4057. [Google Scholar] [CrossRef] [PubMed]
- Cox, D.J.; Smith, M.D.; Fairbanks, A.J. Glycosylation Catalyzed by a Chiral Brønsted Acid. Org. Lett. 2010, 12, 1452–1455. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Kumar, V.; Dere, R.T.; Schmidt, R.R. Glycoside Bond Formation via Acid–Base Catalysis. Org. Lett. 2011, 13, 3612–3615. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.; Galan, M.C. Recent Advances in Organocatalytic Glycosylations. Eur. J. Org. Chem. 2017, 2017, 6247–6264. [Google Scholar] [CrossRef]
- Dubey, A.; Sangwan, R.; Mandal, P.K. N-benzoylglycine/thiourea cooperative catalyzed stereoselective O-glycosidation: Activation of O-glycosyl trichloroacetimidate donors. Catal. Commun. 2019, 125, 123–129. [Google Scholar] [CrossRef]
- Traboni, S.; Bedini, E.; Silipo, A.; Vessella, G.; Iadonisi, A. Solvent-Free Glycosylation from per-O-Acylated Donors Catalyzed by Methanesulfonic Acid. Eur. J. Org. Chem. 2021, 2021, 5669–5676. [Google Scholar] [CrossRef]
- Traboni, S.; Vessella, G.; Bedini, E.; Iadonisi, A. Solvent-free, under air selective synthesis of α-glycosides adopting glycosyl chlorides as donors. Org. Biomol. Chem. 2020, 18, 5157–5163. [Google Scholar] [CrossRef]
- Traboni, S.; Bedini, E.; Vessella, G.; Iadonisi, A. Solvent-Free Approaches in Carbohydrate Synthetic Chemistry: Role of Catalysis in Reactivity and Selectivity. Catalysts 2020, 10, 1142. [Google Scholar] [CrossRef]
- Walsh, P.J.; Li, H.; de Parrodi, C.A. A Green Chemistry Approach to Asymmetric Catalysis: Solvent-Free and Highly Concentrated Reactions. Chem. Rev. 2007, 107, 2503–2545. [Google Scholar] [CrossRef] [PubMed]
- Ričko, S.; Požgan, F.; Štefane, B.; Svete, J.; Golobič, A.; Grošelj, U. Stereodivergent Synthesis of Camphor-Derived Diamines and Their Application as Thiourea Organocatalysts. Molecules 2020, 25, 2978. [Google Scholar] [CrossRef] [PubMed]
- Groselj, U. Camphor-Derivatives in Asymmetric Organocatalysis–Synthesis and Application. Curr. Org. Chem. 2015, 19, 2048–2074. [Google Scholar] [CrossRef]
- Grošelj, U.; Ričko, S.; Svete, J.; Stanovnik, B. Synthesis of 2-(3-(3,5-bis(trifluoromethyl)phenyl)thioureido)-3-((dimethylamino)methyl)camphor organocatalysts. Chirality 2012, 24, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Santacruz, E.; Huelgas, G.; Angulo, S.K.; Mastranzo, V.M.; Hernández-Ortega, S.; Aviña, J.A.; Juaristi, E.; Parrodi, C.A.d.; Walsh, P.J. Catalytic asymmetric hydrosilylation of acetophenone with new chiral thiourea ligands containing the (S)-α-phenylethyl group. Tetrahedron Asymmetry 2009, 20, 2788–2794. [Google Scholar] [CrossRef]
- Periasamy, M.; Sanjeevakumar, N.; Obula Reddy, P. Convenient Methods to Access Chiral Camphanyl Amine Derivatives by Sodium Borohydride Reduction of d-(–)-Camphorquinone Imines. Synthesis 2012, 44, 3185–3190. [Google Scholar] [CrossRef]
- Bucher, C.; Gilmour, R. Fluorine-Directed Glycosylation. Angew. Chem. Int. Ed. 2010, 49, 8724–8728. [Google Scholar] [CrossRef]
- Ditchfield, R.; Hehre, W.J.; Pople, J.A. Self-Consistent Molecular-Orbital Methods. IX. An Extended Gaussian-Type Basis for Molecular-Orbital Studies of Organic Molecules. J. Chem. Phys. 2003, 54, 724–728. [Google Scholar] [CrossRef]
- Zhao, Y.; Truhlar, D.G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: Two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Acc. 2008, 120, 215–241. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16 Rev. C.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Tomasi, J.; Mennucci, B.; Cammi, R. Quantum Mechanical Continuum Solvation Models. Chem. Rev. 2005, 105, 2999–3094. [Google Scholar] [CrossRef] [PubMed]
- Clark, R.; Reid, J. The analytical calculation of absorption in multifaceted crystals. Acta Crystallogr. Sect. A Found. Crystallogr. 1995, 51, 887–897. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Macrae, C.F.; Sovago, I.; Cottrell, S.J.; Galek, P.T.A.; McCabe, P.; Pidcock, E.; Platings, M.; Shields, G.P.; Stevens, J.S.; Towler, M.; et al. Mercury 4.0: From visualization to analysis, design and prediction. J. Appl. Crystallogr. 2020, 53, 226–235. [Google Scholar] [CrossRef]



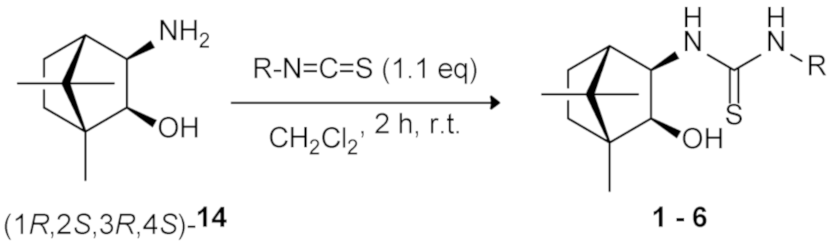 | |||
|---|---|---|---|
| Entry | R-N=C=S | Product | Yield a (%) |
| 1 | 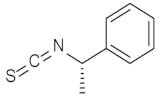 | (1R,2S,3R,4S,12S)-1 | 79 |
| 2 | 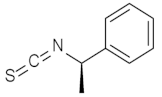 | (1R,2S,3R,4S,12R)-2 | 90 |
| 3 |  | (1R,2S,3R,4S)-3 | 64 |
| 4 | 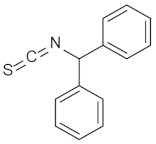 | (1R,2S,3R,4S)-4 | 79 |
| 5 | 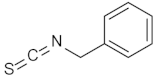 | (1R,2S,3R,4S)-5 | 74 |
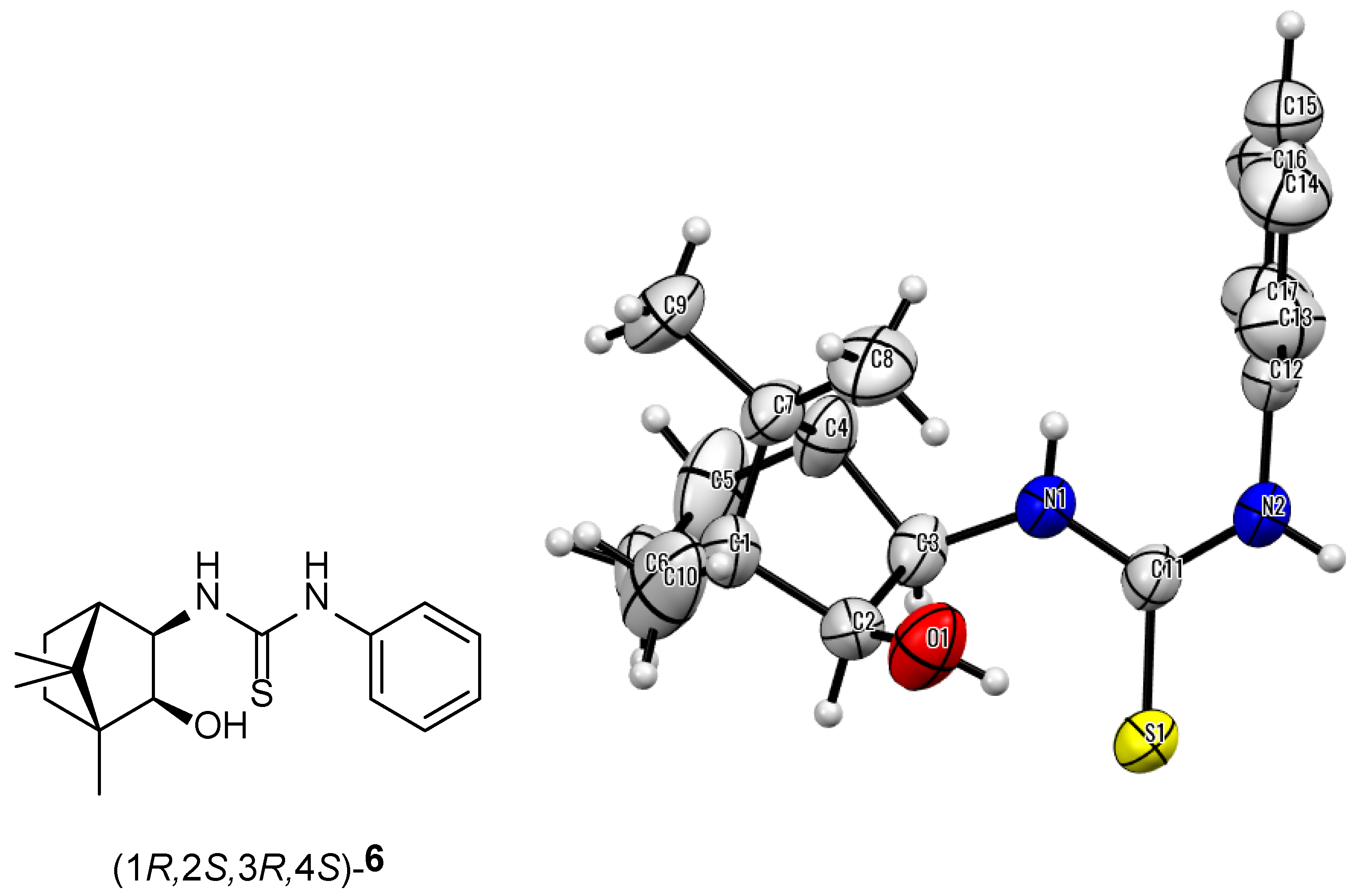
| 6 | 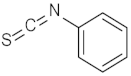 | (1R,2S,3R,4S)-6 | 93 |
 | |||||||
|---|---|---|---|---|---|---|---|
| Entry | Catalyst (mol %) | Additive (2 Equiv) | Solvent (2 mL) | Time (h) | T (°C) | Yield b (%) | α:β c |
| 1 | 1 (15) | None | CH3CN | 120 | r.t. | 15 | 1:8 |
| 2 | 1 (15) | None | CH2Cl2 | 120 | r.t. | 40 | 1:13 |
| 3 | 1 (15) | None | THF | 120 | r.t. | 25 | 1:8 |
| 4 | 1 (15) | None | Et2O | 120 | r.t. | 12 | 1:12 |
| 5 | 1 (15) | None | TBME d | 120 | r.t. | 47 | 1:15 |
| 6 | 1 (15) | None | Toluene | 120 | r.t. | n.r. | - |
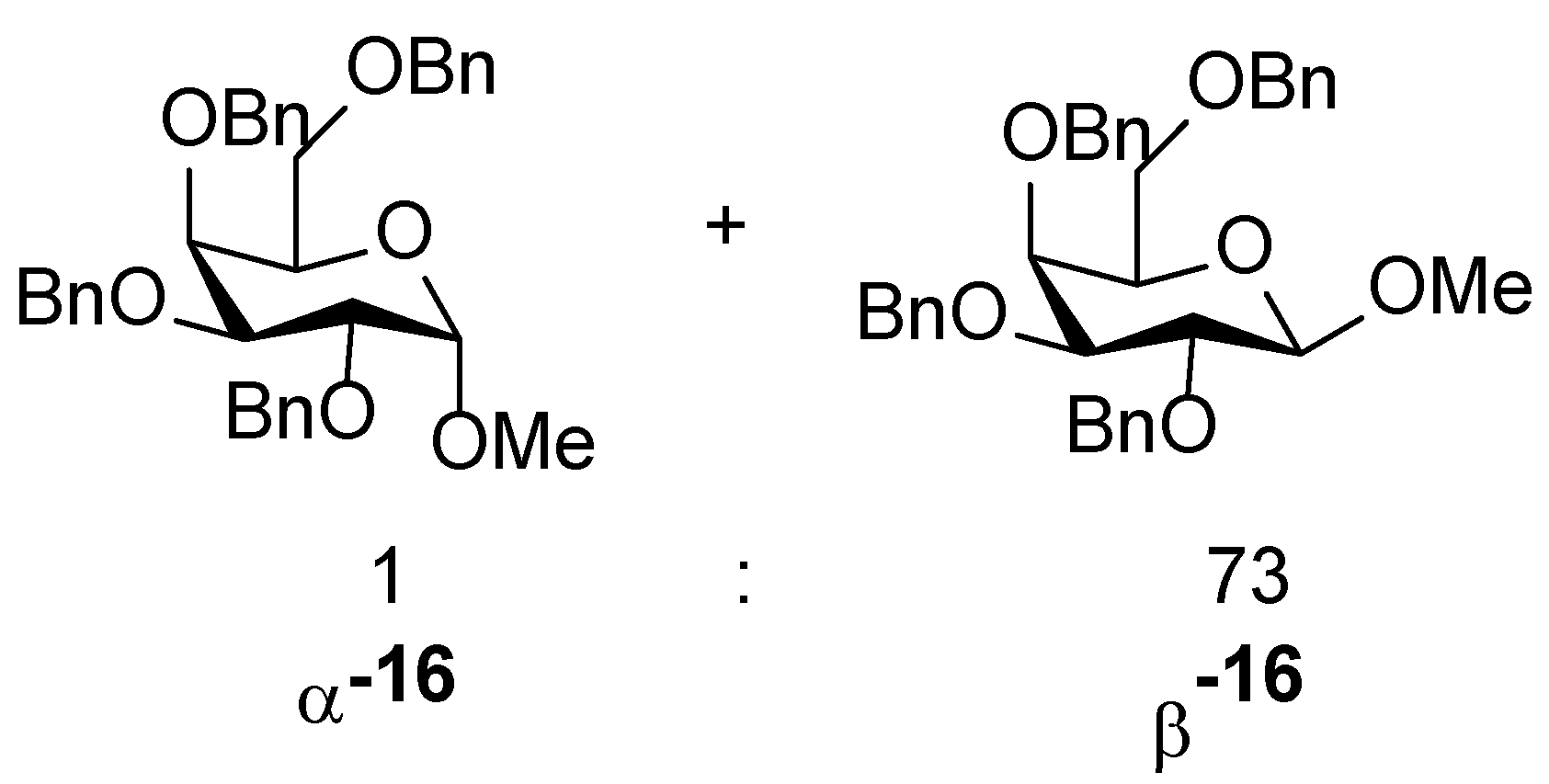
| 7 | 1 (15) | None | solvent free | 1.5 | r.t. | 99 | 1:73 |
| 8 | 1 (15) | None | solvent free | 2 | 0 | 90 | 1:38 |
| 9 | 1 (15) | None | solvent free | 24 | −25 | 70 | 1:41 |
| 10 | 1 (5) | None | solvent free | 1.5 | r.t. | 90 | 1:40 |
| 11 | 1 (10) | None | solvent free | 1.5 | r.t. | 86 | 1:45 |
| 12 | 1 (20) | None | solvent free | 1.5 | r.t. | 80 | 1:40 |
| 13 | 1 (15) | K2CO3 | solvent free | 1.5 | r.t. | n.r. | - |
| 14 | 1 (15) | Molecular sieve | solvent free | 1.5 | r.t. | 92 | 1:40 |
 | |||
|---|---|---|---|
| Entry | Organocatalyst (mol %) | Yield (%) b | α:β c |
| 1 | None | 82 | 1:35 |
| 2 | 1 | 99 | 1:73 |
| 3 | 2 | 81 | 1:42 |
| 4 | 3 | 99 | 1:55 |
| 5 | 4 | 95 | 1:68 |
| 6 | 5 | 99 | 1:56 |
| 7 | 6 | 93 | 1:52 |
 | |||
|---|---|---|---|
| Entry | Organocatalyst | Yield (%) b | (α:β ratio) c |
| 1 | 1 | 81 | 1:58 |
| 2 | 2 | 58 | 1:6 |
| 3 | 3 | 83 | 1:14 |
| 4 | 4 | 92 | 1:53 |
| 5 | 5 | 87 | 1:12 |
| 6 | 6 | 86 | 1:57 |
 | ||||
|---|---|---|---|---|
| Entry | ROH | Reaction Time (h) | Yield (%) b | (α:β ratio) c |
| 1 | Methanol | 1.5 | 99 | 1:73 |
| 2 | Ethanol | 1.5 | 88 | 1:13 |
| 3 | 1-propanol | 4 | 95 | 1:10 |
| 4 | 1-butanol | 4 | 96 | 1:6.5 |
| 5 | 1-octanol | 3 | 76 | 1:8 |
| 6 | iso-propanol | 1.5 | 52 | 1:7 |
| 7 | tert-butanol | 1.5 | 66 | 1:1.1 |
| 8 | Cyclohexanol | 4 | 25 | 1:3 |
| 9 | Phenol d | 4 | 39 | 1:1.3 |
| 10 | 1-naphtol d | 6 | 26 | 1:3 |
| 11 | 2-naphtol d | 6 | 13 | 1:6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López, M.; Huelgas, G.; Sánchez, M.; Armenta, A.; Mendoza, A.; Lozada-Ramírez, J.D.; Anaya de Parrodi, C. Use of Novel Homochiral Thioureas Camphor Derived as Asymmetric Organocatalysts in the Stereoselective Formation of Glycosidic Bonds. Molecules 2024, 29, 811. https://doi.org/10.3390/molecules29040811
López M, Huelgas G, Sánchez M, Armenta A, Mendoza A, Lozada-Ramírez JD, Anaya de Parrodi C. Use of Novel Homochiral Thioureas Camphor Derived as Asymmetric Organocatalysts in the Stereoselective Formation of Glycosidic Bonds. Molecules. 2024; 29(4):811. https://doi.org/10.3390/molecules29040811
Chicago/Turabian StyleLópez, Mildred, Gabriela Huelgas, Mario Sánchez, Adalid Armenta, Angel Mendoza, José Daniel Lozada-Ramírez, and Cecilia Anaya de Parrodi. 2024. "Use of Novel Homochiral Thioureas Camphor Derived as Asymmetric Organocatalysts in the Stereoselective Formation of Glycosidic Bonds" Molecules 29, no. 4: 811. https://doi.org/10.3390/molecules29040811
APA StyleLópez, M., Huelgas, G., Sánchez, M., Armenta, A., Mendoza, A., Lozada-Ramírez, J. D., & Anaya de Parrodi, C. (2024). Use of Novel Homochiral Thioureas Camphor Derived as Asymmetric Organocatalysts in the Stereoselective Formation of Glycosidic Bonds. Molecules, 29(4), 811. https://doi.org/10.3390/molecules29040811







