A Naphthoquinoline-Dione-Based Cu2+ Sensing Probe with Visible Color Change and Fluorescence Quenching in an Aqueous Organic Solution
Abstract
1. Introduction
2. Results and Discussion
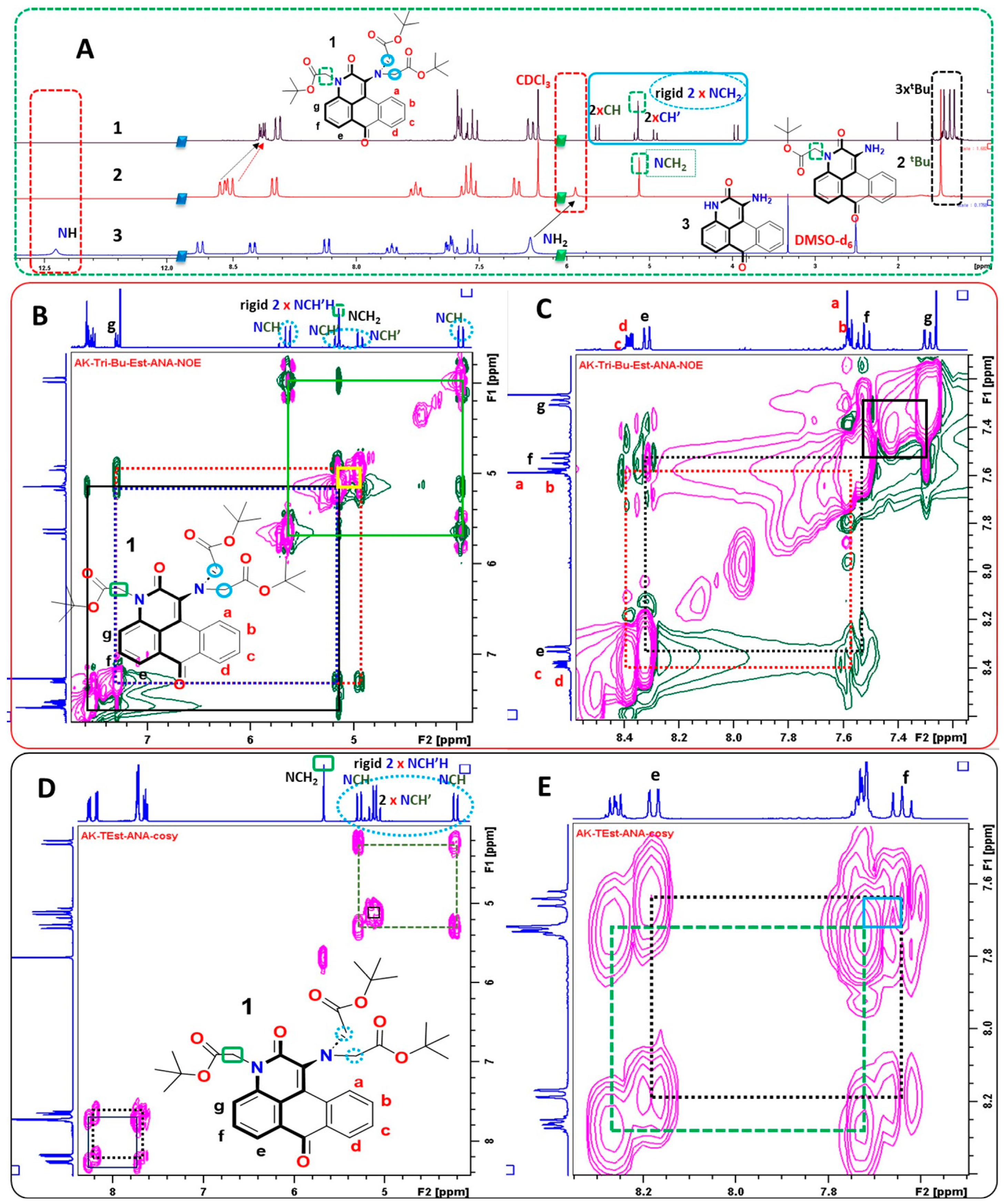
2.1. Synthesis and Characterization of Probe 1
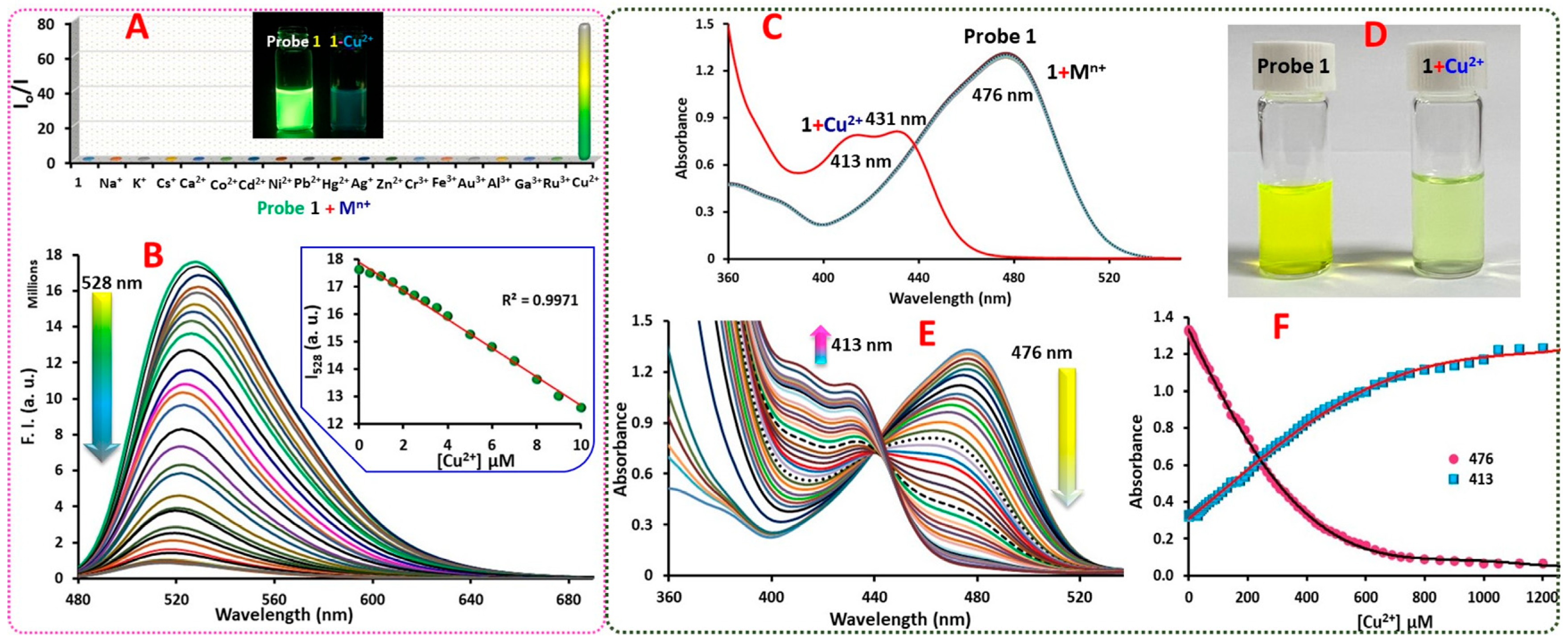
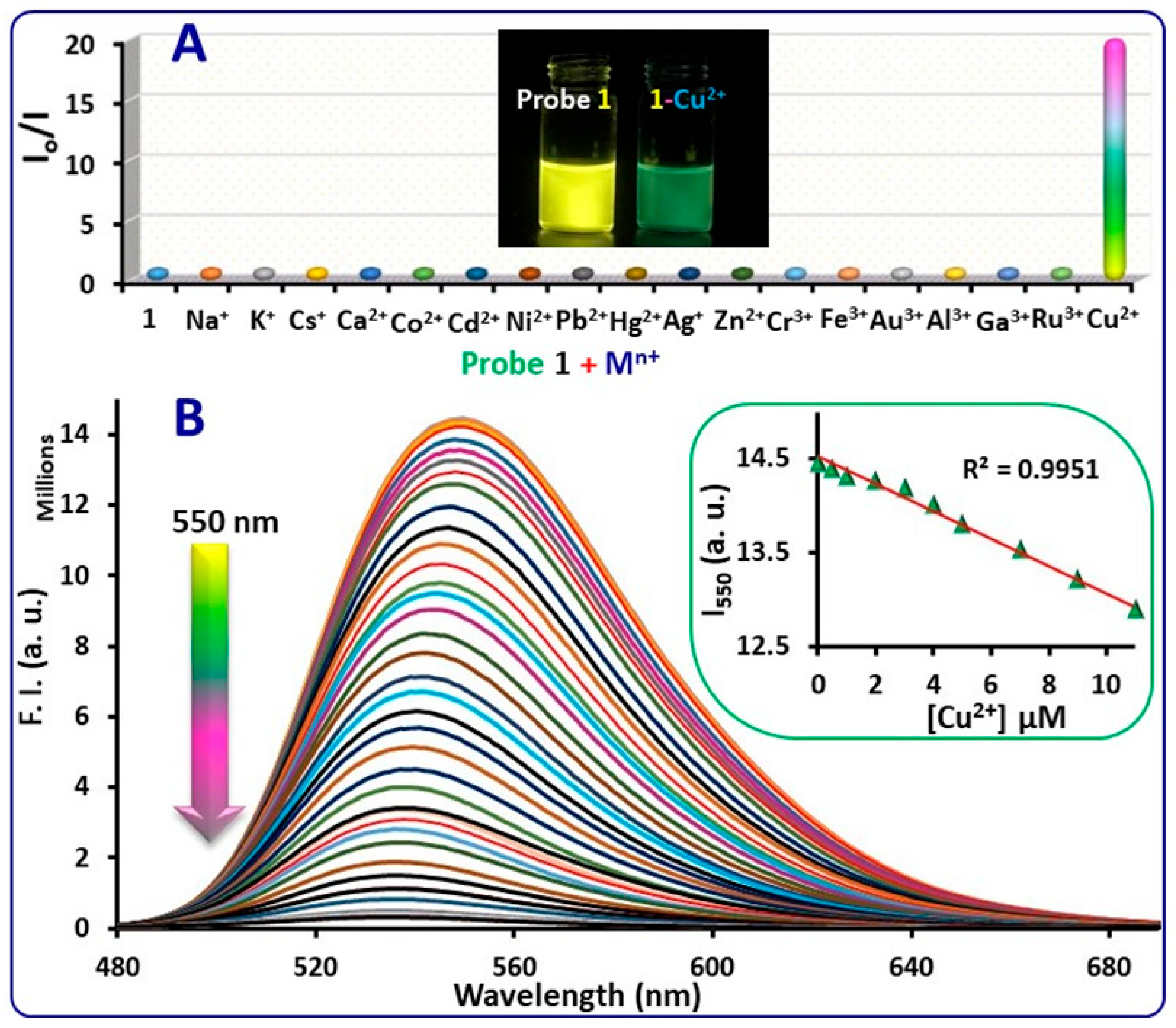
2.2. Spectroscopic Characterization and Sensing Ability of Probe 1
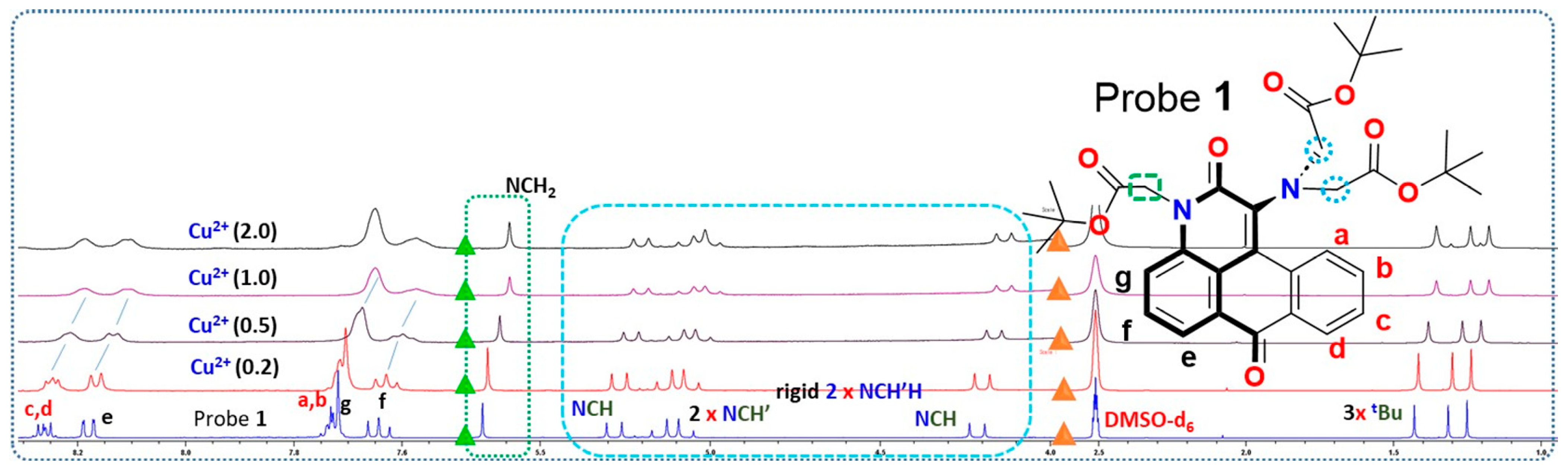
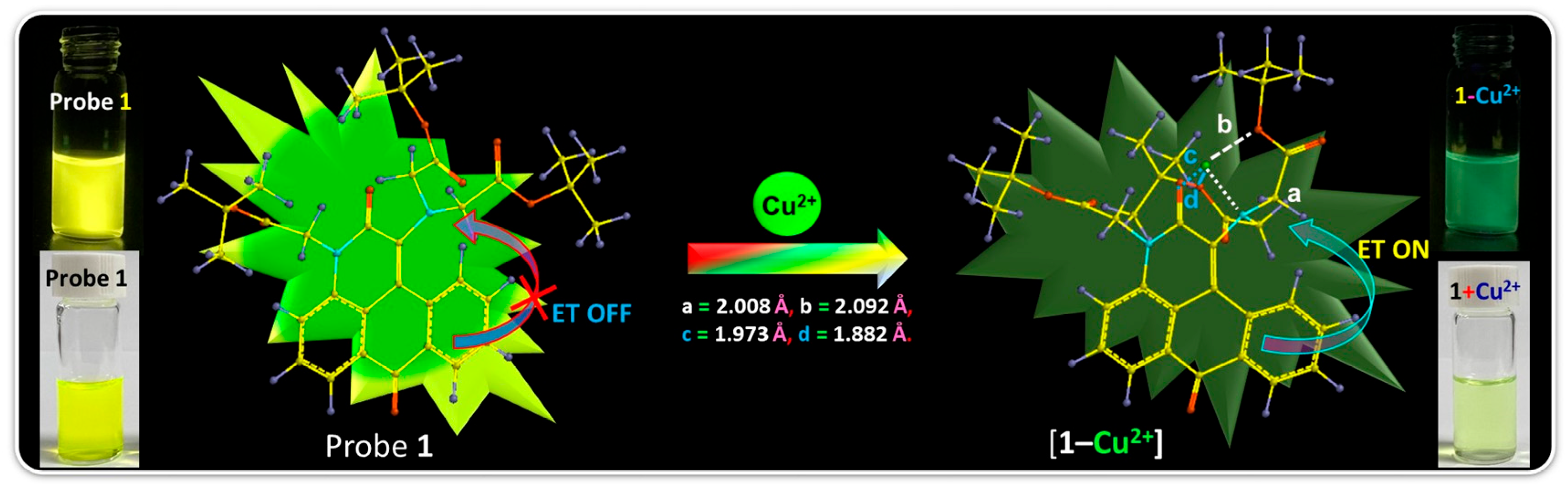
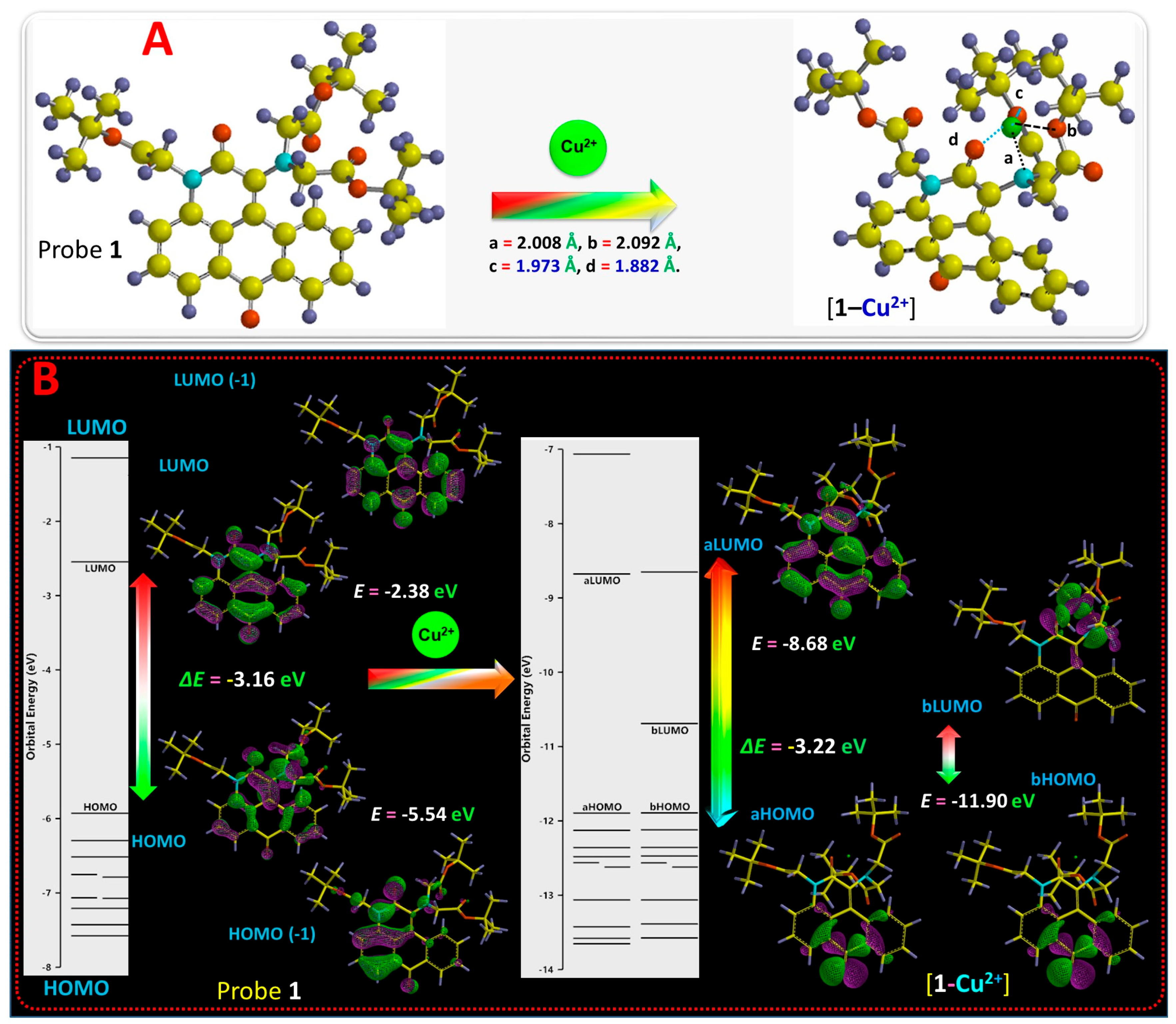
2.3. Mechanism of Cu2+ Interaction with Probe 1
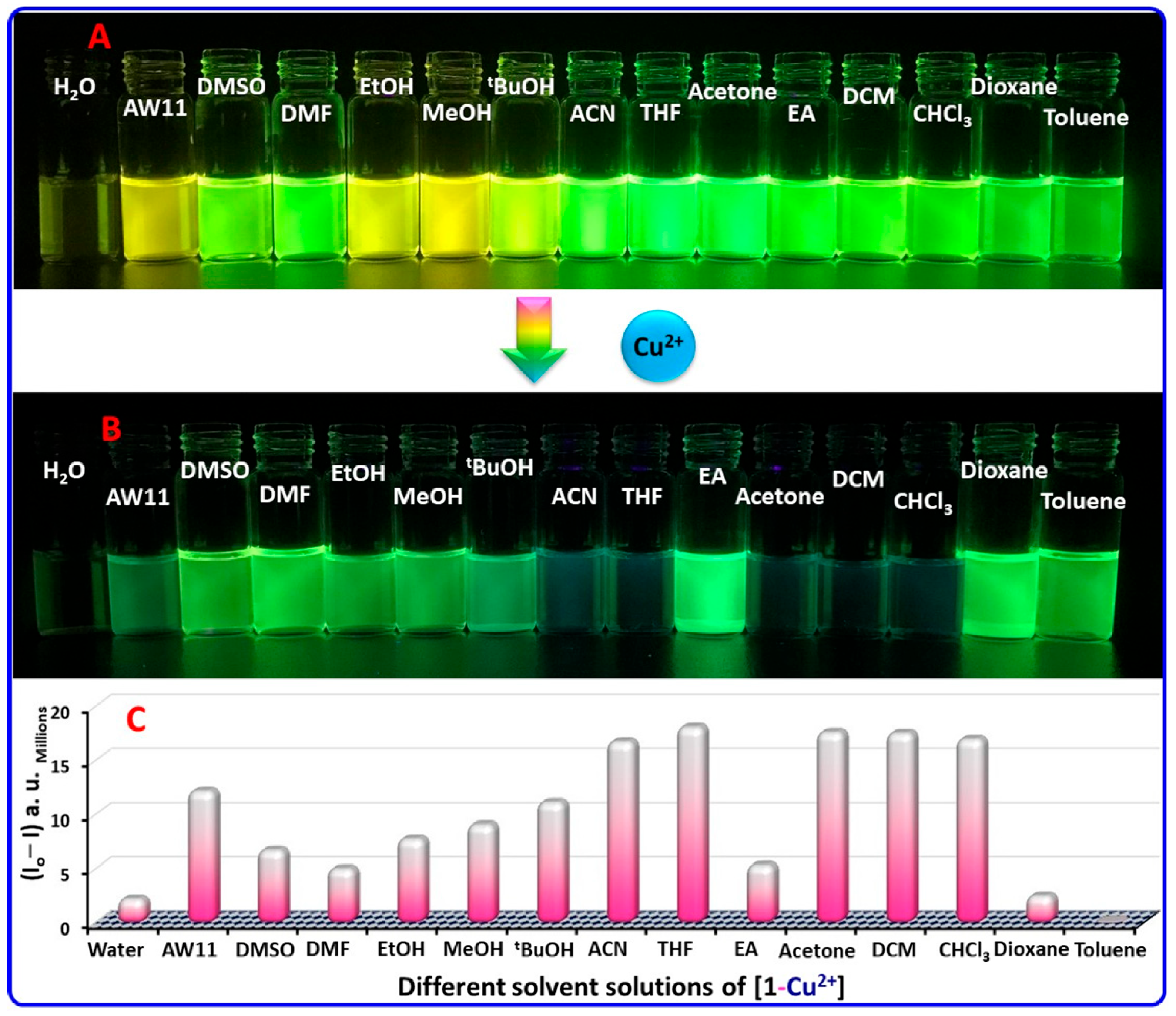
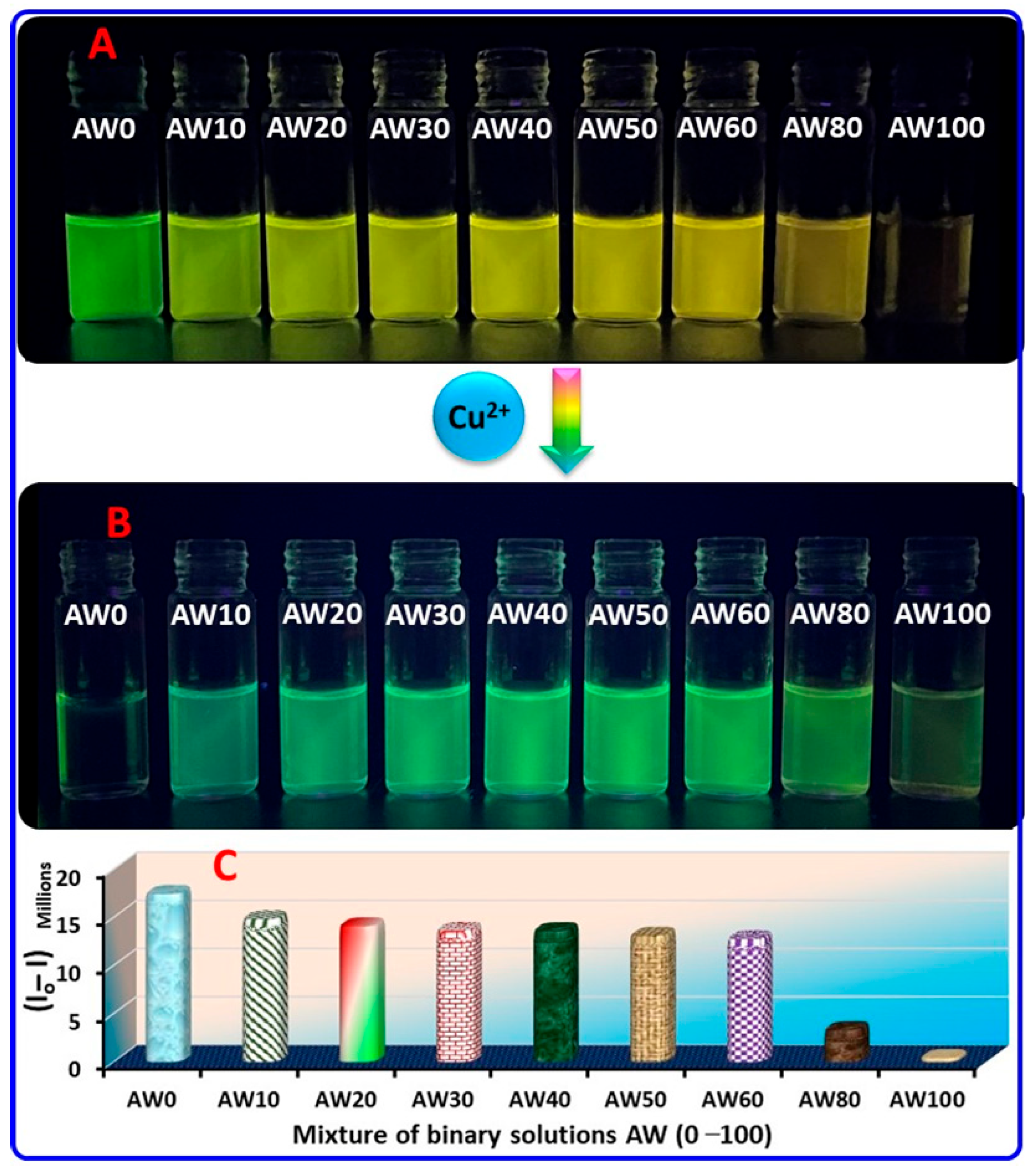
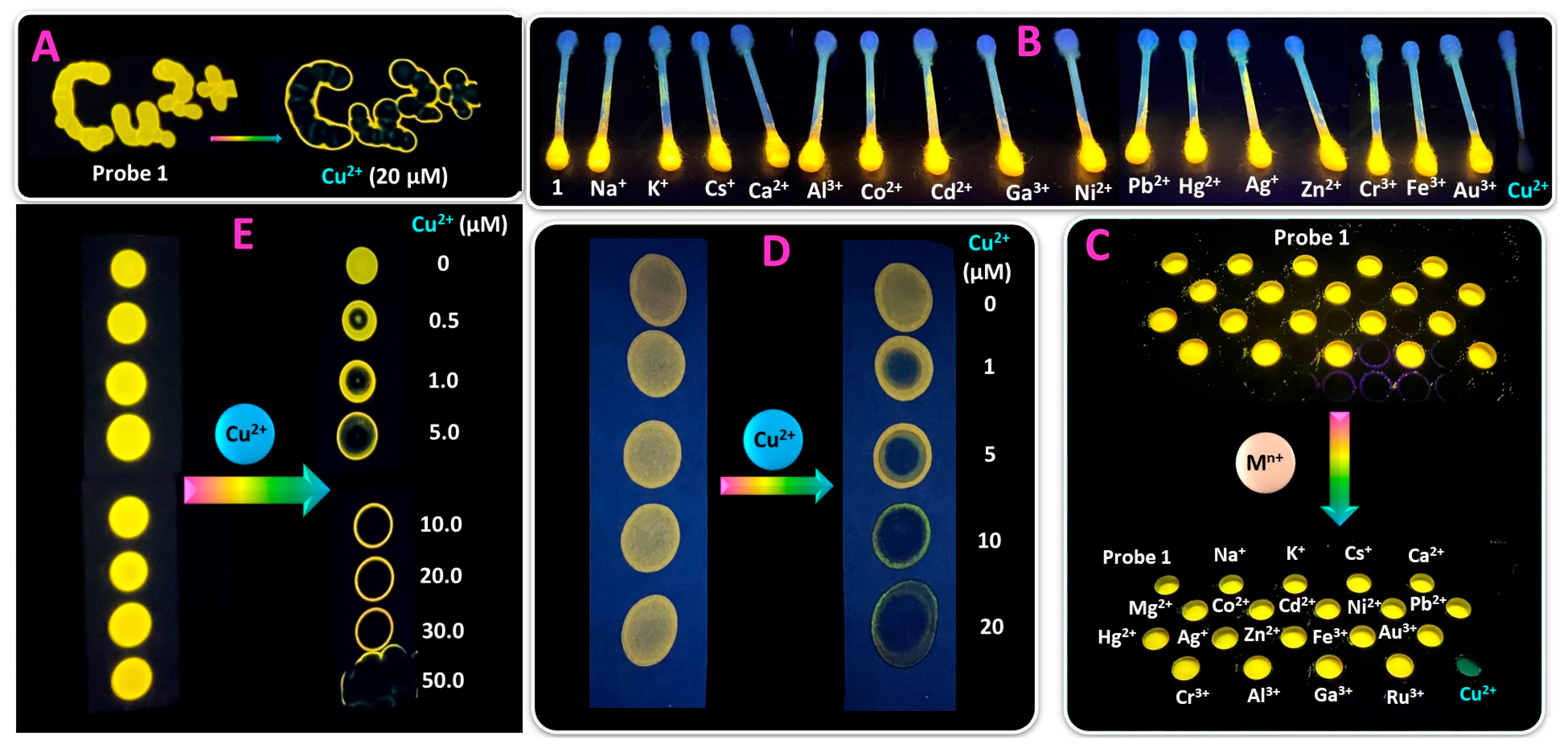
2.4. Practical Application
3. Materials and Methods
3.1. General Chemicals and Materials
3.2. Photo-Physical Studies: Parameters and Conditions
3.3. Theoretical Calculations
3.4. Synthesis of Probe 1
3.4.1. Tert-butyl 2-(1-amino-2,7-dioxo-2,7-dihydro-3H-cnaphtho[1,2,3-de]quinolin-3-yl)acetate (2)
3.4.2. Probe 1
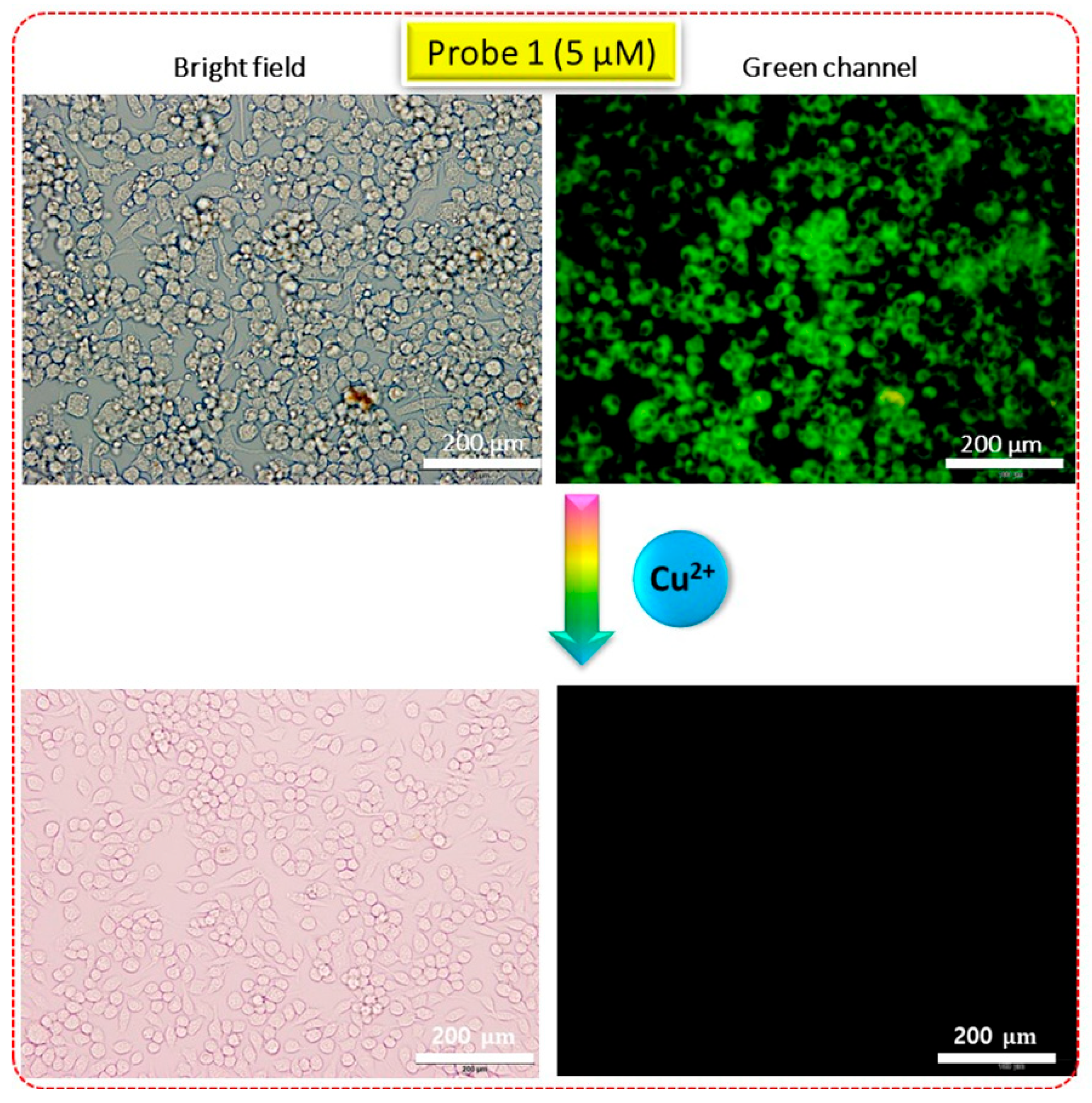
3.5. Live Cell Imaging
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Feng, X.; Wang, X.; Redshaw, C.; Tang, B.Z. Aggregation behaviour of pyrene-based luminescent materials, from molecular design and optical properties to application. Chem. Soc. Rev. 2023, 52, 6715–6753. [Google Scholar] [CrossRef]
- Chanda, K.; Balamurali, M.M. Light emitting probes—Approaches for interdisciplinary applications. Chem. Soc. Rev. 2021, 50, 3706–3719. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Zhang, M.; Jia, M. Optical sensor arrays for the detection and discrimination of natural products. Nat. Prod. Rep. 2023, 40, 628–645. [Google Scholar] [CrossRef]
- Park, S.-H.; Kwon, N.; Lee, J.-H.; Yoon, J.; Shin, I. Synthetic ratiometric fluorescent probes for detection of ions. Chem. Soc. Rev. 2020, 49, 143–179. [Google Scholar] [CrossRef]
- Chua, M.H.; Hui, B.Y.K.; Chin, K.L.O.; Zhu, Q.; Liu, X.; Xu, J. Recent advances in aggregation-induced emission (AIE)-based chemosensors for the detection of organic small molecules. Mater. Chem. Front. 2023, 7, 5561–5660. [Google Scholar] [CrossRef]
- Chyan, W.; Zhang, D.Y.; Lippard, S.J.; Radford, R.J. Reaction-based fluorescent sensor for investigating mobile Zn2+ in mitochondria of healthy versus cancerous prostate cells. Proc. Natl. Acad. Sci. USA 2014, 111, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Chen, L.; Lee, W.; Ko, G.; Yin, J.; Yoon, J. Recent progress in the development of organic dye based near-infrared fluorescence probes for metal ions. Coord. Chem. Rev. 2018, 354, 74–97. [Google Scholar] [CrossRef]
- Peng, S.; He, Q.; Vargas-Zúñiga, G.I.; Qin, L.; Hwang, I.; Kim, S.K.; Heo, N.J.; Lee, C.-H.; Dutta, R.; Sessler, J.L. Strapped calix[4]pyrroles: From syntheses to applications. Chem. Soc. Rev. 2020, 49, 865–907. [Google Scholar] [CrossRef]
- Berg, J.M.; Shi, Y. The galvanization of biology: A growing appreciation for the roles of zinc. Science 1996, 271, 1081–1085. [Google Scholar] [CrossRef]
- Shimizu, T.; Lengalova, A.; Martinek, V.; Martinkova, M. Heme: Emergent roles of heme in signal transduction, functional regulation and as catalytic centres. Chem. Soc. Rev. 2019, 48, 5624–5657. [Google Scholar] [CrossRef]
- Sarpong-Kumankomah, S.; Gibson, M.A.; Gailer, J. Organ damage by toxic metals is critically determined by the bloodstream. Coord. Chem. Rev. 2018, 374, 376–386. [Google Scholar] [CrossRef]
- Goodman, J.E.; Prueitt, R.L.; Dodge, D.G.; Thakali, S. Carcinogenicity assessment of water-soluble nickel compounds. Crit. Rev. Toxicol. 2009, 39, 365–417. [Google Scholar] [CrossRef] [PubMed]
- Carter, K.P.; Young, A.M.; Palmer, A.E. Fluorescent Sensors for Measuring Metal Ions in Living Systems. Chem. Rev. 2014, 114, 4564–4601. [Google Scholar] [CrossRef] [PubMed]
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy Metals Toxicity and the Environment. Mol. Clin. Environ. Toxicol. 2014, 2012, 133–164. [Google Scholar]
- Clarkson, T.W.; Magos, L. The Toxicology of Mercury and Its Chemical Compounds. Crit. Rev. Toxicol. 2006, 36, 609–662. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Chae, P.S.; Kumar, S. A dual-responsive anthrapyridone-triazole-based probe for selective detection of Ni2+ and Cu2+: A mimetic system for molecular logic gates based on color change. Dye. Pigment. 2020, 174, 108092. [Google Scholar] [CrossRef]
- Lippard, S.J.; Berg, J.M. Principles of Bioinorganic Chemistry; University Science Books: Mill Valley, CA, USA, 1994. [Google Scholar]
- Kumar, A.; Kumar, S.; Chae, P.S. A Chromo-Fluorogenic Naphthoquinolinedione-Based Probe for Dual Detection of Cu2+ and Its Use for Various Water Samples. Molecules 2022, 27, 785. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Vanita, V.; Walia, A.; Kumar, S. N,N-dimethylaminoethylaminoanthrone—A chromofluorogenic chemosensor for estimation of Cu2+ in aqueous medium and HeLa cells imaging. Sens. Actuators B 2013, 177, 904–912. [Google Scholar] [CrossRef]
- Arena, G.; Mendola, D.L.; Pappalardo, G.; Sóvágó, I.; Rizzarelli, E. Interactions of Cu2+ with prion family peptide fragments: Considerations on affinity, speciation and coordination. Coord. Chem. Rev. 2012, 256, 2202–2218. [Google Scholar] [CrossRef]
- Kardos, J.; Héja, L.; Simon, Á.; Jablonkai, I.; Kovács, R.; Jemnitz, K. Copper signalling: Causes and consequences. Cell Commun. Signal. 2018, 16, 1–22. [Google Scholar] [CrossRef]
- Zheng, W.; Monnot, A.D. Regulation of brain iron and copper homeostasis by brain barrier systems: Implication in neurodegenerative diseases. Pharmacol. Ther. 2012, 133, 177–188. [Google Scholar] [CrossRef]
- Squitti, R. Copper dysfunction in Alzheimer’s disease: From meta-analysis of biochemical studies to new insight into genetics. J. Trace Elem. Med. Biol. 2012, 26, 93–96. [Google Scholar] [CrossRef]
- Madsen, E.; Gitlin, J.D. Copper and Iron Disorders of the Brain. Annu. Rev. Neurosci. 2007, 30, 317. [Google Scholar] [CrossRef]
- Gaggelli, E.; Kozlowski, H.; Valensin, D.; Valensin, G. Copper Homeostasis and Neurodegenerative Disorders (Alzheimer’s, Prion, and Parkinson’s Diseases and Amyotrophic Lateral Sclerosis). Chem. Rev. 2006, 106, 1995. [Google Scholar] [CrossRef]
- Hordyjewska, A.; Popiołek, Ł.; Kocot, J. The many ”faces” of copper in medicine and treatment. Biometals 2014, 27, 611–621. [Google Scholar] [CrossRef] [PubMed]
- Lamichhane, J.R.; Osdaghi, E.; Behlau, F.; Jones, J.K.J.B.; Aubertot, J.-N. Thirteen decades of antimicrobial copper compounds applied in agriculture. A review. Agron. Sustain. Dev. 2018, 38, 1–18. [Google Scholar] [CrossRef]
- World Health Organization. Guidelines for Drinking Water Quality, 3rd ed.; World Health Organization: Geneva, Switzerland, 2004; p. 188. [Google Scholar]
- Prodi, L.; Bolletta, F.; Montalti, M.; Zaccheroni, N. Luminescent chemosensors for transition metal ions. Coord. Chem. Rev. 2000, 205, 59. [Google Scholar] [CrossRef]
- Kumar, R.; Bawa, R.; Gahlyan, P.; Dalela, M.; Jindal, K.; Jha, P.K.; Tomar, M.; Gupta, V. Pyrene appended bis-triazolylated 1,4-dihydropyridine as a selective fluorogenic sensor for Cu2+. Dye. Pigment. 2019, 161, 162–171. [Google Scholar] [CrossRef]
- Feng, Y.; Yang, Y.; Wang, Y.; Qiu, F.; Song, X.; Tang, X.; Zhang, G.; Liu, W. Dual-functional colorimetric fluorescent probe for sequential Cu2+ and S2− detection in bio-imaging. Sens. Actuators B Chem. 2019, 288, 27–37. [Google Scholar] [CrossRef]
- Kang, D.E.; Lim, C.S.; Kim, J.Y.; Kim, E.S.; Chun, H.J.; Cho, B.R. Two-Photon Probe for Cu2+ with an Internal Reference: Quantitative Estimation of Cu2+ in Human Tissues by Two-Photon Microscopy. Anal. Chem. 2014, 86, 5353–5359. [Google Scholar] [CrossRef]
- Zeng, S.; Liu, X.; Kafuti, Y.S.; Kim, H.; Wang, J.; Peng, X.; Li, H.; Yoon, J. Fluorescent dyes based on rhodamine derivatives for bioimaging and therapeutics: Recent progress, challenges, and prospects. Chem. Soc. Rev. 2023, 52, 5607–5651. [Google Scholar] [CrossRef]
- Huang, Z.; Yang, L.; Kong, L.; Yang, J.-X. Two-photon fluorescent detection of Cu2+ in live cells through ZnS-microhybrid constructed from interfacial coordination bridge of thiocyanate. Dye. Pigment. 2020, 172, 107831. [Google Scholar] [CrossRef]
- Kumar, A.; Vanita, V.; Walia, A.; Chae, P.S.; Kumar, S. Pyridoanthrone-based chromo-fluorogenic amphiphiles for selective CN− detection and their bioimaging application. Sens. Actuators B. Chem. 2020, 304, 127396. [Google Scholar] [CrossRef]
- Anbu, S.; Paul, A.; Surendranath, K.; Solaiman, N.S.; Pombeiro, A.J.L. A benzimidazole-based new fluorogenic differential/sequential chemosensor for Cu2+, Zn2+, CN−, P2O74−, DNA, its live-cell imaging and pyrosequencing applications. Sens. Actuators B 2021, 337, 129785. [Google Scholar] [CrossRef]
- Li, D.; Liu, A.; Xing, Y.; Li, Z.; Luo, Y.; Zhao, S.; Dong, L.; Xie, T.; Guo, K.; Li, J. A smart chemosensor with different response mechanisms to multi-analytes: Chromogenic and fluorogenic recognition of Cu2+, Fe3+, and Zn2+. Dye. Pigment. 2023, 213, 111180. [Google Scholar] [CrossRef]
- Kucukbasmaci, H.; Aydin, D. Optical and quantitative sensing capability of phenolphthalein derived Schiff base chromo-fluorogenic sensor for Cu2+. J. Photochem. Photobiol. A 2023, 437, 114460. [Google Scholar] [CrossRef]
- Kumar, A.; Chae, P.S. A bis(fluorenyl-triazole)-conjugated naphthoquinoline-dione probe for a cascade detection of Cu2+ and F− and its logic circuit with a memory unit. J. Photochem. Photobiol. A 2022, 431, 114048. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, S.; Chae, P.S. A novel anthrapyridone diamine-based probe for selective and distinctive Cu2+ and Hg2+ sensing in aqueous solution; utility as molecular logic gates. Dye. Pigment. 2020, 181, 108522. [Google Scholar] [CrossRef]
- Drisya, V.; Shurooque, K.S.; Das, S.; Chakkumkumarath, L. Fmoc-phenylalanine-based fluorimetric and colorimetric dual-channel probes for the detection of Fe3+, Cu2+ and F−. J. Photochem. Photobiol. A 2023, 442, 114796. [Google Scholar] [CrossRef]
- Sivaraman, G.; Iniya, M.; Anand, T.; Kotla, N.G.; Sunnapu, O.; Singaravadivel, S.; Gulyani, A.; Chellappa, D. Chemically diverse small molecule fluorescent chemosensors for copper ion. Coord. Chem. Rev. 2018, 357, 50–104. [Google Scholar] [CrossRef]
- Singh, G.; Mohit; Pawan; Sharma, S.; Devi, A.; Devi, S.; Yadav, R.; Sehgal, R.; Mohan, B. Click derived organosilane assembled with nano platform for the detection of Cu2+ ions: Biological evaluation and molecular docking approach. Spectrochim. Acta Part A 2023, 295, 122618. [Google Scholar] [CrossRef]
- Kumar, A.; Hur, W.; Seong, G.H.; Kumar, S.; Chae, P.S. Chromofluorogenic naphthoquinolinedione-based probes for sensitive detection and removal of Hg2+ in aqueous solutions. Dye. Pigment. 2022, 198, 110025. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B Condens. Matter 1988, 37, 785–789. [Google Scholar] [CrossRef]
- Schmidt, M.W.; Balbridge, K.K.; Boatz, J.A.; Elbert, S.T.; Gordon, M.S.; Jensen, J.H.; Koseki, S.; Matsunaga, N.; Nguyen, K.A.; Su, S.; et al. General atomic and molecular electronic structure system. J. Comput. Chem. 1993, 14, 1347–1363. [Google Scholar] [CrossRef]
- Huber, R.G.; Margreiter, M.A.; Fuchs, J.E.; Grafenstein, S.V.; Tautermann, C.S.; Liedl, K.R. Heteroaromatic π-Stacking Energy Landscapes. J. Chem. Inf. Model 2014, 54, 1371–1379. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, A.; Chae, P.S. A Naphthoquinoline-Dione-Based Cu2+ Sensing Probe with Visible Color Change and Fluorescence Quenching in an Aqueous Organic Solution. Molecules 2024, 29, 808. https://doi.org/10.3390/molecules29040808
Kumar A, Chae PS. A Naphthoquinoline-Dione-Based Cu2+ Sensing Probe with Visible Color Change and Fluorescence Quenching in an Aqueous Organic Solution. Molecules. 2024; 29(4):808. https://doi.org/10.3390/molecules29040808
Chicago/Turabian StyleKumar, Ashwani, and Pil Seok Chae. 2024. "A Naphthoquinoline-Dione-Based Cu2+ Sensing Probe with Visible Color Change and Fluorescence Quenching in an Aqueous Organic Solution" Molecules 29, no. 4: 808. https://doi.org/10.3390/molecules29040808
APA StyleKumar, A., & Chae, P. S. (2024). A Naphthoquinoline-Dione-Based Cu2+ Sensing Probe with Visible Color Change and Fluorescence Quenching in an Aqueous Organic Solution. Molecules, 29(4), 808. https://doi.org/10.3390/molecules29040808






