Alginate-Based Carriers Loaded with Mulberry (Morus alba L.) Leaf Extract: A Promising Strategy for Prolonging 1-Deoxynojirimicyn (DNJ) Systemic Activity for the Nutraceutical Management of Hyperglycemic Conditions
Abstract
1. Introduction
2. Results and Discussion
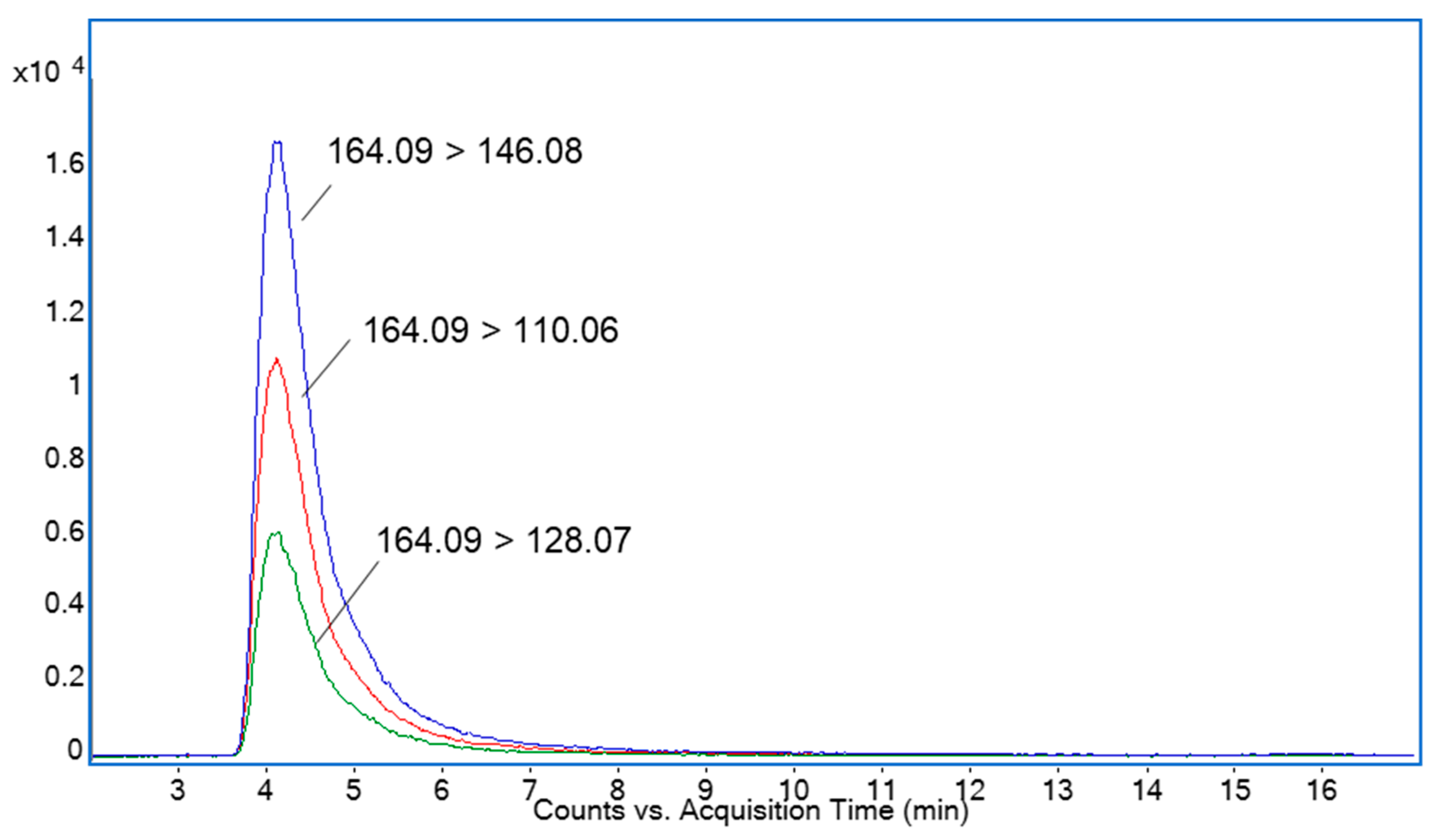
2.1. Determination of DNJ by HPLC-ESI-TQ-MS Analysis
2.2. Particle Size, Morphology, and DNJ Content
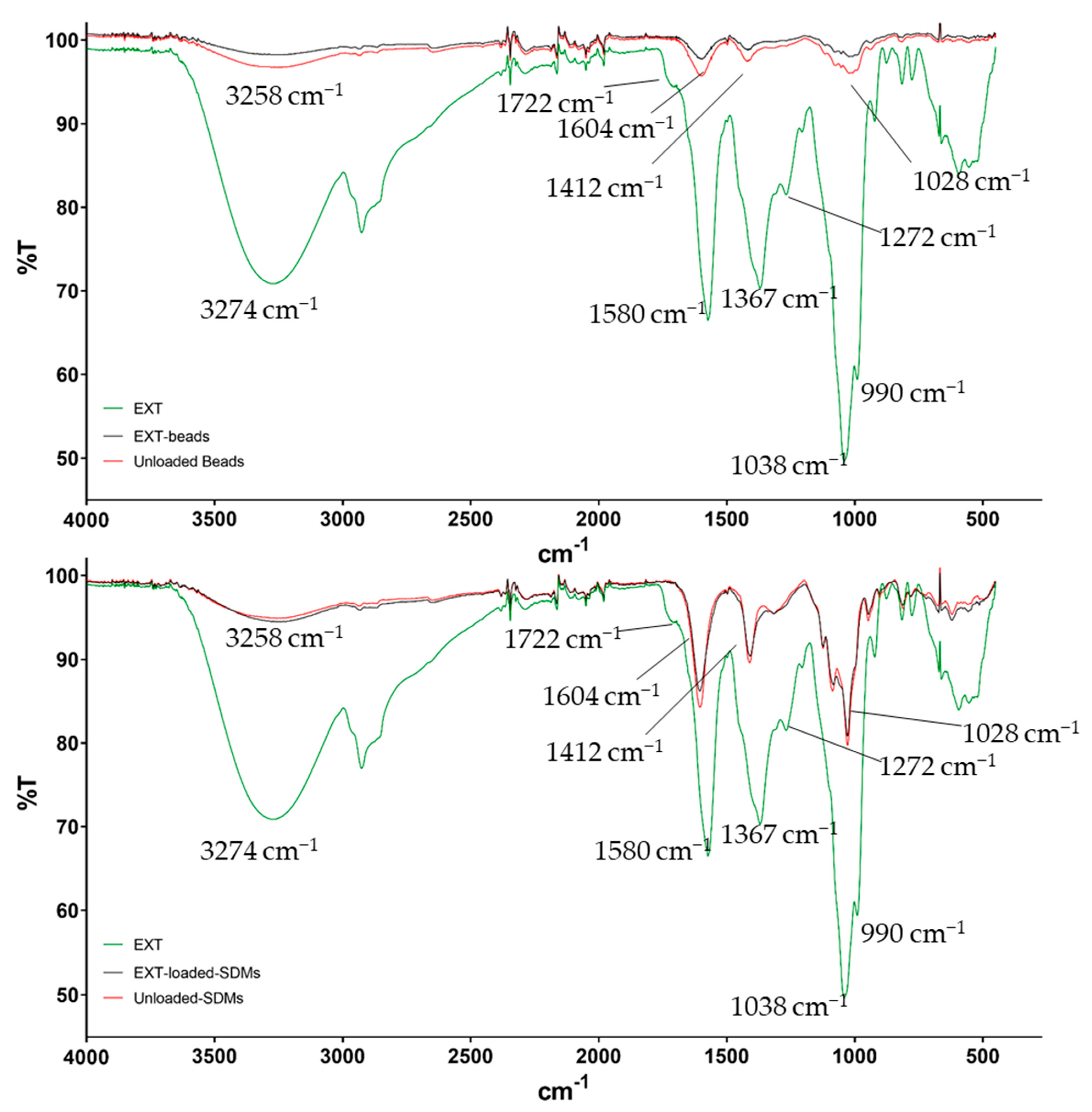
2.3. Fourier-Transform Infrared (FTIR) Spectroscopy
2.4. Interaction Study by NMR Relaxometry
- pf: fraction of the free ligand;
- pb: fraction of the associated ligand;
- Rf: relaxation rate in the free state;
- Rb: relaxation rate in the associated state.
2.5. Ca-ALG Bead Swelling Study
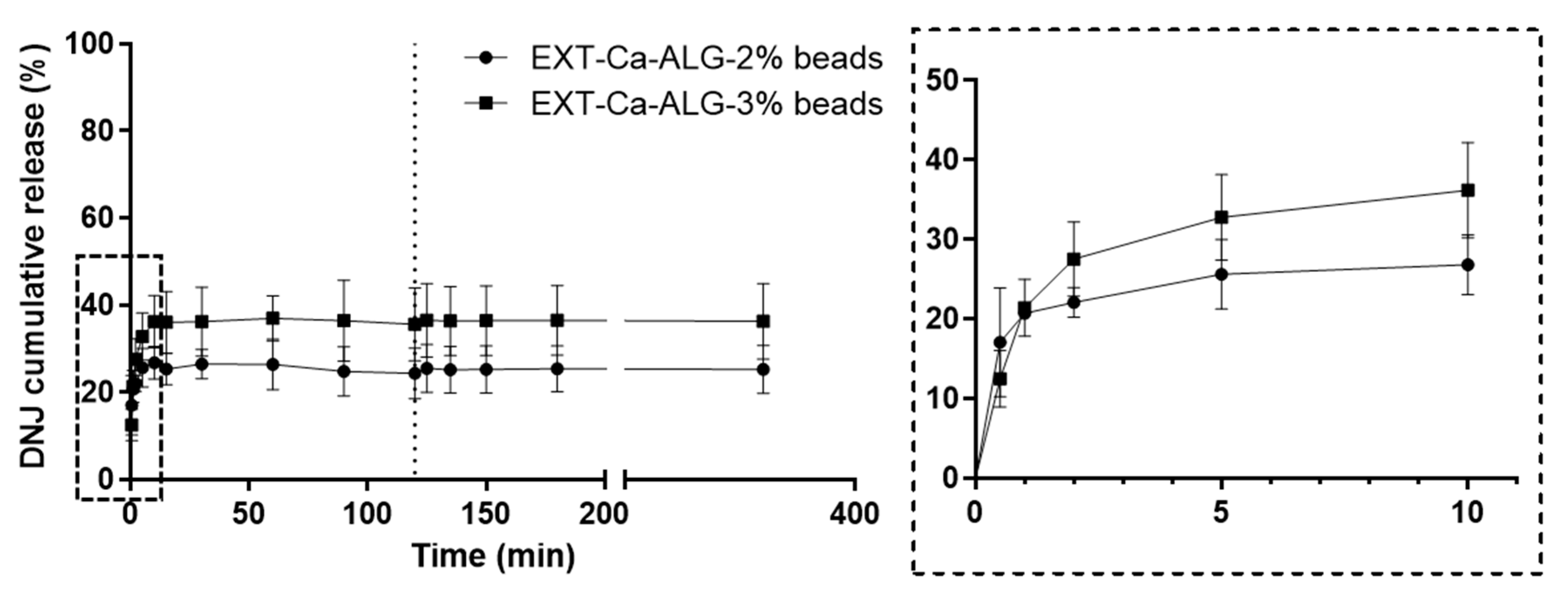
2.6. In Vitro Release of DNJ
3. Materials and Methods
3.1. Chemicals and Solvents
3.2. Plant Material and Extraction of Mulberry Leaves
3.3. Determination of DNJ by HPLC-ESI-TQ-MS Analysis
3.4. Preparation of Ca-ALG Beads
3.5. Preparation of ALG Microparticles by Spray-Drying (SDMs)
3.6. Particle Size Analysis of Ca-ALG Beads and SDMs
3.7. Morphological Analyses
3.8. DNJ Loading and Encapsulation Efficiency
3.9. Fourier-Transform Infrared (FTIR) Spectroscopy
3.10. Nuclear Magnetic Resonance (NMR) Relaxometry
3.11. Ca-ALG Bead Swelling Study
3.12. In Vitro Release of DNJ
3.13. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Global Report on Diabetes; World Health Organization: Geneva, Switzerland, 2016. [Google Scholar]
- Naveen, Y.P.; Urooj, A.; Byrappa, K. A Review on Medicinal Plants Evaluated for Anti-Diabetic Potential in Clinical Trials: Present Status and Future Perspective. J. Herb. Med. 2021, 28, 2210–8033. [Google Scholar] [CrossRef]
- Thakur, K.; Zhang, Y.Y.; Mocan, A.; Zhang, F.; Zhang, J.G.; Wei, Z.J. 1-Deoxynojirimycin, Its Potential for Management of Non-Communicable Metabolic Diseases. Trends Food Sci. Technol. 2019, 89, 88–99. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, J.; Chan, P. Treating Type 2 Diabetes Mellitus with Traditional Chinese and Indian Medicinal Herbs. Evid.-Based Complement. Altern. Med. 2013, 2013, 343594. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-G.; Ji, D.-F.; Zhong, S.; Lin, T.-B.; Lv, Z.-Q.; Hu, G.-Y.; Wang, X. 1-Deoxynojirimycin Inhibits Glucose Absorption and Accelerates Glucose Metabolism in Streptozotocin-Induced Diabetic Mice. Sci. Rep. 2013, 3, 1377. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, L.; Truzzi, E.; Frosi, I.; Papetti, A.; Cappellozza, S.; Saviane, A.; Pellati, F.; Bertelli, D. In Vitro Bioactivity Evaluation of Mulberry Leaf Extracts as Nutraceutical for the Management of Diabetes Mellitus. Food Funct. 2022, 13, 4344–4359. [Google Scholar] [CrossRef]
- Milella, L.; Milazzo, S.; De Leo, M.; Vera Saltos, M.B.; Faraone, I.; Tuccinardi, T.; Lapillo, M.; De Tommasi, N.; Braca, A. α-Glucosidase and α-Amylase Inhibitors from Arcytophyllum Thymifolium. J. Nat. Prod. 2016, 79, 2104–2112. [Google Scholar] [CrossRef] [PubMed]
- Yatsunami, K.; Saito, Y.; Fukuda, E.; Onodera, S.; Oshigane, K. α-Glucosidase Inhibitory Activity in Leaves of Some Mulberry Varieties. Food Sci. Technol. Res. 2003, 9, 392–394. [Google Scholar] [CrossRef]
- Gao, K.; Zheng, C.; Wang, T.; Zhao, H.; Wang, J.; Wang, Z.; Zhai, X.; Jia, Z.; Chen, J.; Zhou, Y.; et al. 1-Deoxynojirimycin: Occurrence, Extraction, Chemistry, Oral Pharmacokinetics, Biological Activities and in Silico Target Fishing. Molecules 2016, 21, 1600. [Google Scholar] [CrossRef]
- Bagheri, L.; Madadlou, A.; Yarmand, M.; Mousavi, M.E. Spray-Dried Alginate Microparticles Carrying Caffeine-Loaded and Potentially Bioactive Nanoparticles. Food Res. Int. 2014, 62, 1113–1119. [Google Scholar] [CrossRef]
- Kurozawa, L.E.; Hubinger, M.D. Hydrophilic Food Compounds Encapsulation by Ionic Gelation. Curr. Opin. Food Sci. 2017, 15, 50–55. [Google Scholar] [CrossRef]
- Sosnik, A. Alginate Particles as Platform for Drug Delivery by the Oral Route: State-of-the-Art. Int. Sch. Res. Not. 2014, 2014, 926157. [Google Scholar] [CrossRef]
- Uyen, N.T.T.; Hamid, Z.A.A.; Tram, N.X.T.; Ahmad, N. Fabrication of Alginate Microspheres for Drug Delivery: A Review. Int. J. Biol. Macromol. 2020, 153, 1035–1046. [Google Scholar] [CrossRef]
- Khorram, M.; Samimi, M.; Samimi, A.; Moghadam, H. Electrospray Preparation of Propranolol-Loaded Alginate Beads: Effect of Matrix Reinforcement on Loading and Release Profile. J. Appl. Polym. Sci. 2015, 132, 41334. [Google Scholar] [CrossRef]
- Østberg, T.; Vesterhus, L.; Graffner, C. Calcium Alginate Matrices for Oral Multiple Unit Administration: II. Effect of Process and Formulation Factors on Matrix Properties. Int. J. Pharm. 1993, 97, 183–193. [Google Scholar] [CrossRef]
- Acartürk, F.; Takka, S. Calcium Alginate Microparticles for Oral Administration: II. Effect of Formulation Factors on Drug Release and Drug Entrapment Efficiency. J. Microencapsul. 1999, 16, 291–301. [Google Scholar] [CrossRef] [PubMed]
- Vichasilp, C.; Nakagawa, K.; Sookwong, P.; Suzuki, Y.; Kimura, F.; Higuchi, O.; Miyazawa, T. Optimization of 1-Deoxynojirimycin Extraction from Mulberry Leaves by Using Response Surface Methodology. Biosci. Biotechnol. Biochem. 2009, 73, 2684–2689. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, L.; Saviane, A.; Paglia, G.; Pellati, F.; Benvenuti, S.; Bertelli, D.; Cappellozza, S. Determination of 1-Deoxynojirimycin (1-DNJ) in Leaves of Italian or Italy-Adapted Cultivars of Mulberry (Morus spp.) by HPLC-MS. Plants 2021, 10, 1553. [Google Scholar] [CrossRef]
- Nakagawa, K.; Ogawa, K.; Higuchi, O.; Kimura, T.; Miyazawa, T.; Hori, M. Determination of Iminosugars in Mulberry Leaves and Silkworms Using Hydrophilic Interaction Chromatography-Tandem Mass Spectrometry. Anal. Biochem. 2010, 404, 217–222. [Google Scholar] [CrossRef]
- Wang, S.B.; Chen, A.Z.; Weng, L.J.; Chen, M.Y.; Xie, X.L. Effect of Drug-Loading Methods on Drug Load, Encapsulation Efficiency and Release Properties of Alginate/Poly-L-Arginine/Chitosan Ternary Complex Microcapsules. Macromol. Biosci. 2004, 4, 27–30. [Google Scholar] [CrossRef]
- Vichasilp, C.; Nakagawa, K.; Sookwong, P.; Higuchi, O.; Kimura, F.; Miyazawa, T. A Novel Gelatin Crosslinking Method Retards Release of Mulberry 1-Deoxynojirimycin Providing a Prolonged Hypoglycaemic Effect. Food Chem. 2012, 134, 1823–1830. [Google Scholar] [CrossRef]
- Essifi, K.; Lakrat, M.; Berraaouan, D.; Fauconnier, M.L.; El Bachiri, A.; Tahani, A. Optimization of Gallic Acid Encapsulation in Calcium Alginate Microbeads Using Box-Behnken Experimental Design. Polym. Bull. 2020, 78, 5789–5814. [Google Scholar] [CrossRef]
- Essifi, K.; Brahmi, M.; Berraaouan, D.; Ed-Daoui, A.; El Bachiri, A.; Fauconnier, M.L.; Tahani, A. Influence of Sodium Alginate Concentration on Microcapsules Properties Foreseeing the Protection and Controlled Release of Bioactive Substances. J. Chem. 2021, 2021, 5531479. [Google Scholar] [CrossRef]
- Sosnik, A.; Seremeta, K.P. Advantages and Challenges of the Spray-Drying Technology for the Production of Pure Drug Particles and Drug-Loaded Polymeric Carriers. Adv. Colloid Interface Sci. 2015, 223, 40–54. [Google Scholar] [CrossRef]
- Esposito, T.; Mencherini, T.; Del Gaudio, P.; Auriemma, G.; Franceschelli, S.; Picerno, P.; Aquino, R.P.; Sansone, F. Design and Development of Spray-Dried Microsystems to Improve Technological and Functional Properties of Bioactive Compounds from Hazelnut Shells. Molecules 2020, 25, 1273. [Google Scholar] [CrossRef]
- Lourenço, S.C.; Moldão-Martins, M.; Alves, V.D. Microencapsulation of Pineapple Peel Extract by Spray Drying Using Maltodextrin, Inulin, and Arabic Gum as Wall Matrices. Foods 2020, 9, 718. [Google Scholar] [CrossRef]
- Ameri, M.; Maa, Y.F. Spray Drying of Biopharmaceuticals: Stability and Process Considerations. Dry. Technol. 2007, 24, 763–768. [Google Scholar] [CrossRef]
- Piñón-Balderrama, C.I.; Leyva-Porras, C.; Terán-Figueroa, Y.; Espinosa-Solís, V.; Álvarez-Salas, C.; Saavedra-Leos, M.Z. Encapsulation of Active Ingredients in Food Industry by Spray-Drying and Nano Spray-Drying Technologies. Processes 2020, 8, 889. [Google Scholar] [CrossRef]
- Coates, J. Interpretation of Infrared Spectra, A Practical Approach. In Encyclopedia of Analytical Chemistry; John Wiley & Sons Ltd.: Chichester, UK, 2006; Volume 1, pp. 10815–10837. [Google Scholar] [CrossRef]
- Das, D.; Ghosh, R.; Mandal, P. Biogenic Synthesis of Silver Nanoparticles Using S1 Genotype of Morus Alba Leaf Extract: Characterization, Antimicrobial and Antioxidant Potential Assessment. SN Appl. Sci. 2019, 1, 1–16. [Google Scholar] [CrossRef]
- Daemi, H.; Barikani, M. Synthesis and Characterization of Calcium Alginate Nanoparticles, Sodium Homopolymannuronate Salt and Its Calcium Nanoparticles. Sci. Iran. 2012, 19, 2023–2028. [Google Scholar] [CrossRef]
- Di Cocco, M.E.; Bianchetti, C.; Chiellini, F. 1H NMR Studies of Alginate Interactions with Amino Acids. J. Bioact. Compat. Polym. 2016, 18, 283–296. [Google Scholar] [CrossRef]
- Mic, M.; Pîrnău, A.; Floare, C.G.; Bogdan, M. Study of the Binding Affinity between Imatinib and α-1 Glycoprotein Using Nuclear Spin Relaxation and Isothermal Titration Calorimetry. Int. J. Biol. Macromol. 2020, 147, 326–332. [Google Scholar] [CrossRef] [PubMed]
- Jaski, J.M.; Barão, C.E.; Morais Lião, L.; da Silva Pinto, V.; Zanoelo, E.F.; Cardozo-Filho, L. β-Cyclodextrin Complexation of Extracts of Olive Leaves Obtained by Pressurized Liquid Extraction. Ind. Crops Prod. 2019, 129, 662–672. [Google Scholar] [CrossRef]
- Fielding, L. Determination of Association Constants (Ka) from Solution NMR Data. Tetrahedron 2000, 56, 6151–6170. [Google Scholar] [CrossRef]
- Oku, K.; Watanabe, H.; Kubota, M.; Fukuda, S.; Kurimoto, M.; Tsujisaka, Y.; Komori, M.; Inoue, Y.; Sakurai, M. NMR and Quantum Chemical Study on the OH⋯π and CH⋯O Interactions between Trehalose and Unsaturated Fatty Acids: Implication for the Mechanism of Antioxidant Function of Trehalose. J. Am. Chem. Soc. 2003, 125, 12739–12748. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, A.S. Hydrogels for Biomedical Applications. Adv. Drug. Deliv. Rev. 2002, 54, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Bajpai, S.K.; Sharma, S. Investigation of Swelling/Degradation Behaviour of Alginate Beads Crosslinked with Ca2+ and Ba2+ Ions. React. Funct. Polym. 2004, 59, 129–140. [Google Scholar] [CrossRef]
- Basu, S.; Mandal, S.; Senthil Kumar, S.; Krishnamoorthy, B.; Kumar Basu, S. Development and Evaluation of Calcium Alginate Beads Prepared by Sequential and Simultaneous Methods. Artic. Braz. J. Pharm. Sci. 2010, 46, 785–793. [Google Scholar]
- Aldawsari, M.F.; Ahmed, M.M.; Fatima, F.; Anwer, M.K.; Katakam, P.; Khan, A. Development and Characterization of Calcium-Alginate Beads of Apigenin: In Vitro Antitumor, Antibacterial, and Antioxidant Activities. Mar. Drugs 2021, 19, 467. [Google Scholar] [CrossRef]
- Pillay, V.; Dangor, C.M.; Govender, T.; Moopanar, K.R.; Hurbans, N. Drug Release Modulation from Cross-Linked Calcium Alginate Microdiscs, 1: Evaluation of the Concentration Dependency of Sodium Alginate on Drug Entrapment Capacity, Morphology, and Dissolution Rate. Drug Deliv. 1998, 5, 25–34. [Google Scholar] [CrossRef]
- Li, Q.; Li, X.; Zhao, C. Strategies to Obtain Encapsulation and Controlled Release of Small Hydrophilic Molecules. Front. Bioeng. Biotechnol. 2020, 8, 437. [Google Scholar] [CrossRef]
- Tønnesen, H.H.; Karlsen, J. Alginate in Drug Delivery Systems. Drug. Dev. Ind. Pharm. 2002, 28, 621–630. [Google Scholar] [CrossRef]
- Hussein, N.; Omer, H.; Ismael, A.; Albed Alhnan, M.; Elhissi, A.; Ahmed, W. Spray-Dried Alginate Microparticles for Potential Intranasal Delivery of Ropinirole Hydrochloride: Development, Characterization and Histopathological Evaluation. Pharm. Dev. Technol. 2020, 25, 290–299. [Google Scholar] [CrossRef]
- Chuang, J.J.; Huang, Y.Y.; Lo, S.H.; Hsu, T.F.; Huang, W.Y.; Huang, S.L.; Lin, Y.S. Effects of PH on the Shape of Alginate Particles and Its Release Behavior. Int. J. Polym. Sci. 2017, 2017, 3902704. [Google Scholar] [CrossRef]
- Gavini, E.; Rassu, G.; Sanna, V.; Cossu, M.; Giunchedi, P. Mucoadhesive Microspheres for Nasal Administration of an Antiemetic Drug, Metoclopramide: In-Vitro/Ex-Vivo Studies. J. Pharm. Pharmacol. 2005, 57, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Iannuccelli, V.; Coppi, G.; Bondi, M.; Pinelli, M.; Mingione, A.; Cameroni, R. Biodegradable Intraoperative System for Bone Infection Treatment II. In Vivo Evaluation. Int. J. Pharm. 1996, 143, 187–194. [Google Scholar] [CrossRef]
- Kock, F.V.C.; Monaretto, T.; Colnago, L.A. Time-Domain NMR Relaxometry as an Alternative Method for Analysis of Chitosan-Paramagnetic Ion Interactions in Solution. Int. J. Biol. Macromol. 2017, 98, 228–232. [Google Scholar] [CrossRef]
- Serai, S.D. Basics of Magnetic Resonance Imaging and Quantitative Parameters T1, T2, T2*, T1rho and Diffusion-Weighted Imaging. Pediatr. Radiol. 2022, 52, 217–227. [Google Scholar] [CrossRef]
- Fieber, W.; Herrmann, A.; Ouali, L.; Velazco, M.I.; Kreutzer, G.; Klok, H.A.; Ternat, C.; Plummer, C.J.G.; Manson, J.A.E.; Sommer, H. NMR Diffusion and Relaxation Studies of the Encapsulation of Fragrances by Amphiphilic Multiarm Star Block Copolymers. Macromolecules 2007, 40, 5372–5378. [Google Scholar] [CrossRef]
- Pasparakis, G.; Bouropoulos, N. Swelling Studies and in Vitro Release of Verapamil from Calcium Alginate and Calcium Alginate–Chitosan Beads. Int. J. Pharm. 2006, 323, 34–42. [Google Scholar] [CrossRef]
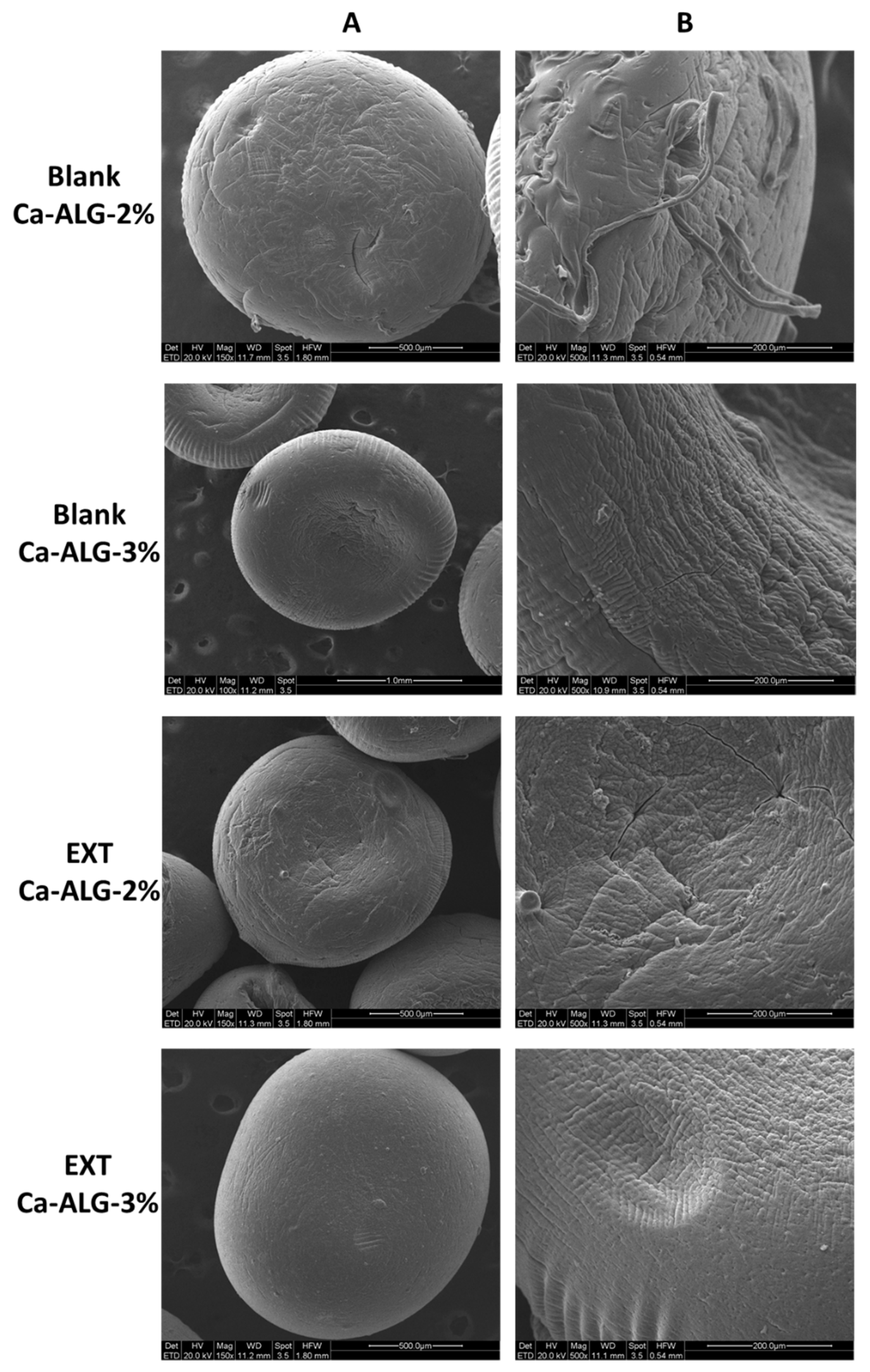



| Formulation | Size | Distribution Width (SPAN Value) | Yield (%) | EE (%) | DL (mg/mg) | |
|---|---|---|---|---|---|---|
| Blank Ca-ALG-2% beads | 1.30 ± 0.19 mm | 0.40 ± 0.02 | 72 ± 2 | - | - | |
| EXT-Ca-ALG-2% beads | 1.10 ± 0.16 mm | 0.35 ± 0.03 | 76.3 ± 1.8 | 54.0 ± 0.4 | 0.52 ± 0.02 | |
| Blank Ca-ALG-3% beads | 1.55 ± 0.15 mm | 0.27 ± 0.01 | 88 ± 3 | - | - | |
| EXT-Ca-ALG-3% beads | 1.5 ± 0.2 mm | 0.34 ± 0.07 | 91.6 ± 1.1 | 55.2 ± 0.3 | 0.43 ± 0.04 | |
| Blank SDMs | 11 ± 6 μm | 3.73 ± 0.15 | 65 ± 3 | - | - | |
| EXT-SDMs | 80% | 11 ± 5 μm | 7.8 ± 1.6 | 66.70 ± 2.51 | 99 ± 3 | 0.63 ± 0.02 |
| 20% | 53 ± 11 μm | |||||
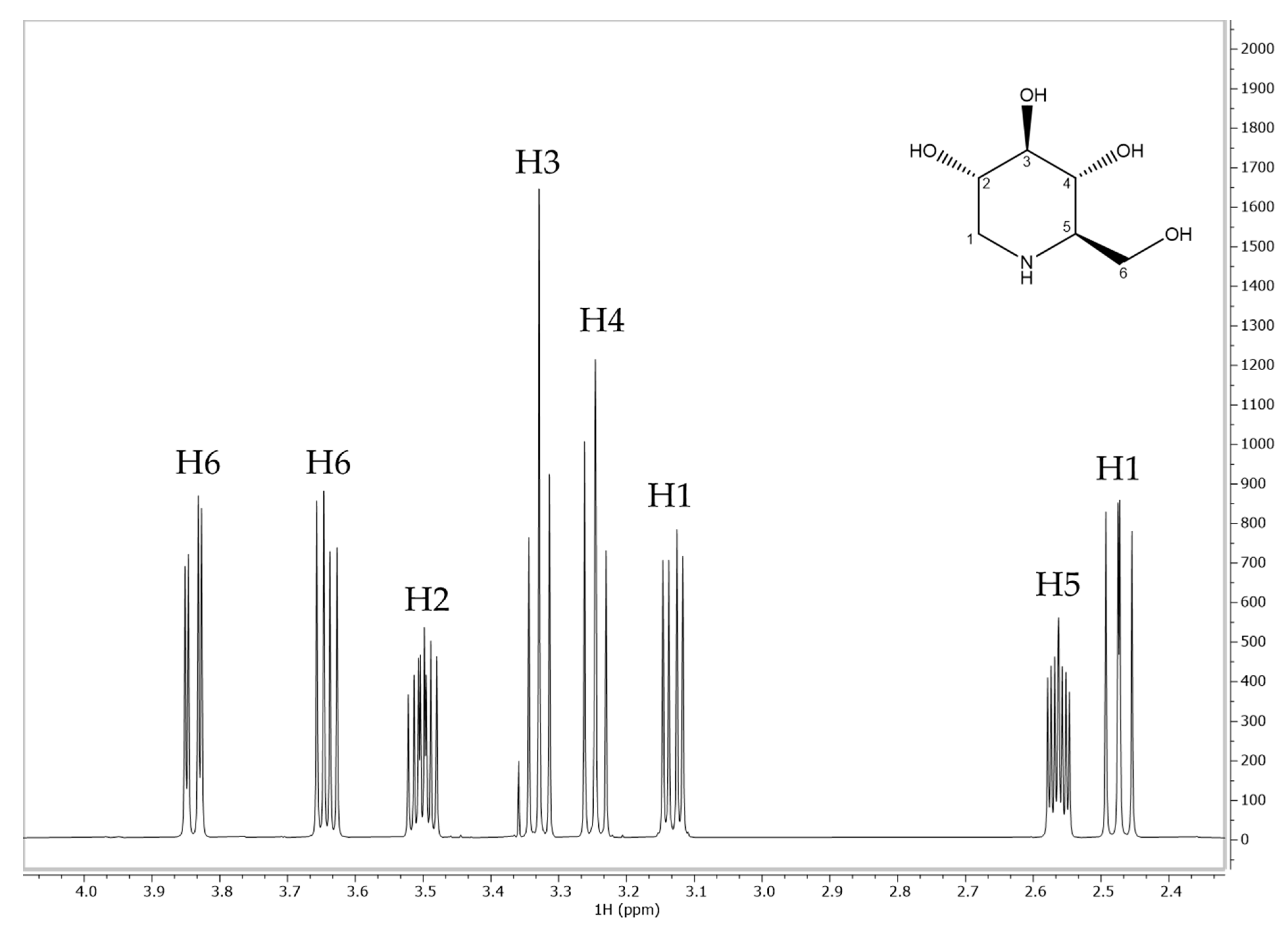
| DNJ Assignment | δ (ppm) | Multiplicity | J (Hz) |
|---|---|---|---|
| H1 | 2.47 | dd | 10.80, 12.50 |
| H1 | 3.13 | dd | 5.05, 12.36 |
| H2 | 3.50 | m | ₋ |
| H3 | 3.33 | t | 9.14 |
| H4 | 3.24 | t | 9.50 |
| H5 | 2.56 | m | ₋ |
| H6 | 3.64 | dd | 6.23, 11.64 |
| H6 | 3.84 | dd | 2.90, 11.60 |
| DNJ | T1ρ DNJ (s) | T1ρ DNJ + ALG (s) | Δ(s) | Δ% |
|---|---|---|---|---|
| H1 | 0.535 | 0.440 | −0.095 | −17.76 |
| H1 | 0.663 | 0.530 | −0.133 | −20.06 |
| H2 | 1.760 | 1.390 | −0.370 | −21.02 |
| H3 | 1.810 | 1.430 | −0.380 | −20.99 |
| H4 | 1.801 | 1.401 | −0.400 | −22.21 |
| H5 | 0.963 | 0.640 | −0.323 | −33.54 |
| H6 | 0.679 | 0.548 | −0.131 | −19.29 |
| H6 | 0.680 | 0.517 | −0.163 | −23.97 |
| DNJ (mM) | T1 H1 2.47 ppm (s) | T1 H1 3.13 ppm (s) | T1 H2 3.50 ppm (s) | T1 H3 3.33 ppm (s) | T1 H4 3.24 ppm (s) | T1 H5 2.56 ppm (s) | T1 H6 3.64 ppm (s) | T1 H6 3.84 ppm (s) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DNJ | DNJ + ALG | DNJ | DNJ + ALG | DNJ | DNJ + ALG | DNJ | DNJ + ALG | DNJ | DNJ + ALG | DNJ | DNJ + ALG | DNJ | DNJ + ALG | DNJ | DNJ + ALG | |
| 4 | 0.671 | 0.545 | 0.781 | 0.597 | 2.422 | 1.438 | 2.505 | 1.934 | 0.818 | 0.523 | 1.468 | 1.048 | 0.827 | 0.618 | 2.542 | 1.742 |
| 5 | 0.670 | 0.538 | 0.784 | 0.560 | 2.382 | 1.346 | 2.501 | 1.837 | 0.817 | 0.523 | 1.469 | 0.963 | 0.820 | 0.592 | 2.533 | 1.569 |
| 10 | 0.662 | 0.559 | 0.771 | 0.604 | 2.337 | 1.532 | 2.440 | 2.036 | 0.806 | 0.579 | 1.432 | 1.061 | 0.808 | 0.630 | 2.463 | 1.810 |
| 15 | 0.666 | 0.580 | 0.774 | 0.607 | 2.347 | 1.607 | 2.459 | 2.075 | 0.811 | 0.597 | 1.435 | 1.071 | 0.814 | 0.637 | 2.468 | 1.861 |
| 20 | 0.661 | 0.584 | 0.770 | 0.605 | 2.324 | 1.739 | 2.431 | 2.124 | 0.806 | 0.616 | 1.422 | 1.080 | 0.806 | 0.650 | 2.440 | 1.893 |
| 25 | 0.654 | 0.584 | 0.760 | 0.637 | 2.212 | 1.780 | 2.323 | 2.090 | 0.794 | 0.669 | 1.403 | 1.070 | 0.787 | 0.695 | 2.386 | 1.860 |
| DNJ (mM) | 1/ΔR (s−1) | |||||||
|---|---|---|---|---|---|---|---|---|
| H1 2.47 ppm | H1 3.13 ppm | H2 3.50 ppm | H3 3.33 ppm | H4 3.24 ppm | H5 2.56 ppm | H6 3.64 ppm | H6 3.84 ppm | |
| 4 | 2.900 | 2.534 | 3.539 | 8.485 | 1.450 | 3.663 | 2.445 | 5.535 |
| 5 | 2.730 | 1.960 | 3.095 | 6.919 | 1.453 | 2.796 | 2.129 | 4.123 |
| 10 | 3.600 | 2.789 | 4.448 | 12.297 | 2.056 | 4.095 | 2.860 | 6.827 |
| 15 | 4.492 | 2.813 | 5.097 | 13.288 | 2.262 | 4.222 | 2.929 | 7.567 |
| 20 | 5.040 | 2.823 | 6.900 | 16.819 | 2.613 | 4.491 | 3.358 | 8.444 |
| 25 | 5.420 | 3.936 | 9.114 | 20.837 | 4.260 | 4.508 | 5.945 | 8.437 |
| −1/Ka | −17.646 | −28.406 | −7.098 | −8.365 | −6.609 | −49.466 | −9.198 | −23.948 |
| Ka | 57 | 35 | 141 | 120 | 151 | 20 | 109 | 42 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marchetti, L.; Truzzi, E.; Rossi, M.C.; Benvenuti, S.; Cappellozza, S.; Saviane, A.; Bogataj, L.; Siligardi, C.; Bertelli, D. Alginate-Based Carriers Loaded with Mulberry (Morus alba L.) Leaf Extract: A Promising Strategy for Prolonging 1-Deoxynojirimicyn (DNJ) Systemic Activity for the Nutraceutical Management of Hyperglycemic Conditions. Molecules 2024, 29, 797. https://doi.org/10.3390/molecules29040797
Marchetti L, Truzzi E, Rossi MC, Benvenuti S, Cappellozza S, Saviane A, Bogataj L, Siligardi C, Bertelli D. Alginate-Based Carriers Loaded with Mulberry (Morus alba L.) Leaf Extract: A Promising Strategy for Prolonging 1-Deoxynojirimicyn (DNJ) Systemic Activity for the Nutraceutical Management of Hyperglycemic Conditions. Molecules. 2024; 29(4):797. https://doi.org/10.3390/molecules29040797
Chicago/Turabian StyleMarchetti, Lucia, Eleonora Truzzi, Maria Cecilia Rossi, Stefania Benvenuti, Silvia Cappellozza, Alessio Saviane, Luca Bogataj, Cristina Siligardi, and Davide Bertelli. 2024. "Alginate-Based Carriers Loaded with Mulberry (Morus alba L.) Leaf Extract: A Promising Strategy for Prolonging 1-Deoxynojirimicyn (DNJ) Systemic Activity for the Nutraceutical Management of Hyperglycemic Conditions" Molecules 29, no. 4: 797. https://doi.org/10.3390/molecules29040797
APA StyleMarchetti, L., Truzzi, E., Rossi, M. C., Benvenuti, S., Cappellozza, S., Saviane, A., Bogataj, L., Siligardi, C., & Bertelli, D. (2024). Alginate-Based Carriers Loaded with Mulberry (Morus alba L.) Leaf Extract: A Promising Strategy for Prolonging 1-Deoxynojirimicyn (DNJ) Systemic Activity for the Nutraceutical Management of Hyperglycemic Conditions. Molecules, 29(4), 797. https://doi.org/10.3390/molecules29040797